Abstract
The vsa genes of Mycoplasma pulmonis encode the V-1 lipoproteins. Most V-1 proteins contain repetitive domains and are thought to be involved in mycoplasma-host cell interactions. Previously, we have reported the isolation and characterization of six vsa genes comprising a 10-kb region of the genome of M. pulmonis strain KD735-15. In the current study, vsa-specific probes were used to clone several fragments from a genomic library of KD735-15 DNA and assemble a single 20-kb contig containing 11 vsa genes. The middle region of the vsa locus contains a large open reading frame (ORF) that is not a vsa gene and has undergone an internal deletion in some strains. The ORF is predicted to encode a membrane protein that may have a role in disease pathogenesis. To examine vsa genes in a strain of M. pulmonis that is unrelated to KD735-15, strain CT was studied. Through Southern hybridization and genomic cloning analyses, CT was found to possess homologs of the KD735-15 vsaA, -C, -E, and -F genes and two unique genes (vsaG and vsaH) that were not found in KD735-15. High-frequency, site-specific DNA inversions serve to regulate the phase-variable production of individual V-1 proteins. As a result of the sequence analysis of vsa recombination products, a model in which DNA inversion arises from strand exchange involving at least six nucleotides of the vrs box is proposed.
Mycoplasmas cause slowly progressive, chronic diseases in human and animals. The mechanisms of mycoplasmal disease pathogenesis are poorly understood, and there are no effective control measures. Mycoplasma pulmonis is the etiologic agent of murine respiratory mycoplasmosis and can also cause genital disease and arthritis in rats and mice (31). Thus, M. pulmonis can colonize a variety of epithelial surfaces. Rat isolates of M. pulmonis such as strains UAB 5782 and UAB 6510 are generally more virulent in rats than in mice (10, 11, 24). In the mouse, UAB 5782 and UAB 6510 colonize the respiratory tract without usually causing lesions (10). In contrast, the mouse isolate strain CT causes severe respiratory disease in the mouse (6, 7, 10, 12). Mycoplasma factors that contribute to the host specificity of disease are unknown. A comparison of the proteins produced by 18 strains of M. pulmonis revealed mostly conserved proteins that were invariant among strains (38). An exception was the V-1 family of surface proteins that are encoded by the vsa (variable surface antigen) genes (4, 21, 33, 35, 39). Variation in the V-1 proteins may contribute to the host specificity of the mycoplasma and to the chronicity and severity of disease.
The chronic nature of mycoplasmal diseases indicates that mycoplasmas can adapt to the rapidly changing conditions in the host. Previous studies had shown that phenotypic variation and genetic recombination occur at high frequencies in M. pulmonis (3). The vsa genes comprise one of the highly recombinogenic loci in this species. Recombination between vsa genes involves site-specific DNA inversions occurring at a 34-bp sequence that defines the vsa recombination site (vrs box) and results in on-off switching of the particular vsa gene that is associated with the vsa expression site (4). The gene that is located in the vsa expression site is transcribed and translated, but all other vsa genes are transcriptionally silent and lack the vsa promoter, ribosome binding site, and first 714 nucleotides of the vsa coding region. The silent vsa genes contain the vrs box at their 5′ end and can become expressed by site-specific recombination (DNA inversion) with the vrs box located at the expression site.
To identify differences in the vsa gene repertoire among rat and mouse isolates of M. pulmonis, the current study describes a comparison of the vsa loci of strains CT and KD735-15, a derivative of UAB 6510 (3, 4). Eleven vsa genes including vsaA, -B, -C, -D, -E, and -F were identified in a 20-kb region of KD735-15. The vsa genes vsaA, -C, -E, -F, -G, and -H were identified in CT. Differences in the vsa repertoire (vsaB and vsaD are absent in CT whereas vsaG and vsaH are absent in KD735-15) may be significant in influencing the pathogenic specificity of the mycoplasma. From a PCR analysis of vrs box-mediated DNA recombination products from KD735-15 and CT, it is concluded that all vsa genes are capable of combining with the vsa expression site and therefore should be functional. A 6-bp sequence within the vrs box is identified as central to the recombination event, and a model for the mechanism of vrs box-mediated DNA inversion is proposed. A comparison of the nucleotide sequences of the vsa locus from a lineage of strains derived from a common ancestor revealed a deletion that may be associated with loss of virulence. The deletion occurred not in a vsa gene but in an open reading frame (ORF) that is embedded within the vsa locus and predicted to encode a membrane protein.
MATERIALS AND METHODS
Strains of M. pulmonis.
Strain UAB 5782 was isolated from the lung of a rat with natural murine respiratory mycoplasmosis (40). Strain UAB 6510 (8) was isolated from the lung of a rat that had been infected with strain UAB 5782. Two stocks of strain UAB 6510 were examined in the current study. The stock designated 6510C is of unknown virulence and was obtained in 1984 from the laboratory of G. H. Cassell. 6510C has remained unpassaged since 1984, but its passage history prior to 1984 is unknown. The other stock of UAB 6510, designated 6510-vir, is highly virulent in rats (32) and was obtained from J. W. Simecka. Strain KD735-15 was derived from 6510C by serial subcloning as described previously (3). Strain X1048 was derived from UAB 6510 by passage through a Lewis rat and is an established rat pathogen (5). Strain CT was isolated from mouse lung and is virulent in mice (10). Mycoplasmas were propagated in mycoplasma broth medium consisting of 2.1% pleuropneumonia like organism broth without crystal violet (Difco Laboratories, Detroit, Mich.) supplemented with 20% whole horse serum (Gibco BRL Life Technologies, Grand Island, N.Y.), 0.5% IsoVitaleX (VWR Scientific Products), 0.02% degraded free-acid DNA (Sigma), 100 μg of ampicillin per ml, and 0.5% glucose.
Library constructions.
Genomic libraries of KD735-15 and CT DNA were constructed using the lambda ZAP II expression vector (Stratagene, La Jolla, Calif.). Genomic DNA was isolated as described previously (14) and partially digested with AluI. Fragments ranging from 2 to 9 kb were purified from an agarose gel and modified by incubation with EcoRI DNA methyltransferase to protect mycoplasmal DNA sequences from cleavage by the EcoRI restriction enzyme. EcoRI linkers were attached to the DNA fragments, and cohesive ends were generated by digestion with EcoRI. The DNA fragments were ligated to the ZAP II vector, and the ligation mixture was packaged in vitro to produce viable phage particles by using the Gigapack III Gold packaging extract according to the directions of the manufacturer (Stratagene). The resulting phage libraries (one library of KD735-15 DNA and another library of CT DNA) were amplified on lawns of Escherichia coli XL1-Blue MRF′. To generate plasmid libraries from the phage libraries, pBluescript SK(−) phagemids were excised according to Stratagene's instructions. E. coli colonies containing the plasmid libraries were scraped from agar plates and stored at −80°C in Luria-Bertani medium supplemented with 10% glycerol. The average insert size of the KD735-15 and CT DNA libraries was determined to be 3.8 and 3.5 kb, respectively.
