Abstract
Purpose
To examine the contemporary epidemiology of adult pituitary tumors with a particular focus on uncommon tumor types, using the 2017 WHO Classification of pituitary tumors.
Methods
Adult patients presenting with a pituitary or sellar tumor between 2004 and 2017 were identified from the U.S. National Cancer Database, with tumor type categorized according to the 2017 WHO classification. Descriptive epidemiological statistics were evaluated and reported for all pituitary tumor types and subtypes.
Results
113,349 adults with pituitary tumors were identified, 53.0% of whom were female. The majority of pituitary tumors were pituitary adenomas (94.0%), followed by craniopharyngiomas (3.8%). Among pituitary adenomas, whereas 71.6% of microadenomas presented in females, only 46.7% of macroadenomas and 41.3% of giant adenomas did (p < 0.001). For craniopharyngiomas, 71.2% were adamantinomatous and 28.8% were papillary, with adamantinomatous tumors associated with Black non-Hispanic race/ethnicity ( ORadj = 2.44 vs. White non-Hispanic, 99.9 %CI = 1.25–4.75, p < 0.001) in multivariable analysis. The remaining 0.7% (n = 676) of pathology-confirmed pituitary tumor types were composed of: 21% tumors of the posterior pituitary, 16% chordomas, 11% pituitary carcinomas (i.e. adenohypophyseal histology with metastasis; herein most frequently to bone), 10% meningiomas, 8% germ cell tumors, 7% hematolymphoid (largely DLBCLs), and 4% neuronal/ paraneuronal (largely gangliogliomas). Pituitary carcinomas and posterior pituitary tumors demonstrated a male predilection (62.2% and 56.0%, respectively), whereas sellar meningiomas predominated in females (84.1%). Age, race/ethnicity, tumor size, and overall survival further varied across uncommon pituitary tumor types.
Conclusions
Our findings provide a detailed contemporary dissection of the epidemiology of common and uncommon adult pituitary tumors in the context of WHO2017.
Keywords: Pituitary tumor, Pituitary adenoma, Craniopharyngioma, Epidemiology, Pituitary carcinoma, Tumor of the posterior pituitary
Introduction
Pituitary tumors comprise approximately 10–15% of all intracranial tumors in the adult population [1–3]. Although generally benign, slow-growing tumors, pituitary tumors can nonetheless cause symptoms due to their hormonal activity and/or sellar mass effect. At least one third of pituitary tumors have been associated with health complications such as diabetes mellitus, accelerated heart disease, infertility, sexual dysfunction, mood disorders, visual disturbances, and hypertension. Furthermore, a minority of pituitary tumor types are clinically aggressive, with a propensity for invasion of nearby structures, apoplexy, and recurrence. Our burgeoning understanding of the wide spectrum of pituitary tumors’ genetics and clinical manifestations catalyzed the 2017 update to the World Health Organization classification of pituitary tumors—together helping to advance a more nuanced diagnosis and management of patients with pituitary tumors [4]. Although sellar tumors are predominated by pituitary adenomas, there are many diverse tumor types that are also encountered in the pituitary region, including craniopharyngiomas, tumors of the posterior pituitary, chordomas, and meningiomas—among several others—which can have a wide range of presentation and morbidity [5]. Reflecting their high prevalence, most epidemiological characterization of pituitary tumors has focused on pituitary adenomas and craniopharyngiomas [3, 6]. Here, we leverage a national cancer database to comprehensively analyze the epidemiology of all pituitary tumor types—with a particular focus on uncommon types—using the contemporary framework of the WHO2017 classification schema. Our study builds on the foundational work by Famini et al., Freda et al., the Central Brain Tumor Registry of the U.S., the German Pituitary Tumor Registry, and others, in order to provide an updated and more detailed, clinically-relevant dissection of pituitary tumor epidemiology—especially for uncommon tumor types whose paucity have limited their study in institutional and even multi-institutional cohorts [3, 5, 7, 8].
Methods
Adult patients who were ≥ 20-years-old and presented from 2004 to 2017 with a newly-diagnosed pituitary region tumor were extracted from the pan-cancer National Cancer Database (NCDB), which encompasses > 70% of newly-diagnosed cancers in the U.S. [9]. In the U.S., multiple cancer registries additionally collect data for intracranial tumors that display benign or borderlines behavior. The methods have been previously described, but briefly, pituitary tumors were defined as either primary benign or malignant tumors originating in the ICD-O-3 topographical codes for the pituitary (C75.1, which includes the sella turcica/pituitary fossa and Rathke pouch) and craniopharyngeal duct (C75.2) [10]. In addition to intrasellar lesions, 13.9% of craniopharyngiomas were encoded in the NCDB with topographical code C71.9 for “Brain, NOS” and were also included herein. Tumor histology was categorized by ICD-O-3 histological codes according to the WHO 4th edition Classification of Tumours of Endocrine Organs into: pituitary adenomas, craniopharyngiomas, tumors of the posterior pituitary (spindle cell oncocytomas, pituicytomas, granular cell tumors, and sellar ependymomas), mesenchymal and stromal tumors (e.g. chordomas, meningiomas, vascular neoplasms, schwannomas, and solitary fibrous tumors), germ cell tumors, hematolymphoid tumors (e.g. lymphomas, Langerhans histiocytosis), neuronal and paraneuronal, or other tumor types (Supplemental Table 1) [4]. As per the WHO 4th edition classification, the rare primary pituitary tumors that were coded as an adenohypophyseal histology (i.e. pituitary adenoma or “pituitary carcinoma”) and demonstrated either craniospinal or distant metastasis at the time of diagnosis were classified herein as pituitary carcinomas (n = 74); whereas the 96 cases that were coded as “pituitary carcinoma” histology (code 8272/3) without evidence of distant metastasis were assigned herein as pituitary adenomas.
