Abstract
Objective:
The aim of the current study was to evaluate energy intake misreporting prevalence, its associated factors and its effects on nutrient intake, in the Portuguese population aged from 18 to 84 years.
Design:
Cross-sectional study.
Setting:
Portugal.
Subjects:
Adults participants from the National Food, Nutrition and Physical Activity Survey, IAN-AF, 2015–2016, who provided two complete 24 h dietary recall and complete covariate information.
Results:
Under, plausible and over-reporters were identified according to the Goldberg method. Total misreporting prevalence was 29·9 %, being 28·5 % of under-reporting and 1·4 % of over-reporting. The current study found higher odds of being classified as an under-reporter especially in participants with higher BMI and in those who self-reported health perception status as non-favourable. Energy intake estimation increases by 853.5 kJ/d (204 kcal/d) when misreporters are excluded, and the same tendency is observed for macro and micronutrients. It is worth mentioning that the prevalence of inadequacy for protein intake decreases by about 5 % when considering plausible reporters.
Conclusions:
The exclusion of misreporters has a small impact on the crude energy and nutrient estimates as well as on assessing the contribution of nutrients to total energy intake. However, a moderate impact was observed in the estimation of nutrient inadequacy prevalence.
Keywords: Misreporting, Energy intake, National dietary survey, Portugal
Nutritional epidemiological studies are important to identify the needs of the population as well as the dietary determinants of health, in order to develop public health programmes and food policies(1).
Self-reported dietary intake data are often used to evaluate food consumption and nutrient intake because of being cost-effective and time-saving. Biochemical methods are a more accurate alternative but usually are less likely to be used owing to higher costs(2). In addition, biomarkers are not available for many of the macronutrient exposures and those available are not feasible to be used in population-based surveys at scale. Additionally, self-reported data provides some information that is not possible to be obtained from a comprehensive set of biomarkers, such as food consumption, food behaviours and eating patterns(1).
Intra-individual variability, seasonal variability and misreporting can affect the reliability of energy intake (EI) dietary results(3). Misreports can be intentional, by voluntary omission of foods consumed, or may result from incomplete recordkeeping on the part of the subjects(4). In dietary assessment, under-eating during the assessment period also affects the reliability of EI results. This under-eating could be by dieting or dietary restraint or due to other factors, such as illness. To better interpret the obtained values, the biological plausibility of an individual’s food intake should be accessed by comparison with energy expenditure based on basal metabolic rate (BMR) and physical activity level (PAL), assuming energy balance during the period of dietary assessment. For example, misreporting might be the explanation for obesity to be increasing while EI is stable or even decreasing(5).
Energy intake under-reporting is considered to be present when reported EI is substantially lower than true intake, compared with the energy expenditure (EE) of individuals. The opposite is considered to be over-reporting. Over-reporting occurs much less often than under-reporting, and for that reason is less studied(6).
The EI misreporting phenomenon, which appears to occur both randomly and non-randomly(7), is still largely overlooked in obesity research(8) and constitutes an important challenge in nutrition epidemiology(3). Specifically, under-reporting affects the estimation of EI and consequently of other nutrients, so may lead to a mis-estimation of nutrient inadequacy depending on the nutrient in question and whether there is systematic under-reporting of specific foods (e.g. those high in fat, sugar or salt). This may be constituted as a bias in the associations between diet and diseases assessed in epidemiological studies(3,9). Bias may also be seen in the estimation of the dietary exposure to chemical or microbiological hazards(10).
For a reliable dietary data analysis, it is important to identify implausible reports so that both under- and over-reports are included. Exclusion of under-reporters (UR) or over-reporters (OvR) from the datasets introduces unknown bias because misreporters are systematically different from plausible reporters (PR) with regard to lifestyle, nutritional status and chronic disease risk. Therefore, some authors maintain that UR and OvR should not be excluded from the sample but should be identified and reported(6).
There is no objective measure of EI, which is the reason why the reference methods used compare EI with total EE, assuming that EI must be equal to EE when weight is stable. The gold standard reference method for assessing total EE is doubly labelled water(3), this has high application costs and is therefore why it is not greatly used in epidemiological studies(9). As an alternative, indirect methods to identify EI misreports are used. The most well-studied method to account for EI misreporting is the Goldberg method, adapted by Black(11,12). In the absence of objective measures of total EE or physical activity, the Goldberg method is revealed to be a reasonable approach to characterize dietary reports(13).
In an up-to-date review of the National Dietary Surveys of the European adult population, misreporters have not been taken into account by the majority of countries involved. Portugal has been identified as one of the countries that has no data on this subject(14). Identifying the prevalence and magnitude of misreporting constitutes the first step to interpreting the results(15). In addition, further research into the characteristics may help the statistical adjustment and the development of correction factors(16).
The aim of this study was to evaluate EI misreporting prevalence, its associated factors and its effects on nutrient intake measurements, in the Portuguese adult population.
Methodology
Survey design and participants
We used data from the National Food, Nutrition and Physical Activity Survey, IAN-AF, 2015–2016. A representative sample of the Portuguese general population, aged between 3 months and 84 years, was selected from the National Health Registry. This was done by multistage sampling, in each of the country’s seven geographical regions. The representativeness of the sample was evaluated by comparison with the information from the Census 2011 (by the National Institute of Statistics), which presented a similar distribution of the resident population in Portugal. The IAN-AF 2015–2016 methodology is described in detail elsewhere(17,18).
Interviews were conducted by nutritionists at Primary Health Care Units or at participants’ homes. The participation rate was approximately 30 % amongst those eligible. A total of 5811 participants completed two interviews and those below the age of 18 were excluded from the analysis. The subtotal of 3857 that remained were further scrutinized against other exclusion criteria. Those who were excluded were pregnant (n 49), breastfeeding (n 47), participants who report unusual intake (n 85) and participants with missing data, both anthropometric objective and self-reported data about weight and height (n 37). Thus, the resulting sample contained 3639 individuals.
Data collection
The data collection followed the guidelines of the EU-Menu, a project promoted by the European Food Safety Agency aimed at harmonizing collection of dietary data in Europe(6).
Data collection was computer-assisted by an electronic platform that was developed for this project: the You Eat & Move platform. This e-platform includes the ‘You’ module to collect sociodemographic and other health-related data; the ‘eAT24’ module (Electronic Assessment Tool for 24-hours recall) for data collection on food consumption; and the ‘MOVE’ module for data collection on physical activity.
