Abstract
Background
Integrase strand transfer inhibitors (INSTIs) are associated with weight gain in people with HIV (PWH). Less is known about the risk of other metabolic outcomes such as diabetes mellitus and hyperglycemia.
Methods
IBM® MarketScan® databases for commercially and Medicaid-insured adults were used to identify PWH newly initiating antiretroviral therapy (ART). The primary outcome was a composite of new-onset diabetes mellitus/hyperglycemia in the 6 months following ART initiation and was identified using International Classification of Disease, Ninth revision, Clinical Modification (ICD-9-CM) and ICD-10-CM diagnosis and procedure codes and Current Procedural Terminology, 4th Edition (CPT-4) codes. To examine the relationship between INSTI use and the composite outcome, we estimated the risk using Cox proportional hazards models with calendar time-specific standardized mortality ratio weights.
Results
Of 42 382 PWH who initiated ART between 1 July 2007 and 30 June 2018, 22 762 (54%) were treated with INSTI-based regimens. Mean age was 38 years, 74% were male, and 19% were Medicaid insured. PWH on INSTIs were 31% more likely to develop new-onset diabetes mellitus/hyperglycemia (hazard ratio [HR], 1.31; 95% confidence interval [CI], 1.15–1.48]) compared with those who initiated non–INSTI-based regimens. When examined individually, the highest risk was associated with elvitegravir (HR, 1.54; 95% CI, 1.32–1.97; P < .001) and the lowest risk with raltegravir (HR, 1.19; 95% CI, 1.03–1.37; P = .02).
Conclusions
INSTI use was associated with increased risk of new-onset diabetes mellitus/hyperglycemia in the 6 months following ART initiation.
Keywords: HIV, antiretroviral therapy, integrase strand transfer inhibitors, diabetes, hyperglycemia
Integrase strand transfer inhibitor (INSTI)-based regimens are associated with increased risk of new-onset diabetes mellitus/hyperglycemia in the 6 months following antiretroviral therapy initiation when compared with non–INSTI-based regimens
The introduction of antiretroviral therapy (ART) has revolutionized HIV care. People with HIV (PWH) treated with ART are surviving longer with life expectancies approaching that of the general population [1]. Despite improvements in virologic outcomes, ART has been associated with numerous metabolic toxicities, including disorders of adipose tissue distribution and/or volume, dyslipidemia, hyperglycemia, and multiorgan insulin resistance. These side effects were classically associated with use of older antiretroviral agents, such as thymidine analogues and protease inhibitors (PIs). Emerging literature suggests that use of integrase strand transfer inhibitor (INSTI), which have become the mainstay of treatment, may be associated with metabolic toxicities including greater initial increases in body weight, greater ongoing weight gain, and potentially worsening glycemic control compared with other modern ART regimens, in particular those that are non-nucleoside reverse transcriptase inhibitor (NNRTI)- based.
Several recent studies have associated the use of INSTIs with excessive weight gain in both the ART-naïve and ART-experienced settings [2–6]. However, studies examining the impact of INSTIs on glycemic control are more limited. Data from the North American AIDS Cohort Collaboration on Research and Design found that in treatment-naïve PWH who started ART, the overall incidence of diabetes mellitus was almost 2-fold that of the general population, at 10.7 cases per 1000 persons/year over a decade. In addition, participants who initiated INSTI-based ART had a 17% greater risk of incident diabetes compared with those starting NNRTI-based ART. This risk was greatest in those taking raltegravir-containing ART (40%). However, the risk of incident diabetes appeared to be attenuated after accounting for 12-month weight gain [7]. In contrast, other cohort studies, including the Women’s Interagency HIV Study (WIHS), did not observe significant increases in the incidence of diabetes mellitus associated with use of INSTI-based ART [8–10]; but, the WIHS did observe significant increases in hemoglobin A1c (HbA1c) associated with use of INSTI-based ART when compared with non–INSTI-based ART, suggesting a possible impact of INSTIs on glycemic control.
