Abstract
Ultrasensitive, quantitative Clostridioides difficile stool toxin measurement demonstrated significantly higher concentrations of toxins A and B in patients infected with the North American pulsed-field gel electrophoresis type 1/ribotype 027 (NAP-1/027) strain compared with other strains, providing in vivo confirmation of the in vitro association between NAP-1/027 and elevated toxin production.
Keywords: NAP-1/027 strain, Clostridioides difficile, toxin concentration, clinical outcomes
The North American pulsed-field gel electrophoresis type 1/ribotype 027 NAP-1/027 strain of Clostridioides difficile has been associated with global disease outbreaks since the early 2000s [1, 2]. The organism has unique pathogenicity features including the capacity for robust toxin production and high-level fluoroquinolone resistance [3]. Prior in vitro work demonstrated approximately 20-fold higher concentrations of C. difficile toxins A and B in NAP-1/027 strain cultures compared with non–NAP-1/027 strain cultures [4]. Enhanced toxin production may contribute to illness severity associated with the strain, although clinical studies have shown conflicting results, with some studies indicating worse clinical outcomes [5] and others finding no association [6]. Using ultrasensitive, quantitative assays for C. difficile toxins A and B, we sought to evaluate stool toxin concentrations in patients infected with NAP-1/027 vs non–NAP-1/027 strains and to characterize clinical severity and disease outcomes according to strain type.
METHODS
Eligible inpatients at Beth Israel Deaconess Medical Center (BIDMC, Boston, MA) and Texas Medical Center Hospitals (TMC, Houston, TX) were prospectively enrolled between 22 June 2016 and 12 July 2019 under protocols approved by the institutional review boards at each institution. Patients were aged ≥18 years with a positive clinical stool C. difficile nucleic acid amplification test (NAAT) result, initiating C. difficile infection (CDI) therapy, and had acute diarrhea [7]. The diagnostic clinical stool sample was captured as a discarded sample.
The study team scored patients using 4 CDI severity scoring guidelines (Society for Healthcare Epidemiology of America [SHEA]/Infectious Diseases Society of America [IDSA], European Society of Clinical Microbiology and Infectious Diseases [ESCMID], Zar et al [8], and Belmares et al [9]) and noted severe outcomes (intensive care unit [ICU] admission, colectomy, or death) and recurrence within 40 days as previously described [10]. Details of diarrhea assessment, patient exclusion, clinical data collection, and outcome definitions/attributions have been previously published [7].
Eligible stool samples (refrigerated) were aliquoted and frozen at −80°C within 72 hours of stool sample collection. NAP-1/027 testing was performed using the Xpert C. difficile/Epi assay (presumptive NAP-1/027 results reported; performed per manufacturer’s recommendation; Cepheid, Sunnyvale, CA) and/or culture with polymerase chain reaction (PCR) ribotyping [11]. For the purposes of this study, presumptive NAP-1/027 results from the Xpert C. difficile/Epi assay were combined with the PCR ribotyping data and are reported in aggregate using the term “NAP-1/027.” All BIDMC samples and 9 of 237 (3.8%) TMC samples were tested using Xpert, and cycle threshold (Ct) value data (tcdB gene) were captured. All TMC samples and 76 of 375 (20.3%) BIDMC samples were tested using PCR ribotyping. Toxin A and toxin B were measured using single molecule array (Simoa) assays at bioMérieux (Lyon, France) [7, 12, 13]. As previously described, a positive toxin result was defined as either toxin A or B ≥20 pg/mL (clinical cutoff).
RESULTS
We enrolled 615 patients; 527 patients had strain typing data available and were included in this analysis. Demographic and clinical characteristics are shown in Supplementary Table 1. There were 72 of 527 patients (13.7%) with NAP-1/027 and 455 of 527 patients (86.3%) with non–NAP-1/027 strains. Patients were equally distributed by sex; mean age was 68 years. Race information was available for 498 patients. White patients were more likely to have non–NAP-1/027 strains when compared with other races (89.6% vs 79.1%; P = .006). The proportions of patients with the NAP-1/027 strain were similar at BIDMC (47 of 374 patients; 12.6%) and TMC (25 of 152 patients; 16.4%; P = .263).
