Abstract
Focal anterior temporal lobe degeneration often preferentially affects the left or right hemisphere. While patients with left-predominant anterior temporal lobe atrophy show severe anomia and verbal semantic deficits and meet criteria for semantic variant primary progressive aphasia and semantic dementia, patients with early right anterior temporal lobe atrophy are more difficult to diagnose as their symptoms are less well understood. Focal right anterior temporal lobe atrophy is associated with prominent emotional and behavioural changes, and patients often meet, or go on to meet, criteria for behavioural variant frontotemporal dementia. Uncertainty around early symptoms and absence of an overarching clinico-anatomical framework continue to hinder proper diagnosis and care of patients with right anterior temporal lobe disease. Here, we examine a large, well-characterized, longitudinal cohort of patients with right anterior temporal lobe-predominant degeneration and propose new criteria and nosology.
We identified individuals from our database with a clinical diagnosis of behavioural variant frontotemporal dementia or semantic variant primary progressive aphasia and a structural MRI (n = 478). On the basis of neuroimaging criteria, we defined three patient groups: right anterior temporal lobe-predominant atrophy with relative sparing of the frontal lobes (n = 46), frontal-predominant atrophy with relative sparing of the right anterior temporal lobe (n = 79) and left-predominant anterior temporal lobe-predominant atrophy with relative sparing of the frontal lobes (n = 75). We compared the clinical, neuropsychological, genetic and pathological profiles of these groups.
In the right anterior temporal lobe-predominant group, the earliest symptoms were loss of empathy (27%), person-specific semantic impairment (23%) and complex compulsions and rigid thought process (18%). On testing, this group exhibited greater impairments in Emotional Theory of Mind, recognition of famous people (from names and faces) and facial affect naming (despite preserved face perception) than the frontal- and left-predominant anterior temporal lobe-predominant groups. The clinical symptoms in the first 3 years of the disease alone were highly sensitive (81%) and specific (84%) differentiating right anterior temporal lobe-predominant from frontal-predominant groups. Frontotemporal lobar degeneration-transactive response DNA binding protein (84%) was the most common pathology of the right anterior temporal lobe-predominant group.
Right anterior temporal lobe-predominant degeneration is characterized by early loss of empathy and person-specific knowledge, deficits that are caused by progressive decline in semantic memory for concepts of socioemotional relevance. Guided by our results, we outline new diagnostic criteria and propose the name, ‘semantic behavioural variant frontotemporal dementia’, which highlights the underlying cognitive mechanism and the predominant symptomatology. These diagnostic criteria will facilitate early identification and care of patients with early, focal right anterior temporal lobe degeneration as well as in vivo prediction of frontotemporal lobar degeneration-transactive response DNA binding protein pathology.
Keywords: right temporal lobe-predominant neurodegeneration, loss of empathy and non-verbal semantics, person-specific knowledge, frontotemporal dementia, FTLD-TDP type C
Younes et al. present diagnostic guidelines for right-predominant anterior temporal lobe degeneration. They show that the condition is characterized by a progressive decline in semantic knowledge for socioemotionally relevant concepts and propose that it should be named ‘semantic behavioural variant FTD’.
Introduction
The term frontotemporal dementia (FTD) was introduced to encapsulate the progressive personality changes, social conduct impairment and language deficits associated with atrophy of the frontal and temporal lobes.1 Within FTD, behavioural symptoms often localize to frontal, temporal, insular and striatopallidal regions in the right hemisphere, whereas language deficits typically localize to structures in the left.2 Currently, the behavioural syndrome associated with FTD is referred to as ‘behavioural variant frontotemporal dementia’ (bvFTD), and the language syndromes are brought together under the term ‘primary progressive aphasia’ (PPA).3,4
Neurodegeneration that targets the anterior temporal lobe (ATL) is often asymmetric in the initial stages of disease—with focal atrophy targeting either the left ATL (lATL) or right ATL (rATL)5,6—and is associated with distinct early linguistic or behavioural clinical presentations. Overtime the disease spreads to the contralateral hemisphere, and language and behavioural symptoms converge.5,7,8 As both lATL- and rATL-predominant degeneration are typically associated with frontotemporal lobar degeneration-transactive response DNA binding protein 43 type C (FTLD-TDP type C) pathology,6 lateralized ATL presentations are thought to reflect different manifestations of a single pathological continuum.5,9–13 Patients with lATL-predominant atrophy typically have notable language deficits with prominent decline in semantic knowledge for objects. The consensus clinical criteria for semantic dementia, and more recently for semantic variant PPA (svPPA),3,14 emphasize verbal semantic deficits that result in anomia, single-word-comprehension deficits and object-identification impairments. Although these criteria do include broader manifestation of semantic processing deficits, such as impairment in identification of visually presented objects and faces, they do not highlight the socioemotional and behavioural deficits that can also arise in the context of ATL degeneration. Thus, the existing diagnostic criteria overlook the main symptoms that result from rATL degeneration.
Patients with focal rATL atrophy exhibit profound changes in emotion and behaviour, symptoms that can be hard to distinguish from those of bvFTD, but may also present with features of svPPA despite lacking early aphasia symptoms.5,7,15–19 Previous studies have shown that patients with rATL-predominant degeneration have difficulties recognizing familiar people and empathizing with others.5,17,18,20,21 Diminished empathy—which can include lack of emotional responsiveness as well as decreased social connection and compassion—is often remarkable when the disease targets the rATL.18,22–25 Neuroimaging studies have associated rATL atrophy with deficits in a wide range of socioemotional functions including empathy,22 non-verbal social cue (e.g. sarcasm) detection26 and facial emotion recognition.27,28 By integrating information from primary and association sensory and motor cortices, the ATLs are considered amodal hubs that represent all categories of semantic knowledge.9,12,13,29–32 Although there is strong evidence for bilateral ATL contributions to semantic knowledge, lateralized specializations of the left and right ATL may reflect divergent inputs from the left and right hemispheres.13,33–36 Whereas the lATL binds verbal features into semantic knowledge through strong connections with linguistic networks, the rATL may be more centrally involved in representing non-verbal semantic knowledge through its prominent connections with right-sided visual and socioemotional networks.10,30,37,38 Thus, by integrating non-verbal (e.g. visual, sensory and visceral) information, the rATL serves as the core hub for socioemotional semantic knowledge.13,39,40 In individuals with typical hemispheric functional lateralization, the model would predict that focal lATL degeneration would disproportionally disrupt verbal semantic knowledge and that rATL degeneration would degrade non-verbal, socioemotional semantic knowledge. Consistent with this hypothesis, non-verbal semantic knowledge tasks, including visual semantic associations41; identification of living beings (animals are recognized mainly by their visual features)37; sound recognition42 and tactile,11 olfactory43 and gustatory stimulus recognition have all been linked to rATL.44 This lateralized specialization is observed in individuals known to have left-hemisphere language dominance, but non-right-handed patients can present with the opposite symptoms in relation to reversed hemispheric dominance.24,45
Despite these advances in our theoretical understanding of right and left ATL functions, patients with rATL-predominant atrophy continue to pose a nosological challenge. The symptoms of the rATL-predominant syndrome have not been clearly linked to theoretical or clinico-anatomical models, and consensus diagnostic criteria are lacking. Patients with rATL-predominant degeneration are often described with terms such as ‘right temporal svPPA’, ‘right temporal semantic dementia’, ‘right temporal bvFTD’ and ‘right temporal variant FTD’5,15–17,46–49 and can have symptoms that overlap with the diagnostic criteria for svPPA and semantic dementia3,14 (which emphasize verbal semantic deficits) and bvFTD (which focus on behavioural and emotional features).4 Furthermore, because loss of empathy may be misinterpreted by families and clinicians as a psychiatric symptom, patients with predominant rATL degeneration may be identified later in the disease course when severe behavioural impairment justifies a diagnosis of bvFTD. These diagnostic challenges may increase uncertainty about the underlying neuropathological changes and, a clinical dilemma that will be increasingly important to resolve as disease-modifying treatment become available. Diagnostic criteria for the rATL-predominant syndrome, therefore, would facilitate early identification, accelerate studies of non-verbal semantics and promote the development of reliable measures that track socioemotional decline in neurodegenerative illnesses.
The goal of the present study was to examine the clinical, neuropsychological, genetic, anatomical and pathological characteristics of a large cohort of patients with rATL-predominant atrophy. Patients were studied within a multidisciplinary project on FTD-spectrum disorders that included comprehensive assessments of both language and socioemotional functioning. We compared rATL-predominant patients to those with frontal-predominant bvFTD and lATL-predominant svPPA, as determined by clinical and neuroimaging criteria. We proposed that patients with rATL-predominant damage would have a clinical profile characterized by early semantic memory loss for socioemotionally relevant, non-verbal concepts (e.g. famous people and emotions) and exhibit behavioural symptoms (e.g. loss of empathy). We expected that loss of empathy would be a prominent feature of patients with rATL-predominant atrophy and that other behavioural symptoms of bvFTD (e.g. disinhibition, apathy/inertia and lack of judgement and dysexecutive symptoms) would be less common. Although we anticipated that patients with rATL-predominant degeneration would have some symptoms of svPPA (e.g. word comprehension and confrontational naming difficulties), we hypothesized these would be comparatively mild and that patients with rATL-predominant atrophy would often not meet diagnostic criteria for PPA (i.e. where aphasia is the most prominent early clinical feature and the principal cause of functional impairment). On the basis of our results, we propose new diagnostic criteria for the rATL-predominant syndrome that is on a continuum with, but qualitatively and quantitatively distinct from, both bvFTD and lATL-dominant svPPA syndromes, as currently defined.
Materials and methods
Participants
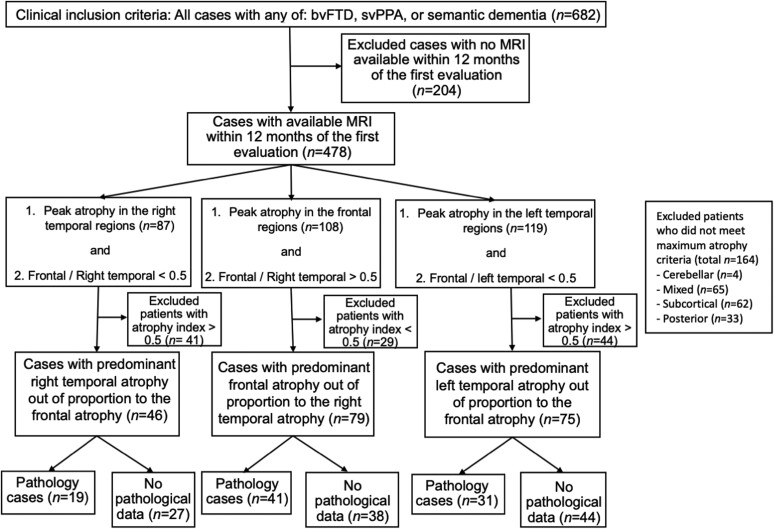
We identified patients who met bvFTD and/or svPPA criteria (see next) and had research visits between 1998 and 2019 (n = 682) at the University of California, San Francisco (UCSF) Memory and Aging Center (MAC) (Fig. 1). As symptoms were often mild at early research visits, scores on the Clinical Dementia Rating scale (CDR) were not used when determining study inclusion.50 Patients who did not have a brain MRI within 1 year of the first research evaluation were excluded (n = 204). From the remaining 478 cases, we used structural neuroimaging measures to identify individuals with predominant rATL atrophy and relative preservation of the frontal lobes (see details next) (n = 46). We also included three other groups for comparison: a group of individuals with lATL-predominant atrophy and relative preservation of the frontal lobes (n = 75), a group of individuals with frontal-predominant atrophy and relative preservation of the rATL (n = 79) and a group of healthy older controls from the MAC Hillblom Healthy Aging Network (n = 59). We used strict clinical and anatomical inclusion criteria to contrast the rATL patients with these three groups and to clarify the distinct cognitive–behavioural phenotype of the rATL-predominant syndrome. Patients or caregivers provided informed consent following procedures aligned with the Declaration of Helsinki, and the study was approved by the UCSF Committee for Human Research.
