Abstract
Long-acting antiretroviral products have the potential to transform human immunodeficiency virus (HIV) prevention and treatment approaches in pediatric populations. Broadly neutralizing antibodies and/or long-acting antiretroviral formulations by injection could dramatically improve provision of HIV prophylaxis and/or early treatment to newborns and infants at risk of HIV infection. Challenges in daily oral antiretroviral administration to toddlers and school age children living with HIV may be relieved by use of long-acting formulations, but the pharmacokinetics and safety of these products in children must be studied before they can enter routine clinical use. Although some initial studies of broadly neutralizing antibodies and injectable long-acting agents in infants and young children are underway, more studies of these and other long-acting products are needed. For many adolescents, compliance with daily medication administration is especially challenging. Long-acting products hold particular promise for adolescents living with HIV as well as those at high risk of HIV acquisition, and adolescents can usually be included in the drug development pipeline simultaneously with adults. Long-acting products have the potential to provide alternatives to lifelong daily oral drug administration across the pediatric age spectrum, leading to more effective prevention and treatment of HIV infection in infants, children, and adolescents.
Keywords: long-acting antiretroviral agents, pediatrics, neonatal physiology, adherence
Long-acting antiviral products have the potential to provide alternatives to lifelong daily oral drug administration across the pediatric age spectrum, leading to more effective prevention and treatment of human immunodeficiency virus (HIV) infection in infants, children, and adolescents.
Despite major scientific advances in the prevention and treatment of pediatric human immunodeficiency virus (HIV) infection, HIV continues to cause substantial pediatric morbidity and mortality, particularly in sub-Saharan Africa. In 2021 there were an estimated 160 000 new pediatric HIV infections and 98 000 child deaths attributed to HIV [1]. Of the estimated 1.7 million children <15 years of age living with HIV infection globally, only just over half (52%) are receiving antiretroviral treatment (ART), and rates of viral suppression have been suboptimal and substantially lower than those reported in adults [1]. In 2020, in 21 focus countries with a high burden of pediatric HIV infection, 40% of children living with HIV infection and 75% of those on ART were virally suppressed [2].
Although multiple factors have contributed to these poor outcomes, the lack of potent agents and regimens in age-appropriate formulations has been a substantial barrier to successful treatment. Pediatric treatment has historically entailed caregivers administering multiple poorly tasting liquid formulations or pills once or twice daily [3, 4]. Current guidelines recommend that infants born to mothers living with HIV receive postnatal HIV prophylaxis with long courses of daily doses of either nevirapine (NVP) oral suspension or zidovudine (ZDV) syrup, both of which are antiquated agents no longer recommended for use in adults [5]. The adherence challenges associated with providing these regimens to infants and children have been well described [6]. Caregivers struggle to give their infants and children daily medications, particularly poorly tasting agents and those requiring special preparation [7, 8]. Toddlers, school age children, and adolescents struggle to take medications daily for a variety of reasons including poor taste, forgetfulness, emerging independence, and the daily reminder that they are living with HIV infection. The recent introduction of once daily dolutegravir promises to address some barriers to adherence, but daily oral medications continue to raise issues of privacy and stigma [9, 10].
Childhood is a period of rapid change, characterized by physical growth and development, organ system maturation, and neuropsychological development, resulting in large pharmacologic changes as children grow from neonates to adolescents. All aspects of drug disposition will be affected. Drug absorption will be impacted by changes in diet and feeding pattern as children grow and mature. Newborns and young children are unable to swallow pills, so special formulations that may have distinct absorption characteristics, such as liquids or dispersible tablets, are needed for them to take oral medications. Drug disposition will be affected by dramatic changes in body size and composition, requiring evaluation of appropriate dose sizes over the course of childhood. Drug metabolism and elimination change during childhood with changes in the activity of drug metabolizing enzymes and maturation of hepatic and renal function. Drug taking behavior is particularly challenging across the pediatric age spectrum, with different issues arising as children grow from infancy through childhood and into adolescence.
Historically it has taken as long as a decade from the time that a new antiretroviral drug (ARV) is introduced for use in adults until it becomes available for children. This includes many of the innovations that have led to measurable improvement in adherence in adults including once daily fixed-dose combination regimens and long-acting injectable agents. New agents need to be tailored to and tested in pediatric populations. Changes across these domains have a direct effect on drug dosing and safety as well as feasibility, acceptability, and adherence of any new agent. It is through this lens that we address the potential of long-acting products to transform HIV treatment and prevention in the pediatric population, focusing the discussion on special considerations for neonates and infants, children, and adolescents.
NEONATES AND INFANTS
HIV treatment and prevention in newborns and young infants using existing daily oral formulations is challenging and could benefit greatly from the features of broadly neutralizing antibodies (bANbs) and long-acting ARV formulations. Given the potential for rapid diagnosis at birth and the recognition of short- and long-term benefits from rapid virologic suppression, there is increased interest in developing highly potent and well-tolerated regimens for neonates [11, 12]. Similarly, there is a longstanding need to optimize approaches to postnatal prophylaxis to reduce the risk of vertical transmission of HIV [13, 14]. An estimated 1–1.5 million newborns and infants require ARVs each year, given from birth through the first months, to reduce the risk of perinatal and postnatal vertical transmission [2]. However, providing prophylaxis or early treatment within the first month of life coincides with a period of dynamic growth and development resulting in dramatic pharmacokinetic (PK) changes in drug absorption, metabolism and excretion, and protein binding. Potential risks of ARV toxicity are also a particular concern during the first month of life when increased drug sensitivity and difficulties in recognition of drug toxicities add to the complexity of ARV use in this population. Premature birth is associated with an increased risk of HIV acquisition, but dosing for preterm infants is especially challenging given the effects of immaturity of gastrointestinal (GI) tract function on drug absorption and of metabolic and renal pathways on drug elimination. There are limited available oral ARVs with suitable infant formulations and established dosing during the first month of life. This list is even shorter for preterm infants where PK and dosing information are only available for 2 ARVs, ZDV and NVP. Challenges in ARV preparation and administration can lead to incomplete dosing, low adherence, and compromised efficacy.
