Abstract
Sleep is involved in many physiological processes and is essential for both physical and mental health. Obesity and sleep deprivation due to sleep disorders are major public health issues. Their incidence is increasing, and they have a wide range of adverse health-related consequences, including life-threatening cardiovascular disease. The impact of sleep on obesity and body composition is well-known, and many studies have shown an association between insufficient or excessive sleep duration and obesity, body fat percentage, and weight gain. However, there is growing evidence of the effects of body composition on sleep and sleep disorders (particularly sleep disordered breathing) through anatomical and physiological mechanisms (nocturnal fluid shift, core body temperature, or diet). Although some research has been conducted on the bidirectional effects of sleep-disordered breathing and body composition, the specific effects of obesity and body composition on sleep and the underlying mechanisms that explain these effects remain unclear. Therefore, this review summarizes the findings on the effects of body composition on sleep and draws conclusions and proposals for future research in this field.
Keywords: body composition, sleep, sleep disorder, weight gain
Introduction
Sleep is involved in many physiological systems and is known to be an essential factor in physical and mental health.1,2 However, sleep disorders are one of the most commonly encountered clinical issues in practice.3 Sleep disorders include several types such as insomnia and sleep-disordered breathing (SDB). For example, SDB is reported to be a problem in approximately 50% of the elderly4 and is twice as common in men than in premenopausal women.5 In the Wisconsin Sleep Cohort Study, the prevalence of SDB was increasing in both men and women, and one possible cause for this is the increase in obesity in the general population.6
The interaction between sleep disturbance and obesity is well established. Cross-sectional studies7,8 and prospective studies9–12 have reported an association between sleep deprivation and risk of obesity, body fat percentage, and weight gain. Short and long sleep also increases the risk of obesity, with 27% and 21% increased risk, respectively, compared to sleepers who sleep for an average amount of time.13
In addition to Body mass index (BMI), cross-sectional studies have shown that several body composition parameters are tightly associated with sleep duration and quality. Body composition includes various parts of the body, especially fat mass (consisting primarily of subcutaneous fat and visceral fat tissue) and lean mass (muscle, bone, viscera, ligaments, and tendons).14 Specifically, lean body mass is positively correlated with sleep duration and sleep quality, while high-fat mass appears to be related to reduced sleep duration and poorer sleep quality.15–17
Numerous researches have explored the impact of sleep on obesity and body composition, with many recommendations proposed for consideration. However, the specific effects of obesity and body composition on sleep and the underlying mechanisms that explain them remain unclear.18,19 This review summarizes the current data on the impact of body composition on sleep and attempts to draw conclusions and recommendations for future research in this area.
This review presents the current reports on the effects of body composition on sleep, focusing on 1) physical and biological aspects, 2) epidemiological aspects, and 3) interventions. There are many reports on the bidirectional influence between body composition and SDB. However, reports on the impact of body composition on sleep disorders/poor sleep quality other than SDB have been scattered through epidemiological studies, and no systematic research has explained the underlying mechanisms. Therefore, studies related to SDB were considered as a standalone item.
Literature Search Methods
This narrative review searched for studies that discussed the specific effects of obesity and body composition on sleep and its underlying mechanisms. Original articles were identified through a systematic search strategy. MEDLINE (PubMed) was searched for eligible studies without specifying the study period. The review covers a wide range of areas, and the key search terms for each item are as follows: “body composition”, “body shape”, “body mass index”, “obesity”, “skinfold measurements”, “waist size”, “waist-to-hip ratio”, “abdominal visceral adiposity”, “body fat mass”, “percentage of body fat”, “lean body mass”, “muscle mass”, “obstructive sleep apnea syndrome”, “sleep-disordered breathing”, “apnea-hypopnea index”, “pharyngeal obstruction”, “nocturnal fluid shift”, “core body temperature”, “sleep parameter”, “total sleep time”, “sleep onset latency”, “wake after sleep onset”, “sleep stage”, “slow-wave sleep”, “rapid eye movement sleep”, “sleep quality”, “appetite”, and “appetite-related hormones”.
This was a narrative review, and the reviewed studies were not evaluated. However, preliminary screening of studies was undertaken by reviewing titles and abstracts to evaluate eligibility, and full-text articles were reviewed to determine eligibility for studies deemed accurate.
Influence of Body Composition on Sleep (Physical and Biological Aspects)
Impact of Body Composition on Obstructive Sleep Apnea Syndrome
Obstructive sleep apnea syndrome (OSAS) is the most frequent SDB disorder with obesity as its most critical pathogenic factor.20–23
Obstructive sleep apnea (OSA) is a sleep-related condition in which the pharyngeal airway is periodically obstructed and reopened.20,23 In terms of body composition and shape, the increase in pharyngeal obstruction may be due to a decreased lung capacity24 and an anatomical imbalance around the pharynx due to increased body size.25
The lungs, although distant from the pharyngeal airway, affect the obstructive potential of the pharyngeal airway. It is thought that the tension that traps pharyngeal air in the caudal longitudinal direction during increased lung volume reduces pharyngeal wall compliance and makes hinders pharyngeal collapse.26 The pharyngeal airway is more likely to be obstructed when the lung capacity is reduced by central obesity. Increased lung capacity in obese OSA patients during sleep improves the apnea-hypopnea index (AHI).
The pharyngeal airway is encircled by soft tissue (the tongue, soft palate, and palatine tonsils) and is surrounded by the maxilla and mandible.27 The pharyngeal airway is the space left over when the soft tissue is stored in the bony structure, and the size of this space is determined by the balance between the capacity of the bony structure and the volume of the soft tissue.28 When soft tissue volume is increased by obesity, the peripharyngeal anatomy is unbalanced, resulting in pharyngeal obstruction.29
OSA is marked by recurrent nocturnal episodes of apnea, the severity of which is expressed as AHI. Although AHI is higher in OSA patients with severe pharyngeal obstruction, the reason for pharyngeal obstruction alone cannot explain the change in AHI in OSA patients whose pharyngeal obstruction is near atmospheric pressure. Instability in respiratory coordination increases the periodicity of OSA.30,31 Leptin secreted from internal fat increases the hypercapnic ventilatory response, resulting in overreaction to abnormal breathing and unstable breathing with repeated hyperventilation and hypopnea. Consequently, obesity acts to increase AHI.32 Furthermore, increased ventilation efficiency due to decreased functional residual capacity associated with obesity, hypometabolism, decreased cardiac output, and hypercarbonatemia can also cause increased AHI.32,33 In addition to obesity, as defined in terms of BMI, the deposition of fatty tissue, especially in the abdomen and chest, leads to decreased compliance of the chest wall and lungs and increased lung elastic recoil pressure, resulting in a high incidence of SDB.34–36 Furthermore, increased visceral adipose tissue may be involved in the secretion of inflammatory cytokines disturb sleep-wake rhythms.35
Impact of the Nocturnal Fluid Shift on OSAS
Fluid accumulates in the lower extremities during the day owing to gravity and is redistributed at night in the supine position.37 Some of the fluid that migrates accumulates in the neck, narrowing the upper airway and causing OSA.38,39 The degree of fluid transfer is closely related to the severity of sleep apnea.40,41 Patients with OSA had a higher fluid index, which indicates “water content” in various parts of the body including both legs. They also had more fluid transfer from the leg to the neck than non-OSA patients (increased neck circumference, P<0.05).42,43 Regarding the correlation between overnight calf circumference and OSA severity, there was a significant association in men but no association in women.44,45 Sex differences in the response to fluid transfer may be related to anatomical differences in fat distribution.45 Furthermore, a study examining body composition using bioelectrical impedance analysis in OSA patients with Type 2 Diabetes (DM) showed that the muscle-to-fat ratio could be helpful in screening for OSA in patients with poorly controlled diabetes.46 This suggests that the muscle-to-fat ratio is likely related to the OSA severity in this population. Although an increase in head and neck fluid volume leads to an increase in neck circumference, and AHI is significantly correlated with changes in neck circumference, it is not directly associated with changes in head and neck fluid volume, and fluid transfer accumulation in the head and neck is not related to OSA.45 Fluid tends to accumulate in the peripharyngeal tissues rather than in the craniofacial soft tissues, leading to upper airway collapse and OSA. The incidence of OSA also varies depending on the location of the fluid accumulation.