Cloning of KD735-15 vsa gene.
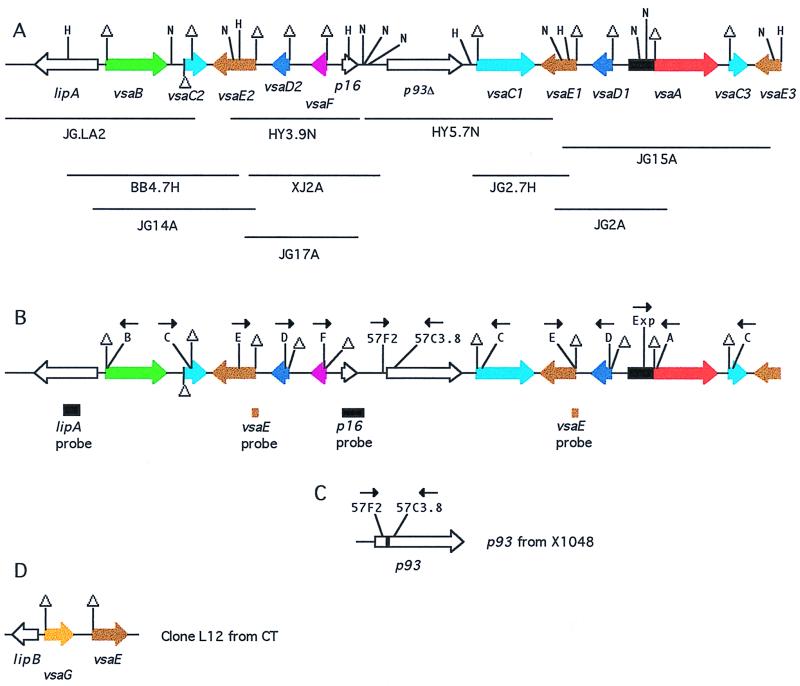
Several strategies were used to clone KD735-15 DNA fragments containing vsa genes (Fig. 1A). The binding sites of several DNA fragments and oligonucleotide primers that were used as probes for cloning are provided in Fig. 1B and Table 1. The clone BB4.7H contains the previously described plasmid pIR49 that was obtained by cloning a 4.7-kb HindIII fragment from KD735-15 that was originally detected as a restriction fragment polymorphism that was present in KD735-15 DNA but absent in DNA from the sibling strain KD735-16 (3). The absence of this 4.7-kb fragment in KD735-15 DNA was subsequently shown to be the result of an example of vrs box-mediated DNA inversion that had occurred in the KD735-16 lineage (4). The clone JG2.7H was obtained by excising from an agarose gel the previously described 2.7-kb HindIII fragment that was originally identified by Southern hybridization using pIR49 as the probe (3). This HindIII fragment was cloned into plasmid pZErO 2.1 (Invitrogen). The clone HY3.9N was obtained by excising a 3.9-kb NsiI fragment of KD735-15 DNA from an agarose gel and inserting the fragment into the NsiI site of plasmid pGEM-11Zf(+) (Promega). The 3.9-kb fragment had originally been identified with the vsaE probe (Fig. 1B). The clone HY5.7N was serendipitously isolated during the process of obtaining clone HY3.9N. The clone JG.LA2 was obtained by screening the KD735-15 genomic library with the lipA probe (Fig. 1B). The clones JG14A, JG17A, JG2A, and JG15A were obtained by screening the KD735-15 library with the vsaE probe. The clone XJ2A was obtained by screening the genomic library with the p16 probe.
FIG. 1.
Schematic diagrams of vsa genes (1 cm = 1,250 bp). (A) Map of the KD735-15 vsa locus. The locations of each of the 10 cloned KD735-15 DNA fragments that were used to assemble the locus are shown. Genes are shown as thick lines with arrows indicating directionality. Homologous vsa genes are shaded in the same color. The region shaded in black represents the vsa expression site encoding the first 714 nucleotides of the 242 amino acids of the Vsa proteins. Triangles denote locations of vrs boxes. Unshaded genes lack vrs boxes and therefore are not vsa genes. HindIII and NsiI restriction sites are denoted by the letters H and N, respectively. (B) Map of the KD735-15 vsa locus providing the locations of hybridization probes used in this study and the locations of the target sites of oligonucleotide primers used for PCR amplification. (C) Map of the p93 gene from UAB 6510-vir. The shaded region is missing in KD735-15 and 6510C. (D) Map of the CT DNA insert in clone L12.
TABLE 1.
Oligonucleotide used in this study
| Name | Orientation | Sequence |
|---|---|---|
| o.358 | − | TGTAAAGGTATTTTTAGTCATAG |
| o.9216 | + | GTGAAAGTTTTACACCATTAGG |
| o.810 | − | AATGACATTAGCTTCATGTAATC |
| LipA Rev 3 | + | CAAAAGTTGTTTCAGTTGTTGGAC |
| 4.9 primer 8 | + | CTTCTAATTTTACTTTTGCATC |
| 4.9 Rev Primer 2 | − | CAATAAGAATTAATTTCAAATAATC |
| Exp. primer (o.6666) | + | GGTCAATCAGATGCTCAAAAAGTA |
| A primer (o.6859) | − | GTAGTTGGAGGAGTCATTG |
| B primer (o.4083) | − | TTGGTTTATTAGGCATAGGG |
| C primer (o.0067) | − | TTACATTTTTGTCTCC(A/G)CTTGC |
| D primer (o.9590) | + | TGGTTTTGTTTGACTACTTCC |
| E primer (o.9272) | + | GATACACTTCCTTCAGCTTGACC |
| F primer | + | CCATTGGTTTATTAGGCAATTGGTTTC |
| G primer | NAa | TGATCATTTGGAGATTTATTAGG |
| H primer | NA | CTGGTGGAGTTTTCAGTTTTGCCAC |
| B repeat oligob | − | CCATCTCTCCTGCATTTGCATC |
| C repeat oligo | − | CCAACAACAGGAGATGCAAACACC |
| D repeat oligo | + | CCTCCAGCAGGTGGAACCATGAAGG |
| F repeat oligo | + | AGATACAATGGTAACTCCTCCTGC |
| G repeat oligo | NA | TGTATCTCCGCCTGGAGCAGGAGG |
| H oligo | NA | CAGCTGACACTCCAGCTCCAGGAGG |
| 57F2 | + | GGTCAAAACAATTAAATCTCTATGC |
| 57C3.8 | − | TGAAATTGAATCACTTTCACTAAAC |
NA, not available.
oligo, oligonucleotide.
