Abstract
Objective:
High sugar-sweetened beverage (SSB) intake has been shown to correlate with a higher risk for CVD and metabolic disorders, while the association between SSB intake and the risk of metabolic syndrome (MetS) remains unclear. The present study aimed to explore the association between SSB intake and MetS among children and adolescents in urban China.
Design:
A cross-sectional study involving 7143 children and adolescents was conducted in urban China. MetS definition proposed by the International Diabetes Federation was adopted. Data on SSB intake, diet, physical activity and family environment factors were obtained through questionnaires. Logistic regression models with multivariable adjustment were adopted to analyse the association between SSB intake and the risk of MetS and its components.
Setting:
Primary and secondary schools in three urban cities of China.
Participants:
Children and adolescents (n 5258) aged 7–18 years.
Results:
Among the participants, 29·9 % of them had high SSB intake (at least 0·3 servings/d) and the overall MetS prevalence was 2·7 %. Participants with high SSB intake were at higher risk for MetS (OR = 1·60; 95 % CI 1·03, 2·54) and abdominal obesity (OR = 1·55; 95 % CI 1·28, 1·83) compared with their counterparts with no SSB intake (0 servings/d).
Conclusions:
High SSB intake is significantly associated with increased MetS and abdominal obesity risk among children and adolescents in urban China. These results suggest that strong policies focusing on controlling SSB intake might be effective in preventing MetS and abdominal obesity.
Keywords: Sugar-sweetened beverages, Metabolic syndrome, Abdominal obesity, Children and adolescents
Sugar-sweetened beverages (SSB) are defined as a wide variety of beverages sweetened with various forms of added sugars(1). These beverages include, but are not limited to, energy drinks, sports drinks, soda drinks, fruit drinks with added sugar, sweetened tea and coffee drinks, which are the main sources of dietary sugar(2,3). Although SSB intake has decreased slightly in the USA and Europe in recent years, it still remains a concern among US children and adolescents who consume a mean of 649 (sd 29) kJ/d (155 (sd 7) kcal/d) from SSB (equal to 350 ml of cola)(4). Although SSB intake is significantly lower in East Asia than in the USA and Europe(5), SSB intake among children and adolescents in China also remains a public health concern. Around one-fifth of Chinese children and adolescents aged 7–18 years consume approximately 120 ml SSB/d and the mean per capita intake is 90·45 (sd 3·55) ml/d(6). With the rapid income growth in China, SSB are becoming more affordable than ever(7,8), which may contribute to the increasing SSB intake in the Chinese population(9).
Metabolic syndrome (MetS), a cluster of metabolic disorders including abdominal obesity, low HDL-cholesterol (HDL-C), hypertriacylglycerolaemia, elevated fasting glucose (FG) and high blood pressure(10), has been recognized as a predictor of type 2 diabetes mellitus and CVD. Although a number of studies indicate that high SSB intake is a potential risk factor for CVD and metabolic disorders (hypertension, dyslipidaemia, CHD(11,12) and type 2 diabetes mellitus(13)), the association is still under debate due to the heterogeneity of research findings. Some cross-sectional studies suggest that both children and adults with the highest SSB intake are at increased risk for MetS(14,15), compared with the lowest SSB intake. However, some researchers claim that no association is found among middle-aged adults(16). Considering the relatively high SSB intake among children and adolescents, it is necessary to explore the association in a child-based population.
On the other hand, both SSB intake and MetS are affected by demographic characteristics and multiple lifestyle factors(17–19). Previous studies have found that SSB intake is positively associated with MetS risk in Korean adult females(20) and Taiwanese teenage males(15), suggesting that the association between SSB intake and MetS might differ by gender and age. Besides, dietary habits help to aggravate or alleviate the risk of MetS development(21,22). Physical activity and sedentary behaviour may also play a role in developing MetS and affecting SSB intake(23,24). In addition, family environment factors, such as parental attitudes towards SSB and the availability of SSB at home, are also likely to affect SSB intake among children and adolescents(25). Although a few research findings note that lifestyle factors mediate the association, analyses are seldom carried out in a lifestyle-adjusted model.
We conducted a cross-sectional study among children and adolescents in eastern, southern and northern urban cities of China, from September 2013 to June 2014. The current study aimed to investigate: (i) the up-to-date prevalence of MetS; (ii) the exact amount and distribution of SSB intake; and (iii) the association between SSB intake and MetS in multivariable-adjusted models.
Methods
Sample size calculation
The present cross-sectional study aimed to explore the association between SSB intake and MetS. According to previous studies, the prevalence of MetS among children and adolescents in China was 2·4–4·1 %(26–28). For logistic regression analysis, the minimum number of cases to be included was calculated as n = 10k/p(29), where k is the number of covariates (estimated to be 10) and p is the smallest estimation of MetS prevalence in the population (estimated to be 2·0 %). The calculated sample size was approximately 5000. To take a 20 % non-response rate into account, the final sample size increased to 6000.
Sampling and data sources overview
The school-based cross-sectional study was conducted among children and adolescents between September 2013 and June 2014, in three large and wealthy cities located in eastern, southern and northern China. A multistage stratified cluster sampling was carried out. We randomly selected several regions from three urban cities in the first stage. In the second stage, eight to twelve schools (primary:secondary = 1:1) from each region were randomly selected. Among the selected schools, two classes of each grade were randomly selected. All students in the selected class were recruited. In total, 7143 children and adolescents aged 7–18 years, who agreed to participate and completed the blood sample collection, were included in data analysis. Participants were excluded if they lacked data on anthropometric measurements (n 336) or questionnaire assessments (dietary intake, n 666; physical activity, n 418; information about their parents, n 465). Finally, the sample size dropped to 5258 (49·0 % boys). Informed consent was obtained from both parents and children, and this study was approved by the Ethical Committee of School of Public Health, Sun Yat-sen University.
Anthropometric measurements
Anthropometric measurements, including height, weight, waist circumference (WC) and blood pressure, were performed by trained nurses and practitioners. All the measurements were conducted in the school where participants studied. A metal column height-measuring stand (precision: 0·1 cm; model TZG, China) was used for measuring height. Children and adolescents stood erect with back, buttocks and heels in continuous contact with the vertical height rod of the stadiometer and head oriented in the Frankfurt plane. The horizontal headpiece was placed on top of the child’s head in order to measure height. When weight was measured, children and adolescents wearing only underwear stood on a lever scale (precision: 0·1 kg; model RGT-140, China). BMI was calculated as weight/height2 (kg/m2). WC was measured to an accuracy of 0·1 cm at 1 cm above the umbilicus using a steel tape, when participants were in the standing position. Blood pressure was measured on the right arm using a validated mercury sphygmomanometer (model XJ1ID, China) after participants were seated comfortably for at least 5 min. All measurements were performed twice and the average numbers were recorded.