Impact of Body Composition on Core Body Temperature
Body composition and shape may affect core body temperature. The relationship between body composition and sleep is mediated by thermoregulation. In general, sleep is triggered with the maximal rate of decrease in core body temperature,47,48 and after sleep onset, integrated sleep with increased slow-wave sleep results from low minimum core body temperature.49,50 Peripheral muscle tissue can act as an insulator, and increased peripheral muscle mass can prevent a decrease in deep-body temperature.51,52 Conversely, heat production by muscles causes an increase in the basal metabolic rate.53–56 Generally, the greater the muscle mass, the greater the heat production.57 Thus, increased muscle mass increased heat production and decreased heat dissipation. Ultimately, an increased muscle mass may decrease sleep quality. It has been reported that the greater the muscle mass, as measured by lean body mass, the shorter the slow-wave sleep proportion.58 Interestingly, the inverse association between muscle mass and sleep quality has been reported to be more pronounced in athletes than in non-athletes. Furthermore, a report that evaluated the relationship between body composition and sleep quality in athletes59 identified a link between body composition parameters and the proportion of N3 sleep in early non-REM sleep, with higher muscle mass indicating lower N3 sleep. This suggests that the opposite may be also true, ie the percentage of N3 sleep in early non-REM sleep is also higher with a higher percentage of fat mass. These findings indicate that muscle mass and fat mass may be the main determinants of objective sleep quality.
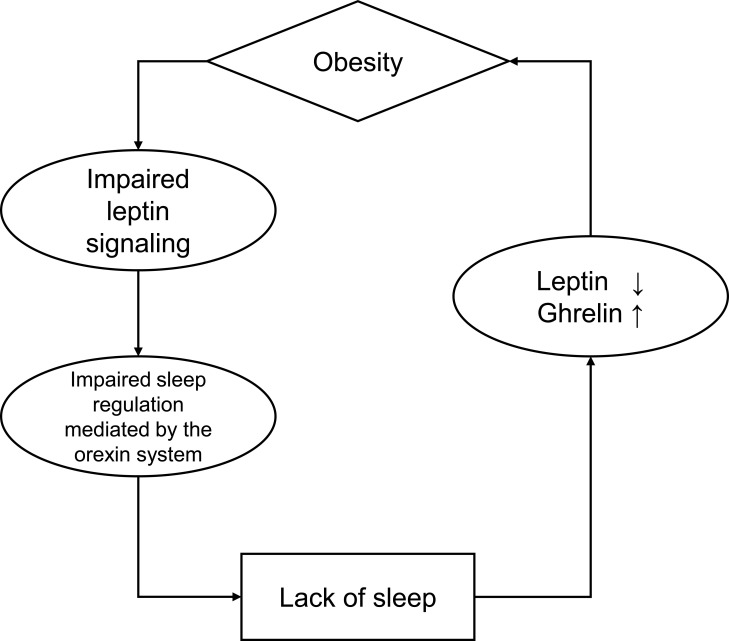
Impact of Body Composition on Appetite-Related Hormones (Figure 1)
Figure 1.
The circle between obesity and sleep.
One mechanism leading to obesity is assumed to be the influence of hormones related to appetite and energy balance, such as leptin and ghrelin.60 Leptin, a metabolic hormone, plays a pivotal role in balancing appetite and satiety by regulating food intake and energy homeostasis.61 Leptin acts synergistically with the metabolic hormone ghrelin. Leptin inhibits food intake and leads weight loss whereas ghrelin increases hunger and food intake.61 Lack of sleep causes a decrease in leptin, an appetite inhibitor secreted by fat cells, and an increase in ghrelin, an appetite stimulant secreted primarily by gastric wall cells.62,63 Additionally, a 30% increase in carbohydrate-rich snack intake was reported in subjects with restricted sleep duration.63 Obesity is not only a consequence of sleep deprivation via appetite and energy balance, but can also cause sleep deprivation. Obesity increases leptin production and secretion and decreases ghrelin production and secretion.64,65 Serum leptin concentration is directly related to fat mass, and increased circulating leptin levels increase leptin resistance and is associated with obesity.66 Leptin has central nervous system-specific effects beyond appetite regulation.67 Leptin levels show a circadian pattern, increasing during the night’s first half and decreasing during the second half.68 Furthermore, leptin maintains deep sleep by opposing the function of orexin neurons in the hypothalamus.69 In animal studies, the orexin (hypocretin) system has been shown to monitor sleep and awakening via interactions with systems that control energy homeostasis. The action of orexin neurons is modulated by leptin, blood glucose, and food intake.69–71 In animal studies using leptin-resistant genetically obese and diabetic mice, disrupted leptin signaling in obesity has been shown to have detrimental effects on sleep regulation.72 Although obesity may be caused by poor sleep quality, as mentioned above, few reports have used clinical data to prove that obesity (increased fat mass), in turn, affects sleep via leptin, and future reports are awaited. In support of this claim, we propose that body composition may influence sleep via appetite-related hormones, as leptin plays an important role in regulating sleep-wake states and metabolism.