Figure 1.
Patient selection. We searched the UCSF MAC database. The first inclusion criterion was the clinical diagnosis; we included all participants who received a clinical diagnosis of bvFTD or svPPA. We then excluded all patients who did not have a brain MRI within 1 year of the first research evaluation. Next, we included participants who had peak atrophy in either the right temporal lobe, frontal lobe or left temporal lobe on a brain MRI W-score map and showed predominant atrophy in their respective lobe based on an atrophy index. Posterior = parietal or occipital lobes.
Diagnostic criteria
Two raters, a behavioural neurologist (K.Y.) and a neuropsychologist (M.M.), reviewed all available medical data for the rATL-predominant patients to determine whether they met the following diagnostic criteria: (i) Neary-FTD14; (ii) Neary-Semantic14; (iii) bvFTD4 and (iv) svPPA.3 We also noted whether patients had semantic variant PPA features (i.e. impaired confrontation naming and single-word comprehension) regardless of meeting PPA general criteria (i.e. aphasia is the most prominent deficit in early disease).3 This allowed us to describe verbal semantic deficits in patients who had predominantly behavioural presentations. The two raters determined whether each of these criteria was met at three different time points: (i) within the first 3 years of disease onset; (ii) at the first MAC research evaluation and (iii) in the years subsequent to the first MAC evaluation.
Detailed symptom taxonomy and chronology
All research participants were evaluated by a behavioural neurologist, a neuropsychologist, a speech and language pathologist and a nurse. A clinical history was obtained from each patient, with corroboration from the caregiver/informant, and began by identifying the nature and onset of the first symptoms. This was followed by a chronological history of how symptoms evolved, and then a detailed inventoried review of the domains of memory, language, executive function, visuospatial abilities, behaviour, sleep, sensory processing and motor function. Patients did not need to present for evaluation at the same stage of the disease in order for the retrospective interview to fully capture the chronology of symptoms.
We documented each patient’s first five symptoms, rather than all symptoms ever noted, because we expected many of the canonical bvFTD symptoms (disinhibition, apathy, loss of empathy, compulsions, hyperorality, and executive deficits) and PPA symptoms (language and semantic impairment) would emerge for most people in the disease’s later stages. In an effort to refine our categorization of the behavioural and emotional symptoms, we catalogued symptoms according to the following taxonomy:
Loss of empathy: difficulty recognizing, understanding or responding to others’ emotions and needs; selfishness; emotional distance from others; reduced or inappropriate emotional expressivity, diminished social interest, interrelatedness or personal warmth.
Words and object semantic loss: loss of knowledge about words, facts, concepts, animate or inanimate objects, places or landmarks. Patients may demonstrate impaired naming, diminished recall, poor identification or reduced feelings of familiarity for these domains.
Person-specific semantic knowledge loss: loss of knowledge about known faces, proper names and people (including biographical information about famous people, close friends and/or family members). Patients may demonstrate impaired naming, diminished recall, poor recognition or reduced feelings of familiarity for previously known people.
Complex compulsions and rigid thought process: adhering to fixed schedules or roles, preoccupation with dogmas (e.g. hyper-religiosity) or health (hypochondriasis), restricted preference for certain colours, clothing or diet, spending hours playing word games and puzzles.
Simple repetitive behaviours, hoarding, or obsessions: repetitive motor (e.g. clicking, tapping, pacing) or verbal stereotypies, hoarding or preoccupation with objects or people.
Apathy/inertia: cognitive (reduced planning and voluntary action), behavioural (reduced self-initiated thoughts and behaviours) and affective (reduced social, emotional, behavioural interest) forms of apathy.51
Disinhibition: impulsivity or socially inappropriate behaviour, loss of manners or decorum.
Lack of judgement and dysexecutive: rash or careless actions, judgement mistakes that are out of character. Of note, in the current bvFTD criteria,3 lack of judgement is considered as a part of disinhibition, but for this study we separated these two symptoms as they may be subserved by different neuroanatomical systems.52
Episodic memory loss: difficulty remembering recent events and autobiographical information.
Hyperorality or dietary changes: altered food preferences, binge eating, increased consumption of alcohol or cigarettes and oral exploration or consumption of inedible objects.
Motor neuron disease signs: bulbar and limb signs of motor neuron disease.
Other symptoms: visuospatial difficulties, declined hygiene, loss of sexual desire, dietary changes (increased or decreased eating), weight gain, weight loss, hypersomnia and insomnia. These symptoms are either common in other neurodegenerative illnesses or not specific for a single neurodegenerative disease.
Functional, cognitive and behavioural assessments
Patients underwent a comprehensive multidisciplinary assessment that included functional, neuropsychological, and socioemotional measures (Tables 1 and 2), as previously described.18,53 A description of the cognitive battery and further details about patients’ performance are presented in the Supplementary material. Verbal semantic knowledge was evaluated with the Peabody Picture Vocabulary Test (patients were asked to choose the picture that best describes a word),54 the abbreviated 15-item Boston Naming Test (patients were asked to name different drawings)55 and semantic verbal fluency (patients generated as many animals as possible in 60 s). Non-verbal semantic knowledge was tested with the picture version of the Pyramids and Palm Trees (PPT-P; patients matched semantically associated pictures).56
Table 1.
Demographics, functional and cognitive scores
| Right temporal | Frontal | Left temporal | Healthy control | |
|---|---|---|---|---|
| Epidemiology and functional scales | ||||
| Handedness (right, left, ambidextrous) | 39, 6, 1 | 72, 3, 3 | 68, 6, 1 | 54, 5, 0 |
| Sex (female, male) | 23, 23 | 39, 40 | 35, 40 | 35, 24 |
| Ethnicity (n) | 42W, 4A | 70W, 8A, 1N | 73W, 3A | 58W, 1A |
| Age of onset, mean (SD, n) | 60.2 (8.2, 46) | 56.7 (10.0, 79) | 58.7 (7.2, 75) | NA |
| Age at evaluation, mean (SD, n) | 65.11 (7.6, 46)a | 60.75 (8.8, 79)b | 63.12 (7.0, 75)c | 74.05 (9.7, 59)a,b,c |
| Years of education, mean (SD, n) | 15.78 (3.0, 46) | 15.59 (2.9, 79)b | 16.43 (2.8, 74) | 17.07 (2.3, 58)b |
| CDR score, mean (SD, n), max = 3 | 0.97 (0.5, 45)a,d | 0.98 (0.7, 79)b,e | 0.67 (0.3, 69)d,e,c | 0.0 (0.0, 59)a,b,c |
| CDR Box Score, mean (SD, n), max = 18 | 5.5 (3.5, 45)a,d | 5.73 (3.9, 79)b,e | 3.551 (2.0, 69)d,e,c | 0.01 (0.1, 59)a,b,c |
| NPI total (severity×frequency), EMM (SEM, n), max = 144 | 36.0 (20.4, 45)a,f,d | 44.7 (21.0, 46)f,b,e | 21.5 (16.2, 24)d,e,c | 8.1 (8.5, 6)a,b,c |
| NPI Caregiver Distress Total, EMM (SEM, n), max = 60 | 14.9 (9.3, 45)a,f,d | 19.5 (8.3, 47)a,f,d | 12.5 (7.3, 22)a,f,d | 2.5 (1.7, 9)a,e,d |
| Global cognition | ||||
| MMSE, EMM (SEM, n), max = 30 | 26.46 (0.87, 46)e,d | 23.39 (0.70, 77)f,b | 21.81 (0.69, 71)d,c | 27.02 (0.95, 58)b,c |
| Visuospatial processing | ||||
| Benson Complex Figure – copy, EMM (SEM, n), max = 17 | 14.72 (0.33, 46) | 13.85 (0.27, 71)e | 15.60 (0.28, 70)e | 14.61 (0.27, 36) |
| VOSP Number Location, EMM (SEM, n), max = 10 | 8.84 (0.26, 42)f | 7.79 (0.22, 62)f,b,e | 9.36 (0.24, 56)e | 9.01 (.27, 59)b |
| Episodic memory | ||||
| CVLT 30’ short delay free recall, EMM (SEM, n), max = 9 | 3.93 (0.29, 43)a,c | 4.70 (0.23, 65)b,e | 2.83 (0.30, 64)d,e,c | 7.30 (0.53, 13)a,b,c |
| CVLT 10’ long delay free recall, EMM (SEM, n), max = 9 | 2.48 (0.32, 43)a | 3.55 (0.26, 65)b,e | 1.84 (0.26, 64)e,c | 7.45 (0.59, 13)a,b,c |
| CVLT Recognition, EMM (SEM, n), max = 9 | 6.44 (0.26, 43)a,f | 7.86 (0.21, 64)f,e | 6.31 (0.22, 62)e,c | 8.28 (0.49, 13)a,c |
| Benson Complex Figure—delay, EMM (SEM, n), max = 17 | 5.62 (0.57, 44)a,f | 8.82 (0.51, 69)f,b | 7.36 (0.48, 75)c | 11.57 (0.75, 36)a,b,c |
| Executive functioning | ||||
| Digit Span—backward, EMM (SEM, n) | 4.97 (0.17, 46)f,d | 3.32 (0.14, 69)f,b,e | 4.73 (1.51, 66)d,e,c | 5.36 (1.90, 55)b,c |
| Stroop (correct in 60 s), EMM (SEM, n) | 42.80 (2.95, 26)f | 29.45 (2.21, 46)f,e | 37.98 (2.12, 49)e | NA |
| Trails (Time), EMM (SEM, n), max = 120” | 61.40 (4.53, 42)a,d | 73.10 (3.95, 59)b,e | 45.23 (3.98, 61)d,e | 32.87 (4.60, 59)a,b |
| Design fluency, EMM (SE, n) | 6.50 (0.48, 42)a | 5.02 (0.40, 65)b,e | 7.89 (0.41, 59)e,c | 11.08 (0.49, 59)a,b,c |
A = Asian; CDR = Clinical Dementia Rating; CVLT = California Verbal Learning Test; EMM = estimated marginal means; FAQ = Functional Activities Questionnaire; N = Native American; NA = Not Applicable; NPI = Neuropsychiatric Inventory; se = standard error; VOSP = Visual Object and Space Perception Battery; W = White.
Right temporal different from health control at <0.05.
Frontal different from health control at <0.05.
Left temporal different from health control at <0.05.
Right temporal different from left temporal at <0.05.
Frontal different from left temporal at <0.05.
Right temporal different from frontal at <0.05.
Table 2.