At baseline, patients with the NAP-1/027 strain were significantly more likely to have elevated white blood cell counts (P = .005), serum creatinine values ≥ 1.5 g/dL (P = .024), and lower median albumin levels (P = .007) compared with patients with non–NAP-1/027 strains. Applying the 2017 IDSA/SHEA C. difficile guidelines, patients with NAP-1/027 had a greater proportion of severe CDI (68.1%) compared with patients with non–NAP-1/027 strains (52.1%; P = .015). Baseline disease severity was also higher according to the Zar severity score, but not by the Belmares or ESCMID severity scores (Supplementary Table 1).
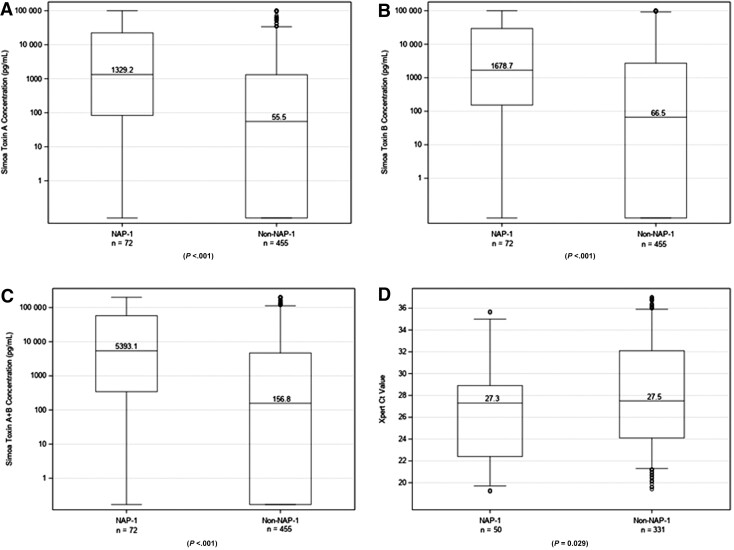
Baseline stool toxin concentrations were assessed using Simoa (see the Methods section). Patients with the NAP-1/027 strain had significantly higher median concentrations of toxin A (1329.2 vs 55.5 pg/mL), toxin B (1678.7 vs 66.5 pg/mL), and toxin A + B (5393.1 vs 156.8 pg/mL; all P values <.001) and lower median Xpert Ct values for tcdB (median, 27.3 and range, 22.4–28.9 vs median, 27.5 and range, 24.1–32.1; P = .029) when compared with patients with non–NAP-1/027 strains (Figure 1, Supplementary Table 1).
Figure 1.
Dot plots showing the distribution of toxin concentrations (measured by Simoa) and Ct values (measured by Xpert nucleic amplification test) in patients infected with the NAP-1/027 strain of Clostridioides difficile (left) and non–NAP-1/027 strains of C. difficile (right). (A) Simoa toxin A concentration. (B) Simoa toxin B concentration. (C) Simoa toxin A plus toxin B concentration. (D) Xpert Ct value for tcdB. The bottom and top edges of the boxes for each cohort indicate the interquartile range, the horizontal line bisecting the boxes indicates the median value, and the whiskers represent 5% and 95% values; outliers are represented by circles. Abbreviations: Ct, cycle threshold; NAP-1, North American pulsed-field gel electrophoresis type 1; Simoa, single molecule array.
