Abstract
Background
Although previous studies have shown that vitamin A deficiency is associated with incident tuberculosis (TB) disease, the direction of the association has not been established. We investigated the impact of vitamin A deficiency on TB disease progression.
Methods
We conducted a longitudinal cohort study nested within a randomized clinical trial among HIV-infected patients in Haiti. We compared serial vitamin A levels in individuals who developed TB disease to controls matched on age, gender, follow-up time, and time to antiretroviral therapy initiation. We also evaluated histopathology, bacterial load, and immune outcomes in TB infection in a guinea pig model of dietary vitamin A deficiency.
Results
Among 773 participants, 96 developed incident TB during follow-up, 62.5% (60) of whom had stored serum samples obtained 90–365 days before TB diagnosis. In age- and sex- adjusted and multivariate analyses, respectively, incident TB cases were 3.99 times (95% confidence interval [CI], 2.41 to 6.60) and 3.59 times (95% CI, 2.05 to 6.29) more likely to have been vitamin A deficient than matched controls. Vitamin A–deficient guinea pigs manifested more extensive pulmonary pathology, atypical granuloma morphology, and increased bacterial growth after experimental TB infection. Reintroduction of dietary vitamin A to deficient guinea pigs after established TB disease successfully abrogated severe disease manifestations and altered cellular immune profiles.
Conclusions
Human and animal studies support the role of baseline vitamin A deficiency as a determinant of future TB disease progression.
Keywords: vitamin A, retinol, Mycobacterium tuberculosis, nutritional deficiency
Among a cohort of individuals living with human immunodeficiency virus, baseline vitamin A deficiency was associated with increased risk of future progression to tuberculosis (TB). Vitamin A–deficient guinea pigs also experienced higher bacillary loads and had distinct histopathological responses to TB exposure.
Tuberculosis (TB) remains a leading cause of death worldwide from an infectious disease. Among the many host risk factors associated with the development of TB disease, undernutrition accounts for a large portion of the disease burden, with population-attributable risks exceeding 60% in some high TB–burden countries [1, 2]. These data emphasize the importance of understanding specific dietary micronutrients that contribute to the risk of TB disease progression after an exposure [3].
One micronutrient that may contribute to TB risk is vitamin A. Vitamin A and, specifically, its bioactive metabolite, retinoic acid, has a well-established pleiotropic role in immune function [4] and known benefit in immunity to viral and diarrheal diseases [5]. Retinoic acid has a direct impact on dendritic cell and macrophage function [6] and contributes to T-cell differentiation [7], including Th1 and Th17 polarization, all of which are critical in the immune pathogenesis of TB. Retinol, the circulating form of vitamin A, is converted to retinaldehyde and then to retinoic acid by retinaldehyde dehydrogenase, the local availability of which underlies cellular and organ capacity for generating bioactive vitamin A. Cross-sectional human studies have demonstrated low serum retinol concentrations in patients with established TB disease [8–12], and more recently, some have identified a limited capacity for retinoic acid generation during Mycobacterium tuberculosis (Mtb) infection in human lung tissue [13].
We and others have conducted longitudinal, prospective studies in which we found that individuals with low baseline serum vitamin A levels have significantly higher risk of developing TB disease during longitudinal follow-up [14,15]. However, because TB is a disease of insidious onset that may remain undetected for months prior to diagnosis, we could not exclude the possibility that low serum retinol levels were the result of early unrecognized TB disease rather than its cause. In our previous prospective study, most TB disease occurred within 3 months of the baseline assessment of vitamin A, making it difficult to rule out a reverse causal effect [14]. It thus remains unclear what role vitamin A deficiency plays in modulating TB risk.
Here, we address this role and the potential bidirectional relationship between TB and vitamin A deficiency through 2 complementary approaches. First, we conducted a longitudinal study to assess the impact on incident TB disease of serum retinol status obtained from participants between 90 and 365 days prior to TB diagnosis. Second, we developed a guinea pig model of vitamin A deficiency and compared the outcomes of experimental Mtb infection in vitamin A– deficient and vitamin A–sufficient animals and after restoring dietary vitamin A in established TB disease. Both approaches provide support for a strong causal impact of vitamin A deficiency in TB disease.
