Abstract
Objective:
Previous studies have shown that the Dietary Approaches to Stop Hypertension (DASH) diet might contribute to managing risk factors of non-alcoholic fatty liver disease (NAFLD), but evidence is limited. We examined the association of DASH diet score (DASH-DS) with NAFLD, as well as the intermediary effects of serum retinol-binding protein-4 (RBP4), serum high-sensitivity C-reactive protein (hs-CRP), serum TAG, homeostasis model assessment of insulin resistance (HOMA-IR) and BMI.
Design:
We performed a cross-sectional analysis of a population-based cohort study. Dietary data and lifestyle factors were assessed by face-to-face interviews and the DASH-DS was then calculated. We assessed serum RBP4, hs-CRP and TAG and calculated HOMA-IR. The presence and degree of NAFLD were determined by abdominal sonography.
Setting:
Guangzhou, China.
Participants:
Guangzhou Nutrition and Health Study participants, aged 40–75 years at baseline (n 3051).
Results:
After adjusting for potential covariates, we found an inverse association between DASH-DS and the presence of NAFLD (Ptrend = 0·009). The OR (95 % CI) of NAFLD for quintiles 2–5 were 0·78 (0·62, 0·98), 0·74 (0·59, 0·94), 0·69 (0·55, 0·86) and 0·77 (0·61, 0·97), respectively. Path analyses indicated that a higher DASH-DS was associated with lower serum RBP4, hs-CRP, TAG, HOMA-IR and BMI, which were positively associated with the degree of NAFLD.
Conclusions:
Adherence to the DASH diet was independently associated with a marked lower prevalence of NAFLD in Chinese adults, especially in women and those without abdominal obesity, and might be mediated by reducing RBP4, hs-CRP, TAG, HOMA-IR and BMI.
Keywords: Non-alcoholic fatty liver disease, Nutrition, Dietary Approaches to Stop Hypertension diet, Cross-sectional study
Non-alcoholic fatty liver disease (NAFLD), the main cause of liver disease worldwide, is the term used to describe a series of pathological findings resulting from the accumulation of fat in the liver. These findings include steatosis, steatohepatitis, steatonecrosis and cirrhosis. In China, the prevalence of fatty liver disease has approximately doubled over the past two decades(1). There is a great public health need to find an efficient and available therapy. Former studies have suggested that insulin resistance (IR) and inflammation induced by imbalance of oxidative stress play significant roles in the pathogenesis of NAFLD(2,3).
Diet is one of the key lifestyle factors involved in the genesis, prevention and control of NAFLD(4,5). The Dietary Approaches to Stop Hypertension (DASH) diet, which advocates high intake of whole grains, fruits and vegetables as well as low-fat dairy products, combined with Na restriction, was initially developed to prevent hypertension(6,7). It discourages the intake of Na, sweetened beverages, and red and processed meats. Many studies have suggested that some DASH components, such as lower intakes of sugar-sweetened beverages(8) and Na(9), are associated with decreasing presence of NAFLD. A meta-analysis showed that the DASH diet score (DASH-DS) is significantly associated with an improvement in insulin sensitivity(10). A few studies have found that the DASH diet was effective in improving circulating serum inflammatory biomarkers(11), in relieving IR(12), and in encouraging weight loss(13) or discouraging fat accumulation(12), all these factors being closely connected with NAFLD.
To date, however, only one case–control study has directly examined the association between the DASH diet and NAFLD; it included 102 patients diagnosed by FibroScan and 204 controls and indicated that participants in the highest (v. lowest) quartile of DASH-DS were 30 % less likely to have NAFLD(14). Another randomised trial, including sixty overweight and obese patients with NAFLD, demonstrated that an intervention with the DASH diet for 8 weeks improved body weight and NAFLD-related metabolic status (alanine aminotransferase and alkaline phosphatase levels)(15). Therefore, the favourable association between the DASH diet and NAFLD in the general population remains inconclusive. To the authors’ knowledge, no study has yet assessed whether or not the DASH diet–NAFLD association is mediated by improving IR, inflammation, body weight or plasma lipids in human subjects.
To address this issue, the present study examined the association between the DASH diet and the prevalence of NAFLD and whether the potentially favourable association was potentially mediated by reducing inflammation, IR, serum TAG and BMI in middle-aged and elderly Chinese adults.
Methods
Study population
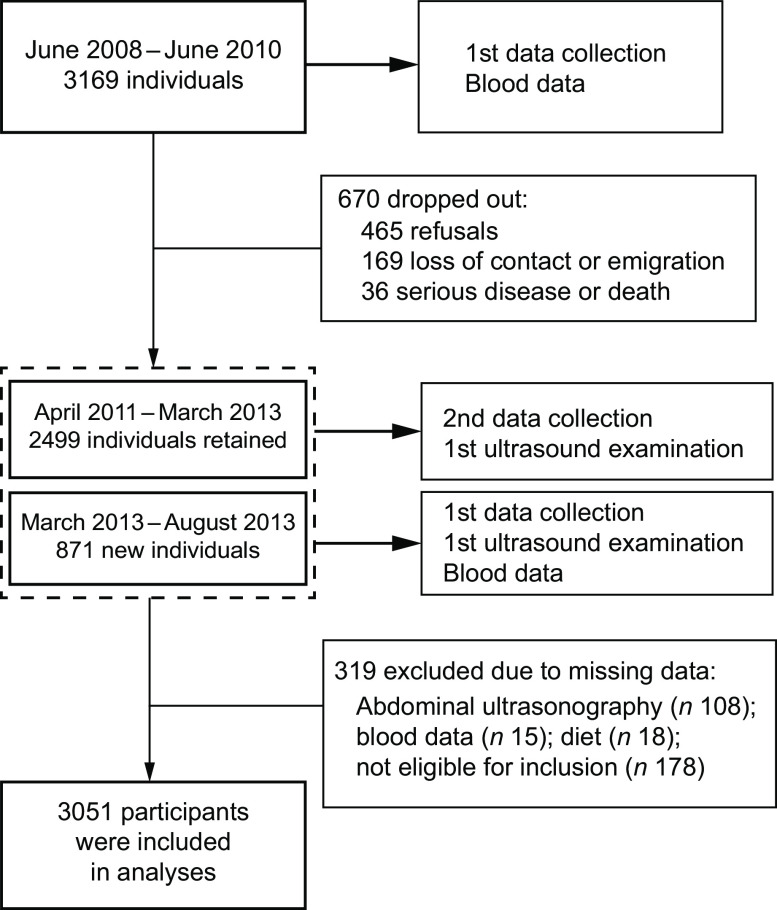
Participants were drawn from a cohort study, the Guangzhou Nutrition and Health Study (GNHS), which was designed to assess the determinants of osteoporosis and cardiometabolic endpoints. Age of 40–75 years at the time of enrolment and residence in Guangzhou for no less than 5 years were the basic inclusion criteria. Participants who reported hospital-confirmed malignancy, stroke, coronary artery disease, liver diseases (e.g. Wilson’s disease, cirrhosis and haemosiderosis), renal failure, and physical and mental disability at the time of enrolment were excluded. Details of the GNHS were described previously(16). As shown in Fig. 1, we recruited a total of 3196 participants aged 40–75 years from 2008 to 2010 in Guangzhou, China. Among these, 2499 were then followed between 2011 and 2013, and then we recruited an additional 871 new participants, in the same way as previously mentioned, in 2013. The calculation of DASH-DS was based on the dietary assessment of the first follow-up, and abdominal ultrasonography for NAFLD was performed at the same time.