Influence of Body Composition on Sleep (Epidemiological Aspects)
Relationship Between Body Composition and SDB
As mentioned above, the hypothesis that body composition, particularly obesity, alters breathing during sleep is based on multiple mechanisms.73 Cross-sectional clinic studies74–82 and population studies83–91 have typically found significant correlations in the relationship between overweight and SDB. In a large-scale population-based, prospective cohort study carried out between July 1989 and January 2000,92 the incidence of SDB was reported to change with increasing or decreasing body weight. A 10% increase in body weight increased AHI by 32% (95% confidence interval [CI], 20%-45), and a 10% decrease in body weight decreased AHI by 26% (95% CI, 18–34%). This trend was even more pronounced when the weight was increased or decreased by 20%. A 20% increase in body weight increased AHI by 70% (95% confidence interval [CI], 42–104%), and a 20% decrease in body weight decreased AHI by 48% (95% CI, 35–58%).92 Furthermore, a 10% increase in body weight was associated with a 6-fold (95% CI, 2.2–17.0) increase in the odds of developing moderate to severe SDB (AHI>15), and a 20% increase in body weight was associated with a 36.6-fold (95% CI, 4.6->50) increase in the odds.92 This shows that even in prospective studies, the incidence of SDB varies with simple weight change. Various other anthropometric parameters have been associated with SDB in cross-sectional studies, including neck morphology22,74–77,80,83,85,86,90 (neck circumference or fat distribution), and obesity78,79,81,82,90 (BMI, skinfold measurements, waist size, waist-to-hip ratio and abdominal visceral adiposity). BMI is an estimate of body fat mass (BFM), an indicator of obesity. However, BMI is limited in its ability to assess actual body composition because it does not precisely reflect true body fat composition and may therefore underestimate health risks of obesity in populations with normal BMI.93 Body composition, such as fat distribution, differs between men and women, with women generally having a higher percentage of body fat (PBF).94 Fat and muscle parameters were correlated in all patients with OSA, with a similar pattern observed in men. However, it is reported that fat parameters were associated with OSA severity in women, but not with muscle parameters.95,96 Furthermore, the type of fat associated with OSA is sex-differentiated, with visceral fat being more implicated in men and total-body fat in women.97 Computed tomography visceral fat area (VFA) assessment shows that men have larger VFA and more severe OSA than women, despite similar BMI and waist circumference, indicating an independent association.98 In a bioelectrical impedance analysis study, AHI was positively correlated with BFM, PBF, and VFA in both women and men, and the correlation was more robust in men.94 Moreover, patients with OSA showed significant correlations with muscle mass (MM), skeletal muscle mass (SMM), and segmental muscle mass. Conversely, female OSA patients only showed correlations with the MM of the trunk, right arm, and left arm, but not with other muscle groups. The authors also reported that the SMM/VFA ratio adjusted for age and BMI showed a significant negative correlation (p=0.015) in men with OSA, but not in women (p=0.354). These reports suggest that the relationship between body composition and OSA is related to both MM and fat mass in men and only to fat mass in women. Muscle and fat distributions may also influence the development of OSA. Furthermore, as mentioned above, OSA is affected by fluid shifts at night. Several epidemiological studies support this hypothesis. In the general population, the prevalence of OSA ranges from 3–14% in men and 4–9% in women.84,99–101 In contrast, the prevalence of OSA is 23–30%102,103 in hypertensive patients and 65–83%104,105 in drug-resistant hypertensive patients. The prevalence of OSA tends to be higher in patients with fluid retention,37 ranging from 31–44%106,107 in patients with end-stage renal disease and 12–26%108,109 in patients with heart failure. In contrast to the general population, the severity of OSA is not associated with a higher BMI in patients with heart failure, suggesting that factors other than obesity are more influential in the development of OSA in this population.110 Moreover, heart failure patients with sleep apnea have higher sodium intake, which enhances fluid retention, than those without sleep apnea, and that AHI correlates with sodium intake.111
Relationship Between Body Composition and Sleep Parameters
Besides BMI, cross-sectional studies have shown that certain body composition parameters are inextricably related to sleep duration and sleep quality.15–17 The combination of reduced SMM and increased fat mass, or sarcopenia-obesity112 is strongly associated with chronic disease,113 especially with short sleep duration.114
Few studies have explicitly investigated whether body composition affects sleep, but a significant correlation between lean body mass and non-REM sleep has been reported. Shapiro et al found a significant positive correlation between lean body mass and the first 7 h of non-REM sleep in men and women (r=0.49, p<0.02) and between lean body mass and slow-wave sleep (r=0.37, p<0.04).115
Although BMI > 25 is considered overweight and the risk of lifestyle-related diseases is a concern, those defined as heavy may include people with shallow fat content, such as bodybuilders. However, people with a high percentage of fat in their body weight may have a BMI that is within the normal range.116 Although it is rare to have difficulty classifying a population’s body size based on BMI alone,117 police officers, firefighters, and other people who require a high level of fitness need to be correctly assessed not only by BMI but also by body fat percentage.118 The relationship between body composition and sleep has been a focus of attention, especially in athletes, who may have a body composition different from the general population, and several reports on the effects of body composition on sleep are acknowledged. In men, lean body mass was negatively correlated with slow-wave sleep (r=−0.41, p<0.05), and these correlations were more pronounced in athletes than in non-athletes (r=−0.54).58 In male and female athletes, the MM percentage was negatively correlated with the rate of slow-wave sleep in the initial non-REM sleep. In contrast, fat mass was reported to be positively correlated with the proportion of slow-wave sleep in initial non-REM sleep.59 Furthermore, this report showed that, while these did not differ significantly between sexes, they did show significant correlations within each sex. Objective sleep quality was poorer in male athletes than in female athletes, indicating that sleep structure may be related to MM.59 Thus, not only obesity but also MM and fat mass may affect sleep structure, indicating that body composition assessment may be meaningful in terms of sleep quality.
Fat distribution may be another factor that defines sleep quality. Based on data from the Korean National Health and Nutrition Examination Survey (2010), a report evaluating the relationship between sleep duration and body composition by dual-energy X-ray absorptiometry in 303 girls revealed that sleep duration was associated with fat distribution and correlated more with fat mass in the trunk than in the head, arms and legs.119 In a report evaluating the association between sleep duration and site-specific fat mass in US adults,120 the authors reported that short sleepers (<7 hours) had a higher trunk fat mass index (dividing the fat mass (kg) by the square of body height (m2)) and higher arm fat mass, arm fat mass index, and leg fat mass than normal sleepers (7–9 hours), but there were no significant differences among those sleeping longer than 9 hours. Similar results were obtained for men, but higher arm fat mass in women was observed only in short-time sleepers. Specifically, short-time sleepers in women had higher arm fat mass and leg fat mass index in the obese group than in normal sleepers, but no association was found in the non-obese group. In US adults, short-time sleep differed from long-time sleep as it was independently related to body fat distribution in different regions, especially in men and obese individuals. In a study whose main objective was to determine the relationship between body composition, including BMI, waist-to-hip ratio, waist-to-height ratio, fat mass index, visceral adipose tissue, and lean BMI (dividing the lean mass [kg] by the square of body height [m2]), and sleep duration and quality, a negative correlation was found between BMI and total sleep time, and the waist-hip ratio was negatively associated with total sleep time and sleep efficiency.121 It also shows that participants with a higher lean mass index have shorter sleep duration and lower sleep efficiency.121 Conversely, no correlation has been reported in objective sleep quality, as indicated by accelerometers, concerning lean BMI.15 Although the difference between these results cannot be definitively stated, Carneiro-Barrera et al argued that their interpretation of the results may be interpreted by the possibility that a lower lean mass index is associated with longer or excessive sleep duration and increased cortisol levels caused by sleep disturbance, which in turn may be associated with muscle deterioration.121
Some studies found that body composition parameters were not associated with subjective sleep quality assessed by the Pittsburgh sleep quality index questionnaire.121 In contrast, others found a negative correlation between lean BMI15 and subjective sleep quality. Subjective sleep quality is highly related to psychological and cognitive functioning.122–124 Body composition parameters are potentially highly relevant to an individual’s intake behavior and daily life and may be due to uncontrollable factors in each study population.
Thus, the effects of the amount and distribution of lean and fat mass on sleep have been reported. Many studies have shown a correlation between sleep parameters and body composition over time. When interpreting the results, it should be considered that it is not possible to clearly show whether sleep affects body composition or whether body composition affects sleep as multiple factors may influence both.
Impact of Interventions on Body Composition on Sleep
Intervention on Nocturnal Fluid Shift
The magnitude and distribution of fluid shift during the night are directly related to the severity of sleep apnea.38–41 A study evaluating the association between fluid shift measurements and respiratory event index in patients hospitalized for ischemic stroke showed a significant association between sitting time and reductions in calf circumference and fluid volume at night.43 The results of this study suggest that activity during the daytime and physical therapy can reduce fluid displacement from the legs overnight. Furthermore, diuretic therapy reportedly decreases AHI, reduces fluid retention in the leg, and increases the neck circumference.125,126 Compression stockings, which prevent lower extremity fluid accumulation during the day and decrease lower extremity fluid volume transfer at night, also decrease AHI in patients with chronic venous insufficiency and OSA.127 Although these reports only involved populations with ischemic stroke43 and cardiovascular disease,125,126 such treatment may be effective in patients with OSA with high baseline fluid retention after body composition analysis.