Diagnosis of the specific type of pituitary tumor was based on radiology, biopsy or surgical resection with histopathological confirmation. As previously described, pathological confirmation was defined as either microscopic diagnosis or biopsy and/or surgical procedure with specimen sent to pathology [10]. The tumor’s maximum dimension was reported from the pathology report if available, or from the radiology report if not. Patient race/ethnicity was categorized as White non-Hispanic, Black non-Hispanic, Asian/Pacific Islander, Hispanic, or other.
Summary statistics were displayed as median with interquartile range for continuous data, or counts and percentages for categorical data. Cell counts < 10 were suppressed. Continuous variables were assessed by t-test and categorical variables by Fisher exact test (or Chi2 approximation). All-cause overall survival (OS) from initial diagnosis was estimated using Kaplan-Meier techniques (reported with 95% confidence intervals [CI]), and was suppressed by the NCDB for patients diagnosed in 2017 due to limited follow-up. Patient and tumor characteristics associated with adamantinomatous vs. papillary craniopharyngioma subtypes were assessed with multivariable logistic regression. Two-sided p values < 0.001 were denoted as significant to reduce false positives. Analyses were conducted in Stata (SEv15.1). This study was approved by the Mass General Brigham Institutional Review Board (2015P002352).
Results
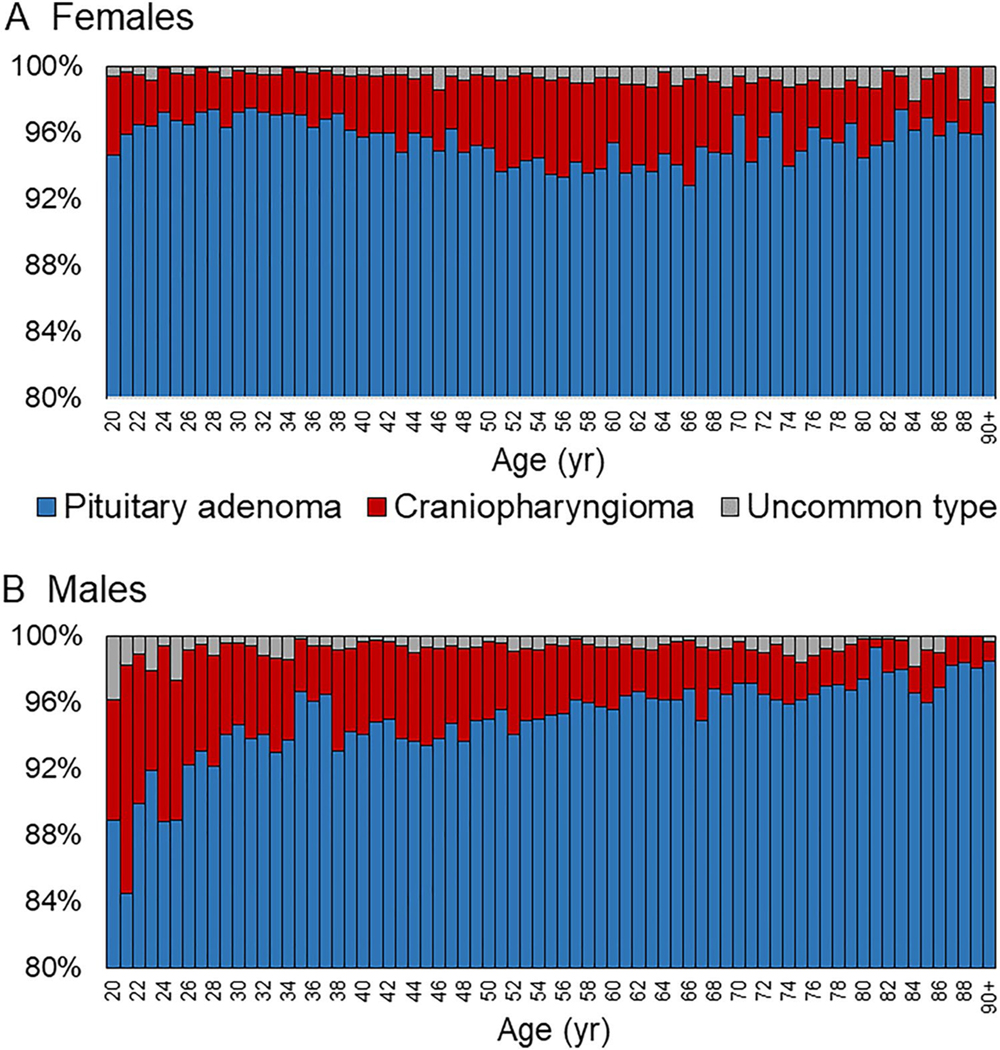
From 2004 to 2017, 113,349 adult patients were diagnosed with a tumor originating in the pituitary region. Overall, 53.0% (n = 60,072) of pituitary tumors occurred in females, and females presented at earlier ages (median 48 years, IQR 35–63; vs. 57 years in males, IQR 45–68 years; p < 0.0001). As diagnosed by either radiographical or pathological assessment, pituitary adenomas (94.0%, n = 106,535) comprised the overwhelming majority of pituitary tumors, followed by craniopharyngiomas (3.8%, n = 4307), and uncommon pituitary tumor types (0.7%, n = 799; Table 1). In 1.5% (n = 1708) of cases, a specific tumor type was not determined. Although pituitary adenomas predominated overall, there were slight differences in distributions by sex and age (Fig. 1). By patients’ race/ethnicity, uncommon pituitary types more frequently occurred in White non-Hispanic patients (69.5%) and less commonly in Black non-Hispanic patients (15.7%), compared to the distribution of overall pituitary tumors (64.3% in White non-Hispanic and 19.9% in Black non-Hispanic patients, respectively) (p < 0.001). The median maximum dimension of pituitary adenomas was 18 mm (IQR 9–26), compared to 26 mm (IQR 19–34) for craniopharyngiomas (p < 0.0001).