Although safety, PK, and dosing studies in newborn infants are intrinsically difficult to conduct, some features of the pharmacology of bNAbs and long-acting formulations are well suited to this population. The dose volumes of bNabs required to treat infants are small, often 1–2 mL, making single injection administration possible, whereas multiple injections are required for older, heavier populations. Although infant absorption following intramuscular (IM) and subcutaneous (SC) administration is not identical to that in adults, it is easier to extrapolate the extent (bioavailablity) and rate of absorption in infants from adults than it is following oral administration. In newborns, unique oral formations are typically needed, which may have different absorption characteristics than in adults. Neonatal gut physiology and function along with diet and food effects can result in altered oral absorption rates and bioavailability in infants [15]. The SC absorption of monoclonal antibodies (mAbs) targeting respiratory syncytial virus is more rapid in infants than adults, but after the first few days the overall shape of the concentration profile is similar [16]. Rapid SC absorption of bNAbs in newborns and infants is a desirable feature as it results in prompt achievement of therapeutic concentrations. Extrapolation from adult data also suggests that the absorption rate of cabotegravir/rilpivirine (CAB/RPV) IM will be faster in infants due to size, but this will require future evaluation [17].
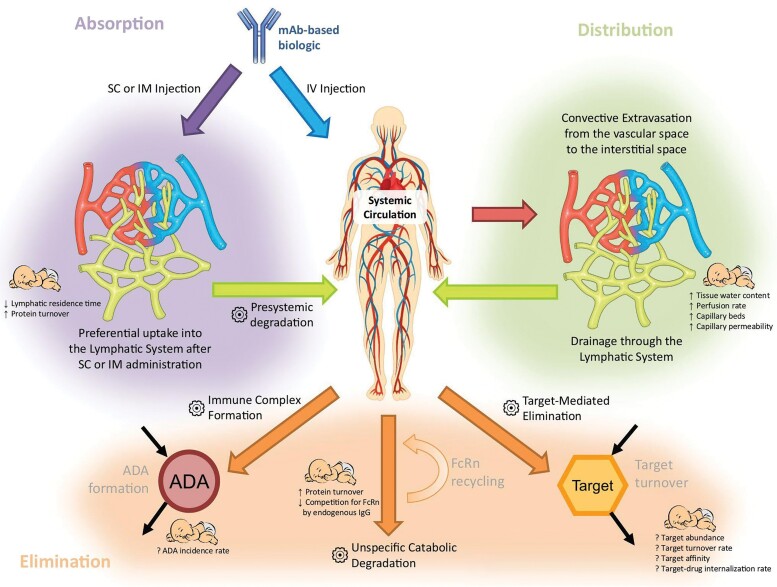
The absorption, distribution, metabolism, and elimination of mAbs differ greatly from small molecules. Many factors influencing mAb PK have complex effects and change from infancy into adulthood as shown in Figure 1. Differences in body composition, capillary architecture, lymphatic flow and protein metabolism, can lead to differences in bioavailability following SC administration between infants and adults. However, cross-study comparisons between infants and adults are confounded by the higher mg/kg doses studied in infants. However, bioavailability differences due to age appear to be modest for bNAbs including VRC01, VRC01LS, and VRC07-53LS [18–22]. Infant concentration profiles are below those seen in adults following IV administration of similar doses suggesting presystemic degradation.
Figure 1.
Graphical summary of the major pharmacokinetic processes determining the disposition of mAbs and their modulation in newborns and infants. The gear symbol indicates elimination processes. Figure from Z. Temrikar et al Paediatr Drugs 2020 [22]. Abbreviations: ADA, anti-drug antibody; FcRn, neonatal Fc receptor; Ig, immunoglobulin; IM, intramuscular; IV, intravenous; mAb, monoclonal antibody; SC, subcutaneous.
The elimination of mAbs is primarily due to unspecific proteolytic catabolism, which is relatively mature at birth with modest increases during childhood [15, 23]. There are additional possible mAB elimination pathways through immune complex formation and target mediated elimination. Although there may be dramatic increases and high variability of typical ARV drug metabolizing enzymes (cytochrome P450, glucuronyl transferases, etc) over the first few weeks of life, large increases are not seen for mAb metabolism. In additional, any very early changes in mAb metabolism do not have a large impact on the shape of concentration profiles for bNAb, because their slow elimination smooths the impact of early change over the first weeks of life.
Certain features of bNAbs and long-acting ARVs could also make their study in infants easier to perform and more informative. Single dose bNab PK studies are very informative and thus multiple dose studies and the risk for accumulation to excessive concentrations avoided. The slow decay of concentrations means that intensive PK collections on a single day are not needed, and PK can be determined by infrequent single collections performed days or weeks apart. However, some features of infancy will make study of bNAbs and long-acting ARVs more challenging. Given the slow elimination of long-acting products, the degree of growth over the first months of life between doses can be significant. This means that the observed decline in plasma concentrations becomes a function of both metabolism and infant growth. Long-acting bNAbs with modified Fc regions can have half-lives approaching 8–12 weeks in adults [21, 22], and weight gain will contribute substantially within a single half-life interval. During the first 12 weeks of life, newborns nearly double in weight creating a dilution effect on bNAb concentrations. Thus, a 10 mg/kg bNAb dose given at birth only represents ∼5 mg/kg of bNAb based on weight at 3 months of age.
