Abstract
Background
Whether human immunodeficiency virus (HIV) infection is associated with the development of nonalcoholic steatohepatitis (NASH) remains unclear. The FibroScan–aspartate aminotransferase (FAST) score was developed to identify patients who have histologic NASH with high nonalcoholic fatty liver disease activity score (NAS ≥4) and significant liver fibrosis (≥F2), which has been associated with higher risk of end-stage liver disease. We examined whether HIV infection is associated with elevated FAST score in a large United States (US) cohort.
Methods
Vibration-controlled transient elastography was performed in 1309 women without history of chronic viral hepatitis enrolled from 10 US sites: 928 women with HIV (WWH) and 381 women without HIV (WWOH). We used multivariable logistic regression to evaluate associations of HIV, demographic, lifestyle, and metabolic factors with an elevated (>0.35) FAST score.
Results
Median age of WWH and WWOH was 51 years and 48 years, respectively. Most (90%) WWH were on antiretroviral therapy and 72% had undetectable HIV RNA. Prevalence of elevated FAST score was higher among WWH compared to WWOH (6.3% vs 1.8%, respectively; P = .001). On multivariable analysis, HIV infection was associated with 3.7-fold higher odds of elevated FAST score (P = .002), and greater waist circumference (per 10 cm) was associated with 1.7-fold higher odds (P < .001). In analysis limited to WWH, undetectable HIV RNA and current protease inhibitor use were independently associated with lower odds of elevated FAST score.
Conclusions
Our findings suggest that HIV is an independent risk factor for NASH with significant activity and fibrosis. Studies validating FAST score in persons with HIV are warranted.
Keywords: human immunodeficiency virus, liver steatosis, nonalcoholic steatohepatitis, VCTE, FAST score
Whether HIV infection increases the risk of nonalcoholic steatohepatitis (NASH) remains unclear. We evaluated the association of HIV with elevated FibroScan-AST (FAST) score, a noninvasive surrogate for NASH with high nonalcoholic fatty liver disease activity score and significant liver fibrosis.
Nonalcoholic fatty liver disease (NAFLD) is a worldwide epidemic with an estimated global prevalence of 25% [1]. NAFLD encompasses a spectrum from simple steatosis to nonalcoholic steatohepatitis (NASH), which is characterized histologically by steatosis with inflammation and hepatocyte ballooning, with or without fibrosis [2]. Approximately 20% of individuals with NAFLD have NASH, which can progress to cirrhosis, hepatocellular carcinoma, and end-stage liver disease [3]. NAFLD is common among persons with human immunodeficiency virus (PWH) [4]. Moreover, nonviral liver diseases, including NASH, have surpassed viral hepatitis–associated liver disease as the leading indications for liver transplant among PWH [5]. HIV has numerous inflammatory effects on the liver and accelerates the natural history of chronic hepatitis C virus (HCV) and hepatitis B virus infections [6–11]. Evidence suggests that human immunodeficiency virus (HIV) infection may be similarly associated with the more severe form of NAFLD. For example, a meta-analysis including studies of PWH without viral hepatitis coinfection and with abnormal ultrasound or elevated liver enzymes found a high prevalence of NASH (42%) and significant fibrosis (22%) on liver biopsy [12]. However, it remains unclear whether HIV infection is independently associated with NASH because prospective histology studies have lacked HIV-seronegative controls.
Although liver biopsy is the gold standard for diagnosing NASH and fibrosis, in clinical practice it is infeasible to perform liver biopsies in everyone with NAFLD. Thus, identifying which patients with NAFLD are at risk for progressive disease remains an important clinical challenge. The FibroScan-AST (FAST) score was developed to noninvasively identify patients who have histologic NASH with elevated NAFLD activity score (NAS ≥4) and significant liver fibrosis (≥F2) and has been validated in multiple large global NAFLD cohorts that excluded persons with HIV [13]. The FAST score incorporates the 2 measurements obtained by vibration-controlled transient elastography (VCTE), liver stiffness (LS), and controlled attenuation parameter (CAP), combined with aspartate aminotransferase (AST). In comparison to other noninvasive measures including the Fibrosis-4 Index for Liver Fibrosis (FIB-4) and the NAFLD fibrosis sore, the FAST score performed significantly better at identifying patients without evidence of HIV infection who had NASH + NAS ≥4 + ≥F2 fibrosis. These histologic criteria were selected because the subgroup of patients with these findings are at the highest risk of adverse liver outcomes [13]. We aimed to determine the association of HIV infection with an elevated FAST score in a large United States (US) cohort of women living with or without HIV infection.
METHODS
Study Population and Design
The Women’s Interagency HIV Study (WIHS, now part of the Multicenter AIDS Cohort Study/WIHS Combined Cohort Study [14]) was a multicenter prospective cohort study established in 1994 to investigate the course of HIV and associated conditions among women living with and without HIV. A total of 4982 women (3678 with HIV and 1304 without HIV) were enrolled from 10 study sites in the US during 4 recruitment waves [15]. Starting in 2013, women aged 35 years and older from the Atlanta, Birmingham/Jackson, Bronx, Brooklyn, Chapel Hill, Chicago, Miami, San Francisco, and Washington, DC sites were enrolled into the Liver Disease and Reproductive Aging (LIVRA) ancillary study. Women with positive hepatitis B surface antigen, hemochromatosis, autoimmune hepatitis, or primary biliary cholangitis were excluded from LIVRA, as were women who reported using medications associated with steatosis (ie, systemic corticosteroids, amiodarone, methotrexate), signs of decompensated cirrhosis, current cancer, or severe renal insufficiency. Women who were pregnant or had an implantable cardiac device were excluded per the VCTE manufacturer (Fibroscan; Echosens, Paris, France).