Questionnaire survey
A standardized questionnaire was designed to collect demographic data (examination date, birth date, gender and parental education level), physical activity and lifestyle (weekly hours of intensive and moderate physical activity, walking and sedentary behaviour) and dietary intake (daily intake of fruits, vegetables, SSB, meat, dairy products, high-energy foods and fried foods). The questionnaire was developed based on the information, motivation and behavioural skills model, which was piloted and revised in the early stages of the project to be feasible for children and teachers. The language of the questionnaire was checked by teachers to ensure that each question could be easily understood by typically developed children above 9 years old. Questionnaires were distributed by teachers in class during the week when anthropometric measurements were performed. Students were instructed to answer the questionnaire immediately if they were in the third grade or above 9 years old. Trained project members interpreted all the questions in detail; in the meantime, trained teachers in each class helped students to understand the questions correctly. The children below third grade (7–8 years old) were instructed to take the questionnaire to their caregiver. A message with questionnaire instructions was sent to the caregiver’s mobile phone with a request to answer the questionnaire and bring it back to school within one week. When all questionnaires were retrieved, the researchers collected them from each class and performed quality control. Questionnaires with five or more answers missing were returned to the children or their caregivers to refill. In addition, questionnaires were repeated within a week for the same participant. Questionnaire items were as follows.
Daily food intake
In the present study, due to the large sample size and limited time for fieldwork, it was difficult to conduct a face-to-face FFQ or 3 d dietary recall/record survey. Thus, a simplified self-reported dietary questionnaire was adopted, by which information about the dietary intake of seven food groups was collected via seven questions, including fruits, vegetables, meat, SSB, dairy products, high-energy foods and fried foods. Hence, this dietary questionnaire cannot estimate the nutrient or energy intake from each food exactly.
Two repeated measurements with the same dietary questionnaire and a 3 d dietary record were used to evaluate the reliability and validity of the dietary questionnaire before the present study commenced. The variables of the dietary questionnaire, including intake and frequency of fruits, vegetables, meat, SSB, dairy products, high-energy foods and fried foods, were used to calculate intraclass correlation coefficients for reliability and Spearman’s correlation coefficients for validity. According to a sample size calculation, 298 pupils (aged 6–12 years) were recruited from a primary school to complete the evaluation. Two repeated measurements with the same dietary questionnaire were conducted with a 7 d interval and the intraclass correlation coefficient was calculated to evaluate reliability, which was from 0·443 to 0·589 (P < 0·05). The dietary questionnaire was also compared with the 3 d dietary record and the Spearman’s correlation coefficient was calculated to evaluate validity, which was from 0·344 to 0·485 (P < 0·05). The dietary questionnaire contents are listed below.
Fruit intake was assessed by the question, ‘How many days did you eat fruit in the past 7 days? How many servings of fruit did you eat per day during the days you ate fruit? One serving of fruit is equal to the size of an adult’s fist (if you are not certain about the size of an adult’s fist, refer to the pictures at the end of the questionnaire)’. Fruits mean the fleshy seed-associated structures of a plant that are sweet or sour and edible in the raw state, including apples, pears, bananas, oranges and so on.
Vegetable intake was assessed by the question, ‘How many days did you eat vegetables in the past 7 days? How many servings of vegetables did you eat per day during the days you ate vegetables? One serving of vegetables is equal to the size of an adult’s fist (if you are not certain about the size of an adult’s fist, refer to the pictures at the end of the questionnaire)’. Vegetables are parts of plants that are consumed by humans as food as part of a meal, including eggplants, tomatoes, carrots, onions, cabbages and so on.
Meat intake was assessed by the question, ‘How many days did you eat meat in the past 7 days? How many servings of meat did you eat per day during the days you ate meat? One serving of meat is equal to the size of an adult’s palm (if you are not certain of the size of an adult’s palm, refer to the pictures at the end of the questionnaire)’. Meats include the flesh of animals (e.g. pigs, cattle, lambs, chicken, duck, fish) and meat products (e.g. bacon and sausages) eaten as food.
SSB intake was assessed by the question, ‘How many days did you drink sugar-sweetened beverages in the past 7 days? How many servings of sugar-sweetened beverages did you drink per day during the days you drank them? One serving of sugar-sweetened beverages is 250 ml’. SSB include energy drinks (e.g. Red Bull®), milk-containing drinks, soda (e.g. Coca-Cola®), fruit drinks with added sugar, and other sugar-added beverages.
Intakes of dairy products, high-energy foods and fried foods were assessed by the question, ‘How many days did you eat dairy products/high-energy foods/fried foods in the past 7 days, respectively?’ Dairy products include milk, yoghurt, milk powder and other dairy products. High-energy foods include cake, chocolate, candy, crisps and other foods with high energy density. Fried foods include fried chicken, fried dough sticks, fried chips, and other foods directly cooked in hot fat or oil.
Daily physical activity and sedentary behaviour
Validity and reliability of the physical activity questionnaire were evaluated before the study commenced. The variables of the physical activity questionnaire, including the frequency of intensive physical activity, moderate physical activity, walking and sedentary behaviour, were used to calculate the intraclass correlation coefficient for reliability and the Spearman’s correlation coefficient for validity. The intraclass correlation coefficients between two repeated measurements with the same questionnaire were from 0·344 to 0·485 (P < 0·05). The Spearman’s correlation coefficients between the physical activity questionnaire and a 3 d physical activity log were from 0·341 to 0·545 (P < 0·05). Questionnaire items were as follows.
Intensive physical activity was assessed by the question, ‘How many hours do you perform intensive physical activity per day? Intensive physical activity, including basketball, football, carrying a heavy load and so on, causes people to be out of breath, perspire and experience extreme exhaustion (requiring 6 metabolic equivalents or more)’.
Moderate physical activity was assessed by the question, ‘How many hours do you perform moderate physical activity per day? Moderate physical activity, including bicycling, playing table tennis, badminton and so on, but not walking, causes people to mildly perspire and experience slight exhaustion (requiring 3 to <6 metabolic equivalents)’.
Walking was assessed by the question, ‘How many hours do you spend walking per day? Walking includes walking at home or school, commuting between school and home, and walking for exercise’.
Sedentary behaviour was assessed by the question, ‘How many hours do you usually spend sitting or lying still at school and home (excluding sleeping) per day?’
Information about family environment factors
In the present study, family environment factors included parental education level, parental attitudes towards SSB and parental SSB intake. Parental education level was categorized into three groups: primary school or below; middle school or high school; and junior college or above. Parental attitudes to children drinking SSB were assessed by the question: ‘Would you purchase sugar-sweetened beverages if your child wants to drink them? Have you ever told your child about the harm of drinking sugar-sweetened beverages?’ The answers were divided into three groups: never, sometimes and always. Parental SSB intake was determined by asking the question: ‘How many days did you drink sugar-sweetened beverages in the past 7 days? How many servings of sugar-sweetened beverages did you drink per day during the days you drank them? One serving of sugar-sweetened beverages is 250 ml’.