Although there is overlap in the utility of long-acting ARVs and bNAbs, there are also important differences in their PK properties. Both long-acting ARVs and bNAbs can cross the placenta and result in fetal exposure. After birth, the long-acting ARV concentration in newborns will decline rapidly like a non–long-acting product because there is no intramuscular depot in the newborn. This decline will be slower than seen with oral administration in adults due to immature metabolism, but because absorption from the maternal long-acting depot ends at delivery, newborn ARV concentrations will disappear over the course of the first few days of life. However, bNAbs administered to the mother during pregnancy that cross the placenta into the fetus will have a continued slow rate of decline in the newborn after birth that will mirror the decline seen following direct infant administration.
There is interest in quickly generating safety and PK data for bANbs and long-acting antiretrovirals in infants. Due to their favorable safety profile in adults, studies of bNAbs have opened in newborns soon after their safety and PK were established in adults. The IMPAACT P1112 study recently completed evaluations of VRC01, VRC01LS, and VRC07-523LS after SC administration in HIV-exposed infants [24]. Overall, infants maintained target troughs with every 4 week (VRC01) or every 12 week (VRC01L AND VRC07-523LS) administration [19, 20, 25]. The IMPAACT 2008 study recently evaluated VRC01 in infants with HIV infection [26]. There are neonatal bNAb studies being designed for newer, more potent, longer acting bNAbs for use in combination in infants [27]. Table 1 is a snapshot of current and planned trials with long-acting agents for infants, children, and adolescents.
Table 1.
Trials of Long-Acting Agents in Newborns, Infants, Children, and Adolescentsa
| Agent | Study | Indication | Objectives | Population | Sample Size | Status |
|---|---|---|---|---|---|---|
| Broadly neutralizing antibodies (bNABs) | ||||||
| SC VRC01, SC VRC01LS, VRC07-523LS | IMPAACT 1112 | Prevention and Treatment | Phase I study to determine safety and Pharmacokinetics (PK) of each agent administered SC in infancy | HIV-exposed infants | 79 | Complete |
| SC VRC07-523LS + CAP256V2LS | PedMAB | Prevention | Phase I/II study, dose finding, PK and safey | HIV-exposed newborns | … | Enrolling |
| SC VRC07-523LS | SAMBUELA | Prevention | Phase II PK and safety in breastfed infants | HIV-exposed, unexposed, and infected newborns | 128 | In development |
| SC VRC01LS | EDCTP Neo bnAb Trial | Prevention | 4 doses from birth to 36 w | HIV-exposed newborns | 2000 | In development |
| SC VRCO1 | IMPAACT 2008 | Cure | Phase I/II RCT of VRC01 with ART to promote clearance of HIV-infected cells in infants with HIV | <12 w, living with HIV | 61 | Complete |
| IV VRC01LS and 10-1074 | Dual bNAb Treatment in Children: Tatelo Study | Treatment | Safety, PK, dosing, and antiviral efficacy of maintenance VRC01LS and 10-1074 immunotherapy on viral suppression in early treated children | 2–5 y, living with HIV | 30 | Ongoing |
| CAP256-V2LS, VRC07-523LS and PGT121.414LS |
ENABLE | Treatment | Safety, PK, reduction in viral load and reservoir size, increase in HIV-specific immune response | 28–180 d, 2–7 y | 20 | In development |
| Antiretroviral Agents | … | … | … | … | ||
| IM CAB/RPV | IMPAACT 2017/MOCHA | HIV Treatment | Phase I/I study of safety, acceptability, tolerability, and PK of CAB/RPV in virologically suppressed children and adolescents | 12≤18 y, youth living with HIV | 155 | Actively enrolling |
| IM CAB/RPV | IMPAACT 2036/CRAYON | HIV Treatment | Phase I/II study of safety, tolerability, PK, and antiviral activity of CAB/RPV in virologically suppressed children | >2≤12 y and weighing >10 kg and <50 kg, children living with HIV | TBD | In development |
| IM CAB/RPV | LATA | HIV Treatment | Open label, non-inferiority study of virologically suppressed participants on first-line ART; primary endpoint: proportion with confirmed virological rebound, HIV-RBA ≥50 copies/mL | 12–19 y, youth living with HIV | 230/arm: CAB/RPV vs DTG/3TC/TDF | Open to enrollment, fall 2022 |
| IM CAB/RPV | IMPAACT 2040/CREATE | HIV Treatment | Phase I/II PK and safety of IM CAB/RPV in pregnancy and postpartum; wash-out PK in exposed neonates | Pregnant people and their newborns through | 45 mother/baby pairs | In development |
Abbreviations: ART, antiretroviral therapy; CAB/RPV, cabotegravir/rilpivirine; CRAYON, Cabotegravir and Rilpivirine Long-Acting Injections in Young Children; CREATE, Cabotegravir & Rilpivirine Antiretroviral Therapy in Pregnancy; EDCTP, European and Developing Countries Clinical Trials Partnership; ENABLE, Early Neutralizing Antibodies children in Earth cohort; HIV, human immunodeficiency virus; IMPAACT, International Maternal Pediatric Adolescent AIDS Clinical Trials Network; LATA, Long Acting Treatment for Adolescents; MOCHA, More Options for Children and Adolescents; PK, pharmacokinetic; RCT, randomly controlled trial; RPV, Rilpivirine.