The present study was conducted within the LIVRA ancillary study. Women were excluded if they had HCV viremia, a history of treated HCV, or were receiving anti-HCV therapy. Women with HCV antibody positivity and undetected HCV RNA at or prior to the VCTE visit with no report of receiving HCV treatment were considered to have spontaneously cleared their HCV and were not excluded. The study was approved by the institutional review boards of all participating sites, and all participants signed informed consent.
Assessment of Hepatic Steatosis and Fibrosis
All LIVRA participants underwent VCTE from December 2013 through December 2018 to assess for hepatic steatosis and fibrosis. Steatosis was estimated in decibels per meter (dB/m) using the VCTE-CAP software, and fibrosis was estimated using LS measurements in kilopascals (kPa). Participants were instructed to fast for at least 3 hours prior to VCTE. Operators were instructed to manually switch from the M probe to the XL probe if images suggested subcutaneous fat interference in the measurement range of the probe, that is, >2.5 cm distance from skin to liver capsule [16]. All examinations had at least 10 successful measurements.
Outcome
The FAST score was calculated using the CAP and LS values and AST level from the same visit as VCTE or, if not available, the visit 6 months prior using the following equation [13]:
FAST scores were categorized as >0.35 and ≥0.67, which are the cutoffs proposed to rule out or rule in NASH with significant fibrosis and elevated NAS, respectively. Specifically, FAST score >0.35 has a sensitivity of 90% and specificity of 50% and FAST score ≥0.67 has a sensitivity of 50% and specificity of 90% in identifying NASH with ≥F2 fibrosis and NAS ≥4 [13].
Covariates
The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using 8-hour fasting insulin and glucose values. HIV infection was defined by documentation of a reactive HIV enzyme immunoassay and a secondary confirmatory test. HCV serostatus was determined by documentation of reactive serum HCV antibody using a commercial second- or third-generation enzyme immunoassay, and HCV RNA was performed using either the COBAS Amplicor Monitor 2.0 or the COBAS TaqMan assay, as previously described (both from Roche Diagnostics, Branchburg, New Jersey) [17]. Undetectable HIV viral load was defined as HIV RNA below the lower limit of quantification (ie <20 copies/mL). Waist circumference was measured in centimeters, and body mass index (BMI) was calculated as kg/m2. Self-reported alcohol consumption was categorized as none; light (>0–7 drinks/week); moderate (>7–12 drinks/week); or heavy (>12 drinks/week). Additional covariates were obtained through self-report, including race/ethnicity, smoking history, current marijuana use, and history of injection drug use.
Statistical Analysis
We compared participant characteristics using t test or Kruskal-Wallis tests for continuous variables and χ2 or Fisher exact tests for categorical variables. To determine the factors associated with FAST score >0.35 and FAST score ≥0.67, we used unadjusted and multivariable adjusted logistic regression models. Candidate covariates for the multivariable models were selected based on associations that were significant at the .10 level with the outcome on univariate analysis and a priori selection (eg, alcohol use), and backward stepwise selection was used for the final multivariable model, which included only a priori and the covariates significant at P < .05. The primary analysis was performed in the entire cohort with HIV serostatus as the primary predictor of interest. The final multivariable logistic regression model was also conducted with HIV serostatus further categorized into HIV seropositive with undetectable viral load or HIV seropositive with detected viral load. Secondary analyses were limited to women with HIV (WWH). Sensitivity analyses were conducted excluding women who self-reported heavy alcohol intake. Next, we evaluated the association of HIV with the components of the FAST score (CAP, LS, or AST, all natural log [ln] transformed to approximate normal distribution) using unadjusted and multivariable linear regression models. The β-coefficients in these models were exponentiated to calculate a percentage difference in the outcome. In models with missing HOMA-IR (17% of study participants), we used multiple imputation with the Markov chain Monte Carlo method with 20 repetitions [18, 19]. The variables used to create the imputation model included all the candidate covariates for multivariable models. Multiple imputation estimates of model parameters were computed by averaging the estimates from imputed models, and the variance and confidence interval (CI) of these estimates were computed using the Rubin combining formula [20]. Finally, to better understand the contribution of each component to the association of HIV with FAST score, we performed separate path analysis [21, 22] for each component of the FAST score (in form of CAP3, ln[LS], and 1/AST as they appeared in the formula for FAST score), where the contribution was measured as the percentage of mediation effect of each component over the total effect of HIV on the FAST score. Analyses were performed using the SAS system, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Study Population
Among the 1576 WIHS women enrolled in LIVRA, 267 were excluded from the current analyses: 136 due to history of HCV RNA positivity, 98 due to cleared HCV with treatment, and 33 due to missing components of the FAST score. In total, 1309 women were included: 928 WWH and 381 women without HIV (WWOH). WWH were older than the WWOH (median age, 51 years [interquartile range {IQR}, 44–54 years] vs 48 years [IQR, 41–54 years]) (Table 1). The majority of participants were black, and race/ethnicity did not differ by serostatus. WWOH reported more alcohol, smoking, and marijuana use and were more likely to have ever used injection drugs than WWH (all P < .05). Median BMI and waist circumference were similar regardless of HIV serostatus, but WWH had higher median HOMA-IR compared to WWOH (2.1 vs 1.8; P = .047).
