Abstract
Antimicrobial-resistant Neisseria gonorrhoeae infections are a threat to public health. Novel strategies for combating such resistance include the development of molecular assays to facilitate real-time prediction of antimicrobial susceptibility. Resistance to ciprofloxacin is determined by the presence of a single mutation at codon 91 of the gyrase A gene; molecular assays to guide therapy are commercially available. Resistance to cefixime is conferred via 1 of 6 critical mutations in either the mosaic penA gene or specific loci in the nonmosaic region. Resistance to ceftriaxone is conferred through mutations in 1 of 4 genes: penA, ponA, penB, and mtr; however, the ability to predict reduced susceptibility based on those genes varies by geographic region. Here, we highlight the work done toward the development of 3 such assays for ciprofloxacin, cefixime, and ceftriaxone, discuss the status of our current understanding and ongoing challenges, and suggest future directions.
Keywords: antimicrobial resistance, cefixime, ceftriaxone, ciprofloxacin, Neisseria gonorrhoeae
Antimicrobial therapy for Neisseria gonorrhoeae can be guided by rapid determination of molecular markers that predict reduced antimicrobial susceptibility. Here, we review the state of resistance-guided therapy for 3 antimicrobials: ciprofloxacin, cefixime, and ceftriaxone.
Antimicrobial-resistant Neisseria gonorrhoeae poses an urgent threat to public health [1]. N. gonorrhoeae has developed resistance to all antibiotics used in its treatment. Recent reports of resistance to third-generation cephalosporins have spurred concerns that we are approaching an era of untreatable infection [2]. Current Centers for Disease Control and Prevention (CDC) guidelines on the treatment of N. gonorrhoeae recommend a single dose of ceftriaxone for all suspected or confirmed infections, without determination of antimicrobial susceptibility [3]. The continued emergence of resistance [4] is thought to be driven by selective pressure conferred by cumulative antibiotic exposure, resulting in the accumulation of genetic mutations that gradually reduce efficacy of various antibiotics even without overt treatment failure [5].
The World Health Organization (WHO) has put forth action plans to combat the emergence of antimicrobial resistance, including calls for the development of molecular assays designed for detection of pathogens and genetic mutations that confer reduced susceptibility to specific antibiotics [6]. Use of genetic markers to guide therapy, known as resistance-guided therapy, is not a new concept; molecular assays detect genetic markers of resistance in Staphylococcus aureus [7], Mycobacterium tuberculosis [8], and many other pathogens. However, as the diagnosis of N. gonorrhoeae infections is primarily achieved through the use of nucleic acid amplification tests, these infections are uniquely suited for the coupling of pathogen detection with molecular resistance assays. Here, we summarize the work done thus far in identifying potential genetic markers associated with resistance for 3 antibiotics: ciprofloxacin, cefixime, and ceftriaxone, progress toward the development of molecular resistance assays, and remaining challenges.
RESISTANCE-GUIDED THERAPY FOR CIPROFLOXACIN
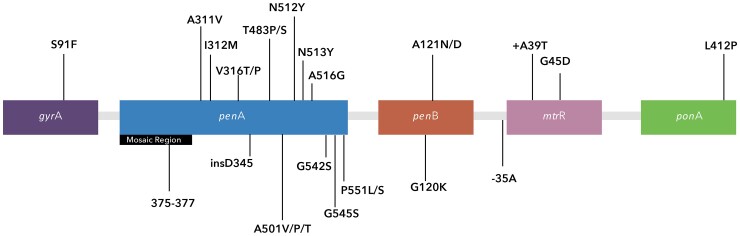
Ciprofloxacin inhibits topoisomerase II (or DNA-gyrase) and topoisomerase IV [9]. Analogously, various specific allelic mutations within the 2 genes that encode DNA-gyrase and topoisomerase IV, gyrase subunit A (gyrA) and parC, respectively, have been associated with ciprofloxacin resistance among N. gonorrhoeae [10]. Notably, despite the association of various mutations with phenotypic ciprofloxacin resistance, it is the absence of a single mutation at the serine 91 codon of the gyrA gene (Figure 1) that has been shown to be both necessary and sufficient to predict susceptibility [11].
Figure 1.