Venous blood collection and biochemical analyses
After a 12 h overnight fast, venous blood samples were collected by drawing from the antecubital vein into EDTA vacuum tubes. The samples were centrifuged at 3000 rpm, aliquoted and stored at −80°C until analysed. Biochemical variables included FG, TAG and HDL-C. A validated biomedical analysis laboratory performed all biochemical analyses of the blood samples. FG was assayed using the glucose oxidase method; TAG was analysed with the enzymatic method; and HDL-C was analysed using the clearance method.
Definition and components of metabolic syndrome
In the current study, MetS was defined according to the International Diabetes Federation criteria which were adapted for children and adolescents(10). The MetS definition divided children and adolescents into the three age groups: <10 years, ≥10–<16 years and ≥16 years old. For children and adolescents aged ≥16 years, the diagnosis of MetS required the presence of abdominal obesity (WC ≥ 90 cm in males and WC ≥ 80 cm in females for Chinese(10)) plus the presence of two or more of the following factors: (i) hypertriacylglycerolaemia (TAG ≥ 1·7 mmol/l); (ii) low HDL-C (HDL-C < 1·03 mmol/l for males and HDL-C < 1·29 mmol/l for females); (iii) elevated FG (FG ≥ 5·6 mmol/l); (iv) high blood pressure (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥85 mmHg). This is also the definition for adults(30). A modified version of the above definition was applied to those aged ≥10–<16 years (WC ≥ 90th percentile cut-off point for Chinese children and adolescents(31); HDL-C < 1·03 mmol/l for both genders). It is suggested by the International Diabetes Federation that MetS cannot be diagnosed among children below the age of 10 years. In the present study, the components were also measured by the International Diabetes Federation criteria described above, hoping to ascertain the epidemic of paediatric MetS and predict the risk of metabolic disorders.
Statistical analysis
Data were inputted using Epidata version 3.0 software (The EpiData Association, Odense, Denmark) and analysed using the statistical software package IBM SPSS Statistics version 21. SSB intake was classified according to tertile cut-off points (non-SSB drinker, 0 servings/d; medium intake, 0–0·3 servings/d; high intake, >0·3 servings/d). Results are shown as means with their standard errors or as medians and interquartile ranges for quantitative variables; and as numbers and percentages for categorical variables. The t test or one-way ANOVA was applied for quantitative variables if normality and homogeneity of variance assumptions were satisfied; otherwise a non-parametric test (the Mann–Whitney U test or Kruskal–Wallis test) was used. Meanwhile, the χ2 test was applied for categorical variables when differences in characteristics between boys and girls or among the three groups of SSB intake were evaluated. The Bonferroni test was used to examine pairwise comparisons. To determine the prevalence of MetS and its components associated with SSB intake, multivariate logistic regression was used in four models. The odds ratios and the corresponding 95 % confidence intervals were presented. Model 1 was an unadjusted model; Model 2 was adjusted for gender and age; Model 3 was additionally adjusted for parental educational level, their attitudes towards SSB and their SSB intake; Model 4 was additionally adjusted for dietary intake (intake and frequency of fruits, vegetables, meat, dairy products, high-energy foods and fried foods) and physical activity (frequency of intensive physical activity, moderate physical activity and walking). P values less than 0·05 were considered statistically significant.
Results
Basic characteristics of participants
The basic characteristics of 5258 participants by gender (49·0 % boys) are presented in Table 1. The mean SSB intake among all participants was 97·27 (se 2·33) ml/d. The overall prevalence of MetS was 2·7 % among children and adolescents aged 7–18 years (3·3 % in boys and 2·1 % in girls). SSB intake and MetS prevalence were significantly higher in boys than in girls (both P < 0·01). All the anthropometric characteristics were also higher in boys (all P < 0·001). HDL-C and TAG levels, sedentary behaviour and high-energy food intake were lower in boys, while FG level, meat and fried food intakes, as well as the frequency of intensive and moderate physical activity, were higher in boys, compared with girls (all P < 0·05). For parental education level and parental attitudes towards SSB, no gender difference was found.
Table 1.
Basic characteristics of the children and adolescents aged 7–18 years (n 5258) from three cities in urban China, September 2013–June 2014
| Total (n 5258) | Boys (n 2578) | Girls (n 2680) | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | se | Mean | se | Mean | se | ||
| Age (years) | 11·45 | 0·05 | 11·38 | 0·07 | 11·52 | 0·06 | 0·119 |
| SSB intake | |||||||
| Participants (ml/d) | 97·27 | 2·33 | 122·79 | 3·87 | 72·72 | 2·27 | <0·001† |
| Parents (ml/d) | 47·28 | 1·40 | 51·43 | 2·19 | 43·29 | 1·76 | <0·001† |
| Anthropometry | |||||||
| Height (cm) | 149·71 | 0·23 | 151·57 | 0·37 | 147·92 | 0·28 | <0·001‡ |
| Weight (kg) | 44·30 | 0·23 | 46·62 | 0·37 | 42·07 | 0·28 | <0·001‡ |
| BMI (kg/m2) | 19·05 | 0·06 | 19·48 | 0·09 | 18·64 | 0·07 | <0·001‡ |
| WC (cm) | 67·20 | 0·16 | 68·75 | 0·25 | 65·71 | 0·19 | <0·001‡ |
| SBP (mmHg) | 103·94 | 0·17 | 105·80 | 0·25 | 102·15 | 0·23 | <0·001‡ |
| DBP (mmHg) | 65·55 | 0·12 | 66·23 | 0·17 | 64·89 | 0·16 | <0·001‡ |
| Biochemistry | |||||||
| TAG (mmol/l) | 0·91 | 0·01 | 0·88 | 0·01 | 0·94 | 0·01 | <0·001‡ |
| HDL-C (mmol/l) | 1·36 | 0·00 | 1·35 | 0·01 | 1·38 | 0·01 | <0·001‡ |
| FG (mmol/l) | 4·82 | 0·01 | 4·89 | 0·01 | 4·75 | 0·01 | <0·001‡ |
| n | % | n | % | n | % | ||
| MetS prevalence | 142 | 2·7 | 85 | 3·3 | 57 | 2·1 | 0·009§ |
| Parental attitudes | 0·116 | ||||||
| Always | 637 | 12·1 | 334 | 13·0 | 303 | 11·3 | 0·067 |
| Sometimes | 2464 | 46·9 | 1213 | 47·1 | 1251 | 46·7 | 0·786 |
| Never | 2157 | 41·0 | 1031 | 40·0 | 1126 | 42·0 | 0·136 |
| Paternal education level | 0·890 | ||||||
| Primary school or below | 230 | 4·4 | 114 | 4·4 | 116 | 4·3 | 0·868 |
| Middle school or high school | 3100 | 59·0 | 1527 | 59·2 | 1537 | 58·7 | 0·692 |
| Junior college or above | 1928 | 36·7 | 937 | 36·3 | 991 | 37·0 | 0·635 |
| Maternal education level | 0·222 | ||||||
| Primary school or below | 386 | 7·3 | 204 | 7·9 | 182 | 6·8 | 0·119 |
| Middle school or High school | 2990 | 56·9 | 1444 | 56·0 | 1546 | 57·7 | 0·220 |
| Junior college or above | 1882 | 35·8 | 930 | 36·1 | 952 | 35·5 | 0·676 |
| Median | IQR | Median | IQR | Median | IQR | ||
| Dietary intake | |||||||
| Fruits (servings/d) | 1·0 | 0·7–2·0 | 1·0 | 0·7–2·0 | 1·0 | 0·7–2·0 | 0·133 |
| Vegetables (servings/d) | 2·0 | 1·0–2·0 | 2·0 | 1·0–2·0 | 2·0 | 1·0–2·0 | 0·701 |
| Meat (servings/d) | 1·0 | 0·6–1·7 | 1·0 | 0·6–1·7 | 1·0 | 0·5–1·1 | <0·001† |
| Dairy products (d/week) | 5·0 | 3·0–5·0 | 5·0 | 3·0–7·0 | 5·0 | 2·0–7·0 | 0·091 |
| High-energy foods (d/week) | 2·0 | 1·0–3·0 | 2·0 | 1·0–3·0 | 2·0 | 1·0–3·0 | <0·004† |
| Fried foods (d/week) | 1·0 | 0·0–2·0 | 1·0 | 0·0–2·0 | 1·0 | 0·0–2·0 | 0·007† |
| Physical activity | |||||||
| Intensive physical activity (h/week) | 2·0 | 0·5–4·2 | 2·3 | 0·9–5·0 | 1·5 | 0·2–3·5 | <0·001† |
| Moderate physical activity (h/week) | 2·0 | 0·8–4·0 | 2·0 | 0·8–4·7 | 2·0 | 0·7–3·5 | <0·001† |
| Walking (h/week) | 3·5 | 1·3–7·0 | 3·5 | 1·5–7·0 | 3·5 | 1·3–7·0 | 0·064 |
| Sedentary behaviour (h/d) | 6·0 | 2·3–9·0 | 6·0 | 2·0–9·0 | 6·2 | 2·5–9·0 | 0·037† |
SSB, sugar-sweetened beverage; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, HDL-cholesterol; FG, fasting glucose; MetS, metabolic syndrome; IQR, interquartile range.
