Abstract
Objective:
To estimate breast-feeding prevalence in Greece in 2007 and 2017, compare breast-feeding indicators and maternity hospital practices between these years, and investigate breast-feeding determinants.
Design:
Two national cross-sectional studies (2007 and 2017) using systematic cluster sampling of babies with the same sampling design, data collection and analysis methodology.
Setting:
Telephone interview with babies’ mothers or fathers.
Participants:
Representative sample of infants who participated in the national neonatal screening programme (n 549 in 2017, n 586 in 2007).
Results:
We found that breast-feeding indicators were higher in 2017 compared with 10 years before. In 2017, 94 % of mothers initiated breast-feeding. Breast-feeding rates were 80, 56 and 45 % by the end of the 1st, 4th and 6th completed month of age, respectively. At the same ages, 40, 25 and <1 % of babies, respectively, were exclusively breast-feeding. We also found early introduction of solid foods (after the 4th month of age). Maternity hospital practices favouring breast-feeding were more prevalent in 2017, but still suboptimal (63 % experienced rooming-in; 51 % experienced skin-to-skin contact in the first hour after birth; 19 % received free sample of infant formula on discharge).
Conclusions:
We observed an increasing trend in all breast-feeding indicators in the past decade in Greece, but breast-feeding rates – particularly rates of exclusive breast-feeding – remain low. Systematic public health initiatives targeted to health professionals and mothers are needed in order to change the prevailing baby feeding ‘culture’ and successfully implement the WHO recommendations for exclusive breast-feeding during the first 6 months of life.
Keywords: Greece, Breast-feeding, Infant formula, Infant food, Prevalence, Trends, Maternity hospitals
The benefits of breast-feeding for mother and child are well established(1). It is estimated that 823 000 child deaths and 20 000 breast cancer deaths per year at a global level could be avoided by implementing breast-feeding promotion policies(2). One of the operational targets outlined in the Global Strategy for Infant and Young Child Feeding is to ensure that health and other relevant sectors protect, promote and support exclusive breast-feeding for 6 months and continued breast-feeding up to 2 years of age or beyond, while providing women access to the support they require – in the family, community and workplace – to achieve this goal(3).
The Baby Friendly Hospital Initiative, launched by WHO and UNICEF in 1991, aims to create a health-care environment where breast-feeding is the norm(4). This is accomplished, inter alia, by enabling mothers to make an informed choice and by supporting early initiation of breast-feeding(4). Compliance of maternity hospitals with the International Code of Marketing of Breast-milk Substitutes is an integral aspect of the Baby Friendly Hospital Initiative(5,6). More than 160 countries have implemented this initiative worldwide(7).
Studies conducted in high-income countries during the last two decades have identified a range of maternal and infant characteristics and hospital practices related to the initiation and duration of breast-feeding. Positive associations have been found for older age, higher educational level and immigrant status of the mothers, and for rooming-in and early skin-to-skin contact at the maternity hospital. Maternal smoking, being a single mother, preterm birth, low birth weight, caesarean section and supplementary feeding with formula milk during the hospital stay, as well as the distribution of free samples of breast-milk substitutes, are recognised negative predictors of breast-feeding(8–11).
The aim of our study was to estimate breast-feeding prevalence in Greece in 2007 and 2017, to compare breast-feeding indicators(12) and maternity hospital practices between these years, and contribute to the evaluation of the impact of changes in breast-feeding policies during this period. In addition, we aimed at investigating breast-feeding determinants for the decade 2007–2017.
Methods
We carried out two national cross-sectional studies, one in 2007(13) and one in 2017(14), by using systematic cluster sampling of babies and carrying out telephone interviews with one of their parents when the babies were 6–9 months old. We used the same sampling design, data collection and analysis methodology in both studies.
Sampling design
As sampling frames we used lists of babies that participated in the neonatal screening programme, which is carried out by the Institute of Child Health and covers more than 98 % of births taking place in Greece(15). In the 2007 study, the sampling frame included babies born from April to July of that year, and in the 2017 study babies born between June and October 2016.
The lists of all babies (one for each study period) that we obtained in order to use them as sampling frames did not include parents’ telephone number(s), and we had to get these by contacting the maternity hospital where each baby in the sample was born. In order to minimize the number of maternity hospitals that had to be contacted, we decided to select our samples of babies using systematic cluster sampling, not simple random sampling(16). As clusters we used ‘artificial’ groupings consisting of babies positioned consecutively in the list of all babies. In these lists newborns were sorted by district (Nomenclature of Territorial Units for Statistics third level region, NUTS-3) and by maternity hospital, while we further sorted them within each hospital assigning them a random order.
Based on assumptions regarding the expected prevalence of study variables (p = 50 %), the desired precision of prevalence estimates (d = 5–6 %) and of confidence intervals of estimates (95 %), the design effect due to cluster sampling (d eff = 2) and the expected participation rate (r = 70–75 %), we calculated the size of the sample that should be drawn for each study period (870 newborns)(16,17). We decided to use clusters of fifteen babies each (i.e. in total fifty-eight clusters in the sample; for 2007 it was feasible to round this to sixty), and divided the number of babies in the list of all babies by the total number of clusters to obtain the sampling interval (k). Subsequently, we carried out systematic sampling of clusters of newborns as follows: we drew a random number (from 1 to k) to identify the first baby of the first cluster to be included in the sample and then repeatedly added k to identify the first babies of all the subsequent clusters of the sample; the clusters consisted of these ‘first’ babies together with the next fourteen in the list of all babies(16).
Using this sampling method, we were able to draw representative samples of babies born in the study periods. In summary, we selected a sample of 900 newborns (in sixty clusters) for the 2007 study and a sample of 870 newborns (in fifty-eight clusters) for the 2017 study.
Information collection
We performed telephone interviews with the mothers – and in a few cases (5 %) the fathers, when the mothers were not available – of the sample infants when they had completed 6 months of age. We used a structured questionnaire designed to obtain information on infant feeding from birth until the day of the interview, which would allow us to calculate the main breast-feeding indicators. The questionnaire also contained questions on practices in the maternity hospitals that affect breast-feeding, on perinatal factors, on maternal and infant health status, and on demographic and socio-economic factors (for the full questionnaire in Greek, see appendices of previous reports(13,14)). The same questionnaire was used in both studies.