Language and socioemotional profile
| Right temporal | Frontal | Left temporal | Healthy control | |
|---|---|---|---|---|
| Language | ||||
| Verbal Agility, EMM (SE, n), max = 5 | 4.94 (0.19, 36) | 4.73 (0.16, 54)a | 5.00 (0.16, 57)a | 5.00 (0.19, 52) |
| Repetition, EMM (SE, n), max = 5 | 4.16 (0.19, 36)b | 3.84 (0.16, 57)c | 3.46 (1.52, 57)d | 4.43 (0.19, 59)b,c,d |
| WRAT Reading, EMM (SE, n), max = 70 | 56.64 (3.02, 21)e | 54.71 (2.18, 41)c | 50.00 (2.26, 38)d | 65.22 (2.11, 58)e,c,d |
| Apraxia of speech rating, EMM (SE, n), max = 7 | 0 (0.00, 27) | 0.14 (0.08, 14) | 0.06 (0.04, 49) | 0.0 (0.00, 9) |
| Dysarthria rating, EMM (SE, n), max = 7 | 0.09 (0.04, 43)f | 1.3 (0.09, 40)a,b,c | 0.06 (0.04, 50)a | 0.14 (0.07, 59)c |
| Syntax comprehension, EMM (SE, n), max = 5 | 4.43 (0.14, 36) | 3.97 (0.12, 56)a | 4.48 (0.12, 56)a | 4.38 (0.14, 52) |
| Lexical fluency—no. in 60 s, EMM (SE, n) | 8.53 (0.64, 43)e,f | 6.16 (0.61, 69)a,b,c | 8.51 (0.53, 66)a,d | 14.09 (0.81, 35)c,d,e |
| Verbal semantics | ||||
| BNT, EMM (SE, n), max = 15 | 6.89 (0.43, 33)e,f,b | 12.02 (0.35, 70)a,b | 5.18 (0.36, 66)a,b,d | 12.99 (0.46, 57)e,d |
| Verbal fluency—semantic—no. in 60 s, EMM (SE, n) | 9.21 (0.76, 43)e | 10.78 (0.74, 69)c | 8.99 (0.64, 66)d | 21.62 (0.78, 35)e,f,d |
| PPVT, EMM (SE, n), max = 16 | 9.56 (0.43, 40)e,f | 13.62 (0.45, 65)a,f | 9.53 (0.39, 53)d,f | 13.97 (0.46, 53)e,d |
| Pyramids and Palm Trees Percent, Pictures, EMM (SE, n), max = 1 | 0.81 (0.02, 27)e,f | 0.90 (0.01, 49)f | 0.86 (0.01, 53) | 0.90 (0.01, 30)e |
| Face perception | ||||
| CATS Face Matching, EMM (SE, n), max = 12 | 11.24 (0.20, 31) | 10.66 (0.18, 42)a,f | 11.79 (0.17, 41)a | 11.55 (0.18, 52)c |
| Person-specific knowledge | ||||
| Famous Faces Naming, EMM (SE, n), max = 20 | 1.26 (0.86, 15)e,f | 6.88 (1.19, 9)a,c,f | 1.75 (1.00, 17)a,d | 12.82 (0.87, 26)c,d,e |
| Famous Faces Familiarity, EMM (SEM, n), max = 20 | 6.85 (0.88, 24)b,e,f | 17.48 (1.70, 7)f | 14.80 (0.92, 32)b | 14.19 (1.10, 26)e |
| Famous Faces Semantic Association, EMM (SE, n), max = 20 | 5.37 (1.13, 12)b,e,f | 16.19 (1.2, 8)a,f | 11.75 (1.37, 10)a,b | 14.71 (0.96, 26)e |
| Famous Faces Name Familiarity, EMM (SE, n), max = 16 | 2.80 (0.78, 14) | 9.80 (1.06, 10) | 2.92 (0.78, 10) | 11.91 (0.94, 29) |
| Social function and emotion | ||||
| CATS Affect Matching, EMM (SE, n), max = 16 | 9.11 (0.42, 35)b,e | 9.67 (0.39, 43)a,c | 12.82 (0.41, 40)a,b | 12.82 (0.46, 52)c,e |
| TASIT–EET, EMM (SE, n), max = 14 | 6.46 (0.48, 27)e | 7.77 (0.40, 45)c | 8.19 (0.42, 40)d | 10.89 (0.40, 57)c,e,f |
| TASIT SI-M Sincere, EMM (SE, n), max = 20 | 15.99 (0.69, 24)f | 13.42 (0.52, 51)a,c,f | 17.16 (0.55, 42)a | 16.64 (0.55, 58)c |
| TASIT SI-M Sarcastic, EMM (SE, n), max = 20 | 4.74 (0.85, 24)b,e,f | 13.49 (0.65, 51)a,c,f | 9.80 (0.67, 42)a,b,d | 17.60 (0.68, 58)e, |
| IRI-Empathetic Concern, EMM (SE, n), max = 24 | 16.09 (1.41, 44)b,e | 14.94 (1.12, 79)a,c | 21.87 (1.14, 75)a,b,d | 27.41 (1.56, 54)c,d,e |
| IRI Perspective Taking, EMM (SE, n), max = 24 | 10.77 (1.10, 44)b,e | 10.22 (0.87, 79)a,c | 14.63 (0.89, 75)a,b,d | 22.86 (1.22, 54)c,d,e |
| Emotional Theory of Mind, EMM (SE, n), max = 16 | 12.25 (0.46, 9)e | 12.44 (0.29, 23)a,c | 13.86 (0.36, 17)a | 14.62 (0.35, 20)c,e |
| Cognitive Theory of Mind, EMM (SE, n), max =16 | 14.79 (0.58, 15)f | 12.04 (0.44, 30)a,c,f | 15.07 (0.44, 33)c | 15.07 (0.35, 59)a |
| Behavioural Inhibition BIS-Total, EMM (SE, n), max = 24 | 17.39 (0.73, 24) | 17.49 (0.55, 54)a | 19.85 (0.59, 41)a,d | 15.95 (0.61, 54)d |
| Behavioural Activation BAS-Drive, EMM (SE, n), max = 24 | 8.56 (0.56, 25) | 10.21 (0.42, 50) | 9.99 (0.44, 38) | 10.40 (0.47, 54) |
| Behavioural Activation BAS-Fun, EMM (SE, n), max = 24 | 7.54 (0.49, 25)e,f | 9.47 (0.39, 50)f | 8.69 (0.42, 36) | 9.50 (0.42, 53)e |
| Behavioural Activation BAS-Reward, EMM (SE, n), max = 24 | 13.13 (0.52, 25)b,e | 13.59 (0.42, 51)a | 15.69 (0.44, 38)a,b | 15.08 (0.45, 54)e |
| IAS—Current Warmth, EMM (SE, n) | 37.59 (3.02, 13)b,c | 38.86 (1.95, 36)a,c | 46.56 (1.84, 44)a,b | 47.89 (2.12, 44)e,f |
| IAS—Current Dominance, EMM (SE, n) | 37.24 (2.59, 13) | 30.86 (1.68, 36)a,c | 37.76 (1.58, 44)a | 42.21 (1.82, 44)c |
| IAS—Current Coldness, EMM (SE, n) | 29.53 (2.27, 13)b,c | 24.77 (1.71, 36)c | 18.88 (1.71, 44)b | 13.72 (1.83, 44)c,e |
| Self-Monitoring RSMS, EMM (SE, n), max = 65 | 36.57 (2.32, 28)b,c | 38.68 (1.78, 55)a,c | 45.14 (1.86, 49)a,b,d | 59.05 (2.24, 47)c,e,f |
| Depression GDS, EMM (SE, n), max = 30 | 7.56 (0.94, 37)e | 8.88 (0.87, 54)c | 8.54 (0.83, 50)d | 3.38 (0.92, 58)c,e,f |
A = Asian; BNT = Boston Naming Test; EC = Empathic Concern; EMM = estimated marginal means; GDS = Geriatric Depression Scale; IAS = Interpersonal Adjective Scales; IRI = Interpersonal Reactivity Index; N = Native American; PPVT = Peabody Picture Vocabulary Test; PT = Perspective Taking; SE = standard error; W = White.
Frontal different from left temporal at <0.05.
Right temporal different from left temporal at <0.05.
Frontal different from health control at <0.05.
Left temporal different from health control at <0.05.
Right temporal different from health control at <0.05.
Right temporal different from frontal at <0.05.
We assessed multiple domains of socioemotional functioning with a battery of task-based measures. Visual face perception was evaluated with the identity-matching subtest of the Comprehensive Affect Testing System (CATS), in which patients determined whether pairs of neutral faces were from the same person or different people.57 The ability to label emotional facial expressions with words was tested with the CATS emotion identification task, in which patients chose the emotion term that matched the facial expression depicted in a photograph from a list of multiple choice options.58 On the abbreviated version of the Emotion Evaluation Test (EET) from The Awareness of Social Inference Test (TASIT), patients identified the target emotion from a list of multiple choice options that were displayed by actors in short video clips. On the TASIT-Social Inference–Minimal Test, patients were asked to detect sarcasm of actors in videos through interpretation of social cues including prosody, facial expression and gesture. Theory of mind—the ability to infer the thoughts, emotions and intentions of others—was tested in cognitive (i.e. the ability to identify first and second order object knowledge of actors in videos) and Emotional modalities (i.e. the ability to identify first and second order emotion knowledge of actors in videos) using the UCSF Theory of Mind Test.59 Person-specific semantic knowledge was evaluated using the UCSF Famous Faces Naming Test (a free response task in which patients named photographs of famous people’s faces), Semantic Famous Face Association Test (patients matched famous faces based on their professions), Semantic Famous Name Association Test (patients matched written names of famous people based on their professions) and Semantic Famous Face Recognition Test (patients chose the famous face among four faces)18,60 Further socioemotional testing details are found in the Supplementary material.
Informant-based measures were also obtained to assess patients’ socioemotional behaviour in everyday life. Informants rated patients’ current cognitive empathy (i.e. perspective taking) and emotional empathy (i.e. empathic concern) using the Interpersonal Reactivity Index (IRI).61 Sensitivity and responsiveness to others’ subtle emotional expressions were rated by informants using the Revised Self-Monitoring Scale (RSMS).62 Interpersonal coldness, warmth and dominance, areas of personality known to be affected in FTD, were evaluated with informant ratings on the Interpersonal Adjective Scales (IAS).63 Behavioural inhibition (i.e. behaviours associated with response avoidance and sensitivity to threat) and behavioural activation (i.e. behaviours associated with approach motivation including reward responsiveness, drive, and fun-seeking) systems were evaluated with informant ratings on the Behavioural Inhibition System/Behavioural Activation System (BIS/BAS) questionnaire.64
Structural neuroimaging analyses
We processed structural T1-weighted images, as previously described.65,66 W-score maps (W-maps) were generated by comparing each patient’s grey matter maps to 534 neurologically healthy older controls from the MAC Hillblom Healthy Aging Network [age range 44–99 years, mean ± standard deviation (SD): 68.7 ± 9.1; 220 male/302 female], adjusted for age, sex, total intracranial volume and magnet field strength. Mean W-score values were extracted for each region of interest in the probabilistic Desikan atlas. W-scores have a mean value of 0 and a SD of 1; values of +1.65 and −1.65 correspond to the 95th and 5th percentiles, and indicate regions with larger and smaller grey matter volume compared to the normative sample, respectively.
Patients were included in the rATL-predominant degeneration group if their lowest three W-scores were in right temporal regions, and they had relative preservations of the frontal lobes based on an atrophy index described as follows. For each patient with rATL maximum atrophy, we calculated the mean W-score of all frontal lobe regions of interest and the mean W-score of all right temporal regions of interest and computed a proportion with the following index: right temporal index = mean whole frontal W-score/mean right temporal W-score. The rATL-predominant degeneration patients who had an index <0.50 were included in this study (n = 46) (Fig. 2A and Supplementary Table 1). A similar approach was used to select the comparison groups. Patients were included in the frontal-predominant group if their lowest three W-scores were in the frontal regions, and they had relative preservation of the right temporal regions based on an atrophy index (mean frontal W-map score/mean right temporal W-map score >0.50). Patients were included in the lATL-predominant group if their lowest three W-map scores were in the left temporal regions, and they had relative preservation of the frontal lobes based on atrophy index (mean frontal W-map score/mean left temporal W-map score <0.50). We implemented this index for the lATL instead of a right/left temporal laterality index to match the rATL and lATL patients based on their degree of accompanying frontal involvement.
Figure 2.
