Abstract
Background
We describe trends in prevalence and identify factors associated with Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), syphilis, and Trichomonas vaginalis (TV) diagnosed in pregnancy among US people with human immunodeficiency virus (PWH) and evaluate associations of sexually transmitted infections (STIs) with preterm birth (PTB).
Methods
We included pregnant PWH enrolled in the Surveillance Monitoring for ART Toxicities dynamic cohort of the Pediatric HIV/AIDS Cohort Study network who delivered between 2010 and 2019. Multivariable log-binomial or Poisson generalized estimating equation models were used to estimate the association of calendar year with each STI, controlling for confounders; the association of demographic and clinical factors with each STI; and the association of each STI with PTB.
Results
The sample included 2241 pregnancies among 1821 PWH. Median age at delivery was 29.2 years; 71% of participants identified as Black or African American. STI prevalence was: CT 7.7%, NG 2.3%, syphilis 2.4%, and TV 14.5%; 30% had unknown TV status. There were no temporal changes in STI prevalence. Younger age and initial HIV viral load ≥400 copies/mL were associated with increased risk of CT, NG, and TV. Recreational substance use was a risk factor for NG, syphilis, and TV. No STI was associated with PTB.
Conclusions
Unlike nationwide trends, no changes in STI prevalence during the study period were observed. The large proportion with unknown TV status underscores the need for increased adherence to screening guidelines. STIs diagnosed during pregnancy in PWH were not associated with risk of PTB.
Keywords: sexually transmitted infections, pregnancy, HIV, preterm birth
Prevalence of sexually transmitted infections was high in pregnant people living with HIV. Coinfection was not associated with risk of preterm birth. One-third of individuals were missing data on Trichomonas vaginalis, underscoring the need for interventions promoting guideline-driven screening.
Sexually transmitted infections (STIs), including Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), syphilis, and Trichomonas vaginalis (TV) are rising in prevalence [1]. In the United States (US) from 2010 to 2019, CT cases increased by 19%, NG cases by 47%, and rates of primary and secondary syphilis by 63% [2]. TV is not a reportable infection in the US, and surveillance data are limited. The 2013–2014 National Health and Nutrition Examination Survey estimated 1.8% prevalence in 18- to 59-year-old women [3].
STIs in pregnancy are associated with maternal and neonatal complications. Untreated CT and NG infections in pregnancy are associated with adverse neonatal conditions (sepsis, pneumonia, conjunctivitis), low birth weight, premature rupture of membranes, preterm labor, chorioamnionitis, and postpartum endometritis [4–6]. Syphilis during pregnancy increases risk of stillbirth, preterm birth, congenital syphilis, and neonatal death [7]. Although the full consequences of TV infection in pregnancy are not entirely known, TV has been associated with preterm birth, low birthweight infants, and preterm prelabor rupture of membranes [8, 9].
Established risk factors for STIs in the general US population include living in the South [10], being born in the US (vs a foreign country), younger age, lower educational level, poverty, lack of condom use, and history of incarceration [11, 12]. Disparities in prevalence are particularly notable by racial and ethnic identity, with prevalence highest among people identifying as non-Hispanic and as Black. For example, prevalence of TV in reproductive-aged US women who were non-Hispanic Black is 8.9% vs 0.8% among women of other racial/ethnic groups [3]. Structural racism and associated inequities in socioeconomic status, healthcare access, and incarceration likely account for this observed difference [13].
Less is known about prevalence and potentially additive effects of STI coinfection in the setting of pregnant people with human immunodeficiency virus (PWH). Coinfection with CT, NG, or TV increases the risk of horizontal and perinatal human immunodeficiency virus (HIV) transmission [14–17], and some evidence suggests that increases in viral shedding can be reversed with STI treatment [18–20]. There remain many gaps in our understanding of prevalence and factors associated with STI coinfection among pregnant PWH, including the role of HIV disease-related factors (eg, viral load, CD4 cell count, and mode of acquisition of HIV, which could affect susceptibility to STI coinfection). In addition, little is known about the association of STI/HIV coinfection with preterm birth (PTB), particularly where combination antiretroviral therapy (ART) is being used. For example, while it has been shown that several STIs and protease inhibitors (PIs) independently increase the risk of PTB [5, 7, 8, 21, 22], it is unclear whether these factors interact to alter risk.
Because the maternal and fetal health effects of STI coinfections may be greater in pregnant PWH than among nonpregnant, HIV-seronegative persons, understanding the epidemiology and trends of STI coinfection is critical. Thus, we sought to (1) describe trends over time in the prevalence of 4 STIs (CT, NG, syphilis, and TV) diagnosed in pregnancy among PWH; (2) identify demographic and HIV-related clinical factors associated with risk of STIs; and (3) evaluate the association of STIs with PTB, exploring potentially interacting effects of exposure to a PI in the first trimester, earliest pregnancy viral load, and perinatally acquired HIV (PHIV).
