Abstract
Wernicke’s area has been assumed since the 1800s to be the primary region supporting word and sentence comprehension. However, in 2015 and 2019, Mesulam and colleagues raised what they termed the ‘Wernicke conundrum’, noting widespread variability in the anatomical definition of this area and presenting data from primary progressive aphasia that challenged this classical assumption. To resolve the conundrum, they posited a ‘double disconnection’ hypothesis: that word and sentence comprehension deficits in stroke-based aphasia result from disconnection of anterior temporal and inferior frontal regions from other parts of the brain due to white matter damage, rather than dysfunction of Wernicke’s area itself.
To test this hypothesis, we performed lesion-deficit correlations, including connectome-based lesion-symptom mapping, in four large, partially overlapping groups of English-speaking chronic left hemisphere stroke survivors.
After removing variance due to object recognition and associative semantic processing, the same middle and posterior temporal lobe regions were implicated in both word comprehension deficits and complex non-canonical sentence comprehension deficits. Connectome lesion-symptom mapping revealed similar temporal-occipital white matter disconnections for impaired word and non-canonical sentence comprehension, including the temporal pole. We found an additional significant temporal-parietal disconnection for non-canonical sentence comprehension deficits, which may indicate a role for phonological working memory in processing complex syntax, but no significant frontal disconnections. Moreover, damage to these middle-posterior temporal lobe regions was associated with both word and non-canonical sentence comprehension deficits even when accounting for variance due to the strongest anterior temporal and inferior frontal white matter disconnections, respectively.
Our results largely agree with the classical notion that Wernicke’s area, defined here as middle superior temporal gyrus and middle-posterior superior temporal sulcus, supports both word and sentence comprehension, suggest a supporting role for temporal pole in both word and sentence comprehension, and speak against the hypothesis that comprehension deficits in Wernicke’s aphasia result from double disconnection.
Keywords: aphasia, lesion-symptom mapping, connectome, comprehension, Wernicke’s area
Using connectome-based lesion-symptom mapping in large groups of people with post-stroke aphasia, Matchin et al. test—and find evidence against—the recent prominent hypothesis that word and sentence comprehension deficits in Wernicke’s aphasia result from ‘double disconnection’.
Introduction
The classical model of language in the brain posits a primary role for Wernicke’s area, roughly thought to be the posterior superior temporal lobe and inferior parietal lobe (with definitions varying widely),1–3 in both word and sentence comprehension,4–7 defined as a set of processes involving the transformation of an auditory signal onto linguistic representations at the lexical and syntactic levels.8 The major motivation for the primacy of Wernicke’s area in word and sentence comprehension is the notable comprehension deficits in Wernicke’s aphasia, typically involving posterior temporal-parietal damage,9,10 which roughly corresponds to the region classically associated with Wernicke’s area.2,3
Recently, the classical view has been questioned from the perspective of primary progressive aphasia (PPA). Mesulam et al.1 analysed patterns of cortical thinning in 72 people with PPA on word and sentence comprehension. Their sentence comprehension task minimized lexical access demands by using sentences with highly frequent words (e.g. boy, girl, dog, cat, kiss, chase) and non-canonical structures (object-first sentences such as passives, which do not conform to expected word order), to emphasize grammatical processing. By contrast, their word comprehension task maximized lexical-semantic demands, and the variance due to object recognition and non-verbal semantics was factored out using the Pyramids and Palm Trees test11 (PPT) as a covariate. They found no association of posterior temporal-parietal atrophy with word comprehension deficits and minimal association of atrophy in this region with deficits in non-canonical sentence comprehension. By contrast, atrophy of the anterior temporal lobe (ATL), primarily the temporal pole, was strongly associated with word comprehension (but not sentence comprehension) impairment, whereas inferior parietal and frontal degeneration was associated with sentence comprehension (but not word comprehension) impairment. Mesulam et al.12 followed up this study by showing that repetition (but not word comprehension) was associated with degeneration of superior posterior temporal and inferior parietal lobes. Overall, they associated the temporal-parietal territory commonly attributed to Wernicke’s area with a phonological working memory function, not critical for basic aspects of linguistic processing.
To explain the discrepancy of their results with the literature on Wernicke’s aphasia, Mesulam et al.1 posited a ‘double disconnection’ hypothesis. Given that strokes often impinge on white matter, and that disconnection due to white matter damage can impair language abilities independently of cortical grey matter damage,13,14 they suggested that lesions to the temporal-parietal cortex may impair both word and sentence comprehension, not through damage to the relevant cortical centres themselves but through disconnection.15 In light of their cortical thinning results in PPA, they proposed that the ATL is the key region underlying word comprehension and that the frontal cortex (primarily Broca’s area or the posterior two-thirds of the inferior frontal gyrus, IFG) is the key region for sentence/syntactic processing, both of which may be disconnected due to posterior temporal-parietal strokes.
There are two problems with this logic. First, functional neuroimaging studies have shown that the spatial location and extent of activation of syntactic processing and lexical access, the processes leading up to and including word retrieval (but not conceptual retrieval), are remarkably similar (for meta-analyses and reviews, compare Hagoort & Indefrey16 for syntactic processing and Lau et al.17 for lexical access). This reliably includes the posterior superior temporal sulcus (STS) and posterior middle temporal gyrus (MTG), overlapping with the traditional Wernicke’s territory (although somewhat more anterior than traditionally depicted). Other regions, including prominently the IFG, ATL and inferior angular gyrus have also been regularly implicated in lexical-semantic processing broadly construed.18,19 Regardless of the explanation for this overlap of lexical and syntactic processing in the middle-posterior temporal lobe,20,21 and whether or not the middle-posterior temporal lobe is uniquely involved in comprehension-related processes relative to other regions, these activations should be accounted for via some mechanism, and suggest (although do not prove) a functional role in comprehension.
Second, a large body of lesion-symptom mapping (LSM) studies in post-stroke aphasia have shown that damage to Broca’s area and surrounding areas is not reliably implicated in sentence or syntactic comprehension deficits.22–27 There may be a minor supporting role for frontal cortex in supporting the processing of particularly difficult sentence structures,28 although the location of the lesion correlates of such deficits is inconsistent22,29–32 (for a review, see Matchin and Hickok21). If non-canonical sentence comprehension deficits in Wernicke’s aphasia are primarily due to disconnection of Broca’s area/posterior IFG from the rest of the language network, then one would expect frontal lesions to also impair syntactic comprehension. However, this is not a pattern that is reliably seen, in contrast to that associated with posterior temporal-parietal damage, which reliably predicts sentence comprehension impairments.22,23,25,26,30,31,33
For these reasons, the dissociation, both in behaviour and lesion correlates, between word and sentence comprehension in PPA reported by Mesulam et al.1 is surprising and deserves scrutiny. We examined these issues, testing the hypothesis of Mesulam et al. that overlap of word and sentence comprehension deficits in post-stroke aphasia is due to distinct disconnection patterns. We analysed data in a large group of people with post-stroke aphasia using LSM as well as connectome-based lesion-symptom mapping (CLSM), which uses diffusion tensor imaging to ascertain the strength of white matter connections between regions associated with behavioural scores.34–36 In a previous report,34 our research group performed LSM and CLSM analyses of word comprehension alone (with PPT as a covariate) in a smaller group of participants (43), finding that impaired word comprehension was associated with damage to the inferior temporal gyrus and disconnection between posterior MTG and inferior temporal gyrus. A follow-up study8 expanded the number of participants (99) and performed LSM analyses, finding the most robust association of word-level deficits (with PPT as a covariate) with damage to middle-posterior superior temporal gyrus (STG) and STS.
Here we expand the number of participants further and add several behavioural measures to directly compare our results to Mesulam et al., crucially including a measure of non-canonical sentence comprehension and corresponding CLSM analyses. We predicted that both word and non-canonical sentence comprehension would primarily involve damage to the middle-posterior temporal lobe and similar disconnection patterns throughout the temporal lobe, and that damage to these areas would predict comprehension impairments above and beyond the contribution of the relevant disconnection patterns. We expected a possible implication of parietal damage and/or disconnection in non-canonical sentence comprehension due to phonological working memory demands that are strongly associated with parietal areas.37–40
Materials and methods
Subjects and measures
We performed behavioural and lesion mapping analyses in four partially overlapping groups of English-speaking participants (Table 1). We collected anatomical images and created lesion maps for all participants, but diffusion tensor data for our CLSM analyses were unavailable for several participants. Group 1 consisted of 218 participants (199 included in CLSM) who were assessed on the Western Aphasia Battery-Revised,41 with subsets of this group being assessed on a variety of other measures. Group 2 consisted of a subset of 180 participants (167 included in CLSM) who were assessed on the PPT.11 Group 3 consisted of a subset of 130 participants (127 included in CLSM) who were assessed on one of two similar tests of sentence comprehension ability involving picture-matching and a variety of semantically reversible canonical and non-canonical sentence structures, the Sentence Comprehension Test subset of the Northwestern Assessment of Verbs and Sentences (NAVS)42 (n = 82) or a task that we label here the Icelandic Task (since its translation was first used in a sample of Icelandic stroke survivors with aphasia31,43) (n = 48). Finally, Group 4 consisted of a subset of 92 participants (90 included in CLSM) who were evaluated for Expressive Agrammatism using consensus perceptual ratings of spontaneous speech samples in one of two studies, den Ouden et al.44 (n = 39) or Matchin et al.45 (n = 53).
Table 1.
Subject demographic data
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Measures | Western Aphasia Battery-Revised | Pyramids and Palm Trees (PPT) | Non-canonical Sentence Comprehension | Perceptual Ratings of Expressive Agrammatism |
| Total number of subjects | n = 218 (199 included in CLSM) | n = 180 (167 included in CLSM) |
n = 130 (127 included in CLSM) Icelandic Task: n = 48 NAVS: n = 82 |
n = 92 (90 included in CLSM) Den Ouden et al.44: n = 39 Matchin et al.45: n = 53 |
| Sex | 133 male, 85 female | 112 male, 68 female | 83 male, 47 female | 60 male, 32 female |
| Mean age at testing (years) | 60.3 (SD = 11.4) | 60.5 (SD = 11.4) | 60.0 (SD = 10.7) | 58.7 (SD = 11.3) |
| Mean months post-first stroke at initial testing | 42.6 (SD = 48.3) | 41.2 (SD = 47.6) | 45.3 (SD = 50.4) | 45.6 (SD = 48.5) |
| Mean number of strokes | 1.2 (SD = 0.5) | 1.2 (SD = 0.5) | 1.2 (SD = 0.5) | 1.1 (SD = 0.4) |
| Mean education (years) | 15.0 (SD = 2.3), an = 210 | 15.0 (SD = 2.3), an = 175 | 15.4 (SD = 2.4), an = 128 | 15.3 (SD = 2.4) |
| Mean lesion volume (mm3) | 120 855 (SD = 97 488) | 128 446 (SD = 97 036) | 111 267 (SD = 92 645) | 96 175 (SD = 84 514) |
| Mean WAB-R AQ | 61.4 (SD = 28.1) | 60.0 (SD = 26.6) | 65.3 (SD = 26.9) | 75.8 (SD = 19.9) |
| Mean WAB-R Auditory Word Recognition | 50.0 (SD = 13.2) | 49.8 (SD = 12.9) | 51.0 (SD = 12.7) | 54.7 (SD = 9.0) |
| Mean WAB-R Sequential Commands | 51.4 (SD = 23.5) | 50.4 (SD = 22.5) | 54.5 (SD = 22.7) | 61.0 (SD = 19.5) |
| Mean WAB-R Repetition | 5.5 (SD = 3.4) | 5.3 (SD = 3.3) | 5.9 (SD = 3.3) | 7.0 (SD = 2.7) |
AQ = aphasia quotient of the WAB-R, a summary measure of overall language ability; WAB-R = Western Aphasia Battery-Revised; SD = standard deviation.