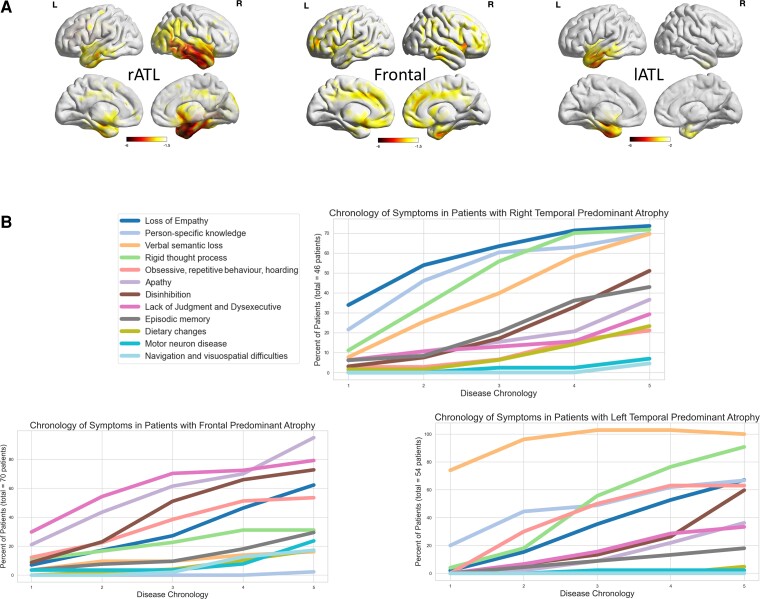
Neuroimaging in right temporal-, left temporal- and frontal-predominant neurodegeneration and chronology of symptoms. (A) Lateral and mesial views. Predominant right temporal, left temporal or frontal atrophy was used as part of the inclusion criteria based on a data-driven neuroimaging approach. The right temporal-predominant group exhibited maximum atrophy in the right temporal lobe more than the left ATL with involvement of the right more than left insula, right caudate and right more than left subgenual anterior cingulate cortex. Notably, there is sparing of the frontal, parietal and occipital lobes. The left temporal-predominant group shows maximum atrophy in the left temporal lobe more than the right ATL with involvement of the left more than right insula, left caudate and left subgenual anterior cingulate cortex. Further, there is sparing of the frontal, parietal and occipital lobes. The frontal group shows bilateral lateral and mesial frontal and left temporal volume loss but relative sparing of the right temporal lobe. (B) Top left: The symptom legend. Top right: Panel shows the symptoms chronology in the right temporal-predominant group; the most common early symptoms in this group are loss of empathy, loss of person-specific knowledge and rigid thought process and complex compulsion. Bottom left: Panel shows symptom chronology in the frontal-predominant group; the most common early symptoms in this group are lack of judgement and dysexecutive symptoms, apathy and disinhibition. The right lower corner shows symptom chronology in the left temporal-predominant group; the most common early symptoms in this group are verbal semantic loss, loss of person-specific knowledge and rigid thought process and complex compulsion.
Patients were excluded if they did not meet either the predominant atrophy or the atrophy index requirements. Patients were excluded if their lowest W-scores were not in the rATL, frontal lobe or lATL (n = 164; four cerebellar, 65 mixed (i.e. lowest three W-scores were in different lobes), 62 subcortical and 33 posterior (i.e. parietal or occipital lobes) or if their greatest atrophy was in the rATL, lATL or frontal lobes but did not meet the atrophy index inclusion threshold (total n = 114; 41 with maximum atrophy in rATL but frontal/right temporal >0.5, 29 with maximum atrophy in the frontal lobe but frontal/right temporal <0.5 and 44 with maximum atrophy in the lATL but frontal/left temporal >0.5).
Recognizing that each of the groups included in this study did not show atrophy merely in one isolated brain region (for instance, patients typically have bilateral ATL volume loss by the time they present for imaging evaluation), we qualify our descriptions by using the term ‘predominant’ to refer to the patient group with maximum atrophy in the one region that is out of proportion to the other regions. Thus, we acknowledge that the brain atrophy pattern of our ‘rATL-predominant’ group also includes frontal and left temporal regions to varying degrees, but these patients unequivocally present with maximum atrophy in the rATL. Similarly, the ‘lATL-predominant’ refers to the patient group with maximum atrophy in the lATL that is out of proportion to the frontal and rATL atrophy. The term ‘frontal-predominant’, likewise, is used to refer to the patient group with maximum atrophy in the frontal lobes that is out of proportion to the ATL atrophy. The atrophy of each group as shown in Fig. 2A extends beyond the regions of maximum atrophy and as such symptoms could be due to atrophy in the other regions involved or multiple parts of the connected networks.
Genetic and neuropathological data
Participants were screened for the following genetic mutations: PGRN, MAPT, TARDBP, C9orf72, APP, PSEN1, PSEN2, FUS and APOE. In the patients who underwent autopsy, brains were processed and analysed according to the UCSF Neurodegenerative Disease Brain Bank protocol.67 In short, eight micro-thick formalin-fixed paraffin-embedded tissue sections from 23 tissue blocks were cut to represent 27 regions of interest. All blocks underwent routine haematoxylin and eosin staining, and subsets underwent immunohistochemistry for hyperphosphorylated tau, amyloid-β, TDP-43, alpha-synuclein and 3R-tau antibodies. Neuropathological diagnoses were based on consensus criteria.68–70
Statistical analysis
Tests of normality for all continuous data were conducted with the Shapiro–Wilk test. Homogeneity of variance was tested by Levene’s test. Statistical differences in the frequency of categorical variables across groups such as clinical symptoms and APOE genotype were performed with the chi-square test. Means of demographic measures (Table 1) were compared across groups with the ANOVA test. Means of functional, neuropsychological, language and socioemotional measures (Tables 1 and 2) were compared with the analysis of covariance test correcting for age, sex and disease severity as measured by the Mini-Mental State Examination (MMSE). Because of the unequal sample sizes and unequal group variances, pairwise post hoc comparisons were done with estimated marginal means and Bonferroni–Sidak adjusted probabilities to correct for multiple comparisons, with P < 0.05 set as the threshold for statistical significance. Data analysis was performed with SPSS (v.27, SPSS/IBM, Chicago, IL, USA). Tables 1 and 2 show estimated marginal means, standard errors and statistical significance after correcting for age, sex and disease severity as measured by MMSE. When using analysis of covariance and estimated marginal means for post hoc between groups analysis, the individual data-points cannot be graphically plotted, for visualization purposes we show in Fig. 3 the uncorrected data-points, means and standard deviations for key socioemotional measures.
Figure 3.
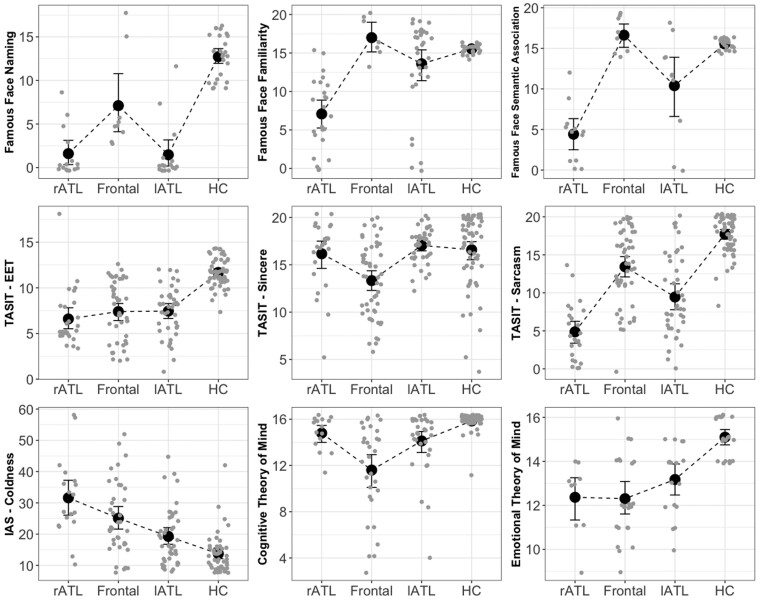
Socioemotional and neuropsychological characteristics. The figure shows the results of the main socioemotional tests that can help distinguish right temporal- from frontal-predominant patients. More details can be found in Table 2. Although all disease groups had difficulties with Famous Faces Naming, only right temporal and a lesser degree left temporal-predominant patients, had difficulties on Famous Face Recognition and Semantic Association. Although all disease groups showed impaired simple and complex social cues recognition on the TASIT–EET and TASIT-Sarcasm, only the frontal-predominant group showed impairment on the control cognitive task, TASIT-Sincere. Right temporal-predominant patients showed significantly worse performance on the complex social cue, TASIT-Sarcasm, compared to the frontal-predominant group. The right temporal-predominant group showed increased coldness compared to the frontal-predominant group. The right and left temporal-predominant groups had difficulty with the Emotional Theory of Mind but not with the Cognitive Theory of Mind task, in contrast to the frontal-predominant group that demonstrated impairment in both Cognitive and Emotional Theory of Mind.
Data availability
The data for this study are available on request. The sensitive nature of patients’ data and the institutional ethics protocols in place at the time these patients gave informed consent do not permit open data sharing. The clinical and neuroimaging data used in the current paper are available from the senior author (M.L.G.T.), on formal request indicating name and affiliation of the researcher as well as a brief description of the intended use for the data. All requests will undergo UCSF-regulated procedure thus requiring submission of a Material Transfer Agreement. No commercial use would be approved.
Results
Demographic features
Table 1 shows the demographic information. Although the sex distribution was not different between healthy control and the patient groups, the healthy controls were older than all of the patient groups. Patients in all cohorts were highly educated with an average over 15.5 years of education. In the rATL-predominant group, 91% were White and 9% were Asians, a proportion that was not different from other groups. In the rATL cohort (n = 46), the average age of onset (60.2 years and SD = 6.8 years). In the rATL-predominant group, 52% of the patients were men and 15% were non-right-handed. On average, rATL-predominant patients were in the mild to moderate range of disease severity; at the first research visit, the average MMSE score (25.7/30; SD = 5.2) was higher than the other disease groups. The CDR for the rATL group (average score 0.9/3; SD = 0.5) was lower than the lATL-predominant group but not different from the frontal-predominant group. We used age, sex and MMSE as confounds in all later analyses.
Diagnostic criteria and clinical symptom chronology
During the first 3 years of the illness, only a minority of patients in the rATL-predominant group met diagnostic criteria for Neary-FTD (13%), Neary-semantic dementia (9%), bvFTD (27%) or svPPA (13%). Approximately one-third of the group had verbal svPPA features (i.e. impaired confrontational naming and object knowledge) but did not meet the general criteria for PPA (36%) because aphasia was not the initial and predominant symptom. At the time of the first research evaluation at the MAC (average 5.3 years after disease onset), these percentages were higher: Neary-FTD (52%), Neary-semantic dementia (11%), bvFTD (83%), svPPA (16%) and semantic variant PPA features (78%) (Supplementary Table 2).
The clinical histories revealed that, when combining all symptoms that emerged during the first 3 years of the illness, the most common symptoms for patients with rATL-predominant degeneration were loss of empathy (27%), loss of person-specific semantic knowledge (23%), complex compulsions and rigid thought process (18%) and loss of verbal semantic knowledge (13%) (Fig. 2B). The sequence of the first two symptoms in rATL-predominant patients is shown in Supplementary Table 3.
Caregiver-reported examples of loss of empathy included decline in the ability to understand and respond to others’ emotions and needs (e.g. not consoling a family member who lost a parent or was diagnosed with a terminal illness, making tactless comments in a funeral, asking a crying child why their eyes were watering and becoming more self-centred). In our experience, often, loss of empathy towards others can be interpreted by caregivers as selfishness. Examples of loss of person-specific semantic knowledge included not recognizing familiar people by the face or voice, not recalling biographical information of a famous person, and not knowing patients’ own relationship to familiar people. Examples of complex compulsions and rigid thought process included adherence to rigid time schedules; dogmatism; hypergraphia; hypochondriasis; restricted colour, clothing, diet, game or puzzle preferences. Less commonly, patients exhibited simple repetitive motor or speech behaviours or hoarding behaviours. Examples of loss of verbal semantic knowledge included difficulty understanding word meaning or recognizing objects.
When rATL-predominant patients had both person-specific and verbal semantic knowledge loss [32 patients (69%)], the person-specific semantic knowledge symptoms were reported to precede the verbal semantic complaints in 24 patients (75%). Five patients (10%) had person-specific semantic knowledge symptoms without verbal semantic complaints, and six patients (14%) had verbal semantic complaints without person-specific semantic knowledge complaints. Only three patients (6%) had neither person-specific nor verbal semantic knowledge complaints.