Given the dynamic scientific landscape, emerging studies may not be included in this table.
The developmental timelines for LA CAB and RPV in infants are following a more deliberate path. They are being evaluated in the classical step down through pediatric age groups in IMPAACT 2017/MOCHA [28] and IMPAACT 2036/CRAYON [29] studies. In addition, a study, IMPAACT 2040/CREATE, is being designed to evaluate LA CAB/RPV PK and safety in mothers during pregnancy and of infant washout PK after birth [30].
TODDLER/SCHOOL AGE CHILDREN
Experience with use of long-acting formulations for other conditions in toddlers and young children is very limited. A recent systematic review of the medical literature for articles published between 1980 and 2018 describing the safety and efficacy of long-acting formulations in children, adolescents, and pregnant women found only 46 articles in infants and children [31]. Of these articles, 36 (78%) were for hormone therapies, generally for treatment of precocious puberty, leaving only 10 for other conditions.
Use of long-acting formulations holds promise for infants and young children living with HIV. Children living with HIV who are unable to swallow pills or capsules generally receive ARVs as liquid formulations, although recently several granule and dispersible tablet products have become available. Liquid formulations are bulky, difficult to store, often have poor palatability and pose supply chain difficulties particularly in low- and middle-income environments. Normal growth may require frequent dose adjustments, and large volumes may be required to dose older children with a liquid formulation. Long-acting formulations offer an attractive alternative for treatment of toddlers and young children that would allow children and their care providers relief from the challenges of daily oral medication administration.
Studies evaluating the dosing, efficacy, and tolerability of long-acting formulations in young children are needed before such formulations can become part of clinical care. Maintenance of adequate circulating ARV concentrations after injection of intramuscular depot formulations is dependent on the rate of release from the depot, which may be different in the muscles and subcutaneous tissues of children than in those of adults. The need for dosing adjustment of such products to account for normal growth and development must be determined, as well as the need to tailor dose size to children with comorbidities, concomitant drugs, and poor or excessive growth. Pharmacokinetic modeling and simulations may play an important role in streamlining and facilitating dose-finding for long acting formulations in children [32] Current long-acting intramuscular depot formulations, such as long-acting CAB/RPV, cannot be removed once they have been administered, so their toxicity profile in children must be well described before they can be routinely used. Provision of repeated intravenous infusions or SC injections in young children poses challenges, and the practicality and tolerability of repeated infusions and/or injections must be evaluated to ensure that children who begin HIV treatment with such products will be willing and able to continue to receive them indefinitely.
Current and planned studies of long-acting antiretroviral formulations in toddlers and young children are limited. The Tatelo study is now underway studying monthly intravenous infusions of dual bNAb treatment using VRC01Ls and 10-1074 in children living with HIV aged 2–5 years who are fully suppressed on standard ART [33]. In a preliminary report, 11 of 25 (44%) children maintained viral suppression over 24 weeks after cessation of their ART regimen while receiving treatment with these bNABs alone. The CRAYON study (IMPAACT 2036) currently under development will be a phase I/II study of long-acting injections of CAB and RPV in virologically suppressed children living with HIV ages 2–12 years [29]. The IMPAACT 1115 study of early intensive treatment of HIV-infected infants includes an arm incorporating monthly treatment with the bNAb VRC01 [34]. The EPIICAL Project is developing a protocol that will involve treatment of infants and children living with HIV with combined administration of 3 bNABs [35]. Other studies of currently available long-acting formulations, including long-acting CAB/RPV and bNAbs, as well as innovative long-acting products and/or alternative delivery methods, are necessary before such products can live up to their promise of improving antiretroviral therapy in young children.
ADOLESCENTS
Long-acting drug and delivery platforms hold particular promise for adolescents living with HIV. Adolescents bear a large burden of the HIV epidemic, especially young women and girls. In 2020, 1.75 million adolescents (10–19 years old) were living with HIV globally [36]; in sub-Saharan Africa, 6 of 7 new HIV infections among adolescents (15–19 years old) were among girls [1]. Adolescents with HIV often have poor treatment outcomes. Estimates of virologic non-suppression among adolescents vary by geography and methodology but range from 11% to as high as 73% [37]. In a recent report from South Africa 11% of pregnant adolescent females and 20% of adolescent males experienced non-suppression over 24 months of follow-up [38]. In the United States, only 54% of adolescents who initiate treatment are estimated to achieve HIV viral suppression [39]. Other studies estimate that poor adherence to the oral treatments is common among adolescents and youth with HIV, estimated at 26% in Africa, 38% Europe, and 47% in North America [40]. A recent systematic review found no patient level interventions that significantly improved adherence or virologic suppression rates in adolescents with HIV [41]. Well-tolerated long-acting treatments hold the potential to address many of the key barriers to adherence for adolescents, which include stigma, drug side-effects, and forgetfulness [42].
This most efficient mechanism for adolescents to gain access to long-acting agents and platforms will be to include them in the development pipeline simultaneously with adults. The pharmacokinetics of most medications in adolescents are similar to adults; although there can be some changes with growth spurts during adolescence, the most dramatic age-related changes in drug metabolism and clearance occur over the first few years life [43, 44]. Accordingly, most of the currently approved antiretrovirals are recommended at the same doses for adolescents as adults, in the United States [45] and by the World Health Organization (WHO) [46]. A strategy to include adolescents in the primary regulatory trials with adults is endorsed by the Pediatric Antiretroviral Working Group of the WHO [47] and the Food and Drug Administration (FDA) [48]. Based on this approach, adolescents were included simultaneously with adults in the primary approval for IM CAB for use as pre-exposure prophylaxis by the FDA in 2021 [49] and the agent is approved using weight (>35 kg) rather than age (traditionally >18 years) to define dose.