Mutations within Neisseria gonorrhoeae implicated in conferring reduced susceptibility to ciprofloxacin, cefixime, and ceftriaxone.
Consequently, molecular assays have been developed for the rapid determination of mutation in codon 91 of N. gonorrhoeae [12], and those assays have been implemented in clinical practice [13]. Further, a multicenter clinical trial evaluating resistance-guided therapy using gyrA genotyping demonstrated 100% treatment efficacy (1-sided 95% confidence interval [CI], 97.5%–100%) of ciprofloxacin where gyrA genotyping predicted ciprofloxacin susceptibility [14]. In light of those findings, the sexually transmitted infections treatment guidelines in the United States, the United Kingdom, and Australia have included statements permissive of the use of ciprofloxacin in certain contexts and when susceptibility has been confirmed through either culture or molecular detection methods [3, 15, 16].
Beyond potentially slowing the emergence of cephalosporin-resistant strains through the sole use of cephalosporins, the use of ciprofloxacin has several other benefits. While treatment with ceftriaxone requires a clinic visit for injection, a single oral dose facilitates care in nonclinical settings [17]. Additionally, expedited partner therapy would be facilitated in regions that allow practitioners to provide extra medication or a prescription for a patient’s sex partner, as currently recommended by the CDC [3]. Both nonclinical care and expedited partner therapy would facilitate increased treatment of individuals who otherwise might not present to care and thereby decrease transmission of N. gonorrhoeae within the community.
Importantly, the prevalence of ciprofloxacin resistance in N. gonorrhoeae continues to rise, with many countries reporting a prevalence of greater than 30% and some greater than 70% [18]. In settings with a prevalence greater than 80%, the utility of predicting ciprofloxacin susceptibility may be reduced. Moreover, the routine use of assays to predict ciprofloxacin resistance in those settings might not be cost-effective. Studies on the cost of such assays have concluded that both the prevalence of ciprofloxacin resistance as well as the frequency of testing will impact the overall cost–benefit ratio of assay implementation [19]. However, none of the above studies have been able to comprehensively account for the systematic costs of rising antimicrobial resistance.
RESISTANCE-GUIDED THERAPY FOR CEPHALOSPORINS
The mechanisms for N. gonorrhoeae resistance to cephalosporins are significantly more complex and heterogeneous than for ciprofloxacin; the 4 principal genes involved in cephalosporin resistance are penA, penB, mtrR, and ponA (Figure 1) [20]. penA encodes penicillin-binding protein 2, for which mutations in 83 amino acid positions have been associated with decreased ceftriaxone susceptibility [20]. Notably, there are 2 regions within the penA gene: a mosaic region that is composed of inserted DNA sequences from other commensal Neisseria subspecies, and a nonmosaic region specific to N. gonorrhoeae. Mutations within each region have been shown to be associated with decreased cephalosporin susceptibility. Further, there are different mosaic strains of N. gonorrhoeae, with penA34 being the most common form in North America [21]. The labeling nomenclature of the penA gene is different from the labeling notation of mutations within the gene that reflect amino acid substitutions.
In addition to penA, penB encodes PorB, an outer membrane porin; amino acid alterations in G120 and A121 sites decrease permeability of antibiotics [20]. mtrR encodes a transcriptional repressor of the gene locus known as mtr, which in turn encodes an efflux pump. Deletion of a single adenine residue from the promoter region of the mtrR gene results in upregulation of the efflux pump and thereby reduced susceptibility to cephalosporins [20]. Finally, the ponA gene, which encodes penicillin-binding protein 1, may contain the amino acid alteration L421P, which has been associated to a lesser extent with resistance to cephalosporins [20]. The resulting multitude of mechanisms for conferring resistance to cephalosporins has posed numerous challenges for predicting resistance phenotypes.