Fig. 1.
Flowchart of participants in the present study: middle-aged and elderly adults aged 40–75 years at baseline, Guangzhou Nutrition and Health Study, China, 2010–2013
Participants with missing data for abdominal ultrasonography (n 108), serum biochemical indices (n 15) or diet (n 18), or who reported excessive alcohol intake (more than 14 drinks/week for men and 7 drinks/week for women; n 12), self-reported viral hepatitis (n 57) or abnormal energy intake (<3347 or >17 573 kJ/d (<800 or >4200 kcal/d) for men and <2510 or >14 644 kJ/d (<600 or >3500 kcal/d) for women; n 109) were all excluded from the study. Finally, a total of 2083 women and 968 men were included in the cross-sectional study.
The present study was conducted according to the guidelines described in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of Sun Yat-sen University’s School of Public Health. Written informed consent was obtained from all participants.
Data collection
Dietary assessment
We used a validated seventy-nine-item quantitative FFQ to recall the usual dietary consumption of eligible participants by means of face-to-face interviews by trained researchers; details were described in a previous study(17). The validity and reproducibility of the FFQ have been evaluated among Chinese people and both measures were acceptable(18). During this interview, we asked participants how often they had consumed a typical portion size over the previous year and consumption was estimated by day, week, month or year, in accordance with the choice of the respondents. The total dietary energy intake was then calculated based on the Chinese Food Composition Table, 2004(19).
DASH score calculation
The FFQ was applied to measure the dietary components of the DASH-DS, which we calculated according to foods and nutrients that were emphasised or minimised in the DASH diet. We concentrated on eight components: high intake of vegetables, fruits, nuts and legumes, dairy products and whole grains (including grain products) and low intake of sweetened beverages, Na, and red and processed meats(20). Due to the lack of data obtained on salt, only data concerning Na in foods were used to assess Na intake. Additionally, as the consumption of low-fat dairy products is quite low in the Chinese population, we replaced this component with general dairy products.
Before calculating the score, we adjusted the dietary intake of each participant for their total energy intake using the residual method(21). For each component, we classified participants into quintiles on the basis of their intake ranking. The component score for vegetables, fruits, nuts and legumes, dairy products, and whole grains and grain products was derived from the participant’s quintile ranking (i.e. quintile 1 was assigned 1 point, while quintile 5 was assigned 5 points), in contrast to components of Na, red and processed meats, and sweetened beverages (5 points was assigned to quintile 1 whereas 1 point was assigned to quintile 5). We summed up the eight component scores to create an overall DASH-DS that ranged from 8 to 40. All the scores were calculated separately for male and female participants.
Abdominal ultrasonography
The diagnosis of NAFLD was based on abdominal ultrasonography conducted with a Doppler sonography machine (Sonoscape SSI-5500, Shenzhen, China) with a 3·5 MHz probe. All abdominal ultrasonography was performed by a single proficient radiologist who was blinded to the participant’s information.
The degree of fatty liver was evaluated semi-quantitatively (absent; mild; moderate to severe) according to hepatorenal echo contrast, liver brightness, deep attenuation and sharpness of the vasculature based on the guidelines for the diagnosis of NAFLD in China(22). The ultrasound criteria for diagnosing fatty liver include the following: (i) diffuse enhancement of near-field echo in the hepatic region (stronger than in the renal and splenic regions) and gradual attenuation of the far-field echo; (ii) unclear display of the intrahepatic lacunae structure; (iii) mild to moderate hepatomegaly with a rounded and blunt border; (iv) reduced or unclear presentation of the blood flow signal in the liver but with a normal distribution of blood flow by colour Doppler ultrasonography; and (v) unclear or non-intact display of the envelope of the right liver lobe and diaphragm. Participants who fit the first criterion and one of the second to fourth criteria were diagnosed with mild NAFLD; participants who fit the first criterion and two of the second to fourth criteria were diagnosed with moderate NAFLD; and participants who fit the first criterion, the fifth criterion and two of the second to fourth criteria were diagnosed with severe NAFLD. Validity, assessed in thirty-four participants with further computed tomography by radiologists who were blinded to the ultrasound results, showed good agreement with the ultrasound findings (κ = 0·691, Spearman’s r = 0·905 and total consistency = 85 %, P < 0·001). Very high precision (κ = 0·875, Spearman’s r = 0·911 and total agreement = 92 %, P < 0·001) of between-operator reliability determined in 100 participants for the ultrasound NAFLD evaluations was observed.