Research on long-acting platforms must navigate the ethics and often country-specific legal guidelines around the inclusion of adolescents in research. Consent from a parent or guardian plus “assent” from the adolescent is often required for participation in research. Strict parental consent requirements can decrease adolescent participation in research, especially from key populations that would benefit the most from inclusion [50]. In Brazil, for example, a 15-year-old girl would not be permitted to participate without explicit parental permission, whereas in Kenya, she could become emancipated from this requirement through marriage or childbirth [51, 52]. Ethical benchmarks for including adolescents in research have been published [53], and other reviews provide guidance about how to enroll adolescents efficiently and safely in research [54]. Other challenges for including adolescents in research include high rates of unplanned pregnancies in some populations of young women and historically greater challenges with uptake and adherence to chronic medications.
The only published research to date about long-acting treatments in adolescents has been for CAB and RPV. The data supporting FDA approval of injectable cabotegravir for pre-exposure prophylaxis was based on efficacy data from trials (HPTN 083 [55] and HPTN 084 [56]) of adults (>18 years of age) but included safety and PK data from 54 adolescents [57]. The ongoing MOCHA study is a phase I/II study of long-acting CAB/RPV for the treatment of virologically suppressed adolescents ages 12 to <18 years (NCT03497676) [28, 58]. Preliminary results suggest that injectable CAB/RPV given every 4–8 weeks is acceptable to youth and parents [59] and achieved target drug levels comparable to adults. The LATA study, an open label, non-inferiority study comparing CAB/RPV to dolutegravir/lamivudine/tenofovir among virologically suppressed youth, ages 12–19 years, with a primary outcome of virological rebound, is expected to open in late 2022 (NCT05154747).
SPECIAL CONSIDERATIONS FOR LONG-ACTING PRODUCTS FOR INFANTS, CHILDREN, AND ADOLESCENTS
There is great enthusiasm for the potential of long-acting products to overcome many of the challenges that have been encountered in bringing new antiretroviral products to children. Historically, the delayed development of specific formulations for children, particularly for infants, have prevented them from having early access to several potent agents [47], whereas the development of inadequate pediatric formulations (poorly tasting, difficult to administer) has had profound impact on adherence and retention in care. Notably this holds true not only for HIV but for other highly prevalent conditions including tuberculosis and hepatitis. Long-acting injectable formulations and alternative delivery platforms could accelerate the study of and access to many of the new agents discussed above for children as well as new products for treatment of tuberculosis and hepatitis. Nonetheless, the optimal approach to delivering specific agents will vary across the age spectrum from birth through adolescence. Recognizing this variation by age, participants at the WHO Pediatric AIDS Drug Optimization-HIV (PADO) 5 meeting discussed and described the advantages and disadvantages of new drug delivery technologies for different pediatric populations, demonstrating preferences for each technology by age [60]. Several of the key findings are reiterated here.
Chronic intramuscular injections of CAB and RPV for HIV treatment have been reported as highly acceptable in adults, and preliminary findings suggest a similar experience for adolescents receiving monthly dosing [58, 61]. Whether the experience changes over time among adolescents (injection fatigue) will need to be evaluated, as will the response of younger children who may be more averse to needles and injections and less well poised to balance the challenges of daily oral medication versus regular injections. Furthermore, intramuscular injections may not be suitable for infants and neonates depending on the injection volume and resulting pain. In many settings, medication injections are highly acceptable and often preferred to oral agents, viewed as more potent and tolerable. Similarly, depot medroxyprogesterone acetate injections given quarterly are the most common form of contraception in adolescents and young women in many countries. In contrast, programs administering monthly intramuscular penicillin G benzathine for secondary prophylaxis to children and adolescents with rheumatic heart disease have reported sub-optimal retention in care and adherence to treatment citing multiple barriers including poor access to health care where injections are administered, travel costs, negative staff interactions, injection site pain and health beliefs of patients, families, and providers [62].
Subcutaneous injections are generally preferable to intramuscular injections, as they may be less painful and easier to administer. They are also more feasible to consider across the pediatric spectrum including neonates and infants. Several of the bNAbs that have been studied in infants are administered subcutaneously, making them attractive candidates for prevention of postnatal HIV transmission. Assuming the availability of a potent, long-acting combination bNAb regimen, repeated subcutaneous injections to breastfeeding infants of mothers living with HIV could possibly be delivered within existing immunization programs. This would be a strategic way to integrate HIV prevention with other routine childhood services but would need to be evaluated for feasibility, cost, and effectiveness.
Other delivery platforms may hold particular promise for pediatric populations. Microarray patches have generated interest and are currently being developed using CAB and RPV as a model for use in children [63]. An approach that avoids daily oral administration as well as the pain of and logistical challenge of injections could lead to significant improvements in quality of life for children with chronic diseases. Several long-acting oral products with less frequent dosing are also in development for prevention and treatment of HIV infection, tuberculosis, and hepatitis in adults. Early attention to the development of pediatric formulations is indicated for products of high interest.