Molecular Determinants of N. gonorrhoeae Reduced Susceptibility to Cefixime
The penA34 mosaic insertion within the penA gene has been repeatedly associated with cefixime resistance in N. gonorrhoeae; that sequence was present in 98% of 270 isolates with reduced cefixime susceptibility in 1 US study [21]. Wong et al developed an assay to detect penA34 mosaicism, which had a high sensitivity and specificity among isolates collected in North America [22]. However, non-penA34 alleles are more common in Europe and Asia [23]. Further, nonmosaic penA mutations are also associated with cephalosporin resistance [24]. Non-penA mutations, such as those in penB (G120K and A121N/D), mtrR (−35A deletion in the promoter region, +A39T, and G45D), and ponA (L412P), likely contribute to the degree of cefixime resistance but are neither necessary nor sufficient to reduce susceptibility independent of penA [25].
Specific loci of mutations within the penA region that appear to be frequently associated with mosaic penA patterns include I312M, V316T, N512Y, and G545S. Importantly, studies using gene transformation techniques demonstrated that those mutations are not sufficient alone to confer reduced susceptibility to cefixime. However, reversion back to the wild type in a strain with cefixime resistance resulted in reduction of the minimum inhibitory concentration (MIC) to levels comparable with those of wild-type penA strains [26]. Thus, other mutations are likely important in addition to those found in the mosaic penA region. Wild-type penA can be distinguished from other mosaic forms of penA (with the exception of penA49) by determining the amino acid sequence of region 375–377 [25]. Nonmosaic penA mutations that appear to be critical to conferring reduced susceptibility to cefixime include point mutations within A501V/P/T, G542S, and P551L/S [25].
Peterson et al developed a multiplex polymerase chain reaction assay for determining N. gonorrhoeae resistance to several antibiotics including cefixime [27]. That assay used the following genetic alterations in penA (A311V, A501V/P/T, N513Y, G545S), ponA (L421P), penB (G120/A121), and mtrR (−35delA) and found a 98.2% (95% CI, 96.8%–99.1%) sensitivity and a 90.1% (95% CI, 88.6%–91.5%) specificity for predicting cefixime susceptibility in the absence of more than 3 mutations. Notably, however, that assay was assessed among strains isolated in Canada, and studies among larger sample sizes and strains from diverse geographic regions are needed.
Deng et al proposed that susceptibility to cefixime could be reliably predicted by detecting the absence of mosaic substitutions within the penA gene amino acid positions 375–377 and the absence of 3 critical mutations in the nonmosaic region of the penA gene: 501, 542, and 551. The authors conclude that an assay that detects any of the above resistance mutations would have a 99.5% (95% CI, 98.3%–99.9%) sensitivity for predicting reduced susceptibility to cefixime [25]. Subsequent work applied an analysis of the same mutations to an external dataset of N. gonorrhoeae strains from the United States and found a 95.9% (95% CI, 97.1%–99.4%) sensitivity for the determination of decreased cefixime susceptibility [28]. Importantly, the reported 95.9% sensitivity equated to a failure of capturing reduced susceptibility to cefixime among 8 strains. Those 8 strains did not contain the expected mosaic penA mutations [28]. Such a finding suggests the potential importance of an additional mutation within a non-penA gene or the importance of an additional locus within the mosaic penA region beyond 375–377.
The assay described above is promising; however, such an assay is still in development, and no clinical trials have been done to confirm test performance. Additionally, while the majority of strains with reduced susceptibility to cefixime may be captured by the assay described above, other mutations are clearly important, and ongoing surveillance and sequencing work is needed to characterize those mutations.
The benefits of an assay with the ability to predict susceptibility to cefixime would be far-reaching. Much like ciprofloxacin, as treatment with cefixime can also be given as a single-dose oral pill, care in nonclinical settings and expedited partner therapy will be greatly facilitated [17]. In fact, expedited partner use of cefixime for treating the sex contacts of a patient with N. gonorrhoeae infection is recommended in the most recent CDC guidelines when partner therapy with ceftriaxone is not an option [3]. Further, cefixime is recommended by the WHO in resource-limited settings when the community prevalence of resistance is low [29]. In addition, oral cefixime is considered safe in pregnancy. Finally, rare but potentially serious side effects of ciprofloxacin may limit its use in some populations, thus favoring cefixime. Importantly, single-dose cefixime may be inadequate for the treatment of pharyngeal N. gonorrhoeae infections [3], while a recent trial demonstrated near 100% microbiologic cure for susceptible pharyngeal infections treated with ciprofloxacin [14].