Intervention on Core Body Temperature
Body composition and body size affect core body temperature through heat production and heat release in the muscle and fat.51–56 Sleep is regulated by core body temperature;47–50 in fact, it has been reported that a higher MM is associated with lower sleep quality.58,59 However, interventions to reduce MM and improve sleep quality are futile. A low core body temperature during the first half of the sleep period is important for sleep quality.128 Furthermore, as the blood flow to the skin increases and body heat is lost, the core body temperature decreases.129 This suggests that manipulation of body temperature may improve sleep quality. Increased sleep quality has been reported with the use of adjusting the temperature of the sleep environment,130 wearing thermosuits that alter body temperature,131,132 wearing socks,133 sleeping on an electric blanket134 and high-heat-capacity mattresses.128 Furthermore, the quality of sleep could be improved not only by manipulating body temperature during sleep but also by adjusting bathing time.135 It has been reported that bathing before bedtime increases the proportion of slow-wave sleep in the first half of sleep136 and decreases the frequency of body movements during sleep,137 thus improving sleep quality. A systematic review and meta-analysis of “water-based passive body heating”138 such as a warm shower or bath before sleep concluded that a nightly shower, foot bath, or body bath for as little as 10 minutes to one-two hours before bedtime can promote a decrease in bedtime core body temperature and optimize sleep onset latency. Based on these reports, it may be possible to improve sleep quality by controlling body temperature before and after bedtime in populations with high MM, such as athletes.
Intervention from the Dietary Aspect
As mentioned above, studies have showed a relationship between sleep quality and obesity.139–141 Several reports exist that, more specifically, evaluate the relationship between sleep and energy intake. People who sleep less have a higher energy intake from fat142,143 and snacking.144 In the National Health and Nutrition Examination Survey data from the United States, populations that sleep less than seven hours per night consume less protein, carbohydrates, fiber, and fat, and to inoculate fewer types of foods, compared to those consumed by normal populations that report sleeping seven to eight hours per night.145 These data are supported by clinical intervention studies, which also showed that restricting sleep increases snacking intake.146 Moreover, participants with sleep restriction tend to prefer foods with high fat.147,148 However, while these studies show a connection between sleep and diet, it is not clear whether sleep influences dietary intake or whether dietary intake affects sleep. Although few studies have evaluated sleep architecture through dietary interventions, Karklin et al reported an increase in sleep onset latency and a decrease in slow-wave sleep duration in nine overweight women following an 800-kcal diet for four weeks.149 One study also reported a decrease in slow-wave sleep and an increase in REM sleep in eight healthy-weight men on a high-carbohydrate, low-fat diet for two days compared to a low-carbohydrate, high-fat diet and a balanced diet for two days.150 Moreover, a study of 26 healthy adults who evaluated sleep architecture through dietary interventions showed that higher fiber intake was associated with less stage N1 sleep (P = 0.0198) and more slow-wave sleep (P = 0.0286), a higher percentage of energy from saturated fatty acids was associated with less slow-wave sleep (P = 0.0422), and a higher rate of energy from sugar and other carbohydrates not considered sugar or fiber was associated with arousal (P = 0.0320 and 0.0481).151 In a review summarizing the effects of food intake on sleep, the authors concluded that a high-carbohydrate diet is associated with decreased sleep onset latency, slow-wave sleep, and increased REM sleep, while a high-fat diet promotes decreased sleep efficiency and REM sleep and increased slow-wave sleep and arousal.152 A similar dietary content for general health (increasing fruit and vegetable intake and choosing whole grains with “high fiber” and vegetable oils with “low saturated fat”) may improve sleep.152
Furthermore, diet may affect body composition and sleep.121 Obesity and central obesity are the most critical pathogenetic factors.20–23 Moreover, obesity may reduce sleep quality through leptin, as shown in Figure 1. Regarding body composition, lean body mass is significantly positively correlated with non-REM and slow-wave sleep115 and sarcopenia-obesity,112 and a combination of reduced skeletal muscle and increased fat mass is strongly associated with reduced sleep duration.114 These findings suggest that a diet that prevents obesity and maintains adequate MM should be implemented. The critical point is that the dietary approach to obesity prevention must also focus on changes in body composition, as the loss of lean body mass can be problematic.153 The Harris-Benedict154 or WHO155 equation should be used to determine the daily energy requirement. Furthermore, the manipulation of diet composition is important.156 Dietary carbohydrates cause excessive insulin secretion157 and promotes body fat accumulation.158,159 Therefore, one way to control obesity is to reduce the carbohydrate ratio in the diet.160 Low-carbohydrate diets for weight loss are long been known.161 Low-carbohydrate diets decrease insulin secretion, promote fat mobilization from adipose tissues, and stimulate the oxidation of free fatty acids.157,162 This results in a decrease in body fat and an increase in energy expenditure.157 Ketogenic diets, a low-carbohydrate diet that maintains a moderate protein intake and consumes at least 70% of its energy from fat while severely limiting carbohydrate intake,163 are considered a strategy for weight loss and improved metabolic control.164 Ketogenic diets are expected to reduce carbohydrate metabolism, increase lipid oxidation, and improve the conversion of free fatty acids to ketone bodies.165–167 In a brief review168 of weight loss methods and lean body mass reduction, 76% of the total weight loss with low-carbohydrate diets was attributed to fat mass and the remaining 24% to lean mass. Conversely, low-fat diets that reduce fat intake similarly result in weight loss.157,169,170 Some meta-analyses have concluded that low-fat diets produce more significant energy expenditure and fat loss than low-carbohydrate diets at the same calories.160 In a brief review168 of weight loss methods and lean body mass reduction, such as low-fat or low-carbohydrate diets, 76% of the total weight loss was attributed to fat mass, and the remaining 24% to lean mass. High-protein diets, with high-fat content and more than 20% of energy derived from protein, prevent obesity by sustaining satiety while maintaining energy expenditure.171 Additionally, proteins, especially the amino acid leucine, can induce muscle protein synthesis and prevent the reduction in lean body mass caused by caloric restriction.172 In a brief review168 of weight loss methods and lean body mass reduction, high-protein diets attributed 89% of the total weight loss to fat mass and the remaining 11% to lean mass, which may be a more appropriate diet from a sleep perspective. Plant-based diets containing plant-based foods (vegetables, fruits, grains, and legumes) also have positive effects on blood lipid levels and body fat percentage.173 These results are generally consistent with the results of epidemiological studies that have evaluated the effects of diet on sleep, and strongly support the possibility that diet mediates body composition and sleep.121 Interventions in body composition based on dietary changes may promote increased sleep quality.
Future Perspectives
The bidirectional effects between SDB, represented by OSAS and obesity, and body composition, have been discussed in terms of epidemiological facts and detailed mechanisms. SDB can demonstrate a bidirectional association between body composition and breathing disorders/nocturnal fluid shifts through an anatomical mechanism. Although there have been epidemiological reports on the effects of obesity and body composition on sleep disturbances and poor sleep quality that differ from those of SDB, few studies have examined the underlying mechanisms. This may be difficult to explain by a single mechanism, as there are other factors that bridge body composition and sleep besides core body temperature and diet, which have been presented in this study. Furthermore, when discussing the impact of body composition on sleep, it is necessary to consider that it is not always possible to indicate whether sleep affects body composition or body composition affects sleep because multiple factors can affect both body composition and sleep.
Further research is needed to demonstrate, with clinical data, that body composition influences sleep via the hypothesized mechanisms (core body temperature and appetite-related hormones). Therefore, it is essential to conduct intervention studies that simultaneously monitor body composition, hypothesized mechanisms (eg, core body temperature and appetite-related hormones), and sleep parameters.
Conclusions
While the epidemiological and mechanistic aspects of the effects of body composition on SDB are being established, there is ambiguity as to whether body composition causes sleep disturbances and poor sleep quality distinct from SDB. Intervention studies to clarify the hypotheses of the mechanisms (core body temperature, diet, something not yet presented) are needed.
Funding Statement
This study was supported by the MEXT*-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (*Ministry of Education, Culture, Sports, Science and Technology), Japanese Center for Research on Women in Sport, Juntendo University, Project for Fostering, Survey Research for the Strategic Strengthening of female athletes 2018–2019 by the Japan Sports Agency. These funding sources did not have any other roles in this study.