Assessment of dietary intake
Dietary intake was obtained by two non-consecutive 24 h dietary recalls (24-hR) from zero to midnight (with 8–15 d interval) during 12 months to minimize seasonal variability. All weekdays were represented similarly(18). Structured interviews were performed by trained nutritionists according to an adapted procedure based on the Automated Multiple-Pass Method for 24-hR (USDA)(19). As part of the 24-hR procedure, participants also indicate whether the reported day represents an usual or unusual intake (including reasons for unusual intakes such as illness or holiday) or if they practise a special diet (for example, a vegetarian or weight loss diet).
Data were included directly on the e-platform, which integrates the detailed European Food Safety Authority (EFSA) FoodEx2 classification system(20) and a nutritional composition database. Therefore, the Portuguese food composition table was used by default(21). A quality control, including the energy and nutrients estimation, was performed at the end of each interview to minimize errors.
Inadequate intake of macronutrients was estimated by comparison with EFSA dietary reference values(22). For protein, EFSA only describes the lower cut off.
Assessment of non-dietary variables
Data on sociodemographic characteristics, health history, health behaviours and food security were collected by interviewer-administered questionnaires, which were included on the e-platform.
Self-reported actual height and weight were asked before the performance of objective measurements. Weight and height were evaluated according to standard procedures(23), by trained personnel. Body weight was measured to the nearest tenth of a kilogram using a digital scale (SECA 813, Hamburg, Germany) and height was measured to the nearest centimetre using a portable wall stadiometer (SECA 213, Hamburg, Germany). During anthropometric measurements, participants were asked to stand, wearing only light clothing and had to be barefoot. For BMI, we assumed the standard cut offs for overweight (25·0–29·9 kg/m2) and obesity (≥30·0 kg/m2)(24). We assumed self-reported values when missing objective values (missing values for measured height: 147 (3·8 %); missing values for measured weight: 11 (0·3 %); correlation between height and weight measured and self-reported on our sample = 0·97 and 0·99, respectively).
Physical activity was accessed by the International Physical Activity Questionnaire (IPAQ) short-form(25). Data cleaning followed the recommended procedures by the IPAQ Research Committee(26). Using the IPAQ’s scoring protocol, it is possible to extract individual frequency and time on vigorous, moderate and walking activities from the previous 7 d and its estimated metabolic equivalent of EE.
Those who were considered active at a higher level were participants who achieved one of the following two criteria: vigorous-intensity activity on at least 3 d and accumulating at least 1500 MET-min/week; or seven or more days of any combination of walking, moderate-intensity or vigorous intensity activities achieving a minimum of at least 3000 MET-min/week. The moderately active were classed as intermediate level participants if they met any of the following three criteria: three or more days of vigorous activity of at least 20 min per day; or five or more days of moderate-intensity activity or walking of at least 30 min per day; or five or more days of any combination of walking, moderate-intensity or vigorous intensity activities achieving a minimum of at least 600 MET-min/week. Participants who did not meet any of the above criteria, were classed as being in the lowest level, which was ‘inactive’.
Classification of EI reports
In this study, participants were classified as UR, PR or OvR, according to the Goldberg method(11) using the coefficients of variation suggested by Black(12). BMR was computed following Schofield age- and gender-specific equations(27). The Goldberg equation calculates the ratio between reported EI and predicted BMR (rEI:pBMR) and compares it with the estimation of the 95 % confidence limits (lower and upper cut-off) for PAL. If the ratio differs from PAL by more than the 2 sd, the EI is determined to be implausible and subjects are defined as UR, PR or OvR depending in whether their individual ratio of rEI:pBMR was below, between or above the confidence limits calculated, respectively(12). To access individual PAL, the categorical score resulting from the analysis of IPAQ short form was used(26). Values of 1·4, 1·6 and 1·8 were applied to inactive, moderately active and active categories, respectively. The lowest physical activity level was assumed for participants with missing value for IPAQ level (n 112). The choice was based on the higher prevalence of this activity level and after a sensitivity analysis that showed similar results of prevalence considering this assumption.
Specific lower and upper cut-off limits were thus calculated for each defined group, by activity against the age category and then compared with rEI:pBMR. Supplementary Table S1 presents the mean pBMR, rEI, rEI:pBMR and the calculated cut-off limits at individual level according to the activity category.
Statistical analysis
Descriptive analysis included frequency, central tendency and dispersion measures. Probabilistic weights were used to compensate for the effect of the complex sampling design. Chi-square was used to compare proportions. Binary logistic regression was performed to identify the risk of being classified as an under-reporter in comparison with a plausible reporter, according to some participants’ characteristics selected a priori as being associated with EI misreporting. Model 1 was the first model, which analysed how single factors affect the prevalence of EI under-reporting. Model 2 was created through adjusting potential covariates relating to sociodemographic characteristics such as gender, age group, geographical region and education, which was based on the results from Model 1. Model 3 was the final model, which shows further adjustments made adjusting to self-reported health perception status and tobacco use. Interactions that were theoretically expected, namely with BMI, were tested. Due to the collinearity between education and the variables occupation, income and food security, no adjustment for education was made in those cases. In addition, there was no adjustment to the variable BMI as there is a relation between this variable and the methodology used to identify misreports.
Energy and nutrient intake means were compared using the Kruskal-Wallis test. Cohen’s d was used to quantify the effect of misreporters exclusion on energy and nutrients estimation. An effect size of 0·01 was considered as a very small effect size(28), 0·2 was considered as a small effect size, 0·5 was considered as a moderate effect size and 0·8 was considered as a large effect size(29).
To access nutrient intake inadequacies, habitual intake distribution was performed using the Statistical Program to Assess Dietary Exposure (SPADE).
A significance level of 95 % was considered
The statistical analyses were carried out using R software version 3.5. and IBM SPSS Statistics 24.
Results
The general sample description and the under-, plausible- and over-reporting prevalence according to different characteristics are presented on Table 1.
Table 1.