METHODS
Study Population
The study population included pregnant PWH enrolled in the Surveillance Monitoring for ART Toxicities (SMARTT) dynamic cohort within the Pediatric HIV/AIDS Cohort Study (PHACS). The purpose of the study was to assess safety of ART use during pregnancy and inform treatment guidelines for pregnant PWH. Details of the cohort and selection have been published elsewhere [23]. In brief, starting in 2007, the SMARTT dynamic cohort enrolled pregnant people from 22 weeks of gestation to 1 week postpartum into an ongoing prospective cohort study at 22 clinical sites in the US, including Puerto Rico. Data were obtained from participant interviews and abstraction of the medical record. STIs were diagnosed utilizing the laboratory method or clinical criteria established at each participating site as part of routine prenatal care. Neither the type nor manufacturer of the diagnostic test was abstracted. STI diagnoses that were made during the current pregnancy were coded as binary variables for each STI (yes/no). This analysis included pregnancies with delivery date from 1 January 2010 to 1 November 2019. Pregnancies with missing delivery date, those missing all pregnancy covariates, and those missing data for all 4 STIs of interest were excluded. As multifetal gestation is an independent risk factor for PTB, individuals with twins and higher-order multiples were excluded from the PTB analysis.
STI Prevalence Over Time
To explore temporal trends in STIs (CT, NG, syphilis, and TV), yearly prevalence and 95% Wilson confidence intervals (CIs) [24, 25] were estimated for each STI and plotted. Self-reported race (Black/African American vs not Black/African American) and ethnicity (Hispanic/Latinx vs non-Hispanic/Latinx) were included in multivariable analyses as a marker for exposure to systemic racism and to account for potential confounding. Race and ethnicity variables were considered as separate variables. Additional potential confounders included maternal age at delivery, PHIV status, and geographic region of delivery (categorized using US Census Bureau region definitions). Log-binomial generalized estimating equation (GEE) models were fit to estimate the association between calendar year and each STI, adjusting for each covariate individually, then all covariates.
Association Between Sociodemographic and Clinical Characteristics and STIs
Multivariable Poisson GEE models were used to model risk of each STI (separately), while controlling for confounding variables. Poisson models were selected due to nonconvergence of log-binomial models. Covariates of interest included those listed above, plus educational attainment, annual household income, trimester of entry into prenatal care, substance use during pregnancy (self-reported tobacco, alcohol, marijuana, or recreational substances), earliest available pregnancy CD4 cell count, and earliest available pregnancy HIV viral load. For model building, covariates associated with the STI in a univariable model with P < .20 were first entered into a full model. Independent variables were then excluded 1 at time, starting with the variable with the largest P value until all remaining independent variables had P values < .20. Dropped covariates were readded 1 at a time in the order they were dropped and retained if P < .20 to produce a final model.
Due to a high proportion of missing data on TV, as a sensitivity analysis, modeling was repeated for TV using a multiple imputation approach. Data were assumed to be missing at random and imputed using multiple imputation by chained equations (MICE) with fully conditional specification [26]. The models for TV were reproduced by combining 1000 imputed datasets according to Rubin’s rules [27]. Model selection for the multiple imputation model proceeded in the same manner as described above.
Association of STIs and Preterm Birth
Univariable log-binomial GEE models were used to estimate the association of STIs with PTB (defined as birth <37 weeks of gestation). Separate models were fit for each STI individually, for any STI during pregnancy, and for number of STIs in pregnancy (0, 1, or ≥2) as the exposure. Additionally, 3 prespecified covariates were considered as potential effect modifiers of the association of STIs with PTB: PI use in the first trimester (given evidence that PI use is independently associated with PTB [21, 22]), viral load ≤400 HIV copies/mL at first measurement in pregnancy, and PHIV status. This was assessed with entry of a multiplicative interaction term of the covariate and STI, in addition to the main effects, into the model with PTB as the outcome.
GEE models with an exchangeable working correlation structure were utilized throughout to account for repeat pregnancies from the same individual. All statistical analyses were carried out using SAS version 9.4 software. Written informed consent was obtained from study participants at enrollment. Institutional review board approval for the SMARTT protocol was obtained at each participating clinical site and at the Harvard T. H. Chan School of Public Health.
RESULTS
From January 2010 to October 2019, there were 2447 pregnancies included in the SMARTT dynamic cohort. After excluding 94 pregnancies missing date of delivery, 52 with no pregnancy information available, 58 missing on all 4 STI variables, and 2 participants who withdrew prior to delivery, there were 2241 pregnancies among 1821 people included in the temporal trend and STI risk factor analyses. Within this group, an additional 94 pregnancies were excluded due to multifetal gestation and 1 due to unknown PTB status, leaving 2146 pregnancies among 1785 people for the PTB analysis.
Sample Characteristics
The median age of participants at delivery was 29.2 years (interquartile range, 24.8–33.7 years). Most participants (70.8%) identified as Black or African American and 27.5% identified as Hispanic or Latinx ethnicity. Half (53.4%) reported annual household income <$10 000 and a minority (29.5%) had not completed high school or attained a General Educational Development diploma. Of note, 678 pregnancies (30.3%) were missing data on TV. Most participants (66.6%) were taking ART at conception or in the first trimester (Table 1).
Table 1.