Table 1.
Characteristics of adult pituitary tumor types
| n | % | Age, year | Size, mm | Female, % | White nonH, % | Black nonH, % | Asian/PI, % | Hispanic, % | OS, % | Pathology confirm,% | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Median | IQR | Median | IQR | 60 mos | 95 %CI | |||||||||
| Total | 113,349 | 100.0 | 53 | (39–65) | 18 | (9–27) | 53.0 | 64.3 | 19.9 | 3.7 | 10.1 | 89.0 | (88.7–89.2) | 58.7 |
| Pituitary adenoma | 106,535 | 94.0 | 53 | (39–66) | 18 | (9–26) | 53.0 | 64.2 | 19.9 | 3.7 | 10.2 | 89.7 | (89.5–89.9) | 57.7 |
| Craniopharyngioma | 4,307 | 3.8 | 52 | (40–63) | 26 | (19–34) | 51.6 | 65.3 | 20.4 | 3.9 | 8.5 | 78.0 | (76.5–79.3) | 85.3 |
| Uncommon tumor types | 799 | 0.7 | 55 | (42–68) | 23 | (17–31) | 52.2 | 69.5 | 15.7 | 4.6 | 8.3 | 74.2 | (70.7–77.4) | 84.6 |
| Tumor type not determined | 1,708 | 1.5 | 55 | (41–70) | 17 | (10–25) | 55.0 | 65.7 | 20.6 | 2.8 | 8.9 | 79.2 | (76.9–81.3) | 36.5 |
IQR interquartile range, nonH non-Hispanic, OS overall survival, CI confdence interval
Fig. 1.
Pituitary tumor type distribution by age and sex. Among females (a) and males (b) presenting with a pituitary tumor, the percent that were pituitary adenomas, craniopharyngiomas, or other uncommon tumor types by age (year)
Among pituitary adenomas, 26.9% were microadenomas (i.e. < 10 mm; median 5 mm, IQR 4–7), 66.9% were macroadenomas (i.e. 10–40 mm; median 21 mm, IQR 16–27), and 6.2% were giant adenomas (i.e. > 40 mm; median 50 mm, IQR 45–64). Whereas 71.6% (n = 16,534) of microadenomas presented in females, only 46.7% (n = 26,805) of macroadenomas and 41.3% (n = 2185) of giant adenomas did (p < 0.001). Although age of presentation for giant adenomas was similar between males and females (median of 52 years, IQR 40–63; and 51 years, IQR 37–63; respectively, p = 0.01); microadenomas presented at a median of 50 years (IQR 38–61) in males and 38 years (IQR 30–49) in females (p < 0.0001). There was also a difference in distributions by race/ethnicity: 67.0% of microadenomas, 63.7% of macroadenomas, and 57.7% of giant adenomas presented in White non-Hispanic patients, while 17.1% of microadenomas, 20.6% of macroadenomas, and 22.9% of giant adenomas were diagnosed in Black non-Hispanic patients; and 10.1% of microadenomas, 9.9% of macroadenomas, and 13.1% of giant adenomas presented in Hispanic patients (p < 0.001). Furthermore, although macroadenomas (5-year OS 87.9%, 95% CI 87.6–88.2) and giant adenomas (5-year OS 87.4%, 95% CI 86.3–88.4) were associated with worse unadjusted OS than microadenomas (5-year OS 95.1%, 95% CI 94.7–95.4; p < 0.0001), age-adjusted 5-year OS rates were ≥ 99.5% for all three size levels.
Epidemiology of pituitary adenomas and craniopharyngiomas with histopathological confirmation
Among newly-diagnosed pituitary tumors, 58.7% had a histopathological diagnosis (n = 66,477), ranging from 57.7% of pituitary adenomas, to 85.3% of craniopharyngiomas and 84.6% of uncommon tumor types (Table 1). Histopathological subtypes of pituitary adenomas were unfortunately only rarely reported by the NCDB (2.4% of pituitary adenomas herein), among which 51.7% were encoded as prolactinomas (n = 767), 38.1% as chromophobic (n = 566), 5.9% as acidophilic (n = 88), and 2.2% as basophilic (n = 33) adenomas. No data regarding pituitary adenoma functionality were reported by the NCDB.
