Abstract
Background:
Several epidemiological studies have reported the protective role of caffeine on health outcomes; however, it remained debatable on caffeine consumption and brain amyloid positivity.
Objective:
We aimed to determine the relationship between caffeine consumption and brain amyloid pathology in cognitively normal older adults.
Methods:
The dataset used for analysis in this cross-sectional study was selected from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) Study. Multivariable logistic regression analyses were performed to explore the association between caffeine consumption and amyloid positivity using odds ratios (ORs) and 95% confidence intervals (CIs).
Results:
In total, 4,394 participants were included in the final analysis. No significant association between caffeine consumption and amyloid positivity was observed in the whole participants (OR, 0.95; 95% CI, 0.78–1.14; p = 0.558). Subgroup analysis showed that caffeine intake was significantly associated with decreased amyloid positivity in males (OR, 0.72; 95% CI, 0.54–0.97; p = 0.032) but not in females (OR, 1.14; 95% CI, 0.90–1.46; p = 0.280), and the association between caffeine and amyloid positivity was not affected by age or APOE genotypes. In addition, different levels of caffeine were not associated with amyloid positivity.
Conclusion:
The findings suggest that caffeine consumption was not significantly associated with amyloid positivity in the whole sample. However, caffeine consumption may be inversely associated with amyloid positivity among males but not females. More studies are needed to explore the mechanisms underlying caffeine consumption and brain amyloid positivity.
Keywords: Amyloid positivity, caffeine, dementia, sex-specific
INTRODUCTION
Caffeine, a central nervous system stimulant of the methylxanthine class, occurs naturally in coffee, tea, and cocoa [1]. It has been consumed for hundreds of years and has become an important part of cultural traditions and social life [2]. It is the most extensively consumed psychoactive substance [3] and is legal and unregulated in almost all parts of the world. It is estimated that adults consume an average of nearly two cups of coffee daily, which is the most typical beverage containing caffeine [4]. Caffeine consumption can affect multiple health outcomes [5], and has beneficial effects on various systems, including cardiology [6], endocrinology [7], psychiatry [8], and neurology [9].
Epidemiological studies have reported protective effects of caffeine against Alzheimer’s disease (AD) [10–12] and cognitive decline [13, 14]. However, there is limited information on the neuropathological evidence supporting the protective effects of caffeine against AD and related cognitive decline in humans. Preclinical findings indicate that caffeine utilization can reduce amyloid-β (Aβ) levels in mammalian animals, particularly in male rats [15–17]. A study indicated that lifetime coffee intake of ≥2 cups/day was significantly associated with a lower Aβ positivity in the human brain, compared to coffee intake of <2 cups/day [18]. Another cohort study also found that higher coffee consumption is associated with slower cognitive decline and less cerebral Aβ accumulation over 126 months [19].
As a biomarker for dementia, detecting Aβ in cognitively normal older adults has clinical implications for the prevention and intervention of cognitive decline and dementia in their early stage [20–22]. Although great progress has been made in exploring the mechanisms underlying the relationship between caffeine and amyloid positivity, the relationship between caffeine intake and amyloid pathology in the human brain in the stratified populations remains unclear. Accordingly, we aimed to determine the relationship between caffeine intake and brain amyloid pathology in cognitively normal older adults. Moreover, we aimed to determine the magnitude of the effect of caffeine intake on amyloid positivity among population-stratified participants.
METHODS
Participants
The dataset used for analysis in this cross-sectional study was selected from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4)/Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study, which was conducted at 67 clinical trial sites in the US, Canada, and Japan [23]. The A4 study is a secondary prevention trial of an anti-amyloid antibody in clinically normal individuals aged 65–85 years with elevated brain Aβ peptides on positron emission tomography (PET), as described elsewhere [24]. The inclusion criteria comprised complete information on caffeine consumption, demographic information, habits, apolipoprotein E (APOE) genotypes and comorbidities, a score between 25 and 30 on the Mini-Mental State Examination [25], and a global Clinical Dementia Rating (CDR) scale score of 0 [26]. This study was approved by the institutional review boards of all participating institutions, and written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. To apply for the use of A4 data, the study was also approved by the Institutional Review Board of the Peking University Sixth Hospital.
Assessment of caffeine intake
Participants were asked to report their daily consumption of caffeine-containing beverages using the average number of cups of caffeine consumed daily. Participants were considered as caffeine drinkers (≥1 cup/day) and non-caffeine drinkers (0 cups/day) based on their daily consumption of caffeine-containing beverages.
Amyloid PET neuroimaging
Amyloid PET neuroimaging was performed at baseline in the A4/LEARN study. Cerebral Aβ levels were determined using post-processed Aβ tracer florbetapir (18F-AV-45) PET imaging data. PET data processing was conducted by Invacare LLC. We used the continuous composite total cerebral amyloid tracer standardized uptake value ratio (SUVR), calculated using the whole cerebellum as the reference region. Aβ positivity was defined as an 18F-florbetapir PET SUVR ≥1.10, while Aβ negativity was defined as an SUVR <1.10, as previously described [27, 28].
Covariates
Covariates contained demographic information, including age, sex, body mass index (BMI), marital status, and educational attainment; habits, including alcohol consumption and smoking; APOE genotypes; and comorbidities, including psychiatric, neurological, cardiovascular, and endocrinological diseases. A summary of covariates is provided in Supplementary Table 1. According to previous literature [29], age was categorized into 5-year age groups as follows: 65–70, 70–75, 75–80, and 80–85 years. Based on the criteria established by the National Institute of Health [30], the BMI cut-off values for underweight, normal weight, overweight, and obesity were <18.5, 18.5–24.9, 25.0–29.9, and ≥30 kg/m2, respectively. The APOE genotypes were ɛ2/ɛ2, ɛ2/ɛ3, ɛ2/ɛ4, ɛ3/ɛ3, ɛ3/ɛ4, and ɛ4/ɛ4. The most common APOE allele, APOE ɛ3, was associated with an average risk of AD, whereas the APOE ɛ4 and APOE ɛ2 alleles lead to higher and lower AD risks, respectively [31, 32].