Characteristics of the Study Population
| Characteristic | No. (%)a (N = 2241) |
|---|---|
| Chlamydia trachomatis (missing n = 44 [2.0%]) | 169 (7.7) |
| Neisseria gonorrhoeae (missing n = 43 [1.9%]) | 50 (2.3) |
| Syphilis (missing n = 77 [3.4%]) | 52 (2.4) |
| Trichomonas vaginalis (missing n = 678 [30.3%]) | 226 (14.5) |
| Maternal age at delivery, median (IQR) (missing n = 0 [0%]) | 29.2 (24.8–33.7) |
| Educational attainment | |
| Less than high school/GED | 648 (29.5) |
| High school/GED, trade school, or some college | 1396 (63.5) |
| College or higher (missing n = 42 [1.9%]) | 155 (7.0) |
| Annual household income | |
| <$10 000 | 1100 (53.4) |
| $10 001–$30 000 | 682 (33.1) |
| ≥$30 001 (missing n = 183 [8.2%]) | 276 (13.4) |
| Black or African American race (missing n = 112 [5.0%]) | 1508 (70.8) |
| Hispanic or Latinx ethnicity (missing n = 5 [0.2%]) | 614 (27.5) |
| Delivery region | |
| Northeast | 449 (20.0) |
| Midwest | 202 (9.0) |
| South | 1018 (45.4) |
| West | 421 (18.8) |
| Puerto Rico (missing n = 0 [0%]) | 151 (6.7) |
| Delivery year | |
| 2010 | 259 (11.6) |
| 2011 | 256 (11.4) |
| 2012 | 232 (10.4) |
| 2013 | 260 (11.6) |
| 2014 | 249 (11.1) |
| 2015 | 244 (10.9) |
| 2016 | 220 (9.8) |
| 2017 | 188 (8.4) |
| 2018 | 198 (8.8) |
| 2019 (missing n = 0 [0%]) | 135 (6.0) |
| Gestational age, wk, at entry into prenatal care, median (IQR) (missing n = 0 [0%]) | 10.7 (8.0–15.8) |
| Alcohol use in pregnancy (missing n = 40 [1.8%]) | 191 (8.7) |
| Marijuana use in pregnancy (missing n = 40 [1.8%]) | 223 (10.1) |
| Tobacco use in pregnancy (missing n = 40 [1.8%]) | 387 (17.6) |
| Recreational substance use in pregnancy (missing n = 48 [2.1%]) | 72 (3.3) |
| Perinatal HIV acquisition (missing n = 31 [1.4%]) | 279 (12.6) |
| Earliest pregnancy CD4 count, cells/µL | |
| 0–250 | 371 (16.8) |
| 251–500 | 738 (33.5) |
| 501–750 | 609 (27.7) |
| 751–1000 | 303 (13.8) |
| >1000 (missing n = 39 [1.7%]) | 181 (8.2) |
| Earliest pregnancy HIV RNA level, copies/mL | |
| 0–400 | 1209 (54.5) |
| 401–1000 | 118 (5.3) |
| 1001–5000 | 266 (12.0) |
| 5001–10 000 | 149 (6.7) |
| >10 000 (missing n = 22 [1.0%]) | 477 (21.5) |
| Trimester of initial ART exposure | |
| At conception | 995 (45.1) |
| First trimester | 475 (21.5) |
| Second trimester | 598 (27.1) |
| Third trimester | 121 (5.5) |
| Unexposed to ART in pregnancy (missing n = 35 [1.6%]) | 17 (0.8) |
| Class of ART in first trimester | |
| No ART | 736 (33.4) |
| INSTI-based regimen | 404 (18.3) |
| PI-based regimen | 755 (34.2) |
| Other regimen (missing n = 35 [1.6%]) | 311 (14.1) |
| Class of ART used anytime during pregnancy (not mutually exclusive) | |
| INSTI | 624 (27.8) |
| PI | 1433 (63.9) |
| NNRTI | 509 (22.7) |
| NRTI | 2166 (96.7) |
| FI (missing n = 35 [1.6%]) | 16 (0.7) |
| Class of ART used anytime during first trimester (not mutually exclusive) | |
| INSTI (missing n = 36 [1.6%]) | 404 (18.0) |
| PI (missing n = 37 [1.7%]) | 868 (38.7) |
| NNRTI (missing n = 36 [1.6%]) | 373 (16.6) |
| NRTI (missing n = 40 [1.8%]) | 1440 (64.3) |
| FI (missing n = 35 [1.6%]) | 13 (0.6) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; FI, fusion inhibitor; GED, General Educational Development; HIV human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Percentage of nonmissing values displayed in this column.