Table 1.
Characteristics of the Study Population by Human Immunodeficiency Virus Serostatus
| Characteristic | WWH (n = 928) |
WWOH (n = 381) |
P Value |
|---|---|---|---|
| Sociodemographic | |||
| Age, y, median (IQR) | 51 (44–54) | 48 (41–54) | .01 |
| Race/ethnicity | .18 | ||
| Black | 74% | 75% | |
| White | 10% | 7% | |
| Hispanic | 12% | 13% | |
| Other | 4% | 5% | |
| Lifestyle | |||
| Alcohol use | <.001 | ||
| None | 52% | 43% | |
| Light | 39% | 41% | |
| Moderate | 3% | 4% | |
| Heavy | 6% | 12% | |
| Current smoking | 36% | 43% | .03 |
| Current marijuana use | 20% | 26% | .02 |
| Ever injection drug use | 2.6% | 5.0% | .03 |
| Metabolic | |||
| BMI, kg/m2, median (IQR) | 30 (26–36) | 32 (27–37) | .15 |
| Waist circumference, cm, median (IQR) | 99 (89–111) | 100 (88–113) | .81 |
| HOMA-IR, median (IQR) | 2.1 (1.3–4.0) | 1.8 (1.1–3.3) | .047 |
| Diabetes mellitus | 19% | 21% | .57 |
| On HTN medication | 47% | 47% | .87 |
| On antidepressant medication | 27% | 17% | <.001 |
| Liver-related | |||
| AST, U/L, median (IQR) | 19 (16–24) | 17 (14–20) | <.001 |
| ALT, U/L, median (IQR) | 15 (12–21) | 14 (11–18) | <.001 |
| CAP, dB/m, median (IQR) | 247 (209–290) | 249 (206–283) | .68 |
| Liver stiffness, kPa, median (IQR) | 5.1 (3.9–6.7) | 4.9 (3.9–6.2) | .21 |
| HCV Ab positivea | 8% | 4% | .01 |
| HIV-related | |||
| Undetectable HIV RNA | 72% | … | |
| CD4 current, cells/µL, median (IQR) | 650 (435–869) | … | |
| CD4 nadir, cells/µL, median (IQR) | 223 (103–360) | … | |
| History of clinical AIDS | 30% | … | |
| Current ARV use | 90% | … | |
| NNRTI | 30% | … | |
| PI | 26% | … | |
| INSTI | 48% | … | |
Data are presented as percentage unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; ARV, antiretroviral; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; HCV Ab, hepatitis C virus antibody; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment of insulin resistance; HTN, hypertension; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; WWH, women with HIV; WWOH, women without HIV.
With no history of positive HCV RNA.
Median CAP was 247 dB/m in WWH (IQR, 209–290 dB/m) and 249 dB/m in WWOH (IQR, 206–283 dB/m). Median LS was also similar and did not differ by serostatus: 5.1 kPa in WWH (IQR, 3.9–6.7 kPa) and 4.9 kPa in WWOH (IQR, 3.9–6.2 kPa). By contrast, median AST and alanine aminotransferase levels were higher in the WWH compared to the WWOH, but median values were in the normal range (<20 U/L). Most WWH were taking antiretroviral therapy (ART) (90%), with undetectable HIV RNA (72%), and 48% were on an integrase strand transfer inhibitor (INSTI)–containing regimen (Table 1). Specific antiviral therapy medications for the WWH can be found in Supplementary Table 1.
Prevalence of Elevated FAST Score
Overall, 65 women (5%) had a FAST score >0.35 and 14 women (1.1%) had a FAST score ≥0.67. The prevalence of FAST score >0.35 was higher in the WWH compared to the WWOH (6.3% vs 1.8%, respectively; P = .001), as was the prevalence of FAST score ≥0.67 (1.4% vs 0.3%, respectively; P = .07). Median FAST score was 0.06 in WWH (IQR, 0.03–0.12) and 0.04 in WWOH (IQR, 0.02–0.07) (P < .001).