For screening, colonies from the pBluescript-based library were subjected to colony hybridization with probes derived from KD735-15 and labeled with 32P by the random primer method (17). The vsaE probe consisted of a PCR product obtained using primers o.9216 and o.358 (Table 1). The primers o.810 and LipA Rev3 were used to PCR amplify the lipA probe. PCR amplification conditions were as described previously (19). The p16 probe corresponded to a 700-bp fragment from the left end (as oriented in Fig. 1A) of the insert in the plasmid from clone HY3.9N and was obtained by digestion of the plasmid with BglII and NsiI.
DNA sequence analysis.
Both strands of cloned DNA fragments were sequenced by the primer walking method using oligonucleotide primers synthesized by Genosys Biotechnologies, Inc., or the Oligonucleotide Synthesis Core Facility at the University of Alabama at Birmingham (UAB). Some DNA fragments were sequenced manually as described previously (4), and other fragments were sequenced by automated dye terminator methods at the Sequencing Core Facility, UAB, or the Iowa State University DNA Synthesis and Sequencing Facility, Ames. Sequence analysis was performed with MacVector, Sequencher, the University of Wisconsin Genetics Computer Group package, and PSI-BLAST (1).
Analysis of vsa gene rearrangements.
A PCR strategy was used to determine whether specific vsa genes were capable of combining with the vsa expression site by vrs box-mediated, site-specific DNA inversion, as previously described (4). The Exp. (expression site) primer (o.6666) and the A, B, C, D, and E primers were previously described (4). The F, G, and H primers were designed from sequence data from the newly identified vsa genes. All primers and their sequences are catalogued in Table 1. Each vsa primer along with the Exp. primer will amplify the target gene if the gene has recombined with the vsa expression site. PCR mixtures were subjected to 3 min at 94°C and 40 cycles of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C. PCR products from KD735-15 and CT templates were directly sequenced.
Southern analysis of vsa genes.
Genomic DNA was digested with HindIII or NsiI, analyzed on 0.8% agarose gels, and blotted onto MagnaCharge nylon transfer membranes (Osmonics Inc.). The blots were probed with oligonucleotides or PCR products specific for particular vsa genes. Oligonucleotide probes consisted of the A and E primers and the repeat primers that are specific for the repeat regions of vsaB, vsaC, vsaD, vsaG, and vsaH (Table 1). Oligonucleotide probes were labeled with 32P using T4 polynucleotide kinase (Gibco BRL) for 60 min at 37°C. PCR products were labeled with 32P by the random primer method. Hybridization with oligonucleotides was at 50°C, and washing conditions were as recommended by Ausubel et al. (2). Hybridization with PCR products used stringent conditions as described previously (3).
Nucleotide sequence accession number.
The nucleotide sequences of the KD735-15 vsa locus (Fig. 1A), the X1048 p93 gene (Fig. 1C), and the CT L12 clone (Fig. 1D) have been deposited in the GenBank database under the accession no. U23947, AF198036, and AF198037, respectively.
RESULTS
The vsa locus of KD735-15.
Previously, we described a 10-kb region of the KD735-15 vsa locus that included one vsaA gene, one vsaB gene, two vsaC genes, one vsaD gene, two vsaE genes (one complete and one partial), and the 5′ end of another gene designated lipA that was predicted to encode a lipoprotein. The current study began as an attempt to clone the complete sequence of the partial lipA and vsaE genes. A DNA fragment corresponding to the 5′-end region of vsaE was used to examine Southern blots of NsiI-digested KD735-15 genomic DNA, revealing a total of three vsaE genes (Fig. 2). Using this vsaE fragment as a probe and other cloning methods described in Materials and Methods, a total of 10 genomic clones (ranging from 2.7 to 5.7 kb) containing vsa genes from KD735-15 were isolated and sequenced. Sequence analysis of these clones permitted the assembly of a single 20-kb vsa locus containing 11 vsa genes and lipA as diagrammed in Fig. 1A. The 20-kb locus contains five vsa genes not identified in the previously described 10-kb region of the vsa locus, and the GenBank accession no. for the 10-kb region (U23947) has been updated. This 20-kb locus probably contains most but perhaps not all of the vsa genes of KD735-15. Southern analyses with the A, B repeat, C repeat, D repeat, and E primers as probes confirmed that there are one vsaA gene, one vsaB gene, two vsaD genes, and three vsaE genes in KD735-15 (representative results are shown in Fig. 2). The vsaE3 gene has not been cloned in its entirety, and the presence of additional vsa genes cannot be excluded at this time.
FIG. 2.
Southern blot analysis of KD735-15 and CT DNA. (A) KD735-15 DNA was digested with HindIII or NsiI and probed with the D repeat oligonucleotide or the vsaE probe. The estimated sizes of the HindIII fragments that hybridized with the D probe are 6 and 3.3 kb. NsiI fragments of 5.5, 3.6, and 2.2 kb hybridized with the E probe. (B) CT DNA was digested with HindIII and probed with the F or G repeat oligonucleotides. Numbers in the margins refer to the estimated size, in kilobases, of the DNA fragment in each band as determined by comparison with molecular size markers consisting of bacteriophage λ DNA digested with HindIII and a 1-kb ladder (Gibco BRL Life Technologies).
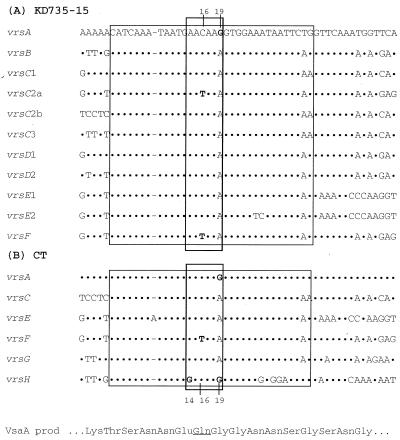
There is a single vsa expression site (the region in Fig. 1A and B that is shaded in black) in the 20-kb contig. All vsa genes are transcriptionally silent except for the particular gene that is associated with the expression site, which in the case of KD735-15 is vsaA (4). The silent genes can recombine with the expression site by site-specific DNA inversion and, hence, become expressed (4). Each of the vsa genes possesses at least one copy of the vrs box that serves as the recombination site for DNA inversion (4). A comparison of the nucleotide sequences of the 11 vrs boxes identified in Fig. 1A reveals a high degree of nucleotide sequence similarity (Fig. 3). Presumably, the vrs box is recognized by an as-yet-unidentified site-specific DNA recombinase that promotes inversions within the vsa locus.
FIG. 3.