While these initial symptoms in the rATL-predominant group emerged within the first 3 years of disease onset, additional symptoms (fifth, sixth and beyond) arose as the disease progressed. Four years after disease onset, common symptoms included apathy and disinhibition. For these two symptoms, differences in reporting created ambiguity. Apathy was explicitly documented in the medical history as a clinical complaint of 11 patients, whereas on the NPI (Supplementary Table 5), apathy was noted in 39 patients, indicating a discrepancy between what caregivers report during the interview with the behavioural neurologist and when answering the NPI questions. Interestingly, the item of apathy on the NPI appeared mainly in the context of affective apathy questions rather than cognitive inertia or autoactivation/behavioural apathy and thus these behaviours could also be interpreted as loss of empathy. This potentially explains the discrepancy between clinical history and NPI with regards to apathy reporting and highlights the need for incorporating loss of empathy questions into the NPI. On history, disinhibition was reported in 23 patients, whereas on the NPI it was coded in 36 patients. In most patients, disinhibition appeared as insensitivity to social context rather than as an impulse-control deficit, for instance, making funny comments in a funeral rather than approaching strangers or engaging in dangerous activities. By history, episodic memory impairment, executive symptoms, dietary changes, motor neuron disease and problems navigating were less frequent and happened later in the disease course. With regards to the less commonly reported symptoms in the rATL-predominant group, five patients (11%) had loss of sexual desire, two as an early symptom. Irritability was reported in eight patients (17%) and as an early symptom only in three patients (6%). Increased eating (7 patients, 8%) did not reach the degree of binge eating, oral exploration or consumption of inedible objects. Sleep changes, increased or decreased sleep, happened in five cases (10%), three of which were in the first year of disease onset.
In comparison, the early symptoms in the frontal-predominant group were lack of judgement and dysexecutive symptoms (24%), apathy (21%) and disinhibition (17%), as shown in Fig. 2B and Supplementary Table 4. In the lATL-predominant patients, the early symptoms were verbal semantic knowledge loss (36%), person-specific knowledge deficits (16%) and rigid thought process (18%). Loss of empathy occurred significantly more often in rATL- compared to frontal- and lATL-predominant patients (χ2 = 22, P < 0.001 and 11.2 P < 0.001, respectively). Deficits in person-specific knowledge were significantly more common in rATL- than frontal-predominant patients (χ2 = 56.1, P < 0.001) but not lATL-predominant patients (χ2 = 3.32, P < 0.68). Similarly, complex compulsions and rigid thought processes were significantly more frequent in rATL- compared to frontal-, but not lATL-predominant groups (χ2 = 19.54, P < 0.001 and χ2 = 1.03, P = 0.3, respectively). In contrast apathy, disinhibition and lack of judgement and dysexecutive symptoms were significantly more common in frontal- than rATL- and lATL-predominant patients (χ2 = 11.5, P < 0.001, χ2 = 5.2, P < 0.02, χ2 = 18.8, P < 0.001, respectively).
Functional, cognitive and behavioural results
Tables 1, 2 and Fig. 3 show the neuropsychological and socioemotional results. Neuropsychological testing demonstrated that, at presentation to the MAC, patients with rATL-predominant degeneration had severe impairment in both verbal semantic knowledge (on the Boston Naming Test and Peabody Picture Vocabulary Test) and non-verbal (visual) semantic knowledge (on the PPT-P). They also had deficits in verbal fluency, with more significant impairment in semantic than in lexical fluency, and on tests of executive functioning. Episodic memory was impaired, and visuospatial processing was intact.
On tests of socioemotional functioning, rATL-predominant degeneration patients had severe deficits in multiple domains. On the CATS, a static face perception test, although they had no difficulty with face identity-matching, their emotion labelling was impaired, suggesting a deficit in emotion recognition. Patients also had difficulty labelling the emotions of others in videos (TASIT–EET) and understanding paralinguistic cues (TASIT-Social Inference–Minimal Test-M). On tests of theory of mind, patients had normal Cognitive Theory of Mind scores but impaired Emotional Theory of Mind scores, indicating poor comprehension of others’ emotional, but not cognitive, states. On the Famous Faces Test, rATL-predominant degeneration patients could not identify the faces, names or occupations, of famous people, indicating loss of person-specific semantic knowledge rather than prosopagnosia. On informant-based measures, rATL degeneration patients had abnormal scores on multiple measures of behaviour and personality. Patients had very low emotional empathy (IRI Empathic Concern), cognitive empathy (IRI Perspective Taking) and socioemotional sensitivity (RSMS). On a personality inventory (IAS), informants rated patients as having low levels of interpersonal warmth and increased interpersonal coldness yet preserved interpersonal dominance.
Although emotion processing was disrupted in frontal-, rATL- and, to a lesser degree, lATL-predominant patients (as measured by IRI-ET, IRI-PT and RSMS), the groups differed in their specific constellations of social and behavioural deficits. While the frontal-predominant patients were impaired on both cognitive and emotional measures, the rATL- and lATL-predominant patients generally showed prominent deficits on the emotional, but not the cognitive, components of socioemotional tasks. Specifically, rATL- and lATL-predominant patients showed preserved Cognitive Theory of Mind but impaired Emotional Theory of Mind, whereas frontal-predominant patients showed impairment on both Cognitive and Emotional Theory of Mind (Fig. 3 and Table 2). Similarly, rATL-predominant patients scored within normal limits, on the TASIT-Sincere task (a cognitive control task that assesses simple comprehension) but below expectations on the TASIT–EET (an emotion naming task) and the TASIT–Simple Sarcasm subscale (a test of paralinguistic cue detection). In contrast, the frontal-predominant group scored below expectations on all three TASIT subsets, suggesting both emotional and cognitive deficits. On informant-based personality measures, the rATL-predominant patients showed increased coldness but preserved dominance, whereas the frontal-predominant patients showed increased coldness (to a lesser degree than rATL-predominant patients) but reduced dominance. Furthermore, rATL-predominant patients showed reduction in both their activation and inhibition systems on the BIS/BAS. Reduced reward sensitivity was associated with reduced drive and fun-seeking in rATL-predominant patients, whereas in the frontal-predominant patients reduced reward sensitivity was associated with higher drive and fun-seeking (Fig. 3 and Table 2). This incongruence in the frontal-predominant group is consistent with the higher rates of impulsivity, such as making sexual comments, in this group as shown on the NPI (Supplementary Table 5).
With regard to face processing and person-specific knowledge, while all disease groups had difficulty with Famous Faces Naming, only rATL-predominant patients (and to a lesser degree lATL-predominant patients) had impaired scores on the Famous Faces Recognition and Semantic Association tests (Fig. 3 and Table 2). On the CATS Face and Affect Matching, whereas the frontal-predominant patients were impaired on both tests, the rATL-predominant patients had preserved face matching but impaired affect matching. The lATL-predominant patients, in contrast, had intact scores on the Face and Affect Matching tests (Fig. 4 and Table 2). The deficits in the rATL group did not appear to be due to broader deficits in visuospatial functioning as only the frontal-predominant patients demonstrated deficits in this area (Benson Figure Copy and Visual Object and Space Perception).
Figure 4.
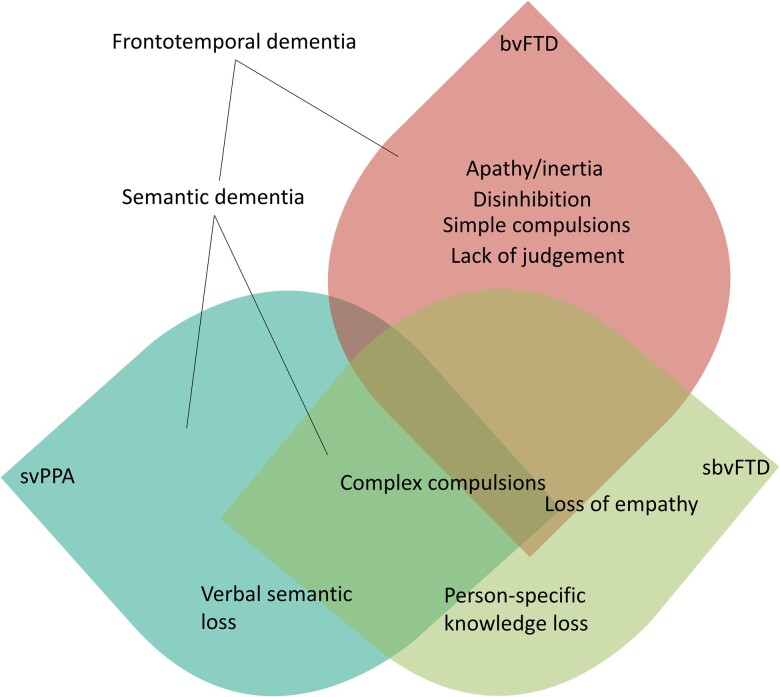
Clinico-anatomical model. Schematic representation of the clinical symptom overlap in FTD between sbvFTD and svPPA (both under the semantic dementia spectrum and often have FTLD-TDP-C pathology) and bvFTD.
With regard to verbal semantics, both rATL- and lATL-predominant patients had greater deficits on the Boston Naming Test than the frontal-predominant group. On episodic memory testing, rATL- and lATL-predominant patients showed worse verbal and visual memory impairment compared to the frontal-predominant group (Fig. 3 and Table 2). On executive function tests, rATL- and lATL-predominant patients showed better executive function performance compared to the frontal-predominant group (Fig. 3 and Table 1).
Genetic and pathology results
Pathology and genetic results are presented in Tables 3 and Supplementary Table 6. Only two of the rATL-predominant patients had a genetic mutation, (one had a MAPT mutation, and one had a possibly pathogenic TARDBP mutation). Seventeen of the frontal-predominant patients (14 C9orf72 and three GRN) and five lATL-predominant patients (three MAPT and two C9orf72) had genetic mutations. APOE data were available in 40 of the rATL-predominant patients (55% E3/E3; 22% E3/E4; 18% E2/E3). No differences in APOE genotypes were found between subgroups with available APOE data (Supplementary Table 7). Most of the rATL-predominant patients with available autopsy data had FTLD-TDP type C pathology (68%). When considering all types of FTLD-TDP cases, regardless of the neuropathological subtype, the percentage increased (84%). Three patients had FTLD-tau (two FTLD-tau Pick’s type and one patient had FTLD-tau unclassifiable 4R tauopathy). In the rATL-predominant group with autopsy data, three patients did not have loss of semantic knowledge on either history or testing and, interestingly, none of these three cases had FTLD-TDP type C (two had FTLD-tau Pick’s type, and one had FTLD-TDP type B). In the lATL-predominant group, there was also large proportion of patients with TDP-43 pathology, in general and TDP-43 type C, specifically. This is in contrast to the frontal-predominant group, which showed more heterogeneity in its underlying pathology (51% tauopathy, 22% FTLD-TDP type B, 12% FTLD-TDP type A and 2% FTLD-TDP type C) (Table 3).
Table 3.
Primary pathology and genetics
| Primary pathology | Frontal, total = 41 n (%) |
Left temporal, total = 31 n (%) |
Right temporal, total = 19 n (%) |
|---|---|---|---|
| Alzheimer’s disease | 1 (2.4) | 1 (3.2) | |
| Argyrophilic grain disease | 1 (2.4) | ||
| FTLD-tau corticobasal degeneration | 9 (21.9) | ||
| FTLD-tau Pick’s Disease | 7 (17.0) | 1 (3.2) | 2 (10.5) |
| FTLD-tau progressive supranuclear palsy | 3 (7.3) | ||
| FTLD-TDP type A | 5 (12.2) | 1 (3.2) | 1 (5.2) |
| FTLD-TDP type B | 9 (21.9) | 4 (12.9) | 1 (5.2) |
| FTLD-TDP type C | 1 (2.4) | 23 (74.1) | 13 (68.4) |
| FTLD-TDP unclassifiable | 4 (9.7) | 1 (5.2) | |
| FTLD-tau unclassifiable | 1 (2.4) | 1 (5.2) | |
| FTLD-tau with MAPT mutation | 1 (3.2) | ||
| Genetics |
Frontal, total = 73
n (%) |
Left temporal, total = 61
n (%) |
Right temporal, total = 38
n (%) |
| MAPT | 3 (4.9) (2 pathogenic and 1 possibly pathogenic) | 1 (2.6) | |
| TARDBP | 1 possibly pathogenic (2.6) | ||
| C9ORF72 | 14 (19.1) | 2 (3.2) | |
| GRN | 3 (4.1) |
Sensitivity and specificity of the proposed diagnostic criteria
Based on the most common early symptoms in the patients with rATL-predominant degeneration, here we propose a new set of diagnostic criteria for this syndrome (Box 1). To test the sensitivity and specificity of these criteria, we contrasted the rATL- and frontal-predominant patients (Supplementary Table 8), groups that are often difficult to disentangle clinically.13,71 To avoid circularity, we did not calculate sensitivity or specificity values based on the neuroimaging data because our groups were anatomically defined. In the first 3 years of the illness, the criteria differentiated the rATL-predominant from the frontal-predominant group with a sensitivity of 81.3% and a specificity of 84.2%. The sensitivity increased to 86.0% at the time of the first clinical visit and to 93.0% when considering symptoms across all visits. The specificity was 82.8% at the first clinical visit and 81.4% when considering all visits. We predict that sensitivity and specificity will increase in prospectively collected samples because of the increased probing of non-verbal socioemotional semantics during patient evaluations, and should improve further with the inclusion of patients’ neuroimaging information.