Laboratory assay
Serum collected from 2011 to 2013 was applied to the measurement of serum retinol-binding protein-4 (RBP4), high-sensitivity C-reactive protein (hs-CRP), TAG, and fasting glucose and insulin.
As a specific carrier of retinol in the blood, RBP4 is recognised as a biomarker of both inflammation(23) and IR(24). An ELISA kit (Adipogen, San Diego, CA, USA), with a microplate spectrophotometer (BIO-TEK, Winooski, VT, USA), was used; the intra-assay CV was 3·59 % for serum RBP4. Serum hs-CRP was detected with a Cobas c 701/702 automatic biochemical analyser and Cardiac C-Reactive Protein (Latex) High Sensitivity (CRPHS) test kit (Roche Diagnostics GmbH, Mannheim, Germany); the intra-assay CV was 1·0 %.
A Hitachi 7600-010 automated analyser was applied to determine overnight fasting serum glucose and TAG by colorimetric methods. Fasting serum insulin was measured by electrochemiluminescence immunoassay using a Roche Cobas 8000/e602® immunoanalyser kit (art. nr. 12017547 122; Roche Diagnostics GmbH). IR was defined by the homeostasis model assessment of insulin resistance (HOMA-IR), calculated as follows(25): [plasma glucose (mg/dl) × plasma insulin (μU/ml)]/405.
Assessment of potential confounders
Trained staff collected participants’ sociodemographic characteristics (e.g. age, sex, household income and education), health-related lifestyle factors, history of menstruation, and history of chronic disease and medications by using structured questionnaires. Weight and height were measured while participants were wearing light clothing and without shoes and then used for calculating BMI. The measurement of waist circumference was performed twice at the midline between the costal margin and iliac crest. A 24 h physical activity questionnaire(26) was used to evaluate participants’ daily physical activity by calculating the metabolic equivalent intensity(27).
Statistical analysis
The data are presented as frequencies and percentages for categorical variables and as means and standard deviations for continuous variables. The t test and the χ2 test were applied to analyse differences in the data stratified by sex or the presence of NAFLD. We used logistic regression analyses to identify the OR and 95 % confidence intervals for the presence of NAFLD with increasing quintile of the DASH-DS, using the lowest quintile as the reference group. We also computed the OR of the individuals with mild and severe NAFLD compared with those without NAFLD. Model 1 was adjusted for age, sex and energy intake. Aimed at investigating the independent association, we additionally adjusted for household income, physical activity, hormone use in postmenopausal women, multivitamin use, smoking, tea intake and alcohol intake in model 2. In model 3, we further adjusted for other potential confounders: heart disease, diabetes and stroke.
Analyses stratified by sex (male/female), abdominal obesity(28) (waist circumference >90 cm for males and >85 cm for females; yes/no) and smoking (yes/no) were conducted to verify whether the associations would be modified by the above factors. All of the statistical procedures were performed using the statistical software package IBM SPSS Statistics version 23.0.
Path analysis is a type of structural equation modelling, which is a multivariate procedure for evaluating relationships between independent variables, either continuous or discrete, and one or more dependent variables(29). We carried out path analyses to test whether the DASH diet was associated with serum RBP4, hs-CRP, TAG, HOMA-IR and BMI, and whether these factors were associated with NAFLD, using the structural equation modelling software IBM SPSS Amos version 24. DASH-DS were fit to the model to test the associations with the mediators (i.e. serum RBP4, HOMA-IR and BMI) and their relationship with the degree of NAFLD in the rudimentary model. We determined the standardised regression coefficients of each identified path, goodness-of-fit index and adjusted goodness-of-fit index of the model to obtain the estimates and evaluate the goodness-of-fit of the models. All reported P values are two-sided and P < 0·05 was considered significant.
Results
The prevalence of NAFLD in the present study, diagnosed by ultrasound, was estimated to be 50·7 %. Participant characteristics are presented in Table 1. The study involved 3051 participants, of whom 2083 were women with a mean age of 62·5 years and 968 were men with a mean age of 65·3 years. Compared with men, women in the cohort had higher intakes of vegetables, fruits and dairy products and lower intakes of energy, whole grains and grain products, and red and processed meats, as well as lower BMI and lower rates of smoking and drinking alcohol and tea (all P < 0·001). Participants with NAFLD were more likely to have lower DASH-DS, physical activity and intake of nuts and legumes, higher BMI and older age and showed a higher intake of sweetened beverages (all P < 0·05). As shown in the online supplementary material, Supplemental Table S1, women consumed more vegetables and fruits and less red and processed meats than men in the same quintile of DASH-DS.
Table 1.
