Abstract
Objective:
The present study compared the age of first solid foods in a cohort of preterm infants with term infants and identified factors influencing timing of solid food introduction.
Design:
Structured interviews on infant feeding practices, growth and medical status at term equivalence and at 3, 6, 9 and 12 months corrected postnatal age. The age of solid food introduction was compared between term and preterm infants, and the influence of maternal, infant and milk feeding factors was assessed.
Setting:
This prospective longitudinal study recruited primary carers of preterm and term infants from a regional metropolitan referral hospital in eastern Australia.
Participants:
One hundred and fifty infants (preterm, n 85; term, n 65).
Results:
When corrected for prematurity, preterm infants received solid foods before the recommended age for the introduction of solid foods for term infants. Median introduction of solid foods for preterm infants was 14 weeks corrected age (range 12–17 weeks). This was significantly less than 19 weeks (range 17–21 weeks) for term infants (P < 0·001). Lower maternal education and male gender were associated with earlier introduction of solid foods among preterm infants.
Conclusions:
Preterm infants are introduced to solid foods earlier than recommended for term infants, taking account of their corrected age. Further research is needed to assess any risk or benefit associated with this pattern and thus to develop clear evidence-based feeding guidelines for preterm infants.
Keywords: Premature infants, Complementary foods, Introduction of solid foods, Prospective longitudinal study
In 2003 the WHO recommended that infants should be exclusively breast-fed for the first 6 months of life to achieve optimal growth, development and health(1). This guideline was adapted in Australia as part of the 2015 Infant Feeding Guidelines, published by the National Health and Medical Research Council(2). Specifically, the guideline states that ‘from around 6 months, infants should be offered a range of foods of an appropriate texture and consistency for their developmental stage’(2). Other authoritative agencies in Australia and abroad have released similar recommendations regarding the timing of solid food introduction. In 2016, based on emerging evidence regarding the introduction of solid foods and allergy prevention(3), the Australasian Society of Clinical Immunology and Allergy revised its infant feeding guidelines to recommend that ‘solid foods be introduced from 4 to 6 months of age, when the infant is developmentally ready to start solid foods’(4) and that allergenic foods should be introduced in the first 12 months of life to prevent the development of allergies. The 2016 Australian Infant Feeding Summit supported this approach, advising that ‘When your infant is ready, at around 6 months, but not before 4 months, start to introduce a variety of solid foods, starting with iron-rich foods’(5). This ‘around 6 months’ recommendation has been officially adopted worldwide, with available data in Australia suggesting that the majority of infants are introduced to solid foods between 5 and 6 months(6).
The timing of solid food introduction is an important issue as research suggests that in addition to prevention of allergies, the timing of solid food introduction may have implications for several health outcomes. Recent studies report that early introduction of solid foods is associated with longer sleep duration, less frequent waking at night and a reduction in serious sleep problems(7). The introduction of solid foods before 4 months is not recommended due to an associated increase in risk of allergy(8) and childhood obesity(9–11). It has also been hypothesised that early microbiota may be affected by the introduction of solids and mediate this potential link to childhood overweight(12).
However, whether current guidelines apply to the preterm infant is not clear, as there is insufficient evidence regarding the timing of solid food introduction to preterm infants and the associated health-related outcomes(5). Preterm infants are more likely to be growth-restricted compared with term infants and to have altered body composition at term-equivalent age compared with the term infant(13). The preterm infant may have increased nutritional requirements, but is also at risk of respiratory problems, gastro-oesophageal reflux and developmental delay. Early introduction of solid foods may assist with meeting these increased nutritional requirements and may assist in oral motor development. Again, however, there may be an association with rapid weight gain and childhood obesity for this group(11).
Recommendations for the introduction of solid foods to the preterm infant vary between sources(14–16). In the UK, the Department of Health suggested that solid foods should be introduced once the infant reaches 5 kg body weight, the extrusion reflex is lost and s/he is able to eat from a spoon(17). Later, the American Academy of Family Physicians suggested that solid foods could commence around 4 to 6 months corrected age (the age of an infant based on due date) and once the infant was oral-motor ready(18). Based on a compromise to balance the nutritional benefits of commencing solid foods from 13 weeks of uncorrected age with the risks of increased eczema development, along with ensuring developmental readiness, Palmer and Makrides(15) suggested that 3 months (13 weeks) corrected age was an appropriate age for introducing solid foods in preterm infants(15). In contrast, Gupta et al.(19) recommended 6 months for introduction of solid foods in preterm infants born at less than 34 weeks’ gestation, based on a higher rate of hospital admissions in a 4-month introduction group(19). That study was conducted in a lower-income country and therefore generalisability to higher-income countries is uncertain.