Notably, several case reports have described new-onset diabetes or acute worsening of well-controlled diabetes in PWH switching to regimens containing INSTIs [11–13]. In all of these reports, diabetic ketoacidosis occurred within weeks to months following initiation of INSTI-based ART. Here, we examined the risk of new-onset diabetes mellitus/hyperglycemia in PWH in the first 6 months following the initiation of INSTI-based regimens using large administrative databases containing claims data for commercially and Medicaid-insured populations.
METHODS
Data Source
We used the IBM MarketScan Commercial Database (2007–2019) and the Medicaid Multi-State Database (2011–2019) (IBM Watson Health, Ann Arbor, MI). These databases contain individual-level healthcare data on health insurance enrollment, inpatient and outpatient diagnoses and procedures, and outpatient pharmacy-dispensed medications for commercially insured persons from participating employers and health plans and individuals covered by Medicaid from US states submitting data. This study was considered exempt from human subjects review by the institutional review board at Washington University in St. Louis.
Study Design and Population
We identified adults who initiated ART from 1 July 2007 through 30 June 2019. We defined new initiators of ART as persons who had at least 6 months of continuous insurance enrollment before initiation of ART. Adults were included if they received a complete 2- or 3-drug ART regimen. Complete 3-drug regimens included one of the following: (1) 2 NRTIs plus a drug from a different class such as INSTI, PI, NNRTI, entry inhibitors, fusion inhibitors; or (2) any combination of 3 drugs from separate drug classes (NRTI, NNRTI, PI, INSTI, entry inhibitors, or fusion inhibitors). Complete 2-drug regimens included one of the following combinations: (1) INSTI (dolutegravir) plus NNRTI (rilpivirine) or NRTI (lamivudine) or (2) PI plus INSTI or NNRTI. INSTIs included raltegravir, elvitegravir, dolutegravir, or bictegravir. We considered all other regimens to be non–INSTI-based. All persons included in the analysis were required to have at least 6 months of follow-up after ART initiation. We excluded persons coded for diabetes mellitus at any time before ART initiation because they had preexisting diabetes.
Outcomes and Covariates
All outcomes and covariates were defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM diagnosis and procedure codes and Current Procedural Terminology, 4th Edition (CPT-4) codes (Supplementary Tables 1 and 2), as appropriate.
The outcome was a composite of new-onset diabetes mellitus/hyperglycemia coded in the 6-month period following ART initiation. Diabetes mellitus was identified using ICD-9-CM diagnosis codes 249.00–250.93 and ICD-10-CM codes E08.00–E13.9 and hyperglycemia was identified using ICD-9-CM diagnosis codes 790.21–790.29 and ICD-10-CM codes R73.01–R73.09 on nondiagnostic/laboratory claims (to avoid identification based on rule-out diagnoses).
We considered a wide range of covariates defined during the baseline 6-month period before ART initiation for inclusion in the propensity score model. These included age, sex, geographical region, Medicaid status, standard comorbidities using the Elixhauser algorithm [14, 15], as well as other important comorbidities such as polycystic ovarian syndrome, pancreatitis, malignancy of the pancreas, cystic fibrosis, Cushing syndrome, malnutrition, hepatitis B and C, and heart disease defined using the Framingham definition [16]. Pregnancy and gestational diabetes in the preceding 6 months were also considered as covariates. Codes used to define covariates are presented in Supplementary Table 1.
Statistical Analysis
To examine the relationship between INSTI use and diabetes/hyperglycemia, we used propensity score-weighted Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI). To address potential confounding resulting from differences in observed covariates between INSTI and non-INSTI recipients, we used multivariable logistic regression models to estimate calendar time-specific propensity scores representing the likelihood of initiating an INSTI-based regimen [17]. The calendar time-specific propensity score models (fit separately for 6 distinct 2-year time segments [beginning 1 July 2007]) were implemented to account for possible channeling bias from changes in use of INSTI-based regimens over time [17, 18]. Channeling refers to selective prescription of a particular medication based on underlying patient characteristics [19]. Then, we attempted to balance the distribution of these potential confounders between our comparison cohorts by assigning standardized mortality ratio (SMR) weights of ps/(1–ps) to INSTI-based regimen recipients. SMR weights standardized users of each type of INSTI to the non-INSTI group. This accounted for differences in INSTI prescribing as users of the individual INSTIs were weighted to be similar to a common standard (non-INSTI). The propensity score models included potential predictors of diabetes or hyperglycemia, including age, sex, and underlying comorbidities, as described previously. Age was modeled using restricted cubic splines. Standardized mean differences were used to assess balance between the INSTI and comparator groups, with absolute differences of 0.1 or less considered evidence of acceptable balance [20].