Factors Associated With Elevated FAST Score in the Entire Cohort
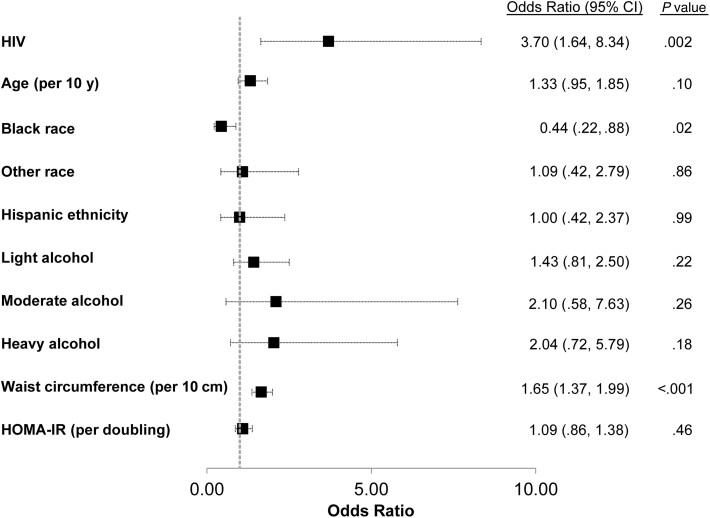
Table 2 shows the unadjusted and adjusted models associated with elevated FAST score in the entire cohort. After adjusting for demographic, metabolic, and lifestyle factors, HIV infection was associated with 3.7-fold higher odds of FAST score >0.35 (95% CI, 1.6–8.3; P = .002) (Figure 1). WWH with undetectable HIV viral load and WWH with detectable HIV viral load had a 2.7-fold (95% CI, 1.2- to 6.3-fold; P = .021) and 6.9-fold (95% CI, 2.9–16.9; P < .001) higher odds of FAST score >0.35 compared to WWOH. Each 10-cm increase in waist circumference was associated with 1.7-fold higher adjusted odds of FAST score >0.35 (95% CI, 1.4–2.0; P < .001), whereas black race was associated with an adjusted odds ratio (OR) of 0.4 (95% CI, .2–.9; P = .021). When the model was repeated with BMI in place of waist circumference due to collinearity between the 2 covariates, BMI was also independently associated with 1.4-fold higher odds of FAST score >0.35 per 5 kg/m2 (95% CI, 1.2–1.6; P < .001) and the other associations were unchanged. After excluding women with self-reported heavy drinking (n = 101), HIV infection remained associated with higher odds of FAST score >0.35 (OR, 4.4 [95% CI, 1.7–11.2]; P = .002). Because only 1 WWOH woman had a FAST score ≥0.67, as compared to 13 WWH, our analysis of factors associated with FAST score ≥0.67 was limited to the WWH.
Table 2.
Factors Associated With FAST Score >0.35 Among All Women (N = 1309)
| Factor | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| HIV infection | 3.56 (1.61–7.88) | .002 | 3.70 (1.64–8.34) | .002 |
| Age (per 10 y) | 1.32 (.96–1.80) | .09 | 1.33 (.95–1.85) | .10 |
| Race/ethnicity (ref = white) | ||||
| Black | .50 (.27–.92) | .03 | .44 (.22–.88) | .02 |
| Other | .96 (.41–2.28) | .93 | 1.09 (.42–2.79) | .86 |
| Hispanic | 1.51 (.77–2.95) | .23 | 1.00 (.42–2.37) | .99 |
| Waist circumference (per 10 cm) | 1.59 (1.36–1.86) | <.001 | 1.65 (1.37–1.99) | <.001 |
| BMI (per 5 kg/m2)b | 1.34 (1.18–1.52) | <.001 | … | |
| HOMA-IR (per doubling) | 1.46 (1.22–1.75) | <.001 | 1.09 (.86–1.38) | .46 |
| Diabetes mellitus (ref = no)c | 2.70 (1.61–4.55) | <.001 | … | |
| On HTN medication (ref = no) | 1.52 (.92–2.51) | .10 | … | |
| On antidepressant medication (ref = no) | 1.47 (.85–2.53) | .17 | … | |
| Alcohol use (ref = none) | ||||
| Light | 1.22 (.72–2.08) | .46 | 1.43 (.81–2.50) | .22 |
| Moderate | 1.56 (.46–5.33) | .48 | 2.10 (.58–7.63) | .26 |
| Heavy | 1.11 (.42–2.94) | .83 | 2.04 (.72–5.79) | .18 |
| Current smoking | 1.15 (.69–1.91) | .59 | … | |
| Current marijuana use | .56 (.27–1.14) | .11 | … | |
| Ever injection drug use | .93 (.22–3.94) | .92 | … | |
Abbreviations: BMI, body mass index; CI, confidence interval; FAST, FibroScan–aspartate aminotransferase; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment of insulin resistance; HTN, hypertension; OR, odds ratio.
Adjusted for covariates included in table.
Not included in final adjusted model due to collinearity with waist circumference. When replaced with waist circumference in the adjusted model, the OR was 1.35 (95% CI, 1.16–1.57), P < .001.
Not included in final adjusted model due to collinearity with HOMA-IR. When replaced with HOMA-IR in the adjusted model, the OR was 1.68 (95% CI, .94–3.01), P = .08.
Figure 1.
Factors associated with FibroScan–aspartate aminotransferase (FAST) score >0.35 among all women (N = 1309) in multivariable analysis. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment of insulin resistance.