In contrast, the histological subtype was encoded for 48.0% (n = 1761) of craniopharyngiomas with histopathological diagnosis, in which 71.2% were adamantinomatous and 28.8% were papillary. Multivariable logistic regression was used to identify any patient or tumor characteristics that were associated with an adamantinomatous vs. papillary subtype, in which only Black non-Hispanic race/ethnicity was significantly associated with increased odds of an adamantinomatous subtype (odds ratio 2.44 compared to White non-Hispanic, 99.9% CI 1.25–4.75, p < 0.001; odds ratio 2.94 compared to Asian/Pacific Islander, 99.9% CI 0.95–9.09, p = 0.002). Only 8.7% of papillary craniopharyngiomas were diagnosed in Black non-Hispanic patients, compared to 20.5% of adamantinomatous craniopharyngiomas—additionally, for reference, 19.9% of pituitary adenomas were diagnosed in Black non-Hispanic patients. Age at diagnosis, sex, and greatest tumor dimension were not associated with craniopharyngioma subtype (all p > 0.10; Supplemental Table 2).
Epidemiology of uncommon pituitary tumor types with histopathological confirmation
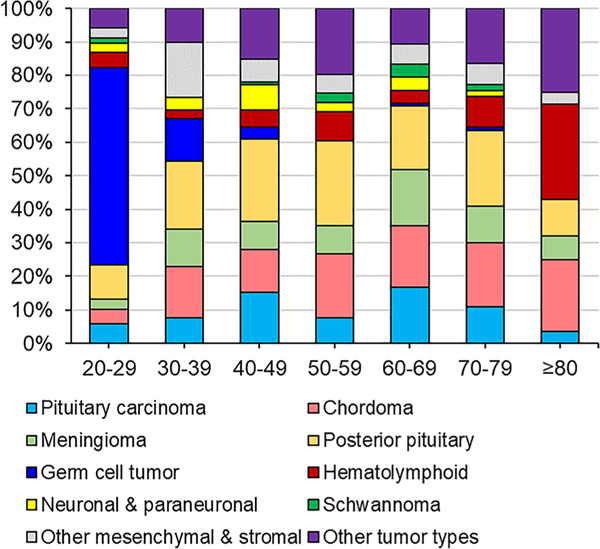
The patient and tumor characteristics of uncommon pituitary tumor types with histopathological diagnosis are reported in Table 2, along with their distributions by age in Fig. 2. Among the uncommon pituitary tumor types, tumors of the posterior pituitary comprised the largest fraction: 20.9% of cases (n = 141, or 0.2% of all pituitary tumors with pathological diagnosis). However, this likely represents a considerable underestimation, given that a distinct ICD-O-3 code for pituicytoma was not incorporated into cancer registries until 2018 and that for spindle cell oncocytomas, the appropriate ICD-O-3 code (i.e. 8290/0) was unconventionally defined for cancer registries as an “oxyphilic adenoma”—consequently, many cases may have been inappropriately encoded as a tumor of unknown type. Furthermore, there may be confusion between the encoding of “granular cell tumour of the sellar region” (i.e. 9582/0; a TTF1 + posterior pituitary tumor posited to arise from granular pituicytes) and “granular cell tumor, NOS” (i.e. 9580/0; a benign tumor with neuroectodermal differentiation and lysosome-rich granular eosinophilic cytoplasm most commonly found in subcutaneous and submucosal sites). Herein, both codes were considered to represent “granular cell tumour of the sellar region”. Overall, tumors of the posterior pituitary presented at a median age of 55 years (IQR 45–67) with a median greatest dimension of 20 mm (IQR 15.5–27.5), and displayed a slight male predominance (56.0% of cases). Additionally, tumors of the posterior pituitary were more likely to be diagnosed in White non-Hispanic patients (71.7%) than pituitary adenomas (64.2%) or craniopharyngiomas (65.3%).
Table 2.
Characteristics of uncommon pituitary tumor types
| n | % | Age, year | Size, mm | Female, % | White nonH, % | OS, % | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Median | IQR | Median | IQR | 60 mos | 95 %CI | |||||
| Total | 676 | 100.0 | 55 | (42–67) | 24 | (18–32) | 51.2 | 71.0 | 74.2 | (70.7–77.4) |
| Posterior pituitary | 141 | 20.9 | 55 | (45–67) | 20 | (15.5–27.5) | 44.0 | 71.7 | 88.0 | (80.1–92.9) |
| Chordoma | 108 | 16.0 | 57.5 | (48.5–69.5) | 30 | (22–41) | 47.2 | 70.8 | 72.3 | (61.7–80.5) |
| Other tumor types | 97 | 14.4 | 56 | (47–70) | 25 | (18–35) | 54.6 | 70.5 | 48.8 | (37.8–59) |
| Pituitary carcinoma | 74 | 11.0 | 58 | (44–67) | 28 | (21–37.5) | 37.8 | 67.6 | 80.2 | (68.2–88.1) |
| Meningioma | 69 | 10.2 | 61 | (45–68) | 23.5 | (20–27) | 84.1 | 67.2 | 85.0 | (73–91.9) |
| Other mesenchymal & stromal | 60 | 8.8 | 51 | (38.5–64.5) | 27 | (20–32) | 63.3 | 74.6 | 72.3 | (57.6–82.7) |
| Germ cell tumor | 56 | 8.3 | 25 | (21.5–32.5) | 20 | (15–31) | 25.0 | 67.9 | 89.7 | (76.9–95.6) |
| Hematolymphoid | 46 | 6.8 | 60 | (51–76) | 12.5 | (11–14) | 56.5 | 75.6 | 40.4 | (24.6–55.6) |
| Neuronal & paraneuronal | 25 | 3.7 | 48 | (42–61) | 24 | (18–30) | 64.0 | 80.0 | 95.8 | (73.9–99.4) |
Counts < 10 are suppressed
IQR interquartile range, nonH non-Hispanic, OS overall survival, CI confidence interval
Fig. 2.