To examine the impact of individual INSTIs, we reperformed the analysis including the 4 INSTI drugs in the same model versus non–INSTI-based regimens.
Sensitivity Analyses
We conducted a sensitivity analysis to assess the impact of excluding events that occurred in the first 2 weeks after initiation of treatment that could potentially be attributed to preexisting condition(s). We selected a 2-week cutoff because cases of diabetic ketoacidosis occurring 3 weeks after INSTI initiation have been reported [12, 13]. We also conducted a sensitivity analysis in which a binary variable indicating the use of tenofovir alafenamide (TAF) in the initial 6-month ART regimen was included in the models. This was performed because TAF has been associated with metabolic abnormalities [21]. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
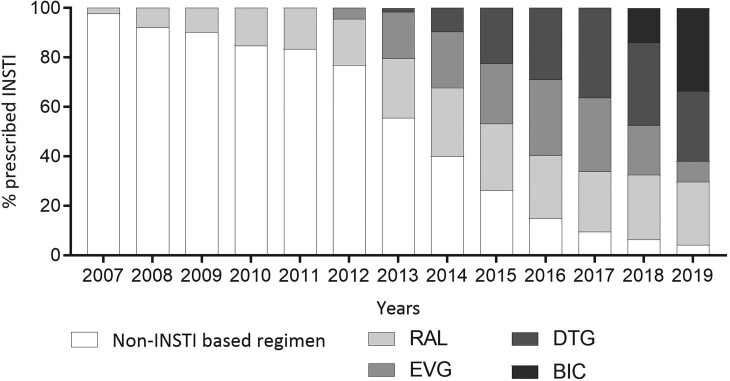
We identified 42 382 individuals who initiated ART between 1 July 2007 and 30 June 2019. The mean age at ART initiation was 38 years (standard deviation, 11.6 years), 31 185 (74%) were male and 7886 (19%) were Medicaid insured. A total of 22 762 (53.7%) persons commenced INSTI-based regimens, with 8999 (39.5%) initiating raltegravir, 6402 (28.1%) elvitegravir, 6071 (26.7%) dolutegravir, and 1290 (5.7%) bictegravir. The proportion of individuals who initiated each specific INSTI changed over time (Figure 1). Of those commencing non–INSTI-based regimens, 11 839 (60.3%) initiated NNRTI-based regimens and 7819 (39.9%) commencing PI-based regimens.
Figure 1.
Proportion of new ART users initiated on INSTIs for July 2007 to July 2019. ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor.
Table 1 presents the proportion of those coded for polycystic ovarian syndrome, pancreatitis, malignancy of the pancreas, cystic fibrosis, Cushing syndrome, malnutrition, hepatitis B and C, cardiovascular disease, hypertension, obesity, pregnancy, and gestational diabetes, before applying the SMR weights. The distribution of observed covariates was well-balanced after SMR weighting, except for Medicaid status (standardized mean difference, 0.17; Supplementary Table 3). In the 6 months before treatment initiation, testing for diabetes (glucose and HBA1c laboratory testing combined) was performed in 64%, 63%, and 67% of non-INSTI users and 71%, 66%, and 67% of INSTI users in the 2007–2009, 2013–2015, and 2017–2019 periods, respectively, indicating that testing volumes remain relatively stable throughout the study period (data for all calendar periods is in Supplementary Table 4). Glucose testing accounted for the majority of testing; however, over time the proportion testing using HBA1c increased, with 4%, 8%, and 14% of non-INSTI users tested and 8%, 9%, and 15% of tests of INSTI users tested in the 2007–2009, 2013–2015, and 2017–2019 periods, respectively. This increased use of HBA1c testing likely followed the addition of HBA1c as a diabetes testing method in the 2010 American Diabetes Association guidelines.
