Abstract
Background
Catastrophic disruptions in care delivery threaten the operational efficiency and potentially the validity of clinical research efforts, in particular randomized clinical trials. Most recently, the COVID-19 pandemic affected essentially all aspects of care delivery and clinical research conduct. While consensus statements and clinical guidance documents have detailed potential mitigation measures, few real-world experiences detailing clinical trial adaptations to the COVID-19 pandemic exist, particularly among, large, global registrational cardiovascular trials.
Methods
We outline the operational impact of COVID-19 and resultant mitigation measures in the Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial, one of the largest and most globally diverse experiences with COVID-19 of any cardiovascular clinical trial to date. Specifically, we address the needed coordination between academic investigators, trial leadership, clinical sites, and the supporting sponsor to ensure the safety of participants and trial staff, to maintain the fidelity of trial operations, and to prospectively adapt statistical analyses plans to evaluate the impact of COVID-19 and the pandemic at large on trial participants. These discussions included key operational issues such as ensuring delivery of study medications, adaptations to study visits, enhanced COVID-19 related endpoint adjudication, and protocol and analytical plan revisions.
Conclusion
Our findings may have important implications for establishing consensus on prospective contingency planning in future clinical trials. Clinicaltrial.gov: NCT03619213.
Clinicaltrial.gov
Key points
-
•
The COVID-19 pandemic levied significant challenges in virtually every aspect of clinical trial conduct and affected trial participants who did and did not contract the virus during their participation in DELIVER.
-
•
Flexible and lean study protocols, the ability to rapidly make necessary amendments to statistical analyses plans, and electronic case report forms may have also helped ensure rapid responses to the pandemic.
-
•
Core learnings from this experience may have direct implications for study sponsors and academic investigators facing future disruptions in clinical trial operations, including but not limited to infectious disease outbreaks, local or international conflict, political turmoil, and economic hardship, among others.
Background
Unexpected disruptions in health care systems may threaten operational efficiency and potentially even the validity of clinical research efforts, particularly randomized clinical trials.1., 2., 3, 4. Most recently, the coronavirus disease-2019 (COVID-19) pandemic presented significant challenges for the conduct of many cardiovascular clinical trials. Guidance documents have detailed potential mitigation strategies,5., 6., 7., 8., 9., 10. however, real-world experiences of cardiovascular clinical trial adaptations have generally been limited to smaller trials enrolling in particular regions.11 , 12 Global, registration trials have important attendant regulatory implications; therefore, ensuring the fidelity of trial operations and data collection is exceedingly important. Mitigation measures in such trials may help inform the larger clinical research community. We outline the operational impact of COVID-19 and resultant mitigation measures in the Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure (DELIVER) trial.
DELIVER and COVID-19
DELIVER was an international, multicenter, double-blind, event-driven clinical trial assessing the safety and efficacy of dapagliflozin 10 mg once daily vs. placebo in 6,263 patients with heart failure and mildly reduced (HFmrEF) or preserved (HFpEF) ejection fraction.13 , 14 Patients with symptomatic heart failure (HF), elevated natriuretic peptides, left ventricular EF>40%, structural heart disease, and at least intermittent diuretic use were randomized. The primary endpoint was a composite of cardiovascular (CV) death or worsening HF event. The first patient was enrolled on August 27, 2018 and the last patient completed the last visit on March 27, 2022. DELIVER was conducted across 353 centers in 20 countries in North America, Latin America, Europe, and Asia.