METHODS
Longitudinal Cohort Study in Individuals in Haiti Living With Human Immunodeficiency Virus
Study Setting and Population
The Comprehensive Program for Research in AIDS (CIPRA) HT-001 trial was an open-label, randomized, controlled trial to evaluate early vs delayed initiation of antiretroviral therapy (ART) in patients in Haiti with human immunodeficiency virus (HIV) with CD4 < 350 cell/mm3 from August 2005 to July 2008. At enrollment, all participants were screened for TB infection using the tuberculin skin test (TST) and for active TB disease. Participants with TST result ≥5 mm were considered to have latent TB infection and given isoniazid preventive therapy [16]. Participants provided baseline serum sample and every 6 months. Samples were stored for later use. Study participants were monitored for incident TB disease with monthly physician evaluation [17]. Those with TB symptoms received chest X-ray and provided 3 sputum samples for smear and mycobacterial culture or a biopsy was obtained for smear and culture if extrapulmonary TB was suspected. The results of this trial have been presented elsewhere [18], and detailed study methods are provided in the Supplementary Materials.
Matched Case-Control Study
We identified individuals who developed TB disease during follow-up and selected from this group those for whom at least 1 blood sample had been obtained 90–365 days prior to TB diagnosis. To account for the possibility that participants with subclinical TB disease may have developed decreased retinol levels, we excluded blood samples obtained less than 90 days before TB diagnosis. For each participant who developed TB disease, we randomly selected up to 5 controls, matching on age, gender, and follow-up time. Among cases who initiated ART before TB diagnosis, we further matched on time from enrollment to ART initiation. If there was more than 1 eligible blood sample for a control participant, we chose the sample obtained at the time closest to when the case sample was obtained. We considered participants with retinol <20 µg/dL to be vitamin A deficient per World Health Organization guidelines [19]. We used a modified Poisson generalized estimating equation to evaluate the association between vitamin A deficiency and incident TB disease. We used customized R codes to evaluate the association between vitamin A deficiency and TB progression using the geepack package in software R 4.0.4.
Case-Only Study Design
Among participants without TB disease at baseline, we identified incident TB cases who had 2 blood samples obtained within 90–730 days before TB diagnosis that were at least 180 days apart. We excluded any TB case who initiated ART between the 2 blood draws. We used the paired Student T test to determine whether incident TB cases had decreasing serum vitamin A levels before the onset of TB disease.
Ethics Statement
The Harvard School of Public Health Institutional Review Board approved this cohort study. The Center of the Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections Institutional Review Board and the Weill Cornell Medical College Institutional Review Board approved the CIPRA HT-001 trial. All participants provided written informed consent.
Impact of Vitamin A Deficiency in Guinea Pigs
Nutritional vitamin A deficiency was established in guinea pigs by feeding a purified diet without retinyl palmitate as the sole source of vitamin A (Supplementary Figures 1, 2). The impact of vitamin A deficiency on TB disease outcome was determined at 60 days after exposure to Mtb in guinea pigs either sufficient or deficient in vitamin A or in deficient guinea pigs with restored dietary vitamin A during established TB disease. Flow cytometry data (Supplementary Table 1) were analyzed using FlowJo analysis software, version 10. Additional details are provided in the Supplementary Materials.
Ethics Statement
All experimental protocols were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals under Animal Welfare Assurance A3572-01. The Animal Care and Usage Committee at Colorado State University approved all protocols under protocol 16-6630AA.
RESULTS
Longitudinal Cohort Study in Individuals in Haiti Living With HIV
We assessed 773 participants free of TB disease at enrollment (Supplementary Table 2), of whom 96 (12.4%) developed TB disease during a median follow-up period of 4.3 years (interquartile range [IQR], 3.5–5.1). The median time to TB disease diagnosis was 494.5 days (IQR, 208.5–998.0). Among the 96 incident TB cases, 33 (34.4%) were diagnosed with TB prior to ART initiation.
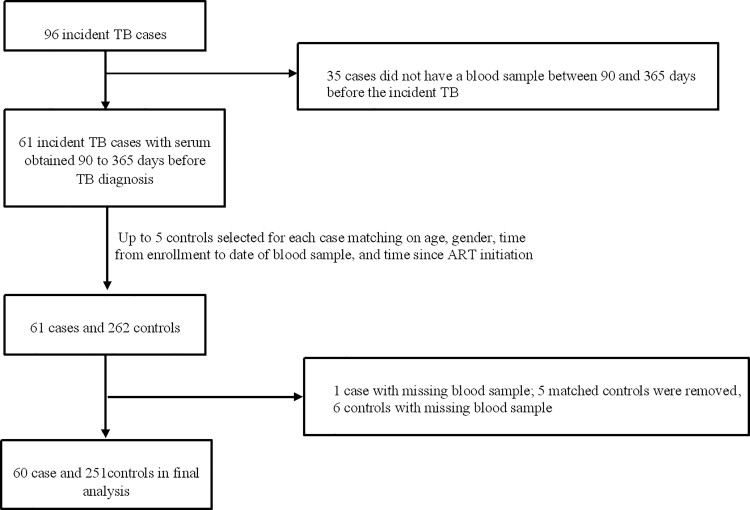
Of 96 incident TB patients, 60 (62.5%) had available serum samples obtained 90–365 days before TB diagnosis. These cases were matched to 251 controls (Figure 1). Baseline characteristics of cases and controls are presented in Table 1. Nineteen (31.7%) TB cases were vitamin A deficient 90–365 days prior to diagnosis compared with 21 (8.5%) controls. In the age- and sex-adjusted analyses, the cases were 3.99 times (95% confidence interval [CI], 2.41 to 6.60; P < .0001) more likely to have been vitamin A deficient than controls (Table 2). After we adjusted for ART use, body mass index, and C-reactive protein, vitamin A deficiency remained associated with incident TB disease (adjusted risk ratio [aRR], 3.59; 95% CI, 2.05 to 6.29; P < .0001; Table 2). These results were robust in sensitivity analyses adjusted for baseline TST status (aRR, 4.04; 95% CI, 2.12 to 7.69; P < .0001) and restricted to samples obtained between 180 and 365 days before TB diagnosis (aRR, 2.56; 95% CI, 1.43 to 4.56; P .001).