Uncommon pituitary tumor type distribution by age. The percent of different tumor types (indicated by different colors) among patients presenting with a pathology-confirmed uncommon pituitary tumor type, by age (year)
Pituitary carcinomas—defined herein as a primary pituitary tumor with adenohypophyseal histology and metastasis at the time of diagnosis—represented 11.0% (n = 74) of uncommon tumor types or 0.12% of adenohypophyseal tumors. Details regarding metastatic sites were only available for half of the patients, among whom bone was the most frequent site of involvement (27.0%). Pituitary carcinomas presented at later ages (median 58 years, IQR 44–67) and with larger tumors (median 28 mm, IQR 21–37.5) than their benign counterparts (median 53 years, IQR 39–66; and median 18 mm, IQR 9–26); and displayed a marked male predominance (62.2%). Estimated OS was 80.2% at 5 years (95CI 68.2–88.1) for all pituitary carcinomas, and 56.3% (95CI 14.7–84.2) for those with bone metastases. The NCDB only reports metastatic disease at the time of diagnosis, and not pituitary adenomas that subsequently metastasize.
Altogether, sellar mesenchymal and stromal tumors accounted for 35.1% (n = 237) of uncommon tumor types, among which chordoma were the most frequent (n = 108). The majority of chordomas were of conventional histology, but 19.4% were chondroid chordomas and none were de-differentiated chordomas. Chordomas were among the largest sellar tumor types at presentation: 30 mm in greatest dimension (IQR 22–41). The 5-year OS rate associated with sellar chordomas was 72.3% (95% CI 61.7–80.5). Unfortunately, the NCDB does not provide the anatomical granularity necessary for distinguishing between parasellar, suprasellar, and intrasellar origins of tumors. Chordomas were followed in prevalence by meningiomas (n = 69), which were marked by a distinct female predominance (84.1%) and older age of presentation (median 61 years, IQR 45–68). Although the overwhelming majority of sellar meningiomas were WHO grade 1, < 15% (n < 10) were reported to be of WHO grade 2 or 3 histopathology. Vascular neoplasms (n = 23), schwannomas (n = 13), and solitary fibrous tumors (i.e. hemangiopericytoma, all WHO grades; n = 11) were exceptionally rare in the sellar compartment.
Additional rare pituitary tumor types in adults included: (1) germ cell tumors (n = 56), which were largely confined to young (median 25 years old, IQR 21.5–32.5), male patients (75.0%)—with 78.6% that were pure germinomas; (2) Primary hematolymphoid tumors (n = 46), 52.2% of which were diffuse large B-cell lymphomas. Sellar hematolymphoid tumors were associated with some of the worst outcomes: an estimated 40.4% survived 5 years after diagnosis (95 CI 24.6–55.6); and (3) Neuronal and para-neuronal tumors, among whom gangliogliomas dominated (64.0%) and the estimated 5-year OS was 95.8% (95% CI 73.9–99.4). Sellar gliomas and embryonal tumors were almost non-existent (approximately n = 10 each) and were grouped into “other tumor types”. Finally, n = 64/97 of the “other tumor types” cases were encoded as “carcinoma, NOS”, neuroendocrine carcinoma, adenocarcinoma, squamous cell carcinoma, and other carcinoma subtypes—with an estimated 5-year OS of 46.0% (95% CI 32.8–58.3) and approximately 15% of which had distant metastases also encoded. These cases could represent metastatic carcinomas from other primary sites (or unknown primary sites) that were erroneously encoded as a pituitary primary, or incorrectly encoded pituitary carcinomas.
Discussion
Pituitary region tumors are relatively common [1–3]. For instance, in a comparative analysis of pituitary tumors between the pediatric and adult populations we previously demonstrated that pituitary tumors comprise 12% and 13% of all intracranial tumors in female and male adult patients, respectively [10]. As has been well-documented in prior cancer registry analyses, pituitary adenomas accounted for the vast majority (94%) of pituitary tumors in adults with an annual age-adjusted incidence of 1.20/100,000, followed by craniopharyngiomas in distant second (4%) with an annual age-adjusted incidence of 0.19/100,000 [3, 6]. In 2017, the WHO classification of tumors arising in the sellar and parasellar regions of the pituitary gland was updated to reflect our growing understanding of their genomics and pathophysiology. In the context of this new classification framework, herein we leverage a national cancer registry to further investigate the epidemiological features of pituitary adenomas and craniopharyngiomas, as well as to define the epidemiology of rarer pituitary tumor types in adults.