In both studies, the questionnaire was piloted by giving it to a random sample of mothers who were not included in the final sample.
Definition of breast-feeding indicators
We calculated breast-feeding indicators based on WHO definitions(12).
Breast-feeding initiation: the percentage of infants who are breast-fed in the first 24 h from birth.
Exclusive breast-feeding (EBF): the percentage of infants who receive only breast milk and no other form of foods or liquids, except for oral rehydration solutions, drops, syrups (vitamins, minerals, medicines).
Breast-feeding: the percentage of infants who receive breast milk with or without any other type of food or drink, including breast-milk substitutes (non-human milk and formula); hereafter referred to as ‘any breast-feeding’.
We also calculated the following indicators, which we considered relevant for assessing feeding practices in Greece.
Breast-feeding without breast-milk substitutes: the percentage of infants who receive breast milk with or without any other type of food or drink, except for breast-milk substitutes (non-human milk and formula).
Introduction of solid/semi-solid foods: the percentage of infants who have received solid/semi-solid foods.
Statistical analysis
We carried out data entry using EpiData version 3.1 (The EpiData Association, Odense, Denmark). The analysis was carried out using the statistical software package Stata version 11.
We calculated descriptive statistics for each study separately and calculated 95 % confidence intervals taking account of the fact that cluster sampling was performed. Further, we created a pooled data set with the data of both studies and performed univariate analysis for selected breast-feeding indicators and selected predictive factors. We calculated prevalence ratios accounting for the sampling design. We performed multiple logistic regression analysis in the pooled data set. Outcome variables were: (i) EBF at 1st completed month of age; and (ii) any breast-feeding at 6th completed month of age. Initial regression models included variables that had a statistically significant association with the selected breast-feeding indicators in the univariate analysis. Maternal age was included as a continuous variable. In the case of collinear variables, we included one of them in the model (low birth weight, not preterm birth; prescription for infant formula, not free sample for infant formula on discharge). We removed variables one at a time from the initial models on the basis of significance testing (P < 0·10) with the adjusted Wald test. Adjusted odds ratios were calculated taking the sampling design into account.
Results
In 2017, 549 dyads (mother/father and infant) participated in the study out of 870 in the sample (response rate: 63 %). The corresponding response rate in the study conducted in 2007 was 65 % (586/900). The infants’ age at the time of the interview was 6–9 completed months. The percentage of non-Greek origin mothers was almost the same in the two studies and in general there were no differences between the two samples regarding geographical region, maternity hospital type (public/private), gestational age, birth weight and mode of delivery. Differences were observed between the two studies regarding percentages of infant gender and maternal characteristics such as age, educational attainment, smoking, private insurance and employment during pregnancy (Table 1).
Table 1.
Characteristics of mothers and infants in the two national breast-feeding prevalence studies in Greece (2007 and 2017), by study year
| 2017 | 2007 | |||||
|---|---|---|---|---|---|---|
| n/N | % | 95 % CI | n/N | % | 95 % CI | |
| Maternal age (years) | ||||||
| 16–24 | 47/542 | 8·7 | 6·2, 12·0 | 52/582 | 8·9 | 6·5, 12·2 |
| 25–34 | 315/542 | 58·1 | 53·9, 62·2 | 389/582 | 66·8 | 63·4, 70·1 |
| ≥35 | 180/542 | 33·2 | 28·9, 37·8 | 141/582 | 24·2 | 20·6, 28·3 |
| Maternal country of origin | ||||||
| Greece | 469/543 | 86·4 | 81·7, 90·0 | 501/583 | 85·9 | 81·3, 89·6 |
| Albania | 39/543 | 7·2 | 5·0, 10·2 | 43/583 | 7·4 | 4·9, 10·9 |
| Other | 35/543 | 6·4 | 4·3, 9·6 | 39/583 | 6·7 | 4·9, 9·0 |
| Region of hospital | ||||||
| Attica | 221/549 | 40·3 | 28·5, 53·3 | 248/586 | 42·3 | 30·3, 55·4 |
| Central Greece | 91/549 | 16·6 | 8·6, 29·6 | 92/586 | 15·7 | 8·4, 27·5 |
| Northern Greece | 180/549 | 32·8 | 21·8, 46·1 | 176/586 | 30·0 | 19·5, 43·3 |
| Aegean islands and Crete | 57/549 | 10·4 | 4·7, 21·3 | 70/586 | 11·9 | 5·6, 23·6 |
| Maternal educational attainment | ||||||
| Primary, secondary and post-secondary education | 271/542 | 50·0 | 45·0, 55·0 | 372/583 | 63·8 | 58·7, 68·6 |
| Tertiary education | 271/542 | 50·0 | 45·0, 55·0 | 211/583 | 36·2 | 31·4, 41·3 |
| Private insurance | ||||||
| Yes | 61/528 | 11·6 | 8·5, 15·6 | 144/583 | 24·7 | 18·8, 31·7 |
| No | 467/528 | 88·4 | 84·4, 91·5 | 439/583 | 75·3 | 68·3, 81·2 |
| Maternal employment during pregnancy | ||||||
| Yes | 320/543 | 58·9 | 54·0, 63·7 | 381/583 | 65·3 | 60·5, 69·9 |
| No | 223/543 | 41·1 | 36·3, 46·0 | 202/583 | 34·7 | 30·1, 39·5 |
| Maternal smoking at the time of interview | ||||||
| Yes | 119/545 | 21·8 | 18·9, 25·1 | 184/584 | 31·5 | 27·9, 35·3 |
| No | 426/545 | 78·2 | 74·9, 81·1 | 400/584 | 68·5 | 64·7, 72·1 |
| Infant gender | ||||||
| Male | 314/549 | 57·2 | 53·5, 60·9 | 291/586 | 49·7 | 45·4, 54·0 |
| Female | 235/549 | 42·8 | 39·1, 46·5 | 295/586 | 50·3 | 46·0, 54·6 |
| Gestational age (weeks) | ||||||
| <33 | 15/521 | 2·9 | 1·3, 6·3 | 6/580 | 1·0 | 0·5, 2·2 |
| 34–36 | 40/521 | 7·7 | 5·5, 10·6 | 52/580 | 9·0 | 6·9, 11·6 |
| ≥37 | 466/521 | 89·4 | 84·8, 92·8 | 522/580 | 90·0 | 87·2, 92·2 |
| Birth weight (g) | ||||||
| <2000 | 14/549 | 2·6 | 1·2, 5·4 | 7/585 | 1·2 | 0·5, 2·7 |
| 2000–2499 | 34/549 | 6·2 | 4·4, 8·6 | 37/585 | 6·3 | 4·4, 8·9 |
| ≥2500 | 501/549 | 91·3 | 87·7, 93·9 | 541/585 | 92·3 | 89·8, 94·5 |
| Maternity hospital type | ||||||
| Public | 268/549 | 48·8 | 36·2, 61·6 | 270/586 | 46·1 | 33·7, 59·0 |
| Private | 281/549 | 51·2 | 38·4, 63·8 | 316/586 | 53·9 | 41·0, 66·3 |
| Mode of delivery | ||||||
| Vaginal birth | 253/548 | 46·2 | 42·2, 50·2 | 296/585 | 50·6 | 46·3, 54·9 |
| Caesarean section | 295/548 | 53·8 | 49·8, 57·8 | 289/585 | 49·4 | 45·1, 53·7 |
In the 2017 study about 95 % of the mothers initiated breast-feeding in the first 24 h from birth. Any breast-feeding remained above 50 % by the end of the 4th month and gradually decreased to 45 % at the 6th completed month of age (Fig. 1(a) and online supplementary material, Supplemental Table S1).