Figure 1.
Flow chart of matched case-control study. Abbreviations: ART, antiretroviral therapy; TB, tuberculosis.
Table 1.
Baseline Characteristics of Cases and Controls
| Characteristic | Case (N = 60) n (%) or Median (IQR) | Na | Control (N = 251) n (%) or Median (IQR) | Na |
|---|---|---|---|---|
| Age in years | 38.0 (31.0–43.5) | 60 | 38.0 (32.0–45.0) | 251 |
| Female | 33 (55.0) | 60 | 149 (59.4) | 251 |
| Living with spouse/partner | 28 (46.7) | 60 | 107 (42.6) | 251 |
| Education | 60 | 251 | ||
| No school | 22 (36.7) | 72 (28.7) | ||
| Primary school | 14 (23.3) | 73 (29.1) | ||
| Secondary school or more | 24 (40.0) | 106 (42.2) | ||
| Annual income ≤$129/year | 39 (65.0) | 60 | 152 (60.6) | 251 |
| History of tuberculosis disease | 16 (26.7) | 60 | 38 (15.1) | 251 |
| Positive tuberculin skin test (≥5 mm) | 22 (44.9) | 49 | 65 (29.0) | 224 |
| Body mass index, kg/m2 | 60 | 251 | ||
| Underweight | 15 (25.0) | 32 (12.8) | ||
| Overweight | 10 (16.7) | 37 (14.7) | ||
| Normal | 35 (58.3) | 182 (72.5) | ||
| Use of antiretroviral therapy | 58 (96.7) | 60 | 239 (95.2) | 251 |
| Human immunodeficiency virus clinical stage | 60 | 251 | ||
| 1 | 12 (20.0) | 66 (26.3) | ||
| 2 | 32 (53.3) | 147 (58.6) | ||
| 3 | 16 (26.7) | 38 (15.1) | ||
| 4 | 0 (0.0) | 0 (0.0) | ||
| Baseline CD4 count (cells/mm3) | 282.0 (239.5–311.0) | 60 | 282.0 (255.0–312.0) | 251 |
| Baseline hemoglobin (g/dL) | 11.5 (10.2–12.5) | 60 | 11.7 (10.6–12.9) | 251 |
| Baseline C-reactive protein (ng/mL) | 1220.5 (671.3) | 60 | 1352.0 (556.0–3790.0) | 245 |
| Highest tertile | 12 728.0 (5590.0–20 957.0) | 21 | 6708.5 (4032.0–10 450.0) | 81 |
| Middle tertile | 1129.5 (899.0–1650.0) | 23 | 1417.0 (1095.0–1789.0) | 79 |
| Lowest tertile | 299.5 (160.0–359.5) | 16 | 282.0 (158.5–566.0) | 85 |
| Vitamin A deficiency (<20 µg/dL) | 19 (31.7) | 60 | 21 (8.4) | 251 |
Abbreviation: IQR, interquartile range.
Number of participants with data for corresponding variable.
Table 2.
Association Between Vitamin A Deficiency and Risk of Incident Tuberculosis Disease
| Variable | Univariate ORa (95% CI) N = 311 | P Value | Multivariate ORb (95% CI) N = 305 |
P Value |
|---|---|---|---|---|
| Body mass index, kg/m2 | ||||
| Underweight | 2.89 (1.42–5.87) | .003 | 2.35 (1.18–4.65) | .01 |
| Overweight | 1.52 (.69–3.32) | .30 | 1.67 (.77–3.65) | .20 |
| Normal | 1.00 | 1.00 | ||
| Use of antiretroviral therapy | 1.02 (.62–1.66) | .95 | 1.24 (.77–2.01) | .38 |
| Baseline C-reactive protein (ng/mL)c | ||||
| Highest tertile | 1.12 (.63–2.00) | .70 | 0.92 (.52–1.63) | .78 |
| Middle tertile | 0.86 (.45–1.65) | .66 | 0.77 (.40–1.46) | .42 |
| Lowest tertile | 1.00 | 1.00 | ||
| Vitamin A deficiency (<20 µg/dL) | 3.99 (2.41–6.60) | <.0001 | 3.59 (2.05–6.29) | <.0001 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for matching factors (age and gender).