A number of factors that influence the timing of solid food introduction in the preterm infant have been described. Maternal factors, such as age, pre-pregnancy BMI and education, along with milk type and infant factors, such as birth weight and gestational age, may influence the age of solid food introduction(14, 20 ). In the term infant, reasons for early introduction of solid foods (<4 months of age) were explored in a study in the USA(21). Maternal perception of infant readiness, hunger, increased interest in solid foods, in addition to advice from a clinician and to improve the infant’s sleep, were all said to be drivers of early introduction of solid foods. Several of these reasons varied depending on the type of milk feeds the infant was receiving (breast-fed, mixed and formula-fed infants)(21). Another US study found that early introduction of solid foods was correlated with mother’s beliefs about early feeding before 6 months of age as well as the influence of infant temperament(22).
There have been no recently published data in Australia on the introduction of solid foods to preterm infants, with previously reported studies coming from the USA, UK and Europe. Historically there are significant variations in the timing reported between countries(14, 20, 23, 24); these vary between 11·5 and 15·1 weeks corrected age. The present study was developed based on previous longitudinal studies(14, 20 ) to update the current feeding practices for preterm infants in a developed country.
The present study thus aimed to determine the age of first solid foods in preterm infants compared with term infants and the factors associated with this, specifically: (i) the age (weeks) of first solid foods in preterm infants compared with term infants; (ii) the proportion of infants introduced to solid foods early (<17 weeks); and (iii) the factors associated with the introduction of first solid foods for preterm infants.
Materials and methods
Study design and participants
This was a prospective, birth cohort study in the Illawarra region of New South Wales, Australia. Prematurity was defined as infants born less than 37 weeks and 0 d of gestation. The introduction of solid foods was defined as the time when solid food (defined as semi-solid, soft or solid foods other than breast milk, formula or other milk) was offered on a daily basis. Both term and preterm infants were recruited from a public, metropolitan, regional referral hospital maternity ward and the associated neonatal unit from June 2014 to March 2016. The neonatal unit is a twenty-bed, level 4 unit providing care to preterm infants from 32 weeks’ gestation. It is a feeder and follow-up unit from higher-level neonatal units, so the majority of preterm infants living in this area are discharged home from this unit. This was a convenience sample of parents and/or carers, dependent on consent and availability of study personnel. Once per week parents of infants who met the research criteria were approached and, if they agreed to comply with the study schedule, consent was obtained.
Exclusion criteria were preterm and term infants with conditions that were likely to interfere with feeding, such as major congenital malformations, neurological conditions or substance withdrawal.
Data collection
Mothers were interviewed in the hospital by a paediatric dietitian shortly after birth (in hospital) and at 3, 6, 9 and 12 months corrected postnatal age (in hospital or by telephone or email). Structured interview questionnaires were developed and tested for face validity prior to implementation. Additional information was extracted from obtained hospital medical records for all time points. Baseline data were taken at birth (for term infants) and term equivalence (for preterm infants), including: maternal age, maternal country of birth, maternal self-reported pre-pregnancy BMI, level of education and infant gender, gestational age at birth, birth weight, medical history and head circumference. Infant feeding practices at birth admission (type of milk, feeding method) were also documented.
Data collected at subsequent time points included growth parameters (weight, length and head circumference), medical diagnoses and current feeding practices. Structured interviews covered milk type, the age of solid food introduction (chronological), and the type (food group) and order of first solid foods. A 24 h recall of the infant’s food intake was also collected at each time point. Additional qualitative information regarding the introduction of solid foods was also obtained, including the attitudes and beliefs that affected the parent’s decision to introduce foods, as well as who guided and/or provided advice concerning timing and type of foods. Participants who were unable to be interviewed in person or over the telephone were contacted via email and provided with an online version of the questionnaire.
Statistical methods
Using proportion being fed solid foods at <17 weeks corrected age from historical studies, a sample size calculation suggested sixty-five infants in each group, using 85 % power and α of 0·05. As completion rates were not known in advance, oversampling was undertaken initially. Final analyses were restricted to those participants who provided data on the primary study outcome, age of solid food introduction. De-identified data were analysed using the statistical software package IBM SPSS Statistics version 21. Mann–Whitney and χ 2 tests were conducted to explore differences in characteristics and outcomes of preterm and term infants, with significance set at P < 0·05.
Univariate analysis of factors associated with early introduction in preterm infants included gender, gestational age (<33 weeks/33–36 weeks), birthweight percentile (<10 %/10–90 %/>90 %), maternal education (<Year 12/≥Year 12), maternal BMI (≤25/>25 kg/m2) maternal age (<30/≥30 years) and milk type (exclusive breast milk/other) at baseline, 3, 6, 9 and 12 months.
Stepwise regression with backward elimination was performed to model the significant influences on the age of solid food introduction in preterm and term groups, respectively. For preterm infants, variables included were milk type at baseline, sex, birth percentile, gestational age, mother’s age, BMI and education. For term infants, variables included were milk type at baseline, sex, birth percentile, and mother’s age, BMI and education.