STI Prevalence Over Time
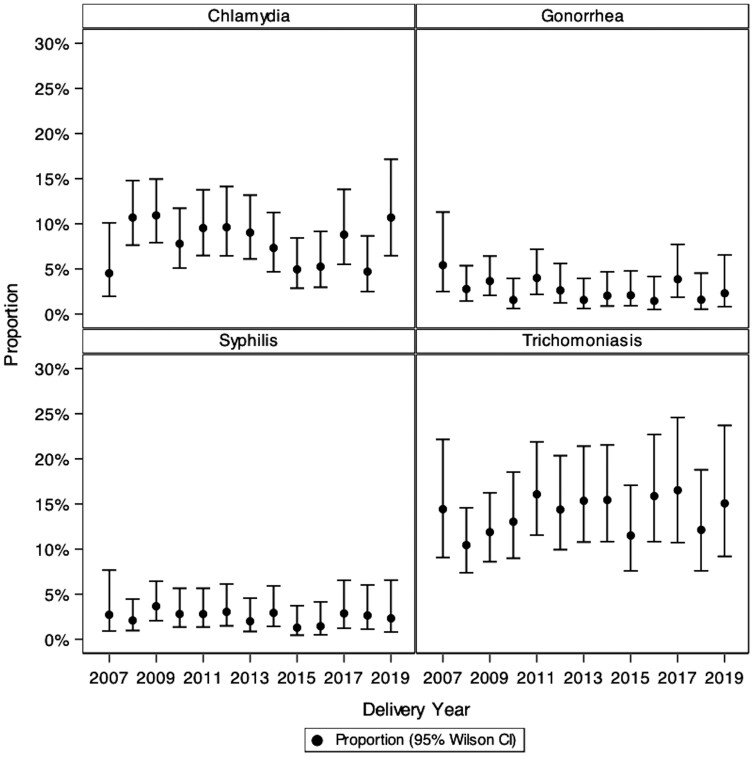
Organism-specific prevalence of each STI over the study period was as follows: CT, 7.7% (95% CI, 6.7%–8.9%); NG, 2.3% (95% CI, 1.7%–3.0%); syphilis, 2.4% (95% CI, 1.8%–3.1%); and TV, 14.5% (95% CI, 12.8%–16.3%). In both unadjusted and adjusted analyses, there was no evidence for linear change in prevalence over time between 2010 and 2019 for any of the 4 STIs ( Figure 1 and Supplementary Table 1).
Figure 1.
Annual prevalence and 95% confidence intervals (CIs) of 4 sexually transmitted infections in the Surveillance Monitoring for Antiretroviral Therapy Toxicities (SMARTT) dynamic cohort, 2007–2019.
Factors Associated With STIs
Univariable model estimates informed multivariable model selection and are shown in Supplementary Table 2. Multivariable model results for CT, NG, syphilis, and TV (complete case analysis and multiple imputation analysis) are presented in Table 2. Age <25 years (risk ratio [RR], 2.27 [95% CI, 1.64–3.14]), entry into prenatal care in the second or third trimester (RR, 1.69 [95% CI, 1.22–2.34]), and earliest viral load ≥400 copies/mL (RR, 1.96 [95% CI, 1.36–2.83]) were associated with CT infection. Younger age (RR, 2.86 [95% CI, 1.55–5.27]), lower annual household income, and recreational substance use (RR, 3.94 [95% CI, 1.45–10.8]) were associated with NG infection. Hispanic/Latinx ethnicity (RR, 0.20 [95% CI, .06–.63]) was associated with lower risk of NG. Later entry into prenatal care (RR, 2.02 [95% CI, 1.11–3.69]), alcohol use (RR, 2.05 [95% CI, 1.05–3.97]), and recreational substance use (RR, 3.81 [95% CI, 1.59–9.16]) were associated with syphilis infection.
Table 2.
Multivariable Poisson Generalized Estimating Equation Models of Demographic and Clinical Factors Associated With Diagnosis of Chlamydia trachomatis, Neisseria gonorrhoeae, Syphilis, and Trichomonas vaginalis During Pregnancy Among Pregnant People With Human Immunodeficiency Virus in the Surveillance Monitoring for Antiretroviral Therapy Toxicities (SMARTT) Dynamic Cohort, 2010–2019a
| Demographic or Clinical Factor | Chlamydia trachomatis, aRRb (95% CI) | Neisseria gonorrhoeae, aRRb (95% CI) | Syphilis, aRRb (95% CI) | Trichomonas vaginalis | |
|---|---|---|---|---|---|
| Complete Case, aRRb (95% CI) | Multiple Imputationc, aRRb (95% CI) | ||||
| Age at delivery, <25 y (vs ≥25 y) | 2.27 (1.64–3.14) | 2.86 (1.55–5.27) | NA | 1.47 (1.14–1.89) | 1.45 (1.12–1.88) |
| Annual household income | |||||
| <$10 000 | 1.78 (.90–3.53) | 5.37 (.69–41.59) | Not included | Not included | Not included |
| $10 001–$30 000 | 1.27 (.62–2.61) | 2.79 (.35–22.60) | |||
| ≥$30 001 | Ref | Ref | |||
| Hispanic or Latinx ethnicity | NA | 0.20 (.06–.63) | NA | Not included | Not included |
| Black or African American race | Not included | Not included | NA | 1.65 (1.14–2.40) | 1.54 (1.08–2.19) |
| Entry into prenatal care in second or third trimester (vs first trimester) | 1.69 (1.22–2.34) | Not included | 2.02 (1.11–3.69) | Not included | Not included |
| Tobacco use in pregnancy | 1.36 (.97–1.92) | NA | Not included | 1.91 (1.46–2.49) | 1.61 (1.18–2.20) |
| Marijuana use in pregnancy | NA | Not included | NA | Not included | 1.27 (.90–1.80) |
| Alcohol use in pregnancy | Not included | NA | 2.05 (1.05–3.97) | Not included | Not included |
| Recreational substance use in pregnancy | NA | 3.94 (1.45–10.75) | 3.81 (1.59–9.16) | 2.00 (1.19–3.37) | 1.71 (1.02–2.87) |
| Earliest CD4 count (cells/µL) in pregnancy | |||||
| 0–250 | 0.65 (.28–1.50) | NA | NA | Not included | NA |
| 251–500 | 1.25 (.60–2.60) | ||||
| 501–750 | 0.92 (.44–1.94) | ||||
| 751–1000 | 1.32 (.60–2.91) | ||||
| >1000 | Ref | ||||
| Earliest HIV RNA level in pregnancy ≥400 copies/mL | 1.96 (1.36–2.83) | 1.60 (.85–3.01) | Not included | 1.37 (1.05–1.79) | 1.48 (1.14–1.92) |
| Geographic region | |||||
| Midwest | Not included | Not included | NA | 2.77 (1.68–4.59) | 2.61 (1.59–4.30) |
| South | 0.62 (.19–2.01) | 2.22 (1.50–3.27) | |||
| West | 2.20 (1.49–3.26) | 1.16 (.69–1.97) | |||
| Puerto Rico | 1.11 (.64–1.90) | 0.60 (.20–1.82) | |||
| Northeast | Ref | Ref | |||
Abbreviations: aRR, adjusted risk ratio; CI, confidence interval; HIV, human immunodeficiency virus; NA, not applicable; Ref, reference group.