Table 1.
Baseline Study Characteristics
| Characteristics | Non-INSTI-Based Regimens n = 19 620 |
INSTI-Based Regimens n = 22 762 |
P |
|---|---|---|---|
| Age, mean (SD), y | 39.6 (11.1) | 37.1 (11.9) | <.001 |
| Males | 14 576 (74.3%) | 16 609 (73.0%) | .002 |
| Medicaid insured | 3254 (16.6%) | 4632 (20.4%) | <.001 |
| Polycystic ovarian syndrome | 23 (0.12%) | 50 (0.22%) | .011 |
| Pancreatitis | 91 (0.46%) | 110 (0.48%) | .78 |
| Pancreatic malignancy | 7 (0.04%) | 5 (0.02%) | .40 |
| Cystic fibrosis | 2 (0.01%) | 1 (0.00%) | .48 |
| Cushing syndrome | 1 (0.01%) | 0 (0.00%) | .28 |
| Hepatitis B infection | 353 (1.8%) | 369 (1.62%) | .16 |
| Hepatitis C infection | 608 (3.1%) | 652 (2.86%) | .16 |
| Pregnancy | 468 (2.39%) | 294 (1.29%) | <.001 |
| Gestational diabetes | 15 (0.08%) | 2 (0.01%) | .001 |
| Malnutrition | 432 (2.2%) | 554 (2.43%) | .11 |
| Cardiovascular disease | 704 (3.59%) | 793 (3.48%) | .34 |
| Congestive heart failure | 95 (0.48%) | 118 (0.52%) | .25 |
| Hypertension | 3552 (18.10%) | 3965 (17.42) | .07 |
| Obesity | 438 (2.23%) | 1067 (4.69%) | <.001 |
INSTI, integrase strand transfer inhibitor; SD, standard deviation.
When we examined the frequency of diabetes testing in the 6 months after ART initiation for each calendar period, our findings were similar to the pre-ART period. Overall diabetes testing was performed in 63%, 69%, and 68% of non-INSTI users and 72%, 64%, and 68% of INSTI users in the 2007–2009, 2013–2015, and 2017–2019 periods, respectively. The proportions tested with HBA1c were also similar to the pre-ART period.
In the 6-month period post-ART initiation, the composite endpoint of new-onset diabetes/hyperglycemia occurred in 2.1% of (882/42 382) people; 2.3% (521/22 762) in the INSTI group; and 1.8% (361/19 620) in the non-INSTI group. Supplementary Figure 1 illustrates the timing of events in each group. There was no difference between the groups in the first 2-week period; thereafter, the rate of events was constant with higher rates in the INSTI group.
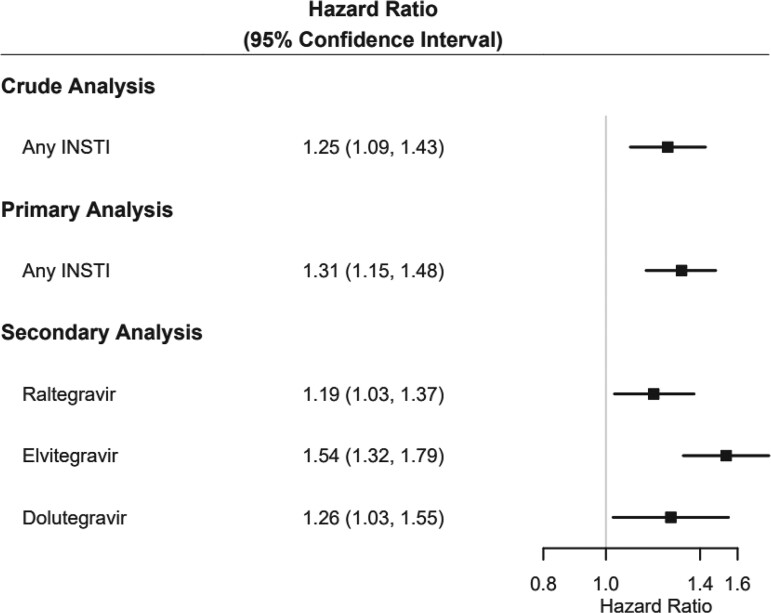
The crude and weighted HRs for the composite outcome of diabetes/hyperglycemia are presented in Figure 2. In the primary analysis model comparing INSTI with non–INSTI-based regimen users, there was a 31% increased risk of new-onset diabetes/hyperglycemia associated with initiation of INSTI-based therapy compared with non–INSTI-based therapy (HR, 1.31; 95% CI, 1.15–1.48; P < .001). After restricting the follow-up period to begin 15 days after ART initiation there was no change in the results of the primary analysis (HR, 1.31; 95% CI, 1.15–1.49; P < .001).