Characteristics of the study participants: middle-aged and elderly adults (n 3051) aged 40–75 years at baseline, Guangzhou Nutrition and Health Study, China, 2010–2013
| Characteristic | Women | Men | P value | Non-NAFLD | NAFLD | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n or mean | % or sd | n or mean | % or sd | n or mean | % or sd | n or mean | % or sd | |||
| n and % | 2083 | 68·3 | 968 | 31·7 | 1503 | 49·3 | 1548 | 50·7 | ||
| Age (years) | 62·5 | 5·4 | 65·3 | 6·3 | <0·001 | 63·1 | 6·0 | 63·6 | 5·7 | 0·008 |
| BMI (kg/m2) | 23·4 | 3·2 | 24·0 | 3·0 | <0·001 | 22·1 | 2·6 | 25·0 | 3·0 | <0·001 |
| Waist circumference (cm) | 84·3 | 8·8 | 86·1 | 8·6 | <0·001 | 81·3 | 7·9 | 88·4 | 8·2 | <0·001 |
| Smoker† | 10 | 0·5 | 341 | 35·2 | <0·001 | 165 | 11·0 | 186 | 12·0 | 0·369 |
| Tea drinker‡ | 1011 | 48·5 | 710 | 73·3 | <0·001 | 786 | 52·3 | 935 | 60·4 | <0·001 |
| Alcohol drinker§ | 78 | 3·7 | 156 | 16·1 | <0·001 | 110 | 7·3 | 124 | 8·0 | 0·473 |
| Oestrogen user∥ | 134 | 6·4 | – | – | – | 84 | 5·6 | 50 | 3·2 | 0·001 |
| Multivitamin user¶ | 448 | 21·5 | 130 | 13·4 | <0·001 | 288 | 19·2 | 290 | 18·7 | 0·763 |
| Household income (yuan/person per month) | 0·119 | 0·936 | ||||||||
| <4000 | 1820 | 87·4 | 825 | 85·2 | 1304 | 86·8 | 1341 | 86·6 | ||
| 4000–6000 | 169 | 8·1 | 98 | 10·1 | 129 | 8·6 | 138 | 8·9 | ||
| >6000 | 94 | 4·5 | 45 | 4·6 | 70 | 4·7 | 69 | 4·5 | ||
| PA (MET-h/d) | 17·6 | 7·0 | 15·7 | 7·18 | <0·001 | 17·6 | 7·0 | 16·4 | 6·7 | <0·001 |
| Serum TAG (mg/dl) | 1·50 | 1·10 | 1·59 | 1·40 | 0·049 | 1·30 | 1·01 | 1·76 | 1·33 | <0·001 |
| Diabetes | 147 | 7·1 | 100 | 10·3 | 0·002 | 98 | 6·5 | 149 | 9·6 | 0·002 |
| Heart disease | 455 | 21·4 | 166 | 17·1 | 0·007 | 294 | 19·6 | 317 | 20·5 | 0·572 |
| Stroke | 55 | 2·6 | 63 | 6·5 | <0·001 | 64 | 4·3 | 54 | 3·5 | 0·270 |
| Components of DASH diet | ||||||||||
| Energy (kJ/d) | 6699 | 1845 | 7862 | 2163 | <0·001 | 7050 | 1992 | 7096 | 2059 | 0·520 |
| Energy (kcal/d) | 1601 | 441 | 1879 | 517 | <0·001 | 1685 | 476 | 1696 | 492 | 0·520 |
| Vegetables (g/d) | 328 | 139 | 278 | 138 | <0·001 | 310 | 139 | 314 | 142 | 0·436 |
| Fruits (g/d) | 148 | 98 | 117 | 85 | <0·001 | 138 | 94 | 137 | 96 | 0·783 |
| Nuts and legumes (g/d) | 27·8 | 22·8 | 28·0 | 24·0 | 0·787 | 28·8 | 23·7 | 26·9 | 22·6 | 0·024 |
| Dairy products (g) | 16·6 | 14·6 | 13·3 | 14·2 | <0·001 | 15·4 | 13·7 | 15·8 | 15·3 | 0·392 |
| Whole grains and grain products (g/d) | 170 | 41 | 229 | 56 | <0·001 | 189 | 53 | 189 | 54 | 0·980 |
| Red and processed meats (g/d) | 152 | 137 | 193 | 151 | <0·001 | 161 | 142 | 168 | 144 | 0·181 |
| Sweetened beverages (ml/d) | 1·38 | 2·00 | 1·51 | 2·06 | 0·128 | 1·32 | 1·94 | 1·53 | 2·09 | 0·005 |
| DASH-DS†† | 24·4 | 4·4 | 24·2 | 4·4 | 0·253 | 24·6 | 4·2 | 24·1 | 4·5 | 0·004 |
NAFLD, non-alcoholic fatty liver disease; PA, physical activity; MET, metabolic equivalent of task; DASH, Dietary Approaches to Stop Hypertension; DASH-DS, DASH diet score.
Values are presented as means and sd (continuous variables) or as n and % (categorical variables).
Smoker: ≥1 cigarette/d in the past year.
Tea drinker: ≥1 cup/week in the past year.
Alcohol drinker: >14 drinks/week for men and 7 drinks/week for women.
Oestrogen user: use of oestrogen in postmenopausal women.
Multivitamin user: ≥30 times in the past year.
DASH-DS: including eight components, range from 8 to 40.
As shown in Table 2, the prevalence of NAFLD was 56·6, 50·3, 49·5, 47·2 and 50·4 % in quintiles 1 to 5 of the DASH-DS, respectively, in all participants. After adjusting for age and sex, the DASH-DS was inversely associated with the prevalence of NAFLD (Ptrend = 0·005). The OR (95 % CI) of NAFLD for quintiles 2−5 (v. quintile 1) were 0·77 (0·62, 0·96), 0·73 (0·58, 0·93), 0·67 (0·54, 0·84) and 0·76 (0·60, 0·95), respectively. Similar associations were observed after further adjusting for other potential confounders (i.e. energy intake, physical activity, household income, multivitamin use, smoking, tea intake, alcohol intake and oestrogen use); the corresponding OR (95 % CI) for quintiles 2–5 (v. quintile 1) were 0·79 (0·63, 0·99), 0·75 (0·59, 0·94), 0·69 (0·55, 0·86) and 0·77 (0·61, 0·97), respectively (Ptrend = 0·008). No obvious change was observed after further adjusting for heart disease, diabetes and stroke in model 3. Compared with non-NAFLD, we found similar results when we calculated the OR for the mild and severe degrees of NAFLD. The results of logistic analyses stratified by sex and abdominal obesity were obtained and appear in Tables 2 and 3. The favourable associations were more pronounced in women and in participants without abdominal obesity, but subgroup analyses indicated no significant interactions when stratified by sex, abdominal obesity and smoking (Pinteraction range = 0·091−0·915).
Table 2.