Comparison of the nucleotide sequences of the vrs boxes from KD735-15 (A) and CT (B) and the encoded amino acid sequence for VsaA. Dots in the sequence indicate identity with the vrsA sequence. Dashes refer to gaps introduced into the sequence to improve alignment. The large rectangle denotes the 34-bp vrs box sequence, and the small rectangle denotes the proposed 6-bp recombination site. The underlined Gln amino acid in the VsaA sequence denotes the location where the protein would terminate when the CAA codon at vrs box positions 16 to 18 is changed to TAA (as in the case of vrsF).
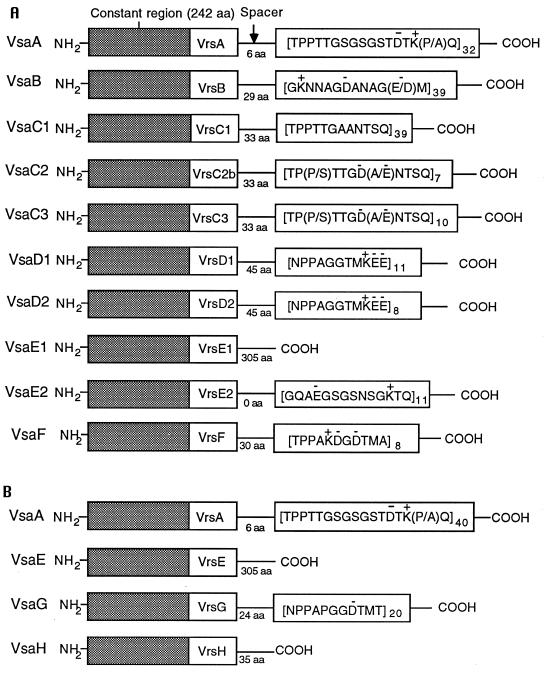
Most of the vsa genes are highly repetitive, and extensive gene duplication must have occurred during the evolution of the locus. Downstream of the vrs box of most vsa genes is a highly reiterated sequence referred to as the tandem repeat domain. The vsaA, vsaB, and vsaF genes are distinct and encode proteins with unique tandem repeat domains (Fig. 4 and Table 2). In contrast, gene duplication is evident from the presence of three vsaC, three vsaE, and two vsaD genes in the 20-kb contig. The gene duplication(s) apparently occurred as a large block because the intergenic regions between the three sets of vsaC and -E genes are highly similar, as are the regions between the sets of vsaE and -D genes. The repeat units of the tandem repeat region of the three vsaC genes are nearly identical, but the number of repeat units per gene is variable. Similarly, the two vsaD genes have identical repeat units and differ only in respect to the number of repeats. The three vsaE genes exhibit the most divergence, and nucleotide differences among these genes aided in assembly of the vsa locus. It is not possible to be definitive about the structure of vsaE3 because the complete sequence of this gene has not been determined. The predicted VsaE1 protein lacks a tandem repeat domain, and in its place is predicted to be a long α-helical domain of about 160 amino acids (4). VsaE2 is predicted to contain 11 tandem copies of a 14-amino-acid repeat (Fig. 4). The vsaE2 and -E3 genes share significant nucleotide similarity for over 700 nucleotides 3′ of the end of each gene's coding region. A single nucleotide substitution in vsaE3 and a single nucleotide deletion in vsaE2 would substantially extend each ORF (by 705 nucleotides for vsaE2 and 711 nucleotides for vsaE3). Each of the extended gene products would have acquired a similar (95% amino acid identity) α-helical domain, as has VsaE1.
FIG. 4.
Schematic diagram of the predicted Vsa proteins of KD735-15 (A) and CT (B). The first 242 amino acids (shaded box) of the Vsa proteins terminate within residues encoded by the vrs box. The tandem repeat domains of the variable carboxy terminus of each Vsa protein are indicated with the subscript denoting the number of repeat units. Charged amino acids are indicated by a + or − above the amino acid. Amino acids in parentheses refer to heterogeneity in the repeat region. For example, the 16th amino acid of the VsaA repeat region is sometimes proline (P) and sometimes alanine (A), depending on the particular repeat unit. aa, amino acid.
TABLE 2.
Predicted Vsa protein characteristics
| Proteina | Repeat length (amino acids) | No. of repeatsb | Repeat pI | Protein pI | Protein mass (kDa) |
|---|---|---|---|---|---|
| VsaA | 17 | 32 | 8.0 | 8.5 | 80 |
| VsaA (CT) | 17 | 40 | 8.0 | 8.7 | 93 |
| VsaB | 13 | 39 | 4.0 | 4.3 | 80 |
| VsaC1 | 12 | 39 | 5.5 | 8.6 | 75 |
| VsaC2 | 12 | 7 | 2.8 | 4.8 | 40 |
| VsaC3 | 12 | 10 | 2.6 | 4.5 | 43 |
| VsaD1 | 11 | 11 | 4.3 | 5.0 | 45 |
| VsaD2 | 11 | 8 | 4.3 | 5.1 | 41 |
| VsaE1 | —c | — | — | 5.4 | 62 |
| VsaE2 | 14 | 11 | 6.1 | 8.8 | 41 |
| VsaE (CT) | — | — | — | 5.5 | 62 |
| VsaF | 11 | 8 | 4.0 | 4.9 | 40 |
| VsaG (CT) | 11 | 20 | 3.4 | 4.5 | 51 |
| VsaH (CT) | — | — | — | 9.1 | 31 |
Predicted proteins of strain CT are indicated by (CT). All other proteins are from KD735-15.
For the large proteins VsaA, VsaB, and VsaC1, the number of repeats was estimated by sizing pertinent restriction fragments and PCR products by agarose gel electrophoresis, as described previously (4). For the other proteins, the number of repeats was determined directly by sequence analysis of the corresponding vsa gene.
—, the protein lacks a tandem repeat domain.
The 20-kb vsa locus contains some ORFs that lack vrs boxes and by definition are not vsa genes. lipA is predicted to encode a lipoprotein (4). p16 is predicted to encode a small basic protein of 16 kDa. Both lipA and p16 are preceded by a typical Shine-Dalgarno (SD) sequence upstream of an ATG start codon and have typical mycoplasmal codon usage (18), indicating that these ORFs are likely to be functional genes. PSI-BLAST analysis indicated that the predicted lipA and p16 gene products had no significant database matches. In contrast to lipA and p16, the ORF p93Δ is probably not a functional gene because it lacks an SD sequence and a typical start codon (ATG, GTG, or TTG).
Identification of a deletion in p93.