Box 1.
Proposed diagnostic criteria for semantic behavioural variant frontotemporal dementia (sbvFTD)
| I. Patient shows gradually progressive deterioration by history and/or testing |
|
II. Clinical diagnosis of sbvFTD:
Patient must have two out of the core features A–C and two out of the supportive features D–F: Core features: A. Loss of empathy (difficulty understanding emotions) B. Difficulty naming and identifying known people C. Complex compulsions or rigid thought process Supportive features: D. Object naming difficulties E. Spared visuospatial functions including preserved perceptual matching and drawing reproduction F. Spared motor speech and phonology |
|
III. Imaging-supported sbvFTD:
All the following must be present: A. Meets criteria for sbvFTD and B. Imaging results consistent with sbvFTD: Laterality is right-sided in right-handed but could be left-sided in non-right-handed individuals. (i) Anterior temporal lobe volume loss and relative sparing of the frontal cortex on MRI or CT; or (ii) Anterior temporal lobe hypometabolism and relative sparing of the frontal cortex on FDG-PET |
|
IV. SbvFTD with definite pathology
All the following must be present: A. Clinical diagnosis of sbvFTD B. Histopathological or genetic evidence: (i) Histopathology of a specific neurodegenerative pathology (e.g. FTLD-TDP, FTLD-tau, other); or (ii) Presence of known pathogenic mutation |
Making the diagnosis of lATL-predominant patients is somewhat less difficult in clinical practice, in part because lATL patients present with early language-centred, word-finding and word-comprehension deficits, instead of early behavioural symptoms, and thus are classified as having a PPA syndrome. However, as the disease progresses and neurodegeneration spreads to the rATL and orbitofrontal regions,5,7,8 the continuum between the two clinical presentations becomes more obvious, as predicted by the same FTLD-TDP pathology. Consistent with this, the proposed criteria showed 76.0% sensitivity and 87.0% specificity in distinguishing rATL- from lATL-predominant patients in the first 2 years of symptoms and 81.3% sensitivity and 68.2% specificity by the third year. The decrease in specificity by the third year highlights the overlap in disease progression between rATL and lATL degeneration. Receiver operator curves and sensitivity and specificity of certain cut-off points of the main socioemotional and neuropsychiatric tests in differentiating the rATL from the frontal and lATL groups is shown in Supplementary Table 9.
Discussion
This research presents the symptom chronology, neuropsychology and socioemotional features of a large cohort of well-characterized patients with predominant rATL degeneration. Cognitive and anatomical data showed that rATL-predominant degeneration disrupts the neural representations sustaining mainly non-verbal semantic knowledge for socioemotional concepts, resulting in early, prominent deficits in empathy, people recognition and social behaviour. This constellation of symptoms reflects dysfunction in the underlying neuroanatomical networks that are anchored by the rATL and that overlap with, but are dissociable from, those involved in frontal-predominant bvFTD and lATL-predominant svPPA.72,73 Guided by our findings, we propose new clinical criteria and nomenclature to facilitate early diagnosis and care of this clinico-anatomical syndrome. Early core symptoms were loss of empathy and person-specific semantic knowledge (mainly face-based, non-verbal semantic knowledge) as well as development of complex compulsions and rigid thinking. Later symptoms included loss of verbal semantic knowledge and, eventually, apathy and disinhibition. As such, this syndrome necessitates a distinct nomenclature, which, herein, we refer to as ‘semantic behavioural variant frontotemporal dementia’ (sbvFTD). This term reflects the underlying cognitive mechanism (loss of semantic knowledge for socioemotional concepts) and the continuum with svPPA (and its original name, semantic dementia) while highlighting that early clinical manifestations are behavioural, and not aphasic (therefore adding ‘semantic’ to ‘bvFTD’). We decided against the use of anatomical terms such ‘left ATL versus right ATL FTD’ or ‘left-predominant versus right-predominant semantic dementia’ as these terms are not consistent with other neurodegenerative disease nomenclature that tend to be descriptive in nature (e.g. non-fluent variant PPA, logopenic variant PPA, bvFTD), do not apply to non-right-handed patients and imply dementia is present although this may not be the case for early-stage patients. It is important to separate the rATL semantic bvFTD syndrome from a general bvFTD diagnosis because its underlying neuropathological aetiology is more consistently FTLD-TDP disease (84%), and most often FTLD-TDP type C, thus placing it on a continuum with its lATL-predominant (aphasic) svPPA counterpart. sbvFTD (behavioural syndrome) and svPPA (language syndrome) can thus be considered the two clinico-anatomical extremes of a ‘semantic dementia spectrum’ (Fig. 4).9,12,13,29–32 The proposed diagnostic criteria capture the notion that loss of non-verbal, socially and emotionally relevant concepts is the central mechanism underlying the clinical deficits (Box 1). The core clinical symptoms include loss of empathy caused by lack of understanding socioemotional cues and diminished emotional experience, difficulty identifying and naming known people and complex compulsions or rigid thought process. Supportive symptoms include object naming difficulties, and relatively spared visuospatial functions and speech production (motor speech and phonology). A diagnosis of imaging-supported sbvFTD also requires neuroimaging evidence of disproportionate rATL atrophy or hypometabolism. The novel diagnostic classification proposed here helps identify early symptoms that are most specifically associated with the non-dominant hemisphere ATL degeneration.
Early semantic dementia descriptions focused on the prominent verbal and object semantic deficits resulting primarily from lATL damage, including surface dyslexia.14,74,75 The recent use of more comprehensive neuropsychological batteries has yielded data clearly delineating the socioemotional and visual semantic impairments that predominate in the setting of rATL degeneration. Depending on the asymmetry of ATL atrophy and the disease stage, there can be variable degrees of clinical and neuropsychological overlap between verbal and non-verbal semantic deficits in patients with ATL degeneration. In the early stages of the disease, sbvFTD patients can show progressive loss of empathy that appear to be their isolated symptom but may be accompanied by other non-verbal semantic deficits that can only be detected with specific tests. Our results indicate that tests of famous people knowledge (visual famous face familiarity judgements such as Famous Face Recognition in our battery), facial expression recognition (CATS), emotion processing (Emotional and Cognitive Theory of Mind and TASIT) and visual semantic associations (PPT-P) as well as personality questionnaires (IAS-Coldness) were the most useful in the differential diagnosis of patients with sbvFTD from those with bvFTD and svPPA. In practice, if a patient presents with relatively isolated loss of empathy or complex compulsion and shows deficits on semantic tests (e.g. people and, later, object knowledge) then our study suggests the most likely diagnosis is sbvFTD.
The rATL is a key hub in neural networks devoted to processing socioemotional semantic knowledge and is critical for binding the sensorimotor activities, visceral changes, encyclopaedic knowledge and subjective experiences into multimodal socially relevant concepts.13,39 Socioemotional semantic deficits not only interfere with patients’ ability to recognize familiar individuals, but also with their ability to attribute meaning to their emotional expressions and cues. This lack of understanding results in a lack of appropriate empathic responses in social contexts.5,7,17,18,22 The term loss of empathy refers to the inability to infer other's emotional states and to accurately predict and appropriately respond to those states in a prosocial manner.76 Processing the emotions of others relies on several steps including understanding the meaning (i.e. socioemotional semantic knowledge) of an observed expression, internally simulating the sentiment of the expressed emotion within the particular social context, assigning the behaviour to the other rather than the self, inhibiting one's own perspective and initiating a prosocial behaviour.76,77 These processes localize to different but interconnected neuroanatomical circuits.77,78 Identification of known people from their face or voice requires person-specific semantic knowledge that incorporates visual, auditory and socioemotional information about what they look and sound like with biographical information about who they are and how they relate to the observer. Patients with sbvFTD displayed severe impairment on all aspects of person-specific knowledge (including Famous Faces Naming, Semantic Association and Recognition tasks), a pattern consistent with previous reports.10,20 These deficits differ from classical prosopagnosia, the visual inability to recognize familiar people from their faces only, as the patients with sbvFTD were also unable to recognize familiar people from their name, voice, biography or information about their relationship to the patient.27,42 Although patients with rATL-predominant degeneration are often described as having ‘prosopagnosia’, this term does not fully capture the deficit because patients cannot recognize familiar people from visual (face), linguistic (name) or auditory cues (voices), which suggests a broader semantic deficit for biographical, person-specific semantic knowledge.46 As the rATL plays an integral role in representing feelings of familiarity for known others,27 even family members and close friends may lose their affective significance to patients with rATL-predominant degeneration. Patients, therefore, may not only have difficulty recognizing known others but may also display diminished interpersonal warmth in their relationships. Caregivers of patients sbvFTD, unlike those of patients with bvFTD and svPPA, often report that difficulty recognizing friends, non-immediate family members, famous people was an early manifestation of the disease. Although patients with svPPA and lATL atrophy often perform below expectations on semantic association and famous face semantic tests,79 caregivers do not usually report early decline in person-specific knowledge, which is probably because with lATL-predominant disease retain a rATL-based sense of visual and emotional ‘familiarity’ with known people.
Understanding others’ feelings (a component of empathy) involves semantic knowledge about non-verbal stimuli (tone of voice, body position, facial expression) as well as access to bodily cues that foster vicarious experience of others’ internal states.76,77 Consistently, informants who had been close to the patients with sbvFTD before disease onset reported changes in behaviour such as a lack of responsiveness to socioemotional signals and increased interpersonal coldness. Typically, sbvFTD family members report that patients who were previously a warm and caring spouse or parent now would show no reaction (or inappropriate reactions) to their loved ones’ feelings. Patients with sbvFTD often seem puzzled by emotionally charged situations, a reaction that resembles the response of patients with svPPA who are faced with a word that they feel they should know but cannot recognize. On our battery of socioemotional functioning tests, patients with sbvFTD had difficulty selecting a label for facial emotional expressions on the CATS emotion identification task despite preserved face perception on CATS face identity-matching. This pattern differed from the frontal-predominant group who exhibited impaired performance on both subtasks, and the lATL-predominant group who showed no impairment on both subtasks. These findings suggest that patients rATL-predominant degeneration have impaired understanding of the meaning of observed facial expressions, as previously described.80 Their difficulty, however, is not limited to facial expression recognition but instead includes multimodal loss of non-verbal emotional cue comprehension, as demonstrated by their difficulty recognizing emotions in videos that include facial, prosodic, postural and gestural emotional cues (TASIT–EET). Moreover, patients with sbvFTD were impaired at interpreting videos that tested Emotional Theory of Mind despite the fact that the emotions of the characters were explicitly verbally labelled for them throughout the task. Notably, patients had no trouble interpreting Cognitive Theory of Mind videos that relied on perspective taking focused on physical objects rather than on others’ emotions. This pattern contrasted with that of the frontal-predominant group who demonstrated impairment on both the cognitive as well as the Emotional Theory of Mind tasks. Taken together, these findings suggest that patients with rATL-predominant sbvFTD have deficits due to underlying impairment in emotion comprehension rather than impairment in task-specific executive functioning demands.