Table 1.
Demographic and clinical characteristics of participants
| Total | Non-caffeine drinkers | Caffeine drinkers | p | |
| Number of observations (N [% ]) | 4,394 (100.0) | 698 (100.0) | 3,696 (100.0) | |
| Sex (N [% ]) | 0.390 | |||
| Male | 17,83 (40.6) | 273 (39.1) | 15,10 (40.9) | |
| Female | 2,611 (59.4) | 425 (60.9) | 2,186 (59.1) | |
| Age (mean [SD]) | 71.29±4.67 | 71.42±4.62 | 71.26±4.68 | 0.425 |
| Age (N [% ]) | 0.932 | |||
| 65–70 y | 2,087 (47.5) | 331 (47.4) | 1,756 (47.5) | |
| 70–75 y | 1,407 (32) | 222 (31.8) | 1,185 (32.1) | |
| 75–80 y | 640 (14.6) | 100 (14.3) | 540 (14.6) | |
| 80–85 y | 260 (5.9) | 45 (6.4) | 215 (5.8) | |
| BMI (mean [SD]) | 27.50±5.10 | 27.45±5.44 | 27.51±5.04 | 0.779 |
| BMI (N [% ]) | 0.352 | |||
| Underweight (<18.5 kg/m2) | 32 (0.7) | 7 (1.0) | 25 (0.7) | |
| Normal weight (18.5–24.9 kg/m2) | 1,469 (33.4) | 249 (35.7) | 1,220 (33.0) | |
| Overweight (25.0–29.9 kg/m2) | 1,766 (40.2) | 264 (37.8) | 1,502 (40.6) | |
| Obesity (≥30.0 kg/m2) | 1,127 (25.6) | 178 (25.5) | 949 (25.7) | |
| Marital status (N [% ]) | 0.614 | |||
| Married | 3,107 (70.7) | 488 (69.9) | 2,619 (70.9) | |
| Not married | 1,287 (29.3) | 210 (30.1) | 1,077 (29.1) | |
| Education (y, mean [SD]) | 16.58±2.84 | 16.55±2.64 | 16.59±2.87 | 0.736 |
| Racial categories (N [% ]) | <0.001 | |||
| American Indian or Alaskan Native | 9 (0.2) | 0 (0) | 9 (0.2) | |
| Asian | 169 (3.8) | 22 (3.2) | 147 (4.0) | |
| Native Hawaiian or other Pacific Islander | 2 (<0.1) | 0 (0) | 2 (0.1) | |
| Black or African American | 155 (3.5) | 47 (6.7) | 108 (2.9) | |
| White | 4,008 (91.2) | 621 (89.0) | 3,387 (91.6) | |
| Unknown or not reported | 51 (1.2) | 8 (1.1) | 43 (1.2) | |
| Alcohol drinking per day (N [% ]) | <0.001 | |||
| None | 2,222 (50.6) | 484 (69.3) | 1,738 (47.0) | |
| At least one cup | 2,172 (49.4) | 214 (30.7) | 1,958 (53.0) | |
| Smoking per day (N [% ]) | 0.086 | |||
| None | 4,340 (98.8) | 694 (99.4) | 3,646 (98.6) | |
| At least one package | 54 (1.2) | 4 (0.6) | 50 (1.4) | |
| APOE genotype (N [% ]) | 0.436 | |||
| ɛ2/ɛ2 | 24 (0.5) | 6 (0.9) | 18 (0.5) | |
| ɛ2/ɛ3 | 446 (10.2) | 65 (9.3) | 381 (10.3) | |
| ɛ2/ɛ4 | 114 (2.6) | 12 (1.7) | 102 (2.8) | |
| ɛ3/ɛ3 | 2,393 (54.5) | 385 (55.2) | 2,008 (54.3) | |
| ɛ3/ɛ4 | 1,279 (29.1) | 209 (29.9) | 1,070 (29.0) | |
| ɛ4/ɛ4 | 138 (3.1) | 21 (3.0) | 117 (3.2) | |
| Psychiatric disorders (N [% ]) | 0.058 | |||
| No | 3,332 (75.8) | 549 (78.7) | 2,783 (75.3) | |
| Yes | 1,062 (24.2) | 149 (21.3) | 913 (24.7) | |
| Neurological diseases (N [% ]) | 0.095 | |||
| No | 3,393 (77.2) | 522 (74.8) | 2,871 (77.7) | |
| Yes | 1,001 (22.8) | 176 (25.2) | 825 (22.3) | |
| Cardiovascular diseases (N [% ]) | 0.180 | |||
| No | 1,705 (38.8) | 255 (36.5) | 1,450 (39.2) | |
| Yes | 2,689 (61.2) | 443 (63.5) | 2,246 (60.8) | |
| Endocrinological diseases (N [% ]) | 0.183 | |||
| No | 2,311 (52.6) | 351 (50.3) | 1,960 (53.0) | |
| Yes | 2,083 (47.4) | 347 (49.7) | 1,736 (47.0) | |
| Amyloid (N [% ]) | 0.599 | |||
| Not elevated | 2,896 (65.9) | 454 (65.0) | 2,442 (66.1) | |
| Elevated | 1,498 (34.1) | 244 (35.0) | 1,254 (33.9) |
APOE, apolipoprotein E; BMI, body mass index; and NA, not available.