General description of the sample and under-, plausible- and over-reporting prevalence according to participants’ characteristics
| All | Under-reporters | Plausible reporters | Over-reporters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | P * | |
| Gender | 3639 | 100 | 1036 | 28·5 | 2551 | 70·1 | 52 | 1·4 | |
| Male | 1778 | 48·9 | 429 | 24·1 | 1312 | 73·8 | 37 | 2·1 | <0·001 |
| Female | 1861 | 51·1 | 607 | 32·6 | 1239 | 66·6 | 15 | 0·8 | |
| Age | |||||||||
| 18–24y | 378 | 10·4 | 89 | 23·5 | 279 | 73·8 | 10 | 2·6 | <0·001 |
| 25–44y | 1240 | 34·1 | 318 | 25·6 | 904 | 72·9 | 18 | 1·5 | |
| 45–64y | 1307 | 35·9 | 432 | 33·1 | 859 | 65·7 | 16 | 1·2 | |
| 65–84y | 714 | 19·6 | 197 | 27·6 | 509 | 71·3 | 8 | 1·1 | |
| Geographical region | |||||||||
| North | 614 | 16·9 | 164 | 26·7 | 439 | 71·5 | 11 | 1·8 | <0·001 |
| Centre | 646 | 17·8 | 178 | 27·6 | 464 | 71·8 | 4 | 0·6 | |
| Lisbon area | 473 | 13·0 | 108 | 22·8 | 355 | 75·1 | 10 | 2·1 | |
| Alentejo | 428 | 11·8 | 76 | 17·8 | 338 | 79·0 | 14 | 3·3 | |
| Algarve | 474 | 13·0 | 101 | 21·3 | 368 | 77·6 | 5 | 1·1 | |
| Madeira | 504 | 13·8 | 222 | 44·0 | 281 | 55·8 | 1 | 0·2 | |
| Azores | 500 | 13·7 | 187 | 37·4 | 306 | 61·2 | 7 | 1·4 | |
| Education | |||||||||
| No education/Basic education (<4 y) | 1921 | 52·9 | 621 | 32·3 | 1272 | 66·2 | 28 | 1·5 | <0·001 |
| Secondary/professional education (4–12 y) | 904 | 24·9 | 238 | 26·3 | 645 | 71·3 | 21 | 2·3 | |
| Higher education (≥12 y) | 806 | 22·2 | 175 | 21·7 | 628 | 77·9 | 3 | 0·4 | |
| Occupation | |||||||||
| Worker for a fee | 1988 | 54·7 | 533 | 26·8 | 1421 | 71·5 | 34 | 1·7 | 0·022 |
| Unemployed | 421 | 11·6 | 142 | 33·7 | 273 | 64·8 | 6 | 1·4 | |
| Other | 1227 | 33·7 | 360 | 29·3 | 855 | 69·7 | 12 | 1·0 | |
| Income | |||||||||
| Less than €485 | 342 | 10·4 | 125 | 36·5 | 214 | 62·6 | 3 | 0·9 | <0·001 |
| €485–1455 | 1792 | 54·6 | 518 | 28·9 | 1246 | 69·5 | 28 | 1·6 | |
| Above €1466 | 1148 | 35·0 | 288 | 25·1 | 843 | 73·4 | 17 | 1·5 | |
| Food security | |||||||||
| Food security | 3254 | 89·7 | 864 | 26·6 | 2342 | 72·0 | 48 | 1·5 | <0·001 |
| Food insecurity | 373 | 10·3 | 167 | 44·8 | 202 | 54·2 | 4 | 1·1 | |
| BMI | |||||||||
| Underweight (<18·5 kg/m2) | 39 | 1·1 | 4 | 10·3 | 33 | 84·6 | 2 | 5·1 | <0·001 |
| Normal weight (18·5–24·9 kg/m2) | 1282 | 35·2 | 228 | 17·8 | 1023 | 79·8 | 31 | 2·4 | |
| Overweight (24·9–29·9 kg/m2) | 1363 | 37·5 | 378 | 27·7 | 974 | 71·5 | 11 | 0·8 | |
| Obese (≥30·0 kg/m2) | 955 | 26·2 | 426 | 44·6 | 521 | 54·6 | 8 | 0·8 | |
| Physical activity level | |||||||||
| Inactive | 1578 | 43·4 | 312 | 19·8 | 1240 | 78·6 | 26 | 1·6 | <0·001 |
| Minimally active | 1037 | 28·5 | 308 | 29·7 | 716 | 69·0 | 13 | 1·3 | |
| Active | 1024 | 28·1 | 416 | 40·6 | 595 | 58·1 | 13 | 1·3 | |
| Disease | |||||||||
| No | 2073 | 57·0 | 528 | 25·5 | 1508 | 72·7 | 37 | 1·8 | <0·001 |
| Yes | 1566 | 43·0 | 508 | 32·4 | 1043 | 66·6 | 15 | 1·0 | |
| Prior depression diagnosis | |||||||||
| No | 1376 | 87·9 | 429 | 31·2 | 933 | 67·8 | 14 | 1·0 | 0·015 |
| Yes | 190 | 12·1 | 79 | 41·6 | 110 | 57·9 | 1 | 0·5 | |
| Self-reported health perception status | |||||||||
| Non favourable | 324 | 8·9 | 143 | 44·1 | 179 | 55·2 | 2 | 0·6 | <0·001 |
| Favourable | 3307 | 91·1 | 888 | 26·9 | 2369 | 71·6 | 50 | 1·5 | |
| Tobacco use | |||||||||
| Never smoked | 1770 | 48·7 | 563 | 31·8 | 1193 | 67·4 | 14 | 0·8 | <0·001 |
| Ex-smoker | 1084 | 29·8 | 271 | 25·0 | 794 | 73·2 | 19 | 1·8 | |
| Actual smoker | 784 | 21·6 | 202 | 25·8 | 563 | 71·8 | 19 | 2·4 | |
P: Chi-square for the proportion of misreporting prevalence within characteristics categories.
Total EI misreporting prevalence according to the Goldberg original method was 29·9 %. We found 28·5 % of under-reporting (n 1036) and 1·4 % of over-reporting (n 52).
The percentage of UR was higher in women (32·6 % v. 24·1 % in men) and the percentage of OvR was higher in men (2·1 % v. 0·8 %). With regards to age, there were more UR among the 45–64 y group (33·1 %), whereas there were more OvR among the youngest group (2·6 %). We observed more UR in the autonomous regions (Madeira and Azores) and more OvR in the Alentejo region (3·3 %). Under-reporting prevalence increases with less education (from 21·7 % in the highest category to 32·3 % in the lowest) and income. Those who experience household food insecurity had a higher percentage of UR (44·8 %), with over-reporting prevalence higher for those who have food security (1·5 %). There were more UR and fewer OvR among overweight and obese subjects (44·6 and 0·8 %, respectively); the over-reporting prevalence was higher among underweight participants (5·1%). For self-reported health perception status, there were more UR among those who were considered non-favourable (44·1 %) and more OvR among those who were considered favourable (1·5 %). Those who had never smoked had a higher percentage of UR (31·8 %); on the other hand, actual smokers had a higher percentage of OvR (2·4 %).
Since over-reporting prevalence was low, the sample size is not sufficient to allow the evaluation of their determinants. So we analysed only the under-reporting associated factors (Table 2). Overall, the adjusted models showed slight variations in the magnitude of the associations when compared with the crude model.