P < 0·05, assessed by the Mann–Whitney U test.
P < 0·05, assessed by the t test.
P < 0·05, assessed by the χ2 test.
Participant characteristics according to sugar-sweetened beverage intake
Relative to non-SSB drinkers, those who had high SSB intake had higher height, weight, BMI, WC, systolic and diastolic blood pressure, TAG and FG, but lower HDL-C (Table 2). In the meantime, they were more likely to develop abdominal obesity and elevated FG (all P < 0·05). As for lifestyle factors, the high intake group consumed less vegetables, but more meat, high-energy foods and fried foods (all P < 0·001), however they had more intensive or moderate activity as well as walking time (all P < 0·05), relative to non-SSB drinkers. For the family environment factors, the high intake group had greater parental SSB intake and more positive attitudes towards SSB, but lower parental education level (all P < 0·05).
Table 2.
Participant characteristics, according to sugar-sweetened beverage (SSB) intake, of the children and adolescents aged 7–18 years (n 5258) from three cities in urban China, September 2013–June 2014
| Non-SSB drinker (0 servings/d) (n 1756) | Medium intake (0–0·3 servings/d) (n 1931) | High intake (>0·3 servings/d) (n 1571) | P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | se | Mean | se | Mean | se | ||
| Anthropometry and biochemistry | |||||||
| Height (cm) | 146·07 | 0·38 | 147·23* | 0·37 | 156·82*** | 0·41 | <0·001† |
| Weight (kg) | 40·86 | 0·37 | 41·90* | 0·36 | 51·10*** | 0·44 | <0·001† |
| BMI (kg/m2) | 18·46 | 0·09 | 18·67 | 0·09 | 20·18*** | 0·10 | <0·001† |
| WC (cm) | 65·17 | 0·26 | 66·04* | 0·25 | 70·89*** | 0·30 | <0·001† |
| SBP (mmHg) | 102·51 | 0·29 | 103·16 | 0·28 | 106·50*** | 0·32 | <0·001† |
| DBP (mmHg) | 64·73 | 0·21 | 65·13 | 0·19 | 66·98*** | 0·22 | <0·001† |
| TAG (mmol/l) | 0·89 | 0·01 | 0·90 | 0·01 | 0·95** | 0·01 | 0·002† |
| HDL-C (mmol/l) | 1·37 | 0·01 | 1·38 | 0·01 | 1·33*** | 0·01 | <0·001† |
| FG (mmol/l) | 4·78 | 0·01 | 4·83** | 0·01 | 4·86*** | 0·01 | <0·001† |
| n | % | n | % | n | % | ||
| MetS components | |||||||
| Abdominal obesity | 395 | 22·5 | 444 | 23·0 | 432 | 27·5** | 0·001‡ |
| Low HDL-C | 273 | 15·5 | 291 | 15·1 | 275 | 17·5 | 0·125 |
| Hypertriacylglycerolaemia | 92 | 5·2 | 104 | 5·4 | 101 | 6·4 | 0·273 |
| Elevated FG | 50 | 2·8 | 69 | 3·6 | 72 | 4·6** | 0·028‡ |
| High blood pressure | 53 | 3·0 | 46 | 2·4 | 66 | 4·2 | 0·008‡ |
| Parental attitudes | <0·001‡ | ||||||
| Always | 137 | 7·8 | 191 | 9·9* | 309 | 19·7*** | <0·001‡ |
| Sometimes | 692 | 39·4 | 977 | 50·6*** | 795 | 50·6*** | <0·001‡ |
| Never | 927 | 52·8 | 763 | 39·5*** | 467 | 29·7*** | <0·001‡ |
| Paternal education level | <0·001‡ | ||||||
| Primary school or below | 82 | 4·7 | 90 | 4·7 | 57 | 3·6 | 0·224 |
| Middle school or high school | 954 | 54·3 | 1148 | 59·4** | 998 | 63·5*** | <0·001‡ |
| Junior college or above | 719 | 40·9 | 493 | 35·9** | 516 | 32·8*** | <0·001‡ |
| Maternal education level | 0·017‡ | ||||||
| Primary school or below | 124 | 7·1 | 164 | 8·5 | 98 | 6·2 | 0·034‡ |
| Middle school or high school | 965 | 55·0 | 1101 | 57·0 | 924 | 58·8* | 0·079 |
| Junior college or above | 667 | 38·0 | 666 | 34·5* | 549 | 34·9 | 0·061 |
| Median | IQR | Median | IQR | Median | IQR | ||
| Dietary intake | |||||||
| Fruits (servings/d) | 1·0 | 0.7–2·0 | 1·0 | 0·7–2·0 | 1·0 | 0·7–2·0 | 0·996 |
| Vegetables (servings/d) | 2·0 | 1·0–2·0 | 1·5 | 1·0–2·0*** | 1·7 | 1·0–2·0** | <0·001§ |
| Meat (servings/d) | 1·0 | 0·5–1·4 | 1·0 | 0·4–1·1* | 1·0 | 0·9–2·0*** | <0·001§ |
| Dairy products (d/week) | 6·0 | 3·0–7·0 | 5·0 | 2·8–7·0* | 5·0 | 2·0–7·0 | 0·049§ |
| High-energy foods (d/week) | 1·0 | 0·0–2·0 | 2·0 | 1·0–3·0*** | 2·0 | 1·0–4·0*** | <0·001§ |
| Fried foods (d/week) | 0·0 | 0·0–1·0 | 1·0 | 0·0–2·0*** | 1·0 | 0·0–3·0*** | <0·001§ |
| Physical activity | |||||||
| Intensive physical activity (h/week) | 1·5 | 0·2–4·0 | 2·0 | 0·5–4·0** | 2·3 | 0·7–5·0*** | <0·001§ |
| Moderate physical activity (h/week) | 2·0 | 0·5–4·0 | 2·0 | 0·8–4·2* | 2·0 | 1·0–4·5** | 0·006§ |
| Walking (h/week) | 3·5 | 1·2–7·0 | 3·5 | 1·5–7·0** | 3·5 | 1·5–7·0* | 0·019§ |
| Sedentary behaviour (h/d) | 6·0 | 2·0–8·7 | 6·0 | 2·0–8·5 | 7·0 | 3·0–9·0*** | <0·001§ |
WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, HDL-cholesterol; FG, fasting glucose; MetS, metabolic syndrome; IQR, interquartile range
*P < 0·05, **P < 0·01, ***P < 0·001, non-SSB drinker v. medium intake or non-SSB drinker v. high intake.