For predictive modeling, P < .20 used for significance level for model entry and retention (see Methods). Covariates with univariable association ≥0.20 (see Supplementary Table 3) are listed as “NA” and covariates with multivariable association ≥0.20 as “not included.”
aRR and 95% CI for association of each factor with each sexually transmitted infection (STI) from multivariable Poisson generalized estimating equation models. Model covariates include those with estimates shown.
Univariable and multivariable models for imputation all included age category, educational level, annual household income, race, ethnicity, geographical region, year of delivery, trimester of entry into prenatal care, substance use during pregnancy, perinatally acquired HIV status, earliest pregnancy CD4 count, earliest pregnancy HIV RNA, any antibiotic treatment for STI in pregnancy, trimester of antiretroviral therapy initiation, and missingness on and infection with other STIs (ie, Chlamydia trachomatis, Neisseria gonorrhoeae, and syphilis).
Missingness on TV was examined across covariates, and several differences were noted. In univariable analysis, missingness on TV was associated with Hispanic/Latinx ethnicity, residence in the Midwest and Puerto Rico, perinatally acquired HIV, and missingness on another STI. Those with lower educational level, self-reported Black race, use of marijuana, tobacco, and recreational substances during pregnancy, those receiving antibiotics for an STI, and those infected with CT were less likely to be missing on TV (Supplementary Table 3). In the complete case analysis, factors associated with TV infection included younger age (RR, 1.47 [95% CI, 1.14–1.89]), Black/African American racial identity (RR, 1.65 [95% CI, 1.14–2.40]), tobacco use in pregnancy (1.91 [95% CI, 1.46–2.49]), recreational substance use in pregnancy (RR, 2.00 [95% CI, 1.19–3.37]), higher earliest HIV viral load (RR, 1.37 [95% CI, 1.05–1.79]), and residence in the Midwest or West vs the Northeast (RR, 2.77 and 2.20, respectively; Table 2). In the multivariable MICE analysis, model parameters and interpretation were largely similar to the complete case analysis, except that geographic residence in the South (vs Northeast; RR, 2.22 [95% CI, 1.50–3.27]) was associated with increased risk of TV and residing in the West (vs the Northeast; RR, 1.16 [95% CI, .69–1.97]) was no longer associated with TV infection (Table 2).
STI and Preterm Birth
Prevalence of PTB was 13.6% of singleton pregnancies (n = 291/2146). In univariable analysis, neither any of the individual STIs nor a composite of any STI, nor number of STI infections, was associated with risk of PTB (Table 3).
Table 3.
Association Between Preterm Birth and Sexually Transmitted Infections in Pregnancy From Univariable Log-Binomial Generalized Estimating Equation Modelsa
| STI | PTB (n = 291), No. (%) | Term Delivery (n = 1855), No. (%) | RR (95% CI) | P Value |
|---|---|---|---|---|
| CT positive | 21 (12) | 148 (88) | 0.91 (.60–1.38) | .67 |
| CT negative (missing n = 41 [2%]) | 267 (14) | 1669 (86) | ||
| NG positive | 5 (10) | 45 (90) | 0.74 (.32–1.69) | .47 |
| NG negative (missing n = 42 [2%]) | 281 (14) | 1773 (86) | ||
| Syphilis positive | 11 (21) | 41 (79) | 1.60 (.90–2.83) | .11 |
| Syphilis negative (missing n = 68 [3%]) | 266 (13) | 1760 (87) | ||
| TV positive | 31 (14) | 185 (86) | 1.04 (.73–1.48) | .82 |
| TV negative (missing n = 642 [30%]) | 176 (14) | 1112 (86) | ||
| Any STI in pregnancy | 58 (15) | 335 (85) | 1.08 (.81–1.44) | .60 |
| No STI in pregnancy (missing n = 604 [28%]) | 154 (13) | 995 (87) | ||
| No. of STIs | ||||
| 0 | 154 (13) | 995 (87) | Ref | .17 |
| 1 | 45 (17) | 223 (83) | 1.23 (.90–1.67) | |
| ≥2 (missing n = 659 [31%]) | 6 (9) | 64 (91) | 0.66 (.31–1.39) |
Abbreviations: CI, confidence interval; CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; PTB, preterm birth; Ref, reference group; RR, risk ratio; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
CT, NG, syphilis, TV, any STI, and number of STIs were each modeled separately.