OR and 95 % CI for non-alcoholic fatty liver disease (NAFLD) according to quintile (Q) of Dietary Approaches to Stop Hypertension (DASH) diet score (DASH-DS) in all participants and stratified by sex: middle-aged and elderly adults (n 3051) aged 40–75 years at baseline, Guangzhou Nutrition and Health Study, China, 2010–2013
| Quintile of DASH-DS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | % | Q2 | % or 95 % CI | Q3 | % or 95 % CI | Q4 | % or 95 % CI | Q5 | % or 95 % CI | P trend | |
| NAFLD compared with non-NAFLD | |||||||||||
| Total | |||||||||||
| Median score | 18·03 | – | 22·09 | – | 24·52 | – | 26·91 | – | 30·55 | – | – |
| NAFLD cases | 347 | 56·6 | 327 | 50·3 | 270 | 49·5 | 318 | 47·2 | 286 | 50·4 | – |
| OR1† | 1·00 | Ref. | 0·77 | 0·62, 0·96 | 0·73 | 0·58, 0·93 | 0·67 | 0·54, 0·84 | 0·76 | 0·60, 0·95 | 0·005 |
| OR2‡ | 1·00 | Ref. | 0·79 | 0·63, 0·99 | 0·75 | 0·59, 0·94 | 0·69 | 0·55, 0·86 | 0·77 | 0·61, 0·97 | 0·008 |
| OR3§ | 1·00 | Ref. | 0·78 | 0·62, 0·98 | 0·74 | 0·59, 0·94 | 0·69 | 0·55, 0·86 | 0·77 | 0·61, 0·97 | 0·009 |
| Women | |||||||||||
| Median score | 17·97 | – | 22·10 | – | 24·52 | – | 26·88 | 30·58 | – | – | |
| NAFLD cases | 228 | 56·4 | 218 | 48·9 | 172 | 47·4 | 224 | 46·8 | 192 | 49·1 | – |
| OR1 | 1·00 | Ref. | 0·72 | 0·55, 0·95 | 0·66 | 0·50, 0·89 | 0·64 | 0·49, 0·84 | 0·70 | 0·53, 0·93 | 0·006 |
| OR2 | 1·00 | Ref. | 0·74 | 0·56, 0·98 | 0·67 | 0·50, 0·89 | 0·66 | 0·50, 0·86 | 0·70 | 0·53, 0·93 | 0·007 |
| OR3 | 1·00 | Ref. | 0·74 | 0·56, 0·98 | 0·67 | 0·50, 0·90 | 0·66 | 0·50, 0·87 | 0·71 | 0·53, 0·94 | 0·009 |
| Men | |||||||||||
| Median score | 18·18 | – | 22·06 | – | 24·51 | – | 26·96 | – | 30·49 | – | – |
| NAFLD cases | 119 | 56·9 | 109 | 53·4 | 98 | 53·6 | 94 | 48·2 | 94 | 53·1 | – |
| OR1 | 1·00 | Ref. | 0·88 | 0·60, 1·30 | 0·90 | 0·60, 1·35 | 0·73 | 0·49, 1·09 | 0·90 | 0·60, 1·34 | 0·369 |
| OR2 | 1·00 | Ref. | 0·90 | 0·60, 1·33 | 0·94 | 0·63, 1·41 | 0·74 | 0·50, 1·11 | 0·92 | 0·61, 1·40 | 0·435 |
| OR3 | 1·00 | Ref. | 0·87 | 0·58, 1·29 | 0·93 | 0·61, 1·40 | 0·72 | 0·48, 1·09 | 0·9 | 0·60, 1·41 | 0·443 |
| Mild and severe NAFLD compared with non-NAFLD | |||||||||||
| Total | |||||||||||
| Median score | 18·08 | – | 22·09 | – | 24·51 | – | 26·93 | – | 30·56 | – | – |
| NAFLD cases | 77 | 22·4 | 66 | 17·0 | 58 | 17·4 | 64 | 15·2 | 59 | 17·3 | – |
| OR1 | 1·00 | Ref. | 0·70 | 0·48, 1·00 | 0·70 | 0·48, 1·02 | 0·60 | 0·41, 0·86 | 0·68 | 0·47, 1·00 | 0·025 |
| OR2 | 1·00 | Ref. | 0·71 | 0·49, 1·03 | 0·71 | 0·48, 1·05 | 0·63 | 0·43, 0·92 | 0·71 | 0·48, 1·05 | 0·053 |
| OR3 | 1·00 | Ref. | 0·68 | 0·47, 1·00 | 0·69 | 0·46, 1·02 | 0·61 | 0·42, 0·89 | 0·70 | 0·47, 1·05 | 0·046 |
| Women | |||||||||||
| Median score | 18·00 | – | 22·12 | – | 24·50 | – | 26·89 | – | 30·54 | – | – |
| NAFLD cases | 54 | 23·5 | 43 | 15·9 | 42 | 18·0 | 41 | 13·9 | 40 | 16·7 | – |
| OR1 | 1·00 | Ref. | 0·59 | 0·37, 0·92 | 0·66 | 0·42, 1·05 | 0·49 | 0·31, 0·77 | 0·60 | 0·38, 0·95 | 0·016 |
| OR2 | 1·00 | Ref. | 0·59 | 0·37, 0·93 | 0·65 | 0·41, 1·03 | 0·50 | 0·32, 0·80 | 0·60 | 0·38, 0·97 | 0·021 |
| OR3 | 1·00 | Ref. | 0·57 | 0·36, 0·91 | 0·64 | 0·40, 1·02 | 0·50 | 0·31, 0·80 | 0·59 | 0·37, 0·95 | 0·020 |
| Men | |||||||||||
| Median score | 18·24 | – | 22·01 | – | 24·52 | – | 27·02 | – | 30·57 | – | – |
| NAFLD cases | 23 | 20·4 | 23 | 19·5 | 16 | 15·8 | 23 | 18·5 | 19 | 18·6 | – |
| OR1 | 1·00 | Ref. | 0·95 | 0·50, 1·81 | 0·73 | 0·37, 1·49 | 0·90 | 0·47, 1·70 | 0·90 | 0·45, 1·78 | 0·702 |
| OR2 | 1·00 | Ref. | 1·00 | 0·53, 2·02 | 0·80 | 0·39, 1·66 | 1·01 | 0·52, 1·96 | 1·07 | 0·53, 2·17 | 0·907 |
| OR3 | 1·00 | Ref. | 1·00 | 0·51, 1·97 | 0·75 | 0·36, 1·57 | 0·92 | 0·46, 1·82 | 1·08 | 0·53, 2·22 | 0·966 |
Ref., reference category.