Figure 2.
Hazard ratio estimates of new-onset diabetes/hyperglycemia in adults initiated on ART from July 2007 to June 2019. ART, antiretroviral therapy.
To examine the impact of individual INSTIs, we modeled the exposure based on initiation of individual INSTI. Compared with those who initiated non–INSTI-based regimens, we observed increased risk of new-onset diabetes/hyperglycemia associated with dolutegravir (HR, 1.26; 95% CI, 1.03–1.55; P = .027), elvitegravir (HR, 1.54; 95% CI, 1.32–1.79; P < .001) and to a lesser extent, raltegravir (HR, 1.19; 95% CI, 1.03–1.37; P = .018). Bictegravir was not associated with increased risk (HR, 1.45; 95% CI, .84–2.51; P = .182); however, the number of people receiving bictegravir was small.
When the individual drugs were examined after restricting the follow-up period to begin 15 days after initiation of treatment, dolutegravir (HR, 1.35; 95% CI, 1.1–1.67; P = .006) and elvitegravir (HR, 1.57; 95% CI, 1.33–1.84; P < .001) were associated with increased risk of new-onset diabetes/hyperglycemia, whereas raltegravir, which had the weakest association in the primary model, was not (HR, 1.15; 95% CI, .99–1.33; P = .07).
We performed a sensitivity analysis to examine the risk of new-onset diabetes/hyperglycemia when TAF was added as a covariate to the models. In the analysis comparing the groups receiving INSTI versus non–INSTI-based ART, although the result was marginal, use of TAF was not associated with an increased risk of the composite outcome (HR, 1.28; 95% CI, .99–1.64; P = .06), adjusting for INSTI use (HR, 1.29; 95% CI, 1.14–1.41). Similarly, use of TAF with individual INSTI’s was not associated with the primary outcome (HR, 1.18; 95% CI, .89–1.58; P = .26). The risk associated with the individual INSTIs remained the same as the model without TAF (raltegravir HR, 1.19; 95% CI, 1.03–1.37; P = .016; dolutegravir HR, 1.25; 95% CI. 1.02–1.54; P = .031; elvitegravir HR, 1.51; 95% CI, 1.29–1.77; P < .001).
DISCUSSION/CONCLUSIONS
We performed a large comparative study to evaluate the effect of ART initiation with an INSTI- versus non–INSTI-based ART regimens on the risk of incident diabetes/hyperglycemia among non-elderly PWH in the United States. Overall, we observed a higher risk of new-onset diabetes/hyperglycemia after accounting for demographic and clinical characteristics using weights derived from a calendar time-specific propensity score model. When individual INSTIs were included in the model, elvitegravir was associated with the greatest risk of new-onset diabetes/hyperglycemia, followed by dolutegravir and then raltegravir.