The potential for interaction between STIs with first trimester use of a PI, earliest pregnancy viral load, and PHIV status was explored. There was no statistically significant interaction between STIs and first trimester PI exposure (Supplementary Table 4). Individuals with syphilis and PHIV had higher risk of PTB in comparison to those who were syphilis negative and not perinatally infected (RR, 3.11 [95% CI, 1.06–9.13]; P = .039). Otherwise, there was no statistically significant interaction between STI and PHIV exposure (Supplementary Table 5). Similarly, those with syphilis and earliest pregnancy viral load ≥400 copies/mL had higher risk of PTB vs syphilis-negative individuals with viral load <400 copies/mL (RR, 2.68 [95% CI, 1.53–4.70]; P = .001). There were no other statistically significant interactions with STI and viral load (Supplementary Table 6).
DISCUSSION
In this large cohort study of pregnant PWH, we found a high prevalence of STIs and no evidence of change in prevalence over time. We identified several risk factors for each STI, which were mostly consistent with factors associated with STIs in the general population. Although we found a high prevalence of PTB, we did not find evidence of an association between STIs and increased risk of PTB. These data fill critical gaps in knowledge of STI patterns, correlates, and impacts in pregnant PWH, an important and underserved group.
In contrast to the general US population, where the prevalence of STIs has been increasing, we did not find a change in the 4 STIs over time with or without adjustment for confounding variables. This may in part reflect the fact that our population was restricted to pregnant people and many of the temporal trends in STIs have been observed primarily in men who have sex with men. Prevalence of CT, NG, syphilis, and TV in this study were 7.5%, 2.2%, 2.3%, and 14.5%, respectively. The Centers for Disease Control and Prevention (CDC) does not publish pregnancy-specific STI rates, and published estimates of prevalence of STIs in pregnancy are limited. An analysis that included nearly 13 000 recently delivered individuals from the Pregnancy Risk Assessment Monitoring System in 5 states between 2009 and 2011 found that self-reported prevalence of CT, NG, syphilis, and TV during pregnancy was 2.4%, 0.5%, 0.2%, and 1%, respectively [28]. Although self-report is likely to underestimate STI diagnoses [29], these figures provide some context for the high rates of STIs observed in this cohort of pregnant PWH. We are aware of only 1 other study publishing estimates of STI coinfection with HIV in pregnancy in the US. A single-center cohort study from Birmingham, Alabama that included 210 pregnant PWH delivering between 2000 and 2014 found prevalence of CT, NG, syphilis, and TV to be 17.1%, 5.7%, 0.5%, and 17.0%, respectively, and found incidence of these infections to be 6.2%, 2.1%, 0%, and 3.6%, respectively [30]. Identification and treatment of STIs is critically important in pregnancy and in the context of HIV to prevent maternal morbidity and adverse perinatal outcomes and to reduce horizontal and perinatal transmission of HIV.
Multiple risk factors for infection were identified, and these varied across STIs. Several risk factors are similar to those for the general population, including younger age, lower socioeconomic status, and tobacco, alcohol, and recreational substance use [31–33]. Higher initial pregnancy viral load was an HIV-specific risk factor that was associated with CT, NG, and TV, but not syphilis. This association may be explained by lower likelihood of being in care prior to pregnancy, unmeasured confounding (eg, higher-risk behaviors, lower adherence to medication), or greater susceptibility to STI acquisition.
The large proportion of values missing for TV infection is noteworthy, given that routine screening for TV is recommended by the CDC in PWH [34]. To account for this missingness, both a complete case approach and a multiple imputation approach were used to model risk factors for TV infection. Although these models resulted in largely similar conclusions, the multiple imputation model (but not the complete case model) identified residing in the South vs the Northeast as a risk factor for TV infection, which is consistent with prior studies [35, 36]. The discrepancies in the models may be due to differential missingness resulting from regional screening patterns. Although the reasons for high rates of missingness in these data are not known, patterns of screening in PWH, especially in pregnancy, deserve further study and, if low rates of screening are confirmed, interventions to improve this metric are warranted.
We did not find evidence of increased risk of PTB with any of the STIs despite the preponderance of available evidence suggesting that CT, NG, syphilis, and TV all are associated with increased risk of PTB in the general population [7, 8, 37–39]. The incidence of PTB in this cohort was high at 13.6% compared to approximately 10% in the US population as a whole [40]. The etiology of PTB is multifactorial. It is possible the effect of STI coinfection with HIV on PTB was not additive in this context of already heightened risk of PTB, or that we were underpowered to measure it. Additionally, we were unable to disentangle the impact of timing of infection and treatment, which may be associated with risk of prematurity. Although individuals with syphilis and higher viral load and those with syphilis and PHIV had an elevated risk of PTB, we interpret these findings with caution, given the small sample size. Overall, we did not find compelling evidence that PI use in the first trimester, higher initial HIV viral load, or PHIV status modified the relationship of STI to PTB.