Fig. 1.
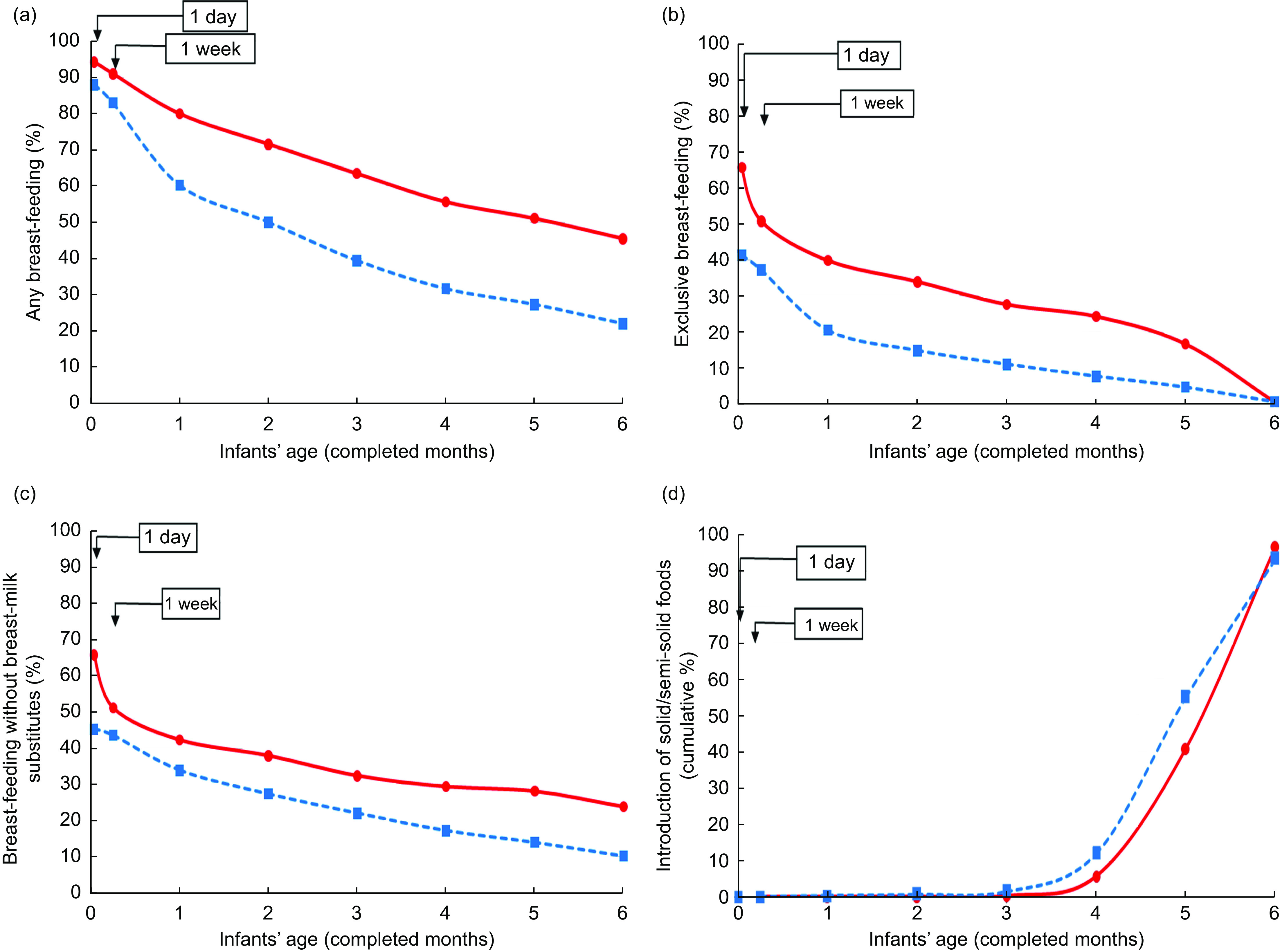
Breast-feeding indicators and introduction of solid/semi-solid foods by infants’ age (in completed months) and study year ( , 2017, N 549;
, 2017, N 549;  , 2007, N 586) in the two national breast-feeding prevalence studies in Greece: (a) any breast-feeding; (b) exclusive breast-feeding; (c) breast-feeding without breast-milk substitutes; and (d) introduction of solid/semi-solid foods (cumulative percentage)
, 2007, N 586) in the two national breast-feeding prevalence studies in Greece: (a) any breast-feeding; (b) exclusive breast-feeding; (c) breast-feeding without breast-milk substitutes; and (d) introduction of solid/semi-solid foods (cumulative percentage)
Almost two-thirds of mothers were exclusively breast-feeding in the first 24 h, but EBF dropped to 50 % by the end of the first week. At the end of the 1st completed month of age 40 % of the infants were exclusively breast-feeding; thereafter there was a continuous decline reaching 25 % at the end of the 4th month, followed by a steeper decline resulting in <1 % at the end of the 6th month (Fig. 1(b) and online supplementary material, Supplemental Table S1). However, one in four infants was breast-feeding without having ever received any breast-milk substitute by the 6th completed month of age (Fig. 1(c) and Supplemental Table S1).