Table 2.
Odds of being classified as an under-reporter compared with a plausible reporter, according to participants’ characteristics
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Gender | ||||||||
| Male | 1741 | 48·5 | 1·00 | – | 1·00 | – | 1·00 | – |
| Female | 1846 | 51·5 | 1·50 | 1·30, 1·73 | 1·56 | 1·34, 1·81 | 1·42 | 1·21, 1·67 |
| Age | ||||||||
| 18–24 | 368 | 10·3 | 0·82 | 0·62, 1·10 | 0·93 | 0·68, 1·28 | 1·04 | 0·75, 1·43 |
| 25–44 | 1222 | 34·1 | 0·91 | 0·74, 1·12 | 1·05 | 0·83, 1·32 | 1·16 | 0·91, 1·48 |
| 45–64 | 1291 | 36·0 | 1·30 | 1·06, 1·59 | 1·35 | 1·09, 1·66 | 1·46 | 1·17, 1·81 |
| 65–84 | 706 | 19·7 | 1·00 | – | 1·00 | – | 1·00 | – |
| Education | ||||||||
| No education/Basic education (<4 y) | 1893 | 52·9 | 1·75 | 1·44, 2·13 | 1·71 | 1·39, 2·12 | 1·57 | 1·26, 1·95 |
| Secondary/professional education (4–12 y) | 883 | 24·7 | 1·32 | 1·06, 1·66 | 1·43 | 1·14, 1·81 | 1·40 | 1·11, 1·76 |
| Higher education (≥12 y) | 803 | 22·4 | 1·00 | – | 1·00 | – | 1·00 | – |
| Occupation | ||||||||
| Worker for a fee | 1954 | 54·5 | 1·00 | – | 1·00 | – | 1·00 | – |
| Unemployed | 415 | 11·6 | 1·39 | 1·11, 1·74 | 1·30 | 1·03, 1·64 | 1·27 | 0·96, 1·68 |
| Other | 1215 | 33·9 | 1·12 | 0·96, 1·32 | 1·18 | 0·94, 1·48 | 1·14 | 0·96, 1·37 |
| Income | ||||||||
| Less than €485 | 339 | 11·3 | 1·71 | 1·32, 2·21 | 1·47 | 1·12, 1·92 | 1·27 | 0·96, 1·68 |
| €485–1455 | 1764 | 58·7 | 1·22 | 1·03, 1·44 | 1·20 | 1·01, 1·43 | 1·14 | 0·96, 1·37 |
| Above €1466 | 903 | 30·0 | 1·00 | – | 1·00 | – | 1·00 | – |
| Food security | ||||||||
| Food security | 3206 | 89·7 | 1·00 | – | 1·00 | – | 1·00 | – |
| Food insecurity | 369 | 10·3 | 2·24 | 1·80, 2·79 | 1·92 | 1·53, 2·41 | 1·75 | 1·39, 2·20 |
| BMI | ||||||||
| <25 kg/m2 | 1288 | 35·9 | 1·00 | – | 1·00 | – | 1·00 | |
| 25·0–29·9 kg/m2 | 1352 | 37·7 | 1·77 | 1·47, 2·13 | 1·89 | 1·55, 2·31 | 1·87 | 1·53, 2·29 |
| ≥30·0 kg/m2 | 947 | 26·4 | 3·72 | 3·07, 4·51 | 3·82 | 3·09, 4·72 | 3·73 | 3·01, 4·63 |
| Chronic disease | ||||||||
| No | 2036 | 56·8 | 1·00 | – | 1·00 | – | 1·00 | – |
| Yes | 1551 | 43·2 | 1·39 | 1·20, 1·61 | 1·31 | 1·11, 1·54 | 1·25 | 1·05, 1·47 |
| Prior depression diagnosis | ||||||||
| No | 1362 | 87·8 | 1·00 | – | 1·00 | – | 1·00 | – |
| Yes | 189 | 12·2 | 1·56 | 1·15, 2·13 | 1·42 | 1·02, 1·97 | 1·27 | 0·90, 1·78 |
| Self-reported health perception status | ||||||||
| Non favourable | 322 | 9·0 | 2·13 | 1·69, 2·69 | 1·96 | 1·52, 2·52 | 1·95 | 1·51, 2·50 |
| Favourable | 3257 | 91·0 | 1·00 | – | 1·00 | – | 1·00 | – |
| Tobacco use | ||||||||
| Never smoked | 1756 | 49·0 | 1·00 | – | 1·00 | – | 1·00 | – |
| Ex-smoker | 1065 | 29·7 | 0·73 | 0·62, 0·86 | 0·82 | 0·68, 0·99 | 0·83 | 0·69, 1·00 |
| Actual smoker | 765 | 21·3 | 0·76 | 0·63, 0·91 | 0·89 | 0·73, 1·09 | 0·91 | 0·70, 1·11 |
Model 1: Crude.
Model 2: Model 1 adjusted for sociodemographic variables: gender, age group, education, geographical region.
Model 3: Model 2 adjusted for self-reported health perception status, tobacco use.
After adjusting for gender, age group, education and geographical region, self-reported health perception status and tobacco use (Model 3), women tend to be classified as UR more often than men (OR: 1·42; 95 % CI: 1·21, 1·67). Participants aged 45–64 years when compared with older participants (65–84 years) (OR: 1·46; 95 % CI: 1·17, 1·81) and participants with lower education compared with those in higher level (OR: 1·57; 95 % CI: 1·26, 1·95) were more likely to be classified as UR. Participants who have some chronic disease (OR: 1·25; 95 % CI: 1·05, 1·47) and who self-reported health perception status such as non-favourable compared with favourable (OR: 1·95; 95 % CI: 1·51, 2·50) presented higher odds of being classified as UR compared with PR. Ex-smokers had lower odds of being classified as under-reporters (OR: 0·83; 95 % CI: 0·69, 1·00). The strongest associations were found for BMI and food security. Overweight (OR: 1·87; 95 % CI: 1·53, 2·29) and obese (OR: 3·73; 95 % CI: 3·01, 4·63) participants and those who experienced food insecurity (OR: 1·75; 95 % CI: 1·39, 2·20) presented higher odds of being classified as UR.
