Abstract
A patient with end-stage renal disease received 2 doses of dalbavancin for methicillin-resistant Staphylococcus aureus (MRSA) arteriovenous fistula infection and presented 5 weeks later with infective endocarditis secondary to vancomycin, daptomycin, and dalbavancin nonsusceptible MRSA. Resistance was associated with walK and scrA mutations, reduced long-chain lipid content, and reduced membrane fluidity.
Keywords: dalbavancin resistance, walK mutation, lipoglycopeptide cross-resistance, dalbavancin relapse
We have demonstrated that the lipoglycopeptide dalbavancin readily selects for cross-resistance to the glycopeptide vancomycin and the lipopeptide daptomycin in vitro using serial passage techniques and clinically relevant pharmacokinetic exposures simulated using in vitro models [1–4]. These strains usually exhibit the seesaw effect with beta-lactams and acquire mutations in genes related to the walKR operon, including atl, which is regulated by WalKR, and stp1, which regulates WalR phosphorylation [2]. However, there are few reported instances of dalbavancin nonsusceptibility reported in the clinical literature [4–7]. Among these reports, only 2 have utilized whole-genome sequencing, and many instances are confounded by the use of dalbavancin in the setting of multiple drug failures. Consequently, the clinical significance of dalbavancin-selected cross-resistance to vancomycin and daptomycin remains unclear. We report a new case in which a patient with end-stage renal disease received 2 doses of dalbavancin for a methicillin-resistant Staphylococcus aureus (MRSA)–infected arteriovenous fistula (AVF) and presented 5 weeks later with aortic valve endocarditis from an isogenic strain that was nonsusceptible to vancomycin, daptomycin, and dalbavancin.
METHODS
Patient Characteristics and Clinical Course
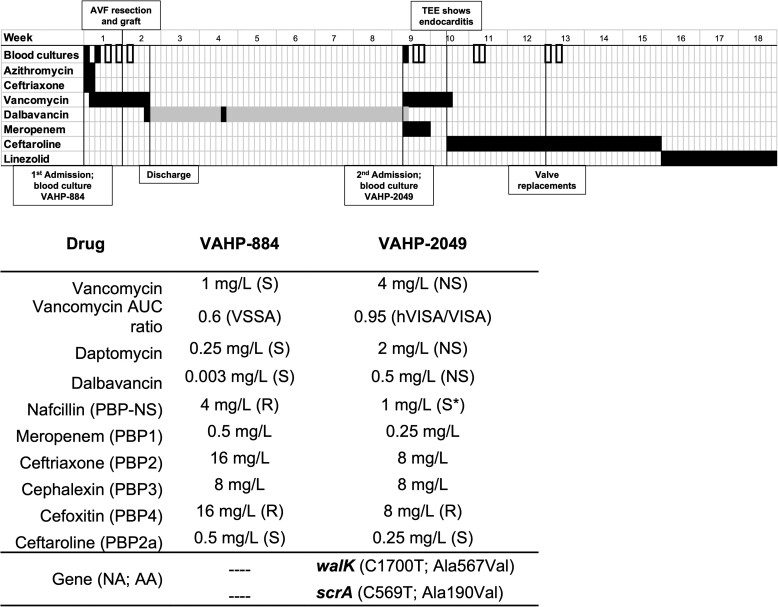
The patient's clinical course is summarized in Figure 1. A 63-year-old man with end-stage renal disease from autosomal dominant polycystic kidney disease, on home hemodialysis (HD) 5 times/week, was admitted on 15 February 2021 to Dayton Veteran’s Affairs Medical Center with shortness of breath and cough, right-sided chest pain, and fever. Chest X-ray disclosed multifocal pneumonia. Blood cultures were taken, and he was empirically treated with ceftriaxone (2 g intravenously [IV] every 24 hours [q24h]) and azithromycin (500 mg IV q24h). On 16 February, blood cultures yielded MRSA (isolate VAHP-884) and treatment switched to renally dosed vancomycin. Computed tomography (CT) angiography of the chest and the left arm revealed several aneurysms or pseudoaneurysms of the left-upper-extremity brachiocephalic fistula venous outflow, multiple pulmonary nodules, and peripheral wedge-shaped opacities concerning for septic emboli. Transesophageal echocardiography was negative for endocarditis. Positron emission tomography (PET)/CT scan revealed avid fluorodeoxyglucose (FDG) uptake into pulmonary nodules, likely representing septic emboli, and mild FDG uptake in the left upper extremity. Repeat blood cultures on 18 February again grew MRSA, but repeat blood cultures on days 19, 21, and 23 February showed no growth. The patient underwent resection of the infected pseudoaneurysm portion of left-arm AVF with interposition bovine graft on 22 February. On 26 February, vancomycin was discontinued, dalbavancin was given, and the patient was discharged home. We elected to treat him with dalbavancin due to his reluctance to have a home IV agency for IV vancomycin and varied duration and number of HD sessions per week. He received a dose of dalbavancin 1500 mg on 26 February and 1000 mg on 15 March.