Adjusted for matching factors (age and gender), body mass index, use of antiretroviral therapy, and baseline C-reactive protein levels.
N = 305.
Case-Only Study
We identified 15 incident TB cases who had repeat blood draws between 90 and 730 days before TB diagnosis (Supplementary Table 3). In 5 of the 15 cases, vitamin A levels fell from sufficient to deficient prior to the diagnosis of TB disease, while none had their vitamin A levels change from deficient to sufficient levels (McNemar test exact P value, .07).
Impact of Vitamin A Deficiency in Guinea Pigs
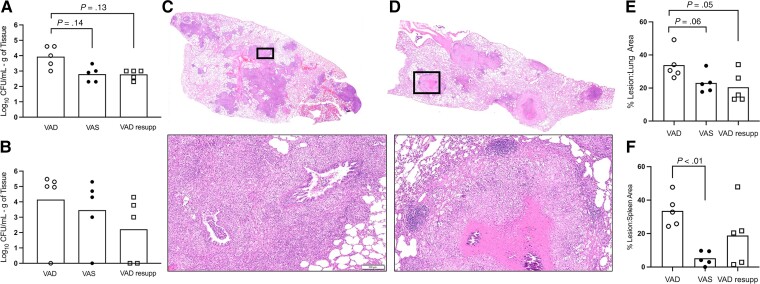
To determine the impact of vitamin A deficiency on the severity of TB disease, vitamin A–deficient guinea pigs were infected with Mtb and compared to guinea pigs that consumed a vitamin A–sufficient diet. Mtb colony-forming units (CFUs) in lung in vitamin A–deficient guinea pigs at day 60 of infection were 1.28 log10 higher (P = .014; 95% CI, .25 to 2.31; Figure 2A) than in vitamin A–sufficient guinea pigs, and we noted a 10.8% (P = .06; 95% CI, −.58 to 22.12) and 28.4% (P < .01; 95% CI, 7.43 to 49.3) mean increase in lung and spleen granuloma lesion area, respectively (Figure 2E, 2F) among vitamin A–deficient guinea pigs. Atypical microscopic morphology of pulmonary granulomas was consistent across vitamin A–deficient guinea pigs. This included a near complete lack of granuloma necrosis and poorly delineated granulomas that lacked development of peripheral lymphoid follicular structures (Figure 2C, 2D; Supplementary Figures 3, 4).
Figure 2.
Impact of vitamin A deficiency on tuberculosis disease outcome in the guinea pig model 60 days after infection with Mycobacterium tuberculosis. Shown are outcomes from VAD (open circle), VAS (closed circle), and VAD guinea pigs resupplemented with vitamin A at 30 days after infection (open square). A, Bacterial burden in lung of VAD, VAS, and VAD resupp. B, Bacterial burden in spleen of VAD, VAS, and VAD resupp. C, Representative hematoxylin and eosin (H&E)–stained histology images of a full lung section from a VAD guinea pig. D, Representative H&E-stained histology images of a full lung section from a VAD guinea pig. Black boxes outline the high magnification images below panels C and D (bar = 100 μm). E, Proportion of lung tissue area affected by inflammatory lesions in VAD, VAS, and VAD resupp guinea pigs. F, Proportion of spleen tissue area affected by inflammatory lesions in VAD, VAS, and VAD resupp guinea pigs. Abbreviations: CFU, colony-forming unit; resupp, resupplemented; VAD, vitamin A deficient; VAS, vitamin A sufficient.