Molecular Determinants of N. gonorrhoeae Decreased Susceptibility to Ceftriaxone
As with cefixime, previous reports have strongly associated the presence of penA mosaicism with decreased susceptibility to ceftriaxone. However, unlike cefixime, a substantial number of strains have been identified in which penA mosaicism is neither necessary nor sufficient to predict reduced susceptibility to ceftriaxone [30]. A series of alterations in both penA and non-penA alleles have been identified as potentially important targets: penA (A311V, A501V/P/T, A516G, N512Y, N513Y, G542S, G545S, I312M, P551L/S, V316T/P, insD345), ponA (L421P), penB (G120/A121), and mtrR (−35delA) [20, 27].
An assay designed against strains isolated from Canada that screened all 4 alleles reported a sensitivity of 98.3% (95% CI, 93.9%–99.8%) and a specificity of 66.7% (95% CI, 57.6%–74.9%) for predicting decreased susceptibility to ceftriaxone when 3 or more mutations were present [31]. However, reports are conflicting about which mutations and in what combination have the most discriminatory predictive performance. Some of the discrepancy is likely due to the heterogeneity in geographic region (and therefore in dominant clonal population) from which the strains originated. de Korne-Elenbaas et al reported strains of N. gonorrhoeae from Amsterdam with and without decreased susceptibility to ceftriaxone [32], noting that all strains with resistance had a penA allele with an A501V mutation and penB G120K/A121D mutations, which were lacking in susceptible strains. Pinto et al reported that strains with reduced susceptibility to ceftriaxone from Portugal contained a nonmosaic penA allele containing mutation G542S as well as penB mutation G120K and A121D [33]. Various mutations such as V316T, G545S, I312M, and N512Y may contribute to reduced susceptibility, as reverting these alterations in vitro reduces the observed MIC [26]. However, they do not appear to be key drivers of resistance among reported strains from epidemiological studies [20].
One molecular assay for the determination of intermediate susceptibility to ceftriaxone among N. gonorrhoeae isolates developed by Peterson et al reported a 99.8% (95% CI, 99.0%–100.0%) sensitivity and an 89.0% (95% CI, 87.5%–90.4%) specificity for the prediction of ceftriaxone susceptibility in the absence of 3 or more of the genetic alterations in penA (A311V, A501V/P/T, N513Y, G545S), ponA (L421P), penB (G120/A121), and mtrR (−35delA) [27]. Doná et al report the development of an assay detecting lack of insD345 and G545S in mosaic penA10 and penA34, finding a near 100% sensitivity (though there was only 1 isolate with cephalosporin resistance and no false negatives) and 81.7% specificity (95% CI, 72.4%–89.0%) among isolates from Switzerland [34].
Lin et al developed 4 testing algorithms from strains isolated in 23 countries (predominantly the United States [30%], Russia [14%], Canada [12%], and New Zealand [11%]) [20]. Two algorithms used penA genes, 1 with and 1 without mosaicism determination; the other 2 algorithms used non-penA genes, 1 with and 1 without mosaicism determination. Among mosaic strains (as determined by amino acid substitutions in the 375–377 region), they highlight penA mutations A311V, T483P/S, A501V/P/T, and P551S, which showed low sensitivities but high specificities for reduced ceftriaxone susceptibility. Further, those mutations have been associated with high-level ceftriaxone resistance when present [26]. Among nonmosaic strains, Lin et al reported A501V/T as being strongly associated with reduced susceptibility to ceftriaxone, while G542S, P551L/S, and insD354 were not, which is in contrast to previous reports [35, 36]. Subsequently, a study using DNA microarray technology was developed for nearly 6000 isolates, concluding that the largest contribution to reduced MIC came from the penA mutations A501P, A311V, G545S, and insD345, as well as porB (G120A) [37].
Regarding non-penA mutations, a consensus appears to be that the non-penA mutations [ponA (L421P), penB (G120/A121), and mtrR (−35delA)] are all important in contributing to reduced ceftriaxone susceptibility specifically among nonmosaic strains [20]. There may be interactions between alleles at those loci and nonmosaic alleles that contribute to the development of decreased ceftriaxone susceptibility, which should be the subject of future work.