Early responses to rising COVID-19 cases
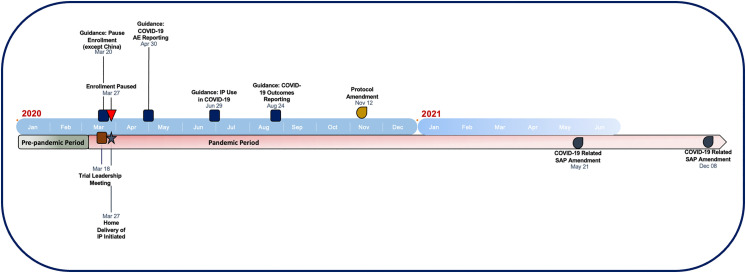
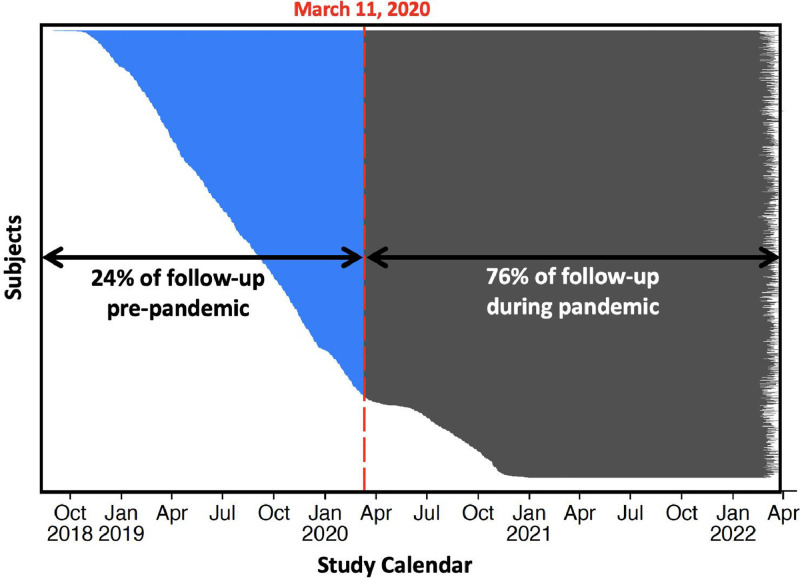
The timeline of events and mitigation measures are outlined in the Figure 1 . On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic. At this time, DELIVER was still actively recruiting; the first reported COVID-19 infection in DELIVER was on March 16, 2020, at which time 5,153 (82.3%) of the total trial population had been randomized. Of a planned target of 1,117 primary endpoint events 363 (32%) had already occurred, and 168 (3%) patients had died. While much of the trial population had been randomized prior to pandemic onset, most (76%) of total follow-up time (by participant years) occurred during the pandemic period (Figure 2 ).
Figure 1.
Timeline of mitigation activities in DELIVER during the COVID-19 pandemic
Figure 2.
Study calendar in relation to the COVID-19 pandemic
Legend: This figure shows enrollment through follow-up for each randomized participant in the DELIVER trial. March 11, 2020, corresponds to the date of the World Health Organization (WHO) declaration of COVID-19 as a pandemic.
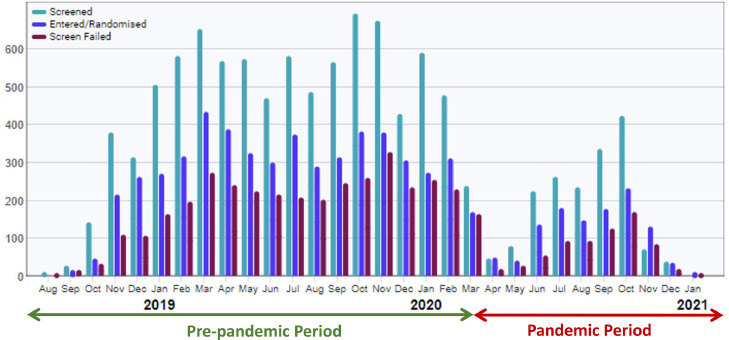
On March 18, 2020, the clinical operations team and the academic executive committee met to discuss efforts to minimize risks to participants, investigators, and clinical trial site staff. On March 20, 2020, trial leadership issued communications to national trial leaders and local study staff indicating sites should pause enrollment; all sites, except those in China (where enrollment was just about to begin and COVID-19 cases were already declining), were instructed to pause enrollment. Enrollment was stopped in all non-Chinese sites on March 27, 2020; randomization continued for patients who had been previously enrolled but not yet randomized in sites which confirmed ability to continue monitoring/follow-up activities. Recruitment restart dates by country are listed in Table I ; screening, recruitment, and screen failures over time is shown in Figure 3 .
Table I.