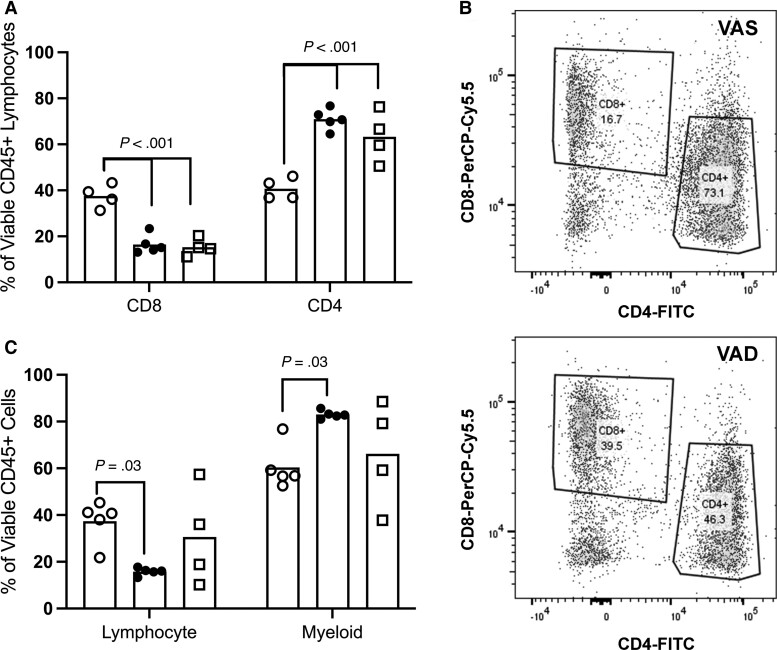
Bronchoalveolar lavage in the vitamin A–deficient guinea pigs (Supplementary Figures 6–8) at day 60 of infection (Figure 3A–3C) demonstrated a higher frequency of lymphocytes (mean increase, 21.6%; 95% CI, 1.4 to 41.7; Supplementary Figure 5), composed of a higher frequency of CD8+ lymphocytes (mean increase, 21.1%; P < .01; 95% CI, 11.7 to 32.8) and lower frequency of CD4+ lymphocytes (mean decrease, 30.2%; P < .01; 95% CI, 20.3 to 40.2), along with reduced myeloid leukocytes (mean decrease, 22.6%; 95% CI, 2.4 to 42.8) compared with vitamin A–sufficient guinea pigs (Supplementary Figure 6). Similar trends in CD4+ and CD8+ cell populations were identified among cells dissociated from lung granulomas (Supplementary Figure 7) without significant difference in total CD3+ cells (Supplementary Figure 8).
Figure 3.
Impact of vitamin A status on responding immune cell phenotypes recovered from infected lung of guinea pigs. Leukocytes recovered by terminal bronchoalveolar lavage at day 60 of infection were phenotyped using flow cytometry. Shown are comparative proportions between VAD (open circle), VAS (closed circle), and VAD guinea pigs resupplemented with vitamin A at 30 days after infection (open square). A, Comparison of CD4+ and CD8+ cells among CD45+ lymphocytes recovered by bronchoalveolar lavage at day 60 of infection. B, Representative flow cytometry contour plots demonstrating CD4+ and CD8+ among gated CD45+ lymphocytes in VAS and VAD guinea pigs. C, Comparison of proportions of lymphocytes and myeloid populations among total CD45+ immune cells recovered by bronchoalveolar lavage at day 60 of infection. Similar differences in immune phenotypes were also identified in cell suspensions from isolated lung granulomas (Supplementary Figure E7). Abbreviations: FITC, fluorescein isothiocyanate; VAD, vitamin A deficient; VAS, vitamin A sufficient.
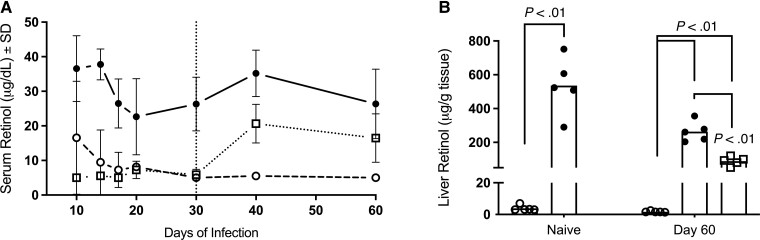
To fully address the question of possible reverse causality, we determined whether Mtb infection leads to a transient reduction in vitamin A regardless of nutritional status by monitoring serum retinol levels over the course of infection in the vitamin A–sufficient guinea pigs and liver retinol concentration at euthanasia end points. Serum retinol concentrations decreased during Mtb infection despite consumption of sufficient dietary vitamin A (Figure 4A). The maximum rate of reduction in serum retinol was observed between day 14 and day 20 of infection, reaching a peak reduction of 40% (13.94 mg/dL ± 6.73, P = .024) at day 20. A reduction in liver retinol was observed at day 60 of infection with a mean decrease of 272 mg/g of tissue (P < .001; 95% CI, 133.3 to 411.7), which was confirmed to be reduced in vitamin A–sufficient guinea pigs in parallel with serum retinol in a separate experiment performed at day 21 of infection (Figure 4B, Supplementary Figure 9).
Figure 4.