In comparison to the 2007 study, all of the above breast-feeding indicators were higher in the 2017 study, across all categories of main known breast-feeding determinants (Fig. 1 and online supplementary material, Supplemental Tables S1 and S3). In both studies an early introduction of solid/semi-solid foods was observed beginning after the 4th completed month of age, although in the 2017 study 41 % of infants received solid/semi-solid foods by the end of the 5th month compared with 55 % in 2007. By the 6th completed month of age almost all infants had already received solid/semi-solid foods (Fig. 1(d) and Supplemental Table S2).
According to mothers’ reporting, about 50 % or more experienced skin-to-skin contact with their babies within the first hour of birth or rooming-in at the maternity hospital in 2017. These percentages are significantly higher compared with those from the 2007 study. In 2017, almost half of the mothers were given a prescription for infant formula on discharge and about a fifth received a free sample. These respective percentages were importantly higher in the previous study (Table 2).
Table 2.
Selected maternity hospital practices in the two national breast-feeding prevalence studies in Greece (2007 and 2017), by study year
| 2017 | 2007 |
P value (χ 2 test) |
|||||
|---|---|---|---|---|---|---|---|
| n/N | % | 95 % CI | n/N | % | 95 % CI | ||
| Skin-to-skin contact within first hour of birth | |||||||
| Yes | 275/541 | 50·8 | 46·0, 55·6 | 157/586 | 26·8 | 22·0, 32·1 | |
| No | 266/541 | 49·2 | 44·4, 54·0 | 429/586 | 73·2 | 67·9, 78·0 | <0·0001 |
| Rooming-in | |||||||
| Yes | 346/546 | 63·4 | 54·2, 71·7 | 284/585 | 48·5 | 36·8, 60·5 | |
| No | 146/546 | 26·7 | 19·3, 35·8 | 265/585 | 45·3 | 34·1, 57·0 | |
| Not applicable* | 54/546 | 9·9 | 6·7, 14·4 | 36/585 | 6·2 | 4·4, 8·5 | 0·0137 |
| Prescription of formula milk on discharge from maternity hospital | |||||||
| Yes | 258/523 | 49·3 | 44·1, 54·6 | 383/577 | 66·4 | 59·3, 72·8 | |
| No | 265/523 | 50·7 | 45·4, 55·9 | 194/577 | 33·6 | 27·2, 40·7 | 0·0002 |
| Free sample of infant formula on discharge from maternity hospital | |||||||
| Yes | 102/527 | 19·4 | 15·9, 23·4 | 207/576 | 35·9 | 28·4, 44·2 | |
| No | 425/527 | 80·6 | 76·6, 84·1 | 369/576 | 64·1 | 55·8, 71·6 | 0·0001 |
Infant in neonatal intensive care unit.
Table 3 presents the results of univariate analysis in the pooled data set for selected predictive factors using as outcome variables EBF at the 1st completed month of age and any breast-feeding at the 6th completed month of age.
Table 3.
Univariate analysis: main breast-feeding indicators by selected predictive factors. Pooled analysis of data from the two national breast-feeding prevalence studies in Greece (2007 and 2017)
| EBF1 | ABF6 | |||||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | PR | 95 % CI | n/N | % | PR | 95 % CI | |
| Study | ||||||||
| 2007 | 119/575 | 20·7 | Ref. | – | 129/586 | 22·0 | Ref. | – |
| 2017 | 204/510 | 40·0 | 1·93 | 1·53, 2·44 | 246/542 | 45·4 | 2·06 | 1·71, 2·49 |
| Mode of delivery | ||||||||
| Vaginal delivery | 189/527 | 35·9 | Ref. | – | 202/545 | 37·1 | Ref. | – |
| Caesarean section | 133/557 | 23·9 | 0·67 | 0·56, 0·80 | 171/581 | 29·4 | 0·79 | 0·68, 0·93 |
| Infant gender | ||||||||
| Male | 154/574 | 26·8 | Ref. | – | 200/599 | 33·4 | Ref. | – |
| Female | 169/511 | 33·1 | 1·23 | 1·03, 1·47 | 175/529 | 33·1 | 0·99 | 0·84, 1·17 |
| Maternal age (years) | ||||||||
| 16–24 | 19/94 | 20·2 | Ref. | – | 30/99 | 30·3 | Ref. | – |
| 25–34 | 197/672 | 29·3 | 1·45 | 0·95, 2·20 | 225/700 | 32·1 | 1·06 | 0·75, 1·50 |
| ≥35 | 105/313 | 33·5 | 1·66 | 1·09, 2·51 | 116/318 | 36·5 | 1·20 | 0·84, 1·72 |
| Maternal country of origin | ||||||||
| Greece | 283/938 | 30·2 | Ref. | – | 296/965 | 30·7 | Ref. | – |
| Albania | 22/77 | 28·6 | 0·95 | 0·63, 1·42 | 47/82 | 57·3 | 1·87 | 1·49, 2·34 |
| Other | 16/67 | 23·9 | 0·79 | 0·50, 1·25 | 28/73 | 38·4 | 1·25 | 0·95, 1·64 |
| Maternal educational attainment | ||||||||
| Primary, secondary and post-secondary education | 146/618 | 23·6 | Ref. | – | 170/639 | 26·6 | Ref. | – |
| Tertiary education | 175/462 | 37·9 | 1·60 | 1·31, 1·97 | 199/479 | 41·5 | 1·56 | 1·30, 1·87 |
| Private insurance | ||||||||
| No | 247/874 | 28·3 | Ref. | – | 308/902 | 34·1 | Ref. | – |
| Yes | 70/198 | 35·4 | 1·25 | 0·97, 1·61 | 58/203 | 28·6 | 0·84 | 0·66, 1·05 |
| Maternal employment during pregnancy | ||||||||
| No | 110/401 | 27·4 | Ref | – | 130/421 | 30·9 | Ref. | – |
| Yes | 210/681 | 30·8 | 1·12 | 0·92, 1·38 | 241/699 | 34·5 | 1·12 | 0·93, 1·34 |
| Maternal smoking at the time of interview | ||||||||
| No | 268/790 | 33·9 | Ref. | – | 336/822 | 40·9 | Ref. | – |
| Yes | 54/294 | 18·4 | 0·54 | 0·41, 0·71 | 37/301 | 12·3 | 0·30 | 0·22, 0·40 |