Disclosure
Takatoshi Kasai is affiliated with a department endowed by Philips Unk onics, ResMed, Fukuda Denshi, and Paramount Bed. The other authors declare no conflicts of interest.
References
- 1.Grandner MA. Sleep, Health, and Society. Sleep Med Clin. 2017;12(1):1–22. doi: 10.1016/j.jsmc.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–592. doi: 10.5664/jcsm.4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karna B, Sankari A, Tatikonda G. Sleep disorder. In: StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 4.Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am. 2015;99(2):431–439. doi: 10.1016/j.mcna.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto T, Chin K. Prevalence of sleep disturbances: sleep disordered breathing, short sleep duration, and non-restorative sleep. Respir Investig. 2019;57(3):227–237. doi: 10.1016/j.resinv.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131 [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Lu BS, Wang B, et al. Short sleep duration and adiposity in Chinese adolescents. Sleep. 2007;30(12):1688–1697. doi: 10.1093/sleep/30.12.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164(10):947–954. doi: 10.1093/aje/kwj280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33(3):289–295. doi: 10.1093/sleep/33.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiura C, Hashimoto H. A 4-year study of the association between short sleep duration and change in body mass index in Japanese male workers. J Epidemiol. 2010;20(5):385–390. doi: 10.2188/jea.je20100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31(4):517–523. doi: 10.1093/sleep/31.4.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan X, Titova OE, Lindberg E, et al. Association between self-reported sleep duration and body composition in middle-aged and older adults. J Clin Sleep Med. 2019;15(3):431–435. doi: 10.5664/jcsm.7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurado-Fasoli L, Amaro-Gahete FJ, De-la OA, Dote-Montero M, Gutiérrez Á, Castillo MJ. Association between sleep quality and body composition in sedentary middle-aged adults. Medicina. 2018;54(5). doi: 10.3390/medicina54050091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Shin D, Jung GU, Lee D, Park SM. Association between sleep duration, fat mass, lean mass and obesity in Korean adults: the fourth and fifth Korea National Health and Nutrition Examination Surveys. J Sleep Res. 2017;26(4):453–460. doi: 10.1111/jsr.12504 [DOI] [PubMed] [Google Scholar]
- 17.Shechter A, Airo M, Valentin J, et al. Effects of continuous positive airway pressure on body composition in individuals with obstructive sleep apnea: a non-randomized, matched before-after study. J Clin Med. 2019;(8):8. doi: 10.3390/jcm8081195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muscogiuri G, Barrea L, Annunziata G, et al. Obesity and sleep disturbance: the chicken or the egg? Crit Rev Food Sci Nutr. 2019;59(13):2158–2165. doi: 10.1080/10408398.2018.1506979 [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Bixler EO, Basta M. Obesity and sleep: a bidirectional association? Sleep. 2010;33(5):573–574. doi: 10.1093/sleep/33.5.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim YH, Choi J, Kim KR, et al. Sex-specific characteristics of anthropometry in patients with obstructive sleep apnea: neck circumference and waist-Hip ratio. Ann Otol Rhinol Laryngol. 2014;123(7):517–523. doi: 10.1177/0003489414526134 [DOI] [PubMed] [Google Scholar]
- 21.Saint Martin M, Roche F, Thomas T, Collet P, Barthélémy JC, Sforza E. Association of body fat composition and obstructive sleep apnea in the elderly: a longitudinal study. Obesity. 2015;23(7):1511–1516. doi: 10.1002/oby.21121 [DOI] [PubMed] [Google Scholar]
- 22.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157(1):280–283. doi: 10.1164/ajrccm.157.1.9703018 [DOI] [PubMed] [Google Scholar]
- 23.Unal Y, Ozturk DA, Tosun K, Kutlu G. Association between obstructive sleep apnea syndrome and waist-to-height ratio. Sleep Breath. 2019;23(2):523–529. doi: 10.1007/s11325-018-1725-4 [DOI] [PubMed] [Google Scholar]
- 24.Yildirim Y, Yilmaz S, Güven M, et al. Evaluation of anthropometric and metabolic parameters in obstructive sleep apnea. Pulm Med. 2015;2015:189761. doi: 10.1155/2015/189761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esaki K. Morphological analysis by lateral cephalography of sleep apnea syndrome in 53 patients. Kurume Med J. 1995;42(4):231–240. doi: 10.2739/kurumemedj.42.231 [DOI] [PubMed] [Google Scholar]
- 26.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg AN, Schwab RJ. Identifying the patient with sleep apnea: upper airway assessment and physical examination. Otolaryngol Clin North Am. 1998;31(6):919–930. doi: 10.1016/s0030-6665(05)70099-2 [DOI] [PubMed] [Google Scholar]
- 28.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162(2 Pt 1):740–748. doi: 10.1164/ajrccm.162.2.9908123 [DOI] [PubMed] [Google Scholar]
- 29.Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108(6):1009–1015. doi: 10.1097/ALN.0b013e318173f103 [DOI] [PubMed] [Google Scholar]
- 30.Khoo MC, Gottschalk A, Pack AI. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol. 1991;70(5):2014–2024. doi: 10.1152/jappl.1991.70.5.2014 [DOI] [PubMed] [Google Scholar]
- 31.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(5):1181–1190. doi: 10.1164/ajrccm.163.5.2007013 [DOI] [PubMed] [Google Scholar]
- 32.Imayama I, Prasad B. Role of leptin in obstructive sleep apnea. Ann Am Thorac Soc. 2017;14(11):1607–1621. doi: 10.1513/AnnalsATS.201702-181FR [DOI] [PubMed] [Google Scholar]
- 33.Kimura Y, Kasai T, Tomita Y, et al. Relationship between metabolic syndrome and hypercapnia among obese patients with sleep apnea. World J Respirol. 2020;10(1):1–10. doi: 10.5320/wjr.v10.i1.1 [DOI] [Google Scholar]
- 34.Kapás L, Szentirmai É. Brown adipose tissue at the intersection of sleep and temperature regulation. Temperature. 2014;1(1):16–17. doi: 10.4161/temp.29120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muscogiuri G, Barrea L, Aprano S, et al. Sleep quality in obesity: does adherence to the Mediterranean diet matter? Nutrients. 2020;12(5):1364. doi: 10.3390/nu12051364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Curr Opin Pulm Med. 2019;25(6):602–608. doi: 10.1097/mcp.0000000000000627 [DOI] [PubMed] [Google Scholar]
- 37.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591(5):1179–1193. doi: 10.1113/jphysiol.2012.245159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174(12):1378–1383. doi: 10.1164/rccm.200607-927OC [DOI] [PubMed] [Google Scholar]
- 39.Su MC, Chiu KL, Ruttanaumpawan P, et al. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir Physiol Neurobiol. 2008;161(3):306–312. doi: 10.1016/j.resp.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 40.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179(3):241–246. doi: 10.1164/rccm.200807-1076OC [DOI] [PubMed] [Google Scholar]
- 41.Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121(14):1598–1605. doi: 10.1161/circulationaha.109.902452 [DOI] [PubMed] [Google Scholar]
- 42.Ding N, Lin W, Zhang XL, et al. Overnight fluid shifts in subjects with and without obstructive sleep apnea. J Thorac Dis. 2014;6(12):1736–1741. doi: 10.3978/j.issn.2072-1439.2014.11.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasai T. Fluid retention and rostral fluid shift in sleep-disordered breathing. Curr Hypertens Rev. 2016;12(1):32–42. doi: 10.2174/1573402112666160114093550 [DOI] [PubMed] [Google Scholar]