OR1: OR and 95 % CI adjusted for age and sex.
OR2: OR and 95 % CI adjusted for variables in OR1 plus energy intake, physical activity, household income, multivitamin use, smoking, tea intake, alcohol intake and oestrogen use.
OR3: OR and 95 % CI adjusted for variables in OR2 plus heart disease, diabetes and stroke.
Table 3.
OR and 95 % CI for non-alcoholic fatty liver disease (NAFLD) according to quintile (Q) of Dietary Approaches to Stop Hypertension (DASH) diet score (DASH-DS) in all participants stratified by waist circumference: middle-aged and elderly adults (n 3051) aged 40–75 years at baseline, Guangzhou Nutrition and Health Study, China, 2010–2013
| Quintile of DASH-DS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | % | Q2 | % or 95 % CI | Q3 | % or 95 % CI | Q4 | % or 95 % CI | Q5 | % or 95 % CI | P trend | |
| NAFLD compared with non-NAFLD | |||||||||||
| Non-abdominal obesity† | |||||||||||
| Median score | 18·01 | – | 22·10 | – | 24·50 | – | 26·93 | – | 30·47 | – | – |
| NAFLD cases | 156 | 45·5 | 136 | 37·0 | 131 | 39·5 | 127 | 31·4 | 119 | 36·6 | – |
| OR1‡ | 1·00 | Ref. | 0·71 | 0·52, 0·96 | 0·78 | 0·58, 1·07 | 0·56 | 0·41, 0·75 | 0·70 | 0·51, 0·95 | 0·005 |
| OR2§ | 1·00 | Ref. | 0·72 | 0·53, 0·97 | 0·79 | 0·58, 1·08 | 0·57 | 0·42, 0·77 | 0·70 | 0·51, 0·96 | 0·006 |
| OR3∥ | 1·00 | Ref. | 0·71 | 0·52, 0·96 | 0·79 | 0·58, 1·08 | 0·56 | 0·41, 0·76 | 0·70 | 0·51, 0·96 | 0·005 |
| Abdominal obesity | |||||||||||
| Median score | 18·07 | – | 22·07 | – | 24·55 | – | 26·87 | – | 30·66 | – | – |
| NAFLD Cases | 191 | 70·7 | 191 | 67·7 | 139 | 65·0 | 191 | 70·7 | 167 | 68·7 | – |
| OR1 | 1·00 | Ref. | 0·87 | 0·61, 1·26 | 0·73 | 0·49, 1·08 | 0·98 | 0·67, 1·43 | 0·88 | 0·60, 1·30 | 0·688 |
| OR2 | 1·00 | Ref. | 0·90 | 0·62, 1·31 | 0·75 | 0·51, 1·12 | 1·00 | 0·68, 1·46 | 0·88 | 0·60, 1·30 | 0·670 |
| OR3 | 1·00 | Ref. | 0·91 | 0·63, 1·32 | 0·75 | 0·50, 1·11 | 1·01 | 0·69, 1·48 | 0·90 | 0·61, 1·33 | 0·736 |
Ref., reference category.
Abdominal obesity: waist circumference >90 cm for males and >85 cm for females.
OR1: OR and 95 % CI adjusted for age and sex.
OR2: OR and 95 % CI adjusted for variables in OR1 plus energy intake, physical activity, household income, multivitamin use, smoking, tea intake, alcohol intake and oestrogen use.
OR3: OR and 95 % CI adjusted for variables in OR2 plus heart disease, diabetes and stroke.
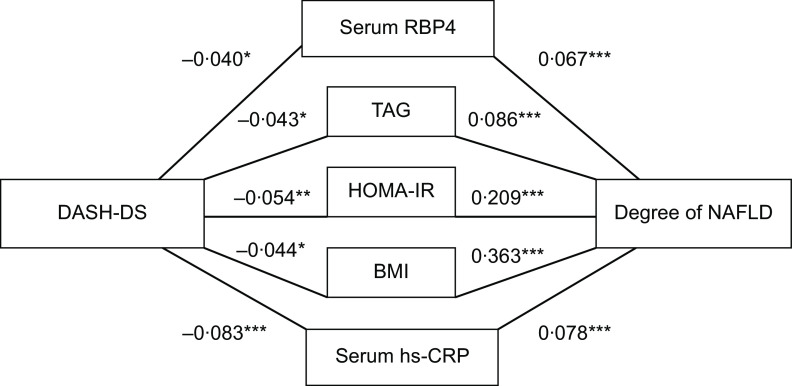
Path analyses were conducted to clarify whether the favourable association of the DASH diet with NAFLD progression might be mediated by inflammatory factors RBP4, TAG, HOMA-IR, BMI and hs-CRP. As shown in Fig. 2, the path models found that the DASH-DS directly affected serum RBP4, TAG, HOMA-IR, BMI and hs-CRP, and their standardised β values were −0·040, −0·043, −0·054, −0·044 and −0·083, respectively (all P < 0·05). All these factors showed a positive association with the degree of NAFLD in the first test, and the standardised β (correlation coefficient) was 0·067 for serum RBP4, 0·086 for TAG, 0·209 for HOMA-IR, 0·363 for BMI and 0·078 for serum hs-CRP (all P < 0·001). This model showed good fit (goodness-of-fit index = 0·957 and adjusted goodness-of-fit index = 0·828).
Fig. 2.