Factors Associated With Elevated FAST Score in the Women With HIV
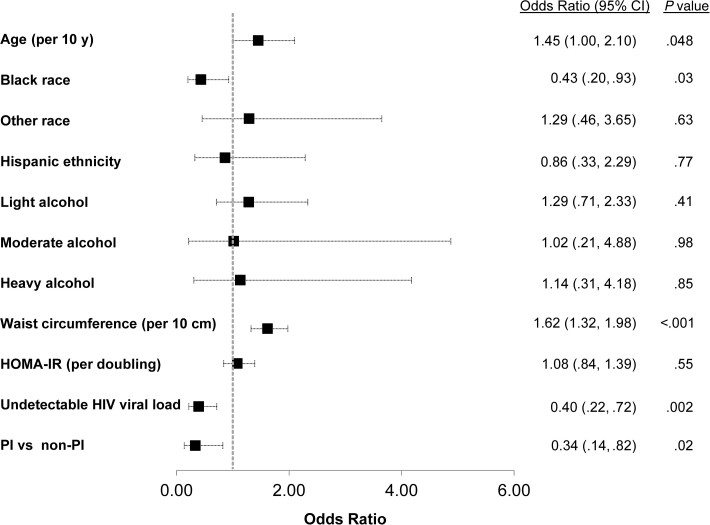
Among the 928 WWH, greater waist circumference, BMI, and HOMA-IR were each associated with higher odds of FAST >0.35 on univariable analysis (Table 3). Conversely, current protease inhibitor (PI) use and undetectable HIV viral load were associated with lower odds of FAST >0.35. INSTI use was associated with a slightly higher odds of FAST >0.35, but this was not statistically significant (OR, 1.4 [95% CI, .8–2.5]; P = .189). On multivariable analysis, greater waist circumference remained significantly associated with higher odds of FAST >0.35 (OR, 1.6 per 10-cm increase [95% CI, 1.3–2.0]; P < .001), and increasing age was also associated with higher odds (OR, 1.5 per 10-year increase [95% CI, 1.0–2.1]; P = .048) (Figure 2). By contrast, black race (OR, 0.4 [95% CI, .2–.9]; P = .031), current PI use (OR, 0.3 [95% CI, .1–.8]; P = .017), and undetectable HIV viral load (OR, 0.4 [95% CI, .2–.7]; P = .002) were independently associated with lower odds. In additional sensitivity analysis, findings were unchanged after excluding women with self-reported heavy alcohol use (data not shown).
Table 3.
Factors Associated With FAST Score >0.35 Among Women With Human Immunodeficiency Virus (n = 928)
| Factor | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (per 10 y) | 1.26 (.89–1.78) | .19 | 1.45 (1.00–2.10) | .048 |
| Race/ethnicity (ref = white) | ||||
| Black | .56 (.29–1.07) | .08 | .43 (.20–.93) | .03 |
| Other | 1.08 (.43–2.73) | .87 | 1.29 (.46–3.65) | .63 |
| Hispanic | 1.43 (.68–3.00) | .34 | .86 (.33–2.29) | .77 |
| Waist circumference (per 10 cm) | 1.56 (1.32–1.85) | <.001 | 1.62 (1.32–1.98) | <.001 |
| BMI (per 5 kg/m2)b | 1.33 (1.17–1.53) | <.001 | … | |
| HOMA-IR (per doubling) | 1.43 (1.17–1.73) | <.001 | 1.08 (.84–1.39) | .55 |
| Diabetes mellitus (ref = no)c | 2.76 (1.58–4.83) | <.001 | … | |
| On HTN medication (ref = no) | 1.36 (.77–2.23) | .33 | … | |
| On antidepressant medication (ref = no) | 1.38 (.78–2.44) | .27 | … | |
| Alcohol use (ref = none) | ||||
| Light | 1.20 (.69–2.10) | .52 | 1.29 (.71–2.33) | .41 |
| Moderate | 1.16 (.26–5.11) | .85 | 1.02 (.21–4.88) | .98 |
| Heavy | .95 (.28–3.25) | .94 | 1.14 (.31–4.18) | .85 |
| Current smoking | 1.35 (.79–2.31) | .28 | … | |
| Current marijuana use | .53 (.23–1.18) | .12 | … | |
| Ever injection drug use | 1.38 (.32–6.00) | .67 | … | |
| Undetectable HIV viral load | .53 (.31–.91) | .02 | .40 (.22–.72) | .002 |
| CD4 current (per 100 cells/µL) | .96 (.88–1.04) | .28 | … | |
| CD4 nadir (per 100 cells/µL) | .95 (.82–1.10) | .50 | … | |
| PI (ref = non-PI ART) | .32 (.13–.81) | .01 | .34 (.14–.82) | .02 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; FAST, FibroScan–aspartate aminotransferase; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment of insulin resistance; HTN, hypertension; OR, odds ratio; PI, protease inhibitor.
Adjusted for covariates included in table.
Not included in final adjusted model due to collinearity with waist circumference. When replaced with waist circumference in the adjusted model, the OR was 1.34 (95% CI, 1.14–1.57), P < .001.
Not included in final adjusted model due to collinearity with HOMA-IR. When replaced with HOMA-IR in the adjusted model, the OR was 1.71 (95% CI, .91–3.22), P = .09.
Figure 2.
Factors associated with FibroScan–aspartate aminotransferase (FAST) score >0.35 among women with human immunodeficiency virus (n = 928) in multivariable analysis. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment of insulin resistance; PI, protease inhibitor.