Recruitment by country in DELIVER during the COVID-19 pandemic
| Country | First subject enrolled | Pause date | Restart date | Last subject enrolled | Number of patients randomized before and during the pause / total number of patients randomized (%) |
|---|---|---|---|---|---|
| Argentina | 12/11/18 | 3/27/20 | 6/25/20 | 10/23/20 | 275/320 (86) |
| Belgium | 1/22/19 | 3/27/20 | 6/8/20 | 10/14/20 | 58/64 (91) |
| Brazil | 2/13/19 | 3/27/20 | 9/18/20 | 10/29/20 | 378/405 (93) |
| Bulgaria | 10/23/18 | 3/27/20 | 5/18/20 | 10/23/20 | 437/493 (89) |
| Canada | 8/27/18 | 3/27/20 | 6/10/20 | 10/30/20 | 236/299 (79) |
| China^ | 3/24/20 | – | – | 12/30/20 | 0/310 (0) |
| Czech Republic | 11/6/18 | – | – | 2/10/20 | 274/274 (100) |
| Hungary* | 11/27/18 | 3/27/20 | 5/18/20 | 9/29/20 | 462/466 (99) |
| Japan | 10/15/18 | 3/27/20 | 6/1/20 | 9/14/20 | 380 /422 (90) |
| Mexico | 7/9/19 | 3/27/20 | 5/26/20 | 10/27/20 | 137 /216 (63) |
| Netherlands | 10/29/18 | 3/27/20 | 6/1/20 | 10/30/20 | 136/176 (77) |
| Peru | 11/22/18 | 3/27/20 | 7/15/20 | 10/30/20 | 201/240 (84) |
| Poland* | 10/15/18 | – | – | 2/7/20 | 572/572 (100) |
| Romania | 1/23/20 | 3/27/20 | 6/9/20 | 10/30/20 | 13/61 (21) |
| Russia* | 10/4/18 | – | – | 2/4/20 | 401/401 (100) |
| Saudi Arabia* | 11/7/18 | 3/27/20 | – | 3/11/20 | 190/190 (100) |
| Spain | 11/2/18 | 3/27/20 | 5/27/20 | 10/30/20 | 252/308 (82) |
| Taiwan | 11/16/18 | 3/27/20 | 5/11/20 | 10/30/20 | 242/318 (76) |
| United States | 8/29/18 | 3/27/20 | 6/5/20 | 11/6/20 | 433/552 (78) |
| Vietnam | 1/7/19 | 3/27/20 | 5/11/20 | 10/30/20 | 158/176 (90) |
| Total | 8/27/18 | – | – | 12/30/20 | 5235/6263 (84) |
Countries with completed recruitment before the pause date.
Enrollment was not paused in China and commenced during the COVID-19 pandemic
Figure 3.
Recruitment over time in DELIVER
Delivery and receipt of investigational products (IP)
On March 27, 2020, protocols for home delivery of IP were initiated to minimize risk to participants and study staff. Home delivery was available to any clinical trial participant with concerns about coming into a study site to receive IP. A courier vendor was contracted by the sponsor to perform home IP delivery to ensure temperature control, product and data security. Once IP was delivered, a patient had to sign Proof of Delivery. Home deliveries of IP were documented and tracked in an electronic case report form (eCRF); delays in receipt of IP were recorded as nonimportant COVID-19-related protocol deviations. Standard IP returns and drug accountability processes were adapted to ensure assessment of compliance and adherence. For example, participants were initially asked to store used IP bottles at their homes, to be returned to study staff at the next in-person site visit. However, given persistent pandemic conditions and the conversion of study visits to virtual formats, pill count information was collected directly from participants during virtual visits and documented in a drug accountability section of the electronic data capture (EDC) platform. Couriers were dispatched to collect used IP bottles to confirm participant-reported drug accountability; discrepancies between participant-reported compliance and information from collected bottles were noted in the eCRF and patient record. With implementation of these strategies, there were no known cases of missed IP delivery during the pandemic; direct patient delivery was used in at least 16 participating countries. Overall treatment compliance was 79% (80% in those randomized to dapagliflozin and 78% in those randomized to placebo). Compliance rates of >80% were observed in 96% of trial participants.
Study visits and procedures
The original eCRF allowed for remote contacts to collect clinical outcomes and safety events. As the pandemic progressed, remote contact was increased for participants with concerns about on-site visits. Types of visits that were permitted included (1) telephone visits, (2) home visits, and (3) collection from other contacts (eg, caregiver, physician, medical record review). Missed visits (defined as ± 14 days outside the visit window) rose from 3.9% prior to pandemic onset to 10.2% during the COVID-19 pandemic. Telephone visits increased from 1.8% prior to pandemic onset to 17.6% during the pandemic (Table II ). Other visit modalities, including home visits and review of records with a family member/caregiver or a primary/treating physician were used in a minority of cases prior to and during the pandemic. Findings from monitoring visits and regional differences were not systematically reported or collated across trial sites in the context of this analysis. Missing data for KCCQ-TSS were balanced between treatment groups, both in the prepandemic and in the pandemic periods.