P < 0·05, assessed by one-way ANOVA.
P < 0·05, assessed by the χ2 test.
P < 0·05, assessed by the Kruskal–Wallis test.
Prevalence of metabolic syndrome and its components according to sugar-sweetened beverage intake
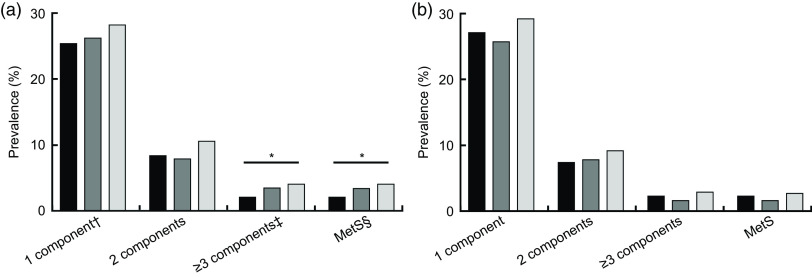
The prevalence of MetS and its components stratified by SSB intake is shown in Table 3. Increasing SSB intake was significantly associated with increasing number of MetS components (Ptrend = 0·006). The prevalence of MetS in non-SSB drinker, medium intake and high intake groups was 0·7, 0·9 and 1·1 %, respectively. Compared with non-SSB drinkers, the MetS prevalence was higher in those who consumed a high amount of SSB (P = 0·038), while no significant association was found after stratification by age. With increasing intake of SSB, the prevalence of MetS and of three or more components of MetS was higher in boys (Ptrend = 0·019 and 0·020, respectively; Fig. 1), while no similar trend was found in girls.
Table 3.
Prevalence of metabolic syndrome (MetS) and number of its components stratified by sugar-sweetened beverage (SSB) intake among children and adolescents aged 7–18 years (n 5258) from three cities in urban China, September 2013–June 2014
| Non-SSB drinker (0 servings/d) (n 1756) | Medium intake (0–0·3 servings/d) (n 1931) | High intake (>0·3 servings/d) (n 1571) | P value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 0 component | 1117 | 21·2 | 1231 | 23·4 | 907 | 18·8 | 0·006† |
| 1 component | 463 | 8·8 | 501 | 9·5 | 449 | 8·5 | |
| 2 components | 137 | 2·6 | 151 | 2·9 | 158 | 3·0 | |
| 3 components | 31 | 0·6 | 42 | 0·8 | 47 | 0·9 | |
| 4 components | 7 | 0·1 | 5 | 0·1 | 10 | 0·2 | |
| 5 components | 1 | 0·0 | 1 | 0·0 | 0 | 0·0 | |
| MetS | |||||||
| Age <10 years | 10 | 0·5 | 8 | 0·4 | 5 | 0·3 | 0·749 |
| Age ≥10–<16 years | 21 | 0·9 | 30 | 1·2 | 34 | 1·4 | 0·526 |
| Age ≥16 years | 8 | 0·9 | 9 | 1·1 | 17 | 2·0 | 0·655 |
| Total | 39 | 0·7 | 47 | 0·9 | 56 | 1·1* | 0·038† |
P < 0·05, non-SSB drinker v. high intake, assessed by the χ2 test.
P < 0·05 among the three groups of SSB intake, assessed by the χ2 test.
Fig. 1.
Prevalence of metabolic syndrome (MetS) and different combinations of MetS components, according to gender (a, boys; b, girls) and sugar-sweetened beverage (SSB) intake ( , non-SSB drinker;
, non-SSB drinker;  , medium intake;
, medium intake;  , high intake), among children and adolescents aged 7–18 years (n 5258) from three cities in urban China, September 2013–June 2014. *Ptrend < 0·05. †Components mean the five factors included in the definition of MetS. ‡Combinations of MetS components: ‘1 component’ means the presence of only one MetS component; ‘2 components’ means the presence of any two of the five MetS components; ‘≥3 components’ means the presence of any three or more of the five MetS components. §MetS: the presence of abdominal obesity plus two or more other components (abdominal obesity is the precondition for diagnosing with MetS)
, high intake), among children and adolescents aged 7–18 years (n 5258) from three cities in urban China, September 2013–June 2014. *Ptrend < 0·05. †Components mean the five factors included in the definition of MetS. ‡Combinations of MetS components: ‘1 component’ means the presence of only one MetS component; ‘2 components’ means the presence of any two of the five MetS components; ‘≥3 components’ means the presence of any three or more of the five MetS components. §MetS: the presence of abdominal obesity plus two or more other components (abdominal obesity is the precondition for diagnosing with MetS)
Association of sugar-sweetened beverage intake with metabolic syndrome and its components
The unadjusted OR for abdominal obesity in the high intake group compared with non-SSB drinkers was 1·31 (95 % CI 1·12, 1·53; P = 0·001). After adjusting for age, gender, dietary intake (intake and frequency of fruits, vegetables, meat, dairy products, high-energy foods and fried foods) and physical activity (frequency of intensive physical activity, moderate physical activity and walking), as well as the family environment factors (parental educational level, their attitudes towards SSB and their SSB intake), the OR for abdominal obesity in those who consumed a high amount of SSB was 1·55 (95 % CI 1·28, 1·83; P < 0·001), compared with non-SSB drinkers. The unadjusted OR and adjusted OR for MetS in the high intake group were 1·63 (95 % CI 1·08, 2·46; P = 0·021) and 1·60 (95 % CI 1·03, 2·54; P = 0·049), relative to non-SSB drinkers, respectively (Table 4). As intake of SSB increased, the crude OR and the adjusted OR of MetS and abdominal obesity increased (all Ptrend < 0·05). No significant association between other MetS components and the three SSB intake groups was found.