- 44.Kasai T, Motwani SS, Yumino D, Mak S, Newton GE, Bradley TD. Differing relationship of nocturnal fluid shifts to sleep apnea in men and women with heart failure. Circ Heart Fail. 2012;5(4):467–474. doi: 10.1161/circheartfailure.111.965814 [DOI] [PubMed] [Google Scholar]
- 45.Brown DL, Yadollahi A, He K, et al. Overnight rostral fluid shifts exacerbate obstructive sleep apnea after stroke. Stroke. 2021;52(10):3176–3183. doi: 10.1161/strokeaha.120.032688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurinami N, Sugiyama S, Ijima H, et al. Clinical usefulness of the body muscle-to-fat ratio for screening obstructive sleep apnea syndrome in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;143:134–139. doi: 10.1016/j.diabres.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 47.Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci. 2010;15(2):604–625. doi: 10.2741/3636 [DOI] [PubMed] [Google Scholar]
- 48.Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8(2):81–93. doi: 10.1016/s1087-0792(03)00023-6 [DOI] [PubMed] [Google Scholar]
- 49.Togo F, Aizawa S, Arai J, et al. Influence on human sleep patterns of lowering and delaying the minimum core body temperature by slow changes in the thermal environment. Sleep. 2007;30(6):797–802. doi: 10.1093/sleep/30.6.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kräuchi K, Fattori E, Giordano A, et al. Sleep on a high heat capacity mattress increases conductive body heat loss and slow wave sleep. Physiol Behav. 2018;185:23–30. doi: 10.1016/j.physbeh.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 51.Anderson GS. Human morphology and temperature regulation. Int J Biometeorol. 1999;43(3):99–109. doi: 10.1007/s004840050123 [DOI] [PubMed] [Google Scholar]
- 52.McClelland JM, Godek SF, Chlad PS, Feairheller DL, Morrison KE. Effects of cardiovascular fitness and body composition on maximal core temperature in collegiate football players during preseason. J Strength Cond Res. 2018;32(6):1662–1670. doi: 10.1519/jsc.0000000000002027 [DOI] [PubMed] [Google Scholar]
- 53.Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(6):1001–1006. doi: 10.1152/jappl.1977.43.6.1001 [DOI] [PubMed] [Google Scholar]
- 54.Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr. 1980;33(11):2372–2374. doi: 10.1093/ajcn/33.11.2372 [DOI] [PubMed] [Google Scholar]
- 55.Thompson J, Manore MM. Predicted and measured resting metabolic rate of male and female endurance athletes. J Am Diet Assoc. 1996;96(1):30–34. doi: 10.1016/s0002-8223(96)00010-7 [DOI] [PubMed] [Google Scholar]
- 56.Taguchi M, Ishikawa-Takata K, Tatsuta W, et al. Resting energy expenditure can be assessed by fat-free mass in female athletes regardless of body size. J Nutr Sci Vitaminol. 2011;57(1):22–29. doi: 10.3177/jnsv.57.22 [DOI] [PubMed] [Google Scholar]
- 57.Buresh R, Berg K, Noble J. Heat production and storage are positively correlated with measures of body size/composition and heart rate drift during vigorous running. Res Q Exerc Sport. 2005;76(3):267–274. doi: 10.1080/02701367.2005.10599298 [DOI] [PubMed] [Google Scholar]
- 58.Paxton SJ, Trinder J, Shapiro CM, Adam K, Oswald I, Gräf KJ. Effect of physical fitness and body composition on sleep and sleep-related hormone concentrations. Sleep. 1984;7(4):339–346. doi: 10.1093/sleep/7.4.339 [DOI] [PubMed] [Google Scholar]
- 59.Kitamura E, Kawasaki Y, Kasai T, et al. The relationship between body composition and sleep architecture in athletes. Sleep Med. 2021;87:92–96. doi: 10.1016/j.sleep.2021.08.028 [DOI] [PubMed] [Google Scholar]
- 60.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91(11):881–884. doi: 10.1136/adc.2005.093013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x [DOI] [PubMed] [Google Scholar]
- 62.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008 [DOI] [PubMed] [Google Scholar]
- 64.Monti V, Carlson JJ, Hunt SC, Adams TD. Relationship of ghrelin and leptin hormones with body mass index and waist circumference in a random sample of adults. J Am Diet Assoc. 2006;106(6):822–8;quiz 829–30. doi: 10.1016/j.jada.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 65.Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87(1):240–244. doi: 10.1210/jcem.87.1.8129 [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7(2):207–222. doi: 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olson CA, Hamilton NA, Somers VK. Percentage of REM sleep is associated with overnight change in leptin. J Sleep Res. 2016;25(4):419–425. doi: 10.1111/jsr.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Licinio J, Mantzoros C, Negrão AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3(5):575–579. doi: 10.1038/nm0597-575 [DOI] [PubMed] [Google Scholar]
- 69.Hirota T, Morioka T, Yoda K, et al. Positive association of plasma leptin with sleep quality in obese type 2 diabetes patients. J Diabetes Investig. 2018;9(5):1100–1105. doi: 10.1111/jdi.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. doi: 10.1016/s0896-6273(03)00331-3 [DOI] [PubMed] [Google Scholar]
- 71.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092 [DOI] [PubMed] [Google Scholar]
- 72.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2059–66. doi: 10.1152/ajpregu.00026.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strobel RJ, Rosen RC. Obesity and weight loss in obstructive sleep apnea: a critical review. Sleep. 1996;19(2):104–115. doi: 10.1093/sleep/19.2.104 [DOI] [PubMed] [Google Scholar]
- 74.Davies R. Stradling. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. European Respiratory Journal. 1990;3(5):509–514. [PubMed] [Google Scholar]
- 75.Katz I, Stradling J, Sljutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141(5_pt_1):1228–1231. doi: 10.1164/ajrccm/141.5_Pt_1.1228 [DOI] [PubMed] [Google Scholar]
- 76.Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J. 1992;5(4):377–381. [PubMed] [Google Scholar]
- 77.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47(2):101–105. doi: 10.1136/thx.47.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17(9):533–540. [PubMed] [Google Scholar]
- 79.Levinson PD, McGarvey ST, Carlisle CC, Eveloff SE, Herbert PN, Millman RP. Adiposity and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 1993;103(5):1336–1342. doi: 10.1378/chest.103.5.1336 [DOI] [PubMed] [Google Scholar]
- 80.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148(2):462–466. doi: 10.1164/ajrccm/148.2.462 [DOI] [PubMed] [Google Scholar]
- 81.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107(2):362–366. doi: 10.1378/chest.107.2.362 [DOI] [PubMed] [Google Scholar]
- 82.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241(1):11–18. doi: 10.1046/j.1365-2796.1997.63889000.x [DOI] [PubMed] [Google Scholar]
- 83.Olson LG, King MT, Hensley MJ, Saunders NA. A community study of snoring and sleep-disordered breathing. Prevalence. Am J Respir Crit Care Med. 1995;152(2):711–716. doi: 10.1164/ajrccm.152.2.7633731 [DOI] [PubMed] [Google Scholar]
- 84.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Eng J Med. 1993;328(17):1230–1235. doi: 10.1056/nejm199304293281704 [DOI] [PubMed] [Google Scholar]
- 85.Ferini-Strambi L, Zucconi M, Palazzi S, et al. Snoring and nocturnal oxygen desaturations in an Italian middle-aged male population. Epidemiologic study with an ambulatory device. Chest. 1994;105(6):1759–1764. doi: 10.1378/chest.105.6.1759 [DOI] [PubMed] [Google Scholar]
- 86.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46(2):85–90. doi: 10.1136/thx.46.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmidt-Nowara WW, Coultas DB, Wiggins C, Skipper BE, Samet JM. Snoring in a Hispanic-American population. Risk factors and association with hypertension and other morbidity. Arch Intern Med. 1990;150(3):597–601. doi: 10.1001/archinte.150.3.597 [DOI] [PubMed] [Google Scholar]