Table II.
Study visits during the COVID 19 pandemic
| Dapagliflozin (N=3131) | Placebo (N=3132) | Total (N=6263) | |
|---|---|---|---|
| Planned study visits | |||
| Prepandemic period | 6,304 | 6,328 | 12,632 |
| Pandemic period | 16,359 | 16,298 | 32,657 |
| Performed visits, n (% of planned) | |||
| Prepandemic period | 6,057 (96.1) | 6,057 (95.7) | 12,114 (95.9) |
| Pandemic period | 14,745 (90.1) | 14,621 (89.7) | 29,366 (89.9) |
| Missed visits, n (% of planned) | |||
| Prepandemic period | 233 (3.7) | 255 (4.0) | 488 (3.9) |
| Pandemic period | 1,628 (10.0) | 1,693 (10.4) | 3,321 (10.2) |
| Visit modality, n (% of performed) | |||
| Prepandemic period | |||
| On-site visit | 5,915 (97.7) | 5,903 (97.5) | 11,818 (97.6) |
| Telephone visit | 113 (1.9) | 109 (1.8) | 222 (1.8) |
| Others* | 29 (0.5) | 45 (0.7) | 74 (0.6) |
| Pandemic period | |||
| On-site visit | 11,616 (78.8) | 11,632 (79.6) | 23,248 (79.2) |
| Telephone visit | 2,673 (18.1) | 2,497 (17.1) | 5,170 (17.6) |
| Others* | 456 (3.1) | 492 (3.4) | 948 (3.2) |
Note: Prepandemic period refers to prior to the World Health Organization's declaration of COVID-19 as a pandemic on March 11, 2020. The pandemic period refers to March 11, 2020, and onward; A visit was included in the time period when it was performed and not when it was planned. Missed visits were defined as ± 14 days outside the visit window.
Other visits include home visits and review of records with a family member/caregiver or a primary/treating physician or medical records review and miscellaneous.
For study visits conducted outside of in-person settings, vital signs and serum creatinine measurements were not recorded; these were reported as nonimportant COVID-19-related protocol deviations. The central laboratory remained open and available during the pandemic. In November 2020, in response to delays in receipt of laboratory kits at specific trial sites, procedures were implemented, allowing (1) transfers of laboratory kits between sites and (2) the use of nonstudy-specific laboratory kits. Electronic Patient Reported Outcomes (ePRO) data were allowed to be collected outside of traditional in-person site visits via telephone visits, though ePRO data were not included in the prespecified secondary endpoint analysis of Kansas City Cardiomyopathy Questionnaire-total symptom score (KCCQ-TSS) assessment. Reasons for this included concerns that phone call related capture of KCCQ-TSS would differ from the more validated direct patient report. In addition, the collateral effects of the pandemic were expected to alter global health status among trial participants. Sites received dedicated training and a guide detailing optimal strategies for ePRO collection, which aligned with European Medicines Agencies (EMA) and US Food and Drug Administration (FDA) guidance.15 The contribution of individual institutional policies or guidelines on remote visit conversion was not systematically available across all trial sites.
Sites were encouraged to schedule on-site study closeout visits, but remote contacts were acceptable if local circumstances or participant preference precluded on-site visits. There were no missed study closeout visits, of which 86.5% were on-site, 10.2% were telephone visits, and the remainder used other platforms. Vital status was available at the end of the study for all but 4 patients, including 1 who withdrew consent.
Endpoint adjudication
Acknowledging the growing contribution of fatal COVID-19 infection to overall mortality in the early months of the pandemic, the steering committee asked the Clinical Endpoint Committee (CEC) in April 2020 to adapt the adjudication process was altered to capture the contribution of COVID-19 illness to deaths occurring during the trial. All deaths, regardless of adjudicated cause, were subsequently adjudicated with regards to their relationship with COVID-19. Deaths as a direct consequence of COVID-19 or those in which COVID-19 was a major contributor to death were classified as “definitely” COVID-19 related. “Definite” cases had symptoms, signs, and clinical trajectory typical of COVID-19 infection in association with confirmatory laboratory testing or diagnostic imaging. Cases in which there was high clinical suspicion for COVID-19 infection in the absence of confirmatory testing were classified as “possibly” COVID-19 related. To ensure consistency in assigning the contribution of COVID-19 to death, all deaths that were potentially related to COVID-19 were reviewed in committee meetings and adjudicated by consensus. As well, all deaths adjudicated prior to the update of the CEC charter were rereviewed by the CEC Chairs to retrospectively assign any possible contribution of COVID-19 to death. Relatedness to COVID-19 for HF hospitalizations was not formally adjudicated.