Prior cross-sectional and retrospective studies have identified a higher incidence of pituitary adenomas in females, particularly during early life [11, 12]. Here, we note only minor differences by patients’ sex and age in the overall distribution of pituitary adenomas among pituitary tumors. However, females presented with pituitary adenomas almost a decade earlier than their male counterparts (median of 48 vs. 57 years). Also consistent with prior reports, pituitary adenomas were twice as likely to present as microadenomas in females compared to males, whereas they were approximately 30–56% more likely to present as either macroadenomas or giant adenomas in males compared to females [13, 14]. This sex-based discrepancy in adenoma size at presentation is likely due to functional manifestations being more clinically pronounced in pre-menopausal females (e.g. infertility, oligomenorrhea, galactorrhea), resulting in an earlier diagnosis of smaller pituitary adenomas in females. For example, prior studies have observed that functional adenomas presenting in adults younger than 50 are more likely to arise in females than males, but that the male-to-female ratio equalizes in older patients [15]. Most giant adenomas are clinically non-functioning, often presenting at a more delayed age with symptoms of sellar mass effect [16]. Although the NCBD does not report data on pituitary adenoma functionality, we found that microadenomas in females presented at earlier ages than those in males (median of 38 years vs. 50 years), whereas giant adenomas presented at a median of 51–52 years-old irrespective of sex, which would be consistent with the finding that slow-growing giant adenomas are more likely clinically non-functioning and so present at older ages.
Overall, craniopharyngiomas represented less than 4% of pituitary tumors in adults. The epidemiology of craniopharyngiomas has been previously described in detail—notably, finding an increased overall incidence of craniopharyngiomas in Black adults (0.29 cases/100,000, compared to 0.18 cases/100,000 in White adults) [3, 17]. Black adults were also shown to have proportionally higher incidence rates of pituitary adenomas (5.82 cases/100,000, compared to 3.25 cases/100,000 in White adults), such that in our analyses craniopharyngiomas comprised 3.9–4.1% of all pituitary tumors in White non-Hispanic, Black non-Hispanic, and Asian/Pacific Islander adults. We further investigated the epidemiology of craniopharyngioma’s subtypes, for which—as has been well-described—the adamantinomatous subtype was more than twice as common as the papillary subtype in adults. Age at diagnosis, patient sex, and tumor size were similar between adamantinomatous and papillary tumors. However, we found that craniopharyngiomas in Black non-Hispanic patients were significantly more likely to be of the adamantinomatous subtype. Although our understanding of the genetics and molecular drivers of adamantinomatous and papillary craniopharyngiomas has dramatically improved in recent years, there remains limited data regarding their molecular epidemiology.
Together, pituitary adenomas and craniopharyngiomas represent 98% of newly-diagnosed pituitary region tumors (both in those with pathological confirmation and those without), whereas all other pituitary tumor types comprise just 1%. Most frequent among the uncommon pituitary tumor types were mesenchymal and stromal tumors, namely conventional chordomas and meningiomas—both of which were likely underestimated herein due to the inexact coding of anatomical location in cancer registry data (e.g. sellar and parasellar cases may have been coded to a skull primary site or a meningeal NOS site) [18, 19]. In the German Pituitary Tumor Registry, meningiomas and chordomas represented 0.9% and 0.5% of all pituitary tumors, whereas herein they amounted to 0.1% and 0.2% of pituitary tumors with pathological confirmation, respectively [5]. Similar to conventional chordomas arising in other sites, a slight male predominance was identified for sellar chordomas and patients often presented in their 50 and 60 s [20]. A fifth of sellar chordomas were encoded as the chondroid histological subtype, but expression of the notochordal marker brachyury is not reported by the NCDB, so some cases could have actually been low-grade chondrosarcomas. No sellar dedifferentiated chordomas were diagnosed, which is analogous to extracranial sites, where they account for only 1% of chordomas. Sellar meningiomas, however, overwhelmingly arose in females (84%), and < 15% were reported as WHO grade 2 or 3. Beyond chordomas and meningiomas, other mesenchymal and stromal tumor types—such as vascular neoplasms, schwannomas, and solitary fibrous tumors (i.e. hemangiopericytoma)—were all incredibly rare in the sellae, as underscored by the paucity of such cases reported in the literature [5, 21–27].
A sizable fraction of uncommon pituitary tumor types were tumors of the posterior pituitary: pituicytomas, granular cell tumors of the sellar region, spindle cell oncocytomas, and sellar ependymomas. Initially thought of as distinct entities, they are now recognized to likely represent a low-grade, histological spectrum defined by TTF1 expression, whose management and ccorresponding clinical outcomes have been described in multiple case series [28–35]. Together they exhibited a slight male predilection (56% of cases) and were diagnosed at a median age of 55 years. Also demonstrating a male predominance were patients presenting with pituitary carcinoma, now distinctly defined in the WHO 2017 as a pituitary tumor with adenohypophyseal histology with metastasis—in contrast to some small institutional case series that reported a female predominance. Furthermore, the estimated 5-year overall survival (80%) associated with pituitary carcinoma is somewhat higher than has been previously reported from single-digit case series; although those cases with bone metastases (the most common metastatic site herein) were associated with an estimated 5-year overall survival of just 56% [36–39]. In the pituitary region, germ cell tumors (overwhelmingly pure germinomas in young adult males), hematolymphoid (largely diffuse large B-cell lymphomas), and neuronal and paraneuronal tumors (largely gangliogliomas) were exceptionally rare in adults, with their epidemiological characterization only permitted by national cancer registries such as the German Pituitary Tumor Registry and the NCDB [5].