Statistical analysis
Descriptive statistics were used to present the demographic and clinical characteristics of participants according to status of caffeine drinking. Independent sample t-tests and χ2 tests were used to compare continuous and categorical variables, respectively.
Multivariable logistic regression analyses were performed to explore the association between caffeine consumption and amyloid positivity. Model 1 included sex, age, and APOE genotypes as covariates, whereas model 2 included demographic information, habits, APOE genotypes, and comorbidities as covariates. To explore the sex, age, and APOE genotypes effect of caffeine consumption on amyloid positivity, interactions included sex×caffeine, age×caffeine, APOE genotypes×caffeine, were further added to the multivariable logistic regression analysis (model 3). When the interaction was significant, a multivariable logistic regression analysis was further conducted to assess the caffeine consumption and amyloid positivity in subgroup participants. Similarly, we performed the multivariable logistic regression models mentioned above to explore the association between cups of caffeine consumption and amyloid positivity in all participants. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for all regression analysis. Two-sided Wald tests were performed to determine whether the ORs in the regression models were significant. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software version 22 (IBM Corp).
RESULTS
Demographic and clinical characteristics of participants
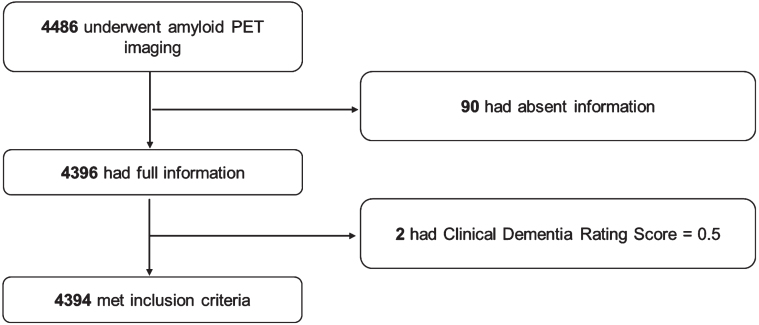
A total of 4,486 participants underwent PET. Of this sample, 4,396 had available information on caffeine consumption, demographic information, habits, APOE genotypes, and comorbidities. Two participants were excluded for preclinical dementia with a CDR of 0.5, and 4,394 participants were included in the present analysis. The detailed process of sample inclusion is shown in Fig. 1.
Fig. 1.
Flow chart of the process of sample inclusion.
Among the included participants, 3,696 were caffeine drinkers, and 698 were non-caffeine drinkers. The mean age of included participants was 71.29±4.67 years. The following factors were found to be significantly different between caffeine-drinkers and non-caffeine drinkers: racial category, and alcohol consumption per day. The detailed demographic and clinical characteristics of participants according to caffeine-drinkers are shown in Table 1.
Relationship between caffeine consumption and amyloid positivity
Table 2 presents the results of relationship between caffeine consumption and amyloid positivity in all participants. The proportions of amyloid positivity in caffeine and non-caffeine drinkers were 33.9% and 35.0%, respectively, in all participants. No significant association was found between caffeine consumption and amyloid positivity (model 1: OR, 0.96; 95% CI, 0.80–1.15; p = 0.627; and model 2: OR, 0.95; 95% CI, 0.79–1.14; p = 0.558).
Table 2.
Relationship between caffeine consumption and amyloid positivity in all participants
| Factors | Model 1 adjusted OR (95% CI) | p | Model 2 adjusted OR (95% CI) | p | Model 3 adjusted OR (95% CI) | p |
| Caffeine consumption (ref: no) | 0.96 (0.80, 1.15) | 0.627 | 0.95 (0.78, 1.14) | 0.558 | 0.67 (0.05, 8.30) | 0.752 |
| Female (ref: male) | 1.09 (0.95, 1.25) | 0.217 | 1.09 (0.93, 1.26) | 0.286 | 0.78 (0.55, 1.10) | 0.160 |
| 70–75 y (ref: 65–70 y) | 1.54 (1.31, 1.80) | <0.001 | 1.54 (1.31, 1.80) | <0.001 | 1.56 (1.06, 2.29) | 0.026 |
| 75–80 y (ref: 65–70 y) | 2.30 (1.89, 2.81) | <0.001 | 2.31 (1.88, 2.83) | <0.001 | 2.06 (1.24, 3.40) | 0.005 |
| 80–85 y (ref: 65–70 y) | 2.70 (2.04, 3.58) | <0.001 | 2.72 (2.05, 3.63) | <0.001 | 3.33 (1.71, 6.50) | <0.001 |
| ɛ2/ɛ3 (ref: ɛ2/ɛ2) | 1.06 (0.35, 3.21) | 0.921 | 1.07 (0.35, 3.26) | 0.907 | 1.24 (0.13, 11.66) | 0.853 |
| ɛ2/ɛ4 (ref: ɛ2/ɛ2) | 3.54 (1.12, 11.15) | 0.031 | 3.56 (1.13, 11.25) | 0.032 | 4.12 (0.35, 48.22) | 0.259 |
| ɛ3/ɛ3 (ref: ɛ2/ɛ2) | 1.50 (0.51, 4.44) | 0.467 | 1.51 (0.51, 4.49) | 0.462 | 1.47 (0.17, 12.95) | 0.730 |
| ɛ3/ɛ4 (ref: ɛ2/ɛ2) | 6.41 (2.16, 19.03) | 0.001 | 6.43 (2.16, 19.15) | 0.001 | 5.03 (0.57, 44.54) | 0.146 |