Education information was not available for all subjects, the number for which education information indicated here.
All participants provided informed consent to participate in this study, which was approved by the Institutional Review Boards at the University of South Carolina and the Medical University of South Carolina. All participants were native speakers of American English and had at least one ischaemic stroke to the left hemisphere at least 6 months before study inclusion.
To compare our study effectively with the studies of Mesulam et al. in PPA,1,12 we included a similar set of six behavioural measures: the WAB-R Auditory Word Comprehension subtest, the PPT, the Philadelphia Naming Test, Non-canonical Sentence Comprehension, the WAB-R Repetition subtest and our perceptual ratings of Expressive Agrammatism. Each of these measures is described next. Out of these six measures, we ultimately performed four lesion mapping analyses, described in the section ‘Analyses’.
The Auditory Word Recognition subtest of the WAB-R41 involves asking the participant to point to real-world objects or printed images (n = 60). Importantly, the test does not require syntactic parsing to perform correctly, as the participant only needs to identify the lexical item presented in each item. Our Word Comprehension measure used for lesion analyses consisted of this measure incorporating the PPT measure described next as a covariate.
The WAB-R Repetition subtest41 (referred to subsequently as Repetition) involves presenting a series of increasingly complex utterances and requiring participants to repeat them verbatim. Scoring is based on the number of words correctly recalled in the correct order.
The PPT11 involves presenting a target picture with two candidate pictures below that are possible associates of the target picture (e.g. the target picture could be a pyramid, with candidate pictures of palm trees and pine trees). The participant is required to point to the candidate picture that is more related to the target picture (e.g. the palm trees). There are a total of 52 trials. This task was included to provide a control for object recognition and non-verbal semantic processing in the WAB-R word comprehension test (as in Mesulam et al.1 and Bonilha et al.34).
The Philadelphia Naming Test46 is a 175-item assessment of picture naming, involving the presentation of a number of simple line drawings. Here we used the total number of items correctly named on the task (rather than phonological or semantic errors).
Our Non-canonical Sentence Comprehension measure was derived from either the NAVS (n = 82) or the Icelandic Task (n = 48). The NAVS Sentence Comprehension Test42 involves testing the comprehension of a variety of canonical and non-canonical sentence types, each with five total trials, assessed via pointing to the correct picture. Mesulam et al. (2015) assessed the comprehension performance on the three non-canonical sentence types: passives (with a by-phrase) e.g. ‘the dog is chased by the cat’, object-extracted WH-questions, e.g. ‘who is the cat chasing?’ and object-relatives, e.g. ‘Pete saw the boy who the girl is pulling’, for a maximum score of 15. The Icelandic Task includes a similar set of canonical and non-canonical sentence types, each with five total trials: passives (with a by-phrase), e.g. ‘the boy is painted by the girl’, object-extracted WH-questions, e.g. ‘which boy is the girl painting?’ and object clefts, e.g. ‘it is the girl that the boy paints’. The sentence types across the two tasks are not strictly identical, but involve essentially the same structures with the same degree of complexity, including the key factor of non-canonical object-first word order. Therefore, for participants who were not assessed with the NAVS, we calculated the equivalent scores on the Icelandic Task (correct non-canonical trials, out of 15 points).
The Expressive Agrammatism measure was a perceptual measure of grammatical deficits in speech production. We derived this measure from samples of connected speech production elicited either by describing the Cookie Theft picture47 (n = 39) as reported in den Ouden et al.44 or retelling the story of Cinderella in their own words48 (n = 53), as reported in Matchin et al.45 Production samples were rated independently by speech and language experts for the systematic simplification of sentence structure and omission of function words and morphemes. This resulted in a categorical assessment for each subject, either agrammatic or not.
Brain imaging and lesion mapping
We acquired anatomical MRI and diffusion tensor imaging (DTI) data using the same parameters and procedures as described in previous studies.30,34,44 High-resolution neuroimaging data (T1- and T2-weighted images) were collected at the University of South Carolina and the Medical University of South Carolina on a 3 T Siemens Trio scanner with a 12-element head coil. Lesions were demarcated onto each subject’s T2 image by an expert neurologist (L.B.) or an expert cognitive neuroscientist (Dr Roger Newman-Norlund) extensively trained by L.B. (with consultation as needed with an expert on lesion mapping, Dr Chris Rorden), both blind to the behavioural data. Lesion maps were then aligned to the high-resolution T1 image. Lesions were replaced with the corresponding brain structure from the intact hemisphere, and this image as well as the lesion map in subject space were subsequently warped to Montreal Neurological Institute (MNI) space using SPM12.49 The warped lesion map was then binarized with a 50% probability threshold, which was used to perform voxel-wise and region of interest (ROI) analyses.
Lesion overlap maps for both groups are shown in Fig. 1. Overall, there was good coverage in perisylvian cortex, covering all relevant language-related regions (see lesion overlap maps in Fig. 1). We used the JHU atlas50 (depicted in Supplementary Fig. 2, a full list of relevant region abbreviation definitions is in Supplementary Table 1) for LSM and CLSM analyses. The parcellations of the JHU atlas provide good alignment with language-relevant brain regions and have roughly equivalent parcel sizes relative to parcellations included in other atlases, such as the AICHA atlas.51 The JHU atlas contains two ROI containing the polar and lateral anterior components of the STG and MTG, the STG pole and MTG pole, which correspond to key components of the ATL. We note that Mesulam et al.1,12 define the ATL as including regions more inferior and posterior to these two ROIs. However, we also note that Mesulam et al.1 attributed a dominant role to the polar part of the ATL (p. 2431): ‘…the ATL peak atrophy sites associated with severe word comprehension impairment consistently included the temporal pole…ATL neuronal loss causes major word comprehension impairments only if it extends anteriorly all the way into the polar region’, thus we consider these two ROIs as well-defined to test the disconnection claim. However, as described later in the section ‘LSM and CLSM combined’, we accommodated the possibility that more posterior portions of the ATL might be relevant to the disconnection claims in our combined LSM and CLSM analyses.
Figure 1.
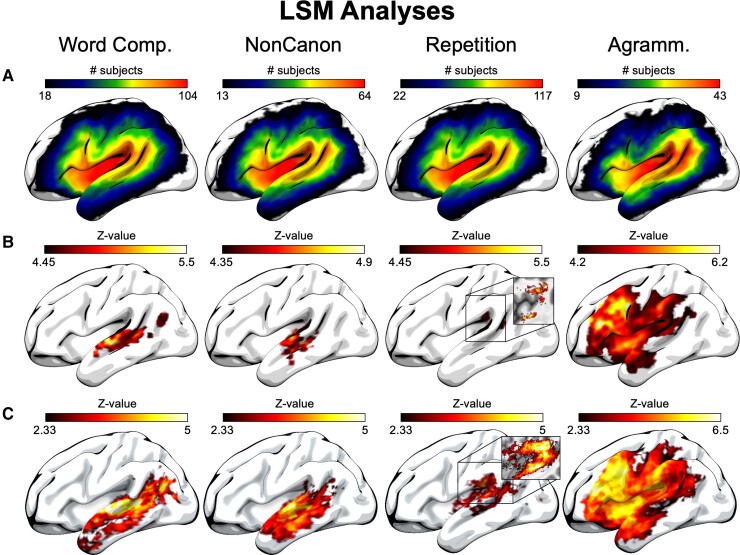
LSM analyses. (A) Lesion overlap maps for each group associated with the behavioural measures for which we analysed lesion-deficit correlations. Note that the lower value in each colour spectrum indicates the minimum number of subjects with damage to each region that was required for our 10% lesion load threshold. (B) Voxel-wise univariate LSM analyses for each behavioural measure (incorporating lesion volume as a covariate), with a permutation correction for multiple comparisons (10 000 permutations, corrected P < 0.05), with results spatially smoothed for improved visibility. (C) Uncorrected (voxel-wise P < 0.01) and unsmoothed voxel-wise univariate LSM analyses for each behavioural measure (incorporating lesion volume as a covariate). Insets show white matter damage in a medial slice of the volumetric data underlying the cortical surface not adequately depicted in the surface rendering. Agramm. = Expressive Agrammatism; Comp = Comprehension; NonCanon = Non-canonical Sentence Comprehension.
Analyses
Behavioural
To examine the relationship among the six total behavioural variables, we performed non-parametric Spearman correlations using JASP.52 Missing data were accounted for with pairwise deletion. Non-parametric Spearman correlations were used given that the behavioural variables did not have pairwise normal distributions. Lesion volume is strongly correlated with aphasia severity in chronic stroke,53–55 including in our data (one-tailed Spearman’s rho correlating WAB-R AQ and lesion volume = −0.694, P = 6.254 × 10−33), and therefore is a potential confound,56,57 which we controlled by using lesion volume as a covariate in our LSM analyses. Similarly, we report behavioural correlations both with and without lesion volume as a covariate to evaluate overall severity effects. Mesulam et al.1 did not correct their behavioural correlation analyses for multiple comparisons, but given the large number of behavioural correlations we performed (15 pairwise correlations without lesion volume as a covariate, 15 pairwise correlations with lesion volume as a covariate), we report whether or not each correlation survived a Bonferroni correction for multiple comparisons, treating each set of 15 correlations (with and without the lesion volume covariate) as a separate family of tests, using an adjusted alpha level of P < 0.003.