ART prescribing patterns can change rapidly when newer ART agents are Food and Drug Administration (FDA) approved, usually driven by promising data from registration trials related to improved tolerability, safety and/or convenient dosing parameters. The rapid adoption of new ART agents is apparent in this study from 2008 to 2019. For example, dolutegravir was FDA approved for the treatment of HIV in 2013, and by 2014 was included in 10% of all regimens initiated and over one-third of all regimens by 2017. These drug utilization patterns underscore the importance of accounting for calendar time when performing longitudinal analyses examining outcomes related to ART, as we did in this study. In addition, as we previously reported [22], newer agents perceived as having fewer side effects can be preferentially selected for treatment of those with increased comorbidities soon after becoming available, emphasizing the importance of using study design and analytic methods to account for differences in underlying conditions between treatment groups.
In this study, we estimated the risk of new-onset diabetes/hyperglycemia in the first 6 months after ART initiation. We chose this timeframe specifically to capture early changes in glycemic control in light of a number of reports of diabetic ketoacidosis soon after switch to INSTIs [11–13]. Because our analysis focused on the first 6 months after INSTI initiation, we selected a composite endpoint of hyperglycemia and/or new-onset diabetes mellitus because HIV clinic follow-up visits typically occur every 3–6 months, we included hyperglycemia in the composite outcome to capture patients with incident dysglycemia who did not have a complete diagnostic workup within that period. Conversely, whereas in the previously mentioned analysis of the North American AIDS Cohort Collaboration on Research and Design cohort [7], there was an attenuation of the total effect of INSTIs initiation on incident diabetes after accounting for potential mediation of weight gain at 12 months (attenuation of an absolute amount of 5%) there was still a 3% increased risk of diabetes not accounted for by weight gain. Moreover, in the WIHS study, which showed a greater increase in HbA1c in patients on INSTIs, there was also a lower incidence of insulin resistance among this group compared with the non-INSTI group [8]. These findings, along with the higher incidence of hyperglycemia and/or early-onset diabetes shown in this study suggests a possible distinct underlying mechanism(s) in addition to the weight gain as potential contributors to the development of diabetes associated with INSTI initiation.
Notably, in our study, the proportion assessed for diabetes via glucose and HBA1c testing remained relatively stable throughout the study period.
This study has limitations. First, the results of this nonrandomized study are subject to potential confounding. For example, even though the presence of obesity was well-balanced between exposure groups, it is possible that time-varying confounding by weight gain may account for the observed increase in risk of new-onset diabetes/hyperglycemia among INSTI-regimen users. Unfortunately, because these administrative data were collected for the purpose of insurance reimbursement, certain clinically relevant variables such as weight are not available in the database, whereas others such as obesity may be undercoded, as has been previously demonstrated [23, 24]; but because the duration of follow-up was restricted to 6 months after initiation, we expect the bias from weight gain to be small. However, residual confounding from unmeasured or poorly measured variables remains a possibility. Similarly, other important demographic variables such as race and ethnicity are not available. Second, bictegravir, one of the most widely used INSTIs currently, is underrepresented in our data because this drug was only recently FDA approved in 2018. In our study, bictegravir accounted for approximately 5% of INSTI use overall, and this use was restricted to the final 2 years of the study period. Given its structural similarities to dolutegravir, it is possible that similar effects will be observed with use of bictegravir, but further analyses will be required. Last, we used administrative data for commercially and Medicaid-insured adult PWH; thus, results may not be generalizable to all PWH.
Understanding the degree to which newer ART medications are associated with metabolic toxicities remains a challenge. Here, we demonstrate increased short-term risk of diabetes mellitus/hyperglycemia after initiation of an INSTI-containing ART regimen. Use of tenofovir alafenamide was not associated with increased risk of new-onset diabetes/hyperglycemia in our study. These results add to a growing body of evidence to suggest that INSTIs may impact glycemic control. INSTI-based regimens are well tolerated on a daily basis by PWH, which helps to optimize adherence to ART and achieve viral suppression, a major goal in HIV treatment. However, providers caring for PWH on these treatments should remain vigilant for unwanted toxicities particularly among subgroups of PWH known to be at increased risk of metabolic toxicities.
Supplementary Material
Contributor Information
Jane A O’Halloran, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA.
John Sahrmann, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA.