Table 4.
Multivariate logistic regression analysis of metabolic syndrome (MetS) and its components with sugar-sweetened beverage (SSB) intake among children and adolescents aged 7–18 years (n 5258) from three cities in urban China, September 2013–June 2014
| Non-SSB drinker (0 servings/d) (n 1756) | Medium intake (0–0·3 servings/d) (n 1931) | High intake (>0·3 servings/d) (n 1571) | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Abdominal obesity | ||||||
| Model 1 | 1·00 | Ref. | 1·03 | 0·88, 1·20 | 1·31* | 1·12, 1·53 |
| Model 2 | 1·00 | Ref. | 1·03 | 0·88, 1·20 | 1·35* | 1·14, 1·59 |
| Model 3 | 1·00 | Ref. | 1·03 | 0·88, 1·20 | 1·36* | 1·15, 1·61 |
| Model 4 | 1·00 | Ref. | 1·09 | 0·93, 1·28 | 1·55* | 1·28, 1·83 |
| Low HDL-C | ||||||
| Model 1 | 1·00 | Ref. | 0·96 | 0·81, 1·15 | 1·15 | 0·96, 1·39 |
| Model 2 | 1·00 | Ref. | 0·94 | 0·78, 1·13 | 0·88 | 0·73, 1·07 |
| Model 3 | 1·00 | Ref. | 0·91 | 0·76, 1·09 | 0·86 | 0·71, 1·05 |
| Model 4 | 1·00 | Ref. | 0·93 | 0·77, 1·13 | 0·94 | 0·76, 1·15 |
| Hypertriacylglycerolaemia | ||||||
| Model 1 | 1·00 | Ref. | 1·03 | 0·71, 1·37 | 1·24 | 0·93, 1·66 |
| Model 2 | 1·00 | Ref. | 1·02 | 0·76, 1·36 | 1·08 | 0·79, 1·46 |
| Model 3 | 1·00 | Ref. | 1·02 | 0·76, 1·37 | 1·10 | 0·80, 1·50 |
| Model 4 | 1·00 | Ref. | 1·06 | 0·79, 1·43 | 1·17 | 0·85, 1·63 |
| Elevated FG | ||||||
| Model 1 | 1·00 | Ref. | 1·26 | 0·87, 1·83 | 1·64* | 1·14, 2·37 |
| Model 2 | 1·00 | Ref. | 1·22 | 0·84, 1·76 | 1·11 | 0·76, 1·63 |
| Model 3 | 1·00 | Ref. | 1·19 | 0·82, 1·74 | 1·10 | 0·74, 1·64 |
| Model 4 | 1·00 | Ref. | 1·18 | 0·80, 1·73 | 1·11 | 0·73, 1·68 |
| High blood pressure | ||||||
| Model 1 | 1·00 | Ref. | 0·78 | 0·53, 1·17 | 1·41 | 0·98, 2·04 |
| Model 2 | 1·00 | Ref. | 0·73 | 0·48, 1·09 | 0·75 | 0·51, 1·10 |
| Model 3 | 1·00 | Ref. | 0·69 | 0·45, 1·02 | 0·70 | 0·47, 1·05 |
| Model 4 | 1·00 | Ref· | 0·69 | 0·46, 1·04 | 0·76 | 0·50, 1·17 |
| MetS | ||||||
| Model 1 | 1·00 | Ref. | 1·10 | 0·75, 1·69 | 1·63* | 1·08, 2·46 |
| Model 2 | 1·00 | Ref. | 1·06 | 0·69, 1·54 | 1·21 | 0·79, 1·86 |
| Model 3 | 1·00 | Ref. | 1·12 | 0·73, 1·73 | 1·35 | 0·87, 2·12 |
| Model 4 | 1·00 | Ref. | 1·18 | 0·76, 1·83 | 1·60* | 1·03, 2·54 |
HDL-C, HDL-cholesterol; FG, fasting glucose; Ref., reference category.
Model 1, crude model; Model 2, adjusted for age and gender; Model 3, Model 2 + adjusted for parental educational level, their attitudes towards SSB and their SSB intake; Model 4, Model 3 + adjusted for dietary intake (intake and frequency of fruits, vegetables, meat, dairy products, high-energy foods and fried foods), physical activity (frequency of intensive physical activity, moderate physical activity and walking).
P < 0·05, assessed by multivariate logistic regression.
Discussion
Childhood MetS has become an international epidemic which may jeopardize the well-being of the whole population in the coming decade(32). Considering the long-term potential complications of MetS, it is urgent to identify its different aetiologies. SSB intake is associated with MetS(33) and affected by several factors including age, gender, dietary intake, physical activity and social economic factors(34). However, available information in this area is quite limited, especially among children and adolescents. By conducting a cross-sectional survey on 5258 children and adolescents in urban China, we found that the prevalence of MetS among Chinese children aged 7–18 years was 2·7 % and it was higher in boys than in girls. Most importantly, SSB intake was positively associated with the risk of MetS and abdominal obesity, implying that sugar-related diet control could be a potential target for MetS prevention among children and adolescents.
As one of the potential risk factors for MetS, SSB intake is increasing among Chinese children and adolescents according to our regional survey in 2013(6). Although we found the mean SSB intake among children in South China (97·27 ml/d) was lower than that of children in the UK (314 ml/d)(35) and the USA (411 ml/d)(36), it was still higher than that of children from South Korea (63 ml/d)(37), a country with a diet pattern similar to China. In addition, according to the WHO recommendation, no SSB intake is suggested for children, and the energy coming from free sugar should not be above 10 % of total daily energy intake(38). Fortunately, a significant decrease in SSB intake was observed from 2003 to 2014 in the USA, partly due to the current strategies focusing on SSB control(39). Thus, similar measures should be taken to reverse the trend in China as well.
Excessive SSB intake is associated with CVD and metabolic disorders(35,40). It is well known that MetS is a cluster of cardiovascular risk factors. In our study, a positive association between SSB intake and MetS was observed, which was consistent with a study performed among children and adolescents in Tehran(14) and with other studies performed among adults in the USA and Europe(41). However, no significant association between SSB intake and MetS was found in American middle-aged adults(16) and Iranian adults(42). Inconsistent results may be explained by the difference in age, race and adjusted factors. Sugar intake could be a stressor for the internal environment which may jeopardize homeostasis and lead to oxidative stress. Our finding supports the hypothesis that SSB may act as a mediator in the pathway to MetS which increases the possibility for CVD and metabolic disorders(43). Hence, SSB intake control is critically important for the long-term health of children and adolescents.