Limitations
Several limitations of the NCDB constrained this study. Namely, the NCDB lacked data regarding the symptomatology, endocrine test results, radiographic details, or hereditary syndromes associated with pituitary tumors. For pituitary adenomas, the NCDB did not report either details about tumor functionality nor pituitary transcription factor and adenohypophyseal hormone immunohistochemistry results, and only had histopathological subtypes encoded for a small fraction of pituitary adenomas. The development of multi-institutional pituitary tumor-specific registry databases, such as the German Pituitary Tumor Registry, will help improve the robust collection of clinical and tumor data relevant for pituitary tumors. For tumors of the posterior pituitary, a distinct ICD-O-3 code for pituicytoma was not used by cancer registries until 2018, and the ICD-O-3 code for spindle cell oncocytoma was defined for registries as “oxyphilic adenoma”—likely resulting in an undercounting of tumors of the posterior pituitary. Furthermore, several newly recognized entities are not yet encoded by cancer registries (e.g. pituitary blastoma), nor are novel recognized genomic features captured by the NCDB. For pituitary carcinomas, although the NCDB reported the presence of metastasis at time of presentation, it did not report subsequent metastasis. The NCDB also lacked pituitary tumors’ precise neuroanatomical details (i.e. parasellar vs. suprasellar vs. intrasellar) and extent of local invasion. In terms of survival outcomes, the NCDB is restricted to all-cause OS, which is of limited usefulness for patients with benign pituitary tumors. The NCDB is hospital-based and not population-based, precluding estimation of incidence or adjustment for baseline population characteristics.
Conclusions
Reflecting our growing understanding of the genetics and biology of pituitary tumors, the 2017 WHO update has established a more histopathologically and clinically relevant classification of pituitary tumors. Herein, we leveraged data from a cancer registry to dissect the epidemiology of adult pituitary tumors in the U.S., with a particular focus on defining the epidemiology of uncommon tumor types (i.e. for diagnoses beyond pituitary adenomas and craniopharyngiomas) according to the 2017 WHO classification. Our findings shed light on the characteristics of uncommon pituitary tumor types and help inform the diverse differential posed by a new pituitary mass in adults.
Supplementary Material
Funding
JBI gratefully acknowledges funding support from the National Cancer Institute (K12CA090354) and Conquer Cancer Foundation. LEC acknowledges funding support from the NIH (T32DK007028). CG is an NCI F31 Diversity Individual Predoctoral Fellow.
Footnotes
Code availability Not applicable.
Declarations
Conflict of interest The authors report no conflicts of interest.
Consent to participate/publication Not applicable.
Ethical approval This study was approved by the MGB institutional review board.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11102-021-01189-6.
Data availability
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Data available by NCDB application.
References
- 1.Melmed S (2020) Pituitary-tumor endocrinopathies. N Engl J Med 382:937–950. 10.1056/NEJMra1810772 [DOI] [PubMed] [Google Scholar]
- 2.Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266. 10.1038/nrendo.2011.40 [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Patil N, Cioffi G et al. (2020) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncol 22:iv1–iv96. 10.1093/neuonc/noaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd RV, Osamura RY, Klöppel G, Rosai J (2017) WHO Classification of tumours of endocrine organs, 4th edn. IARC, Lyon [Google Scholar]
- 5.Saeger W, Lüdecke DK, Buchfelder M et al. (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 156:203–216. 10.1530/eje.1.02326 [DOI] [PubMed] [Google Scholar]
- 6.Gittleman H, Ostrom QT, Farah PD et al. (2014) Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J Neurosurg 121:527–535. 10.3171/2014.5.JNS131819 [DOI] [PubMed] [Google Scholar]
- 7.Famini P, Maya MM, Melmed S (2011) Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab 96:1633–1641. 10.1210/jc.2011-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freda PU, Bruce JN, Khandji AG et al. (2020) Presenting features in 269 patients with clinically nonfunctioning pituitary adenomas enrolled in a prospective study. J Endocr Soc 4:bvaa021. 10.1210/jendso/bvaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boffa DJ, Rosen JE, Mallin K et al. (2017) Using the National Cancer Database for outcomes research: a review. JAMA Oncol 3:1722–1728. 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 10.Castellanos LE, Misra M, Smith TR et al. (2021) The epidemiology and management patterns of pediatric pituitary tumors in the United States. Pituitary 24:412–419. 10.1007/s11102-020-01120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDowell BD, Wallace RB, Carnahan RM et al. (2011) Demographic differences in incidence for pituitary adenoma. Pituitary 14:23–30. 10.1007/s11102-010-0253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Hu Y, Lyu L et al. (2021) Incidence, demographics, and survival of patients with primary pituitary tumors: a SEER database study in 2004–2016. Sci Rep 11:15155. 10.1038/s41598-021-94658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agustsson TT, Baldvinsdottir T, Jonasson JG et al. (2015) The epidemiology of pituitary adenomas in Iceland, 1955–2012: a nationwide population-based study. Eur J Endocrinol 173:655–664. 10.1530/EJE-15-0189 [DOI] [PubMed] [Google Scholar]