| ɛ4/ɛ4 (ref: ɛ2/ɛ2) | 27.18 (8.43, 87.61) | <0.001 | 27.62 (8.54, 89.31) | <0.001 | 46.66 (3.42, 636.48) | 0.004 |
| Normal weight (ref: underweight) | 1.24 (0.55, 2.80) | 0.605 | 1.22 (0.54, 2.75) | 0.634 | ||
| Overweight (ref: underweight) | 1.20 (0.53, 2.70) | 0.661 | 1.18 (0.53, 2.67) | 0.684 | ||
| Obesity (ref: underweight) | 1.24 (0.55, 2.81) | 0.595 | 1.23 (0.54, 2.78) | 0.621 | ||
| Married (ref: not married) | 1.02 (0.88, 1.19) | 0.772 | 1.02 (0.88, 1.20) | 0.757 | ||
| Education years | 1.01 (0.99, 1.04) | 0.337 | 1.01 (0.99, 1.04) | 0.345 | ||
| Alcohol drinking (ref: no) | 1.05 (0.92, 1.21) | 0.444 | 1.06 (0.92, 1.22) | 0.427 | ||
| Smoking (ref: no) | 0.99 (0.54, 1.82) | 0.999 | 1.00 (0.54, 1.83) | 0.995 | ||
| Psychiatric disorders (ref: no) | 1.11 (0.95, 1.30) | 0.214 | 1.11 (0.94, 1.30) | 0.211 | ||
| Neurological diseases (ref: no) | 1.13 (0.96, 1.33) | 0.147 | 1.13 (0.96, 1.32) | 0.149 | ||
| Cardiovascular diseases (ref: no) | 1.05 (0.91, 1.21) | 0.539 | 1.05 (0.90, 1.21) | 0.551 | ||
| Endocrinological diseases (ref: no) | 1.08 (0.94, 1.24) | 0.287 | 1.08 (0.94, 1.23) | 0.305 | ||
| Caffeine×female | 1.49 (1.02, 2.17) | 0.037 | ||||
| Caffeine×70–75 y | 0.99 (0.65, 1.52) | 0.969 | ||||
| Caffeine×75–80 y | 1.15 (0.66, 1.98) | 0.626 | ||||
| Caffeine×80–85 y | 0.78 (0.37, 1.63) | 0.510 | ||||
| Caffeine×ɛ2/ɛ3 | 0.85 (0.06, 11.30) | 0.903 | ||||
| Caffeine×ɛ2/ɛ4 | 0.85 (0.05, 13.93) | 0.912 | ||||
| Caffeine×ɛ3/ɛ3 | 1.05 (0.08, 12.94) | 0.972 | ||||
| Caffeine×ɛ3/ɛ4 | 1.36 (0.11, 16.83) | 0.813 | ||||
| Caffeine×ɛ4/ɛ4 | 0.56 (0.03, 10.54) | 0.698 |
OR, odds ratio.
Interactions included sex×caffeine, age×caffeine, APOE genotypes×caffeine were further added to the multivariable logistic regression analysis to explore the effect of caffeine consumption on amyloid positivity in subgroups, and the interaction between sex×caffeine was statistically significant (OR, 1.49; 95% CI, 1.02–2.17; p = 0.037), while age×caffeine, and APOE genotypes×caffeine were not statistically significant.
Relationship between caffeine consumption and amyloid positivity in male and female participants
The proportions of participants with amyloid positivity among caffeine and non-caffeine drinkers were 39.9% and 33.0% in male participants and 31.8% and 34.6% in female participants. Caffeine drinkers had lower ORs for elevated amyloid positivity than non-caffeine drinkers in multivariable adjusted logistic regression models (model 1: OR, 0.74; 95% CI, 0.55–0.99; p = 0.040; and model 2: OR, 0.72; 95% CI, 0.54–0.97; p = 0.032) in male participants. The association between caffeine consumption and amyloid positivity was not statistically significant among female participants. The relationship between caffeine consumption and amyloid positivity in male and female participants is presented in Table 3.
Table 3.
Relationship between caffeine consumption and amyloid positivity in male and female participants
| Model 1 | ||||
| Factors | Male | Female | ||
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Caffeine consumption (ref: no) | 0.74 (0.55, 0.99) | 0.040 | 1.14 (0.90, 1.45) | 0.287 |
| 70–75 y (ref: 65–70 y) | 1.48 (1.14, 1.91) | 0.003 | 1.59 (1.31, 1.94) | <0.001 |
| 75–80 y (ref: 65–70 y) | 2.58 (1.91, 3.49) | <0.001 | 2.08 (1.59, 2.73) | <0.001 |
| 80–85 y (ref: 65–70 y) | 2.74 (1.84, 4.09) | <0.001 | 2.71 (1.80, 4.06) | <0.001 |
| ɛ2/ɛ3 (ref: ɛ2/ɛ2) | 1.76 (0.21, 14.77) | 0.602 | 0.83 (0.22, 3.10) | 0.784 |
| ɛ2/ɛ4 (ref: ɛ2/ɛ2) | 7.09 (0.80, 62.45) | 0.078 | 2.39 (0.61, 9.38) | 0.213 |
| ɛ3/ɛ3 (ref: ɛ2/ɛ2) | 2.54 (0.31, 20.74) | 0.385 | 1.16 (0.32, 4.17) | 0.822 |
| ɛ3/ɛ4 (ref: ɛ2/ɛ2) | 11.39 (1.39, 93.37) | 0.023 | 4.81 (1.33, 17.35) | 0.016 |
| ɛ4/ɛ4 (ref: ɛ2/ɛ2) | 50.62 (5.57, 460.14) | <0.001 | 19.62 (4.83, 79.61) | <0.001 |
| Model 2 | ||||
| Factors | Male | Female | ||
| Caffeine consumption (ref: no) | 0.72 (0.54, 0.97) | 0.032 | 1.14 (0.90, 1.46) | 0.280 |
| 70–75 y (ref: 65–70 y) | 1.48 (1.14, 1.92) | 0.003 | 1.59 (1.30, 1.95) | <0.001 |
| 75–80 y (ref: 65–70 y) | 2.59 (1.91, 3.51) | <0.001 | 2.09 (1.58, 2.76) | <0.001 |