Results
Maternal and infant characteristics
Baseline characteristics of the preterm and term infants and their mothers are described in Table 1. There was no significant difference between groups by mother’s age or formal education. There was an unexpected difference in reported pre-pregnancy BMI, with the preterm group having more mothers in the healthy weight category (P = 0·046). As expected, there was a larger proportion of male infants than females in each group, reaching significance for the preterm group (P = 0·046), but the proportion of males was not significantly different between the term and preterm groups. There was a significantly lower birth weight percentile for the preterm group, reflecting the pathologies leading to preterm delivery.
Table 1.
Baseline characteristics of mothers and infants from a regional metropolitan referral hospital in the Illawarra region of New South Wales, Australia, June 2014–March 2016
| Preterm (n 85) | Term (n 65) | ||||
|---|---|---|---|---|---|
| Median or n | IQR or % | Median or n | IQR or % | P value | |
| Mother | |||||
| Age (years) | 30·7 | 28·4–35·5 | 30·2 | 26·1–35·6 | 0·39 |
| BMI†,‡ (kg/m2) | 22·0 | 20·3–27·2 | 24·9 | 21·1–27·8 | 0·03 |
| Healthy (<25 kg/m2) | 52 | 61 | 29 | 45 | |
| Overweight (≥25 kg/m2) | 25 | 29 | 30 | 46 | |
| Maternal education‡,§ | 0·57 | ||||
| <Year 12 | 44 | 52 | 31 | 48 | |
| ≥Year 12 | 40 | 47 | 34 | 52 | |
| Maternal country of birth∥ | 0·42 | ||||
| Australia | 80 | 94 | 60 | 92 | |
| China | 2 | 2 | 1 | 2 | |
| Europe | 2 | 2 | 1 | 2 | |
| Indonesia | 1 | 1 | 0 | 0 | |
| India or Pakistan | 0 | 0 | 2 | 3 | |
| Palestine | 0 | 0 | 1 | 2 | |
| Infant | |||||
| Gestational age (weeks) | 33·5 | 32·0–35·2 | 39·0 | 38·1–40·1 | 0·001 |
| Birth weight (kg) | 2·0 | 1·6–2·3 | 3·3 | 3·0–3·7 | 0·001 |
| Birth length (cm) | 43·5 | 41·0–46·0 | 50·0 | 48·0–51·0 | 0·001 |
| Birth percentile∥ | 28·0 | 14·0–53·0 | 55·0 | 25·0–83·0 | 0·001 |
| SGA (<10 %) | 13 | 15 | 10 | 15 | |
| AGA (10–90 %) | 70 | 82 | 47 | 72 | |
| LGA (>90 %) | 2 | 2 | 8 | 12 | |
| Gender | 0·14 | ||||
| Male | 56 | 66 | 35 | 54 | |
| Female | 29 | 34 | 30 | 46 | |
IQR, interquartile range; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
Continuous data are presented as median and IQR, categorical data as n and %.
Preterm, n 77; term, n 59.
The percentages for maternal BMI sub-categories (healthy and overweight) and maternal education (preterm infants) do not add up to 100 % due to missing data.
Preterm, n 84.
The percentages for maternal country of birth and birth percentile do not add up to 100 % due to rounding of percentage values.
Age at introduction of solid foods
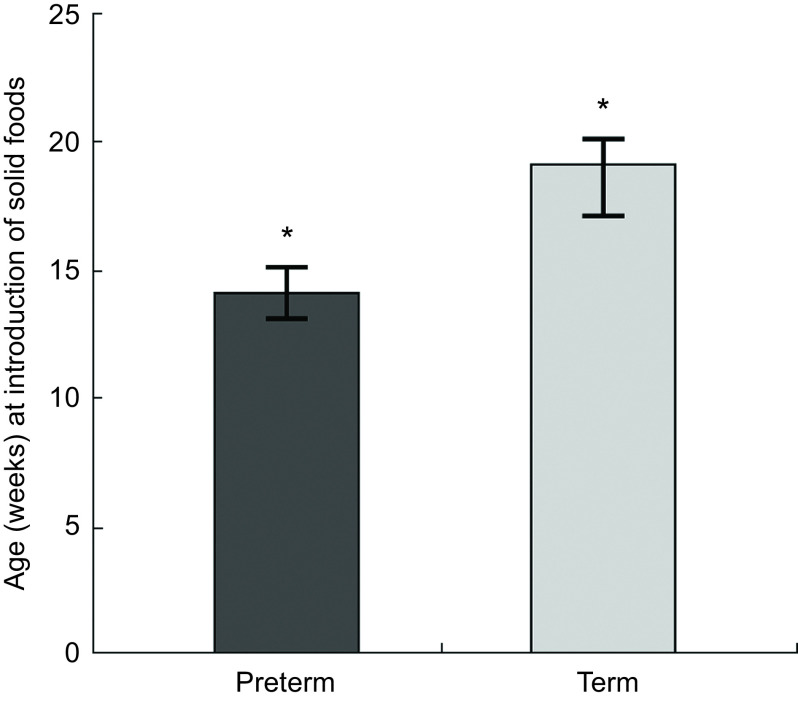
Solid foods were introduced to preterm infants at a median of 21 weeks post-delivery (range 18–24 weeks) which, although significantly later than the median of 19 weeks (range 17–21 weeks) of term infants (P < 0·002; Fig. 1), was found to be significantly earlier than for term infants when corrected for prematurity at birth: 14 weeks (range 12–17 weeks; P < 0·0001; Fig. 1). Seventy-one (83 %) preterm infants were introduced to solid foods before 17 weeks corrected age, compared with twenty-six (40 %) term infants (P = 0·001). By 6 months corrected age nearly all infants (96·7 %) were receiving solid foods, with no difference in the proportion being fed solid foods between groups.