LSM
With respect to LSM, using NiiStat (https://www.nitrc.org/projects/niistat/) we performed both voxel-based and ROI-based analyses relating each of the four selected behavioural variables to lesion location. ROI analyses were included to maximize statistical power and afford an opportunity to combine the LSM and CLSM data to test the hypothesis of Mesulam et al. that disconnection accounts for the substantial association of damage to middle-posterior temporal lobe regions and comprehension impairments. For voxel-based analyses, we performed one-tailed t-tests within each voxel comparing the magnitude of the behavioural measure for subjects with and without damage to that voxel (results were converted to Z-values for ease of interpretation). For ROI analyses, we performed univariate linear regression analyses relating proportion damage within the grey matter of each parcellated region (the number of voxels that were damaged divided by the total number of voxels in each region) contained within the JHU atlas (depicted in Supplementary Fig. 2), except for Expressive Agrammatism, which was logistic regression.50 Both voxel- and ROI-based analyses were only performed within voxels/regions that had at least 10% of participants with damage located there57,58 and were corrected for multiple comparisons using permutation tests (10 000 permutations) with a corrected alpha threshold of P < 0.05. We performed analyses for four behavioural measures that provided maximum comparison to the analyses of Mesulam et al.1,12: WAB-R Auditory Word Recognition with PPT as a covariate (Word Comprehension), Non-canonical Sentence Comprehension, WAB-R Repetition (Repetition) and Expressive Agrammatism. Lesion volume was included as a covariate in all LSM analyses to ensure accurate localization56,57 (we also report correlations between each major behavioural measure and overall lesion volume to assess the likelihood of Type 2 errors of including lesion volume as a covariate, that is, of overly conversative LSM analyses resulting from the lesion volume covariate). Univariate analyses were performed as opposed to multivariate approaches such as support vector regression to match the univariate analyses of Mesulam et al.,1 for straightforward interpretation of statistical results, and because univariate approaches have been shown to outperform multivariate approaches with large sample sizes and adequate lesion load restrictions.57 To supplement our voxel-based analyses, we also report reduced threshold (voxel-wise P < 0.01) lesion maps for all behavioural variables to provide the fullest picture of the lesion data without obscuring near-threshold results.
To address the question of regions that cause both word and sentence comprehension impairment, we calculated the overlap of the corrected voxel-based lesion maps for the Word Comprehension and Non-canonical Sentence Comprehension measures. Regions with significant overlap could be considered candidates for an updated anatomical definition of ‘Wernicke’s area’ according to its functional definition as causing both of these impairments.
Finally, an additional issue concerns the fact that our Word Comprehension and Non-canonical Sentence Comprehension measures do not control for prelexical speech perception impairments. We performed analyses of two additional measures including the Repetition score as an additional covariate to attempt to control for these impairments. We first combined the behavioural scores with these covariates in a linear regression model (WAB-R Auditory Word Recognition with both PPT and Repetition, and Non-canonical Sentence Comprehension with Repetition), saved the residual values and then performed LSM analyses of the two resulting measures in the same manner as described before.
CLSM
For CLSM, we analysed the diffusion-weighted images that were acquired for each participant and estimated the pairwise connection strength between all regions within the JHU atlas, including both the left and right hemispheres50 (see Supplementary Fig. 2 for a depiction of a subset of the regions contained within the JHU atlas). First, each participant’s T1 image was used to register white matter and region parcellation to the diffusion-weighted images. Next, we estimated connection strength between regions using fibre count (corrected for distance and region volume) for each of the pairwise connections by using probabilistic tractography as implemented in FSL’s Diffusion Toolbox (FDT) method,59 using an approach that minimizes the potential distorting effects of brain damage on fibre tracking.13 Pairwise connectivity was calculated as the fibre count arriving in one region when another region was seeded and averaged with the fibre count calculated in the reverse direction. The lesioned tissue was removed from all tractography tracings to maximize accuracy, which also minimizes the effect of lesion volume on the final analyses. The estimated number of streamlines from a completely destroyed region was set to zero. Overall, the number of streamlines that can be estimated between a pair of regions is constrained but not determined by the amount of damage to each region, as the damage to intervening white matter pathways is crucial to estimating the intact connections between these regions. The number of estimated tracts connecting pairs of regions was divided by the total volume of both regions, controlling for unequal region sizes. For full details of the methodological approach, see other publications from our research group using the same method.34,36
We then performed linear regression analyses relating scores on each behavioural measure with the estimated connection strength for each connection using NiiStat (https://www.nitrc.org/projects/niistat/), correcting for multiple comparisons using permutation tests (10 000 permutations) and a corrected alpha threshold of P < 0.05 for each of the four behavioural measures (Word Comprehension, Non-canonical Sentence Comprehension, Repetition and Expressive Agrammatism). When including lesion volume as a covariate, we identified very few significant disconnections associated with non-canonical sentence comprehension deficits and no significant disconnections associated with any of the other three behavioural measures we assessed (Word Comprehension, Repetition and Expressive Agrammatism). It is as yet unclear whether lesion volume is critical for accurate localization as in LSM,56,57 although as noted before, lesion volume was already factored into our analyses by removing damaged tissue from estimated tractographies. Therefore, we focus our results on analyses that did not include lesion volume as a covariate in our CLSM analyses, in line with previous reports from our research group.34,44
LSM and CLSM combined
The ‘double disconnection’ hypothesis of Mesulam et al. entails that the association between damage to temporal-parietal regions (roughly Wernicke’s area) with word and sentence comprehension deficits in Wernicke’s aphasia can be explained via disrupted connections due to damage to underlying white matter between the ATL and frontal cortex to potentially various other regions of the brain (Mesulam, personal communication). If this were true, then there should be no robust independent contribution of damage to these regions above and beyond the extent of disruption to these connections. To test this, we combined the LSM and CLSM data by assessing whether lesion-deficit correlations for these behavioural measures would still be statistically robust when incorporating connection strength as a covariate.
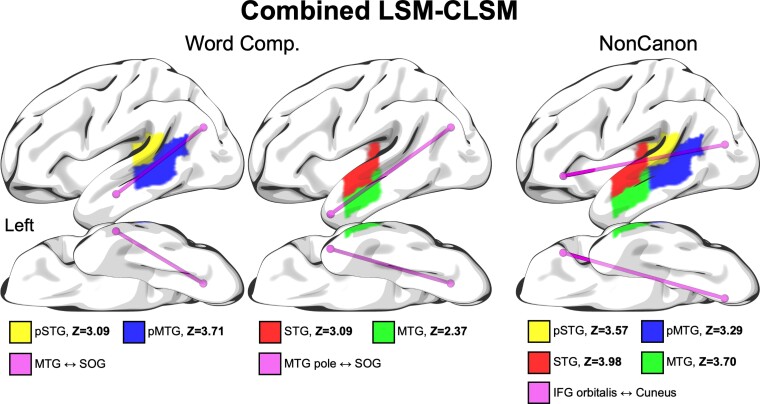
First, we identified the relevant regions associated with Word Comprehension and Non-canonical Sentence Comprehension using the ROI-based LSM analyses described in the section ‘LSM’. Both measures identified the same set of regions: superior temporal gyrus, middle portion (STG), posterior superior temporal gyrus (pSTG), MTG, middle portion and posterior MTG (pMTG). For Word Comprehension, for the more posterior of these regions (pSTG and pMTG) we identified the single strongest disconnection involving a more ATL region associated with behavioural deficits, MTG ↔︎ superior occipital gyrus (SOG) (Z = 4.04). We then did the same for the middle temporal regions, STG and MTG, identifying the MTG pole ↔︎ SOG (Z = 3.98). For Non-canonical Sentence Comprehension, we identified the single strongest disconnections involving all four of these regions identified in the ROI-based LSM analyses and a frontal lobe region. Given that no frontal disconnections were identified that survived the multiple comparisons correction in the CLSM analyses, we selected the single strongest subthreshold frontal disconnection for these regions out of the set of IFG pars opercularis (IFG opercularis), IFG pars triangularis (IFG triangularis), IFG pars orbitalis (IFG orbitalis) and posterior middle frontal gyrus (pMFG), given that the IFG regions are classically associated with grammatical processing and the pMFG was identified in our ROI-based LSM analysis of Expressive Agrammatism. This identified the connection between IFG orbitalis and the cuneus (Z = 3.66) as the most strongly disconnected. The selected regions and connections for these analyses are depicted in Fig. 4.
Figure 4.
Combined LSM–CLSM analyses. Selected regions and connections for the analyses combining LSM and CLSM data, in which connection strength was included as a covariate for the linear regression analysis relating proportion damage within each region to the behavioural variable, including total lesion volume as a covariate.21Z-scores for the association of damage to each region with comprehension deficits, after taking into account variance associated with connection strength, are shown at the bottom. All Z-scores are >2.33, which corresponds to P < 0.01. Comp. = Comprehension; NonCanon = Non-canonical Sentence Comprehension; Z = Z-score.
We then performed linear regression analyses to predict behavioural scores using proportion damage to each ROI, incorporating both lesion volume and the relevant connection for that region as covariates. In this way, we tested whether the strongest anterior temporal disconnection could account for the lesion-deficit correlations in the posterior temporal lobe for Word Comprehension, and whether the strongest frontal disconnection could account for the lesion-deficit correlations in the temporal lobe for Non-canonical Sentence Comprehension. Under the Mesulam et al. hypothesis, we would expect that there would no longer be a significant association between the behavioural scores and damage to middle-posterior temporal lobe regions when these disconnections are considered.
Data availability
All of the lesion maps and behavioural data for participants enrolled in this study are available for download at https://www.dropbox.com/sh/3w4aeizgypfs7sd/AAB-W8Yn5qDUFeBj90WKsBqAa?dl=0 (use ‘wernickeConundrumRevisited_CLSM.xlsx’ file).
Spearman correlations found significant correlations among most of our behavioural variables when lesion volume was not included as a covariate (Table 2, top). In fact, the only correlations that were not significant were between Non-canonical Sentence Comprehension and PPT (although this nearly survived the Bonferroni correction for multiple comparisons), and between Expressive Agrammatism and three measures: Non-canonical Sentence Comprehension, Repetition and PPT. When lesion volume was included as a covariate, we found overall weaker correlations among the behavioural measures and Expressive Agrammatism was no longer significantly correlated with any of the other measures (Table 2, bottom).
Table 2.
Behavioural analyses
| PNT | NonCanon | Repetition | PPT | Agramm. | |
|---|---|---|---|---|---|
| Analyses with no covariates | |||||
| Word Comp. | n = 162 | n = 130 | n = 218 | n = 181 | n = 92 |
| ρ = 0.565 | ρ = 0.415 | ρ = 0.738 | ρ = 0.622 | ρ = 0.377 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
| PNT | n = 102 | n = 162 | n = 155 | n = 73 | |
| ρ = 0.451 | ρ = 0.802 | ρ = 0.408 | ρ = 0.350 | ||
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| NonCanon | n = 130 | n = 108 | n = 79 | ||
| ρ = 0.441 | ρ = 0.236 | ρ = 0.101 | |||
| P < 0.001 | P = 0.007 | P = 0.197 | |||
| Repetition | n = 181 | n = 92 | |||
| ρ = 0.502 | ρ = 0.205 | ||||
| P < 0.001 | P = 0.039 | ||||
| PPT | n = 75 | ||||
| ρ = 0.112 | |||||
| P = 0.164 | |||||
| Analyses with lesion volume covariate | |||||
| Word Comp. | n = 162 | n = 130 | n = 218 | n = 181 | n = 92 |
| ρ = 0.433 | ρ = 0.281 | ρ = 0.549 | ρ = 0.523 | ρ = 0.038 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.359 | |
| PNT | n = 102 | n = 162 | n = 155 | n = 73 | |
| ρ = 0.364 | ρ = 0.756 | ρ = 0.306 | ρ =−0.051 | ||
| P < 0.001 | P < 0.001 | P < 0.001 | P = 0.683 | ||
| NonCanon | n = 130 | n = 108 | n = 79 | ||
| ρ = 0.317 | ρ = 0.146 | ρ =−0.137 | |||
| P < 0.001 | P = 0.067 | P = 0.875 | |||
| Repetition | n = 181 | n = 92 | |||
| ρ = 0.359 | ρ = 0.121 | ||||
| P < 0.001 | P = 0.152 | ||||
| PPT | n = 75 | ||||
| ρ =−0.076 | |||||
| P = 0.747 | |||||
Correlation analyses that survived the Bonferroni correction for multiple comparisons within each family of tests (with/without lesion volume covariate) (adjusted alpha of P < 0.003) indicated in bold. ρ = Spearman’s rho. Agramm. = Expressive Agrammatism; Comp. = Comprehension; NonCanon = Non-canonical Sentence Comprehension; PNT = Philadelphia Naming Task.