Figure 1.
Top. Summary of clinical course over 18 weeks from initial presentation with shortness of breath and fever. Positive blood cultures (black filled), negative blood cultures (black outlined). The duration of each antibiotic is indicated in black, dalbavancin doses are indicated in black, and duration is highlighted in gray. Vertical lines are labeled with important events. Bottom. Susceptibility of parent strain VAHP-884 and post–dalbavancin exposure isolate VAHP-2049 measured by broth microdilution and vancomycin population analysis profile (AUC ratio). Minimum inhibitory concentrations are listed in mg/L, interpretation is based on established breakpoints from the Clinical Laboratory Standards Institute. *nafcillin MIC <4 mg/L is considered susceptible in methicillin-susceptible strains that do not harbor the mecA gene. No interpretive criteria exist for meropenem, ceftriaxone, or cephalexin against Staphylococci. WalK Ala567Val affects the histidine kinase domain close to where WalR is presumed to interact. Abbreviations: AA, amino acid change; AUC, area under the curve; AVF, arteriovenous fistula; hVISA, heterogeneous–vancomycin-intermediate Staphylococcus aureus; MIC, minimum inhibitory concentration; NA, nucleic acid change; NS, nonsusceptible; PBP, penicillin binding protein; R, resistant; S, susceptible; TEE, transesophageal echocardiogram; VISA, vancomycin-intermediate Staphylococcus aureus.
On 14 April, 33 days after the second dose of dalbavancin, the patient was readmitted to the hospital for shortness of breath and chills and he was empirically given vancomycin and meropenem. Blood cultures from admission grew MRSA (VAHP-2049), later identified as nonsusceptible to vancomycin, daptomycin, and dalbavancin. Transthoracic echocardiogram was negative for endocarditis, and PET/CT scan on 20 April disclosed no FDG avid lesions and pulmonary nodules were mostly resolved. Repeat blood cultures on 15 April and 17 April showed no growth. Transesophageal echocardiogram on 22 April revealed 2-cm aortic valve vegetation with moderate-to-severe aortic regurgitation, moderate mitral regurgitation, moderate-to-severe tricuspid regurgitation, and prolapse of the noncoronary cusp with preserved left ventricular systolic function.
The patient was transferred to an outside hospital for consideration of valve surgery and antibiotics were changed to ceftaroline-fosamil (200 mg IV every 12 hours [q12h]). On 5 October, the patient underwent median sternotomy, bioprosthetic aortic valve replacement, bioprosthetic mitral valve replacement, tricuspid valve repair using annuloplasty ring, and replacement of the ascending aorta using a woven aortic surgical graft. Histopathology of the valves revealed evidence of calcification and fibrosis but no signs of vegetation, inflammatory infiltrates, or microorganisms; however, the valves were not cultured. Postoperatively, he completed 3 weeks of ceftaroline-fosamil 200 mg IV q12h followed by 3 weeks of linezolid 600 mg orally q12h with no further positive cultures after 1 year of follow-up.
Susceptibility Testing
Susceptibilities to dalbavancin, daptomycin, vancomycin, nafcillin, meropenem, ceftriaxone, cephalexin, cefoxitin, and ceftaroline were determined in duplicate by broth microdilution per Clinical Laboratory Standards Institute guidelines. Vancomycin susceptibility by the modified population analysis profile method was performed as previously described to determine if the initial isolate was positive for the heterogeneous–vancomycin-intermediate Staphylococcus aureus (hVISA) phenotype [8].
Whole-Genome Sequencing
Whole-genome sequencing was performed on the patient’s blood isolates using the MiSeq platform, sequence reads were mapped to Genbank accession CP043392.1, and variant calling performed as previously described [9]. Sequence features were annotated using SnpEFF [10]. Sequence data are available from the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra; PRJNA808066).
Dalbavancin Plasma Concentration (see Supplementary Methods)
One plasma sample drawn when VAHP-2049 was isolated was available for dalbavancin quantification. The sample and calibration curve were processed as described previously but using daptomycin as the internal standard. The free fraction was estimated based on 99% protein binding reported typically reported for dalbavancin [11].