The sbvFTD and svPPA groups both exhibited complex, compulsive behaviours as well as cognitive rigidity. Previous studies have indicated that repetitive behaviours can be more linguistic (i.e. word games) or visual (i.e. visual puzzles) in nature depending on the relative preservation of rATL or lATL functions.5 Patients with frontal-predominant bvFTD, in contrast, show more simple motor repetitive behaviours (e.g. tapping and pacing), hoarding and echolalia that localize to frontal subcortical networks and the left lateral temporal lobe.81,82 However, more complex compulsions (e.g. preoccupation with certain ideas or activities, following fixed schedules, parsimony and complex rituals), in contrast, localize to the rATL.83 Consistent with previous studies, we found that patients with sbvFTD most commonly exhibited complex, goal-oriented and time-consuming repetitive behaviours and rigid thought processes. Restricted dietary routines (i.e. eating only vegetables or only meat or eating yoghurt every day at 11 a.m.) were also common in sbvFTD, although patients rarely showed overeating or preference for high sugar and high fat foods, as previously reported in bvFTD.
In the later phases of disease, patients with ATL-predominant degeneration, regardless of their early sbvFTD or svPPA presentations, also exhibited apathy and disinhibition, symptoms that are cardinal early features of the frontal-predominant group and probably relate to further spreading of atrophy to frontal regions. Conversely, in the frontal-predominant group, apathy, disinhibition and lack of judgement and dysexecutive symptoms were the most common early symptoms. This suggests that early symptoms can help distinguish sbvFTD from bvFTD. In FTD-spectrum disorders, apathy and disinhibition can reflect underlying deficits in cognitive, behavioural or affective systems that are anchored by the frontal lobes.51 In our cohort, the clinical histories of patients with rATL-predominant atrophy suggested that symptoms recorded by clinicians as apathy and disinhibition differed from typical examples reported in the frontal-predominant group and often in bvFTD. For instance, lack of participation in activities with family or making tactless comments were due to socioemotional semantic deficits rather than apathy or disinhibition, respectively. By history, the patients with sbvFTD had early loss of interest in friends and family, were less affectionate and made tactless comments, which indicates a lack of understanding for social cues, but they did not show deficits in impulse control until the fourth year of illness.
The high frequency of the first two symptoms (loss of empathy and person-specific semantic knowledge) in the sbvFTD cohort and the deficits on both facial emotion recognition and Famous Faces tests suggest that the regions subserving face and emotion processing are interlinked and possibly undergo interdependent development during maturation and concordant degeneration during neurodegeneration. Neurodevelopmentally, the ability to acquire and respond to social and emotional concepts is shown to be linked to accurate interpretation of emotional expressions during early childhood.84 In fact, recognition of emotional facial expressions is a fundamental aspect of human behavioural neurodevelopment, as infants prefer to look at faces from a very early age and regulate their actions based on maternal emotional facial expressions.84 Furthermore, impairment in recognizing emotional facial expressions is presumed to be one of the mechanisms underpinning the behavioural symptoms in autism spectrum disorder, which involves the rATL.85–88 Recent work proposes that developmental factors might influence vulnerability to specific neurodegenerative illnesses and links to specific phenotypic presentations.89,90 In particular, previous studies suggest that non-right-handedness is over-represented in svPPA compared to other PPA variants and to the general population.89 In our sbvFTD cohort, there was also a relatively high prevalence of non-right-handedness (15%) compared to the 10% reported in the general population.91 Furthermore, a previous case report described a behavioural presentation in a non-right-handed patient who had left temporal-predominant atrophy.45 Taken together, this evidence suggests that handedness, and, thus, lateralization of language and emotion processing, might influence how linguistic and behavioural symptoms associate with ATL atrophy, contributing to phenotypic variation.89,90
Although loss of empathy is the most common symptom used by clinicians and caregivers to describe the early stages of sbvFTD, a previous study suggested a prodromal phase of irritability, emotional distance and changes in sleep, appetite and libido.5 In the present study, we considered the subtle early emotional changes such as becoming more selfish and emotionally distant as part of loss of empathy as these symptoms are probably the subtle early manifestations of socioemotional semantic loss. Libido changes and irritability happened in the context of loss of empathy. Similarly, appetite changes happened in the context of other complex compulsions. Sleep changes happened as a prodromal symptom only in a minority of patients. It is possible that the prevalence of these symptoms is underestimated and masked by the more pressing symptoms by the time patients present for evaluation. A recently proposed diagnostic framework for focal rATL degeneration identified memory symptoms and prosopagnosia as key features but did not distinguish between episodic or semantic memory.92 Our results indicate the deficits in the rATL-predominant group extend beyond classical prosopagnosia and represent a multimodal semantic loss for person-specific concepts, but it is also possible that some rATL-predominant patients may have selective prosopagnosia (without person-specific knowledge) in the very early stage of their illness.93 We believe that our large sample size and comprehensive language and socioemotional testing battery enabled us to derive a more complete and precise depiction of symptoms, while at the same time highlighting a semantic memory deficit as the common underlying mechanism in both sbvFTD and svPPA. Defining the nature of memory loss in these patients as mainly semantic rather than episodic is particularly relevant, because including episodic memory deficits as a core diagnostic criterion for rATL degeneration syndrome is likely to cause diagnostic confusion with clinical Alzheimer’s disease, particularly in settings where Alzheimer’s disease biomarkers are unavailable.
This study has several limitations. Although our cohorts of patients were studied prospectively by a multidisciplinary team that included experts in both language and behaviour, the retrospective nature of chart reviews is heavily informed by the clinician writing the original report. Consistent with most criteria in the field, the classification proposed here is not based on cut-off scores on specific tests but on clinical judgement regarding the pattern of impairment, an approach that allows each medical centre to apply their preferred diagnostic tools. Further, the interpretation of the symptoms experienced by patients and their families entails, if inadvertently, an element of subjectivity. Given our reliance on patients’ and caregivers’ recollections of the symptom chronology, it is possible that recall bias may have influenced our findings (although our large sample size makes this less likely). Although it is difficult to ascertain the natural history of early symptoms in rare diseases such as the ones we study here, future collaborative, prospective cohort studies with shared measures and approaches will be imperative for making strides in this area of medicine and neuroscience. Another limitation pertains to our imaging-based selection criteria, which focused on identifying rATL-predominant cases and excluded cases that had both concomitant severe frontal or lATL atrophy. Additional studies are needed to phenotype the subset of patients with FTD who have other atrophy patterns, such as those with co-occurring right frontal and bilateral temporal damage. In addition, although the rATL-predominant group we describe includes prominent atrophy in rATL atrophy, these patients also have atrophy in a network of regions (e.g. lATL and right insula) connected to the rATL. The atrophy of each group (as shown in Fig. 2A) extends beyond the regions of maximum atrophy and, thus, symptoms could be due to atrophy in the other regions involved or multiple parts of the connected networks. Future work is needed to further elucidate how structural and functional damage in the rATL and its associated networks relate to the symptoms we detected in this group. Finally, we acknowledge the serious limitation that patients included in this study are mostly white, highly educated and native English speakers. Further studies that include more diverse patient populations are needed to shed light on the cultural and environmental variability of socioemotional and linguistic presentations in patients with focal ATL degeneration.94 These future studies will provide clinical tools that use culturally sensitive language and stimuli. While the sensitivity and specificity might appear lower than what is expected given the depth and breadth of the neuropsychological, socioemotional and imaging data included in our study, we emphasize that the sensitivity and specificity values we present were based only on the clinical symptoms reported by patients and caregivers and did not include any neuropsychological, socioemotional or neuroimaging data. Although including neuroimaging data would undoubtedly increase the sensitivity and specificity of the proposed criteria, this would introduce circularity into our methods. To obtain more precise metrics of sensitivity and specificity, our findings call for replication and validation of the proposed criteria in larger, independent datasets.
In conclusion, we show that patients with sbvFTD show early loss of empathy and person-specific knowledge in relation to rATL-predominant degeneration and decline in the neural networks that support non-verbal, socioemotional semantic knowledge. Disease progression is accompanied by language-based semantic loss, which highlights the continuum between sbvFTD and svPPA (and the original description of semantic dementia). Specific neuropsychological tests that investigate knowledge of emotions, social concepts and biographical information for known people are important for capturing early sbvFTD symptoms and should be included in standard evaluations. Accurate identification of patients with sbvFTD will pave the way to better prognostication and therapeutics and will help to advance our understanding of the role of non-verbal semantics in human social behaviour.
Supplementary Material
Acknowledgements
The authors thank the research patients and their families for the time and effort they dedicated to research at UCSF’s Memory and Aging Center. VBM analyses were performed using the Brainsight system, developed at UCSF by Katherine P. Rankin, Cosmo Mielke and Paul Sukhanov and powered by the VLSM script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the UCSF Chancellors Fund for Precision Medicine. We thank Anna Karydas and Jennifer Yokoyama (UCSF Memory and Aging Center genetics experts) for assistance with genetic data, as well as Dr John Trojanowski (Department of Pathology and Laboratory Medicine, University of Pennsylvania. Pennsylvania, PA, USA) for assistance with autopsy data.
Abbreviations
- bvFTD
behavioural variant frontotemporal dementia
- CATS
Comprehensive Affect Testing System
- EET
Emotion Evaluation Test
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- IAS
Interpersonal Adjective Scales
- l/rATL
left/right anterior temporal lobe
- MMSE
Mini-Mental State Examination
- sbvFTD
semantic behavioural variant frontotemporal dementia
- svPPA
semantic variant primary progressive aphasia
- TASIT
The Awareness of Social Inference Test
- TDP-43
transactive response DNA binding protein 43
Contributor Information
Kyan Younes, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA; Department of Neurology and Neurological Sciences, Stanford University, Stanford, CA 94304, USA.
Valentina Borghesani, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Maxime Montembeault, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Salvatore Spina, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Maria Luisa Mandelli, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Ariane E Welch, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Elizabeth Weis, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Patrick Callahan, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Fanny M Elahi, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Alice Y Hua, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
David C Perry, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Anna Karydas, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Daniel Geschwind, Neurogenetics Program, Department of Neurology and Semel Institute for Neuroscience and Human Behavior, David Geffen School of Medicine, University of California, Los Angeles, CA 90024, USA.
Eric Huang, Department of Pathology, University of California, San Francisco, CA 94143, USA.
Lea T Grinberg, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA; Department of Pathology, University of California, San Francisco, CA 94143, USA.
Joel H Kramer, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Adam L Boxer, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Gil D Rabinovici, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Howard J Rosen, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
William W Seeley, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA; Department of Pathology, University of California, San Francisco, CA 94143, USA.
Zachary A Miller, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Bruce L Miller, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Virginia E Sturm, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Katherine P Rankin, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA.
Maria Luisa Gorno-Tempini, Memory and Aging Center, Department of Neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco, CA 94158, USA; Dyslexia Center, University of California, San Francisco, CA 94158, USA.