Similar to Mesulam et al.,1 we found that WAB-R Auditory Word Recognition and PPT scores were strongly correlated, justifying our use of PPT scores as a covariate with WAB-R Auditory Word Recognition to create the Word Comprehension measure, removing variance due to object recognition and non-verbal semantic processing in our LSM analyses. We also found that Non-canonical Sentence Comprehension and Repetition scores were correlated, suggesting that at least some of the variance in sentence comprehension scores could be due to phonological working memory abilities.
There were notable differences in our analyses from the behavioural correlations reported by Mesulam et al.1 Importantly, we found robust correlations between Non-canonical Sentence Comprehension and WAB-R Auditory Word Recognition, whether or not lesion volume was included as a covariate, unlike Mesulam et al., who found that word and sentence comprehension were not correlated. This is consistent with the classical findings in stroke-based aphasia that word and sentence comprehension deficits coincide. These results underscore the ‘Wernicke conundrum’: apparent differences in the patterns of word and sentence comprehension deficits between PPA and stroke-based aphasia. We also found that WAB-R Repetition was very robustly correlated with WAB-R Auditory Word Recognition, whereas Mesulam et al. found that Word Comprehension and Repetition were not correlated.
Finally, we also found that Non-canonical Sentence Comprehension was not correlated with Expressive Agrammatism, whether or not lesion volume was included as a covariate. Although correlations between Expressive Agrammatism and other behavioural variables were not assessed in Mesulam et al.,1,12 this lack of a relationship in our data speaks against one of the major ideas promoted in this work. Namely, they suggested that sentence comprehension deficits in both PPA and stroke-based aphasia are due to degeneration and/or disconnection of a frontal-based grammatical processing centre that affects both production and comprehension. Under this perspective, one would expect that Expressive Agrammatism and Non-canonical Sentence Comprehension would be correlated, but they were not. Note that we did find that Expressive Agrammatism correlated with WAB-R Auditory Word Recognition and picture naming when lesion volume was not included as a covariate; thus, the failure to identify a correlation between Expressive Agrammatism and Non-canonical Sentence Comprehension cannot be merely due to a lack of statistical power.
LSM
Voxel-based LSM results are shown in Fig. 1 and Supplementary Fig. 1. ROI-based LSM results are listed in Table 3. Spearman correlations between each behavioural measure and overall lesion volume are as follows: Word Comprehension (WAB-R Auditory Word Recognition deficits, with PPT scores as a covariate) and lesion volume, r = −0.354, P = 2.851 x 10−6; Non-canonical Sentence Comprehension and lesion volume, r = −0.314, P = 2.734 x 10−4; Repetition and lesion volume, r = −0.577, P = 9.824 x 10−21; Expressive Agrammatism, r = 0.639, P = 6.965 x 10−12.
Table 3.
Significant regions from ROI-based LSM analyses
| ROI | R 2 | βu (SE) | βs | Z | BFm (null) |
|---|---|---|---|---|---|
| Word Comprehension | |||||
| STG | 0.523 | −0.009 (0.002) | −0.236 | 4.18 | 1.685 × 10−26 |
| MTG | 0.349 | −0.008 (0.002) | −0.236 | 3.60 | 8.853 × 10−15 |
| pSTG | 0.465 | −0.010 (0.002) | −0.231 | 3.89 | 3.544 × 10−22 |
| pMTG | 0.409 | −0.012 (0.002) | −0.309 | 4.88 | 2.146 × 10−18 |
| Non-canonical Sentence Comprehension | |||||
| STG | 0.551 | −0.030 (0.007) | −0.284 | 4.36 | 4.284 × 10−20 |
| MTG | 0.410 | −0.028 (0.007) | −0.305 | 4.10 | 8.183 × 10−13 |
| pSTG | 0.491 | −0.033 (0.008) | −0.282 | 4.08 | 9.572 × 10−17 |
| pMTG | 0.388 | −0.030 (0.008) | −0.282 | 3.75 | 8.102 × 10−12 |
| Repetition | |||||
| pSTG | 0.503 | −0.029 (0.007) | −0.247 | 4.11 | 1.570 × 10−30 |
| pInsula | 0.460 | −0.025 (0.007) | −0.209 | 3.35 | 9.765 × 10−27 |
| SLF/AF | 0.634 | −0.029 (0.005) | −0.300 | 5.70 | 1.319 × 10−44 |
| Expressive Agrammatism | |||||
| pMFG | 0.510 | 0.768 (0.179) | 0.487 | 4.07 | 3.936 × 10−12 |
| Word Comprehension with Repetition covariate | |||||
| pMTG | 0.370 | −0.009 (0.003) | −0.220 | 3.56 | 4.961 × 10−16 |
| Non-canonical Sentence Comprehension with Repetition covariate | |||||
| MTG | 0.417 | −0.031 (0.007) | −0.302 | 4.28 | 3.880 × 10−13 |
| STG | 0.532 | −0.028 (0.007) | −0.234 | 3.73 | 5.030 × 10−19 |
See Supplementary Table 1 for region abbreviation definitions. All regions are left hemisphere. βu = unstandardized estimated beta coefficient; βs = standardized estimated beta coefficient; BFm (null) = Bayes factor index indicating support for the null hypothesis; SE = standard error; Z = Z-score.
Focusing on the corrected voxel-based analyses (Fig. 1B), Word Comprehension deficits were associated with damage to middle and posterior STG (pSTG) and posterior MTG (pMTG) and to a lesser extent inferior angular gyrus. Non-canonical Sentence Comprehension deficits were associated with a similar lesion distribution, although without including damage to the inferior angular gyrus. The corrected ROI analyses revealed that damage to the same set of four regions was associated with both Word Comprehension and Non-canonical Sentence Comprehension deficits: STG (middle), MTG (middle), pSTG and pMTG. The supplementary analyses that included Repetition as a covariate for both of these measures revealed largely similar results with substantially reduced statistical strength (Supplementary Fig. 1), with a subset of the same regions significantly identified in the ROI analyses (Table 3).
By contrast, the corrected voxel-based LSM analyses revealed that Repetition deficits were primarily associated with damage to posterior STG and superior longitudinal/arcuate fasciculus. The corrected ROI-based analyses revealed similar results, including damage to those regions as well as the posterior insula. Expressive Agrammatism was associated with a widespread pattern of damage in the corrected voxel-based analyses, including most prominently the posterior middle frontal gyrus (pMFG) and superior IFG, although this lesion map extended contiguously into the anterior insula, supramarginal gyrus, inferior angular gyrus and ATL. The corrected ROI-based analyses of Expressive Agrammatism only revealed significant damage to the pMFG.
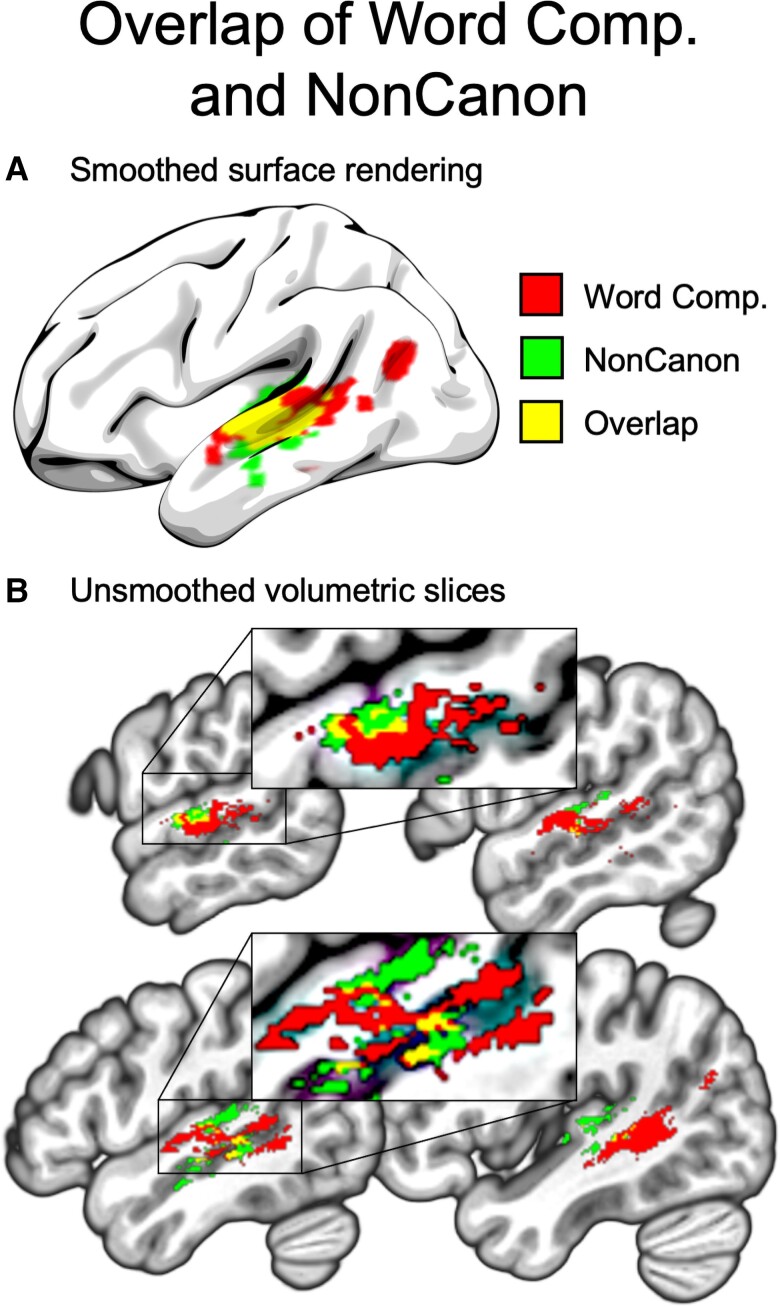
The voxel-based overlap analyses of Word Comprehension and Non-canonical Sentence Comprehension (Fig. 2) showed substantial overlap of the lesion correlates of these two measures in the middle STG and middle-posterior STS. The centres of mass for the primary clusters of overlapping voxels were located at the following sets of Montreal Neurological Institute (MNI) coordinates:
Figure 2.
Wernicke’s area localized through the convergence of the lesion maps for word and sentence comprehension deficits. (A) Smoothed surface rendering of the overlap (yellow) of the corrected lesion maps for Word Comprehension (red) and Non-canonical Sentence Comprehension (green). (B) Unsmoothed slices of the volumetric (non-surface-rendered) overlap. Insets show zoomed-in views of the portions of the slices surrounded by rectangles. Comp = Comprehension; NonCanon = Non-canonical Sentence Comprehension.