COVID-19 adverse event reporting
COVID-19 infections were requested to be reported as SAEs. Recording of COVID-19 testing results from Visit 2 onwards was added to safety assessments; the type and results of tests were added to the eCRF. COVID-19 diagnosis was investigator reported and based on local regulations; central lab or protocol was not specified. Furthermore, adverse event reporting included data collection of the COVID-19 events resulting in or prolonging hospitalization and COVID-19 events with a fatal outcome. On June 29, 2020, the executive committee issued guidance to sites regarding using sodium-glucose cotransporter 2 inhibitors (including IP) in patients at risk for COVID-19 infections. Investigators were recommended to consider interruption of IP in patients at risk or with COVID-19 who had diabetes and known DKA risk factors or prior DKA. Sites were instructed to continue IP in patients with or at risk for COVID-19 who were nondiabetic patients and without risk factors for DKA. Diabetic ketoacidosis was collected as an adverse event throughout the study, including during or following COVID-19 event; an independent DKA adjudication committee reviewed all potential cases. There were no adjudicated cases of DKA among trial participants who developed COVID-19 during DELIVER in either study arm.
Adaptations to the statistical analysis plan (SAP)
There were 4 SAP amendments (November 6, 2020, December 9, 2020, May 21, 2021, and December 8, 2021) during the pandemic prior to unblinding; 2 SAP amendments were specifically COVID-19 related. On May 21, 2021, an SAP amendment redefined the population considered for prespecified analysis of the impact of therapy on Kansas City Cardiomyopathy Questionnaire-Total Symptom Score (KCCQ-TSS) as those participants with planned or completed 8-month assessment prior to March 11, 2020. The SAP amendment on December 8, 2021 (prior to unblinding), instituted a formal COVID-19 sensitivity analysis, evaluating the effect of therapy on the clinical endpoints in the full study population with censoring at the time of the first recorded AE associated with COVID-19.
Protocol deviations related to COVID-19
Protocol deviations related to COVID-19 were defined and determined to be either important or nonimportant. Important COVID-19-related protocol deviations included randomization despite failing inclusion criteria and receipt of different IP than allocated by randomization, which each occurred in one trial participant. Important COVID-19-related protocol deviations occurred infrequently, affecting only 5 (0.1%) trial participants. Nonimportant COVID-19-related protocol deviations included visits outside the ± 14-day window and serious adverse event (SAE) reporting >24 hours of awareness, among others. Nonimportant COVID-19-related protocol deviations were common, affecting 2,571 (41.1%) trial participants; relatedness to COVID-19 was based on sponsor-led monitor reporting. The most common reason for a nonimportant COVID-19 protocol deviation was missed study procedures and assessments, occurring in 2,310 (36.9%) trial participant. Nonimportant COVID-19 related protocol deviations were balanced by treatment assignment (Table III).
Table III.
COVID-19-related protocol deviations
| Number of participants (%) |
|||
|---|---|---|---|
| Dapagliflozin (N=3131) | Placebo (N=3132) | Total (N=6263) | |
| COVID-19-related important protocol deviations | |||
| ≥1 important deviation | 4 (0.1) | 1 (0.0) | 5 (0.1) |
| Failed inclusion criteria | 1 (0.0) | – | 1 (0.0) |
| Fulfilled exclusion criteria | – | 1 (0.0) | 1 (0.0) |
| Received IP not allocated by randomization | 1 (0.0) | – | 1 (0.0) |
| Other important deviations | 2 (0.1) | – | 2 (0.0) |
| COVID-19-related nonimportant protocol deviations | |||
| ≥ 1 nonimportant deviation | 1,270 (40.6) | 1,301 (41.5) | 2,571 (41.1) |
| Study procedures and assessments | 1,147 (36.6) | 1,163 (37.1) | 2,310 (36.9) |
| Visits performed outside ± 14-day window | 384 (12.3) | 424 (13.5) | 808 (12.9) |
| SAE reported > 24 hours after awareness | 42 (1.3) | 45 (1.4) | 87 (1.4) |
| Inappropriate ICF administration | 41 (1.3) | 28 (0.9) | 69 (1.1) |
| Study sample management | 6 (0.2) | 10 (0.3) | 16 (0.3) |
| Incomplete AE/SAE reporting | 5 (0.2) | 4 (0.1) | 9 (0.1) |
| Nonimportant inclusion/exclusion criteria deviation | 2 (0.1) | 3 (0.1) | 5 (0.1) |
| Inappropriate concomitant medication use | – | 2 (0.1) | 2 (0.0) |
Relatedness to COVID-19 determined by investigators.