| 80–85 y (ref: 65–70 y) | 2.73 (1.82, 4.10) | <0.001 | 2.74 (1.81, 4.14) | <0.001 |
| Normal weight (ref: underweight) | 0.58 (0.10, 3.31) | 0.537 | 1.43 (0.58, 3.52) | 0.437 |
| Overweight (ref: underweight) | 0.53 (0.09, 3.04) | 0.480 | 1.41 (0.57, 3.47) | 0.459 |
| Obesity (ref: underweight) | 0.55 (0.09, 3.14) | 0.497 | 1.47 (0.59, 3.65) | 0.406 |
| ɛ2/ɛ3 (ref: ɛ2/ɛ2) | 1.61 (0.19, 13.68) | 0.660 | 0.88 (0.23, 3.29) | 0.847 |
| ɛ2/ɛ4 (ref: ɛ2/ɛ2) | 6.38 (0.72, 56.88) | 0.097 | 2.56 (0.65, 10.15) | 0.180 |
| ɛ3/ɛ3 (ref: ɛ2/ɛ2) | 2.33 (0.28, 19.26) | 0.431 | 1.21 (0.33, 4.39) | 0.769 |
| ɛ3/ɛ4 (ref: ɛ2/ɛ2) | 10.45 (1.26, 86.45) | 0.030 | 5.07 (1.40, 18.39) | 0.014 |
| ɛ4/ɛ4 (ref: ɛ2/ɛ2) | 47.92 (5.22, 440.23) | 0.001 | 21.21 (5.19, 86.65) | <0.001 |
| Married (ref: not married) | 1.05 (0.78, 1.42) | 0.758 | 1.02 (0.84, 1.22) | 0.870 |
| Education years | 0.99 (0.95, 1.03) | 0.716 | 1.02 (0.99, 1.06) | 0.147 |
| Alcohol drinking (ref: no) | 1.20 (0.96, 1.50) | 0.110 | 0.97 (0.81, 1.17) | 0.783 |
| Smoking (ref: no) | 0.83 (0.37, 1.85) | 0.651 | 1.22 (0.47, 3.13) | 0.683 |
| Psychiatric disorders (ref: no) | 1.32 (0.99, 1.77) | 0.060 | 1.02 (0.84, 1.24) | 0.841 |
| Neurological diseases (ref: no) | 1.39 (1.07, 1.80) | 0.013 | 1.00 (0.81, 1.22) | 0.983 |
| Cardiovascular diseases (ref: no) | 1.06 (0.83, 1.34) | 0.646 | 1.05 (0.87, 1.26) | 0.618 |
| Endocrinological diseases (ref: no) | 1.01 (0.81, 1.26) | 0.946 | 1.13 (0.95, 1.35) | 0.177 |
OR, odds ratio.
Relationship between different levels of caffeine consumption and amyloid positivity
Table 4 presents the results of different cups of caffeine consumption and amyloid positivity in all participants. No significant association was found between different cups of caffeine consumption and amyloid in all participants (model 1: OR, 1.00; 95% CI, 0.96–1.03; p = 0.793; and model 2: OR, 1.00; 95% CI, 0.96–1.03; p = 0.840). Interactions included sex×caffeine levels, age×caffeine levels, APOE genotypes×caffeine levels were further added to the multivariable logistic regression analysis to explore the effect of caffeine levels on amyloid positivity in subgroups, and no statistically significant interaction was found.
Table 4.
Relationship between levels of caffeine consumption and amyloid positivity in all participants
| Factors | Model 1 adjusted OR (95% CI) | p | Model 2 adjusted OR (95% CI) | p | Model 3 adjusted OR (95% CI) | p |
| Cups of caffeine consumption (ref: no) | 1.00 (0.96, 1.03) | 0.793 | 1.00 (0.96, 1.03) | 0.840 | 0.78 (0.27, 2.28) | 0.654 |
| Female (ref: male) | 1.09 (0.95, 1.25) | 0.222 | 1.08 (0.93, 1.26) | 0.292 | 1.05 (0.85, 1.31) | 0.629 |
| 70–75 y (ref: 65–70 y) | 1.54 (1.31, 1.80) | <0.001 | 1.54 (1.31, 1.80) | <0.001 | 1.51 (1.19, 1.92) | 0.001 |
| 75–80 y (ref: 65–70 y) | 2.30 (1.89, 2.81) | <0.001 | 2.30 (1.88, 2.82) | <0.001 | 2.08 (1.56, 2.78) | <0.001 |
| 80–85 y (ref: 65–70 y) | 2.70 (2.03, 3.58) | <0.001 | 2.72 (2.04, 3.63) | <0.001 | 2.49 (1.61, 3.83) | <0.001 |
| ɛ2/ɛ3 (ref: ɛ2/ɛ2) | 1.06 (0.35, 3.21) | 0.921 | 1.07 (0.35, 3.26) | 0.905 | 0.83 (0.14, 4.84) | 0.838 |
| ɛ2/ɛ4 (ref: ɛ2/ɛ2) | 3.53 (1.12, 11.12) | 0.031 | 3.54 (1.12, 11.2) | 0.031 | 2.81 (0.45, 17.59) | 0.270 |
| ɛ3/ɛ3 (ref: ɛ2/ɛ2) | 1.50 (0.50, 4.44) | 0.467 | 1.51 (0.51, 4.48) | 0.462 | 1.16 (0.21, 6.55) | 0.862 |
| ɛ3/ɛ4 (ref: ɛ2/ɛ2) | 6.41 (2.16, 19.04) | 0.001 | 6.42 (2.15, 19.12) | 0.001 | 4.56 (0.81, 25.68) | 0.085 |
| ɛ4/ɛ4 (ref: ɛ2/ɛ2) | 27.16 (8.42, 87.56) | <0.001 | 27.54 (8.52, 89.08) | <0.001 | 28.21 (4.33, 183.79) | <0.001 |
| Normal weight (ref: underweight) | 1.24 (0.55, 2.79) | 0.609 | 1.24 (0.55, 2.80) | 0.600 | ||
| Overweight (ref: underweight) | 1.19 (0.53, 2.69) | 0.669 | 1.20 (0.53, 2.71) | 0.658 | ||
| Obesity (ref: underweight) | 1.24 (0.55, 2.80) | 0.611 | 1.24 (0.55, 2.82) | 0.602 | ||
| Married (ref: not married) | 1.02 (0.88, 1.19) | 0.781 | 1.03 (0.88, 1.20) | 0.739 | ||
| Education years | 1.01 (0.99, 1.04) | 0.337 | 1.01 (0.99, 1.04) | 0.346 | ||
| Alcohol drinking (ref: no) | 1.05 (0.91, 1.20) | 0.521 | 1.05 (0.91, 1.20) | 0.530 | ||
| Smoking (ref: no) | 0.99 (0.54, 1.82) | 0.973 | 0.98 (0.53, 1.81) | 0.955 | ||