Membrane Analysis (see Supplementary Methods)
We performed comprehensive lipidomic analysis of the strain pair as described previously to evaluate the changes in cell envelope physiology and correlate membrane composition with changes in antimicrobial susceptibility and observed mutations [1, 3]. Briefly, VAHP-884 (wild-type) and VAHP-2049 (dalbavancin-resistant) were grown overnight in tryptic soy broth at 37°C with shaking, harvested by centrifugation, washed in sterile PBS, and dried by SpeedVac (Thermo Scientific). Lipids were extracted by the Bligh and Dyer method, and analyzed by hydrophilic interaction liquid chromatography coupled with ion mobility–mass spectrometry. Data were normalized to all compounds and reported as relative quantities of all lipid species including free fatty acids (FFAs), diglucosyl-diacylglycerol (DGDG), phosphatidylglycerol (PG), plasmalogen-phosphatidylglycerols (PGps), cardiolipins (CLs), and lysyl-phosphatidylglycerol (LysylPG), with fatty acyl compositions ranging from 25:0 to 38:0 (total carbons:total degree unsaturation). Membrane fluidity was measured in octuplicate by polarizing spectrofluorometry.
RESULTS
Susceptibility Testing, Whole-Genome Sequencing, and Dalbavancin Concentration
Susceptibility changes and mutations differentiating VAHP-844 and VAHP-2049 are summarized in Figure 1. There were 2 single nucleotide mutations that arose in VAHP-2049, one in walK and one in scrA. VAHP-844 was fully susceptible to vancomycin, daptomycin, and dalbavancin and was a non-hVISA by vancomycin population analysis (area under the curve [AUC] ratio <0.9). VAHP-2049 was vancomycin-, daptomycin-, and dalbavancin-nonsusceptible with 4-fold, 8-fold, and 128-fold increases in each respective minimum inhibitory concentration (MIC). VAHP-2049 exhibited a modest beta-lactam seesaw effect, with MICs dropping by 1–2 log2 dilutions. At the time VAHP-2049 was isolated from the patient, the dalbavancin plasma concentration was 24 mg/L. Assuming 99% average protein binding, the circulating unbound concentration was 0.24 mg/L or approximately 0.5 times the MIC of VAHP-2049.
Lipidomics and Membrane Fluidity
Changes in lipid composition and membrane fluidity are illustrated in Supplementary Figure 1. For all lipid classes, VAHP-2049 exhibited decreased levels of long-chain species (≥C33 total carbon number for those with 2 fatty acyl chains: PGs, DGDGs, LysylPGs, PGps, and PAs; ≥C19 for FFAs) (Supplementary Figure 1). Except for LysylPGs, all lipid classes also displayed slightly increased levels in short-chain species (C29–C31). The levels of all species of LysylPGs decreased significantly in VAHP-2049 relative to the parent strain. These changes together result in a more rigid cell membrane in VAHP-2049 as indicated by a higher degree of fluorescence polarity (Supplementary Figure 1).
DISCUSSION
Off-label dalbavancin use has been increasing and is likely fueled by coronavirus disease 2019 (COVID-19)–related pressures to reduce healthcare exposures. This is the first report of a clinically derived strain of dalbavancin-nonsusceptible MRSA with cross-resistance to daptomycin and a mutation in the walKR operon similar to the strains we selected for in our in vitro models [2]. We sequenced more than 50 strains evolved under dalbavancin exposure and 75% acquired mutations in walKR-associated genes and most exhibited cross-resistance to vancomycin and daptomycin, suggesting that clinicians should exercise caution when considering dalbavancin for invasive MRSA infections. Since dalbavancin inhibits peptidoglycan cross-linking like vancomycin and anchors in the membrane somewhat like daptomycin, it may be well suited to select for cross-resistance to both agents [12].
WalKR is an essential 2-component signal transduction system that regulates autolytic activity, which is known to affect glycopeptide susceptibility, and coordinates aspects of cell division [13, 14]. Previous studies have linked walKR mutations with vancomycin and daptomycin nonsusceptibility, but of the 2 case reports of dalbavancin resistance with genomic data available neither directly implicated walKR [4, 5]. We performed comprehensive lipidomic analysis to understand the link between dalbavancin and daptomycin cross-resistance. The role of WalKR in modulating lipid metabolism in S. aureus has not yet been reported, but in Staphylococcus pneumoniae, walR induction led to increased expression of fatty acid synthesis genes (eg, fabKDGF and accABC) [15, 16]. Thus, it is plausible that a reduced-function walK mutation could downregulate fatty acid synthesis, consistent with the observed decreases in long-chain fatty acids and lipids observed in this study. The reduction in PGs, which daptomycin targets, coupled with increased membrane rigidity may best explain cross-resistance to daptomycin in VAHP-2049. Mutations in walK have also been found to lead to changes to amino acid, purine, and pyrimidine metabolism, but the contribution of these changes to the antimicrobial susceptibility remains unclear [17].