Similarly, greater waist circumference, BMI, and HOMA-IR were each associated with higher odds of FAST ≥0.67 on univariable analysis (Supplementary Table 2); however, current PI use and undetectable HIV viral load were not. On multivariable analysis, greater waist circumference (OR, 1.6 per 10-cm increase [95% CI, 1.1–2.4]; P = .015) and older age (OR, 2.9 per 10 years [95% CI, 1.3–6.1]; P = .008) remained associated with FAST ≥0.67. These findings were unchanged after excluding women with self-reported heavy alcohol use (data not shown).
Association of HIV Infection With Components of FAST Score
To understand how the components of the FAST score may influence the relationship of HIV with elevated FAST, we evaluated unadjusted and adjusted associations of HIV serostatus with CAP, LS, AST, and FAST modeled continuously. On univariable analysis, HIV was associated with 0.5% higher CAP and 4.2% higher LS, but this was not statistically significant (P = .752 and P = .116, respectively). HIV was associated with significantly higher AST (13% higher, P < .001), and FAST score (43% higher, P < .001). In multivariable models adjusted for demographic, behavioral, and metabolic factors, these associations remained similar, with HIV associated with 0.1% higher CAP (P = .951), 3.8% higher LS (P = .144), 13% higher AST (P < .001), and 42% higher FAST score (P < .001). We next used separate path analysis to assess the individual contributions of AST, LS, or CAP to the association of HIV with FAST score. In the path analysis where AST was a mediator between HIV and FAST score, the indirect effect of AST explained 98% of the total effect between HIV and FAST score and the direct effect explained only 2%; LS explained 14% of the total effect, and CAP explained 2% of the total effect.
DISCUSSION
In the largest study to date of VCTE in WWH and a comparison group of WWOH with similar sociodemographic and metabolic risk factors for NAFLD, we found that HIV infection is associated with 3.7-fold higher odds of elevated FAST scores, even after adjustment for sociodemographic, behavioral, and metabolic factors. As expected, greater waist circumference, a marker of visceral obesity, was also strongly associated with elevated FAST. The use of a noninvasive measure that can serve as a surrogate for histologic findings that are associated with significantly higher risk of developing cirrhosis and end-stage liver disease [23] is greatly needed in PWH. However, our finding that the association of HIV infection with elevated FAST was mainly driven by the AST component of the score indicates that further investigation of FAST and other noninvasive measures in the setting of HIV is warranted.
Prior liver biopsy studies in PWH without viral hepatitis coinfection have demonstrated relatively high prevalence of NASH and ≥F2 fibrosis [12, 24, 25]. However, these studies have been limited by small sample size, potential bias in who is referred for liver biopsy, and lack of HIV-seronegative controls. While our findings are informative, when we examined the association of HIV with the different components that make up the FAST score, we found no association of HIV with CAP values, a finding that is consistent with prior studies [26–28]. A possible reason is that CAP detects steatosis but does not differentiate NASH from simple steatosis, and the FAST score was developed to identify an even more high-risk population of patients with histologic NASH, ≥F2 fibrosis, and NAS ≥ 4 [13]. Interestingly, HIV was also minimally associated with increased LS, a surrogate of fibrosis, and this was not statistically significant. However [29], whether HIV is an independent risk factor for more advanced fatty liver disease pathology needs further study.
Some have postulated that HIV replication and immune activation may potentiate the pathogenesis of NASH [30]. In our study, undetectable HIV viral load was protective against elevated FAST score. HIV proteins can directly activate Kupffer cells and hepatic stellate cells [31–33] and induce hepatocyte apoptosis [34, 35]. HIV also depletes gut mucosal CD4 T cells, resulting in increased intestinal microbial translocation [36, 37]. Both microbial translocation and HIV-related chronic immune activation lead to Kupffer cell activation and release of proinflammatory cytokines [31, 36, 38]. Circulating levels of soluble CD163 (sCD163), a marker of macrophage activation, are increased in HIV infection (despite effective ART) [39] and are independently associated with greater LS and biopsy-confirmed ≥F2 fibrosis in PWH in the absence of viral hepatitis [40, 41]. Elevations of sCD163 have also been associated with development of NASH and advanced fibrosis in patients without HIV [42].
The role of ART in the development of steatosis and NASH remains complicated. Older ART agents associated with mitochondrial toxicity and lipoatrophy may have irreversible “legacy effects” on risk of NAFLD [4]. In PWH, reduced leg subcutaneous adipose tissue, a feature of lipoatrophy, is associated with decreases in adiponectin, which are also observed in NASH [43, 44]. Contemporary ART may impact hepatic steatosis and NASH indirectly through metabolic effects rather than direct hepatoxicity. While weight gain is frequently observed after starting ART, presumably as part of a “return-to-health” effect, this is more pronounced with INSTI use, and switching to an INSTI-based regimen has been associated with weight gain and increasing visceral adiposity [45]. We found a strong independent association of waist circumference, a marker of visceral adiposity, with elevated FAST score, but did not find an association of INSTI use. Instead, we found an inverse association of current PI use with elevated FAST score. The reason for this is unclear. Due to the observational nature of our cohort, we cannot exclude the possibility of selection bias, as providers may have avoided prescribing PIs in women with greater metabolic comorbidities.