Limitations of this study include those inherent to clinical records abstraction including variations in type and quality of clinical documentation. Additionally, STI testing was not standardized and data relating to mode of STI diagnosis, timing of infection, and type and timing of treatment were not collected. Nearly one-third of individuals had missing data for TV and reason for missingness is unknown, although we suspect that tests were not performed. Finally, the indication for PTB was not recorded and therefore we were not able to exclude iatrogenic PTB. Strengths include a large, geographically diverse, unique cohort with robust statistical methods including MICE utilized to explore the effect of missingness within the TV variable.
Pregnant PWH are an underserved population. STI coinfection in this group may increase risk to their own health as well as risk of adverse perinatal outcomes and has the potential to accelerate vertical and horizontal HIV transmission. In this large cohort of pregnant PWH delivering over a 10-year period, we found high but temporally stable rates of STIs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Marisa R Young, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA.
Carly Broadwell, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Deborah Kacanek, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Ellen G Chadwick, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Jennifer Jao, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Anna-Barbara Moscicki, Department of Pediatrics, University of California, Los Angeles, Los Angeles, California, USA.
Kathleen Powis, Departments of Internal Medicine and Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, USA; Department of Immunology and Infectious Diseases, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Katherine Tassiopoulos, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Lynn M Yee, Department of Obstetrics and Gynecology, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Lisa B Haddad, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia, USA; Population Council, Center for Biomedical Research, New York, New York, USA.
Notes
Acknowledgments. The authors thank the participants, caregivers, and families for their participation in the Pediatric HIV/AIDS Cohort Study (PHACS), and the individuals and institutions involved in the conduct of PHACS. The following institutions, clinical site investigators, and staff participated in conducting PHACS Surveillance Monitoring for Antiretroviral Therapy Toxicities (SMARTT) in 2019, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, Yoonsun Pyun; Baylor College of Medicine: Mary Paul, Shelley Buschur, Chivon McMullen-Jackson, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahoobullah Mirza Baig, Alma Villegas; Children’s Diagnostic and Treatment Center: Lisa-Gaye Robinson, Jawara Dia Cooley, James Blood, Patricia Garvie; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Jennifer Lewis; Rutgers–New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson, Karen Surowiec; St Jude Children’s Research Hospital: Katherine Knapp, Jamie Russell-Bell, Megan Wilkins, Stephanie Love; San Juan Hospital Research Unit/Department of Pediatrics, San Juan, Puerto Rico: Nicolas Rosario, Lourdes Angeli-Nieves, Vivian Olivera; State University of New York Downstate Medical Center: Stephan Kohlhoff, Ava Dennie, Jean Kaye, Jenny Wallier; Tulane University School of Medicine: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama at Birmingham: Cecelia Hutto, Paige Hickman, Julie Huldtquist, Dan Marullo; University of California, San Diego: Stephen A. Spector, Veronica Figueroa, Megan Loughran, Sharon Nichols; University of Colorado, Denver: Elizabeth McFarland, Emily Barr, Christine Kwon, Carrie Glenny; University of Florida, Center for HIV/AIDS Research, Education and Service: Mobeen Rathore, Saniyyah Mahmoudi, Rosita Almira, Jamilah Tejan; University of Illinois, Chicago: Karen Hayani, Lourdes Richardson, Renee Smith, Alina Miller; University of Miami: Gwendolyn Scott, Maria Mogollon, Gabriel Fernandez, Anai Cuadra; Keck Medicine of the University of Southern California: Toni Frederick, Mariam Davtyan, Dalia Regos Stewart, Guadalupe Morales-Avendano; University of Puerto Rico School of Medicine, Medical Science Campus: Zoe M. Rodriguez, Lizmarie Torres, Nydia Scalley.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
Financial support. The PHACS network was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health Office of the Director, National Institute of Dental and Craniofacial Research, National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke, National Institute on Deafness and Other Communication Disorders, National Institute of Mental Health, National Institute on Drug Abuse, National Cancer Institute, National Institute on Alcohol Abuse and Alcoholism, and the National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102), Tulane University School of Medicine (HD052104), and Harvard T. H. Chan School of Public Health for the PHACS 2020 network (P01HD103133).
References
- 1. Eisinger RW, Erbelding E, Fauci AS. Refocusing research on sexually transmitted infections. J Infect Dis 2020; 222:1432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Sexually transmitted disease surveillance 2019. 2021. Available at: https://www.cdc.gov/std/statistics/2019/default.htm. Accessed 6 December 2021. [Google Scholar]
- 3. Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis 2018; 67:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . STDs and pregnancy—CDC fact sheet. Available at: www.cdc.gov/std/pregnancy/stdfact-pregnancy.htm. Accessed 8 May 2022.