In follow-up studies, Lin et al applied the findings by Peterson et al with regard to detecting decreased ceftriaxone susceptibility to their global dataset and reported a comparable sensitivity (98.4%; 95% CI, 97.1%–99.2%) but lower specificity (67.3%; 95% CI, 65.6%–68.9%) [38]. Likewise, Lin et al reported application of their algorithms among strains reported by de Korne-Elenbaas et al and also reported a high sensitivity but low specificity [39]. Such discrepant specificities likely reflect consequences of heterogeneity in molecular markers associated with decreased susceptibility across various geographic locations. That heterogeneity poses an important practical challenge for implementation of such assays. In areas with low prevalence of decreased susceptibility, the positive predictive value of an assay with a low specificity would be exceedingly low. Thus, tailoring of the molecular targets used in any such assay should be done with consideration of local differences in the genetic epidemiology of N. gonorrhoeae. Importantly, such work will be greatly facilitated by increased genomic epidemiological surveillance through increased laboratory and research capacity as well as increased reporting of the complete genomic sequences in the scientific literature.
FUTURE DIRECTIONS
The utility of resistance-guided therapy will be directly impacted by the frequency of antimicrobial resistance in the population and by how effective reduction of antibiotic use is in alleviating the selective pressure toward the emergence of resistance. The latter, importantly, is relatively unknown, with limited data from other settings suggesting a potential benefit [40], thus warranting further study. For areas in which the prevalence of resistance to ciprofloxacin is low, implementation of gyrA genotyping in conjunction with pathogen detection may prove to be cost-effective. Such efforts can be facilitated by the incorporation of resistant marker determination into molecular point-of-care tests [41, 42].
For assays that are used to determine N. gonorrhoeae reduced susceptibility to cefixime, simultaneous mutation detection of at least 6 amino acid locations will be essential. It will be important to assess the validity of such assays in geographically distinct regions with strains of N. gonorrhoeae carrying different mosaic alleles. Combining such an assay with pathogen detection would facilitate tailored treatment decisions at the time of diagnosis. Notably, there appear to be a minority of circulating strains that are not captured by the 6 amino acid mutations proposed. Toward the development of an assay to predict susceptibility to ceftriaxone, increased capacity and funding for and reporting of genetic epidemiology will be essential. It may be necessary for future studies to evaluate country-level characteristics of the dominant circulating mosaic strain.
For all of those assays, real-world analyses among varying specimen types will be essential in order to validate performance metrics. Further, the context in which such implementation occurs will likely vary depending on the intended use of the assay. For example, a point-of-care assay will be most useful for symptomatic patients, while reflex molecular diagnostics might be of greater use for populations that present for screening in a setting that is already reliant on nucleic acid amplification tests. Additionally, careful consideration of systematic costs and continued monitoring for the development of resistance will be important for evaluation of the potential benefit of those assays.
CONCLUSIONS
In summary, a growing body of research has laid the groundwork for the development of molecular assays capable of predicting susceptibility of N. gonorrhoeae to several antibiotics. The development of such assays is an important tool for combating the emergence of multidrug-resistant N. gonorrhoeae strains. Assays that predict N. gonorrhoeae susceptibility to ciprofloxacin by way of identifying the absence of mutation at codon 91 of the gyrase A gene are commercially available in some areas. Prediction of susceptibility to cefixime is thought to be possible by determining the absence of mutation at any of 6 critical loci, and a molecular assay is currently in development; however, there are likely a subset of strains that will not be identified with determination of those mutations alone. Reduced susceptibility for ceftriaxone appears to be determined by various mutations in 1 of 4 genes; the precise mutations, however, likely vary geographically. Any molecular assay aimed at determining susceptibility to ceftriaxone must account for local genetic epidemiology.
Contributor Information
Lao-Tzu Allan-Blitz, Division of Global Health Equity, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Department of Pediatrics, Boston Children’s Hospital, Boston, Massachusetts, USA.
Paul C Adamson, Division of Infectious Diseases, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, California, USA.
Jeffrey D Klausner, Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Notes
Financial support. P. C. is supported by the National Institutes of Health (NIH; award K01TW012170). This research was also supported in part by a gift to the University of Southern California Keck School of Medicine by the W. M. Keck Foundation. J. D. K. reports support for this work (NIH R21 AI157817).