Because our study used a noninvasive surrogate for NASH with significant activity and fibrosis, our findings should be interpreted with caution. Furthermore, the FAST score was derived weighing AST, CAP, and LS in a cohort of patients with suspected NAFLD and a relatively high prevalence of NASH [13]. As noted in the original publication that described FAST, the score may need to be recalibrated in populations of low NASH prevalence, such as our cohort. Interestingly, in our cohort, although median AST values were in the normal range for WWH and WWOH, we found a strong independent association of HIV with higher AST values that explained most of the observed association of HIV and FAST, whereas the association of HIV with LS mediated a smaller proportion. It is possible that HIV may be associated with higher AST values independent of NASH with significant activity and fibrosis. Therefore, it will be important to validate the FAST score in other populations of people with HIV with a low prevalence of NASH.
Other study limitations include the cross-sectional design, which prevents us from demonstrating causal associations, and the inability to generalize our findings to men with and without HIV. We included women who reported moderate or heavy alcohol consumption because we wanted the characteristics of the cohort to be comparable to those of women with and without HIV in the US [14]. Only a small proportion of our cohort reported moderate or greater alcohol use, and excluding women with heavy alcohol consumption did not impact our findings.
In conclusion, we found that HIV infection is independently associated with elevated FAST score and that among WWH, HIV viral suppression may be important in reducing the odds of a high FAST score. While our findings suggest that HIV may be a risk factor for more advanced NAFLD histology and liver disease progression, validation of the FAST score in other cohorts of PWH is needed. This is especially important given the high contribution of AST to the association of HIV with elevated FAST. Finally, our findings underscore the importance of longitudinal studies to assess long-term outcomes of NAFLD in PWH and continued emphasis on ART adherence and weight reduction in HIV clinical management.
Supplementary Material
Contributor Information
Jennifer C Price, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Yifei Ma, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Mark H Kuniholm, Department of Epidemiology and Biostatistics, University at Albany, State University of New York, Rensselaer, New York, USA.
Adaora A Adimora, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Margaret Fischl, Department of Medicine, University of Miami Miller School of Medicine, Miami, Florida, USA.
Audrey L French, Department of Medicine, CORE Center/Stroger Hospital of Cook County, Chicago, Illinois, USA.
Elizabeth T Golub, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Deborah Konkle-Parker, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Howard Minkoff, Department of Obstetrics and Gynecology, State University of New York Downstate Health Sciences University, Brooklyn, New York, USA.
Ighovwerha Ofotokun, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Michael Plankey, Department of Medicine, Georgetown University Medical Center, Washington, District of Columbia, USA.
Anjali Sharma, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Phyllis C Tien, Department of Medicine, University of California, San Francisco, San Francisco, California, USA; Department of Veterans Affairs, San Francisco, California, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Financial support. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS), now the Multicenter AIDS Cohort Study/WIHS Combined Cohort Study (MWCCS). MWCCS (Principal Investigators): Atlanta Clinical Research Site (CRS) (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange, and Elizabeth Golub), U01-HL146193; Chicago–Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; University of Alabama at Birmingham–MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; and University of North Carolina CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Nursing Research, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and National Institute on Minority Health and Health Disparities, and in coordination and alignment with the research priorities of the NIH, Office of AIDS Research. MWCCS data collection is also supported by UL1-TR000004 (University of California, San Francisco, Clinical and Translational Science Award [CTSA]), UL1-TR003098 (Johns Hopkins University, Institute for Clinical and Translational Research [ICTR]), UL1-TR001881 (University of California, Los Angeles, Clinical and Translational Science Institute [CTSI]), P30-AI-050409 (Atlanta, Center for AIDS Research [CFAR]), P30-AI-073961 (Miami CFAR), P30-AI-050410 (University of North Carolina CFAR), P30-AI-027767 (University of Alabama at Birmingham CFAR), and P30-MH-116867 (Miami, Center for HIV and Research in Mental Health [CHARM]). The study was also supported by the NIAID (K24 AI 108516 to P. C. T.), the NIDDK (5R01DK109823 to P. C. T.), and an American College of Gastroenterology Junior Faculty Development Award (to J. C. P.). P. C. T. also reports the following grant made to institution in support of this work: R01 DA 044111.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–57. [DOI] [PubMed] [Google Scholar]
- 3. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA 2020; 323:1175–83. [DOI] [PubMed] [Google Scholar]
- 4. Lake JE, Overton T, Naggie S, et al. . Expert panel review on non-alcoholic fatty liver disease in persons with human immunodeficiency virus. Clin Gastroenterol Hepatol 2022; 20:256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campos-Varela I, Dodge JL, Terrault NA, Brandman D, Price JC. Non-viral liver disease is the leading indication for liver transplant in the U.S. in persons living with human immunodeficiency virus. Am J Transplant 2021; 21:3148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Telfer P, Sabin C, Devereux H, Scott F, Dusheiko G, Lee C. The progression of HCV-associated liver disease in a cohort of haemophilic patients. Br J Haematol 1994; 87:555–61. [DOI] [PubMed] [Google Scholar]
- 7. Soto B, Sanchez-Quijano A, Rodrigo L, et al. . Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 1997; 26:1–5. [DOI] [PubMed] [Google Scholar]