Fig. 1.
Age (weeks) at introduction of solid foods in preterm† (n 85) and term infants (n 65) from a regional metropolitan referral hospital in the Illawarra region of New South Wales, Australia, June 2014–March 2016. Values are medians, with their 95 % CI represented by vertical bars. *Significant difference between groups: P < 0·05. †Corrected for prematurity in weeks post due date
Factors associated with age of solid food introduction
In our preterm population of infants, lower maternal education level and male gender were associated on univariate analysis with earlier introduction of solid foods (Table 2). Neither milk type nor birth percentile was associated with the age at introduction of solid foods (data not shown).
Table 2.
Univariate maternal, infant and feeding factors in relation to age in weeks at introduction of solid foods in preterm† and term infants from a regional metropolitan referral hospital in the Illawarra region of New South Wales, Australia, June 2014–March 2016
| Preterm (n 85) | Term (n 65) | |||||
|---|---|---|---|---|---|---|
| Factor | Median | IQR | P value | Median | IQR | P value |
| Gender | ||||||
| Male | 13·0 | 11·0–16·0 | 0·019* | 18·0 | 17·0–20·0 | 0·58 |
| Female | 15·0 | 13·0–18·0 | 19·5 | 17·0–22·0 | ||
| Gestational age | ||||||
| <33 weeks | 14·0 | 10·0–17·0 | 0·812 | N/A | N/A | |
| 33–36 weeks | 14·0 | 12·0–16·0 | N/A | N/A | ||
| Birth percentile | ||||||
| SGA (<10 %) | 15·0 | 13·0–17·0 | 0·930 | 18·5 | 17·0–21·0 | 0·159 |
| AGA (10–90 %) | 14·0 | 12·0–16·0 | 19·0 | 17·0–21·0 | ||
| LGA (>90 %) | 14·0 | 11·0–17·0 | 22·0 | 19·0–22·5 | ||
| Maternal education | ||||||
| <Year 12 | 13·0 | 10·0–16·0 | 0·012* | 17·0 | 16·0–20·0 | 0·009* |
| ≥Year 12 | 14·0 | 13·0–18·0 | 19·5 | 17·0–22·0 | ||
| Maternal BMI | ||||||
| Healthy weight | 14·0 | 11·5–17·0 | 0·320 | 19·0 | 17·0–21·0 | 0·516 |
| Overweight or obese | 15·0 | 13·0–17·0 | 19·0 | 16·0–21·0 | ||
| Maternal age | ||||||
| <30 years | 14·0 | 11·0–16·5 | 0·569 | 17·0 | 17·0–19·0 | 0·001* |
| ≥30 years | 14·0 | 12·0–17·0 | 21·0 | 18·0–23·0 | ||
IQR, interquartile range; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; N/A, not applicable.
Significant at P < 0.05.
Corrected for prematurity in weeks post due date.
In term infants, lower maternal education (<Year 12) and younger maternal age (<30 years) were associated on univariate analysis with earlier introduction of solid foods (Table 2).
Stepwise regression models were conducted to determine which factors predicted the age (weeks) of introduction of solid foods while controlling for all other variables. Only male gender and lower maternal education (<Year 12) were significant factors predicting earlier introduction of solid foods for preterm infants (Table 3). Similarly, for term infants, male gender (borderline significance), lower maternal education (<Year 12) as well as younger maternal age were significantly associated with the earlier introduction of solid foods.
Table 3.