- 88.Jennum P, Hein HO, Suadicani P, Gyntelberg F. Cardiovascular risk factors in snorers. A cross-sectional study of 3323 men aged 54 to 74 years: the Copenhagen Male Study. Chest. 1992;102(5):1371–1376. doi: 10.1378/chest.102.5.1371 [DOI] [PubMed] [Google Scholar]
- 89.Jennum P, Sjøl A. Snoring, sleep apnoea and cardiovascular risk factors: the MONICA II Study. Int J Epidemiol. 1993;22(3):439–444. doi: 10.1093/ije/22.3.439 [DOI] [PubMed] [Google Scholar]
- 90.Bearpark H, Elliott L, Grunstein R, et al. Occurrence and correlates of sleep disordered breathing in the Australian town of Busselton: a preliminary analysis. Sleep. 1993;16(8 Suppl):S3–5. [PubMed] [Google Scholar]
- 91.Enright PL, Newman AB, Wahl PW, Manolio TA, Haponik EF, Boyle PJ. Prevalence and correlates of snoring and observed apneas in 5201 older adults. Sleep. 1996;19(7):531–538. doi: 10.1093/sleep/19.7.531 [DOI] [PubMed] [Google Scholar]
- 92.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015 [DOI] [PubMed] [Google Scholar]
- 93.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox”. J Am Coll Cardiol. 2012;60(15):1374–1380. doi: 10.1016/j.jacc.2012.05.037 [DOI] [PubMed] [Google Scholar]
- 94.Kim JR, Song P, Joo EY. Sex differences in obstructive sleep apnea by bioelectrical impedance analysis. J Clin Neurol. 2021;17(2):283–289. doi: 10.3988/jcn.2021.17.2.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem. 2008;114(4):211–223. doi: 10.1080/13813450802364627 [DOI] [PubMed] [Google Scholar]
- 96.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484 [DOI] [PubMed] [Google Scholar]
- 97.Kritikou I, Basta M, Tappouni R, et al. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J. 2013;41(3):601–609. doi: 10.1183/09031936.00183411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harada Y, Oga T, Chihara Y, et al. Differences in associations between visceral fat accumulation and obstructive sleep apnea by sex. Ann Am Thorac Soc. 2014;11(3):383–391. doi: 10.1513/AnnalsATS.201306-182OC [DOI] [PubMed] [Google Scholar]
- 99.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. doi: 10.1378/chest.119.1.62 [DOI] [PubMed] [Google Scholar]
- 100.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065 [DOI] [PubMed] [Google Scholar]
- 101.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: i. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079 [DOI] [PubMed] [Google Scholar]
- 102.Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103(2):190–195. doi: 10.7326/0003-4819-103-2-190 [DOI] [PubMed] [Google Scholar]
- 103.Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med. 1998;157(1):111–115. doi: 10.1164/ajrccm.157.1.9609063 [DOI] [PubMed] [Google Scholar]
- 104.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022 [DOI] [PubMed] [Google Scholar]
- 105.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131(2):453–459. doi: 10.1378/chest.06-1442 [DOI] [PubMed] [Google Scholar]
- 106.de Oliveira Rodrigues CJ, Marson O, Tufic S, et al. Relationship among end-stage renal disease, hypertension, and sleep apnea in nondiabetic dialysis patients. Am J Hypertens. 2005;18(2 Pt 1):152–157. doi: 10.1016/j.amjhyper.2004.08.028 [DOI] [PubMed] [Google Scholar]
- 107.Jurado-Gamez B, Martin-Malo A, Alvarez-Lara MA, Muñoz L, Cosano A, Aljama P. Sleep disorders are underdiagnosed in patients on maintenance hemodialysis. Nephron Clin Pract. 2007;105(1):c35–42. doi: 10.1159/000096982 [DOI] [PubMed] [Google Scholar]
- 108.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106(1):21–28. doi: 10.1016/j.ijcard.2004.12.068 [DOI] [PubMed] [Google Scholar]
- 109.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15(4):279–285. doi: 10.1016/j.cardfail.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 110.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716–1722. doi: 10.1001/archinte.166.16.1716 [DOI] [PubMed] [Google Scholar]
- 111.Kasai T, Arcand J, Allard JP, et al. Relationship between sodium intake and sleep apnea in patients with heart failure. J Am Coll Cardiol. 2011;58(19):1970–1974. doi: 10.1016/j.jacc.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 112.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. 2019;22(1):13–19. doi: 10.1097/mco.0000000000000519 [DOI] [PubMed] [Google Scholar]
- 114.Hu X, Jiang J, Wang H, Zhang L, Dong B, Yang M. Association between sleep duration and sarcopenia among community-dwelling older adults: a cross-sectional study. Medicine. 2017;96(10):e6268. doi: 10.1097/md.0000000000006268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shapiro CM, Catterall J, Warren P, et al. Lean body mass and non-rapid eye movement sleep. Br Med J. 1987;294(6563):22. doi: 10.1136/bmj.294.6563.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. doi: 10.1093/ajcn/72.3.694 [DOI] [PubMed] [Google Scholar]
- 117.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. Obes Res. 1998;6(Suppl 2):51s–209s. [PubMed] [Google Scholar]
- 118.Gledhill N, Jamnik VK. Development and validation of a fitness screening protocol for firefighter applicants. Can J Sport Sci. 1992;17(3):199–206. [PubMed] [Google Scholar]
- 119.Park HK, Kim J, Shim YS. Association between sleep duration and body composition in girls ten to eighteen years of age: a population-based study. Child Obes. 2020;16(4):281–290. doi: 10.1089/chi.2019.0191 [DOI] [PubMed] [Google Scholar]
- 120.Xu C, Zhao S, Yu S, et al. Short sleep duration was associated with increased regional body fat in US adults: the NHANES from 2011–2018. Nutrients. 2022;14(14). doi: 10.3390/nu14142840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carneiro-Barrera A, Amaro-Gahete FJ, Acosta FM, Ruiz JR. Body composition impact on sleep in young adults: the mediating role of sedentariness, physical activity, and diet. J Clin Med. 2020;9(5). doi: 10.3390/jcm9051560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Medicine Reviews. 2010;14(3):179–189. doi: 10.1016/j.smrv.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 123.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571. [PMC free article] [PubMed] [Google Scholar]
- 124.Brandolim Becker N, Jesus SN, Viseu JN, Stobäus CD, Guerreiro M, Domingues RB. Depression and quality of life in older adults: mediation effect of sleep quality. Int J Clin Health Psychol. 2018;18(1):8–17. doi: 10.1016/j.ijchp.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32(3):673–680. doi: 10.1097/hjh.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 126.Bucca CB, Brussino L, Battisti A, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest. 2007;132(2):440–446. doi: 10.1378/chest.07-0311 [DOI] [PubMed] [Google Scholar]
- 127.Redolfi S, Arnulf I, Pottier M, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med. 2011;184(9):1062–1066. doi: 10.1164/rccm.201102-0350OC [DOI] [PubMed] [Google Scholar]
- 128.Reid KJ, Kräuchi K, Grimaldi D, et al. Effects of manipulating body temperature on sleep in postmenopausal women. Sleep Med. 2021;81:109–115. doi: 10.1016/j.sleep.2021.01.064 [DOI] [PubMed] [Google Scholar]
- 129.Kräuchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11(6):439–451. doi: 10.1016/j.smrv.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 130.Dewasmes G, Nicolas A, Rodriguez D, et al. Human core temperature minimum can be modified by ambient thermal transients. Neurosci Lett. 1994;173(1–2):151–154. doi: 10.1016/0304-3940(94 [DOI] [PubMed] [Google Scholar]