Conclusions
This case validates in vitro findings suggesting that clinical exposures of dalbavancin can readily select for cross-resistance to vancomycin and daptomycin via walK mutation associated with alterations in cell membrane metabolism.
Supplementary Material
Contributor Information
Rutan Zhang, Department of Medicinal Chemistry, University of Washington School of Pharmacy, Seattle, Washington, USA.
Hari Polenakovik, Veterans Affairs Medical Center, Dayton, Ohio, USA; Department of Medicine, Wright State University Boonshoft School of Medicine, Dayton, Ohio, USA.
Ismael A Barreras Beltran, Department of Pharmacy, University of Washington School of Pharmacy, Seattle, Washington, USA.
Adam Waalkes, Department of Laboratory Medicine, University of Washington, Seattle, Washington, USA.
Stephen J Salipante, Department of Laboratory Medicine, University of Washington, Seattle, Washington, USA.
Libin Xu, Department of Medicinal Chemistry, University of Washington School of Pharmacy, Seattle, Washington, USA.
Brian J Werth, Department of Pharmacy, University of Washington School of Pharmacy, Seattle, Washington, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI136979. S. J. S. is supported in part by SINGH19R0 from the Cystic Fibrosis Foundation. A. W. also reports support for this project from the Cystic Fibrosis Foundation.
References
- 1. Zhang R, Barreras Beltran IA, Ashford NK, et al. Synergy between beta-lactams and lipo-, glyco-, and lipoglycopeptides, is independent of the seesaw effect in methicillin-resistant Staphylococcus aureus. Front Mol Biosci 2021; 8:688357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werth BJ, Ashford NK, Penewit K, et al. Dalbavancin exposure in vitro selects for dalbavancin-non-susceptible and vancomycin-intermediate strains of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 2021; 27:910.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hines KM, Shen T, Ashford NK, et al. Occurrence of cross-resistance and beta-lactam seesaw effect in glycopeptide-, lipopeptide- and lipoglycopeptide-resistant MRSA correlates with membrane phosphatidylglycerol levels. J Antimicrob Chemother 2020; 75:1182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werth BJ, Jain R, Hahn A, et al. Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen. Clin Microbiol Infect 2018; 24:429.e1–e5. [DOI] [PubMed] [Google Scholar]
- 5. Kussmann M, Karer M, Obermueller M, et al. Emergence of a dalbavancin induced glycopeptide/lipoglycopeptide non-susceptible Staphylococcus aureus during treatment of a cardiac device-related endocarditis. Emerg Microbes Infect 2018; 7:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steele JM, Seabury RW, Hale CM, Mogle BT. Unsuccessful treatment of methicillin-resistant Staphylococcus aureus endocarditis with dalbavancin. J Clin Pharm Ther 2018; 43:101–3. [DOI] [PubMed] [Google Scholar]
- 7. Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the general hospital of Vienna. Clin Infect Dis 2018; 67:795–8. [DOI] [PubMed] [Google Scholar]
- 8. Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 2001; 47:399–403. [DOI] [PubMed] [Google Scholar]
- 9. Salipante SJ, SenGupta DJ, Cummings LA, Land TA, Hoogestraat DR, Cookson BT. Application of whole-genome sequencing for bacterial strain typing in molecular epidemiology. J Clin Microbiol 2015; 53:1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cingolani P, Platts A, Wang le L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012; 6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leighton A, Gottlieb AB, Dorr MB, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 2004; 48:940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng D, Debabov D, Hartsell TL, et al. Approved glycopeptide antibacterial drugs: mechanism of action and resistance. Cold Spring Harb Perspect Med 2016; 6:a026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubrac S, Msadek T. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J Bacteriol 2004; 186:1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubrac S, Boneca IG, Poupel O, Msadek T. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol 2007; 189:8257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohedano ML, Amblar M, de la Fuente A, Wells JM, Lopez P. The response regulator YycF inhibits expression of the fatty acid biosynthesis repressor FabT in Streptococcus pneumoniae. Front Microbiol 2016; 7:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohedano ML, Overweg K, de la Fuente A, et al. Evidence that the essential response regulator YycF in Streptococcus pneumoniae modulates expression of fatty acid biosynthesis genes and alters membrane composition. J Bacteriol 2005; 187:2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howden BP, McEvoy CR, Allen DL, et al. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog 2011; 7:e1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.