CONCLUSIONS
The history of antiretroviral development is punctuated with multiple failed efforts to bring potent, child-friendly agents to the pediatric population, resulting in generations of children using suboptimal regimens leading to suboptimal outcomes. Although better, more potent medications are increasingly available, the challenge of taking medication each day for the remainder of one's life remains daunting. Long-acting formulations, those discussed above as well as others in development, have the potential to decrease the adherence burden on children and families, and improve quality of life and rates of sustained viral suppression among children and adolescents living with HIV. The introduction of potent, safe long-acting products like injectable CAB or an efficacious bNAb combination regimen for postnatal prevention would leapfrog efforts to end the pediatric epidemic. The recent accelerated development and introduction of a pediatric dolutegravir oral formulation provides a roadmap for how to more rapidly develop, test, and scale-up a product for children [9, 10, 15, 64]. It will be critical to follow this roadmap and to routinely include infants, children, and adolescents in the new long-acting product pipeline for HIV and other highly prevalent diseases including tuberculosis and hepatitis, to ensure that the full transformative potential of these products is achieved.
Contributor Information
Elaine J Abrams, ICAP at Columbia University, Mailman School of Public Health, Columbia University, New York, New York, USA; Department of Pediatrics, Vagelos College of Physicians and Surgeons, Columbia University, New York, New York, USA; Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York, USA.
Edmund Capparelli, Department of Pediatrics and Clinical Pharmacy, University of California San Diego, La Jolla, California, USA.
Theodore Ruel, Department of Pediatrics, University of California San Francisco, San Francisco, California, USA.
Mark Mirochnick, Department of Pediatrics, Boston University, Boston, Massachusetts, USA.
Notes
Acknowledgments. E. J. A., M. M., E. C., and T. R. all receive support from the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT). Support for the IMPAACT Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under award numbers UM1AI068632-15 (IMPAACT LOC), UM1AI068616-15 (IMPAACT SDMC), and UM1AI106716-15 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplement sponsorship. This article appears as part of the supplement “Long-Acting and Extended-Release Formulations for the Treatment and Prevention of Infectious Diseases,” sponsored by the Long-Acting/Extended Release Antiretroviral Research Resource Program (LEAP).
References
- 1. UNAIDS . 2022 UNAIDS Global AIDS Update—In Danger. 2022. Available at: https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf. Accessed 28 August 2022.
- 2. UNAIDS . Start free, stay free, AIDS free, Final report on 2020 targets. 2021 July 2021. Available at: https://www.unaids.org/en/resources/documents/2021/start-free-stay-free-aids-free-final-report-on-2020-targets. Accessed 25 May 2022.
- 3. Schlatter AF, Deathe AR, Vreeman RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treat 2016; 2016:1654938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penazzato M, Lee J, Capparelli E, et al. Optimizing drugs to reach treatment targets for children and adolescents living with HIV. J Int AIDS Soc 2015; 18(Suppl 6):20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO . Policy Brief: comprehensive package of care for infants and young children exposed to HIV. 2021. Available at: https://apps.who.int/iris/bitstream/handle/10665/350214/9789240040236-eng.pdf. Accessed 25 May 2022.
- 6. Penazzato M, Kasirye I, Ruel T, et al. Antiretroviral postnatal prophylaxis to prevent HIV vertical transmission: present and future strategies. In press. JIAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasipanodya B, Kuwengwa R, Prust ML, et al. Assessing the adoption of lopinavir/ritonavir oral pellets for HIV-positive children in Zimbabwe. J Int AIDS Soc 2018; 21:e25214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teasdale CA, Abrams EJ, Coovadia A, et al. Adherence and viral suppression among infants and young children initiating protease inhibitor-based antiretroviral therapy. Pediatr Infect Dis J 2013; 32:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruel TD, Acosta EP, Liu JP, et al. Pharmacokinetics, safety, tolerability, and antiviral activity of dolutegravir dispersible tablets in infants and children with HIV-1 (IMPAACT P1093): results of an open-label, phase 1-2 trial. Lancet HIV 2022; 9:e332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turkova A, White E, Mujuru HA, et al. Dolutegravir as first- or second-line treatment for HIV-1 infection in children. ODYSSEY trial team. N Engl J Med 2021; 385:2531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: prospects for cure. Curr Opin HIV AIDS 2015; 10:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiau S, Abrams EJ, Arpadi SM, Kuhn L. Early antiretroviral therapy in HIV-infected infants: can it lead to HIV remission? Lancet HIV 2018; 5:e250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beste S, Essajee S, Siberry G, et al. Optimal antiretroviral prophylaxis in infants at high risk of acquiring HIV: a systematic review. Pediatr Infect Dis J 2018; 37:169–75. [DOI] [PubMed] [Google Scholar]
- 14. Jacobs TG, Schouwenburg S, Penazzato M, et al. What babies need: accelerating access to current and novel antiretroviral drugs in neonates through pharmacokinetic studies. Lancet HIV. 2022 Sep;9(9):e649-e657. [DOI] [PubMed] [Google Scholar]
- 15. Wollmer E, Ungell AL, Nicolas JM, Klein S. Review of paediatric gastrointestinal physiology relevant to the absorption of orally administered medicines. Adv Drug Deliv Rev 2022; 181:114084. [DOI] [PubMed] [Google Scholar]
- 16. Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother 2012; 56:4927–36. Erratum in: Antimicrob Agents Chemother. 2012 Oct; 56(10):5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bunglawala F, Mirochnick M, Capparelli E, Siccardi M. Dose optimisation of long-acting injectables in neonates via PBPK modelling. Abstract 606. CROI 2021. Virtual. 6-10 March 2021. Available at:https://www.croiconference.org/abstract/dose-optimisation-of-long-acting-injectables-in-neonates-via-pbpk-modelling/. Accessed 25 May 2022.