Impact of diet and Mycobacterium tuberculosis infection on serum and liver retinol concentrations in the guinea pig model. Impact is shown in vitamin A–deficient (open circle), vitamin A–sufficient (closed circle), and vitamin A–deficient guinea pigs resupplemented with vitamin A at 30 days after infection (VAD resupp; open square). A, Serum retinol concentrations measured in duplicative experiments over the course of infection. B, Liver retinol stores measured at day 60 of infection compared with liver retinol concentrations in age-matched naive guinea pigs. Resupplementation at day 30 of infection (VAD resupp) is designated by the vertical dotted line. Abbreviation: SD, standard deviation.
To determine whether vitamin A supplementation could resolve the severity of TB disease seen in vitamin A deficiency, we allowed vitamin A–deficient guinea pigs to progress to day 30 of Mtb infection, at which time dietary vitamin A was restored. At day 60 of infection, we observed no difference in the area morphometry of the granuloma lesions in lung or spleen (Figure 2, Supplementary Figure 4) between the vitamin A–sufficient and resupplemented guinea pigs or in the bacterial burden in the lung (mean difference, 0.18 log10 CFU/g; 95% CI, −1.21 to .85) and spleen (Figure 2A, 2B). In parallel with improved TB disease outcome, pulmonary T-cell populations were restored in vitamin A–deficient, resupplemented guinea pigs to levels similar to levels in sufficient guinea pigs with a 7.7% mean difference in CD4+ lymphocytes (95% CI, −17.7 to 2.3) and 1.2% mean difference in CD8+ lymphocytes (95% CI, −11.2 to 8.8; Figure 3A).
DISCUSSION
In previous prospective studies, we and others have noted an association between low serum retinol levels and the risk of developing TB disease. However, because acute infection is known to transiently reduce retinol levels, the possibility of a reverse causal effect could not be excluded, whereby patients with incipient, but undetected, TB had reduced retinol levels as a result of their disease [14, 15]. In this study, we provide support for the causal impact of vitamin A deficiency on incident TB disease in 2 ways. First, in a cohort of patients with HIV, we showed that low retinol levels measured between 3 and 12 months prior to a diagnosis of TB strongly predict subsequent disease. When we further measured serum retinol at least 6 months prior to disease diagnosis, this association was mildly attenuated but remained statistically significant. In support of these data, we demonstrate here that a lack of vitamin A, through nutritional deficiency in the guinea pig model, promotes more severe TB disease outcomes reflected by impaired control of bacterial growth, altered immunity, and extensive pathology with altered histomorphological patterns. Addressing the potential for reverse causality in the guinea pig model, we have also established a bidirectional relationship between TB and vitamin A deficiency by showing that active TB disease reduces serum vitamin A even in the presence of sufficient vitamin A intake, as previously suggested in human studies [2, 8–12]. The timing of this infection-induced serum deficiency between days 10 and 40 of infection raises the question of whether vitamin A deficiency during the development of adaptive immunity affects TB outcome, even in the face of sufficient nutrition. We hypothesize that such an impact would depend on retinol availability to the lung and the ability of the lung immune response to generate bioactive all-trans retinoic acid (ATRA) from retinol.
Our findings are consistent with those from previous studies in both human populations and animal models. We and others have shown that low serum retinol levels in high-risk groups with and without HIV are strongly associated with incident TB disease [14, 15]. While the short time span between ascertainment of vitamin A status and TB diagnosis raises the possibility of reverse causation among people who might have had undiagnosed incipient TB at baseline, few studies have implicated low vitamin A as a determinant of later TB progression. For example, a study conducted in Philadelphia in the 1930s noted increased risk of TB among participants with baseline low vitamin A who were followed for up to 7 years [20]. Similarly, Soh and colleagues showed that participants in the highest quartile of vitamin A intake at baseline were modestly protected compared with those in the lowest quartile from developing TB disease over an average of 16.9 years of follow-up [21].
Our animal data also align with that from previous work, although recent in vivo studies have not compared TB outcomes in animal models with nutritional vitamin A deficiency to those with sufficient intake. Yamada and colleagues noted a reduction in CFUs at 3 and 5 weeks after Mtb infection in both lung and spleen and less severe histopathology in vitamin A–sufficient rats treated with pharmacological doses of ATRA [22]. In that study, CD4-positive and CD8-positive T cells, natural killer cells, and CD163-positive macrophages were increased in infected lung tissues of retinoic acid–treated rats at week 3 after infection, but these differences were no longer significant by week 5. Similarly, O’Connor and colleagues showed that treatment of Mtb-infected BALB/c mice with an inhalable formulation of ATRA reduced the bacterial burden and pulmonary pathology [23]. Our results indicated that dietary vitamin A deficiency worsened TB disease outcome and bacterial burden. In contrast to these previous studies, we also noted a shift toward a dominant CD8 T-cell response over CD4 after 60 days of infection, which may reflect the higher disease burden at this late point in the course of infection in response to higher bacterial burden rather than any meaningful discrepancies in immune response to vitamin A.