Path model showing the mediation of the association between the Dietary Approaches to Stop Hypertension (DASH) diet score (DASH-DS) and non-alcoholic fatty liver disease (NAFLD) by serum retinol-binding protein-4 (RBP4), serum TAG, homeostasis model assessment of insulin resistance (HOMA-IR), BMI and serum high-sensitivity C-reactive protein (hs-CRP) among middle-aged and elderly adults (n 3051) aged 40–75 years at baseline, Guangzhou Nutrition and Health Study, China, 2010–2013 (goodness-of-fit index = 0·957; adjusted goodness-of-fit index = 0·828). *P < 0·05, **P < 0·01, ***P < 0·001
Discussion
An inverse association was found between the DASH-DS and the prevalence of NAFLD in a relatively large, community-based, middle-aged and elderly population. Participants who showed more adherence to the DASH diet, especially women and participants without abdominal obesity, had a lower prevalence of NAFLD. The reason why the relationship was significant only among women and those without abdominal obesity might be that female participants were more prevalent than male participants in our study and more willing to adopt a healthy diet, and participants with abdominal obesity were more prone to have NAFLD and could not easily lose weight by changing their diet. The favourable associations might be mediated by alleviating inflammation and IR, lowering TAG and losing body weight.
As shown in the path analysis, the possible potential pathophysiological mechanisms linking the DASH-DS to NAFLD are the reduction in IR, serum TAG, inflammation and fat accumulation. These variables weaken the ability of insulin to inhibit lipolysis, namely by inhibiting the release of NEFA, result in deficiency of the insulin-sensitising cytokine adiponectin and, therefore, promote synthesis of intrahepatocellular TAG(30), leading to hepatic injury, inflammation and fibrosis.
The DASH diet is known to advocate a diet low in fat and added sugars. Experiments done by Enriori et al. showed that by cutting down the fat content of a mouse’s diet, both leptin responsiveness of neuropeptide Y/agouti-related peptide and proopiomelanocortin neurons recovered synchronously, accompanied by recovery of normal leptin sensitivity and achievement of glycaemic control(31). Excess glucose, fructose and amino acids are converted to TAG in the liver via de novo lipogenesis, which increases in NAFLD(32). A significant association between sugar-sweetened soda intake and NAFLD was demonstrated by a recent meta-analysis(33). Another dormant pathophysiological mechanism explaining the association between the DASH diet and NAFLD is the counterbalance of oxidative stress and reduction of inflammation. Participants with a high DASH-DS tend to consume more fruits and vegetables, which are rich in antioxidants such as carotenoids, and have been suggested to be associated with a lower presence of NAFLD(34,35).
As for population studies, a case–control study with 102 patients diagnosed by FibroScan and 204 controls indicated that individuals in the highest quintile of DASH-DS were 30 % less likely to have NAFLD (OR = 0·70; 95 % CI 0·61, 0·80)(14). In a randomised controlled trial aimed at determining the effects of the DASH diet on losing weight and improving metabolism in sixty overweight patients with NAFLD(15), Razavi Zade et al. found that weight (P = 0·006), BMI (P = 0·01), alkaline phosphatase level (P = 0·001), alanine aminotransferase level (P = 0·02), insulin level (P = 0·01) and IR estimated by HOMA-IR (P = 0·01) decreased significantly in those who adhered to the DASH diet. The results of that study are consistent with our own, as the DASH diet was associated with decreased BMI, reduced IR and improved liver function. A meta-analysis (including twenty randomised controlled trials)(10) showed that the DASH diet can significantly reduce fasting insulin concentration (mean difference = −0·15 mIU/l; 95 % CI −0·22, −0·08 mIU/l). Additionally, evidence on the beneficial effects of the DASH diet on relieving inflammation(11), decreasing plasma lipids(36,37) and weight loss(13,15) is also abundant. As mentioned before, these factors are closely related to the occurrence and development of fatty liver.
Strengths of the present study are that the large community-based study sample afforded us sufficient power to probe relatively small effects. Abdominal ultrasonography, rather than aminotransferase level, was used for the classification of NAFLD; and it allowed careful assessment of confounding. We also validated the ultrasonography method; compared with the computed tomography method in blinded assessments, we observed high reliability (κ = 0·875) by test–retest and good validity (κ = 0·691).
Some limitations merit consideration. First, although we used a validated FFQ to measure dietary intake, recall bias and measurement error cannot be easily ruled out in our study. Second, since some of the participants with changes in metabolic features, like NAFLD or other chronic diseases, tended to have healthy lifestyles, following physician suggestions, some NAFLD patients might have achieved a high DASH-DS. This kind of tendency would lessen the observed favourable association between healthy diet and NAFLD, but we still observed an inverse association in our study. Moreover, Na intake was considered only in foods with intrinsic Na because of difficulty in assessment, which could only partially represent salt intake for the participants and might weaken the association between DASH-DS and NAFLD; but we still observed an inverse correlation between DASH-DS and NAFLD. Furthermore, despite adjusting all analyses for the known risk factors for NAFLD, there were still some unknown or unmeasured risk factors that remained unadjusted. Finally, a relatively high prevalence of NAFLD (50·7 %) was observed in the present study, although a similarly high prevalence of NAFLD (52·6 %) was found in a total of 2612 participants (1091 men and 1521 women, mean age of 53·6 years) from a community-based Chinese population in Tangshan city (north China)(38).
Conclusion
In conclusion, adherence to the DASH diet was independently associated with a marked lower prevalence of non-alcoholic fatty liver in middle-aged and elderly Chinese adults, possibly via the reduction of inflammation (RBP4 and hs-CRP), TAG, IR and BMI. Our findings support the hypothesis that the DASH diet is helpful for the prevention of NAFLD among the Chinese population. Further prospective studies are needed to verify our results.