Of 72 patients with the NAP-1/027 strain, 18 (25.0%) required ICU admission; 6 of 72 (8.3%) had ICU admissions primarily attributable to CDI. This was significantly higher than for patients with non–NAP-1/027 strains (69 of 455, 15.2% ICU admissions, P = .041 and 8 of 455, 1.8% primarily attributable ICU admissions, P = .006, respectively). Colectomy rates were low (<2%) and comparable in both groups (P = 1.000). The proportion of patients who died within 40 days was higher in the NAP-1/027 cohort (16.7% vs 6.2%; P = .006); however, there were no statistically significant differences in the proportion of deaths primarily attributed to CDI (P = .092). Using logistic regression (data not shown), when NAP-1/027 was contrasted to an aggregate of all other ribotypes, it was independently associated with ICU admission and any severe outcome primarily attributable to CDI. When adjusted for the NAP-1/027 strain, toxin A, toxin B, and Simoa Ct values for tcdB were not significantly associated with the severe outcomes. The proportion of patients with recurrence was similar in the NAP-1/027 cohort (6 of 72; 8.3%) and the non–NAP-1/027 cohort (29 of 455; 6.4%; P = .6). CDI treatment, duration of CDI treatment, and antimicrobial treatment prior to sample collection were similar between groups (Supplementary Table 1).
DISCUSSION
The NAP-1/027 strain of C. difficile has been associated with disease outbreaks and severe clinical outcomes since the early 2000s [1, 2]. It has unique features including in vitro resistance to fluoroquinolones, a partial deletion of the tcdC gene responsible for downregulation of toxin, production of an additional binary toxin, and enhanced in vitro production of toxins A and B [3]. Prior to this study, there has been no in vivo quantification of C. difficile toxins A and B in patients infected with the NAP-1/027 strain of C. difficile. In this multicenter study of 2 large, geographically diverse, tertiary care medical centers with 527 patients who had C. difficile strain typing data available, we compared clinical characteristics and stool toxin concentrations in those infected with the NAP-1/027 strain vs other strain types. We observed substantially higher median stool concentrations of C. difficile toxin A (23.9 times higher) and toxin B (25.2 times higher) in patients infected with the NAP-1/027 strain vs non–NAP-1/027 strains. Prior in vitro work evaluated the impact of strain type on toxin levels produced by C. difficile isolates in culture [4], measuring concentrations of toxins A and B using capture enzyme-linked immunosorbent assay. NAP-1/027 strains were found to have approximately 16 times higher concentrations of toxin A and 23 times higher concentrations of toxin B when compared with control strains. Our study verifies these observations and extends that work by quantifying toxin concentrations in a real-world scenario, thus providing further evidence that NAP-1/027 may be associated with hyperproduction of toxins A and B in vivo. One unexpected finding was an uneven distribution of the NAP-1/027 strain across race. It is not clear whether these differences are due to differential use of healthcare resources or related to an underlying genetic predisposition. Future epidemiologic studies may address these important questions.
Uncertainty remains as to whether strain type directly impacts clinical severity or disease outcomes in CDI. A prior study of 250 CDI patients found no association between CDI severity and strain type [6]. This contrasts with research from the Centers for Disease Control and Prevention Emerging Infections Program on C. difficile surveillance, which noted more severe clinical outcomes in patients with NAP-1/027 strains, even after controlling for comorbidities and advanced age [5]. In our study, we observed significant differences in laboratory markers of severity and more severe baseline disease by some (but not all) severity scores in those with NAP-1/027. Among the primarily attributable severe clinical outcomes evaluated, only ICU stay was found to be significantly different in patients with NAP-1/027 strains. Based on our prior work [7], we hypothesized that the increased severity in those with NAP-1/027 infection might be due to increased stool toxin concentrations. However, when compared with other ribotypes, we observed an independent association between NAP-1/027 and both ICU admission and any severe outcome primarily attributable to CDI. When adjusted for the NAP-1/027 strain, toxin A, toxin B, and NAAT Ct values for tcdB were not significantly associated with the severe outcomes. This observation raises interesting questions about the pathogenesis of NAP-1 in vivo, including whether some of the observed differences may be due to strain differences such as the presence of binary toxin. Our study was limited by a small sample size of participants with the NAP-1/027 strain (n = 72). This may result in the study being underpowered to detect differences in the severe clinical outcomes within 40 days and recurrence (see Supplementary Text and Results).
In this study of 527 patients with CDI, we found significantly higher stool concentrations of toxins A and B in vivo in patients with the NAP-1/027 strain of C. difficile. These findings verify prior in vitro data that suggest that hyperproduction of toxin by NAP-1 strains and point to a mechanism for strain-specific increased pathogenicity.