Although the precise mechanisms by which vitamin A exerts its protective effect against Mtb are not clear, retinol is known to modulate multiple immune responses relevant to TB disease, including epithelial barrier maintenance [6], innate phagocyte response, and adaptive T-cell immunity [7]. Studies have also demonstrated retinoic acid–enhanced macrophage activation in response to in vitro Mtb infection and antimicrobial activity in ATRA-stimulated, Mtb-infected macrophages that was mediated through an ATRA-induced reduction in cellular cholesterol [22, 24]. This ATRA-mediated killing of Mtb is dependent on the NPC2 gene, which regulates cellular cholesterol transport [24]; recent transcriptional profiling studies have identified upregulation of the NPC2 gene in TB disease [25, 26]. Recent studies also suggest a role for retinoic acid in promoting Th1 and Th17 activation during inflammation [7]. Last, retinol is known to induce ILC3 (type 3 innate lyphoid cell) differentiation and homing to the gut [27]. While ILC3 appears to play a role in protection against pulmonary TB, it is unclear whether ILC3 activity in the lung is affected by retinol status. In this study, we show that vitamin A deficiency leads to marked histopathological changes that stray from the typical granuloma structure that defines the guinea pig model [28]. This finding combined with alterations in lymphoid and myeloid immune phenotypes suggests a requirement for vitamin A in the coordinated immune development of the granuloma. Vitamin A is likely to be involved in multiple immune compartments in the response to Mtb infection.
In conclusion, both human studies and animal models support the role of vitamin A in protection against TB disease progression. The prevalence of vitamin A deficiency in children aged <5 years remains high in low- and middle-income countries, regions with disproportionately high TB burden [5]. Our cohort study was limited to adults with advanced HIV, and it is unclear how HIV status exerts specific effects on vitamin A metabolism. Data on population vitamin A levels in adults are sparse, and it is thus difficult to estimate the contribution of vitamin A deficiency to TB disease burden. There is an urgent need to better understand the prevalence of vitamin A deficiency among adults with and without HIV and to explore the impact of vitamin A supplementation as an inexpensive, safe, and effective means of preventing progression from TB infection to TB disease.
Supplementary Material
Contributor Information
Brendan K Podell, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
Omowunmi Aibana, Department of Internal Medicine, McGovern Medical School, Houston, Texas, USA.
Chuan-Chin Huang, Department of Global Health and Social Medicine, Harvard Medical School, Boston, Massachusetts, USA.
James E DiLisio, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
Macallister C Harris, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
David F Ackart, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
Kody Armann, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
Alexander Grover, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
Patrice Severe, Haitian group for the study of Kaposi's Sarcoma and Opportunistic Infections (GHESKIO) Centers, Port au Prince, Haiti.
Marc Antoine Jean Juste, Haitian group for the study of Kaposi's Sarcoma and Opportunistic Infections (GHESKIO) Centers, Port au Prince, Haiti.
Kathryn Dupnik, Department of Medicine, Center for Global Health, Weill Cornell Medicine, New York, New York, USA.
Randall J Basaraba, Mycobacteria Research Laboratories, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colorado, USA.
Megan B Murray, Department of Global Health and Social Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Dr Dan Fitzgerald, MD, at the Haitian group for the study of Kaposi's Sarcoma and Opportunistic Infections (GHESKIO) Centers, for his contributions to the longitudinal cohort studies. The authors acknowledge the staff at the facilities that supported the technical aspects of this study: Dr Chris Allen of the Flow Cytometry Facility, staff members of the Experimental Pathology Facility at Colorado State University, and Cheryl Engfehr at the Nutrition Laboratory at the Michigan State University Veterinary Diagnostic Laboratory.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (U01AI057786 and U19AI076217 to M. B. M. and C.-C. H., K01OD016997 and R21AI144662 to B. K. P., U19AI111224 to M. B. M., C.-C. H., and R. J. B.), Centers of Excellence for Translational Research (U19AI109755 to M. B. M. and C.-C. H.), National Institute on Drug Abuse (T32DA013911 to O. A.); and National Institute of Mental Health (R25MH083620 to O. A.).