Multiple regression of factors associated with the age in weeks at introduction of solid foods in preterm† and term infants from a regional metropolitan referral hospital in the Illawarra region of New South Wales, Australia, June 2014–March 2016
| Cohort | Factor | B | se (B) | β | P value |
|---|---|---|---|---|---|
| Preterm | Gender | 1·9 | 0·9 | 0·2 | 0·038* |
| Maternal education | 1·9 | 0·9 | 0·3 | 0·026* | |
| Term | Gender | 1·6 | 0·8 | 0·2 | 0·053 |
| Maternal age | 2·3 | 0·9 | 0·3 | 0·012* | |
| Maternal education | 1·8 | 0·9 | 0·3 | 0·043* |
B, unstandardised regression coefficient; se(B), standard error of the coefficient; β, standardised coefficient.
Significant at P < 0·05.
Corrected for prematurity in weeks post due date.
Rice cereal (68 %) was the most common first solid food offered to the infant, with fruit (18 %) and vegetables (12 %) also offered as a first foods. When parents were asked about the main governors of solid food introduction for their infant, the most cited influences were perception of milk not satisfying the infant; the infant appeared developmentally ready for solid foods; and from advice of health professionals (paediatricians and dietitians ranked most frequently).
Discussion
This cohort of preterm infants (n 85) in Australia commenced solid foods at 14 weeks (corrected age), corresponding to 21 weeks (chronological age), which was significantly earlier than the 19 weeks for term infants. This finding is similar to other studies conducted previously in the UK, Europe and the USA(14, 20, 23, 25 ). The US longitudinal study conducted in 2001 (but reported after our study commenced) determined that in the USA solid foods were introduced to preterm infants at a corrected age of 13 weeks and to term infants at 17 weeks(23). Two previous observational studies, restricted to preterm infants, showed both earlier and later introduction of solid foods: a UK study estimated that complementary feeding among preterm infants commenced at 11·5 weeks corrected age(20); and a similar preterm study from Italy reported that solid foods were offered slightly later, at 15 weeks corrected age, in their population(14). A recent German national survey of parents of very-low-birth-weight infants had an average of 15 weeks, 6·5 weeks earlier than term infants(25). These timing differences have been attributed to differing local recommendations, environmental factors, parental cultural beliefs and attitudes towards introducing solid foods specific to each country(14, 23, 26, 27 ). Regardless of the variations in the timing between countries, research thus consistently confirms our findings, with large proportions of the preterm population, ranging from 40 to 98 %, commencing solid foods before the recommended age when adjusting age for prematurity(14, 20, 23–26, 28 ).
Parents of preterm infants, or their professional advisors, appear not to be adjusting for prematurity when introducing solid foods. Most guidelines state that preterm infants should be corrected for prematurity until 2 years of age(29, 30 ), as the development of fine and gross motor skills, cognition and growth among the preterm population is dependent on corrected age and this continues into early childhood(29, 31, 32 ). Introducing solid foods to premature infants before they have developed the necessary fine and gross motor skills may be unsafe and may contribute to the long-term abnormal feeding behaviours more commonly seen in these populations(33–35). This failure to correct for prematurity has been documented among health-care providers. D’Agostino et al. (36) found for the majority of child care visits that primary care providers were not adjusting for prematurity and almost one-third of paediatricians were not informing parents of their infant’s corrected age(37). Thus, advice to parents on age of commencing solid foods was earlier than recommended guidelines(36, 37 ).
Suggestions that introducing solid foods before 4 months of age may increase the risk of chronic conditions such as obesity are currently being explored(11), with recent research finding no significant difference in BMI Z-score at 1 year between early and later introduction of solid foods in preterm infants( 11 ). Another study, by Spiegler et al.(25), also found the early introduction of solid foods did not negatively influence length or weight gain in very-low-birth-weight infants(25). In contrast, Pluymen et al.(38) found the prevalence of overweight throughout childhood was higher in children with introduction of solid foods before 4 months (with a higher risk in formula-fed infants and those breast-fed for <4 months)(38). It has thus been suggested that any obesity association may be more of a risk in older gestational age preterm infants with no co-morbidities than in those of earlier gestational age(39). More recently, researchers have identified that preterm infants have altered body composition, characterised predominantly by less fat-free mass but a maintained fat mass, leading to a greater percentage total body fat(13, 40, 41 ). Further research into the childhood and later-life obesity risks of timing of the introduction of solid foods is required in these at-risk populations.
The appropriate time to commence solid foods in the preterm infant is unclear(5) and may not be the same as for the term infant, as there are a number of preterm-specific factors to be considered. Fetal nutrient accretion peaks during the third trimester of pregnancy. Premature infants thus have limited nutrient stores at birth(42). Some infant feeding guidelines recommend that meat, fish, poultry, tofu, legumes or iron-enriched infant cereal should be among the first foods introduced, prior to fruit, vegetables and dairy, to address the perceived inadequacy of milk diets in meeting the nutritional requirements of premature infants, in particular for iron and zinc(2). The potential consequence of preterm infant deficiencies, such as anaemia of prematurity, are known but also apply to other tissue growth, including the brain(42). Despite this, both our study and others suggest the first foods offered are consistently poor sources of energy, protein, iron and zinc(14, 20 ). Recent studies in both lower- and higher-income countries also identified that first foods are often less energy-dense and require the infant to consume more to meet energy needs(19, 25 ). Furthermore, it has been suggested that these compositional dietary differences, rather than timing, may be more of an obesity risk(25). High energy and dairy protein in infancy have been associated with increased infant fat mass and these changes may be mediated by differences in gut microbiota(13).