Supplementary Material
Contributor Information
Carolyn D Alonso, Division of Infectious Disease, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Nira R Pollock, Division of Infectious Disease, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Department of Laboratory Medicine, Boston Children’s Hospital, Boston, Massachusetts, USA.
Kevin W Garey, Department of Pharmacy Practice and Translational Research, University of Houston College of Pharmacy, Houston, Texas, USA.
Anne J Gonzales-Luna, Department of Pharmacy Practice and Translational Research, University of Houston College of Pharmacy, Houston, Texas, USA.
David N Williams, Institutional Centers for Clinical and Translational Research, Boston Children’s Hospital, Boston, Massachusetts, USA.
Kaitlyn Daugherty, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Christine Cuddemi, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Javier Villafuerte-Gálvez, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Nicole C White, Division of Infectious Disease, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Xinhua Chen, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Hua Xu, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Rebecca Sprague, Division of Infectious Disease, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Caitlin Barrett, Division of Infectious Disease, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Mark Miller, bioMérieux, Marcy L'Etoile, France.
Agnès Foussadier, bioMérieux, Marcy L'Etoile, France.
Aude Lantz, bioMérieux, Marcy L'Etoile, France.
Alice Banz, bioMérieux, Marcy L'Etoile, France.
Ciarán P Kelly, Harvard Medical School, Boston, Massachusetts, USA; Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. P. K. and N. R. P. conceived and designed the study. All members of the study group acquired the data. C. D. A., C. P. K., K. W. G., A. J. G.-L., D. W., K. D., J. V.-G., X. C., H. X., M. M., A. B., and N. R. P. analyzed and interpreted the data. C. D. A., C. P. K., and N. R. P. drafted the manuscript. Critical revisions to the manuscript were made by all members of the study group. C. P. K. and N. R. P. obtained the funds for the study. C. D. A., C. P. K., D. W., K. D., and N. R. P. verified all data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments. The authors thank Alice Cui and Matthew Perrotta for their assistance with sample collections during the study.
Financial support . This study was funded by a grant from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (grant 5R01AI116596-05 to N. R. P. and C. P. K.). C. D. A. has Division of Loan Repayment, National Institute of Health/National Institute of Allergy and Infectious Diseases. Single molecule array assays were provided as an in-kind service by bioMérieux.
References
- 1. McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005; 353:2433–41. [DOI] [PubMed] [Google Scholar]
- 2. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442–9. [DOI] [PubMed] [Google Scholar]
- 3. Burnham CA, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev 2013; 26:604–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005; 366:1079–84. [DOI] [PubMed] [Google Scholar]
- 5. See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis 2014; 58:1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cloud J, Noddin L, Pressman A, Hu M, Kelly C. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin Gastroenterol Hepatol 2009; 7:868–73 e2. [DOI] [PubMed] [Google Scholar]
- 7. Alonso CD, Pollock NR, Garey KW, et al. Higher in vivo fecal concentrations of Clostridioides difficile toxins A and B in patients with North American pulsed-field gel electrophoresis type 1/ribotype 027 strain infection. Clin Infect Dis 2022; 75:2019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB . A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 9. Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S . Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect 2007; 55:495–501. [DOI] [PubMed] [Google Scholar]
- 10. White NC, Mendo-Lopez R, Papamichael K, et al. Laxative use does not preclude diagnosis or reduce disease severity in Clostridiodes difficile infection. Clin Infect Dis 2020; 71:1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martinson JN, Broadaway S, Lohman E, et al. Evaluation of portability and cost of a fluorescent PCR ribotyping protocol for Clostridium difficile epidemiology. J Clin Microbiol 2015; 53:1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollock NR, Banz A, Chen X, et al. Comparison of Clostridioides difficile stool toxin concentrations in adults with symptomatic infection and asymptomatic carriage using an ultrasensitive quantitative immunoassay. Clin Infect Dis 2019; 68:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelly CP, Chen X, Williams D, et al. Host immune markers distinguish Clostridioides difficile infection from asymptomatic carriage and non-C. difficile diarrhea. Clin Infect Dis 2020; 70:1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.