| Psychiatric disorders (ref: no) | 1.11 (0.94, 1.30) | 0.208 | 1.11 (0.94, 1.30) | 0.209 | ||
| Neurological diseases (ref: no) | 1.13 (0.96, 1.33) | 0.131 | 1.13 (0.96, 1.32) | 0.141 | ||
| Cardiovascular diseases (ref: no) | 1.05 (0.91, 1.21) | 0.521 | 1.04 (0.90, 1.21) | 0.555 | ||
| Endocrinological diseases (ref: no) | 1.08 (0.94, 1.24) | 0.291 | 1.08 (0.94, 1.24) | 0.303 | ||
| Cups of caffeine×female | 1.01 (0.94, 1.09) | 0.712 | ||||
| Cups of caffeine×70–75 y | 1.01 (0.93, 1.10) | 0.822 | ||||
| Cups of caffeine×75–80 y | 1.05 (0.95, 1.16) | 0.318 | ||||
| Cups of caffeine×80–85 y | 1.05 (0.88, 1.24) | 0.591 | ||||
| Cups of caffeine×ɛ2/ɛ3 | 1.23 (0.42, 3.59) | 0.708 | ||||
| Cups of caffeine×ɛ2/ɛ4 | 1.22 (0.41, 3.63) | 0.725 | ||||
| Cups of caffeine×ɛ3/ɛ3 | 1.23 (0.42, 3.58) | 0.702 | ||||
| Cups of caffeine×ɛ3/ɛ4 | 1.28 (0.44, 3.72) | 0.650 | ||||
| Cups of caffeine×ɛ4/ɛ4 | 1.09 (0.37, 3.26) | 0.872 |
OR, odds ratio.
DISCUSSION
The present study found no significant association between caffeine consumption and amyloid positivity in all participants. Subgroup analysis showed that caffeine consumption may be inversely associated with amyloid positivity, predominantly among males but not females. Moreover, caffeine consumption level was not associated with amyloid positivity. These findings suggest that caffeine consumption is associated with a reduced pathological cerebral amyloid positivity in older male adults, possibly through its contribution to lowering the risk of AD or related cognitive decline.
The null association between caffeine consumption and amyloid positivity in all participants in this study was not consistent with previous findings, which indicated that high caffeine consumption is associated with lower amyloid accumulation [23]. Similar to studies that explored the association between caffeine consumption and other health outcomes (cognitive decline or dementia), the effect of caffeine did not seem to be robust. As indicated in the literature [10], the effect of caffeine was generally affected by the chronicity of exposure, categories, and doses of caffeine-containing beverages, as well as the characteristics of drinkers, which possibly explains the discrepancy in findings between studies.
In the meantime, an explicit explanation for the effect of caffeine consumption on amyloid burden in older male adults needs to be explored in future research. Preclinical studies on male aged rats have supported that caffeine intake contributes to reducing pathological cerebral amyloid deposition, possibly by mitigating Aβ-induced antioxidant activity [33, 34], inhibiting enzymes for Aβ production [17, 35, 36], and reducing Aβ-induced mitochondrial dysfunction [37, 38]. However, the precise neuronal cellular mechanisms underlying sex differences in caffeine and amyloid burdens should be further explored in the future.
The present findings also provide a neuropathological explanation for the negative association between caffeine and lower risk of AD or cognitive decline in males [11, 13]. Several meta-analyses have supported caffeine consumption as a protective factor against dementia or cognitive impairment [39–41], while a few studies have shown that caffeine may not be effective in delaying cognitive decline [42]. Whether there are sex-specific effects of caffeine consumption on cognitive impairment remains unclear. The inconclusive results regarding caffeine and cognitive impairment may be explained by the characteristics of different individuals since we found that caffeine intake had a distinct effect on amyloid positivity in participants with different demographic characteristics (e.g., sex, age, and BMI). Although the current findings provided an explanation for the association between caffeine intake and reduced cognitive decline in men, the results that support the benefits of caffeine in women have not been well interpreted [43]. Further results from well-designed and well-conducted cohort studies are required to derive robust evidence regarding caffeine consumption and cognitive indicators in male and female participants with different demographic characteristics.