Although the association between SSB intake and MetS has been reported by many studies, the underlying mechanism is not fully studied yet. Sugar induces a fast increase in blood glucose and may lead to oxidative stress, as a consequence of which vascular damage and abnormal blood pressure appear(44). On the other hand, a molecular pathway involving carbohydrate responsive element-binding protein and the metabolic hormone fibroblast growth factor-21 may influence sugar metabolism, thereby explaining the fructose-induced metabolic disease(45). In the present study, children and adolescents in the high SSB intake group had higher FG relative to non-SSB drinkers (Table 2), which further supported the mechanism mentioned above.
As one of the components of MetS, a higher risk for abdominal obesity was associated with higher SSB intake (Table 4). This was consistent with a study involving children from six provinces of China(46) as well as a study in Tehranian children(14). High SSB intake increases additional sugar intake and energy intake(35). The excess energy will store in visceral fat, which may contribute to abdominal obesity. At the same time, SSB intake reduces circulating insulin and leptin, decreases insulin sensitivity and attenuates postprandial suppression of ghrelin(47,48), consequently activating appetite and leading children to eat more food. As another mechanism of abdominal obesity suggested, SSB intake alters lipid metabolism and lipoprotein remodelling(49). Our study showed a higher rate of low HDL-C and hypertriacylglycerolaemia in the high SSB intake group (Table 2). Above all, the endocrine and metabolic effects of SSB intake result in abnormal lipid and glucose metabolism, ultimately contributing to weight gain and abdominal obesity.
Interestingly, although we found the high SSB intake group had increased abnormal rates for high blood pressure, low HDL-C, hypertriacylglycerolaemia and elevated FG glucose (Table 2), the OR were not significant in multivariable-adjusted models (Table 4). According to the laboratory studies mentioned above, sugar intake is the cause of oxidative stress which leads to vascular endothelial cell damage and dysregulation of lipid and glucose metabolism(44). In this case, our negative findings may be attributed to the relatively low prevalence of the four MetS components which compromised the statistical power. On the other hand, we classified >0·3 servings/d as high SSB intake; however, this is still much lower than the average intake in Western countries(4). Considering the dose-dependent effect that sugar has, it is possible that the damage for high intake of SSB was not as severe as that in other research findings.
The association between SSB intake and MetS was mediated by unhealthy lifestyle factors, such as improper dietary intake(21) and lack of physical activity(23). We found participants in the high SSB intake group consumed fewer vegetables and more high-energy foods (Table 2), but also performed more physical activity (Table 2). Similarly, one study conducted in American children and adults also reported that SSB consumers were more likely to snack and consume more energy(50). In this case, we found a significant association between SSB intake and MetS after additionally adjusting for dietary intake, physical activity and family environment factors. By collecting the dietary intake data, we can only know the main sources of energy and the rough estimation of energy intake. Energy intake cannot be estimated precisely or taken into consideration as a confounding factor. It was not clear whether the association of SSB intake with MetS and abdominal obesity was independent of energy intake or not.
The present study clarified the association between SSB intake and MetS among a large number of children and adolescents in urban China, with the consideration of many potential confounding factors. There were several limitations. First, because the present study was a cross-sectional study, the underlying causation between SSB intake and MetS cannot be inferred. Second, the data on SSB intake were collected by self-reported questionnaire, which may cause recall bias. Meanwhile, total energy intake cannot be estimated independently. We could not evaluate the potential effect of total energy intake on the relationship between SSB intake and MetS. Instead, the dietary intake, including frequency and quantity of fruits, vegetables, meat, dairy products, high-energy foods and fried foods, was considered and adjusted for when analysing the data, which to some extent can represent the total energy intake and reflect the influence of it. Third, children younger than 10 years old cannot be diagnosed with MetS as suggested by the International Diabetes Federation. In the present study, measurements of the five MetS components were also made for them, which may help to ascertain the epidemic of paediatric MetS and predict the risk of metabolic disorders in the future.
Conclusions
SSB intake was significantly associated with the risk of MetS among children and adolescents in urban China after adjustment for age, gender, dietary intake, physical activity and family environment factors. Our results suggest that high SSB intake leads to MetS, which can be mediated by dietary intake and physical activity. In addition, high SSB intake also contributes to the development of abdominal obesity. Efforts need to be made to control SSB intake among children and adolescents in order to prevent the development of MetS. Further research should clarify the impact of multiple lifestyle factors on the association between SSB intake and MetS, as well as explore the underlying mechanisms.
Acknowledgements
Acknowledgements: The authors thank the children and their caregivers for their continuous and enthusiastic collaboration in this study. They also acknowledge all team members, local education and health staffs for their support. Financial support: This research was funded by Guangdong Provincial Natural Science Foundation (grant numbers 2015A030313175 and.2017A030313844); Sanming Project of Medicine in Shenzhen (grant number SZSM201803061); and the Fundamental Research Funds for the Central Universities in SYSU (grant number 15ykpy09). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: Y.Z. contributed to formulate the research questions and design the study. C.Y. and H.Z. participated in carrying out the experiments and collecting the data. S.L. analysed the data. Y.Z., M.C. and S.L. were involved in writing the paper. All authors had read and approved the final version of this manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Committee of School of Public Health, Sun Yat-sen University. Written informed consent was obtained from all participants.
References
- 1. The Dietary Guidelines Advisory Committee (2015) Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. http://www.health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf (accessed May 2019).