AE, Adverse events; COVID-19, Coronavirus disease 2019; ICF, Informed consent form; SAE, Serious adverse event
Site monitoring
Risk-based monitoring continued during the pandemic; on-site monitoring was frequently replaced by remote monitoring due to pandemic-related challenges to conducting on-site visits. In addition, centralized monitoring activities were increased to evaluate completeness of adverse event reporting and assessments of study drug compliance. There were instances where video conferencing capabilities were implemented in addition to accessing source documentation from electronic medical records. The trial sponsor ensured privacy remained in accordance with local regulations. Appropriate informed consent was obtained from trial participants to ensure privacy of participant data when sharing via electronic means instead of in-person study visits. As on-site monitoring resumed, priority items included ensuring appropriate documentation of informed consent and primary efficacy and safety endpoint documentation.
Conclusions
The COVID-19 pandemic levied significant challenges in virtually every aspect of clinical trial conduct and affected trial participants who did and did not contract the virus during their participation in DELIVER. Prior reports have indicated post hoc analyses or changes in statistical analysis plans describing the impact of the pandemic on clinical events and treatment effects11 , 16; however, to our knowledge, this represents one of the first and largest descriptions of comprehensive responses to a global pandemic in the context of a large, multinational cardiovascular randomized clinical trial. Early mitigation strategies focused on the safety of trial participants and study staff, while maintaining the study integrity. Ensuring timely delivery of IP to participants, maintaining drug accountability, and ascertaining the effects of the pandemic on data collection and analyses minimized the impact of the pandemic on threatening the validity of clinical trial findings; vital status was known at the end of the trial in all but 2 patients in the dapagliflozin group and 2 patients in the placebo group. Furthermore, flexible and lean study protocols, the ability to rapidly make necessary amendments to statistical analyses plans, and electronic case report forms may have also helped ensure rapid responses to the pandemic. In addition, prospective attempts to improve flexibility in statistical analyses, including the use of adaptative clinical trial platform with prespecified action plans in response to changes in enrollment and event rates, may help make trials more resilient. The emerging use of technology and remote-based operational aspects may have important implications for the design and conduct of future clinical trials, particularly those operating under fully virtual protocols. Of note, many of mitigation efforts in DELIVER were reactive to the unique, unexpected demands of the pandemic. Moving forward, core learnings from this experience may have direct implications for study sponsors and academic investigators facing future disruptions in clinical trial operations, including but not limited to infectious disease outbreaks, local or international conflict, political turmoil, and economic hardship, among others. Codifying successful strategies and establishing consensus across study sponsors, academic investigators, and regulators on important mitigation features that can be prespecified in clinical trial design may aid in prospective contingency planning for future clinical trials.