| Maternity hospital type | ||||||||
| Public | 147/503 | 29·2 | Ref. | – | 185/532 | 34·8 | Ref. | – |
| Private | 176/582 | 30·2 | 1·04 | 0·82, 1·31 | 190/596 | 31·9 | 0·92 | 0·74, 1·13 |
| Preterm birth | ||||||||
| No (≥37 weeks) | 291/950 | 30·6 | Ref. | – | 331/984 | 33·6 | Ref. | – |
| Yes (<37 weeks) | 20/109 | 18·3 | 0·60 | 0·42, 0·87 | 27/112 | 24·1 | 0·72 | 0·49, 1·04 |
| Low birth weight | ||||||||
| No (≥2500 g) | 313/997 | 31·4 | Ref. | – | 362/1037 | 34·9 | Ref. | – |
| Yes (<2500 g) | 10/88 | 11·4 | 0·36 | 0·18, 0·71 | 13/91 | 14·3 | 0·41 | 0·25, 0·67 |
| Skin-to-skin contact within first hour of birth | ||||||||
| No | 137/605 | 22·6 | Ref. | – | 185/628 | 29·5 | Ref. | – |
| Yes | 185/415 | 44·6 | 1·97 | 1·62, 2·39 | 186/430 | 43·3 | 1·47 | 1·27, 1·70 |
| Rooming-in | ||||||||
| No | 115/488 | 23·6 | Ref. | – | 135/499 | 27·1 | Ref. | – |
| Yes | 208/595 | 35·0 | 1·48 | 1·18, 1·87 | 238/625 | 38·1 | 1·41 | 1·12, 1·77 |
| Prescription for infant formula on discharge from maternity hospital | ||||||||
| No | 180/442 | 40·7 | Ref. | – | 201/455 | 44·2 | Ref. | – |
| Yes | 136/619 | 22·0 | 0·54 | 0·44, 0·66 | 161/639 | 25·2 | 0·57 | 0·47, 0·69 |
| Free sample of infant formula on discharge from maternity hospital | ||||||||
| No | 263/765 | 34·4 | Ref. | – | 285/788 | 36·2 | Ref. | – |
| Yes | 54/298 | 18·1 | 0·53 | 0·40, 0·69 | 76/309 | 24·6 | 0·68 | 0·54, 0·85 |
EBF1, exclusive breast-feeding at the 1st completed month of age; ABF6, any breast-feeding at the 6th completed month of age; PR, prevalence ratio; ref., reference category.
In the multivariable analysis of the pooled data, female infant gender, higher maternal age, maternal tertiary education, maternal private insurance and skin-to-skin contact within the first hour of birth were found to have an independent positive association with EBF at the 1st completed month, while caesarean section, maternal smoking, low birth weight and prescription for infant formula had a negative association. Any breast-feeding at the 6th completed month was positively associated with maternal tertiary education, Albanian origin and skin-to-skin contact; maternal smoking, low birth weight and prescription for infant formula were negatively associated. Of note, participation in the 2017 study (compared with participation in the 2007 study) was found to increase by a factor of about two the odds of breast-feeding as expressed by the above indicators, independently of all the other factors studied (Table 4).
Table 4.
Multivariable analysis: main breast-feeding indicators by selected predictive factors. Pooled analysis of data from the two national breast-feeding prevalence studies in Greece (2007 and 2017)
| Predictive factor | EBF1 (N 1041*) | ABF6 (N 1080*) | ||
|---|---|---|---|---|
| Adjusted OR | 95 % CI | Adjusted OR | 95 % CI | |
| Study | ||||
| 2007 | Ref. | – | Ref. | – |
| 2017 | 1·97 | 1·37, 2·84 | 2·19 | 1·65, 2·90 |
| Maternal age | ||||
| Continuous variable | 1·03 | 1·00, 1·06 | NA† | |
| Infant gender | ||||
| Male | Ref. | – | NA† | |
| Female | 1·49 | 1·11, 2·00 | ||
| Maternal educational attainment | ||||
| Primary, secondary and post-secondary education | Ref. | – | Ref. | – |
| Tertiary education | 1·64 | 1·15, 2·35 | 1·81 | 1·30, 2·51 |
| Caesarean section | ||||
| No | Ref. | – | NA† | |
| Yes | 0·68 | 0·50, 0·92 | ||
| Maternal smoking at the time of interview | ||||
| No | Ref. | – | Ref. | – |
| Yes | 0·57 | 0·39, 0·83 | 0·23 | 0·15, 0·34 |
| Private insurance | ||||
| No | Ref. | – | NA† | |
| Yes | 2·01 | 1·30, 3·11 | ||
| Low birth weight | ||||
| No | Ref. | – | Ref. | – |
| Yes | 0·36 | 0·16, 0·80 | 0·26 | 0·13, 0·53 |
| Skin to skin contact within first hour from birth | ||||
| No | Ref. | – | Ref. | – |
| Yes | 2·23 | 1·55, 3·22 | 1·54 | 1·18, 2·00 |
| Prescription for infant formula on discharge from maternity hospital | ||||
| No | Ref. | – | Ref. | – |
| Yes | 0·48 | 0·35, 0·66 | 0·49 | 0·36, 0·65 |
| Maternal country of origin | ||||
| Greece | NA† | Ref. | – | |
| Albania | 3·75 | 2·01, 7·00 | ||
| Other | 1·58 | 0·93, 2·67 | ||
EBF, exclusive breast-feeding at the 1st completed month of age; ABF6, any breast-feeding at the 6th completed month of age; ref., reference category.
Participants with no missing values for any of the variables were included in the model.
Not applicable (variable not included in the final regression model, see ‘Methods’ section).
Discussion
We report here the results of two national cross-sectional studies on breast-feeding prevalence and maternity hospital practices in Greece, carried out a decade apart (2007 and 2017), using the same sampling, data collection and analysis methodology. To our knowledge, these are the only nationwide studies on these issues conducted in Greece.