−48, −34, −1
−59, −16, 2
−53, −20, −3.
This overlap is consistent with the classic picture from stroke-based aphasia in which word and sentence comprehension coincide in Wernicke’s aphasia following damage to posterior temporal lobe, although we note that the strongest overlap occurred in middle STG and posterior middle STS for both measures. Combined with the robust ROI results in both posterior and middle temporal regions, these results indicate that the middle temporal lobe is crucial to an anatomical definition of Wernicke’s area. The overlap of the supplemental analyses including Repetition as a covariate showed very similar regions but with no strict overlap of Word Comprehension and Non-canonical Sentence Comprehension at the voxel level (Supplementary Fig. 1), probably due to the greatly reduced statistical power of these analyses.
CLSM
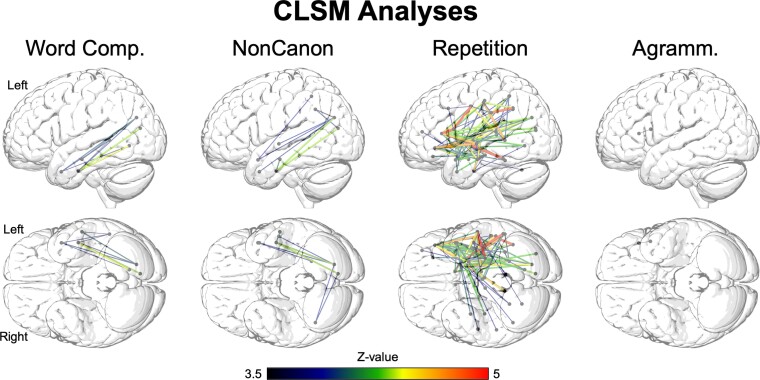
CLSM results are shown in Fig. 3, and the top ten significant disconnections for our primary analyses are listed in Table 4 (the full set of significant disconnections for the CLSM analysis of Repetition are listed in Supplementary Table 3, as are the significant disconnections for our supplementary analyses of Word and Non-canonical Sentence Comprehension including Repetition as a covariate). Both impaired Word Comprehension (WAB-R Auditory Word Recognition with PPT as a covariate) and Non-canonical Sentence Comprehension were associated with several disconnections within the temporal lobe and between temporal and occipital areas, including temporal pole regions. Impaired Non-canonical Sentence Comprehension was associated with one significant disconnection between the superior parietal lobe and the temporal pole, as well as two right hemisphere angular gyrus disconnections involving the left visual cortex. There were no significant frontal disconnections associated with either Word or Non-canonical Sentence Comprehension deficits, and there were no right hemisphere disconnections associated with Word Comprehension deficits. This speaks against the hypothesis of Mesulam et al.1 that impaired non-canonical sentence comprehension in stroke-based aphasia results from impaired disconnection of a frontal-based grammatical processing system and that impaired word comprehension might result from disconnection of the left ATL from right hemisphere regions.
Figure 3.
CLSM analyses. Significant disconnections between ROIs based on the JHU atlas ascertained by diffusion tensor imaging, with a permutation correction for multiple comparisons (10 000 permutations, corrected P < 0.05). Dots indicate regions at the endpoints of significant disconnections. Agramm. = Expressive Agrammatism; Comp. = Comprehension; NonCanon = Non-canonical Sentence Comprehension.
Table 4.
Top ten significant connections in the CLSM analyses
| Connection | R 2 | βu (SE) | βs | Z | BFm (null) |
|---|---|---|---|---|---|
| Word Comprehension | |||||
| ITG ↔ LG | 0.111 | 0.519 (0.115) | 0.333 | 4.38 | 0.0007 |
| ITG ↔ cuneus | 0.108 | 0.202 (0.045) | 0.329 | 4.32 | 0.0009 |
| MTG ↔ SOG | 0.095 | 0.241 (0.058) | 0.309 | 4.04 | 0.003 |
| MTG pole ↔ SOG | 0.093 | 0.068 (0.017) | 0.304 | 3.98 | 0.003 |
| ITG ↔ SOG | 0.091 | 0.225 (0.056) | 0.301 | 3.94 | 0.004 |
| MTG pole ↔ pMTG | 0.085 | 0.317 (0.082) | 0.291 | 3.80 | 0.007 |
| MTG pole ↔ LG | 0.084 | 0.206 (0.053) | 0.290 | 3.79 | 0.007 |
| ITG ↔ pMTG | 0.081 | 0.692 (0.182) | 0.285 | 3.72 | 0.009 |
| MTG ↔ pMTG | 0.081 | 0.743 (0.196) | 0.284 | 3.71 | 0.009 |
| Non-canonical Sentence Comprehension | |||||
| ITG ↔ cuneus | 0.141 | 0.604 (0.134) | 0.375 | 4.33 | 0.0008 |
| ITG ↔ SOG | 0.140 | 0.785 (0.175) | 0.374 | 4.31 | 0.0009 |
| MTG ↔ ITG | 0.126 | 0.760 (0.180) | 0.355 | 4.08 | 0.002 |
| AG (RH) ↔ cuneus | 0.118 | 1.282 (0.315) | 0.343 | 3.93 | 0.004 |
| MTG pole ↔ SOG | 0.118 | 0.207 (0.051) | 0.343 | 3.93 | 0.004 |
| STG ↔ SOG | 0.116 | 1.082 (0.268) | 0.341 | 3.91 | 0.004 |
| AG (RH) ↔ SOG | 0.115 | 0.544 (0.135) | 0.339 | 3.89 | 0.005 |
| STG pole ↔ SOG | 0.115 | 0.214 (0.053) | 0.339 | 3.88 | 0.005 |
| SPL ↔ STG pole | 0.114 | 0.064 (0.016) | 0.338 | 3.87 | 0.005 |
| Repetition | |||||
| MTG ↔ Hippocampus | 0.125 | 0.713 (0.135) | 0.353 | 5.10 | 0.00003 |
| IFG opercularis ↔ IFG triangularis | 0.119 | 1.296 (0.252) | 0.345 | 4.97 | 0.00005 |
| IFG opercularis ↔ PrCG | 0.117 | 0.742 (0.146) | 0.342 | 4.94 | 0.00006 |
| Putamen ↔ pITG | 0.116 | 1.215 (0.239) | 0.341 | 4.92 | 0.00006 |
| SMG ↔ AG | 0.115 | 1.278 (0.253) | 0.339 | 4.89 | 0.00007 |
| PoCG ↔ pMTG | 0.107 | 0.419 (0.086) | 0.328 | 4.71 | 0.0002 |
| IFG orbitalis ↔ STG | 0.107 | 0.367 (0.076) | 0.327 | 4.71 | 0.0002 |
| IFG orbitalis ↔ Putamen | 0.107 | 0.065 (0.013) | 0.327 | 4.70 | 0.0002 |
| STG ↔ Caudate | 0.106 | 1.268 (0.262) | 0.326 | 4.69 | 0.0002 |
| IFG triangularis ↔ STG | 0.106 | 0.184 (0.038) | 0.326 | 4.68 | 0.0002 |
| Expressive Agrammatism | |||||
| IFG opercularis ↔ IFG triangularis | 0.135 | −0.011 (0.003) | −0.368 | 3.57 | 0.014 |
See Supplementary Table 1 for region abbreviation definitions. All regions are left hemisphere, unless indicated as right hemisphere with (RH). βu = unstandardized estimated beta coefficient; βs = standardized estimated beta coefficient; BFm (null) = Bayes Factor index indicating support for the null hypothesis; SE = standard error; Z = Z-score.
By contrast, impaired Repetition was associated with a very large number of significant disconnections, within the frontal and temporal lobes as well as between frontal lobe and all other lobes, and between temporal lobe and all other lobes (there were no significant disconnections between parietal and occipital lobes). Expressive Agrammatism was associated with one significant disconnection in the frontal lobe, between IFG pars triangularis and IFG pars opercularis. These results underscore that the lack of significant frontal-based disconnections for Non-canonical Sentence Comprehension was not due to lack of statistical power or other methodological issues.
LSM and CLSM combined
Figure 4 and Supplementary Table 1 show the results of the combined analyses. The lesion-deficit correlations for all four middle and posterior temporal lobe regions (STG, MTG, pSTG, pMTG) for Word Comprehension remained statistically robust even when including the strongest relevant disconnections involving a more ATL region, MTG ↔ SOG for the pSTG and pMTG and MTG pole ↔ SOG for the STG and MTG. Likewise, the lesion-deficit correlations for all the same middle and posterior temporal lobe regions (STG, MTG, pSTG, pMTG) for Non-canonical Sentence Comprehension remained statistically robust even when including the strongest relevant frontal disconnection (IFG orbitalis ↔ cuneus). Thus, there remains a strong independent association between damage to middle-posterior temporal lobe regions and comprehension deficits even when accounting for the strongest disconnections of ATL and the frontal lobe.
Discussion
Consistent with previous research, the lesion correlates of Word Comprehension (WAB-R Auditory Word Recognition with PPT as a covariate) and Non-canonical Sentence Comprehension deficits in chronic post-stroke aphasia converged on the middle-posterior temporal lobe in our LSM analyses, overlapping in the middle STG and middle-posterior STS. Because we (and Mesulam et al.1) used the PPT as a covariate in our analyses of Word Comprehension, variance associated with conceptual-semantic processing was probably greatly reduced, and thus this measure should highlight mechanisms up to and including lexical access. Given previous functional neuroimaging studies, we suggest that the middle STG is associated with phonetic and/or phonological mechanisms60,61 and the middle-posterior STS with lexical access.17 The supplementary analyses of these measures including Repetition as a covariate resulted in similar lesion maps but with greatly reduced statistical power, without significant overlap but in nearly identical locations. The overlap of word and sentence comprehension deficits observed in this study is roughly consistent with the historical definition of Wernicke’s area, although it should be noted that robust overlap occurred more anteriorly than as often depicted (although see Wernicke62 for localization remarkably similar to what we observed here, resembling the results of functional neuroimaging studies of lexical and syntactic processing). In general, we agree with others who have argued3,63 that the term ‘Wernicke’s area’ should be avoided because of potential confusion about its localization stemming from the scattered historical anatomical localization of Wernicke’s area that most often focuses on more posterior temporal-parietal regions.1,2 However, our results do reinforce the mostly uncontroversial conclusion that the temporal lobe regions associated with both word and sentence comprehension deficits in stroke-based aphasia are clearly posterior to the anterior locus of word comprehension deficits identified in the papers reported by Mesulam et al.1,12
One of the main claims of Mesulam et al.1 is that damage to the territory associated with Wernicke’s area involves ‘double disconnection’ in Wernicke’s aphasia. That is, they posited that word comprehension deficits follow from disconnection of the ATL, and sentence comprehension deficits follow from disconnection of frontal cortex, to various other regions of the brain, potentially including the right hemisphere. However, while our CLSM analyses found that there were significant disconnections involving the temporal pole for both word and non-canonical sentence comprehension deficits, no significant frontal disconnections were associated with non-canonical sentence comprehension deficits. Our combined LSM and CLSM analyses further showed that, using the strongest subthreshold frontal-based connection as a covariate, there were still robust effects of damage to middle and posterior temporal lobe regions. Thus, the fundamental prediction of Mesulam et al. that sentence comprehension deficits associated with damage to Wernicke’s area are explained by a frontal disconnection pattern was disconfirmed. Note that our results are unlikely to be due to lack of statistical power, as we identified significant frontal-temporal disconnections associated with speech repetition deficits, and a significant LSM effect of Expressive Agrammatism in frontal areas (particularly pMFG) as well as somewhat weaker effects in the ATL, with a significantly damaged connection between IFG pars opercularis and IFG pars triangularis.