Comparing the EI mean value between total sample and only among PR (after excluding misreporters), this value increases as expected (difference: +853.5 kJ/d (+204 kcal/d) (Table 3). In addition, all nutrient mean values increased after exclusion of misreporters. As predicted, absolute intakes of all macro and micronutrients were lower in UR and higher in OvR. However, after an adjustment for EI by the nutrient density model, the percentage of energy from each macronutrient differs between UR, OvR and PR, although the values among PR are similar to the values including all individuals. The results identified a higher percentage of energy from protein and total carbohydrate, as well as a lower percentage of total fat and alcohol in UR, compared with PR. It is noteworthy that UR showed a tendency of higher nutritional densities for micronutrients. The reverse happens with OvR. The Kruskal-Wallis test showed significant differences for all variables under study between UR, PR and OvR. The exclusion of misreporters resulted in a small to medium effect on EI estimation and a very small to small effect on macro and micronutrient contribution estimates.
Table 3.
Energy intake, predicted basal metabolic rate and nutrient intake, weighted for the Portuguese population distribution, by total and misreporting categories
| Total | Under-reporters | Plausible reporters | |||||
|---|---|---|---|---|---|---|---|
| Sample (n) | 3639 | 1036 | 2551 | ||||
| Estimated population (N) | 8 637 392 | 2 272 728 | 6 245 452 | ||||
| Mean | sd | Mean | sd | Mean | sd | Cohen’s d * | |
| EI (kJ/d) |
8230 | 740·5 | 5347 | 339·9 | 9083 | 618·6 | 0·28 |
| pBMR (kJ/d) |
6460 | 252·6 | 6602 | 260·4 | 6410 | 248·4 | 0·05 |
| rEI:pBMR | 1·27 | 0·44 | 0·81 | 0·17 | 1·42 | 0·33 | 0·32 |
| Protein (g/d) |
86·1 | 35·71 | 60·5 | 22·08 | 93·9 | 33·07 | 0·22 |
| Protein (%TEI) |
17·7 | 4·20 | 18·9 | 4·75 | 17·4 | 3·90 | 0·07 |
| Total carbohydrate (g/d) |
230·3 | 90·54 | 156·6 | 49·46 | 252·4 | 81·50 | 0·24 |
| Total carbohydrate (%TEI) |
47·6 | 9·66 | 49·3 | 10·12 | 46·9 | 9·40 | 0·07 |
| Fibre (g/d) |
18·4 | 7·50 | 13·7 | 5·09 | 19·8 | 7·09 | 0·19 |
| Fibre density g/1000kJ |
2·32 | 0·77 | 2·63 | 0·87 | 2·22 | 0·70 | 0·12 |
| Total fat (g/d) |
68·4 | 34·07 | 40·8 | 16·05 | 76·5 | 30·09 | 0·24 |
| Total fat (%TEI) |
30·8 | 7·47 | 28·6 | 7·38 | 31·5 | 7·33 | 0·09 |
| Alcohol (g/d) |
11·4 | 20·04 | 5·4 | 10·96 | 13·2 | 21·34 | 0·09 |
| Alcohol (%TEI) |
3·7 | 6·13 | 2·7 | 5·39 | 4·0 | 6·35 | 0·05 |
| Sodium (mg/d) |
3098 | 1360·5 | 2092 | 768·6 | 3402 | 1269·3 | 0·22 |
| Sodium density (mg/kJ) |
6·65 | 1·607 | 6·86 | 1·703 | 6·57 | 1·569 | 0·05 |
| Calcium (mg/d) |
763 | 369 | 581·1 | 285 | 820 | 368·7 | 0·15 |
| Calcium density (mg/kJ) |
1·72 | 0·841 | 1·97 | 0·950 | 1·63 | 0·787 | 0·10 |
| Vitamin A (µg/d) |
851 | 1278·2 | 672 | 847·5 | 908 | 1403·7 | 0·04 |
| Vitamin A density (μg/kJ) |
1·97 | 3·305 | 2·30 | 3·075 | 1·84 | 2·987 | 0·04 |
| Folate (µg/d) |
251 | 229·3 | 191 | 170·6 | 268 | 243·0 | 0·07 |
| Folate density (μg/kJ) |
0·54 | 0·607 | 0·63 | 0·611 | 0·54 | 0·607 | 0·00 |
rEI: reported energy intake; pBMR: predicted basal metabolic rate; %TEI: percentage of total energy intake.
Cohen’s d quantifies the effect of misreporter exclusion on energy and nutrients estimation.
Figure 1 shows the inadequacy prevalence decreasing according to the EFSA dietary reference values after misreporters exclusion. For protein there was observed a decrease of the inadequacy below dietary reference values (5·8 % to 0·9 %) whereas for total fat there was observed a decrease of the inadequacy above dietary reference values (16·5 % to 13·7 %). An increase in inadequacy prevalence below the dietary reference values for total carbohydrates (47·0–50·2 %) is observed. There appears to be no difference in the estimation of the prevalence of inadequacy for free sugars and saturated fat when excluding misreporters.
Fig. 1.