- 18. Li J, Nikanjam M, Cunningham CK, et al. Model informed development of VRC01 in newborn infants using a population pharmacokinetics approach. Clin Pharmacol Ther 2021; 109:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McFarland EJ, Cunningham CK, Muresan P, et al. Safety, tolerability, and pharmacokinetics of a long-acting broadly neutralizing human immunodeficiency virus type 1 (HIV-1) monoclonal antibody VRC01LS in HIV-1-exposed newborn infants. J Infect Dis 2021; 224:1916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunningham CK, Capparelli E, McFarland EJ, et al. CROI 2022. Virtual. 12–16 February 2021. Oral abstract 732. Available at: https://www.croiconference.org/abstract/extended-safety-and-pk-of-anti-hiv-monoclonal-ab-vrc07-523ls-in-hiv-exposed-infants/ . Accessed 25 May 2022.
- 21. Gaudinski MR, Houser KV, Doria-Rose NA, et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV 2019; 6:e667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15:e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Temrikar ZH, Suryawanshi S, Meibohm B. Pharmacokinetics and clinical pharmacology of monoclonal antibodies in pediatric patients. Paediatr Drugs 2020; 22:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ClinicalTrials.gov . National Library of Medicine (U.S.). (30 June 2015–6 December 2021). Evaluating the Safety and Pharmacokinetics of VRC01, VRC01LS, and VRC07-523LS, Potent Anti-HIV Neutralizing Monoclonal Antibodies, in HIV-1-Exposed Infants. Identifier NCT02256631. 22 April 2022. Available at:https://www.clinicaltrials.gov/ct2/show/NCT02256631. Accessed 25 May 2022.
- 25. Cunningham CK, McFarland EJ, Morrison RL, et al. Safety, tolerability, and pharmacokinetics of the broadly neutralizing human immunodeficiency virus (HIV)-1 monoclonal antibody VRC01 in HIV-exposed newborn infants. J Infect Dis 2020; 222:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ClinicalTrials.gov . National Library of Medicine (U.S.). (8 May 2018–11 February 2021). Evaluating the Safety and Antiviral Activity of Monoclonal Antibody VRC01 in HIV-Infected Infants Receiving Combination Antiretroviral Therapy. Identifier NCT03208231. 22 April 2022. Available at:https://clinicaltrials.gov/ct2/show/NCT03208231. Accessed 25 May 2022.
- 27. University of Bergen: Centre for International Health. PedMAb: a project with paradigm-shift potential. 22 June 2021. Available at: https://www.uib.no/en/cih/146061/pedmab-project-paradigm-shift-potential. Accessed 26 May 2022.
- 28. ClinicalTrials.gov . National Library of Medicine (U.S.). (19 May 2019 –). More Options for Children and Adolescents (MOCHA): Oral and long-acting injectable cabotegravir and rilpivirine in HIV-infected children and adolescents (MOCHA). Identifier NCT03497676. 22 April 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT03497676. Accessed 25 May 2022.
- 29. IMPAACT. IMPAACT 2036: Phase I/II study of the safety, tolerability, pharmacokinetics, and antiviral activity of oral and long-acting injectable cabotegravir and rilpivirine in virologically suppressed HIV-infected children >2 to >12 years of age and weighing >10 kgs and >50 kgs. DAIDS Number 38932. Available at: https://www.impaactnetwork.org/studies/impaact2036. Accessed 25 May 2022.
- 30. IMPAACT. IMPAACT 2040: pharmacokinetics and safety of long-acting injectable cabotegravir and rilpivirine in virally suppressed people with HIV-1 during pregnancy and postpartum. Available at: https://www.impaactnetwork.org/studies/impaact2040. Accessed 25 May 2022.
- 31. Bertagnolli L. LEAP Systematic reviews of LA formulation use in pediatrics and pregnancy. Presented at LEAP Resource Program Annual Workshop. 12 Feb 2022. Available at: https://longactinghiv.org/files/inline-files/LynnBertagnolli-LEAP2022.mp4. Accessed 14 April 2022.
- 32. Rajoli RKR, Back DJ, Rannard S, et al. In silico dose prediction for long-acting rilpivirine and cabotegravir administration to children and adolescents. Clin Pharmacokinetics 2018; 57:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shapiro RL, Maswabi K, Ajibola G, et al. Treatment with broadly neutralizing antibodies in children with HIV in Botswana. CROI 2022. 12–16 February. Virtual. Oral abstract 32. Available at: https://www.croiconference.org/abstract/treatment-with-broadly-neutralizing-antibodies-in-children-with-hiv-in-botswana/ . Accessed 25 May 2022.
- 34. ClinicalTrials.gov . National Library of Medicine (U.S.). (23 January 2015 –). Very early intensive treatment of HIV-infected infants to achieve HIV remission. Identifier NCT02140255. 4 November 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02140255. Accessed 25 May 2022.
- 35. EPIICAL Project . The HIV clinical and experimental platform. Available at: https://www.epiical.org/. Accessed 13 April 2022.
- 36. UNICEF . Turning the tide against AIDS will require more concentrated focus on adolescents and young people. July 2021. Available at: https://data.unicef.org/topic/adolescents/hiv-aids/. Accessed 13 April 2022.