Funding
This work was funded by the National Institutes of Health (NINDS R01NS050915, NIDCD K24DC015544, NIA P50AG023501, NIA P01AG019724, NIA R01AG038791, NINDS U54NS092089, NIA K08AG052648, NIA R01AG029577, NIA P50AG023501), Alzheimer’s Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer’s Foundation; Koret Family Foundation; Consortium for Frontotemporal Dementia Research and McBean Family Foundation, and The Iqbal Farrukh and Asad Jamal Fund. These supporting sources were not involved in the study design, collection, analysis or interpretation of data, nor were they involved in writing the paper or in the decision to submit this report for publication.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Neary D, Brun A, Englund B, et al. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: Clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4:204–213. [DOI] [PubMed] [Google Scholar]
- 3. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011; 134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borghesani V, Battistella G, Mandelli ML, et al. Regional and hemispheric susceptibility of the temporal lobe to FTLD-TDP type C pathology. NeuroImage Clin. 2020;28:102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of left-versus right-lateralized semantic dementia. Brain. 2016;139:986–998. [DOI] [PubMed] [Google Scholar]
- 8. Brambati SM, Amici S, Racine CA, et al. Longitudinal gray matter contraction in three variants of primary progressive aphasia: A tenser-based morphometry study. NeuroImage Clin. 2015;8:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding J, Chen K, Liu H, et al. A unified neurocognitive model of semantics language social behaviour and face recognition in semantic dementia. Nat Commun. 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snowden JS, Harris JM, Thompson JC, et al. Semantic dementia and the left and right temporal lobes. Cortex. 2018;107:188–203. [DOI] [PubMed] [Google Scholar]
- 11. Hoffman P, Jones RW, Ralph MAL. The degraded concept representation system in semantic dementia: Damage to pan-modal hub, then visual spoke. Brain. 2012;135:3770–3780. [DOI] [PubMed] [Google Scholar]
- 12. Rice GE, Ralph MAL, Hoffman P. The roles of left versus right anterior temporal lobes in conceptual knowledge: An ALE meta-analysis of 97 functional neuroimaging studies. Cereb Cortex. 2015;25:4374–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ralph MAL, Jefferies E, Patterson K. The neural and computational bases of semantic cognition. Nat Publ Gr. 2016;18:42–55. [DOI] [PubMed] [Google Scholar]
- 14. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 15. Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt):1027–1040. [DOI] [PubMed] [Google Scholar]
- 16. Chan D, Anderson V, Pijnenburg Y, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132:1287–1298. [DOI] [PubMed] [Google Scholar]
- 17. Josephs KA, Whitwell JL, Knopman DS, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73(18):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004;40:631–644. [DOI] [PubMed] [Google Scholar]
- 19. Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–2284. [DOI] [PubMed] [Google Scholar]
- 20. Binney RJ, Henry ML, Babiak M, et al. Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex. 2016;82:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henry ML, Wilson SM, Ogar JM, et al. Neuropsychological, behavioral, and anatomical evolution in right temporal variant frontotemporal dementia: A longitudinal and post-mortem single case analysis. Neurocase. 2014;20:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rankin KP, Gorno-Tempini ML, Allison SC, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rankin KP, Kramer JH, Mychack P, Miller B. Differential patterns of empathy loss in frontal and temporal variants of FTLD. J Int Neuropsychol Soc. 2002;8:2. [Google Scholar]
- 24. Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, Miller BL. Hemispheric dominance for emotions, empathy and social behaviour: Evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–160. [DOI] [PubMed] [Google Scholar]
- 25. Rosen HJ, Perry RJ, Murphy J, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125:2286–2295. [DOI] [PubMed] [Google Scholar]
- 26. Rankin KP, Salazar A, Gorno-Tempini ML, et al. Detecting sarcasm from paralinguistic cues: Anatomic and cognitive correlates in neurodegenerative disease. Neuroimage. 2009;47:2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: A systematic review. Neuropsychologia. 2007;45:1591–1607. [DOI] [PubMed] [Google Scholar]
- 28. Irish M, Kumfor F, Hodges JR, Piguet O. A tale of two hemispheres: Contrasting socioemotional dysfunction in right- versus left-lateralised semantic dementia. Dement Neuropsychol. 2013;7:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci. 2001;13:341–356. [DOI] [PubMed] [Google Scholar]
- 30. Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. [DOI] [PubMed] [Google Scholar]
- 31. Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. [DOI] [PubMed] [Google Scholar]
- 32. Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994:372:669–672. [DOI] [PubMed] [Google Scholar]
- 33. Guo CC, Gorno-Tempini ML, Gesierich B, et al. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain. 2013;136:2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 35. Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. [DOI] [PubMed] [Google Scholar]
- 36. Hodges JR, Patterson K. Semantic memory disorders. Trends Cogn Sci. 1997;1:68–72. [DOI] [PubMed] [Google Scholar]
- 37. Brambati SM, Myers D, Wilson A, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–1653. [DOI] [PubMed] [Google Scholar]
- 38. Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML. The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cogn Behav Neurol. 2009;22:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winkielman P, Coulson S, Niedenthal P. Dynamic grounding of emotion concepts. Philos Trans R Soc B Biol Sci. 2018;373:20170127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Binney RJ, Ramsey R. Social semantics: The role of conceptual knowledge and cognitive control in a neurobiological model of the social brain. Neurosci Biobehav Rev. 2020;112:28–38. [DOI] [PubMed] [Google Scholar]
- 41. Snowden JS, Griffiths HL, Neary D. Semantic-episodic memory interactions in semantic dementia: Implications for retrograde memory function. Cogn Neuropsychol. 1996;13:1101–1139. [Google Scholar]
- 42. Luzzi S, Baldinelli S, Ranaldi V, et al. Famous faces and voices: Differential profiles in early right and left semantic dementia and in Alzheimer’s disease. Neuropsychologia. 2017;94:118–128. [DOI] [PubMed] [Google Scholar]
- 43. Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. [DOI] [PubMed] [Google Scholar]
- 44. Piwnica-Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex. 2010;46:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller ZA, Hinkley LB, Herman A, et al. Anomalous functional language lateralization in semantic variant PPA. Neurology. 2015;84:204–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joubert S, Felician O, Barbeau E, et al. The right temporal lobe variant of frontotemporal dementia: Cognitive and neuroanatomical profile of three patients. J Neurol. 2006;253:1447–1458. [DOI] [PubMed] [Google Scholar]
- 47. Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: An examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry. 2015;86:1082–1088. [DOI] [PubMed] [Google Scholar]
- 48. Coon EA, Whitwell JL, Parisi JE, Dickson DW, Josephs KA. Right temporal variant frontotemporal dementia with motor neuron disease. J Clin Neurosci. 2012;19:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. González-Caballero G, Abellán-Miralles I, Sáenz-Sanjuan MJ. Right temporal lobe variant of frontotemporal dementia. J Clin Neurosci. 2015;22:1139–1143. [DOI] [PubMed] [Google Scholar]
- 50. Morris JC. The clinical dementia rating (CDR): Current version and scoring rules [see comments]. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 51. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. [DOI] [PubMed] [Google Scholar]
- 52. Guimet N M, Miller BL, Allegri RF, Rankin KP. What do we mean by behavioral disinhibition in frontotemporal dementia? Front Neurol. 2021;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vonk JMJ, Borghesani V, Battistella G, et al. Verbal semantics and the left dorsolateral anterior temporal lobe: A longitudinal case of bilateral temporal degeneration. Aphasiology. 2019;34:865–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dunn LM, Dunn D. Peabody Picture Vocabulary Test - Revised. American Guidance Service; 1981. [Google Scholar]
- 55. Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: Shortened versions for use in Alzheimer’s disease. J Gerontol. 1992;47:P154–P158. [DOI] [PubMed] [Google Scholar]
- 56. Howard D, Patterson K. Pyramids and 7l palm trees: A test of semantic access form pictures and words. Thames Valley Publishing Company; 1992. [Google Scholar]
- 57. Froming KB, Ekman P, Levy M. Comprehensive Affect Testing System. Published online 2001.
- 58. McDonald S. Exploring the process of inference generation in sarcasm: A review of normal and clinical studies. Brain Lang. 1999;68:486–506. [DOI] [PubMed] [Google Scholar]
- 59. Shany-Ur T, Poorzand P, Grossman SN, et al. Comprehension of insincere communication in neurodegenerative disease: Lies, sarcasm, and theory of mind. Cortex. 2012;48:1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gorno-Tempini ML, Price CJ. Identification of famous faces and buildings. A functional neuroimaging study of semantically unique items. Brain. 2001;124:2087–2097. [DOI] [PubMed] [Google Scholar]
- 61. Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Personal Soc Psychol. 1983;44:113–126. [Google Scholar]
- 62. Lennox RD, Wolfe RN. Revision of the self-monitoring scale. J Pers Soc Psychol. 1984;46:1349–1364. [DOI] [PubMed] [Google Scholar]
- 63. Wiggins JS. Interpersonal adjectives scale: Professional manual. Psychological Assessment Resources Inc.; 1995. [Google Scholar]
- 64. Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 65. Ossenkoppele R, Cohn-Sheehy BI, La Joie R, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36:4421–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. La Joie R, Perrotin A, Barré L, et al. Region-specific hierarchy between atrophy, hypometabolism, and 2-amyloid (aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012;32:16265–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spina S, Brown JA, Deng J, et al. Neuropathological correlates of structural and functional imaging biomarkers in 4-repeat tauopathies. Brain. 2019;142:2068–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. J Alzheimers Dis. 2006;9(3 Suppl):417–423. [DOI] [PubMed] [Google Scholar]
- 69. Mackenzie IRA, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montine TJ, Phelps CH, Beach TG, et al. National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140:3329–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rankin KP. Brain networks supporting social cognition in dementia. Curr Behav Neurosci Reports. 2020;7:203–211. [Google Scholar]
- 73. Yang WFZ, Toller G, Shdo S, et al. Resting functional connectivity in the semantic appraisal network predicts accuracy of emotion identification. NeuroImage Clin. 2021;31:102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Snowden J, Goulding P, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–182. [Google Scholar]
- 75. Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt):1783–1806. [DOI] [PubMed] [Google Scholar]
- 76. Decety J, Jackson PL. A social-neuroscience perspective on empathy. Curr Dir Psychol Sci. 2006;15:54–58. [Google Scholar]
- 77. Shdo SM, Ranasinghe KG, Gola KA, et al. Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia. 2018;116:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zaki J, Ochsner K. The neuroscience of empathy: Progress, pitfalls and promise. Nat Neurosci. 2012;15:675–680. [DOI] [PubMed] [Google Scholar]
- 79. Graham KS, Lee ACH, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: New evidence from three PET studies. Cogn Affect Behav Neurosci. 2003;3:234–254. [DOI] [PubMed] [Google Scholar]
- 80. Hutchings R, Palermo R, Piguet O, Kumfor F. Disrupted face processing in frontotemporal dementia: A review of the clinical and neuroanatomical evidence. Neuropsychol Rev. 2017;27:18–30. [DOI] [PubMed] [Google Scholar]
- 81. Moheb N, Charuworn K, Ashla MM, Desarzant R, Chavez D, Mendez MF. Repetitive behaviors in frontotemporal dementia: Compulsions or impulsions? J Neuropsychiatry Clin Neurosci 31:132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Perry DC, Whitwell JL, Boeve BF, et al. Voxel-based morphometry in patients with obsessive-compulsive behaviors in behavioral variant frontotemporal dementia. Eur J Neurol. 2012;19:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mitchell E, Tavares TP, Palaniyappan L, Finger EC. Hoarding and obsessive–compulsive behaviours in frontotemporal dementia: clinical and neuroanatomic associations. Cortex. 2019;121:443–453. [DOI] [PubMed] [Google Scholar]
- 84. Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36:408–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ecker C, Suckling J, Deoni SC, et al. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: A multicenter magnetic resonance imaging study. Arch Gen Psychiatry. 2012;69:195–209. [DOI] [PubMed] [Google Scholar]
- 86. Zilbovicius M, Boddaert N, Belin P, et al. Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. Am J Psychiatry. 2000;157:1988–1993. [DOI] [PubMed] [Google Scholar]
- 87. Abell F, Krams M, Ashburner J, et al. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. [DOI] [PubMed] [Google Scholar]
- 88. Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. [DOI] [PubMed] [Google Scholar]
- 89. Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136(Pt):3461–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rogalski E, Weintraub S, Mesulam MM. Are there susceptibility factors for primary progressive aphasia? Brain Lang. 2013;127:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McManus C. Half a century of handedness research: Myths, truths; fictions, facts; backwards, but mostly forwards. Brain Neurosci Adv. 2019;3:239821281882051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Erkoyun HU, Groot C, Heilbron R, et al. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain;143:2831–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Joubert S, Felician O, Barbeau E, et al. Progressive prosopagnosia. Neurology. 2004;63:1962–1965. [DOI] [PubMed] [Google Scholar]
- 94. Rozzini L, Bianchetti A, Lussignoli G, Cappa S, Trabucchi M. Surface dyslexia in an Italian patient with semantic dementia. Neurocase. 1997;3:307–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study are available on request. The sensitive nature of patients’ data and the institutional ethics protocols in place at the time these patients gave informed consent do not permit open data sharing. The clinical and neuroimaging data used in the current paper are available from the senior author (M.L.G.T.), on formal request indicating name and affiliation of the researcher as well as a brief description of the intended use for the data. All requests will undergo UCSF-regulated procedure thus requiring submission of a Material Transfer Agreement. No commercial use would be approved.