Dose-specific analysis also suggested that no significant association between different levels of caffeine consumption and amyloid positivity. The association between doses of caffeine intake and health outcomes remained debatable. Pervious findings indicated that moderate intake was the most efficacious [13, 44]; nevertheless, two studies have pointed that high level of caffeine consumption could reduce amyloid pathology [18, 19]. As for the amount of caffeine consumed daily, noteworthily, excessive caffeine consumption can cause caffeine poisoning, the symptoms of which include anxiety, agitation, insomnia, gastrointestinal disorders, and mental disorders [45]. Thus, the effects of different amount caffeine on amyloid pathology need further investigated.
The strength of this study is its large population-based sample size; however, several limitations exist. First, the self-reported number of cups of caffeine-containing beverages per day and lack of clarity of chronicity of exposure to caffeine consumption may not reflect the actual magnitude of caffeine intake. Thus, the association between different levels of caffeine consumption and amyloid positivity should be cautiously interpreted as measurement errors may occur. Second, this study was limited to participants aged >65 years without cognitive impairment, thereby limiting the analyses to the outcome of the emerging Aβ pathology in the absence of significant cognitive dysfunction. Third, the vast majority of A4 study participants were white and not representative of the population at risk for AD. Fourth, this is a cross-sectional study. Therefore, associations between caffeine consumption and amyloid positivity cannot necessarily be considered causal relationships. Fifth, the current results should be cautiously read, as it merely reflects the association between caffeine consumption and amyloid positivity, while no significant sex-specific effect on the association of caffeine consumption and amyloid levels using continuous measures.
Conclusions
The present study found no significant association between caffeine consumption and amyloid positivity in the whole participants. Caffeine consumption was inversely associated with amyloid positivity predominantly among males, but not females. Well-established studies are required to explore and validate the effects of caffeine consumption on brain amyloid positivity in the future.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220591.
FUNDING
This work was supported by the National Key Research and Development Program of China (grant number: 2020YFC2003600), Natural Science Foundation of China (grant numbers: 82271527 and 81521063), Young Elite Scientists Sponsorship Program by CAST (grant number: 2019QNRC001), and PKU-Baidu Fund (grant number: 2020BD011).
CONFLICT OF INTEREST
All authors declared no conflict of interest.
DATA AVAILABILITY
The data used in this study were extracted from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4)/Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study.
REFERENCES
- [1]. Nehlig A, Daval JL, Debry G (1992) Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17, 139–170. [DOI] [PubMed] [Google Scholar]
- [2]. van Dam RM, Hu FB, Willett WC (2020) Coffee, caffeine, and health. N Engl J Med 383, 369–378. [DOI] [PubMed] [Google Scholar]
- [3]. Karuppagounder SS, Uthaythas S, Govindarajulu M, Ramesh S, Parameshwaran K, Dhanasekaran M (2021) Caffeine, a natural methylxanthine nutraceutical, exerts dopaminergic neuroprotection. Neurochem Int 148, 105066. [DOI] [PubMed] [Google Scholar]
- [4]. Lieberman HR, Agarwal S, Fulgoni VL, 3rd (2019) Daily patterns of caffeine intake and the association of intake with multiple sociodemographic and lifestyle factors in US adults based on the NHANES 2007-2012 surveys. J Acad Nutr Diet 119, 106–114. [DOI] [PubMed] [Google Scholar]
- [5]. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J (2017) Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 359, j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB (2014) Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129, 643–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Santos RM, Lima DR (2016) Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur J Nutr 55, 1345–1358. [DOI] [PubMed] [Google Scholar]
- [8]. Wang L, Shen X, Wu Y, Zhang D (2016) Coffee and caffeine consumption and depression: A meta-analysis of observational studies. Aust N Z J Psychiatry 50, 228–242. [DOI] [PubMed] [Google Scholar]
- [9]. Hong CT, Chan L, Bai CH (2020) The effect of caffeine on the risk and progression of Parkinson’s disease: A meta-analysis. Nutrients 12, 1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Eskelinen MH, Kivipelto M (2010) Caffeine as a protective factor in dementia and Alzheimer’s disease. J Alzheimers Dis 20(Suppl 1), S167–S174. [DOI] [PubMed] [Google Scholar]
- [11]. Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M (2009) Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J Alzheimers Dis 16, 85–91. [DOI] [PubMed] [Google Scholar]
- [12]. Sugiyama K, Tomata Y, Kaiho Y, Honkura K, Sugawara Y, Tsuji I (2016) Association between coffee consumption and incident risk of disabling dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis 50, 491–500. [DOI] [PubMed] [Google Scholar]
- [13]. van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D (2007) Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur J Clin Nutr 61, 226–232. [DOI] [PubMed] [Google Scholar]
- [14]. Vercambre MN, Berr C, Ritchie K, Kang JH (2013) Caffeine and cognitive decline in elderly women at high vascular risk. J Alzheimers Dis 35, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J, Citron BA, Lin X, Echeverria V, Potter H (2009) Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J Alzheimers Dis 17, 661–680. [DOI] [PubMed] [Google Scholar]
- [16]. Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J (2006) Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 142, 941–952. [DOI] [PubMed] [Google Scholar]