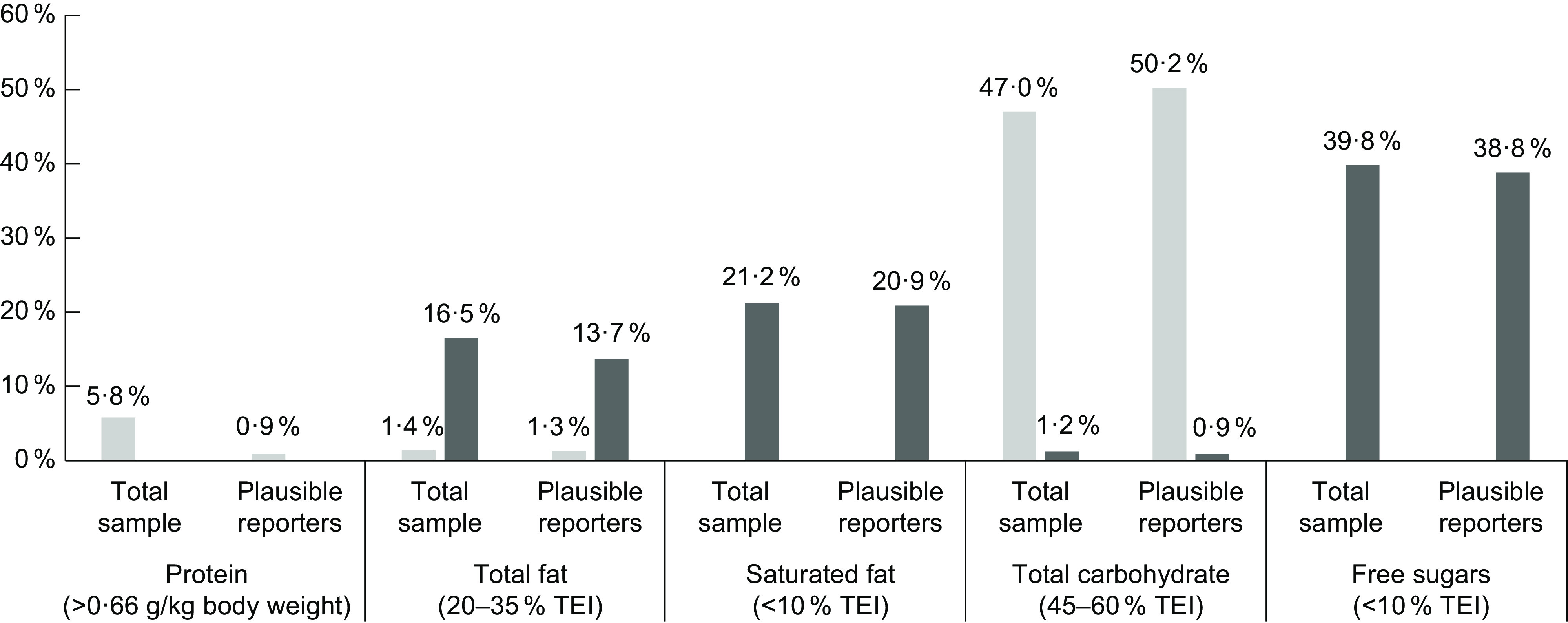
Macronutrient inadequacy ( below and
below and  above dietary reference values), on total sample and among plausible reporters, after adjustment to usual intake and weighting for the Portuguese population distribution
above dietary reference values), on total sample and among plausible reporters, after adjustment to usual intake and weighting for the Portuguese population distribution
Discussion
This is the first study examining EI misreporting prevalence and characteristics of UR in a Portuguese representative sample. Globally, the EI misreporting prevalence found in this study is in agreement with other studies carried out with different populations. The current study found 28·5 and 1·4 % of under- and over-reporting and higher odds of being classified as an under-reporter in females, in the 45–64 age group (compared with the older age group), in less educated participants, in those who reported household food insecurity, with higher BMI, with chronic disease, with non-favourable self-reported health perception status, and in those who have never smoked.
A systematic literature search(15), which included 37 relevant studies on misreporting of EI in adults by 24-hR or by estimated or weighed food records, found about 30 % of participants classified as UR, with reported energy intake estimated to be 15 % less than energy needs. Different study designs, including different methods to evaluate EI and different approaches to identify misreporting may impact the comparison of the results. Under-reporting is prevalent and persists in diverse dietary assessment methods, although in different proportions(9,30).
Only a few recent national surveys have examined misreporting of EI among adults(7,31-37); in addition, under-reporting has been more studied than over-reporting. The prevalence of under-reporting ranged from 3·2 % in Japan(35) to 34·1 % in Belgium(31). Japan was noted for having a much lower value compared with the others, which could be due to cultural differences. In general, under-reporting was associated with higher BMI, female gender, and also with lower levels of education, such as in the present study. Several studies reported that older individuals were more likely to under-report(7,34), contrary to what is seen in this study and in Japanese adults(35). In the current study, being in an unemployment situation was associated with under-reporting, as in Sweden, where blue-collar workers had higher odds of being classified as UR(33). To the best of our knowledge, no investigation has been done until now on the relationship between EI misreporting and food insecurity. Participants who reported experiencing household food insecurity – which means insufficient or inadequate food access, availability and utilization due to limited financial resources – were more likely to be classified as UR. It may reflect real situations of under-eating that we are unable to distinguish and could lead to an overestimation of UR. The adjustment for BMI maintained this effect. We found a higher probability of under-reporting in individuals with BMI below 25 kg/m2 with chronic disease, which was not observed in individuals with BMI equal to or above 25 kg/m2 with chronic disease, which may also reflect real under-eating cases.
As in the present study, the study on South Korea(37) also found a positive relationship between non-favourable self-reported health perception status and under-reporting. We found a positive association between prior depression diagnosis and under-reporting that became not significant after adjustment. The same was observed in Irish women(38). However, in Greek women, low depression scores were positively associated with under-reporting(39) and a study by Davison found 1·3 times more prevalence of under-reporting in those taking mood stabilizers compared with those not taking this psychiatric medication(40). A review from 2006(4) reported that there are insufficient data to conclude if depression or anxiety are related with EI misreporting.
It is known that there is a selective misreporting of food and drinks. For example, UR tend to underestimate energy-dense food(41). It has been showed that among UR, the prevalence of individuals in the healthier pattern cluster is high(42). The existence of differences in the reporting according to the characteristics of participants results in a differential reporting of nutrients. Energy under-reporting has been more related to the under-reporting of total fat, total carbohydrates and alcohol, rather than protein(43) – a tendency observed in the current study as well as in American adults(44).
However, Hirvonen et al.(45) concluded that estimates of nutrients expressed as a percentage of EI are not affected by the exclusion of UR, which is in accord with the current study. In this study, the percentage of energy from protein, total carbohydrates, total fat and alcohol is similar between all individuals and among PR. We expected less contributions to total energy intake across all macronutrients in UR. Nevertheless, the percentage of energy from protein and total carbohydrate was higher among UR, compared with PR; for total fat and alcohol it was lower. With the exception of alcohol, in which we found a higher contribution of alcohol among OvR, the results are in line with The Survey of Lifestyle, Attitudes and Nutrition (Ireland)(38). There was also a tendency for dietary micronutrient density to be higher in UR, as seen in another studies(15). The explanation could be the omission or under-estimating of portion sizes of food groups such as oils and fats, whereas food groups such as cereals, fruit and vegetables portions were over-estimated. UR may report a higher intake of vegetable proteins such as legumes and less from meat and meat products. This hypothesis is supported by the highest value of fibre density among UR. In the case of alcohol, the lower values on UR may be related to social desirability.
Although the percentage of nutrients to total energy intake is similar, including or excluding misreporters, when studying nutrient inadequacy prevalence the exclusion leads to different results. The effect of exclusion of EI misreporters and the adjustment for the usual intake leads to a distribution with higher kurtosis, meaning less extreme values were seen, therefore the number of individuals below or above recommendations diminished.