References
- 1. Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2013. Atlanta, GA: CDC, 2013. [Google Scholar]
- 2. Kirkcaldy RD, HookEW, 3rd, Soge OO, et al. Trends in Neisseria gonorrhoeae susceptibility to cephalosporins in the United States, 2006–2014. JAMA 2015; 314:1869–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021; 70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin X, Qin X, Wu X, et al. Markedly increasing antibiotic resistance and dual treatment of Neisseria gonorrhoeae isolates in Guangdong, China, from 2013 to 2020. Antimicrob Agents Chemother 2022; 66:e0229421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Global health sector strategy on sexually transmitted infections 2016–2021. Available at: https://apps.who.int/iris/bitstream/handle/10665/246296/WHO-RHR-16.09-eng.pdf?sequence=1. Accessed 2 February 2022.
- 7. Malhotra-Kumar S, Van Heirstraeten L, Lee A, et al. Evaluation of molecular assays for rapid detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2010; 48:4598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen TNA, Anton-Le Berre V, Banuls AL, Nguyen TVA. Molecular diagnosis of drug-resistant tuberculosis; a literature review. Front Microbiol 2019; 10:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988; 35:373–447. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka M, Takahashi K, Saika T, Kobayashi I, Ueno T, Kumazawa J. Development of fluoroquinolone resistance and mutations involving GyrA and ParC proteins among Neisseria gonorrhoeae isolates in Japan. J Urol 1998; 159:2215–9. [DOI] [PubMed] [Google Scholar]
- 11. Allan-Blitz LT, Wang X, Klausner JD. Wild-type gyrase A genotype of Neisseria gonorrhoeae predicts in vitro susceptibility to ciprofloxacin: a systematic review of the literature and meta-analysis. Sex Transm Dis 2017; 44:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siedner MJ, Pandori M, Castro L, et al. Real-time PCR assay for detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J Clin Microbiol 2007; 45:1250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allan-Blitz LT, Humphries RM, Hemarajata P, et al. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clin Infect Dis 2017; 64:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klausner JD, Bristow CC, Soge OO, et al. Resistance-guided treatment of gonorrhea: a prospective clinical study. Clin Infect Dis 2021; 73:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020; 31:4–15. [DOI] [PubMed] [Google Scholar]
- 16. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine . Australian STI management guidelines for use in primary care: gonorrhoeae. Available at: https://sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea/. Accessed 3 February 2022.
- 17. Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 2005; 352:676–85. [DOI] [PubMed] [Google Scholar]
- 18. Unemo M, Lahra MM, Cole M, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health 2019; 16:412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harding-Esch EM, Huntington SE, Harvey MJ, et al. Antimicrobial resistance point-of-care testing for gonorrhoea treatment regimens: cost-effectiveness and impact on ceftriaxone use of five hypothetical strategies compared with standard care in England sexual health clinics. Euro Surveill 2020; 25:1900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin EY, Adamson PC, Deng X, Klausner JD. Establishing novel molecular algorithms to predict decreased susceptibility to ceftriaxone in Neisseria gonorrhoeae strains. J Infect Dis 2021; 223:1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grad YH, Harris SR, Kirkcaldy RD, et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 2016; 214:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong LK, Hemarajata P, Soge OO, Humphries RM, Klausner JD. Real-time PCR targeting the penA mosaic XXXIV type for prediction of extended-spectrum-cephalosporin susceptibility in clinical Neisseria gonorrhoeae isolates. Antimicrob Agents Chemother 2017; 61:e01339-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen SC, Yin YP, Dai XQ, Unemo M, Chen XS. First nationwide study regarding ceftriaxone resistance and molecular epidemiology of Neisseria gonorrhoeae in China. J Antimicrob Chemother 2016; 71:92–9. [DOI] [PubMed] [Google Scholar]