Acknowledgements
Acknowledgements: The authors are grateful to other team staff for their contribution in the data collection and for facilitating both the recruitment of participants and the interviews. Financial support: This study was jointly supported by the National Natural Science Foundation of China (grant numbers 81472965 and 81372976) and the 5010 Program for Clinical Researches (grant number 2007032) of the Sun Yat-sen University, Guangzhou, China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflict of interest: None of the authors reported a conflict of interest related to the study. Authorship: M.-L.X. and J.-S.L. contributed equally to this article. Y.-M.C. conceived and designed the research; M.-L.X., J.-S.L., Y.-H.L., M.L. and Y.-Y.D. collected the data; M.-L.X. performed the statistical analysis; M.-L.X. wrote the paper; C.-Y.W. and Y.-M.C. revised the paper and had primary responsibility for final content. All authors read and approved the final manuscript. Ethics of human subject participation: The GNHS protocols and procedures were authorised by the Ethics Committee of the School of Public Health of Sun Yat-Sen University. Written informed consent was obtained from all participants at each visit. Trial registration: This study has been registered at http://www.clinicaltrials.gov as NCT03179657.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019002568.
click here to view supplementary material
References
- 1.Fan JG (2013) Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol 28, Suppl. 1, 11–17. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Sullivan S & Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han X, Liu C, Xue Y et al. (2016) Long-term fatty liver-induced insulin resistance in orotic acid-induced nonalcoholic fatty liver rats. Biosci Biotechnol Biochem 80, 735–743. [DOI] [PubMed] [Google Scholar]
- 4.de Wit NJ, Afman LA, Mensink M et al. (2012) Phenotyping the effect of diet on non-alcoholic fatty liver disease. J Hepatol 57, 1370–1373. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Rodas MC, Valenzuela R & Videla LA (2015) Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int J Mol Sci 16, 25168–25198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM et al. (2001) Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344, 3–10. [DOI] [PubMed] [Google Scholar]
- 7.Parikh A, Lipsitz SR & Natarajan S (2009) Association between a DASH-like diet and mortality in adults with hypertension: findings from a population-based follow-up study. Am J Hypertens 22, 409–416. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Fox CS, Jacques PF et al. (2015) Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol 63, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huh JH, Lee KJ, Lim JS et al. (2015) High dietary sodium intake assessed by estimated 24-h urinary sodium excretion is associated with NAFLD and hepatic fibrosis. PLoS One 10, e0143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirani F, Salehi-Abargouei A & Azadbakht L (2013) Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 29, 939–947. [DOI] [PubMed] [Google Scholar]
- 11.Soltani S, Chitsazi MJ & Salehi-Abargouei A (2018) The effect of Dietary Approaches to Stop Hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr 37, 542–550. [DOI] [PubMed] [Google Scholar]
- 12.Asemi Z & Esmaillzadeh A (2015) DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res 47, 232–238. [DOI] [PubMed] [Google Scholar]
- 13.Shenoy SF, Poston WS, Reeves RS et al. (2010) Weight loss in individuals with metabolic syndrome given DASH diet counseling when provided a low sodium vegetable juice: a randomized controlled trial. Nutr J 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hekmatdoost A, Shamsipour A, Meibodi M et al. (2016) Adherence to the Dietary Approaches to Stop Hypertension (DASH) and risk of nonalcoholic fatty liver disease. Int J Food Sci Nutr 67, 1024–1029. [DOI] [PubMed] [Google Scholar]
- 15.Razavi Zade M, Telkabadi MH, Bahmani F et al. (2016) The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int 36, 563–571. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Shi WQ, Cao Y et al. (2014) Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br J Nutr 112, 2041–2048. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Lin X-L, Fan Y-Y et al. (2016) Diet quality scores and risk of nasopharyngeal carcinoma in Chinese adults: a case–control study. Nutrients 8, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang CX & Ho SC (2009) Validity and reproducibility of a food frequency questionnaire among Chinese women in Guangdong province. Asia Pac J Clin Nutr 18, 240–250. [PubMed] [Google Scholar]
- 19.Yang YX, Wang GY & Pan XC (2002) China Food Composition Table. Beijing: Peking University Medical Press. [Google Scholar]
- 20.Fung TT, Chiuve SE, McCullough ML et al. (2008) Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 168, 713–720. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Howe GR & Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65, 1220S–1228S; discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 22.Zeng MD, Fan JG, Lu LG et al. (2008) Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis 9, 108–112. [DOI] [PubMed] [Google Scholar]
- 23.Balagopal P, Graham TE, Kahn BB et al. (2007) Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 92, 1971–1974. [DOI] [PubMed] [Google Scholar]
- 24.Yao-Borengasser A, Varma V, Bodles AM et al. (2007) Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 92, 2590–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiura M, Nakamura M, Ikoma Y et al. (2006) The homeostasis model assessment-insulin resistance index is inversely associated with serum carotenoids in non-diabetic subjects. J Epidemiol 16, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attard SM, Howard AG, Herring AH et al. (2015) Differential associations of urbanicity and income with physical activity in adults in urbanizing China: findings from the population-based China Health and Nutrition Survey 1991–2009. Int J Behav Nutr Phys Act 12, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Herrmann SD et al. (2011) 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43, 1575–1581. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Zhai F, Ma G et al. (2009) Abdominal obesity and the prevalence of diabetes and intermediate hyperglycaemia in Chinese adults. Public Health Nutr 12, 1078–1084. [DOI] [PubMed] [Google Scholar]
- 29.Straus J, Chang H & Hong C-Y (2016) An exploratory path analysis of attitudes, behaviors and summer water consumption in the Portland Metropolitan Area. Sustain Cities Soc 23, 68–77. [Google Scholar]
- 30.Yki-Jarvinen H (2014) Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2, 901–910. [DOI] [PubMed] [Google Scholar]
- 31.Enriori PJ, Evans AE, Sinnayah P et al. (2007) Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5, 181–194. [DOI] [PubMed] [Google Scholar]
- 32.Lambert JE, Ramos-Roman MA, Browning JD et al. (2014) Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijarnpreecha K, Thongprayoon C, Edmonds PJ et al. (2016) Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: a systematic review and meta-analysis. QJM 109, 461–466. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz B, Sahin K, Bilen H et al. (2015) Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr 4, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, Wang C, Liu J et al. (2015) Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci Rep 5, 12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asemi Z, Samimi M, Tabassi Z et al. (2014) Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized clinical trial. Nutrition 30, 1287–1293. [DOI] [PubMed] [Google Scholar]
- 37.Chiu S, Bergeron N, Williams PT et al. (2016) Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr 103, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y-C, Zhou Y, Gao X et al. (2018) Association between nonalcoholic fatty liver disease and carotid artery disease in a community-based Chinese population: a cross-sectional study. Chin Med J 131, 2269–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019002568.
click here to view supplementary material