The current study presents some strengths and limitations that should be addressed if further research is carried out. Data from a national representative sample were used and participation was independent of the regular use of the National Health System. This is because all individuals should be registered. Although the participation rate was low, the characteristics of the participants are similar to non-participants. The similarities found were the prevalence of obesity, fruit and vegetable consumption and in physical activity. However, participants were younger and more educated(17). Our interviews were conducted by highly trained observers with a nutrition background, according to standardized procedures, including objectively measured anthropometry. The e-platform, specifically designed for this project, allowed more accurate and easier data inclusion. The multiple-pass dietary interviews minimized the omission of possible forgotten foods and standardized the level of detail for describing foods. The method of elicit specific details of certain food items and the photographs of different portions helped to reduce portion-size measurement error. The distribution of the dietary report days was satisfactory. This study included only 2 d of dietary assessment and it is known that longer reporting periods, above 3 d and ideally 7 d, are preferable – namely because of the implications on nutrient estimations with higher intra-variability. However, using 2 d, it was possible to adjust for this intra-individual variability. We also have all the limitations inherent in the Goldberg method, namely the use of predictive equations. Schofield equations overestimate BMR in obese people, which may lead to larger values of under-reporting prevalence(46) and there might be some differences between our population and the reference population used. This method takes into account the variation over shorter recording periods, in that the cut-off for a 2-d recording period is lower than the cut off for a 7-d period, but it assumes that EIs are the same for each day of the week(47). EE was not objectively measured, physical activity was assessed by questionnaire. The majority of similar studies do not have an individual estimation of physical activity, so they use a fixed value for all the sample. On this study, each participant was assigned to an individual PAL, according to IPAQ, which allows to better classification of physical activity(11,12). Over-reporting of physical activity is probably the primary concern in surveys and this may introduce positive bias(10). The method implies that participants are maintaining body weight. We assumed that everyone in the sample is in body weight maintenance because we have no information about whether people are losing or gaining weight. Although we have information about dietary special days, we are unable to distinguish between under- and over-eaters from UR and OvR because information about eating less, the same or more than the usual amount, is missing. However, to minimize this possible bias, participants reporting unusual intakes were excluded. The definitions used to identify EI misreporting might not be applicable in special circumstances. For example, ill-patients or undernourished people have nutritional requirements generally different from the normal population.
Conclusion
EI misreporting is present in a considerable proportion of dietary reports, and misreporters have different characteristics when compared with PR. The current study found 28·5 and 1·4 % of under- and over-reporting, respectively. A higher risk of being classified as an under-reporter comparing with a plausible reporter was found in the following groups: female gender, 45–64 years (compared with older age), less educated participants, food insecurity, higher BMI, chronic disease, non-favourable self-reported health perception status, and those who never smoked.
UR, PR and OvR differ on nutrient intake, although in population studies of nutritional intake assessment, the exclusion of misreporters has no implication in the estimates. On the other hand, when the topic under study is nutritional inadequacy prevalence, an analysis excluding these cases is recommended, in particular for protein. In order to better interpret the results and to ensure sound nutritional policy, misreporters should be identified and used in sensitivity analysis but should not be excluded from the datasets.
Acknowledgements
Acknowledgements: The researchers acknowledge the IAN-AF Consortium and all institutions and persons involved in all phases of the Survey, as well as participants. IAN-AF 2015–2016 had the institutional support of the General Directorate for Health, the Central Administration of the Health System, the Regional Health Administrations, the Regional Health Secretariats of the Azores and Madeira and the European Food Safety Authority. Financial support: IAN-AF 2015–2016 received funding from the European Economic Area from Iceland, Liechtenstein and Norway through the EEA Grants Program – Public Health Initiatives, Area of Health Information Systems (PT06 – 000088SI3). Conflict of interest: None. Authorship: V.M. conceived the present aim, analysed and interpreted this data with M.S. support. M.S. also verified the analytical methods and gave additional inputs to the study design. E.R. collaborated on the planning of data collection and in the interpretation of results. C.L. and D.T. coordinated the IAN-AF 2015–2016 survey, formulating the main research questions and were also involved in the design and in the interpretation of results of the current study. V.M. wrote the manuscript and all authors discussed the results, contributing to the final document. Ethics of human subject participation: The study was approved by the National Commission for Data Protection, the Ethical Committee of the Institute of Public Health of the University of Porto and from the Ethical Commissions of each one of the Regional Administrations of Health. Informed consent was obtained from all participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019002465.
click here to view supplementary material
References
- 1. Subar AF, Freedman LS, Tooze JA et al. (2015) Addressing current criticism regarding the value of self-report dietary data. J Nutr 145, 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banna JC, McCrory MA, Fialkowski MK et al. (2017) Examining plausibility of self-reported energy intake data: considerations for method selection. Front Nutr 4, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castro-Quezada I, Ruano-Rodriguez C, Ribas-Barba L et al. (2015) Misreporting in nutritional surveys: methodological implications. Nutr Hosp 31, 119–127. [DOI] [PubMed] [Google Scholar]
- 4. Maurer J, Taren DL, Teixeira PJ et al. (2006) The psychosocial and behavioral characteristics related to energy misreporting. Nutr Rev 64, 53–66. [DOI] [PubMed] [Google Scholar]
- 5. Willett W (2013) Nutritional Epidemiology. 3rd ed. Oxford: Oxford University Press. [Google Scholar]
- 6. European Food Safety Authority (2014) Guidance on the EU Menu methodology. EFSA J 12, 3944. [Google Scholar]
- 7. Murakami K & Livingstone MB (2015) Prevalence and characteristics of misreporting of energy intake in US adults: NHANES 2003–2012. Br J Nutr 114, 1294–303. [DOI] [PubMed] [Google Scholar]
- 8. Jessri M, Lou WY & L’Abbe MR (2016) Evaluation of different methods to handle misreporting in obesity research: evidence from the Canadian national nutrition survey. Br J Nutr 115, 147–59. [DOI] [PubMed] [Google Scholar]
- 9. Livingstone MB & Black AE (2003) Markers of the validity of reported energy intake. J Nutr 133, 895s–920s. [DOI] [PubMed] [Google Scholar]
- 10. Rennie KL, Coward A & Jebb SA (2007) Estimating under-reporting of energy intake in dietary surveys using an individualised method. Br J Nutr 97, 1169–1176. [DOI] [PubMed] [Google Scholar]
- 11. Goldberg GR, Black AE, Jebb SA et al. (1991) Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 45, 569–581. [PubMed] [Google Scholar]
- 12. Black AE (2000) Critical evaluation of energy intake using the Goldberg cut-off for energy intake-basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord 24, 1119–1130. [DOI] [PubMed] [Google Scholar]
- 13. Tooze JA, Krebs-Smith SM, Troiano RP et al. (2012) The accuracy of the Goldberg method for classifying misreporters of energy intake on a food frequency questionnaire and 24-h recalls: comparison with doubly labeled water. Eur J Clin Nutr 66, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rippin HL, Hutchinson J, Jewell J et al. (2017) Adult nutrient intakes from current national dietary surveys of European populations. Nutrients 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poslusna K, Ruprich J, de Vries JH et al. (2009) Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr 101, S73–S85. [DOI] [PubMed] [Google Scholar]
- 16. Trijsburg L, Geelen A, Hollman PC et al. (2017) BMI was found to be a consistent determinant related to misreporting of energy, protein and potassium intake using self-report and duplicate portion methods. Public Health Nutr, 20, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopes C, Torres D, Oliveira A et al. (2018) National food, nutrition, and physical activity survey of the Portuguese General Population (2015–2016): protocol for design and development. JMIR Res Protoc 7, e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopes C, Torres D, Oliveira A et al. (2017) National Food, Nutrition and Physical Activity Survey of the Portuguese General Population. EN-1341, p. 37, EFSA supporting publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raper N, Perloff B, Ingwersen L et al. (2004) An overview of USDA’s dietary intake data system. J Food Compost Anal 17, 545–555. [Google Scholar]
- 20. European Food Safety Authority (2011) Report on the development of a food classification and description system for exposure assessment and guidance on its implementation and use. EFSA J 9, 2489. doi: 10.2903/j.efsa.2011.2489. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- 21. National Health Institute Doctor Ricardo Jorge (2007) Portuguese Food Composition Table. Lisbon.