- 24. Liao M, Gu WM, Yang Y, Dillon JA. Analysis of mutations in multiple loci of Neisseria gonorrhoeae isolates reveals effects of PIB, PBP2 and MtrR on reduced susceptibility to ceftriaxone. J Antimicrob Chemother 2011; 66:1016–23. [DOI] [PubMed] [Google Scholar]
- 25. Deng X, Allan-Blitz LT, Klausner JD. Using the genetic characteristics of Neisseria gonorrhoeae strains with decreased susceptibility to cefixime to develop a molecular assay to predict cefixime susceptibility. Sex Health 2019; 16:488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tomberg J, Unemo M, Davies C, Nicholas RA. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 2010; 49:8062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peterson SW, Martin I, Demczuk W, et al. Multiplex real-time PCR assays for the prediction of cephalosporin, ciprofloxacin and azithromycin antimicrobial susceptibility of positive Neisseria gonorrhoeae nucleic acid amplification test samples. J Antimicrob Chemother 2020; 75:3485–90. [DOI] [PubMed] [Google Scholar]
- 28. Deng X, Klausner JD. Six penA codons accurately and reliably predict cefixime-decreased susceptibility in Neisseria gonorrhoeae. J Infect Dis 2020; 221:851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization . WHO guidelines for the treatment of Neisseria gonorrhoeae. 2016. Available at: https://apps.who.int/iris/handle/10665/246114. Accessed February 2, 2022. [PubMed]
- 30. Gianecini RA, Golparian D, Zittermann S, et al. Genome-based epidemiology and antimicrobial resistance determinants of Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina in 2011–16. J Antimicrob Chemother 2019; 74:1551–9. [DOI] [PubMed] [Google Scholar]
- 31. Peterson SW, Martin I, Demczuk W, et al. Molecular assay for detection of genetic markers associated with decreased susceptibility to cephalosporins in Neisseria gonorrhoeae. J Clin Microbiol 2015; 53:2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Korne-Elenbaas J, Bruisten SM, de Vries HJC, Van Dam AP. Emergence of a Neisseria gonorrhoeae clone with reduced cephalosporin susceptibility between 2014 and 2019 in Amsterdam, the Netherlands, revealed by genomic population analysis. J Antimicrob Chemother 2021; 76:1759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pinto M, Matias R, Rodrigues JC, et al. Cephalosporin-resistant Neisseria gonorrhoeae isolated in Portugal, 2019. Sex Transm Dis 2020; 47:e54–6. [DOI] [PubMed] [Google Scholar]
- 34. Doná V, Smid JH, Kasraian S, et al. Mismatch amplification mutation assay-based real-time PCR for rapid detection of Neisseria gonorrhoeae and antimicrobial resistance determinants in clinical specimens. J Clin Microbiol 2018; 56:e00365-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whiley DM, Goire N, Lambert SB, et al. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J Antimicrob Chemother 2010; 65:1615–8. [DOI] [PubMed] [Google Scholar]
- 36. Shaskolskiy B, Dementieva E, Kandinov I, et al. Resistance of Neisseria gonorrhoeae isolates to beta-lactam antibiotics (benzylpenicillin and ceftriaxone) in Russia, 2015–2017. PLoS One 2019; 14:e0220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shaskolskiy B, Kandinov I, Kravtsov D, et al. Prediction of ceftriaxone MIC in Neisseria gonorrhoeae using DNA microarray technology and regression analysis. J Antimicrob Chemother 2021; 76:3151–8. [DOI] [PubMed] [Google Scholar]
- 38. Lin EY, Adamson PC, Klausner JD. Evaluating the generalizability of a multiplex real-time PCR assay for predicting decreased susceptibility to ceftriaxone in a global set of Neisseria gonorrhoeae sequences. J Antimicrob Chemother 2021; 76:1104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin EY, Adamson PC, Klausner JD. Applying molecular algorithms to predict decreased susceptibility to ceftriaxone from a report of strains of Neisseria gonorrhoeae in Amsterdam, the Netherlands. J Antimicrob Chemother 2022; 77:534–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seppala H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med 1997; 337:441–6. [DOI] [PubMed] [Google Scholar]
- 41. Van Der Pol B, Taylor SN, Mena L, et al. Evaluation of the performance of a point-of-care test for chlamydia and gonorrhea. JAMA Netw Open 2020; 3:e204819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morris SR, Bristow CC, Wierzbicki MR, et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis 2021; 21:668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]