- [17]. Li S, Geiger NH, Soliman ML, Hui L, Geiger JD, Chen X (2015) Caffeine, through adenosine A3 receptor-mediated actions, suppresses amyloid-β protein precursor internalization and amyloid-β generation. J Alzheimers Dis 47, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Kim JW, Byun MS, Yi D, Lee JH, Jeon SY, Jung G, Lee HN, Sohn BK, Lee JY, Kim YK, Shin SA, Sohn CH, Lee DY (2019) Coffee intake and decreased amyloid pathology in human brain. Transl Psychiatry 9, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Gardener SL, Rainey-Smith SR, Villemagne VL, Fripp J, Doré V, Bourgeat P, Taddei K, Fowler C, Masters CL, Maruff P, Rowe CC, Ames D, Martins RN (2021) Higher coffee consumption is associated with slower cognitive decline and less cerebral Aβ-amyloid accumulation over 126 months: Data from the Australian Imaging, Biomarkers, and Lifestyle Study. Front Aging Neurosci 13, 744872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. de Wolf F, Ghanbari M, Licher S, McRae-McKee K, Gras L, Weverling GJ, Wermeling P, Sedaghat S, Ikram MK, Waziry R, Koudstaal W, Klap J, Kostense S, Hofman A, Anderson R, Goudsmit J, Ikram MA (2020) Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 143, 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Nabers A, Perna L, Lange J, Mons U, Schartner J, Güldenhaupt J, Saum KU, Janelidze S, Holleczek B, Rujescu D, Hansson O, Gerwert K, Brenner H (2018) Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol Med 10, e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, Shi J, Vitiello MV, Lu L (2018) Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev 40, 4–16. [DOI] [PubMed] [Google Scholar]
- [23]. Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P (2014) The A4 study: Stopping AD before symptoms begin? Sci Transl Med 6, 228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Sperling RA, Donohue MC, Raman R, Sun CK, Yaari R, Holdridge K, Siemers E, Johnson KA, Aisen PS (2020) Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol 77, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [26]. Morris JC (1997) Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9(Suppl 1), 173–176; discussion 177-178. [DOI] [PubMed] [Google Scholar]
- [27]. Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Arora A, Carpenter AP, Flitter ML, Joshi AD, Krautkramer MJ, Lu M, Mintun MA, Skovronsky DM (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: A prospective cohort study. Lancet Neurol 11, 669–678. [DOI] [PubMed] [Google Scholar]
- [28]. Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM (2012) Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. J Nucl Med 53, 378–384. [DOI] [PubMed] [Google Scholar]
- [29]. Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Förster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gómez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Köhler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleó A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonça A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Møllergård HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodríguez-Rodríguez E, Roe CM, Rot U, Rowe CC, Rüther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schröder J, Schütte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin ÅK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H (2015) Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 313, 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Weir CB, Jan A (2021) BMI classification percentile and cut off points. In StatPearls, StatPearls Publishing LLC., Treasure Island (FL). [PubMed] [Google Scholar]
- [31]. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Shen L, Jia J (2016) An overview of genome-wide association studies in Alzheimer’s disease. Neurosci Bull 32, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Schimidt HL, Carrazoni GS, Garcia A, Izquierdo I, Mello-Carpes PB, Carpes FP (2021) Strength training or green tea prevent memory deficits in a β-amyloid peptide-mediated Alzheimer’s disease model. Exp Gerontol 143, 111186. [DOI] [PubMed] [Google Scholar]
- [34]. Natella F, Nardini M, Giannetti I, Dattilo C, Scaccini C (2002) Coffee drinking influences plasma antioxidant capacity in humans. J Agric Food Chem 50, 6211–6216. [DOI] [PubMed] [Google Scholar]
- [35]. Arendash GW, Cao C (2010) Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis 20(Suppl 1), S117–126. [DOI] [PubMed] [Google Scholar]
- [36]. Ishida K, Yamamoto M, Misawa K, Nishimura H, Misawa K, Ota N, Shimotoyodome A (2020) Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci Res 154, 35–44. [DOI] [PubMed] [Google Scholar]
- [37]. Dragicevic N, Smith A, Lin X, Yuan F, Copes N, Delic V, Tan J, Cao C, Shytle RD, Bradshaw PC (2011) Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J Alzheimers Dis 26, 507–521. [DOI] [PubMed] [Google Scholar]
- [38]. Polito CA, Cai ZY, Shi YL, Li XM, Yang R, Shi M, Li QS, Ma SC, Xiang LP, Wang KR, Ye JH, Lu JL, Zheng XQ, Liang YR (2018) Association of tea consumption with risk of Alzheimer’s disease and anti-beta-amyloid effects of tea. Nutrients 10, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Zhao QF, Li JQ, Wang J, Yu JT (2015) Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 86, 1299–1306. [DOI] [PubMed] [Google Scholar]
- [40]. Kakutani S, Watanabe H, Murayama N (2019) Green tea intake and risks for dementia, Alzheimer’s disease, mild cognitive impairment, and cognitive impairment: A systematic review. Nutrients 11, 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N (2010) Caffeine intake and dementia: Systematic review and meta-analysis. J Alzheimers Dis 20(Suppl 1), S187–S204. [DOI] [PubMed] [Google Scholar]
- [42]. Kwok MK, Leung GM, Schooling CM (2016) Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer’s disease: A Mendelian randomization study. Sci Rep 6, 36500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Arab L, Khan F, Lam H (2013) Epidemiologic evidence of a relationship between tea, coffee, or caffeine consumption and cognitive decline. Adv Nutr 4, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Chen JQA, Scheltens P, Groot C, Ossenkoppele R (2020) Associations between caffeine consumption, cognitive decline, and dementia: A systematic review. J Alzheimers Dis 78, 1519–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Willson C (2018) The clinical toxicology of caffeine: A review and case study. Toxicol Rep 5, 1140–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study were extracted from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4)/Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study.