- 8. Benhamou Y, Bochet M, Di Martino V, et al. . Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30:1054–8. [DOI] [PubMed] [Google Scholar]
- 9. Colin JF, Cazals-Hatem D, Loriot MA, et al. . Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999; 29:1306–10. [DOI] [PubMed] [Google Scholar]
- 10. Thio CL, Seaberg EC, SkolaskyR, Jr, et al. . HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–6. [DOI] [PubMed] [Google Scholar]
- 11. Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology 2009; 137:795–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. [DOI] [PubMed] [Google Scholar]
- 13. Newsome PN, Sasso M, Deeks JJ, et al. . FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020; 5:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Souza G, Bhondoekhan F, Benning L, et al. . Characteristics of the MACS/WIHS combined cohort study: opportunities for research on aging with HIV in the longest US observational study of HIV. Am J Epidemiol 2021; 190:1457–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adimora AA, Ramirez C, Benning L, et al. . Cohort profile: the Women's Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–4i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Ledinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int 2010; 30:1043–8. [DOI] [PubMed] [Google Scholar]
- 17. Al-Harthi L, Voris J, Du W, et al. . Evaluating the impact of hepatitis C virus (HCV) on highly active antiretroviral therapy-mediated immune responses in HCV/HIV-coinfected women: role of HCV on expression of primed/memory T cells. J Infect Dis 2006; 193:1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bodner TE. What improves with increased missing data imputations? Struct Equ Model 2008; 15:651–75. [Google Scholar]
- 19. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30:377–99. [DOI] [PubMed] [Google Scholar]
- 20. Rubin DB. Multiple imputation for nonresponse in surveys. Wiley Series in Probability and Statistics. New York: John Wiley & Sons, Inc.,1987. [Google Scholar]
- 21. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51:1173–82. [DOI] [PubMed] [Google Scholar]
- 22. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Instrum Comput 2004; 36:717–31. [DOI] [PubMed] [Google Scholar]
- 23. US Food and Drug Administration . Draft guidance. Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment guidance for industry. Silver Spring, MD: FDA, 2018. [Google Scholar]
- 24. Maurice JB, Goldin R, Hall A, et al. . Increased BMI and type 2 diabetes are the main predictors of NAFLD and advanced fibrosis in liver biopsies of patients with HIV mono-infection. Clin Infect Dis 2021;73:e2184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lemoine M, Assoumou L, De Wit S, et al. . Diagnostic accuracy of noninvasive markers of steatosis, NASH, and liver fibrosis in HIV-monoinfected individuals at risk of nonalcoholic fatty liver disease (NAFLD): results from the ECHAM study. J Acquir Immune Defic Syndr 2019; 80:e86–94. [DOI] [PubMed] [Google Scholar]
- 26. Price JC, Seaberg EC, Latanich R, et al. . Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol 2014; 109:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torgersen J, So-Armah K, Freiberg MS, et al. . Comparison of the prevalence, severity, and risk factors for hepatic steatosis in HIV-infected and uninfected people. BMC Gastroenterol 2019; 19:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirkegaard-Klitbo DM, Fuchs A, Stender S, et al. . Prevalence and risk factors of moderate-to-severe hepatic steatosis in human immunodeficiency virus infection: the Copenhagen Co-morbidity Liver Study. J Infect Dis 2020; 222:1353–62. [DOI] [PubMed] [Google Scholar]
- 29. Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther 2015; 41:368–78. [DOI] [PubMed] [Google Scholar]
- 30. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010; 52:1836–46. [DOI] [PubMed] [Google Scholar]
- 31. Crane M, Iser D, Lewin SR. Human immunodeficiency virus infection and the liver. World J Hepatol 2012; 4:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiao P, Usami O, Suzuki Y, et al. . Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. AIDS 2008; 22:1749–57. [DOI] [PubMed] [Google Scholar]
- 33. Tuyama AC, Hong F, Saiman Y, et al. . Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 2010; 52:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vlahakis SR. Human immunodeficiency virus and hepatitis C virus co-infection. J Med Liban 2006; 54:106–10. [PubMed] [Google Scholar]
- 35. Bruno R, Galastri S, Sacchi P, et al. . Gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut 2010; 59:513–20. [DOI] [PubMed] [Google Scholar]
- 36. Brenchley JM, Price DA, Schacker TW, et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 37. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. . Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balagopal A, Philp FH, Astemborski J, et al. . Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 2008; 135:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burdo TH, Lentz MR, Autissier P, et al. . Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lemoine M, Lacombe K, Bastard JP, et al. . Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients: results of the METAFIB study. AIDS 2017; 31:1955–64. [DOI] [PubMed] [Google Scholar]
- 41. Maurice JB, Garvey L, Tsochatzis EA, et al. . Monocyte-macrophage activation is associated with nonalcoholic fatty liver disease and liver fibrosis in HIV monoinfection independently of the gut microbiome and bacterial translocation. AIDS 2019; 33:805–14. [DOI] [PubMed] [Google Scholar]
- 42. Mueller JL, Feeney ER, Zheng H, et al. . Circulating soluble CD163 is associated with steatohepatitis and advanced fibrosis in nonalcoholic fatty liver disease. Clin Transl Gastroenterol 2015; 6:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kosmiski LA, Bacchetti P, Kotler DP, et al. . Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab 2008; 93:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stojsavljevic S, Gomercic Palcic M, Virovic Jukic L, Smircic Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20:18070–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eckard AR, McComsey GA. Weight gain and integrase inhibitors. Curr Opin Infect Dis 2020; 33:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.