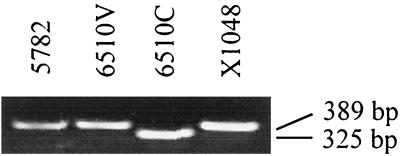
Because features in the sequence of the 5′-end region of p93Δ suggested that it may not be a functional gene, the nucleotide sequence was reexamined by directly sequencing PCR products containing this region. The primers used for this amplification were 57F2 and 57C3.8 (Table 1). Using these primers and DNA from strains KD735-15 and 6510C as template, the sequence of p93Δ was confirmed. However, a PCR product containing an additional 64 bp was obtained with DNA from strains 6510-vir, X1048, and 5782 as template (Fig. 5). The frameshift that resulted from inclusion of these nucleotides in the gene sequence extended the ORFs 5′ end (Fig. 1C). The new ORF, p93, begins with an ATG start codon preceded by a strong SD sequence and is predicted to encode a 93-kDa protein. PSI-BLAST analysis failed to reveal a significant match between P93 and sequences deposited in the protein and nucleotide databases. Amino acids 5 through 28 from the N terminus of P93 are hydrophobic and may represent a signal peptide sequence or a transmembrane domain. The remaining portion of P93 is remarkably hydrophilic, leading to the prediction that P93 is a surface membrane protein. Strains 6510-vir (having an intact p93 gene) and 6510C (having p93Δ) both have the vsaA gene at the vsa expression site and are not known to have any genetic differences other than in p93. Preliminary data from experiments in progress indicate that 6510-vir is more virulent than 6510C in rats (N. Zou and K. Dybvig, unpublished data), but the genetic defect in the p93 gene of 6510C must be complemented before a definitive statement regarding the role of p93 in virulence can be made.
FIG. 5.
Agarose gel analysis of the PCR products obtained by amplification of the region containing the 5′ end of p93Δ, revealing that the p93Δ gene of 6510C is 64 bp shorter than p93 from UAB 5782, 6510-vir, and X1048.
Comparison of vsa genes between strains KD735-15 and CT.
Previously, CT was shown to have a homolog of the KD735-15 vsaA gene and also another vsa gene that was produced by cells displaying a hemadsorption-positive phenotype (33). We refer to this second gene as vsaH. The ends of the inserts of numerous random clones from the CT DNA library were sequenced as part of an effort to initiate nucleotide sequencing of the complete CT genome. The resulting sequence data provided partial nucleotide sequences of the CT vsaA (confirming the previously described sequence of this gene), vsaC, and vsaE genes and the complete (confirmatory) sequence of vsaH. Clone L12 (Fig. 1D) was isolated by screening the library with the vsaE probe. Sequence analysis of the L12 cloned insert revealed the complete nucleotide sequence of the CT vsaE and -G genes as well as another gene referred to as lipB. The nucleotide sequences of the vrs box of the CT and KD735-15 vsa genes are very similar (Fig. 3). The tandem repeat regions of the CT VsaA and VsaC proteins have essentially the same predicted amino acid sequences as their counterparts in KD735-15. The CT VsaE protein is very similar (three amino acid differences) to the VsaE1 protein of KD735-15. The CT VsaG and VsaH proteins are predicted to have a tandem repeat region unlike any of the known KD735-15 proteins (Fig. 4). LipB protein is predicted to be a lipoprotein with essentially the same signal peptide sequence as that of LipA from KD735-15, and the overall amino acid identity shared by these two proteins is 51% (calculated using the University of Wisconsin Genetics Computer Group program GAP). Southern hybridizations using oligonucleotides as probes were used to compare the vsa repertoire of KD735-15 and CT. Probes specific for each of the vsa genes confirmed that KD735-15 has the vsa genes vsaA to vsaF but not vsaG and vsaH. CT was found to have the vsa genes vsaA, -C, -E, -F, -G, and -H but lacked vsaB and -D. The results of representative Southern hybridization experiments are shown in Fig. 2.
Identification of a 6-bp recombination site in the vrs box.
A copy of the 34-bp vrs box is present in all vsa genes (Fig. 1A and 3). PCR analysis was used to assess whether specific vsa genes could undergo vrs box-mediated recombination with the vsa expression site. The strategy was to pair the expression site primer (Exp. primer) with individual primers (e.g., F primer) that were specific for each vsa gene (Table 1 and Fig. 1B). If recombination with the expression site occurred, a PCR product of about 300 bp (the precise size being dependent on the particular vsa gene that was examined) would be obtained. In each case, the nucleotide sequence of the PCR product was determined to verify its identity. Using this strategy, we previously demonstrated that the vsa genes vsaA, -B, -C, -D, and -E were recombinogenic in KD735-15 (4). In the current study, we confirmed the earlier results with vsaA to vsaE and also found that vsaF can recombine with the expression site in strain KD735-15 as expected. Similarly, the vsa genes vsaA, -C, -E, -F, -G, and -H were found to be capable of undergoing vrs box-mediated recombination with the expression site in strain CT. PCR analysis failed to detect recombination involving the vsaB and -D genes in CT and the vsaG and -H genes in KD735-15, which is consistent with the Southern data indicating that vsaB and -D are absent in CT and vsaG and -H are absent in KD735-15.
It should perhaps be noted that vrs box-mediated DNA inversion can occur between vrs box copies that are oppositely oriented in the chromosome but not between copies that are oriented in the same direction. When the vsa locus is configured as illustrated in Fig. 1A, two DNA inversions are required for some vsa genes, e.g., vsaB, to recombine with the expression site. The first DNA inversion, e.g., inversion between vrsB and vrsD1, would serve to orient vsaB in the chromosome in the opposite direction from the vsa expression site. A second DNA inversion could then incorporate vsaB into the expression site. Such double inversion events resulting in incorporation of vsaB into the expression site have been described previously (4).