- 2. Sanchez-Pimienta TG, Batis C, Lutter CK et al. (2016) Sugar-sweetened beverages are the main sources of added sugar intake in the Mexican population. J Nutr 146, issue 9, 1888S–1896S. [DOI] [PubMed] [Google Scholar]
- 3. Boulton J, Hashem KM, Jenner KH et al. (2016) How much sugar is hidden in drinks marketed to children? A survey of fruit juices, juice drinks and smoothies. BMJ Open 6, e10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kit BK, Fakhouri TH, Park S et al. (2013) Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr 98, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh GM, Micha R, Khatibzadeh S et al. (2015) Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS One 10, e124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He B, Long W, Li X et al. (2017) Sugar-sweetened beverages consumption positively associated with the risks of obesity and hypertriglyceridemia among children aged 7–18 years in South China. J Atheroscler Thromb 25, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blecher E, Liber AC, Drope JM et al. (2017) Global trends in the affordability of sugar-sweetened beverages, 1990–2016. Prev Chronic Dis 14, E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han E, Kim TH & Powell LM (2013) Beverage consumption and individual-level associations in South Korea. BMC Public Health 13, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li D, Yu D & Zhao L (2014) Trend of sugar-sweetened beverage consumption and intake of added sugar in China nine provinces among adults. Wei Sheng Yan Jiu 43, 70–72. [PubMed] [Google Scholar]
- 10. Zimmet P, Alberti KG, Kaufman F et al. (2007) The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes 8, 299–306. [DOI] [PubMed] [Google Scholar]
- 11. Malik VS & Hu FB (2015) Fructose and cardiometabolic health. J Am Coll Cardiol 66, 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richelsen B (2013) Sugar-sweetened beverages and cardio-metabolic disease risks. Curr Opin Clin Nutr Metab Care 16, 478–484. [DOI] [PubMed] [Google Scholar]
- 13. Imamura F, O’Connor L, Ye Z et al. (2016) Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br J Sport Med 50, 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mirmiran P, Yuzbashian E, Asghari G et al. (2015) Consumption of sugar sweetened beverage is associated with incidence of metabolic syndrome in Tehranian children and adolescents. Nutr Metab (Lond) 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan TF, Lin WT, Huang HL et al. (2014) Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents. Nutrients 6, 2088–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lutsey PL, Steffen LM & Stevens J (2008) Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 117, 754–761. [DOI] [PubMed] [Google Scholar]
- 17. Mazarello Paes V, Hesketh K, O’Malley C et al. (2015) Determinants of sugar-sweetened beverage consumption in young children: a systematic review. Obes Rev 16, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Young TK, Zinman B et al. (2006) Lifestyle variables, non-traditional cardiovascular risk factors, and the metabolic syndrome in an aboriginal Canadian population. Obesity (Silver Spring) 14, 500–508. [DOI] [PubMed] [Google Scholar]
- 19. Buckland G, Salas-Salvadó J, Roure E et al. (2008) Sociodemographic risk factors associated with metabolic syndrome in a Mediterranean population. Public Health Nutr 11, 1372–1378. [DOI] [PubMed] [Google Scholar]
- 20. Chung S, Ha K, Lee H et al. (2015) Soft drink consumption is positively associated with metabolic syndrome risk factors only in Korean women: data from the 2007–2011 Korea National Health and Nutrition Examination Survey. Metabolism 64, 1477–1484. [DOI] [PubMed] [Google Scholar]
- 21. Wang D, Hawley NL, Thompson AA et al. (2017) Dietary patterns are associated with metabolic outcomes among adult Samoans in a cross-sectional study. J Nutr 147, 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steffen LM, Van Horn L, Daviglus ML et al. (2014) A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br J Nutr 112, 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tucker JM, Welk GJ, Beyler NK et al. (2016) Associations between physical activity and metabolic syndrome: comparison between self-report and accelerometry. Am J Health Promot 30, 155–162. [DOI] [PubMed] [Google Scholar]
- 24. Sampasa-Kanyinga H & Chaput JP (2016) Consumption of sugar-sweetened beverages and energy drinks and adherence to physical activity and screen time recommendations among adolescents. Int J Adolesc Med Health 29, issue 5, doi: 10.1515/ijamh-2015-0098. [DOI] [PubMed] [Google Scholar]
- 25. Bogart LM, Elliott MN, Ober AJ et al. (2017) Home sweet home: parent and home environmental factors in adolescent consumption of sugar-sweetened beverages. Acad Pediatr 17, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang ZH, Zou ZY, Wang S et al. (2017) Analysis of the epidemiological characteristics of metabolic syndrome among 10–16 adolescents in 7 provinces in China, 2012. Zhonghua Yu Fang Yi Xue Za Zhi 51, 295–299. [DOI] [PubMed] [Google Scholar]
- 27. He YN, Zhao WH, Zhao LY et al. (2017) The epidemic status of metabolic syndrome among Chinese adolescents aged 10–17 years in 2010–2012. Zhonghua Yu Fang Yi Xue Za Zhi 51, 513–518. [DOI] [PubMed] [Google Scholar]
- 28. Song P, Yu J, Chang X et al. (2017) Prevalence and correlates of metabolic syndrome in Chinese children: the China Health and Nutrition Survey. Nutrients 9, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peduzzi P, Concato J, Kemper E et al. (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49, 1373–1379. [DOI] [PubMed] [Google Scholar]
- 30. Alberti KGMM, Zimmet P & Shaw J (2006) Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 23, 469–480. [DOI] [PubMed] [Google Scholar]
- 31. Ma GS, Ji CY, Ma J et al. (2010) Waist circumference reference values for screening cardiovascular risk factors in Chinese children and adolescents. Biomed Environ Sci 23, 21–31. [DOI] [PubMed] [Google Scholar]
- 32. Seo J & Kim JH (2017) Validation of surrogate markers for metabolic syndrome and cardiometabolic risk factor clustering in children and adolescents: a nationwide population-based study. PLoS One 12, e186050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Narain A, Kwok CS & Mamas MA (2017) Soft drink intake and the risk of metabolic syndrome: a systematic review and meta-analysis. Int J Clin Pract 71, e12927. [DOI] [PubMed] [Google Scholar]
- 34. Vaughan CA, Collins R, Ghosh-Dastidar M et al. (2017) Does where you shop or who you are predict what you eat? The role of stores and individual characteristics in dietary intake. Prev Med 100, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seferidi P, Millett C, Laverty AA et al. (2018) Sweetened beverage intake in association to energy and sugar consumption and cardiometabolic markers in children. Pediatr Obes 13, 195–203. [DOI] [PubMed] [Google Scholar]
- 36. Rehm C & Drewnowski A (2016) Trends in consumption of solid fats, added sugars, sodium, sugar-sweetened beverages, and fruit from fast food restaurants and by fast food restaurant type among US children, 2003–2010. Nutrients 8, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ha K, Chung S, Lee HS et al. (2016) Association of dietary sugars and sugar-sweetened beverage intake with obesity in Korean children and adolescents. Nutrients 8, E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization (2015) Guideline: Sugars Intake for Adults and Children. http://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed March 2018). [PubMed]
- 39. Bleich SN, Vercammen KA, Koma JW et al. (2018) Trends in beverage consumption among children and adults, 2003–2014. Obesity (Silver Spring) 26, 432–441. [DOI] [PubMed] [Google Scholar]
- 40. Cohen L, Curhan G & Forman J (2012) Association of sweetened beverage intake with incident hypertension. J Gen Intern Med 27, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crichton G, Alkerwi A & Elias M (2015) Diet soft drink consumption is associated with the metabolic syndrome: a two sample comparison. Nutrients 7, 3569–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khosravi-Boroujeni H, Sarrafzadegan N, Mohammadifard N et al. (2012) Consumption of sugar-sweetened beverages in relation to the metabolic syndrome among Iranian adults. Obes Facts 5, 527–537. [DOI] [PubMed] [Google Scholar]
- 43. Azimi P, Ghiasvand R, Feizi A et al. (2014) Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud 11, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saisho Y (2014) Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci 15, 18381–18406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKeown NM, Dashti HS, Ma J et al. (2018) Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: a meta-analysis. Diabetologia 61, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shang XW, Liu AL, Zhang Q et al. (2012) Report on childhood obesity in China (9): sugar-sweetened beverages consumption and obesity. Biomed Environ Sci 25, 125–132. [DOI] [PubMed] [Google Scholar]
- 47. Stanhope KL & Havel PJ (2008) Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr 88, issue 6, 1733S–1737S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teff KL, Elliott SS, Tschöp M et al. (2004) Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 89, 2963–2972. [DOI] [PubMed] [Google Scholar]
- 49. Stanhope KL, Schwarz JM, Keim NL et al. (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119, 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bleich SN & Wolfson JA (2015) US adults and child snacking patterns among sugar-sweetened beverage drinkers and non-drinkers. Prev Med 72, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]