We used systematic samples of babies born in the country in the respective study periods and, due to the common methodology used in 2007 and 2017, we were able to derive comparable indices regarding breast-feeding (WHO indicators) and related practices. We sought information on the babies’ age at the time when they started or stopped the feeding practices under investigation retrospectively. The relevant information was collected when the babies were 6–9 months of age. This is a limitation of the present study.
We showed that during the decade 2007–2017 a substantial improvement in all breast-feeding indicators studied took place. In 2017 the great majority of mothers initiated breast-feeding in the first 24 h from birth, while rates of any breast-feeding remained above 50 % by the end of the 4th month. EBF was found to be consistently higher compared with 2007, with the exception of the 6th month indicator. Early introduction of solid/semi-solid foods may explain the almost zero levels of EBF at 6 months of age, given the finding that about 25 % of the infants were breast-feeding without having ever received any breast-milk substitute by this age. The increasing trend in all breast-feeding rates was observed across all levels of main predictive factors.
The results of recent local studies in Greece are in line with our findings. High rates of breast-feeding initiation (80–95 %) and rates of any breast-feeding (about 85 % during the 1st month, reaching 20 % at the end of the 6th month postpartum) have been found. The reported rates for EBF during the 1st month have ranged from about 20 to 40 % and dropped to almost zero by 6 months of age(18–20).
Increasing breast-feeding rates have been observed in other European countries during the last 20 years, such as Scotland(21), France(22), Ireland(23), England(24) and Germany(25). In Sweden, where breast-feeding rates were already high, an opposite trend has been observed(26). The breast-feeding rates we found in Greece in 2017 tend to be higher than those in some other European countries like France or the UK, but remain lower than in Scandinavian countries or Japan(8). EBF at the age of 6 months is lower than the median estimate for WHO European Region countries(27) and lags behind WHO targets(28), although consistent with findings reported in other European countries(29–31). Low prevalence of EBF after the age of 4 months in the WHO European Region has been attributed to early introduction of complementary feeding(27).
Mothers participating in our 2017 study reported significantly higher rates of early skin-to-skin contact with their babies and rooming-in, compared with the 2007 findings. Regarding use of breast-milk substitutes, we noted a significant decreasing trend, although there is a lot of room for further improvement, as indicated by the steep decline we found in EBF during the first week of age. Improvements in hospital practices have been observed in other European countries(32) but the use of breast-milk substitutes in the maternity ward continues to be an obstacle in achieving higher breast-feeding rates(33).
We found that maternal educational attainment was a significant predictive factor for breast-feeding; this association is in fact one of the most consistent associations in the relevant literature(8,10,34,35). We identified private insurance of the mother, an indicator of socio-economic status, to be independently associated with higher EBF at the 1st month. While education and social class are usually correlated, education has been found to have a more direct influence on breast-feeding outcomes than occupation-based social class(36). We found that certain maternity hospital practices influence breast-feeding uptake, in line with findings of other studies. Early skin-to-skin contact has been shown to have a positive association with breast-feeding(10), while caesarean section(18,37) and supplying breast-milk substitutes adversely affect breast-feeding initiation and duration(10). In a previous study in Greece, maternity hospital practices were found to be more significant breast-feeding predictors than sociodemographic factors(37).
The association we observed between female infant gender and EBF at the 1st month of age has been also observed in other studies, but is not a consistent finding(10,11,38,39). In our study, Albanian mothers were found to have three times the odds of any breast-feeding at 6 months compared with Greek mothers. In many studies, immigrant mothers were found to breast-feed more than natives(11,19,40); country of origin has also been found to affect breast-feeding practices(40). The advantage of immigrant women regarding breast-feeding may be due to cultural differences(40) and has been found to attenuate with acculturation(8).
Our finding that the time of the study (2017 compared with 2007) was independently associated with the main breast-feeding indicators is of particular importance. It suggests that, apart from changes in maternity hospital practices or maternal characteristics (such as the improvement in educational level or the decrease in smoking rates), factors that have been accounted for in the multivariable analysis, other changes have occurred in Greece during this decade which also played a role in the increasing trend in breast-feeding rates.
In the period 2007–2017, several breast-feeding promotion activities took place. A national breast-feeding promotion programme named ‘Alkyoni’ was implemented by the Institute of Child Health(41), which included national awareness campaigns, educational activities for health professionals and parents, and a breast-feeding helpline. In addition, technical support for the Baby Friendly Hospital Initiative was provided to health-care facilities and at this time five maternity hospitals in Greece have been designated as baby friendly, while in several others, practices promoting breast-feeding (such as rooming-in and skin-to-skin contact during the first hour from birth) have been introduced gradually. Due to an organized effort to promote breast-feeding in the community, more than 250 businesses (restaurants, shops, pharmacies) have also been designated as baby friendly(41). Further, during the past decade numerous important local initiatives to promote breast-feeding have been taken across the country.
With regard to policy changes, new legislation was set up by the Ministry of Health and the Ministry of Labour. It includes laws aiming at maternity leave protection, breast-feeding promotion in the workplace, and the introduction of a written informed consent signed by the mother for the provision of a breast-milk substitute prior to discharge from the maternity hospital(14). A new child health booklet(42) (in which the WHO growth charts were adopted) was developed and new guidelines for the follow-up of children in primary health care were issued(43). In 2018 the Ministry of Health published guidelines with regard to complementary feeding initiation, recommending EBF for the first 6 months of age(44).
Our study provides evidence that the above initiatives and policies had a positive effect on breast-feeding uptake, while additional factors may have also contributed. During the past decade the values of a more ‘natural way of life’ have become more prevalent in Greece, which has probably favoured breast-feeding uptake. In addition, the study period (2007–2017) coincides with the recent financial crisis in Greece (2008 onwards), which might have also affected breast-feeding positively, as has been shown elsewhere(45).
Implications
In conclusion, our study shows that despite the increasing trend observed in all breast-feeding indicators in the past decade in Greece, the WHO recommendation for EBF during the first 6 months of life has not been adopted by mothers and promoted adequately by health-care professionals. Instead, introduction of human milk substitutes in the first months of life is predominant and early introduction of solids after the 4th month of age is considered the ‘norm’. To change the prevailing baby feeding ‘culture’, a variety of policies, initiatives and educational activities targeted to health professionals and mothers are required.