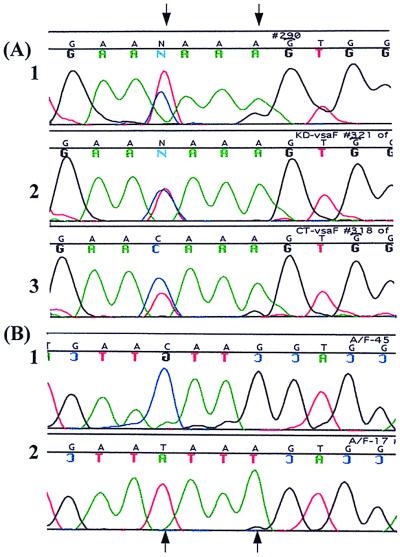
Nucleotide polymorphisms in the vrs box sequences of the vsa genes were instrumental in identifying the site of strand exchange during DNA inversion. In both KD735-15 and CT, the 19th nucleotide of vrsA is G instead of the usual A and the 16th nucleotide of vrsF is T instead of C (Fig. 3). When the Exp. primer and the F primer were paired to amplify the recombination product arising by DNA inversion between vrsA and vrsF, the sequence of the resulting PCR product (sequenced directly without cloning) revealed a mixed population (close to a 50:50 mixture) of T and C in the 16th position and a mixture of A and G in the 19th position (Fig. 6A). This heterogeneity in the vrs box sequence did not exist prior to the recombination event. When vrsA was PCR amplified using the Exp. primer and the A primer, the 16th and 19th nucleotides of the PCR product were C and G, respectively, with no indication of a subpopulation having T and A at these respective positions. Similarly, when vrsF was PCR amplified, the sequence of the resulting PCR product had T and A at the 16th and 19th nucleotide positions, respectively, with no indication of a C and a G at these positions, respectively. The C/T (16th position) and G/A (19th position) heterogeneity exhibited by sequence analysis of the vrsA-vrsF recombination product was reproducible (observed in four independent experiments). To confirm the C/T heterogeneity, the PCR product containing the vrsA-vrsF recombination product from KD735-15 was cloned. Sequence analysis of multiple clones revealed that some clones had T and A at the 16th and 19th positions, respectively, and other clones had C and G, respectively (Fig. 6B). PCR and sequence analysis of several recombination products arising from rearrangements between vrsA and other vrs boxes also revealed G/A heterogeneity at the 19th nucleotide. The vsaH gene in CT has a G instead of an A at the 14th nucleotide, and this G nucleotide was found to be retained by vsaH even after vrsH recombined with the expression site. Therefore, strand exchange leading to DNA inversion must have occurred 5′ of the G. Collectively, these data suggest that the six bases at positions 14 to 19 are involved in strand exchange.
FIG. 6.
Sequence analysis of vrs boxes after DNA inversion. (A) Direct sequencing of PCR products. (1) Sequence of the KD735-15 vrsA after a vsaA-vsaF inversion; (2) KD735-15 vrsF after vsaA-vsaF inversion; (3) CT vrsF after a vsaA-vsaF inversion. (B) Sequences of two cloned PCR products containing the KD735-15 vrsF after vsaA-vsaF inversion. Nucleotide heterogeneity identified by direct sequencing in panel A was resolved by cloning of the PCR products. Arrows refer to the 16th and 19th nucleotides of the vrs box (see Fig. 3) that vary.
The PCR products described above arose from genuine recombination events and were not artifacts resulting from annealing of incomplete amplicons at the homologous vrs sequences. Artifactual PCR products that were the result of annealing at vrs sequences were generated as follows. A DNA template was prepared that contained a single vrs box, vrsA, and a second DNA template was prepared that contained a single vrs box, vrsF. These two DNAs were mixed and used as template for PCR amplification using the Exp. primer and the F primer. Because the Exp. primer could bind only to the template containing vrsA and the F primer could bind only to the template containing vrsF, any resulting PCR product would be artifactual. By using a high level of template concentration and a high number of amplification cycles, artifactual PCR products were obtained. The nucleotide sequence of these products revealed polymorphisms in the vrs box sequence at positions 33, 42, 44, and 46 (data not shown), as would be expected from products generated by annealing of incomplete amplicons at vrs sequences. These sequence polymorphisms were not observed in the PCR products obtained by amplification of genomic DNA to detect recombination products, indicating that the recombination products were indeed not artifacts.
DISCUSSION
Phase-variable surface proteins with repetitive domains are common in mycoplasmas. Although their function(s) is often unknown, many of these proteins are thought to be adhesins or involved with other host-mycoplasma interactions (9, 22, 27, 28, 36, 42). The V-1 proteins of M. pulmonis, encoded by the vsa genes, influence several properties of the mycoplasma. Colonies of CT that produce VsaA are hemadsorption negative whereas CT colonies producing VsaH are hemadsorption positive (33), indicating that the Vsa proteins may influence ligand binding properties. KD735-15 cells that produce VsaA are susceptible to infection by mycoplasma virus P1, but cells producing VsaB are virus resistant (15). VsaA may be the phage receptor, or perhaps, VsaB interferes with the binding of P1 to its receptor. The growth properties of M. pulmonis are also affected by the Vsa proteins. KD735-15 and CT cells producing VsaA proteins with a high number of tandem repeat units have a growth advantage in broth but a growth disadvantage on agar compared to cells having VsaA proteins with fewer repeat units (16). The issue of how the number of repeat units would measurably affect cell growth is intriguing. The ability of the Vsa proteins to modulate interactions between the mycoplasma and the environment and the ability of M. pulmonis to colonize and cause disease on numerous epithelial surfaces suggest the possibility that Vsa protein variation may have a role in tissue tropism.
For the most part, the mechanisms of surface protein variation in mycoplasmas are poorly understood. In Mycoplasma hyorhinis, variation in the Vlp (variable lipoprotein) surface proteins results from high-frequency changes in the length of a poly(A) sequence that is located upstream of each vlp gene's transcription start site (41). The mechanism by which the length of the poly(A) sequence affects vlp gene transcription is unknown. In Mycoplasma pneumoniae and Mycoplasma genitalium, there is evidence supporting the contention that some surface protein genes can vary by DNA recombination with repetitive elements scattered around the mycoplasma chromosome (23, 29, 30). DNA rearrangements involving these repetitive elements have not been well characterized but may resemble gene conversion. In Mycoplasma gallisepticum, the phase-variable expression of members of the pMGA gene family is apparently controlled by the length of the trinucleotide GAA tandem repeat region located upstream of each gene's transcription start site (20, 25). The mechanism by which the GAA repeats influence pMGA gene expression is unknown. The vsp genes of Mycoplasma bovis vary by DNA rearrangements that have not been well characterized (26). The vsa locus of M. pulmonis is probably the best-understood DNA rearrangement system in mycoplasmas. Site-specific DNA inversions regulating expression of the vsa genes occur by recombination between copies of the vrs box sequence. In the current study, a specific 6-nucleotide segment (Fig. 3, nucleotides 14 to 19) of the vrs box was identified as central to the recombination events.