The Mesulam et al. frontal-temporal disconnection proposal regarding sentence comprehension deficits has logical force if a primary cortical locus of syntactic abilities were in the frontal lobe, as suggested by many authors.64–68 Accordingly, we did find that agrammatic production deficits are associated with damage to the frontal lobe, including Broca’s area/posterior IFG, consistent with previous work.32,44,45,69–73 In addition, functional neuroimaging studies of syntactic processing do reliably identify frontal activations including Broca’s area as indicated by meta-analyses of these studies.74,75 However, recent theories propose that the systems for building hierarchical syntactic structure in comprehension are in the posterior temporal lobe, and not the frontal lobe.16,65 The lack of a significant association between non-canonical sentence comprehension deficits and damage to the inferior or middle frontal lobe, and the lack of an association with significant frontal-temporal disconnections, provides evidence against the view that syntactic computations associated with sentence comprehension are processed in or around Broca’s area. Matchin and Hickok21 proposed that hierarchical syntax and lexical access are both subserved by the posterior temporal lobe, intimately intertwined, which is consistent with approaches to syntax positing ‘lexicalized’ syntactic representations. Under this view, coincidence of the lesion correlates of lexical and syntactic processing in the posterior temporal lobe is expected. However, we must also keep in mind that the lack of frontal effects for syntactic comprehension might also be due to functional reorganization in chronic aphasia, and there is some evidence that people with chronic aphasia show enhanced activity for language processing in right IFG relative to healthy controls, as revealed by a recent meta-analysis.76
Importantly, our analyses of non-canonical sentence comprehension were matched to Mesulam et al.1 in assessing overall comprehension ability. Previous studies from our group in stroke-based aphasia that have examined residual performance on non-canonical structures after controlling for performance to canonical structures have associated deficits with damage to frontal regions30,77 (with other studies showing conflicting results25,44,45). Residual performance on non-canonical sentence comprehension after controlling for canonical sentence comprehension probably highlights executive function resources that are necessary for processing particularly complex structures and revising sentence interpretation,78–84 thus some implication of frontal regions that are associated with these supporting mechanisms is expected.21,85–88 The existence of subthreshold frontal disconnections provides some support for this account.
One prediction of Mesulam et al.1 was partially confirmed: word comprehension deficits involved disconnection of anterior temporal regions. The disconnections we found between anterior temporal regions and other temporal lobe regions and visual cortex were not unique to word comprehension deficits, as similar disconnections were associated with non-canonical sentence comprehension deficits. However, we take the primary claim of Mesulam et al. to be not that disconnections of ATL are associated with word comprehension impairments, but rather that these disconnections ‘explain’ the association of posterior temporal lobe damage with these impairments. Critically, our combined LSM–CLSM analyses revealed that damage to middle and posterior temporal lobe regions was robustly associated with word comprehension impairments even when including the strongest disconnections involving a more ATL region. In addition, we also tested the possibility raised by Mesulam et al. that word comprehension impairments are associated with disconnection of right hemisphere regions, but no significant right hemisphere disconnections were identified.
The Mesulam et al. hypothesis would also predict that damage to the ATL itself should be associated with word comprehension deficits. However, we note that the corrected voxel-based LSM analyses of Word Comprehension (when controlling for conceptual-semantic processing via the PPT covariate) did not reveal an association with ATL damage. This lack of a robust ATL effect for Word Comprehension is unlikely to be due to statistical power, as we had adequate lesion coverage in this area and did identify significant effects for Expressive Agrammatism there. Overall, these combined results speak against the disconnection hypothesis of Mesulam et al. They suggest that the ATL may play a supporting role for word comprehension by retrieving conceptual-semantic representations that are necessary to perform a picture identification task in some contexts, and thus ATL disconnection contributes in a minor way to the impaired word comprehension performance seen in many patients with post-stroke aphasia involving damage to the posterior temporal lobe.
Under Mesulam et al.’s hypothesis,12 Wernicke’s area (broadly construed) plays a key role in phonological short-term/working memory, which is in line with their reported association between repetition deficits and posterior temporal cortical degeneration. Consistent with this, in our study, repetition deficits were associated with arcuate fasciculus and posterior temporal damage and multiple frontal-parietal-temporal disconnections. Furthermore, deficits in non-canonical sentence comprehension (but not word comprehension) were associated with a significant disconnection between the parietal and temporal lobe. This converges with previous reports of associations between deficits in complex sentence comprehension and inferior parietal lobe damage in chronic stroke patients with aphasia,22,23,25,26,31,44,77,89 as well as functional neuroimaging studies finding activation for sensory-motor integration in this vicinity.37,39,90 It is possible that these disconnections reflect the additional phonological short-term memory resources that are useful in parsing sentence structure but are not typically required for word comprehension,79–82,91,92 which may explain why the significant correlation between word and sentence comprehension abilities was only moderate in strength.
Task difference is a major factor that helps explain the discrepancy between the lesion localization for word comprehension deficits we report here (and generally in the stroke aphasia literature) and those of Mesulam et al.1,12 The word comprehension task used by Mesulam et al. consists of moderately difficult items of the Peabody Picture Vocabulary Task,94 which involve much more complex semantic inference than the task we used, the Auditory Word Recognition subtest of the WAB-R,41 probably resulting in considerable variance due to conceptual-semantic processing despite including the PPT as a covariate. Wernicke and Lichtheim thought of Wernicke’s area as the key node for ‘auditory word memories’,6,7,62 which can be interpreted in modern linguistic terms as phonological and lexical processing, with conceptual-semantic representations widely distributed elsewhere in the brain. The WAB-R Auditory Word Recognition test is therefore more appropriate to assess this idea, as it does not require fine-grained visual processing and object recognition, and limits semantic processing demands, unlike the Peabody Picture Vocabulary Task. Future studies should examine the relationship between these word comprehension measures and the results obtained in LSM analyses in both PPA and stroke-based aphasia.
In addition, PPA is a progressive neurodegenerative disease that has key differences from stroke-based aphasia, evolving to ultimately affect a number of cognitive domains.95,96 Functional neuroimaging studies of people with the semantic variant of PPA, associated primarily with ATL atrophy, have shown abnormal activation patterns and functional connectivity in regions outside the ATL.97–103 This suggests that the neuropathology of semantic PPA is more widespread than what shows up in gross measures of grey matter atrophy and may affect a network (potentially including middle and posterior temporal cortex) that contributes to word comprehension impairments. Individuals with PPA show neural degeneration far beyond the areas of peak atrophy (as seen in the figures in Mesulam et al.1), as well as disrupted functional networks beyond the sites of major neural degeneration. Longitudinal MRI and word comprehension testing reveals that deterioration in auditory word comprehension over time in PPA is associated with within-individual atrophy in left middle temporal cortex, left angular gyrus and right inferior and middle temporal cortex.104 Moreover, the word-level comprehension deficits seen in stroke patients and the semantic variant of PPA might have qualitative differences. Word comprehension deficits in stroke may be more commonly due to deficits in basic linguistic processing, i.e. at the phonological and lexical/lemma levels, whereas word comprehension deficits in PPA may be more commonly due to deficits in accessing fine-grained semantic features of words downstream from lexical access. These may be more completely disrupted in semantic PPA due to the pattern of neurodegeneration than is seen with strokes impinging on this area, or in resections.
Under our account, in which the middle-posterior temporal lobe plays a dominant causal role in both lexical access and syntactic comprehension, one would expect a strong association between word and sentence comprehension deficits and degeneration of the middle and posterior temporal lobe in PPA. However, this is not what is seen, as patients with the logopenic variant of PPA and posterior temporal-parietal degeneration do not typically show notable word comprehension deficits1,105–107 (but see Bonner and Grossman108). Also, Mesulam et al.1 do not report a notable association of atrophy in middle-posterior temporal lobe with deficits in non-canonical sentence comprehension. Why not? Some of the present authors have previously suggested that logopenic PPA does not necessarily involve complete degeneration of posterior temporal-parietal cortex, and that remaining neurons may be sufficient to perform the basic phonological and lexical access functions.8 Additionally, some models posit an important role for the right hemisphere in phonological processing and lexical access,109–111 which could sufficiently compensate for left hemisphere degeneration. This is consistent with recent demonstrations of small but significant word comprehension deficits in right hemisphere stroke.112
One area of agreement between Mesulam et al. and our data is that damage or degeneration of the ATL is not implicated in non-canonical sentence comprehension deficits. However, we did find significant disconnections of the temporal pole ROI for impaired non-canonical sentence comprehension deficits. This is consistent with previous reports implicating damage to ATL in grammatical processing deficits.22,31 Nevertheless, we suggest that the (relatively less common) implication of ATL damage or disconnection in sentence comprehension deficits probably reflects semantic rather than syntactic deficits,21,63 which is consistent with functional neuroimaging data.113–117
Overall, our combined LSM and CLSM results speak against the hypothesis of Mesulam et al.1 of a double disconnection syndrome underlying word and sentence/syntactic comprehension deficits in post-stroke aphasia. Rather, our results support the classical concept of Wernicke’s area as directly supporting both word and sentence comprehension, although our results do suggest that ATL and inferior parietal networks bolster core linguistic processing through conceptual-semantic processing and phonological working memory resources, respectively. The discrepancy between our results and those of Mesulam et al. from PPA might be explained by differences between language deficits in post-stroke aphasia versus PPA or differences in tests used to assess comprehension, or both. Alternatively, the conclusions based on PPA might be unfounded because they were based on regions of peak atrophy associated with errors, rather than considering other areas of degeneration or dysfunction that might be responsible for deficits. Irrespective of the account of the discrepant results, our data provide strong evidence for the major role of the middle STG and middle-posterior STS in both word and sentence comprehension.
Supplementary Material
Acknowledgements
We would like to thank Alexandra Basilakos and Brielle C. Stark for assistance with rating of speech samples, Leigh Ann Spell, Allison Croxton, Anna Doyle, Michele Martin, Katie Murphy and Sara Sayers for their assistance with data collection, and graduate student clinicians in the Aphasia Laboratory for transcribing and coding speech samples. We would also like to thank two anonymous reviewers for their fruitful analysis suggestions.
Contributor Information
William Matchin, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Dirk-Bart den Ouden, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Gregory Hickok, Department of Cognitive Sciences, University of California, Irvine, Irvine, CA 92697, USA; Department of Language Science, University of California, Irvine, Irvine, CA 92697, USA.