The sharp decline in breast-feeding rates during the first week of life, along with the high percentage of mothers given free samples of formula milk, underlines the importance of compliance with the provisions of the International Code for the Marketing of Breast-milk Substitutes. In addition, the Baby Friendly Hospital Initiative in Greece needs to be expanded to more maternity clinics and include mother-friendly care services. A breast-feeding supportive environment in primary health care with baby-friendly physicians’ offices is essential, together with projects aiming at baby-friendly communities using a whole-of-society approach. The development of an operational action plan on breast-feeding promotion, with objectives aligned with the Sustainable Development Goals(46), is instrumental.
Acknowledgements
Acknowledgements: The authors would like to thank Antigoni Souli for the valuable secretarial support she provided; Konstantinos Koutentakis and Giasemi Sarafidou for their contribution to data analysis; and express their gratitude to the mothers and other family members of the study infants for their participation in the study. Financial support: The study was supported financially by the Institute of Child Health, Athens, Greece and by the Ministry of Health, Greece. The funders had no role in the design, analysis or writing of this article. Conflict of interest: I.A. is the coordinator of, and C.E., E.N. and S.Z. have participated in ‘Alkyoni’, a national breast-feeding promotion programme run by the Institute of Child Health, Athens, Greece. I.A., T.P. and T.S. are or have been members of the Greek National Breastfeeding Committee. Z.I. has received remuneration by ELPEN Pharma for covering registration fees of an international conference. The other authors have no conflict of interest. Authorship: I.A. conceived the idea of the study. T.P. and D.P. designed the sampling scheme, and T.P., D.P., S.Z. and Z.I. adapted it for the 2017 study. I.A., T.P., E.G. and D.P. designed the questionnaire, and I.A., T.P., Z.I. and D.P. adapted it for the 2017 study. E.G. collected the data for the 2007 study, and Z.I., C.E., E.N. and P.M. for the 2017 study. D.P. and T.P. carried out the analysis for the 2007 study, and I.Z., D.P., T.S. and, T.P. for the 2017 study. I.Z., D.P., T.S. and T.P. wrote the manuscript. All authors contributed to the interpretation of the findings, critically revised the paper for important intellectual content and approved the final version. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving study participants were approved by the Medical Ethics Committee of the Institute of Child Health, Athens, Greece. Verbal informed consent was obtained from all participants at the beginning of the telephone interviews; verbal consent was witnessed by at least one other person and formally recorded.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019003719.
click here to view supplementary material
References
- 1. American Academy of Pediatrics (2019) Benefits of Breastfeeding. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Breastfeeding/Pages/Benefits-of-Breastfeeding.aspx (accessed January 2019).
- 2. Victora CG, Bahl R, Barros AJD et al. (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization & UNICEF (2007) Planning Guide for National Implementation of the Global Strategy for Infant and Young Child Feeding. Geneva: WHO. [Google Scholar]
- 4. World Health Organization & UNICEF (2009) Baby-Friendly Hospital Initiative: Revised, Updated and Expanded for Integrated Care. Geneva: WHO. [PubMed] [Google Scholar]
- 5. World Health Organization (1981) International Code of Marketing of Breast-Milk Substitutes. Geneva: WHO. [Google Scholar]
- 6. World Health Organization (2018) Ten steps to successful breastfeeding (revised 2018). http://www.who.int/nutrition/bfhi/ten-steps/en/ (accessed January 2019).
- 7. Pérez-Escamilla R, Martinez JL & Segura-Pérez S (2016) Impact of the Baby-friendly Hospital Initiative on breastfeeding and child health outcomes: a systematic review. Matern Child Nutr 12, 402–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ibanez G, Martin N, Denantes M et al. (2012) Prevalence of breastfeeding in industrialized countries. Rev Epidemiol Sante Publique 60, 305–320. [DOI] [PubMed] [Google Scholar]
- 9. Dennis C-L (2002) Breastfeeding initiation and duration: a 1990–2000 literature review. J Obstet Gynecol Neonatal Nurs 31, 12–32. [DOI] [PubMed] [Google Scholar]
- 10. Yngve A & Sjöström M (2001) Breastfeeding determinants and a suggested framework for action in Europe. Public Health Nutr 4, 729–739. [DOI] [PubMed] [Google Scholar]
- 11. Ajetunmobi O, Whyte B, Chalmers J et al. (2014) Informing the ‘early years’ agenda in Scotland: understanding infant feeding patterns using linked datasets. J Epidemiol Community Health 68, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization (2008) Indicators for Assessing Infant and Young Child Feeding Practices. Part 1: Definitions. Conclusions of a Consensus Meeting held 6–8 November 2007 in Washington, DC, USA. Geneva: WHO. [Google Scholar]
- 13. Gaki E, Papamichail D, Sarafidou G et al. (2009) National Study of Prevalence and Determinants of Breastfeeding in Greece. Athens: Institute of Child Health; available at http://epilegothilasmo.gr/wp-content/uploads/2018/07/Ekthesi_Ethnikhs_Meleths_Thilasmou.pdf (accessed December 2019). [Google Scholar]
- 14. Iliodromiti Z, Papamichail D, Ekizoglou C et al. (2018) National Study of Prevalence and Determinants of Breastfeeding in Greece. Athens: Institute of Child Health; available at http://epilegothilasmo.gr/wp-content/uploads/2018/03/meleti_breastfeeding_-2018_17_final.pdf (accessed December 2019). [Google Scholar]
- 15. Rodwell C & Aymé S (editors) (2014) 2014 Report on the State of the Art of Rare Disease Activities in Europe – Part V: Activities of European Member States and other European countries in the field of rare diseases. EUCERD, European Union; available at http://www.eucerd.eu/upload/file/Reports/2014ReportStateofArtRDActivitiesV.pdf (accessed January 2020). [Google Scholar]
- 16. Lemeshow S, Hosmer DW Jr, Klar J et al. (1992) Adequacy of Sample Size in Health Studies. Chichester: Wiley. [Google Scholar]