A number of maternal factors have been identified in the literature as predictors of early introduction of solid foods, including younger age, a lower level of educational attainment and higher BMI(14, 20, 23, 43, 44 ). In the present study only lower maternal education was a significant predictor of earlier introduction of solid foods. Other factors identified in the literature to predict early introduction of solid foods include smoking, low socio-economic status, previous children and milk feeding practices(14, 23, 26, 28, 44 ), all of which are known to be associated with maternal education level. The influence of milk feeding practices in the present study was found to be similar to other studies, with breast-fed infants more likely to have solid foods introduced later. Consistent with other studies the only infant characteristic that was significantly associated with the timing of solid food introduction was gender. Males were significantly more likely to commence solid foods earlier than females, but it is not known if this is due to cultural or biological influences(14, 20 ).
The main reasons given by mothers in our study for when solid foods were introduced were the perception that milk was not satisfying the infant, that the infant was developmentally ready or following the advice given by a health professional. These reasons were similar to those given in studies of term infants(22). There is some evidence in the literature of a perception of the importance of ‘catch-up’ growth for the preterm population and a need to meet developmental milestones similar to that of term babies, i.e. a failure to correct temporal expectations for the degree of prematurity(45).
There are some limitations to our study. The data were limited to a convenience sample within our health district and thus might not be generalisable to the larger Australian or international population. Our area is, however, highly representative of national cohorts(46) and our results are largely consistent with international findings. Maternal demographic data and infant feeding practices were self-reported and so subject to the usual limits of recall bias. Study strengths include prospective recruitment(47, 48 ) in a public hospital environment, thus including those born over a full range of socio-economic circumstances. Our level 4 neonatal unit accepts preterm infants from higher level 5/6 units and therefore we were able to obtain a representative sample of preterm infants living in the Illawarra area. Our study is in a non-US, non-European environment but consistent with findings from these areas. There is little information to guide the application of these findings to emerging economies, where the risks of increasing obesity and other non-communicable diseases are escalating and where weaning practices are starting to be examined for these populations(19).
Our study demonstrates the current feeding practices of preterm infants at weaning. We have confirmed that infants born prematurely are still introduced to solid foods well before the recommended age for term infants, without clear evidence of the benefits or risks associated with this practice. Further larger studies into the specific characteristics of preterm infants that might affect the introduction of solids, such as medical conditions at birth and at the time of solid food introduction, Apgar scores and days to reach full enteral feeds, are required. The ideal timing of introduction of solid foods to the preterm infant needs to be studied further in respect to allergy prevention, reducing the risk of childhood or later-life obesity, reducing the risk of hospitalisation and optimisation of the microbiota. In addition, studies should be conducted on nutritional composition of first foods, as this may also affect obesity risk, fat mass, nutrient intakes and changes in microbiota. Finally, these studies need also to be undertaken in the emerging economic areas, where the potential effects of future disease are huge.
The present study highlights some of the maternal and infant predictors of early solid food introduction, which could be used in practice to identify those at risk of early solid food introduction. It also highlights the need to educate health professionals in the use of prematurity corrected age so that advice provided for preterm infants is consistent and developmentally appropriate. Although the lack of evidence for specific feeding practices for preterm infants leads clinicians to base recommendations on the degree of prematurity, growth patterns, nutritional requirements and developmental readiness, the present study highlights the need for further research to determine an evidence base for the recommendations for preterm infants.
Acknowledgements
Acknowledgments: The authors thank Steven Bowden, Amanda Owers, Renee Jennings, Marijka Batternham, Yasmin Probst and all the nurses, midwives, parents and infants who helped with the Feeding Your Infant study. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None declared. Authorship: J.C. and I.M.W. designed the study; J.C., A.H. and S.M.C.D. conducted the study; J.C., A.H., S.M.C.D. and I.M.W. analysed and interpreted the results; all authors drafted the paper and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the University of Wollongong & Illawarra Shoalhaven Local Health District Human Research Ethics Committee. Written informed consent was obtained from all subjects.
References
- 1. World Health Organization & UNICEF (2003) Global strategy for infant and young child feeding. https://www.who.int/nutrition/publications/infantfeeding/9241562218/en/ (accessed August 2019).
- 2. National Health and Medical Research Council (2012) Infant Feeding Guidelines: information for health workers. http://nhmrc.gov.au/about-us/publications/infant-feeding-guidelines-information-health-workers (accessed August 2019).