- 5. Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol 2000; 183:662–8. [DOI] [PubMed] [Google Scholar]
- 6. Alger LS, Lovchik JC, Hebel JR, Blackmon LR, Crenshaw MC. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol 1988; 159:397–404. [DOI] [PubMed] [Google Scholar]
- 7. Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ 2013; 91:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis 2014; 41:369–76. [DOI] [PubMed] [Google Scholar]
- 9. Cotch MF, Pastorek JG. 2nd, Nugent RP, et al.Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 1997; 24:353–60. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention . Sexually transmitted disease surveillance 2018. 2019. Available at: https://www.cdc.gov/std/stats. Accessed 14 October 2020. [Google Scholar]
- 11. Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45:1319–26. [DOI] [PubMed] [Google Scholar]
- 12. Freeman AH, Katz KA, Pandori MW, et al. Prevalence and correlates of Trichomonas vaginalis among incarcerated persons assessed using a highly sensitive molecular assay. Sex Transm Dis 2010; 37:165–8. [DOI] [PubMed] [Google Scholar]
- 13. Hogben M, Leichliter JS. Social determinants and sexually transmitted disease disparities. Sex Transm Dis 2008; 35(12 Suppl):S13–8. [DOI] [PubMed] [Google Scholar]
- 14. Fastring DR, Amedee A, Gatski M, et al. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis and vaginal shedding of HIV-1 RNA. Sex Transm Dis 2014; 41:173–9. [DOI] [PubMed] [Google Scholar]
- 15. Low AJ, Konate I, Nagot N, et al. Neisseria gonorrhoeae and Chlamydia trachomatis infection in HIV-1-infected women taking antiretroviral therapy: a prospective cohort study from Burkina Faso. Sex Transm Infect 2014; 90:100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanton C, Weiss HA, Le Goff J, et al. Correlates of HIV-1 genital shedding in Tanzanian women. PLoS One 2011; 6:e17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adachi K, Xu J, Yeganeh N, et al. Combined evaluation of sexually transmitted infections in HIV-infected pregnant women and infant HIV transmission. PLoS One 2018; 13:e0189851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis 2009; 36:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS 2001; 15:105–10. [DOI] [PubMed] [Google Scholar]
- 20. Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis 2001; 183:1017–22. [DOI] [PubMed] [Google Scholar]
- 21. Powis KM, Kitch D, Ogwu A, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis 2011; 204:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watts DH, Williams PL, Kacanek D, et al. Combination antiretroviral use and preterm birth. J Infect Dis 2013; 207:612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams PL, Seage GR 3rd, Van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol 2012; 175:950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22:209–12. [Google Scholar]
- 25. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 1998; 52:119–26. [Google Scholar]
- 26. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007; 16:219–42. [DOI] [PubMed] [Google Scholar]
- 27. Little RJA, Rubin DB. Statistical analysis with missing data. In: Wiley series in probability and statistics. 3rd ed. Hoboken, NJ: Wiley, 2020. [Google Scholar]
- 28. Williams CL, Harrison LL, Llata E, Smith RA, Meites E. Sexually transmitted diseases among pregnant women: 5 states, United States, 2009–2011. Matern Child Health J 2018; 22:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iritani BJ, Ford CA, Miller WC, Hallfors DD, Halpern CT. Comparison of self-reported and test-identified chlamydial infections among young adults in the United States of America. Sex Health 2006; 3:245–51. [DOI] [PubMed] [Google Scholar]
- 30. Dionne-Odom J, Khan MJ, Jauk VC, et al. HIV status and other risk factors for prevalent and incident sexually transmitted infection during pregnancy (2000–2014). Infect Dis Obstet Gynecol 2019; 2019:6584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim TG, Young MR, Goggins ER, et al. Trichomonas vaginalis in pregnancy: patterns and predictors of testing, infection, and treatment. Obstet Gynecol 2020; 135:1136–44. [DOI] [PubMed] [Google Scholar]
- 32. Gregory ECW, Ely DM. Trends and characteristics of sexually transmitted infections during pregnancy: United States, 2016–2018. Natl Vital Stat Rep 2020; 69:1–11. [PubMed] [Google Scholar]
- 33. Goggins ER, Chamberlain AT, Kim TG, Young MR, Jamieson DJ, Haddad LB. Patterns of screening, infection, and treatment of Chlamydia trachomatis and Neisseria gonorrhoeae in pregnancy. Obstet Gynecol 2020; 135:799–807. [DOI] [PubMed] [Google Scholar]
- 34. Workowski KA, Bolan GA; Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 35. Miller WC, Swygard H, Hobbs MM, et al. The prevalence of trichomoniasis in young adults in the United States. Sex Transm Dis 2005; 32:593–8. [DOI] [PubMed] [Google Scholar]
- 36. Stemmer SM, Mordechai E, Adelson ME, Gygax SE, Hilbert DW. Trichomonas vaginalis is most frequently detected in women at the age of peri-/premenopause: an unusual pattern for a sexually transmitted pathogen. Am J Obstet Gynecol 2018; 218:328.e1–13. [DOI] [PubMed] [Google Scholar]
- 37. Qin J, Yang T, Xiao S, Tan H, Feng T, Fu H. Reported estimates of adverse pregnancy outcomes among women with and without syphilis: a systematic review and meta-analysis. PLoS One 2014; 9:e102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahmadi A, Ramazanzadeh R, Sayehmiri K, Sayehmiri F, Amirmozafari N. Association of Chlamydia trachomatis infections with preterm delivery; a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018; 18:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vallely LM, Egli-Gany D, Wand H, et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: systematic review and meta-analysis. Sex Transm Infect 2021; 97:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol 2017; 41:387–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.