Argye E Hillis, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD 21218, USA; Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD 21218, USA; Department of Cognitive Science, Johns Hopkins University, Baltimore, MD 21218, USA.
Leonardo Bonilha, Department of Neurology, Emory University School of Medicine, Atlanta, GA 30322, USA.
Julius Fridriksson, Department of Communication Sciences and Disorders, University of South Carolina, Columbia, SC 29208, USA.
Funding
This research was supported by National Institute on Deafness and Other Communication Disorders grants P50 DC014664 and U01 DC011739 awarded to J.F., and grant R01 DC014021 awarded to L.B.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Mesulam M-M, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138:2423–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bogen JE, Bogen GM. Wernicke’s region—Where is it? Ann N Y Acad Sci. 1976;280:834–843. [DOI] [PubMed] [Google Scholar]
- 3. Tremblay P, Dick AS. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang. 2016;162:60–71. [DOI] [PubMed] [Google Scholar]
- 4. Geschwind N. Language and the brain. Sci Am. 1972;226:76–83. [DOI] [PubMed] [Google Scholar]
- 5. Geschwind N. Specializations of the human brain. Sci Am. 1979;241:180–199. [DOI] [PubMed] [Google Scholar]
- 6. Lichtheim L. On aphasia. Brain. 1885;7:433–484. [Google Scholar]
- 7. Wernicke C. The symptom complex of aphasia: A psychological study on an anatomical basis. In: Cohen RS and Wartofsky MW, eds. Boston studies in the philosophy of science: D. Reidel Publishing Company; 1874. p 34–97. [Google Scholar]
- 8. Hillis AE, Rorden C, Fridriksson J. Brain regions essential for word comprehension: Drawing inferences from patients: Lesion-deficit mapping. Ann Neurol. 2017;81:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damasio AR. Aphasia. N Engl J Med. 1992;326:531–539. [DOI] [PubMed] [Google Scholar]
- 10. Hillis AE. Aphasia: Progress in the last quarter of a century. Neurology. 2007;69:200–213. [DOI] [PubMed] [Google Scholar]
- 11. Howard D, Patterson K. The pyramids and palm trees test: A test of semantic access from words and pictures: Harcourt Assessment; 1992. [Google Scholar]
- 12. Mesulam MM, Rader BM, Sridhar J, et al. Word comprehension in temporal cortex and Wernicke area: A PPA perspective. Neurology. 2019;92:e224–e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fridriksson J, Bonilha L, Rorden C. Severe Broca’s aphasia without Broca’s area damage. Behav Neurol. 2007;18:237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hagoort P, Indefrey P. The neurobiology of language beyond single words. Annu Rev Neurosci. 2014;37:347–362. [DOI] [PubMed] [Google Scholar]
- 17. Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (De)constructing the N400. Nat Rev Neurosci. 2008;9:920–933. [DOI] [PubMed] [Google Scholar]
- 18. Jackson RL. The neural correlates of semantic control revisited. NeuroImage. 2021;224:117444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fedorenko E, Blank IA, Siegelman M, Mineroff Z. Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition. 2020;203:104348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matchin W, Hickok G. The cortical organization of syntax. Cereb Cortex. 2020;30:1481–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. [DOI] [PubMed] [Google Scholar]
- 23. Pillay SB, Binder JR, Humphries C, Gross WL, Book DS. Lesion localization of speech comprehension deficits in chronic aphasia. Neurology. 2017;88:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Race DS, Ochfeld E, Leigh R, Hillis AE. Lesion analysis of cortical regions associated with the comprehension of nonreversible and reversible yes/no questions. Neuropsychologia. 2012;50:1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogalsky C, LaCroix AN, Chen K-H, et al. The neurobiology of agrammatic sentence comprehension: A lesion study. J Cogn Neurosci. 2018;30:234–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thothathiri M, Kimberg DY, Schwartz MF. The neural basis of reversible sentence comprehension: Evidence from voxel-based lesion symptom mapping in aphasia. J Cogn Neurosci. 2012;24:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson SM, Saygın AP. Grammaticality judgment in aphasia: Deficits are not specific to syntactic structures, aphasic syndromes, or lesion sites. J Cogn Neurosci. 2004;16:238–252. [DOI] [PubMed] [Google Scholar]
- 28. den Ouden D-B, Saur D, Mader W, et al. Network modulation during complex syntactic processing. NeuroImage. 2012;59:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amici S, Brambati SM, Wilkins DP, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. J Neurosci. 2007;27:6282–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fridriksson J, den Ouden D-B, Hillis AE, et al. Anatomy of aphasia revisited. Brain. 2018;141:848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magnusdottir S, Fillmore P, den Ouden D-B, et al. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study: ATL damage impairs complex syntax processing. Hum Brain Mapp. 2013;34:2715–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson SM, Galantucci S, Tartaglia MC, et al. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baldo JV, Dronkers NF. Neural correlates of arithmetic and language comprehension: A common substrate? Neuropsychologia. 2007;45:229–235. [DOI] [PubMed] [Google Scholar]
- 34. Bonilha L, Hillis AE, Hickok G, den Ouden D-B, Rorden C, Fridriksson J. Temporal lobe networks supporting the comprehension of spoken words. Brain. 2017;140:2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Del Gaizo J, Fridriksson J, Yourganov G, et al. Mapping language networks using the structural and dynamic brain connectomes. eNeuro. 2017;4:ENEURO.0204-17.2017. doi: 10.1523/ENEURO.0204-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate connectome-based symptom mapping in post-stroke patients: Networks supporting language and speech. J Neurosci. 2016;36:6668–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buchsbaum BR, Baldo J, Okada K, et al. Conduction aphasia, sensory-motor integration, and phonological short-term memory – An aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buchsbaum BR, D’Esposito M. A sensorimotor view of verbal working memory. Cortex. 2019;112:134–148. [DOI] [PubMed] [Google Scholar]
- 39. Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory–motor interaction revealed by fMRI: Speech, music, and working memory in area SPT. J Cogn Neurosci. 2003;15:673–682. [DOI] [PubMed] [Google Scholar]
- 40. Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. NeuroImage. 2004;22:562–573. [DOI] [PubMed] [Google Scholar]
- 41. Kertesz A. Western aphasia battery-revised: Grune and Stratton; 2007. [Google Scholar]
- 42. Cho-Reyes S, Thompson CK. Verb and sentence production and comprehension in aphasia: Northwestern assessment of verbs and sentences (NAVS). Aphasiology. 2012;26:1250–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Magnusdottir S. Setningafræðipróf (Test of syntax). Landspítali University Hospital; 2005. [Google Scholar]
- 44. den Ouden D-B, Malyutina S, Basilakos A, et al. Cortical and structural-connectivity damage correlated with impaired syntactic processing in aphasia. Hum Brain Mapp. 2019;40:2153–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matchin W, Basilakos A, Stark BC, den Ouden D-B, Fridriksson J, Hickok G. Agrammatism and paragrammatism: A cortical double dissociation revealed by lesion-symptom mapping. Neurobiol Lang. 2020;14:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia naming test: Scoring and rationale. Clin Aphasiol. 1996;24:121–133. [Google Scholar]
- 47. Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE). 2nd edn. Lippincott Williams & Wilkins; 1983. [Google Scholar]
- 48. MacWhinney B, Fromm D, Forbes M, Holland A. Aphasiabank: Methods for studying discourse. Aphasiology. 2011;25:1286–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 50. Faria AV, Joel SE, Zhang Y, et al. Atlas-based analysis of resting-state functional connectivity: Evaluation for reproducibility and multi-modal anatomy–function correlation studies. NeuroImage. 2012;61:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joliot M, Jobard G, Naveau M, et al. AICHA: An atlas of intrinsic connectivity of homotopic areas. J Neurosci Methods. 2015;254:46–59. [DOI] [PubMed] [Google Scholar]
- 52. JASP Team . JASP.; 2020.