- 17. Carlin JB & Hocking J (1999) Design of cross-sectional surveys using cluster sampling: an overview with Australian case studies. Aust N Z J Public Health 23, 546–551. [DOI] [PubMed] [Google Scholar]
- 18. Vassilaki M, Chatzi L, Bagkeris E et al. (2014) Smoking and caesarean deliveries: major negative predictors for breastfeeding in the mother–child cohort in Crete, Greece (Rhea study). Matern Child Nutr 10, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tavoulari E-F (2015) Immigrant status as important determinant of breastfeeding practice in Southern Europe. Cent Eur J Public Health 23, 39–44. [DOI] [PubMed] [Google Scholar]
- 20. Bakoula C, Nicolaidou P, Veltsista A et al. (2007) Does exclusive breastfeeding increase after hospital discharge? A Greek study. J Hum Lact 23, 165–173. [DOI] [PubMed] [Google Scholar]
- 21. Skafida V (2014) Change in breastfeeding patterns in Scotland between 2004 and 2011 and the role of health policy. Eur J Public Health 24, 1033–1041. [DOI] [PubMed] [Google Scholar]
- 22. Bonet M, Kaminski M & Blondel B (2007) Differential trends in breastfeeding according to maternal and hospital characteristics: results from the French National Perinatal Surveys. Acta Paediatr 96, 1290–1295. [DOI] [PubMed] [Google Scholar]
- 23. Brick A & Nolan A (2014) Explaining the increase in breastfeeding at hospital discharge in Ireland, 2004–2010. Ir J Med Sci 183, 333–339. [DOI] [PubMed] [Google Scholar]
- 24. Oakley LL, Kurinczuk JJ, Renfrew MJ et al. (2016) Breastfeeding in England: time trends 2005–2006 to 2012–2013 and inequalities by area profile: breastfeeding time trends and inequalities. Matern Child Nutr 12, 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Libuda L, Bolzenius K & Alexy U (2017) Breastfeeding trends in healthy infants since 1990 – results of the DONALD study. Eur J Clin Nutr 71, 1016–1018. [DOI] [PubMed] [Google Scholar]
- 26. Magnusson M, Lagerberg D & Wallby T (2016) No widening socioeconomic gap within a general decline in Swedish breastfeeding: no widening socioeconomic gap in Swedish breastfeeding. Child Care Health Dev 42, 415–423. [DOI] [PubMed] [Google Scholar]
- 27. Bagci Bosi AT, Eriksen KG, Sobko T et al. (2016) Breastfeeding practices and policies in WHO European region member states. Public Health Nutr 19, 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization & UNICEF (2014) Global Nutrition Targets 2025: Breastfeeding Policy Brief. Geneva: WHO. [Google Scholar]
- 29. Economou M, Kolokotroni O, Paphiti-Demetriou I et al. (2018) Prevalence of breast-feeding and exclusive breast-feeding at 48 h after birth and up to the sixth month in Cyprus: the BrEaST start in life project. Public Health Nutr 21, 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erkkola M, Salmenhaara M, Kronberg-Kippilä C et al. (2010) Determinants of breast-feeding in a Finnish birth cohort. Public Health Nutr 13, 504–513. [DOI] [PubMed] [Google Scholar]
- 31. Grimshaw KEC, Aksoy B, Palmer A et al. (2015) Prospective food diaries demonstrate breastfeeding characteristics in a UK birth cohort: prospective diaries of breastfeeding nature. Matern Child Nutr 11, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forrester-Knauss C, Merten S, Weiss C et al. (2013) The baby-friendly hospital initiative in Switzerland: trends over a 9-year period. J Hum Lact 29, 510–516. [DOI] [PubMed] [Google Scholar]
- 33. Biggs K, Hurrell K, Matthews E et al. (2018) Formula milk supplementation on the postnatal ward: a cross-sectional analytical study. Nutrients 10, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Theofilogiannakou M, Skouroliakou M, Gounaris A et al. (2006) Breast-feeding in Athens, Greece: factors associated with its initiation and duration. J Pediatr Gastroenterol Nutr 43, 379–384. [DOI] [PubMed] [Google Scholar]
- 35. Bouras G, Mexi-Bourna P, Bournas N et al. (2013) Mothers’ expectations and other factors affecting breastfeeding at six months in Greece. J Child Health Care 17, 387–396. [DOI] [PubMed] [Google Scholar]
- 36. Skafida V (2009) The relative importance of social class and maternal education for breast-feeding initiation. Public Health Nutr 12, 2285–2292. [DOI] [PubMed] [Google Scholar]
- 37. Pechlivani F, Vassilakou T, Sarafidou J et al. (2007) Prevalence and determinants of exclusive breastfeeding during hospital stay in the area of Athens, Greece: exclusive breastfeeding initiation in Greece. Acta Paediatr 94, 928–934. [DOI] [PubMed] [Google Scholar]
- 38. Vanderlinden K, Levecque K & Van Rossem R (2015) Breastfeeding or bottled milk? Poverty and feeding choices in the native and immigrant population in Belgium. J Immigr Minor Health 17, 319–324. [DOI] [PubMed] [Google Scholar]
- 39. Lande B, Andersen L, Baerug A et al. (2007) Infant feeding practices and associated factors in the first six months of life: the Norwegian infant nutrition survey. Acta Paediatr 92, 152–161. [DOI] [PubMed] [Google Scholar]
- 40. Kelly YJ, Watt RG & Nazroo JY (2006) Racial/ethnic differences in breastfeeding initiation and continuation in the United Kingdom and comparison with findings in the United States. Pediatrics 118, e1428–e1435. [DOI] [PubMed] [Google Scholar]
- 41. Institute of Child Health (2013) Breastfeeding – Alkyoni. http://epilegothilasmo.gr/ (accessed January 2019).
- 42. Institute of Child Health (2015) Child Health Booklet. Athens: Ministry of Health. [Google Scholar]
- 43. Antoniadou-Koumatou I, Panagiotopoulos T & Attilakos A (2015) Guidelines for the Follow Up of Children in Primary Health Care. Athens: Institute of Child Health. [Google Scholar]
- 44. Directorate of Public Health, Department of Non Communicable Diseases and Nutrition (2018) Guidelines for Introduction of Solid Foods at the First Year of Life. Athens: Ministry of Health. [Google Scholar]
- 45. Elo IT & Grummer-Strawn LM (1993) Changes in breastfeeding initiation and duration in Peru, 1977–1986. Soc Biol 40, 224–243. [PubMed] [Google Scholar]
- 46. United Nations (2015) Transforming Our World: The 2030 Agenda for Sustainable Development. New York: UN.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019003719.
click here to view supplementary material