Conflict of Interest
ASB has no relevant disclosures to the content of this manuscript. DL was an AstraZeneca employee when the DELIVER trial was conducted, AN, NZ, AML, and MP are employees and shareholders of AstraZeneca. BLC has received consulting fees from Amgen, Cardurion, Corvia, and Novartis. MV has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, speaker engagements with AstraZeneca, Novartis, and Roche Diagnostics, and participates on clinical trial committees for studies sponsored by Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. MNK has received research grant support from AstraZeneca, and Boehringer Ingelheim; has served as a consultant or on an advisory board for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma; has received other research support from AstraZeneca; and has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. CSPL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Bayer and Roche Diagnostics; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Actelion, Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Eli Lilly, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and serves as co-founder & nonexecutive director of Us2.ai. AFH has received research grants from American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Somologic and Verily; and has served as a consultant or on the Advisory Board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cytokinetics, Eidos, Intercept, Merck, and Novartis. FAM has received consultation fees and research grants from: AstraZeneca, Baliarda, Bayer, Boheringer Ingelheim, Bristol Meirs Squibb, Gador, Milestone, Novartis, Pfizer, and St Lukes University. SEI has served on clinical trial committees or as a consultant to AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, Pfizer, vTv Therapeutics, Abbott, and Esperion; and has given lectures sponsored by AstraZeneca and Boehringer Ingelheim. SJS has received research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer, and has received consulting fees from and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, GSK, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. RAdB has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals GmbH, Ionis Pharmaceuticals, Inc., Novo Nordisk, and Roche; and has had speaker engagements with Abbott, AstraZeneca, Bayer, Bristol Myers Squibb, Novartis, and Roche. AD reports institutional grant support from Abbott, Alnylam, AstraZeneca, Bayer, Novartis, and Consulting Fees from Abbott, Alnylam, AstraZeneca, Avidity, Axon Therapeutics, Bayer, Biofourmis, Boston Scientific, Cytokinetics, GlaxoSmithKline, Merck, Novartis, Parxel, Regeneron, Roche, and Verily. PSJ reports speakers’ fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim, Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc. PSJ's employer the University of Glasgow has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and NovoNordisk; Director Global Clinical Trial Partners (GCTP). JJVM has received payments through Glasgow University for work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, Theracos Personal lecture fees: the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, Global Clinical Trial Partners (GCTP). SDS has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros.
References
- 1.Lunt H, Heenan H. Mitigating the impact of disasters and emergencies on clinical trials site conduct: a site perspective following major and minor unforeseen events. Contemp Clin Trials Commun. 2019;16 doi: 10.1016/j.conctc.2019.100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin R. Clinical trials disrupted during war in Ukraine. JAMA. 2022;327:1535–1536. doi: 10.1001/jama.2022.5571. [DOI] [PubMed] [Google Scholar]
- 3.Kurihara C, Crawley FP, Baroutsou V, et al. The continuation of clinical trials in times of war: a need to develop ethics and situationally adaptive clinical research guidelines. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.966220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington J, Felker GM, Mentz RJ. Catastrophic disruptions in clinical trials. Circulation. 2022;146:369–371. doi: 10.1161/CIRCULATIONAHA.122.060541. [DOI] [PubMed] [Google Scholar]
- 5.Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA. 2021;326:257–265. doi: 10.1001/jama.2021.9941. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Newman AB. Preserving clinical trial integrity during the coronavirus pandemic. JAMA. 2020;323:2135–2136. doi: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 7.Lansky A, Shah T, Wijns W, et al. Immediate and long-term impact of the COVID-19 pandemic on cardiovascular clinical trials: considerations for study conduct and endpoint determination. EuroIntervention. 2020;16:787–793. doi: 10.4244/EIJV16I10A147. [DOI] [PubMed] [Google Scholar]
- 8.Lunardi M, Mylotte D, Wijns W, et al. Maintaining high standards of clinical research during the Covid-19 pandemic: insights from an excellence clinical research centre. Eur Heart J. 2021;42:4202–4205. doi: 10.1093/eurheartj/ehab409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Psotka MA, Abraham WT, Fiuzat M, et al. Conduct of clinical trials in the era of COVID-19: JACC scientific expert panel. J Am Coll Cardiol. 2020;76:2368–2378. doi: 10.1016/j.jacc.2020.09.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anker SD, Butler J, Khan MS, et al. Conducting clinical trials in heart failure during (and after) the COVID-19 pandemic: an expert consensus position paper from the heart failure association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:2109–2117. doi: 10.1093/eurheartj/ehaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zile MR, Desai AS, Costanzo MR, et al. The GUIDE-HF trial of pulmonary artery pressure monitoring in heart failure: impact of the COVID-19 pandemic. Eur Heart J. 2022;43:2603–2618. doi: 10.1093/eurheartj/ehac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowie MR, Cleland JGF. The COVID-19 pandemic and heart failure: lessons from GUIDE-HF. Eur Heart J. 2022;43:2619–2621. doi: 10.1093/eurheartj/ehac226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon SD, de Boer RA, DeMets D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217–1225. doi: 10.1002/ejhf.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon SD, McMurray JJV, Claggett B, et al. DELIVER trial committees and investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 15.United Stated Food and Drug Administration (FDA). FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic. 2021.
- 16.Ponikowski P, Kirwan B-A, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396:1895–1904. doi: 10.1016/S0140-6736(20)32339-4. [DOI] [PubMed] [Google Scholar]