- 53. Basso A, Capitani E, Laiacona M, Luzzatti C. Factors influencing type and severity of aphasia. Cortex. 1980;16:631–636. [DOI] [PubMed] [Google Scholar]
- 54. Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang. 1979;8:34–50. [DOI] [PubMed] [Google Scholar]
- 55. Lahiri D, Dubey S, Ardila A, Ray BK. Factors affecting vascular aphasia severity. Aphasiology. 2021;35:633–641. [Google Scholar]
- 56. DeMarco AT, Turkeltaub PE. A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Hum Brain Mapp. 2018;39:4169–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ivanova MV, Herron TJ, Dronkers NF, Baldo JV. An empirical comparison of univariate versus multivariate methods for the analysis of brain–behavior mapping. Hum Brain Mapp. 2021;42:1070–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baldo JV, Wilson SM, Dronkers NF. Uncovering the neural substrates of language: a voxel-based lesion-symptom mapping approach. In: Faust M, ed. The handbook of the neuropsychology of language. Wiley-Blackwell; 2012:582–594. [Google Scholar]
- 59. Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci U S A. 2012;109:E505–E514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yi HG, Leonard MK, Chang EF. The encoding of speech sounds in the superior temporal gyrus. Neuron. 2019;102:1096–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wernicke C. Lehrbuch Der Gehirnkrankheiten. Theodore Fischer; 1881. [Google Scholar]
- 63. Binder JR. Current controversies on Wernicke’s area and its role in language. Curr Neurol Neurosci Rep. 2017;17:58. [DOI] [PubMed] [Google Scholar]
- 64. Friederici AD. Language in our brain: The origins of a uniquely human capacity. MIT Press; 2017. [Google Scholar]
- 65. Grodzinsky Y, Pieperhoff P, Thompson C. Stable brain loci for the processing of complex syntax: A review of the current neuroimaging evidence. Cortex. 2021;142:252–271. [DOI] [PubMed] [Google Scholar]
- 66. Hagoort P. The neurobiology of language beyond single-word processing. Science. 2019;366:55–58. [DOI] [PubMed] [Google Scholar]
- 67. Tyler LK, Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans R Soc B Biol Sci. 2008;363:1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ullman MT. The declarative/procedural model. In: Neurobiology of language. Elsevier; 2016:953–968. [Google Scholar]
- 69. Fridriksson J, Fillmore P, Guo D, Rorden C. Chronic Broca’s aphasia is caused by damage to Broca’s and Wernicke’s areas. Cereb Cortex. 2015;25:4689–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mesulam MM. Primary progressive aphasia and the language network: The 2013 H. Houston Merritt lecture. Neurology. 2013;81:456–462. [DOI] [PubMed] [Google Scholar]
- 71. Sapolsky D, Bakkour A, Negreira A, et al. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wilson SM, Dronkers NF, Ogar JM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci. 2010;30:16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zaccarella E, Schell M, Friederici AD. Reviewing the functional basis of the syntactic merge mechanism for language: A coordinate-based activation likelihood estimation meta-analysis. Neurosci Biobehav Rev. 2017;80:646–656. [DOI] [PubMed] [Google Scholar]
- 75. Meyer L, Friederici AD. Neural systems underlying the processing of complex sentences. In: Hickok G, Small SL, eds. Neurobiology of language. Elsevier; 2016:597–606. [Google Scholar]
- 76. Wilson SM, Schneck SM. Neuroplasticity in post-stroke aphasia: A systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiol Lang. 2020;2:22–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kristinsson S, Thors H, Yourganov G, et al. Brain damage associated with impaired sentence processing in acute aphasia. J Cogn Neurosci. 2020;32:256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Caplan D, Waters G. Memory mechanisms supporting syntactic comprehension. Psychon Bull Rev. 2013;20:243–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gibson E. Linguistic complexity: Locality of syntactic dependencies. Cognition. 1998;68:1–76. [DOI] [PubMed] [Google Scholar]
- 80. Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychol Rev. 1992;99:122–149. [DOI] [PubMed] [Google Scholar]
- 81. Lewis RL, Vasishth S, Van Dyke JA. Computational principles of working memory in sentence comprehension. Trends Cogn Sci. 2006;10:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miller GA, Chomsky N. Finitary models of language users. In: Handbook of mathematical psychology. Vol. 2. Wiley; 1963:419–491. [Google Scholar]
- 83. Novick JM, Trueswell JC, Thompson-Schill SL. Cognitive control and parsing: Reexamining the role of Broca’s area in sentence comprehension. Cogn Affect Behav Neurosci. 2005;5:263–281. [DOI] [PubMed] [Google Scholar]
- 84. Rogalsky C, Matchin W, Hickok G. Broca’s area, sentence comprehension, and working memory: An fMRI study. Front Hum Neurosci. 2008;2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6:350–356. [DOI] [PubMed] [Google Scholar]
- 86. Matchin W. A neuronal retuning hypothesis of sentence-specificity in Broca’s area. Psychon Bull Rev. 2018;25:1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rogalsky C, Hickok G. The role of Broca's area in sentence comprehension. J Cogn Neurosci. 2011;23:1664–1680. [DOI] [PubMed] [Google Scholar]
- 88. Stowe LA, Haverkort M, Zwarts F. Rethinking the neurological basis of language. Lingua. 2005;115:997–1042. [Google Scholar]
- 89. Pettigrew C, Hillis AE. Role for memory capacity in sentence comprehension: Evidence from acute stroke. Aphasiology. 2014;28:1258–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci. 2001;25:663–678. [Google Scholar]
- 91. Baddeley A, Eldridge M, Lewis V. The role of subvocalisation in reading. Q J Exp Psychol Sect A. 1981;33:439–454. [Google Scholar]
- 92. Caplan D, Waters GS. Verbal working memory and sentence comprehension. Behav Brain Sci. 1999;22:77–126. [DOI] [PubMed] [Google Scholar]
- 93. Caspari I, Parkinson SR, LaPointe LL, Katz RC. Working memory and aphasia. Brain Cogn. 1998;37:205–223. [DOI] [PubMed] [Google Scholar]
- 94. Dunn L, Dunn L. Peabody Picture Vocabulary Test-4. Pearson; 2007. [Google Scholar]
- 95. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mesulam MM, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Battistella G, Henry M, Gesierich B, et al. Differential intrinsic functional connectivity changes in semantic variant primary progressive aphasia. NeuroImage Clin. 2019;22:101797. doi: 10.1016/j.nicl.2019.101797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kielar A, Deschamps T, Jokel R, Meltzer JA. Abnormal language-related oscillatory responses in primary progressive aphasia. NeuroImage Clin. 2018;18:560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mummery CJ, Patterson K, Wise RJS, Vandenbergh R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. [DOI] [PubMed] [Google Scholar]
- 100. Ranasinghe KG, Hinkley LB, Beagle AJ, et al. Distinct spatiotemporal patterns of neuronal functional connectivity in primary progressive aphasia variants. Brain. 2017;140:2737–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sonty SP, Mesulam M-M, Thompson CK, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. [DOI] [PubMed] [Google Scholar]
- 102. Sonty SP, Mesulam M-M, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wilson SM, Brambati SM, Henry RG, et al. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Faria AV, Sebastian R, Newhart M, Mori S, Hillis AE. Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: Potential clinical significance. Aphasiology. 2014;28:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rohrer JD, Caso F, Mahoney C, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang. 2013;127:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bonner MF, Grossman M. Gray matter density of auditory association cortex relates to knowledge of sound concepts in primary progressive aphasia. J Neurosci. 2012;32:7986–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131–138. [DOI] [PubMed] [Google Scholar]
- 110. Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. [DOI] [PubMed] [Google Scholar]
- 111. Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. [DOI] [PubMed] [Google Scholar]
- 112. Rogalsky C, Basilakos A, Rorden C, et al. The neuroanatomy of speech processing: A large-scale lesion study. J Cogn Neurosci. 2022:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Goucha T, Friederici AD. The language skeleton after dissecting meaning: A functional segregation within Broca’s area. NeuroImage. 2015;114:294–302. [DOI] [PubMed] [Google Scholar]
- 114. Matchin W, Hammerly C, Lau E. The role of the IFG and pSTS in syntactic prediction: Evidence from a parametric study of hierarchical structure in fMRI. Cortex. 2017;88:106–123. [DOI] [PubMed] [Google Scholar]
- 115. Matchin W, Brodbeck C, Hammerly C, Lau E. The temporal dynamics of structure and content in sentence comprehension: Evidence from fMRI-constrained MEG. Hum Brain Mapp. 2019;40:663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pallier C, Devauchelle AD, Dehaene S. Cortical representation of the constituent structure of sentences. Proc Natl Acad Sci U S A. 2011;108:2522–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pylkkänen L. Neural basis of basic composition: What we have learned from the red–boat studies and their extensions. Philos Trans R Soc B Biol Sci. 2020;375:20190299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the lesion maps and behavioural data for participants enrolled in this study are available for download at https://www.dropbox.com/sh/3w4aeizgypfs7sd/AAB-W8Yn5qDUFeBj90WKsBqAa?dl=0 (use ‘wernickeConundrumRevisited_CLSM.xlsx’ file).
Spearman correlations found significant correlations among most of our behavioural variables when lesion volume was not included as a covariate (Table 2, top). In fact, the only correlations that were not significant were between Non-canonical Sentence Comprehension and PPT (although this nearly survived the Bonferroni correction for multiple comparisons), and between Expressive Agrammatism and three measures: Non-canonical Sentence Comprehension, Repetition and PPT. When lesion volume was included as a covariate, we found overall weaker correlations among the behavioural measures and Expressive Agrammatism was no longer significantly correlated with any of the other measures (Table 2, bottom).
Table 2.
Behavioural analyses
| PNT | NonCanon | Repetition | PPT | Agramm. | |
|---|---|---|---|---|---|
| Analyses with no covariates | |||||
| Word Comp. | n = 162 | n = 130 | n = 218 | n = 181 | n = 92 |
| ρ = 0.565 | ρ = 0.415 | ρ = 0.738 | ρ = 0.622 | ρ = 0.377 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |
| PNT | n = 102 | n = 162 | n = 155 | n = 73 | |
| ρ = 0.451 | ρ = 0.802 | ρ = 0.408 | ρ = 0.350 | ||
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||
| NonCanon | n = 130 | n = 108 | n = 79 | ||
| ρ = 0.441 | ρ = 0.236 | ρ = 0.101 | |||
| P < 0.001 | P = 0.007 | P = 0.197 | |||
| Repetition | n = 181 | n = 92 | |||
| ρ = 0.502 | ρ = 0.205 | ||||
| P < 0.001 | P = 0.039 | ||||
| PPT | n = 75 | ||||
| ρ = 0.112 | |||||
| P = 0.164 | |||||
| Analyses with lesion volume covariate | |||||
| Word Comp. | n = 162 | n = 130 | n = 218 | n = 181 | n = 92 |
| ρ = 0.433 | ρ = 0.281 | ρ = 0.549 | ρ = 0.523 | ρ = 0.038 | |
| P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.359 | |
| PNT | n = 102 | n = 162 | n = 155 | n = 73 | |
| ρ = 0.364 | ρ = 0.756 | ρ = 0.306 | ρ =−0.051 | ||
| P < 0.001 | P < 0.001 | P < 0.001 | P = 0.683 | ||
| NonCanon | n = 130 | n = 108 | n = 79 | ||
| ρ = 0.317 | ρ = 0.146 | ρ =−0.137 | |||
| P < 0.001 | P = 0.067 | P = 0.875 | |||
| Repetition | n = 181 | n = 92 | |||
| ρ = 0.359 | ρ = 0.121 | ||||
| P < 0.001 | P = 0.152 | ||||
| PPT | n = 75 | ||||
| ρ =−0.076 | |||||
| P = 0.747 | |||||
Correlation analyses that survived the Bonferroni correction for multiple comparisons within each family of tests (with/without lesion volume covariate) (adjusted alpha of P < 0.003) indicated in bold. ρ = Spearman’s rho. Agramm. = Expressive Agrammatism; Comp. = Comprehension; NonCanon = Non-canonical Sentence Comprehension; PNT = Philadelphia Naming Task.
Similar to Mesulam et al.,1 we found that WAB-R Auditory Word Recognition and PPT scores were strongly correlated, justifying our use of PPT scores as a covariate with WAB-R Auditory Word Recognition to create the Word Comprehension measure, removing variance due to object recognition and non-verbal semantic processing in our LSM analyses. We also found that Non-canonical Sentence Comprehension and Repetition scores were correlated, suggesting that at least some of the variance in sentence comprehension scores could be due to phonological working memory abilities.
There were notable differences in our analyses from the behavioural correlations reported by Mesulam et al.1 Importantly, we found robust correlations between Non-canonical Sentence Comprehension and WAB-R Auditory Word Recognition, whether or not lesion volume was included as a covariate, unlike Mesulam et al., who found that word and sentence comprehension were not correlated. This is consistent with the classical findings in stroke-based aphasia that word and sentence comprehension deficits coincide. These results underscore the ‘Wernicke conundrum’: apparent differences in the patterns of word and sentence comprehension deficits between PPA and stroke-based aphasia. We also found that WAB-R Repetition was very robustly correlated with WAB-R Auditory Word Recognition, whereas Mesulam et al. found that Word Comprehension and Repetition were not correlated.
Finally, we also found that Non-canonical Sentence Comprehension was not correlated with Expressive Agrammatism, whether or not lesion volume was included as a covariate. Although correlations between Expressive Agrammatism and other behavioural variables were not assessed in Mesulam et al.,1,12 this lack of a relationship in our data speaks against one of the major ideas promoted in this work. Namely, they suggested that sentence comprehension deficits in both PPA and stroke-based aphasia are due to degeneration and/or disconnection of a frontal-based grammatical processing centre that affects both production and comprehension. Under this perspective, one would expect that Expressive Agrammatism and Non-canonical Sentence Comprehension would be correlated, but they were not. Note that we did find that Expressive Agrammatism correlated with WAB-R Auditory Word Recognition and picture naming when lesion volume was not included as a covariate; thus, the failure to identify a correlation between Expressive Agrammatism and Non-canonical Sentence Comprehension cannot be merely due to a lack of statistical power.