- 131.Fronczek R, Raymann RJ, Overeem S, et al. Manipulation of skin temperature improves nocturnal sleep in narcolepsy. J Neurol Neurosurg Psychiatry. 2008;79(12):1354–1357. doi: 10.1136/jnnp.2008.143610 [DOI] [PubMed] [Google Scholar]
- 132.Raymann RJ, Swaab DF, Van Someren EJ. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131(Pt 2):500–513. doi: 10.1093/brain/awm315 [DOI] [PubMed] [Google Scholar]
- 133.Ko Y, Lee JY. Effects of feet warming using bed socks on sleep quality and thermoregulatory responses in a cool environment. J Physiol Anthropol. 2018;37(1):13. doi: 10.1186/s40101-018-0172-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fletcher A, van den Heuvel C, Dawson D. Sleeping with an electric blanket: effects on core temperature, sleep, and melatonin in young adults. Sleep. 1999;22(3):313–318. doi: 10.1093/sleep/22.3.313 [DOI] [PubMed] [Google Scholar]
- 135.Kawasaki Y, Kasai T, Koikawa N, et al. Sex differences in factors associated with poor subjective sleep quality in athletes. J Sports Med Phys Fitness. 2020;60(1):140–151. doi: 10.23736/s0022-4707.19.09875-x [DOI] [PubMed] [Google Scholar]
- 136.Horne JA, Reid AJ. Night-time sleep EEG changes following body heating in a warm bath. Electroencephalogr Clin Neurophysiol. 1985;60(2):154–157. doi: 10.1016/0013-4694(85)90022-7 [DOI] [PubMed] [Google Scholar]
- 137.Kanda K, Tochihara Y, Ohnaka T. Bathing before sleep in the young and in the elderly. Eur J Appl Physiol Occup Physiol. 1999;80(2):71–75. doi: 10.1007/s004210050560 [DOI] [PubMed] [Google Scholar]
- 138.Haghayegh S, Khoshnevis S, Smolensky MH, Diller KR, Castriotta RJ. Before-bedtime passive body heating by warm shower or bath to improve sleep: a systematic review and meta-analysis. Sleep Med Rev. 2019;46:124–135. doi: 10.1016/j.smrv.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 139.Patel SR. Reduced sleep as an obesity risk factor. Obes Rev. 2009;10(Suppl 2):61–68. doi: 10.1111/j.1467-789X.2009.00664.x [DOI] [PubMed] [Google Scholar]
- 140.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16(3):643–653. doi: 10.1038/oby.2007.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Keith SW, Redden DT, Katzmarzyk PT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes. 2006;30(11):1585–1594. doi: 10.1038/sj.ijo.0803326 [DOI] [PubMed] [Google Scholar]
- 142.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33(9):1201–1209. doi: 10.1093/sleep/33.9.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci. 2002;21(2):115–120. doi: 10.2114/jpa.21.115 [DOI] [PubMed] [Google Scholar]
- 144.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11(2):180–184. doi: 10.1016/j.sleep.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;(64):71–80. doi: 10.1016/j.appet.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–133. doi: 10.3945/ajcn.2008.26574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90(6):1476–1482. doi: 10.3945/ajcn.2009.27984 [DOI] [PubMed] [Google Scholar]
- 149.Karklin A, Driver HS, Buffenstein R. Restricted energy intake affects nocturnal body temperature and sleep patterns. Am J Clin Nutr. 1994;59(2):346–349. doi: 10.1093/ajcn/59.2.346 [DOI] [PubMed] [Google Scholar]
- 150.Phillips F, Chen CN, Crisp AH, et al. Isocaloric diet changes and electroencephalographic sleep. Lancet. 1975;2(7938):723–725. doi: 10.1016/s0140-6736(75)90718-7 [DOI] [PubMed] [Google Scholar]
- 151.St-Onge MP, Roberts A, Shechter A, Choudhury AR. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med. 2016;12(1):19–24. doi: 10.5664/jcsm.5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.St-Onge MP, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. 2016;7(5):938–949. doi: 10.3945/an.116.012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aragon AA, Schoenfeld BJ, Wildman R, et al. International society of sports nutrition position stand: diets and body composition. J Int Soc Sports Nutr. 2017;14:16. doi: 10.1186/s12970-017-0174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harris JA, Benedict FG, Biometric A. Study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4(12):370–373. doi: 10.1073/pnas.4.12.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.World Health Organization. Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. World Health Organ Tech Rep Ser. 1985;724:1–206. [PubMed] [Google Scholar]
- 156.Atakan MM, Koşar ŞN, Güzel Y, Tin HT, Yan X. The role of exercise, diet, and cytokines in preventing obesity and improving adipose tissue. Nutrients. 2021;13(5). doi: 10.3390/nu13051459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104(2):324–333. doi: 10.3945/ajcn.116.133561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167–2168. doi: 10.1001/jama.2014.4133 [DOI] [PubMed] [Google Scholar]
- 159.Taubes G. The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. BMJ. 2013;346:f1050. doi: 10.1136/bmj.f1050 [DOI] [PubMed] [Google Scholar]
- 160.Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718–1727.e3. doi: 10.1053/j.gastro.2017.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Banting W. Letter on corpulence, addressed to the public. Obes Res. 1993;1(2):153–163. doi: 10.1002/j.1550-8528.1993.tb00605.x [DOI] [PubMed] [Google Scholar]
- 162.Fleming JA, Kris-Etherton PM. Macronutrient content of the diet: what do we know about energy balance and weight maintenance? Curr Obes Rep. 2016;5(2):208–213. doi: 10.1007/s13679-016-0209-8 [DOI] [PubMed] [Google Scholar]
- 163.Noakes TD, Windt J. Evidence that supports the prescription of low-carbohydrate high-fat diets: a narrative review. Br J Sports Med. 2017;51(2):133–139. doi: 10.1136/bjsports-2016-096491 [DOI] [PubMed] [Google Scholar]
- 164.Stubbs BJ, Newman JC. Ketogenic diet and adipose tissue inflammation-a simple story? Fat chance! Nat Metab. 2020;2(1):3–4. doi: 10.1038/s42255-019-0164-2 [DOI] [PubMed] [Google Scholar]
- 165.Dyson PA, Beatty S, Matthews DR. A low-carbohydrate diet is more effective in reducing body weight than healthy eating in both diabetic and non-diabetic subjects. Diabet Med. 2007;24(12):1430–1435. doi: 10.1111/j.1464-5491.2007.02290.x [DOI] [PubMed] [Google Scholar]
- 166.Goday A, Bellido D, Sajoux I, et al. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. 2016;6(9):e230. doi: 10.1038/nutd.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harvey C, Schofield GM, Zinn C, Thornley SJ, Crofts C, Merien FLR. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: a randomised clinical trial. PeerJ. 2019;7:e6273. doi: 10.7717/peerj.6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Willoughby D, Hewlings S, Kalman D. Body composition changes in weight loss: strategies and supplementation for maintaining lean body mass, a brief review. Nutrients. 2018;10(12). doi: 10.3390/nu10121876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147–157. doi: 10.7326/0003-4819-153-3-201008030-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627–2634. doi: 10.1001/jama.2012.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr. 2012;108(Suppl 2):S105–12. doi: 10.1017/s0007114512002589 [DOI] [PubMed] [Google Scholar]
- 172.Hector AJ, Phillips SM. Protein recommendations for weight loss in elite athletes: a focus on body composition and performance. Int J Sport Nutr Exerc Metab. 2018;28(2):170–177. doi: 10.1123/ijsnem.2017-0273 [DOI] [PubMed] [Google Scholar]
- 173.Turner-McGrievy G, Mandes T, Crimarco A. A plant-based diet for overweight and obesity prevention and treatment. J Geriatr Cardiol. 2017;14(5):369–374. doi: 10.11909/j.issn.1671-5411.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]