- 3. Du Toit G, Roberts G, Sayre PH et al. (2015) Randomized trial of peanut consumption in infants at risk for peanut allergy. New Engl J Med 372, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Australasian Society of Clinical Immunology and Allergy (2016) ASCIA Guidelines – Infant feeding and allergy prevention. https://www.allergy.org.au/hp/papers/infant-feeding-and-allergy-prevention (accessed December 2018).
- 5. Netting MJ, Beck KM, Dharmage SC et al. (2017) An Australian consensus of infant feeding guidelines ot prevent food allergy: outcomes from the Australian infant feeding summit. J Allergy Clin Immunol Pract 5, 1617–1624. [DOI] [PubMed] [Google Scholar]
- 6. Australian Institute of Health and Welfare (2010) Australian National Infant Feeding Survey: indicator results. https://www.aihw.gov.au/getmedia/af2fe025-637e-4c09-ba03-33e69f49aba7/13632.pdf.aspx?inline=true (accessed August 2019).
- 7. Perkin MR, Bahnson HT, Logan K et al. (2018) Association of early introduction of solid foods with infant sleep: a secondary analysis of a randomized clinical trial. JAMA Pediatr 172, e180739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fewtrell M, Bronsky J, Campoy C et al. (2017) Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr 64, 119–132. [DOI] [PubMed] [Google Scholar]
- 9. Pearce J, Taylor MA, Langley-Evans SC et al. (2013) Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond) 37, 1295–1306. [DOI] [PubMed] [Google Scholar]
- 10. Weng SF, Redsell SA, Swifth JA et al. (2012) Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child 97, 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vissers KM, Feskens EJM, van Goudoever JB et al. (2018) The timing of initiating complementary feeding in preterm infants and its effect on overweight: a systematic review. Ann Nutr Metab 72, 307–315. [DOI] [PubMed] [Google Scholar]
- 12. Iozzo P & Sanguinetti E (2018) Early dietary patterns and microbiota development: still a way to go from descriptive interactions to health-relevant solutions. Front Nutr 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson MJ, Wootton SA, Leaf AA et al. (2012) Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 130, e640–e649. [DOI] [PubMed] [Google Scholar]
- 14. Fanaro S & Vigi V (2007) Weaning preterm infants: an open issue. J Pediatr Gastroenterol Nutr 45, Suppl. 3, S204–S209. [DOI] [PubMed] [Google Scholar]
- 15. Palmer DJ & Makrides M (2012) Introducing solid foods to preterm infants in developed countries. Ann Nutr Metab 60, 31–38. [DOI] [PubMed] [Google Scholar]
- 16. Fewtrell MS, Morgan JB, Duggan C et al. (2007) Optimal duration of exclusive breastfeeding: what is the evidence to support current recommendations? Am J Clin Nutr 85, issue 2, 635S–638S. [DOI] [PubMed] [Google Scholar]
- 17. Department of Health (1994) Weaning and The Weaning Diet: Report of the Working Group on the Weaning Diet of the Committee on Medical Aspects of Food Policy. Report on Health and Social Subjects no. 45. London: HMSO. [PubMed] [Google Scholar]
- 18. LaHood A & Bryant CA (2007) Outpatient care of the premature infant. Am Fam Physician 76, 1159–1165. [PubMed] [Google Scholar]
- 19. Gupta S, Agarwal R, Aggarwal KC et al. (2017) Complementary feeding at 4 versus 6 months of age for preterm infants born at less than 34 weeks of gestation: a randomised, open-label, multicentre trial. Lancet Glob Health 5, e501–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norris FJ, Larkin MS, Williams CM et al. (2002) Factors affecting the introduction of complementary foods in the preterm infant. Eur J Clin Nutr 56, 448–454. [DOI] [PubMed] [Google Scholar]
- 21. Clayton HB, Li R, Perrine CG et al. (2013) Prevalence and reasons for introducing infants early to solid foods: variations by milk feeding type. Pediatrics 131, e1108–e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doub AE, Moding KJ & Stifter CA (2015) Infant and maternal predictors of early life feeding decisions. The timing of solid food introduction. Appetite 92, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braid S, Harvey EM, Bernstein J et al. (2015) Early introduction of complementary foods in preterm infants. J Pediatr Gastroenterol Nutr 60, 811–818. [DOI] [PubMed] [Google Scholar]
- 24. Yee J, Smith A, O’Connor D et al. (2001) Introduction of complementary foods in preterm infants varies among countries. J Am Diet Assoc 101, A76. [Google Scholar]
- 25. Spiegler J, Eisemann N, Ehlers S et al. (2015) Length and weight of very low birth weight infants in Germany at 2 years of age: does it matter at what age they start complementary food? Eur J Clin Nutr 69, 662–667. [DOI] [PubMed] [Google Scholar]