- 14.Song Y-J, Chen M-T, Lian W et al. (2017) Surgical treatment for male prolactinoma. Med (Baltim) 96:e5833. 10.1097/MD.0000000000005833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin SO (2020) Epidemiology of functioning pituitary adenomas. Endocrinol Metab 35:237–242. 10.3803/EnM.2020.35.2.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iglesias P, Arcano K, Triviño V et al. (2017) Prevalence, clinical features, and natural history of incidental clinically nonfunctioning pituitary adenomas. Horm Metab Res 49:654–659. 10.1055/s-0043-115645 [DOI] [PubMed] [Google Scholar]
- 17.Momin AA, Recinos MA, Cioffi G et al. (2021) Descriptive epidemiology of craniopharyngiomas in the United States. Pituitary 24:517–522. 10.1007/s11102-021-01127-6 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A-K, Dawood HY, Arnaout OM et al. (2018) Presentation, treatment, and long-term outcome of intrasellar chordoma: a pooled analysis of institutional, SEER (surveillance epidemiology and end results), and published data. World Neurosurg 109:e676–e683. 10.1016/j.wneu.2017.10.054 [DOI] [PubMed] [Google Scholar]
- 19.Bohman L-E, Koch M, Bailey RL et al. (2014) Skull base chordoma and chondrosarcoma: influence of clinical and demographic factors on prognosis: a SEER analysis. World Neurosurg 82:806–814. 10.1016/j.wneu.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Das P, Soni P, Jones J et al. (2020) Descriptive epidemiology of chordomas in the United States. J Neurooncol 148:173–178. 10.1007/s11060-020-03511-x [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Iorgulescu JB, Hoffman S et al. (2020) The diagnosis and management of primary and iatrogenic soft tissue sarcomas of the sella. Pituitary 23:558–572. 10.1007/s11102-020-01062-y [DOI] [PubMed] [Google Scholar]
- 22.Maartens NF, Ellegala DB, Vance ML et al. (2003) Intrasellar schwannomas: report of two cases. Neurosurgery 52:1200–1205; discussion 1205–1206 [PubMed] [Google Scholar]
- 23.Cugati G, Singh M, Symss NP et al. (2012) Primary intrasellar schwannoma. J Clin Neurosci Off J Neurosurg Soc Australas 19:1584–1585. 10.1016/j.jocn.2011.09.041 [DOI] [PubMed] [Google Scholar]
- 24.Honegger J, Koerbel A, Psaras T et al. (2005) Primary intrasellar schwannoma: clinical, aetiopathological and surgical considerations. Br J Neurosurg 19:432–438. 10.1080/02688690500390391 [DOI] [PubMed] [Google Scholar]
- 25.Kong X, Wu H, Ma W et al. (2016) Schwannoma in sellar region mimics invasive pituitary macroadenoma: literature review with one case report. Med (Baltim) 95:e2931. 10.1097/MD.0000000000002931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammed S, Kovacs K, Munoz D, Cusimano MD (2010) A short illustrated review of sellar region schwannomas. Acta Neurochir (Wien) 152:885–891. 10.1007/s00701-009-0527-7 [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Xu S, Liu Q et al. (2016) Intrasellar and suprasellar schwannoma misdiagnosed as pituitary macroadenoma: a case report and review of the literature. World Neurosurg 96:612. 10.1016/j.wneu.2016.08.128 [DOI] [PubMed] [Google Scholar]
- 28.Giantini Larsen AM, Cote DJ, Zaidi HA et al. (2018) Spindle cell oncocytoma of the pituitary gland. J Neurosurg 131:517–525. 10.3171/2018.4.JNS18211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinschmidt-DeMasters BK, Lopes MBS (2013) Update on hypophysitis and TTF-1 expressing sellar region masses. Brain Pathol Zurich Switz 23:495–514. 10.1111/bpa.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefevre E, Bouazza S, Bielle F, Boch A-L (2018) Management of pituicytomas: a multicenter series of eight cases. Pituitary 21:507–514. 10.1007/s11102-018-0905-3 [DOI] [PubMed] [Google Scholar]
- 31.Viaene AN, Lee EB, Rosenbaum JN et al. (2019) Histologic, immunohistochemical, and molecular features of pituicytomas and atypical pituicytomas. Acta Neuropathol Commun 7:69. 10.1186/s40478-019-0722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole TS, Potla S, Sarris CE et al. (2019) Rare thyroid transcription factor 1-positive tumors of the sellar region: barrow neurological institute retrospective case series. World Neurosurg 129:e294–e302. 10.1016/j.wneu.2019.05.132 [DOI] [PubMed] [Google Scholar]
- 33.Guerrero-Pérez F, Vidal N, Marengo AP et al. (2019) Posterior pituitary tumours: the spectrum of a unique entity. A clinical and histological study of a large case series. Endocrine 63:36–43. 10.1007/s12020-018-1774-2 [DOI] [PubMed] [Google Scholar]
- 34.Whipple SG, Savardekar AR, Rao S et al. (2021) Primary tumors of the posterior pituitary gland: a systematic review of the literature in light of the new 2017 World Health Organization classification of pituitary tumors. World Neurosurg 145:148–158. 10.1016/j.wneu.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 35.Ahmed A-K, Dawood HY, Cote DJ et al. (2019) Surgical resection of granular cell tumor of the sellar region: three indications. Pituitary 22:633–639. 10.1007/s11102-019-00999-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pernicone PJ, Scheithauer BW, Sebo TJ, et al. (1997) Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer 79:804–812 [DOI] [PubMed] [Google Scholar]
- 37.Hansen TM, Batra S, Lim M et al. (2014) Invasive adenoma and pituitary carcinoma: a SEER database analysis. Neurosurg Rev 37:279–285. 10.1007/s10143-014-0525-y discussion 285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey RM, Kuan EC, Workman AD et al. (2020) A population-level analysis of pituitary carcinoma from the National Cancer Database. J Neurol Surg Part B Skull Base 81:180–186. 10.1055/s-0039-1683435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenders NF, Inder WJ, McCormack AI (2021) Towards precision medicine for clinically non-functioning pituitary tumours. Clin Endocrinol (Oxf). 10.1111/cen.14472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Data available by NCDB application.