Luis Parra-Rodriguez, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA.
Daniel T Vo, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA.
Anne M Butler, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA; Department of Surgery, Washington University School of Medicine, St. Louis, Missouri, USA.
Margaret A Olsen, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA; Department of Surgery, Washington University School of Medicine, St. Louis, Missouri, USA.
William G Powderly, Department of Medicine, Washington University School of Medicine, St. Louis, Missouri, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support . Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences of the National Institutes of Health. A. M. B. received salary support from the National Center for Advancing Translational Sciences (NCATS), National Institute of Health (NIH) under award number KL2 TR002346.
References
- 1. May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4 + cell count and viral load response to antiretroviral therapy. AIDS 2014; 28(8):1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71(6):1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70(7):1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381(9):803–15. [DOI] [PubMed] [Google Scholar]
- 5. Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76(5):527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lake JE, Wu K, Bares SH, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020; 71(9):e471-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with HIV in the US and Canada. Clin Infect Dis 2020; 73(7):e2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Summers NA, Lahiri CD, Angert CD, et al. Metabolic changes associated with the use of integrase strand transfer inhibitors among virally controlled women. J Acquir Immune Defic Syndr 2020; 85(3):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu R, Brunet L, Fusco JS, et al. Incident type 2 diabetes mellitus after initiation of common HIV antiretroviral drugs. AIDS 2021; 35(1):81–90. [DOI] [PubMed] [Google Scholar]
- 10. Ursenbach A, Max V, Maurel M, et al. Incidence of diabetes in HIV-infected patients treated with first-line integrase strand transfer inhibitors: a French multicentre retrospective study. J Antimicrob Chemother 2020; 75(11):3344–8. [DOI] [PubMed] [Google Scholar]
- 11. Fong PS, Flynn DM, Evans CD, et al. Integrase strand transfer inhibitor-associated diabetes mellitus: a case report. Int J STD AIDS 2017; 28(6):626–8. [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin M, Walsh S, Galvin S. Dolutegravir-induced hyperglycaemia in a patient living with HIV. J Antimicrob Chemother 2018; 73(1):258–60. [DOI] [PubMed] [Google Scholar]
- 13. Nolan NS, Adamson S, Reeds D, O'Halloran JA. Bictegravir-based antiretroviral therapy-associated accelerated hyperglycemia and diabetes mellitus. Open Forum Infect Dis 2021; 8(5):ofab077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 15. HCUP Databases . Healthcare Cost and Utilization Project (HCUP). 2006–2009. Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/databases.jsp. Accessed December 19, 2021. [PubMed]
- 16. Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history. A life course analysis of the original Framingham Heart Study cohort. Eur Heart J 2002; 23(6):458–66. [DOI] [PubMed] [Google Scholar]
- 17. Mack CD, Glynn RJ, Brookhart MA, et al. Calendar time-specific propensity scores and comparative effectiveness research for stage III colon cancer chemotherapy. Pharmacoepidemiol Drug Saf 2013; 22(8):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes MD, Williams PL. Challenges in using observational studies to evaluate adverse effects of treatment. N Engl J Med 2007; 356(17):1705–7. [DOI] [PubMed] [Google Scholar]
- 19. Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med 1991; 10(4):577–81. [DOI] [PubMed] [Google Scholar]
- 20. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28(25):3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallon PW, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc 2021; 24(4):e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Halloran JA, Sahrmann J, Butler AM, Olsen MA, Powderly WG. Brief report: integrase strand transfer inhibitors are associated with lower risk of incident cardiovascular disease in people living with HIV. J Acquir Immune Defic Syndr 2020; 84(4):396–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suissa K, Schneeweiss S, Lin KJ, Brill G, Kim SC, Patorno E. Validation of obesity-related diagnosis codes in claims data. Diabetes Obes Metab 2021; 23(12):2623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nickel KB, Wallace AE, Warren DK, et al. Modification of claims-based measures improves identification of comorbidities in non-elderly women undergoing mastectomy for breast cancer: a retrospective cohort study. BMC Health Serv Res 2016; 16(a):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.