- 26. Fewtrell MS, Lucas A & Morgan JB (2003) Factors associated with weaning in full term and preterm infants. Arch Dis Child Fetal Neonatal Ed 88, F296–F301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamilton K, Daniels L, White KM et al. (2011) Predicting mothers’ decisions to introduce complementary feeding at 6 months. An investigation using an extended theory of planned behaviour. Appetite 56, 674–681. [DOI] [PubMed] [Google Scholar]
- 28. Morgan JB, Williams P, Foote KD et al. (2006) Do mothers understand healthy eating principles for low-birth-weight infants? Public Health Nutr 9, 700–706. [DOI] [PubMed] [Google Scholar]
- 29. Rickards AL, Kitchen WH, Doyle LW et al. (1989) Correction of developmental and intelligence test scores for premature birth. J Paediatr Child Health 25, 127–129. [DOI] [PubMed] [Google Scholar]
- 30. Department of Education and Early Childhood Development, Maternal and Child Health Service, State Government of Victoria (2009) Standard 2: optimal health and development. In Maternal and Child Health Service Program Standards, pp. 20–27. Melbourne: Programs and Partnerships Division, Office for Children and Portfolio Coordination. [Google Scholar]
- 31. King C (2009) An evidence based guide to weaning preterm infants. Paediatr Child Health 19, 405–414. [Google Scholar]
- 32. Brandt I, Sticker EJ, Gausche R et al. (2005) Catch-up growth of supine length/height of very low birth weight, small for gestational age preterm infants to adulthood. Pediatrics 147, 662–668. [DOI] [PubMed] [Google Scholar]
- 33. Hawdon JM, Beauregard N, Slattery J et al. (2000) Identification of neonates at risk of developing feeding problems in infancy. Dev Med Child Neurol 42, 235–239. [DOI] [PubMed] [Google Scholar]
- 34. Escobar GJ, Joffe S, Gardner MN et al. (1999) Rehospitalization in the first two weeks after discharge from the neonatal intensive care unit. Pediatrics 104, e2. [DOI] [PubMed] [Google Scholar]
- 35. Demauro SB, Patel PR, Medoff-Cooper B et al. (2011) Postdischarge feeding patterns in early- and late-preterm infants. Clin Pediatr 50, 957–962. [DOI] [PubMed] [Google Scholar]
- 36. D’Agostino JA, Gerdes M, Hoffman C et al. (2013) Provider use of corrected age during health supervision visits for premature infants. J Pediatr Health Care 27, 172–179. [DOI] [PubMed] [Google Scholar]
- 37. Chung J, Lee J, Spinazzola R et al. (2014) Parental perception of premature infant growth and feeding behaviors: use of gestation-adjusted age and assessing for developmental readiness during solid food introduction. Clin Pediatr 53, 1271–1277. [DOI] [PubMed] [Google Scholar]
- 38. Pluymen LP, Wijga AH, Gehring U et al. (2018) Early introduction of complementary foods and childhood overweight in breastfed and formula-fed infants in the Netherlands: the PIAMA birth cohort study. Eur J Nutr 57, 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasylyeva TL, Barche A, Chennasamudram SP et al. (2013) Obesity in prematurely born children and adolescents: follow up in pediatric clinic. Nutr J 12, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gianni ML, Roggero P, Liotto N et al. (2012) Postnatal catch-up fat after late preterm birth. Pediatr Res 72, 637–640. [DOI] [PubMed] [Google Scholar]
- 41. Al-Theyab N (2018) Body composition in very preterm infants during their hospital stay. PhD Theses, The University of Queensland. [Google Scholar]
- 42. Shah MD & Shah SR (2009) Nutrient deficiencies in the premature infant. Pediatr Clin North Am 56, 1069–1083. [DOI] [PubMed] [Google Scholar]
- 43. Grummer-Strawn LM, Li R, Perrine CG et al. (2014) Infant feeding and long-term outcomes: results from the year 6 follow-up of children in the Infant Feeding Practices Study II. Pediatrics 134, Suppl. 1, S1–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greer FR, Sicherer SH, Burks AW et al. (2008) Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121, 183–191. [DOI] [PubMed] [Google Scholar]
- 45. Scott JA, Binns CW, Graham K et al. (2009) Predictors of the early introduction of solid foods in infants: results of a cohort study. BMC Pediatr 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moses RG, Wong VCK, Lambert K et al. (2016) The prevalence of hyperglycaemia in pregnancy in Australia. Aust N Z J Obstet Gynaecol 56, 341–345. [DOI] [PubMed] [Google Scholar]
- 47. Newby RM & Davies PS (2015) A prospective study of the introduction of complementary foods in contemporary Australian infants: what, when and why? J Paediatr Child Health 51, 186–191. [DOI] [PubMed] [Google Scholar]
- 48. Li R, Scanlon KS & Serdula MK (2005) The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev 63, 103–110. [DOI] [PubMed] [Google Scholar]