- 22. European Food Safety Authority (2017) Dietary reference values for nutrients: summary report. EFSA Supp Publ 14, e15121E. doi: 10.2903/sp.efsa.2017.e15121. [DOI] [Google Scholar]
- 23. The International Society for the Advancement of Kinanthropometry (2001) International Standards for Anthropometric Assessment. Potchefstroom, South Africa: International Society for the Advancement of Kinanthropometry. [Google Scholar]
- 24. World Health Organization (1998) Obesity: Preventing and managing the global epidemic. Report on a WHO Consultation on Obesity. Geneva: WHO. [PubMed] [Google Scholar]
- 25. Bassett DR Jr (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35, 8, 1396. [DOI] [PubMed] [Google Scholar]
- 26. IPAQ Research Committee (2005) Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) - Short Form.
- 27. James WPT & Schofield EC (1990) Human Energy Requirements. USA.
- 28. Sawilowsky S (2009) New effect size rules of thumb. J Mod Appl Stat Methods 8, 467–474. [Google Scholar]
- 29. Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 30. Ribas-Barba L, Serra-Majem L, Roman-Vinas B et al. (2009) Effects of dietary assessment methods on assessing risk of nutrient intake adequacy at the population level: from theory to practice. Br J Nutr 101, S64–S72. [DOI] [PubMed] [Google Scholar]
- 31. De Ridder KBS, Brocatus L, Cuypers K, [Bel S, Tafforeau J et al. (2016) La consommation alimentaire. Dans: Enquête de Consommation Alimentaire 2014-2015, éditors] Rapport 4. Brussel: WIV-ISP. [Google Scholar]
- 32. Berta Vanrullen I, Volatier JL, Bertaut A et al. (2014) Characteristics of energy intake under-reporting in French adults. Br J Nutr 111, 1292–1302. [DOI] [PubMed] [Google Scholar]
- 33. Mattisson I, Wirfalt E, Aronsson CA et al. (2005) Misreporting of energy: prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmo Diet and Cancer cohort. Br J Nutr 94, 832–842. [DOI] [PubMed] [Google Scholar]
- 34. Johansson L, Solvoll K, Bjorneboe GE et al. (1998) Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr 68, 266–274. [DOI] [PubMed] [Google Scholar]
- 35. Murakami K, Livingstone MBE, Okubo H et al. (2018) Prevalence and characteristics of misreporting of energy intake in Japanese adults: the 2012 National Health and Nutrition Survey. Asia Pac J Clin Nutr 27, 441–450. [DOI] [PubMed] [Google Scholar]
- 36. Gemming L, Jiang Y, Swinburn B et al. (2014) Under-reporting remains a key limitation of self-reported dietary intake: an analysis of the 2008/09 New Zealand Adult Nutrition Survey. Eur J Clin Nutr 68, 259–264. [DOI] [PubMed] [Google Scholar]
- 37. Kye S, Kwon SO, Lee SY et al. (2014) Under-reporting of energy intake from 24-hour dietary recalls in the Korean National Health and Nutrition Examination Survey. Osong Public Health Res Perspect 5, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lutomski JE, van den Broeck J, Harrington J et al. (2011) Sociodemographic, lifestyle, mental health and dietary factors associated with direction of misreporting of energy intake. Public Health Nutr 14, 532–541. [DOI] [PubMed] [Google Scholar]
- 39. Yannakoulia M, Panagiotakos DB, Pitsavos C et al. (2007) Low energy reporting related to lifestyle, clinical, and psychosocial factors in a randomly selected population sample of Greek adults: the ATTICA Study. J Am Coll Nutr 26, 327–333. [DOI] [PubMed] [Google Scholar]
- 40. Davison KM (2013) Energy under-reporting in adults with mood disorders: prevalence and associated factors. Eat Weight Disord 18, 323–327. [DOI] [PubMed] [Google Scholar]
- 41. Lissner L (2002) Measuring food intake in studies of obesity. Public Health Nutr 5, 889–892. [DOI] [PubMed] [Google Scholar]
- 42. Scagliusi FB, Ferriolli E, Pfrimer K et al. (2008) Under-reporting of energy intake is more prevalent in a healthy dietary pattern cluster. Br J Nutr 100, 1060–1068. [DOI] [PubMed] [Google Scholar]
- 43. Subar AF, Kipnis V, Troiano RP et al. (2003) Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 158, 1–13. [DOI] [PubMed] [Google Scholar]
- 44. Lafay L, Mennen L, Basdevant A et al. (2000) Does energy intake underreporting involve all kinds of food or only specific food items? Results from the Fleurbaix Laventie Ville Sante (FLVS) study. Int J Obes Relat Metab Disord 24, 1500–1506. [DOI] [PubMed] [Google Scholar]
- 45. Hirvonen T, Mannisto S, Roos E et al. (1997) Increasing prevalence of underreporting does not necessarily distort dietary surveys. Eur J Clin Nutr 51, 297–301. [DOI] [PubMed] [Google Scholar]
- 46. Horgan GW & Stubbs J (2003) Predicting basal metabolic rate in the obese is difficult. Eur J Clin Nutr 57, 335–340. [DOI] [PubMed] [Google Scholar]
- 47. Whybrow S, Horgan G & Stubbs RJ (2008) Low-energy reporting and duration of recording period. Eur J Clin Nutr 62, 1148–1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019002465.
click here to view supplementary material