- 37. Ferrand RA, Briggs D, Ferguson J, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health 2016; 21:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyakato P, Schomaker M, Fatti G, et al. Virologic non-suppression and early loss to follow up among pregnant and non-pregnant adolescents aged 15–19 years initiating antiretroviral therapy in South Africa: a retrospective cohort study. J Int AIDS Soc 2022; 25:e25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014; 28:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS 2014; 28:1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reif LK, Abrams EJ, Arpadi S, et al. Interventions to improve antiretroviral therapy adherence among adolescents and youth in low- and middle-income countries: a systematic review 2015-2019. AIDS Behav 2020; 24:2797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ammon N, Mason S, Corkery JM. Factors impacting antiretroviral therapy adherence among human immunodeficiency virus-positive adolescents in sub-Saharan Africa: a systematic review. Public Health 2018; 157:20–31. [DOI] [PubMed] [Google Scholar]
- 43. Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 2014; 19:262–76. https://pubmed.ncbi.nlm.nih.gov/25762871/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Momper JD, Mulugeta Y, Green DJ, et al. Adolescent dosing and labeling since the Food and Drug Administration Amendments Act of 2007. JAMA Pediatr 2013; 167:926–32. [DOI] [PubMed] [Google Scholar]
- 45. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed 14 April 2022.
- 46. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring recommendations for a public health approach. 16 July 2021. Available at: https://www.who.int/publications/i/item/9789240031593. Accessed 25 May 2022. [PubMed]
- 47. Penazzato M, Gnanashanmugam D, Rojo P, et al. Optimizing research to speed up availability of pediatric antiretroviral drugs and formulations. Clin Infec Dis 2017; 64:1597–603. https://pubmed.ncbi.nlm.nih.gov/32869500/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. U.S. Food & Drug Administration . Pediatric HIV infection: drug product development for treatment: guidance for industry. March 2019. FDA-2018-D-1638. Available at: https://www.fda.gov/media/113319/download. Accessed 14 April 2022.
- 49. U.S. Food & Drug Administration . FDA approves first injectable treatment for HIV pre-exposure prevention. News Release. 20 December 2021. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-injectable-treatment-hiv-pre-exposure-prevention. Accessed 14 April 2022.
- 50. Singh JA, Karim SS, Karim QA, et al. Enrolling adolescents in research on HIV and other sensitive issues: lessons from South Africa. PLoS Med 2006; 3:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. National Institute for Allergy and Infectious Diseases . Brazil: informed consent—children/minors. 12 May 2022. Available at: https://clinregs.niaid.nih.gov/country/brazil#children/minors. Accessed 24 May 2022.
- 52. Kenya Medical Research Institute . Standard operating procedure for general requirements of informed consent and documentation of informed consent. 18 October 2017. Available at:https://www.kemri.go.ke/wp-content/uploads/2019/11/PI_SOP-6_General-Requirements-of-Informed-Consent-and-Documentation.pdf. Accessed 14 April 2022.
- 53. Bekker LG, Slack C, Lee S, Shah S, Kapogiannis B. Ethical issues in adolescent HIV research in resource-limited countries. J Acquir Immune Defic Syndr 2014; 65(Suppl 1):S24–8. https://pubmed.ncbi.nlm.nih.gov/24321980/ [DOI] [PubMed] [Google Scholar]
- 54. Day S, Kapogiannis BG, Shah SK, et al. Adolescent participation in HIV research: consortium experience in low and middle-income countries and scoping review. Lancet HIV 2020; 7:e844–52. https://pubmed.ncbi.nlm.nih.gov/33275917/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Landovitz R, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomized clinical trial. Lancet 2022; 399:1779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. U.S. Food and Drug Administration . Apretude Label (Reference ID: 4908014). 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf. Accessed 25 May 2022.
- 58. Moore CB, Capparelli E, Calabrese K, et al. Safety and PK of long -acting cabotegravir and rilpivirine in adolescents. Abstract 738. CROI 2022. Virtual. 12–16 February 2022. Available at: https://www.croiconference.org/abstract/safety-and-pk-of-long-acting-cabotegravir-and-rilpivirine-in-adolescents/ . Accessed 25 May 2022.
- 59. Lowenthal ED, Chapman J, Calabrese K, et al. Adolescent and parent experiences with long acting injectables in the MOCHA study. Abstract 739. CROI 2022. Virtual. 12–16 February 2022. Available at: https://www.croiconference.org/abstract/adolescent-and-parent-experiences-with-long-acting-injectables-in-the-mocha-study/. Accesses 25 May 2022.
- 60. Penazzato M, Townsend CL, Sam-Agudu NA. Advancing the prevention and treatment of HIV in children: priorities for research and development. Lancet HIV. 2022 Sep;9(9):e658-e666. [DOI] [PubMed] [Google Scholar]
- 61. Murray M, Pulido F, Mills A, et al. Patient-reported tolerability and acceptability of cabotegravir + rilpivirine long-acting injections for the treatment of HIV-1 infection: 96-week results from the randomized LATTE-2 study. HIV Res Clin Pract 2019; 20(4-5):111–22. [DOI] [PubMed] [Google Scholar]
- 62. Kevat PM, Reeves BM, Ruben AR, Gunnarsson R. Adherence to secondary prophylaxis for acute rheumatic fever and rheumatic heart disease: a systematic review. Curr Cardiol Rev 2017; 13:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crudden MTC M, Larrañeta E, Clark A, et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release 2018; 292:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Penazzato M, Watkins M, Morin S, et al. Catalysing the development and introduction of paediatric drug formulations for children living with HIV: a new global collaborative framework for action. Lancet HIV 2018; 5:e259–64. [DOI] [PubMed] [Google Scholar]