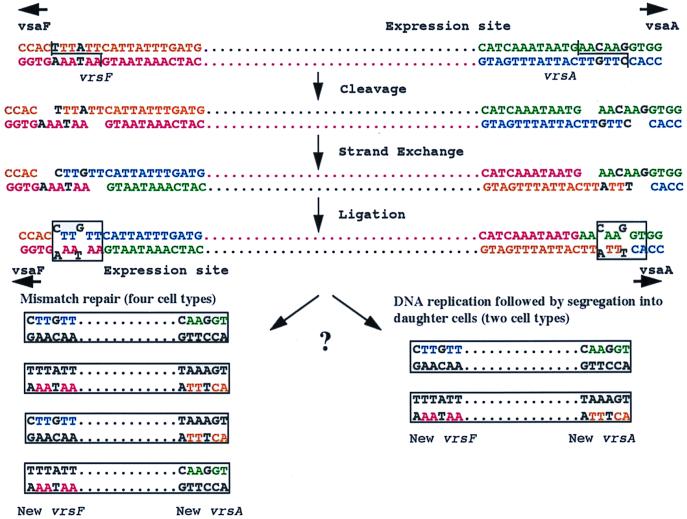
We propose a model (Fig. 7) using recombination between vrsA and vrsF as an example to account for the observed heterogeneity in the sequence of the vsa recombination products obtained by PCR amplification. The model proposes a crossover event involving a staggered cleavage reaction centering on the six nucleotides at positions 14 to 19 of the vrs box. Recombination results in mismatched nucleotides (explaining the sequence heterogeneity of the PCR products) that may be resolved by either mismatch repair or DNA replication followed by segregation into daughter cells. The λ integrase and Tn3 resolvase families of site-specific recombinases catalyze reactions involving breakage sites having six- to eight-base 5′ overhangs and two-base 3′ overhangs, respectively (34). Because at least six nucleotides of the vrs box are apparently involved with strand breakage, we predict that the putative site-specific recombinase that promotes vrs box-mediated DNA inversion is a member of the λ integrase family.
FIG. 7.
Model of site-specific vsa gene rearrangements leading to nucleotide variation in vrs box sequences. A staggered DNA cleavage reaction leads to strand exchange involving mismatched nucleotides that are resolved either by mismatch repair or by DNA replication followed by segregation into daughter cells.
Variation in positions 14, 16, and 19 of the vrs box may have important phenotypic consequences. When the 16th nucleotide at the expression site is C, the CAA codon at positions 16 to 18 encodes glutamine (Fig. 3). When the 16th nucleotide is replaced by T, an in-frame TAA stop codon is formed that would result in a truncated Vsa protein. Thus, C/T variation at the 16th position would determine the phase-variable production of the Vsa repeat region. G/A variation at the 19th nucleotide of the vrs box switches the codon from GGT (glycine) to AGT (serine). Similarly, A/G variation at the 14th position switches the codon from GAA (glutamic acid) to GGA (glycine). Growth conditions and host defenses may, therefore, apply selective pressure on the cell population to maintain certain nucleotides at vrs box positions 14 to 19.
Strains of M. pulmonis tend to lose virulence when propagated in culture. Virulence can usually be reacquired by infection of rats or mice followed by isolation of mycoplasmas from infected tissues. For example, strain UAB T was originally isolated from mouse lung but lost virulence after passage in mycoplasmal growth medium. The relatively avirulent UAB T strain was used to inoculate mice, and the virulent CT strain was isolated from the lung of one of the infected animals (13). A comparison of the proteins of UAB T and CT revealed no differences other than the V-1 protein profile (37). We propose that, during growth in culture, the vsa locus is not under selective pressure to maintain a particular gene configuration and the vsa tandem repeat regions freely undergo expansion and construction, resulting in genes of sizes that are suboptimal for animal infection. After inoculation into the animal host, selective pressure is restored and virulent subpopulations of M. pulmonis cells that can produce the appropriate type(s) of Vsa proteins of the correct size for colonization can be recovered from infected tissue. In culture, there is no selective pressure to maintain genes that are important for virulence but not for growth. Such genes may be rendered inactive due to spontaneous mutation. An example may be p93, which has apparently undergone a 64-bp deletion in strain 6510C. Although it has not yet been shown that the deletion in p93 was associated with loss of virulence, the chromosomal location of p93 in the middle of the vsa locus is interesting and suggests that P93 may function in mycoplasma-host cell interactions.
ACKNOWLEDGMENTS
We thank Portia Caldwell and Tajuana Johnson for technical assistance and Ramakrishnan Sitaraman for helpful comments.
This work was supported by Public Health Service grant AI41113 to K.D. and training grant award AI07041 from the National Institutes of Health. The UAB oligonucleotide synthesis core facility is supported by NIH grant 5P50 CA13148.
Database searches using the PSI-BLAST program were provided free of charge by the National Center for Biotechnology Information at the website http://www.ncbi.nlm.nih.gov.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1999. [Google Scholar]
- 3.Bhugra B, Dybvig K. High-frequency rearrangements in the chromosome of Mycoplasma pulmonis correlate with phenotypic switching. Mol Microbiol. 1992;6:1149–1154. doi: 10.1111/j.1365-2958.1992.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhugra B, Voelker L L, Zou N, Yu H, Dybvig K. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol Microbiol. 1995;18:703–714. doi: 10.1111/j.1365-2958.1995.mmi_18040703.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown M B, Steiner D A. Experimental genital mycoplasmosis: time of infection influences pregnancy outcome. Infect Immun. 1996;64:2315–2321. doi: 10.1128/iai.64.6.2315-2321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartner S C, Lindsey J R, Gibbs-Erwin J, Cassell G H, Simecka J W. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect Immun. 1998;66:3485–3491. doi: 10.1128/iai.66.8.3485-3491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartner S C, Simecka J W, Lindsey J R, Cassell G H, Davis J K. Chronic respiratory mycoplasmosis in C3H/HeN and C57BL/6N mice: lesion severity and antibody response. Infect Immun. 1995;63:4138–4142. doi: 10.1128/iai.63.10.4138-4142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassell G H, Davis J K. Protective effect of vaccination against Mycoplasma pulmonis respiratory disease in rats. Infect Immun. 1978;21:69–75. doi: 10.1128/iai.21.1.69-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citti C, Kim M F, Wise K S. Elongated versions of Vlp surface lipoproteins protect Mycoplasma hyorhinis escape variants from growth-inhibiting host antibodies. Infect Immun. 1997;65:1773–1785. doi: 10.1128/iai.65.5.1773-1785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson M K, Lindsey J R, Parker R F, Tully J G, Cassell G H. Differences in virulence for mice among strains of Mycoplasma pulmonis. Infect Immun. 1988;56:2156–2162. doi: 10.1128/iai.56.8.2156-2162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis J K, Cassell G H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982;19:280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- 12.Davis J K, Parker R F, White H, Dziedzic D, Taylor G, Davidson M K, Cox N R, Cassell G H. Strain differences in susceptibility to murine respiratory mycoplasmosis in C57BL/6 and C3H/HeN mice. Infect Immun. 1985;50:647–654. doi: 10.1128/iai.50.3.647-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis J K, Thorp R B, Parker R F, White H, Dziedzic D, D'Arcy J, Cassell G H. Development of an aerosol model of murine respiratory mycoplasmosis in mice. Infect Immun. 1986;54:194–201. doi: 10.1128/iai.54.1.194-201.