References
- 1. Lonnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–55. [DOI] [PubMed] [Google Scholar]
- 2. Sinha P, Davis J, Saag L, et al. Undernutrition and tuberculosis: public health implications. J Infect Dis 2019; 219:1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8:286–98. [PubMed] [Google Scholar]
- 4. Larange A, Cheroutre H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Annu Rev Immunol 2016; 34:369–94. [DOI] [PubMed] [Google Scholar]
- 5. Stevens GA, Bennett JE, Hennocq Q, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health 2015; 3:e528–36. [DOI] [PubMed] [Google Scholar]
- 6. Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol 2009; 21:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bono MR, Tejon G, Flores-Santibañez F, Fernandez D, Rosemblatt M, Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients 2016; 8:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanekom WA, Potgieter S, Hughes EJ, Malan H, Kessow G, Hussey GD. Vitamin A status and therapy in childhood pulmonary tuberculosis. J Pediatr 1997; 131:925–7. [DOI] [PubMed] [Google Scholar]
- 9. Karyadi E, Schultink W, Nelwan RHH, et al. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. J Nutr 2000; 130:2953–8. [DOI] [PubMed] [Google Scholar]
- 10. Oh J, Choi R, Park HD, et al. Evaluation of vitamin status in patients with pulmonary tuberculosis. J Infect 2017; 74:272–80. [DOI] [PubMed] [Google Scholar]
- 11. Qrafli M, El Kari K, Aguenaou H, Bourkadi JE, Sadki K, El Mzibri M. Low plasma vitamin A concentration is associated with tuberculosis in Moroccan population: a preliminary case control study. BMC Res Notes 2017; 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramachandran G, Santha T, Garg R, et al. Vitamin A levels in sputum-positive pulmonary tuberculosis patients in comparison with household contacts and healthy ‘normals.’ Int J Tuberc Lung Dis 2004; 8:1130–3. [PubMed] [Google Scholar]
- 13. Kim EW, De Leon A, Jiang Z, et al. Vitamin A metabolism by dendritic cells triggers an antimicrobial response against Mycobacterium tuberculosis. mSphere 2019; 4:e00327-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aibana O, Franke M, Huang CC, et al. Impact of vitamin A and carotenoids on the risk of tuberculosis progression. Clin Infect Dis 2017; 65:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tenforde MW, Yadav A, Dowdy DW, et al. Vitamin A and D deficiencies associated with incident tuberculosis in HIV-infected patients initiating antiretroviral therapy in multinational case-cohort study. J Acquir Immune Defic Syndr 2017; 75:e71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet 1993; 342:268–72. [DOI] [PubMed] [Google Scholar]
- 17. Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167:603–62. [DOI] [PubMed] [Google Scholar]
- 18. Collins SE, Jean Juste MA, Koenig SP, et al. CD4 deficit and tuberculosis risk persist with delayed antiretroviral therapy: 5-year data from CIPRA HT-001. Int J Tuberc Lung Dis 2015; 19:50–7. [DOI] [PubMed] [Google Scholar]
- 19. Rice AL, West KP Jr, Black RE. Vitamin A deficiency: global and regional burden of disease attributable to selected major risk factors. Vol 1, Chapter 4. World Health Organization, 2004. Available at:http://www.who.int/publications/cra/chapters/volume1/0211-0256.pdf? ua=1. Accessed 24 January 2022. [Google Scholar]
- 20. Getz HR, Long ER, Henderson HJ. A study of the relation of nutrition to the development of tuberculosis; influence of ascorbic acid and vitamin A. Am Rev Tuberc 1951; 64:381–93. [DOI] [PubMed] [Google Scholar]
- 21. Soh AZ, Chee CBE, Wang YT, Yuan JM, Koh WP. Dietary intake of antioxidant vitamins and carotenoids and risk of developing active tuberculosis in a prospective population-based cohort study. Am J Epidemiol 2017; 186:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamada H, Mizuno S, Ross AC, Sugawara I. Retinoic acid therapy attenuates the severity of tuberculosis while altering lymphocyte and macrophage numbers and cytokine expression in rats infected with Mycobacterium tuberculosis. J Nutr 2007; 137:2696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Connor G, Krishnan N, Fagan-Murphy A, et al. Inhalable poly(lactic-co-glycolic acid) (PLGA) microparticles encapsulating all-trans-retinoic acid (ATRA) as a host-directed, adjunctive treatment for Mycobacterium tuberculosis infection. Eur J Pharm Biopharm 2019; 134:153–65. [DOI] [PubMed] [Google Scholar]
- 24. Wheelwright M, Kim EW, Inkeles MS, et al. All-trans retinoic acid-triggered antimicrobial activity against Mycobacterium tuberculosis is dependent on NPC2. J Immunol 2014; 192:2280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Araujo LS, Vaas LAI, Ribeiro-Alves M, et al. Transcriptomic biomarkers for tuberculosis: evaluation of DOCK9, EPHA4, and NPC2 mRNA expression in peripheral blood. Front Microbiol 2016; 7:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta RK, Turner CT, Venturini C, et al. Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis. Lancet Respir Med 2020; 8:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilhelm C, Harrison OJ, Schmitt V, et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J Exp Med 2016; 213:1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect Immun 2003; 71:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.