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dybvig K, Alderete J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid. 1988;20:33–41. doi: 10.1016/0147-619x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 15.Dybvig K, Alderete J, Cassell G H. Adsorption of Mycoplasma pulmonis virus P1 to host cells. J Bacteriol. 1988;170:4373–4375. doi: 10.1128/jb.170.9.4373-4375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dybvig K, Simecka J W, Watson H L, Cassell G H. High-frequency variation in Mycoplasma pulmonis colony size. J Bacteriol. 1989;171:5165–5168. doi: 10.1128/jb.171.9.5165-5168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dybvig K, Sitaraman R, French C T. A family of phase-variable restriction enzymes with differing specificities generated by high-frequency gene rearrangements. Proc Natl Acad Sci USA. 1998;95:13923–13928. doi: 10.1073/pnas.95.23.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dybvig K, Voelker L L. Molecular biology of mycoplasmas. Annu Rev Microbiol. 1996;50:25–57. doi: 10.1146/annurev.micro.50.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Dybvig K, Woodard A. Cloning and DNA sequence of a mycoplasmal recA gene. J Bacteriol. 1992;174:778–784. doi: 10.1128/jb.174.3.778-784.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glew M D, Baseggio N, Markham P F, Browning G F, Walker I D. Expression of the pMGA genes of Mycoplasma gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5′ noncoding region. Infect Immun. 1998;66:5833–5841. doi: 10.1128/iai.66.12.5833-5841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz S A, Garret B, Davis J K, Cassell G H. Isolation of Mycoplasma pulmonis membranes and identification of surface antigens. Infect Immun. 1987;55:1314–1320. doi: 10.1128/iai.55.5.1314-1320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu T, Minion F C. Identification of the cilium binding epitope of the Mycoplasma hyopneumoniae P97 adhesin. Infect Immun. 1998;66:4762–4766. doi: 10.1128/iai.66.10.4762-4766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenri T, Taniguchi R, Sasaki Y, Okazaki N, Narita M, Izumikawa K, Umetsu M, Sasaki T. Identification of a new variable sequence in the P1 cytadhesin gene of Mycoplasma pneumoniae: evidence for the generation of antigenic variation by DNA recombination between repetitive sequences. Infect Immun. 1999;67:4557–4562. doi: 10.1128/iai.67.9.4557-4562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey J R, Baker H J, Overcash R G, Cassell G H, Hunt C E. Murine chronic respiratory disease: significance as a research complication and experimental production with M. pulmonis. Am J Pathol. 1971;64:675–707. [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Dybvig K, Panangala V S, van Santen V L, French C T. GAA trinucleotide repeat region regulates M9/pMGA gene expression in Mycoplasma gallisepticum. Infect Immun. 2000;68:871–876. doi: 10.1128/iai.68.2.871-876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lysnyansky I, Rosengarten R, Yogev D. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J Bacteriol. 1996;178:5395–5401. doi: 10.1128/jb.178.18.5395-5401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markham P F, Glew M D, Browning G F, Whithear K G, Walker I D. Expression of two members of the pMGA gene family of Mycoplasma gallisepticum oscillates and is influenced by pMGA-specific antibodies. Infect Immun. 1998;66:2845–2853. doi: 10.1128/iai.66.6.2845-2853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markham P F, Glew M D, Whithear K G, Walker I D. Molecular cloning of a member of the gene family that encodes pMGA, a hemagglutinin of Mycoplasma gallisepticum. Infect Immun. 1993;61:903–909. doi: 10.1128/iai.61.3.903-909.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson S N, Bailey C C, Jensen J S, Borre M B, King E S, Bott K F, Hutchison C A., III Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc Natl Acad Sci USA. 1995;92:11829–11833. doi: 10.1073/pnas.92.25.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruland K, Himmelreich R, Herrmann R. Sequence divergence in the ORF6 gene of Mycoplasma pneumoniae. J Bacteriol. 1994;176:5202–5209. doi: 10.1128/jb.176.17.5202-5209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtlander C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–415. [Google Scholar]
- 32.Simecka J W, Patel P, Davis J K, Ross S E, Otwell P, Cassell G H. Specific and nonspecific antibody responses in different segments of the respiratory tract in rats infected with Mycoplasma pulmonis. Infect Immun. 1991;59:3715–3721. doi: 10.1128/iai.59.10.3715-3721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons W L, Zuhua C, Glass J I, Simecka J W, Cassell G H, Watson H L. Sequence analysis of the chromosomal region around and within the V-1-encoding gene of Mycoplasma pulmonis: evidence for DNA inversion as a mechanism for V-1 variation. Infect Immun. 1996;64:472–479. doi: 10.1128/iai.64.2.472-479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark W M, Boocock M R, Sherratt D J. Catalysis by site-specific recombinases. Trends Genet. 1992;8:432–439. [PubMed] [Google Scholar]
- 35.Talkington D F, Fallon M T, Watson H L, Thorp R K, Cassell G H. Mycoplasma pulmonis V-1 antigen surface protein variation: occurrence in vivo and association with lung lesions. Microb Pathog. 1989;7:429–436. doi: 10.1016/0882-4010(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 36.Washburn L R, Weaver K E, Weaver E J, Donelan W, Al-Sheboul S. Molecular characterization of Mycoplasma arthritidis variable surface protein MAA2. Infect Immun. 1998;66:2576–2586. doi: 10.1128/iai.66.6.2576-2586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson H L, Blalock D K, Cassell G H. Characterization of the variable V-1 antigen of Mycoplasma pulmonis. In: Stanek G, Cassell G H, Tully J G, Whitcomb R F, editors. Recent advances in mycoplasmology. New York, N.Y: Gustav Fischer Verlag; 1990. pp. 529–534. [Google Scholar]
- 38.Watson H L, Davidson M K, Cox N R, Davis J K, Dybvig K, Cassell G H. Protein variability among strains of Mycoplasma pulmonis. Infect Immun. 1987;55:2838–2840. doi: 10.1128/iai.55.11.2838-2840.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson H L, McDaniel L S, Blalock D K, Fallon M T, Cassell G H. Heterogeneity among strains and a high rate of variation within strains of a major surface antigen of Mycoplasma pulmonis. Infect Immun. 1989;56:1358–1363. doi: 10.1128/iai.56.5.1358-1363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson J S P, Davis J K, Cassell G H. Polyclonal inactivation of rat splenic lymphocytes after in vivo administration of Mycoplasma pulmonis and its relation to in vitro response. Infect Immun. 1986;52:594–599. doi: 10.1128/iai.52.2.594-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yogev D, Rosengarten R, Watson-McKown R, Wise K S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 1991;10:4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Wise K S. Molecular basis of size and antigenic variation of a Mycoplasma hominis adhesin encoded by divergent vaa genes. Infect Immun. 1996;64:2737–2744. doi: 10.1128/iai.64.7.2737-2744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]