Abstract
Objective:
Due to the growing interest in the role of dietary patterns (DPs) on chronic diseases, we assessed the association between a posteriori identified DPs in the Seguimiento Universidad de Navarra (SUN) Project – a prospective cohort study in a Mediterranean country – and breast cancer (BC) risk.
Design:
DPs were ascertained through a principal component analysis based on 31 predefined food groups. BC cases were initially identified through self-report or, if deceased, from death certificates or by notification by the next kin. Women reporting BC were asked to provide a copy of their medical report and diagnoses for confirmation purposes. We fitted Cox regression models to assess the association between adherence to the identified DPs and BC risk.
Setting:
Spanish university graduates.
Participants:
We included 10 713 young and middle-aged – mainly premenopausal – women.
Results:
After a median follow-up of 10·3 years, we identified 100 confirmed and 168 probable incident BC cases. We described two major DPs: ‘Western dietary pattern’ (WDP) and ‘Mediterranean dietary pattern’ (MDP). A higher adherence to a WDP was associated with an increased risk of overall BC (multivariable-adjusted HR for confirmed BC Q4 v. Q1 1·70; 95 % CI 0·93, 3·12; P for trend = 0·045). Contrarily, adherence to a MDP was inversely associated with premenopausal BC (multivariable-adjusted HR Q4 v. Q1 0·33; 95 % CI 0·12, 0·91). No significant associations were observed for postmenopausal BC.
Conclusions:
Whereas a higher adherence to the WDP may increase the risk of BC, a higher adherence to the MDP may decrease the risk of premenopausal BC.
Keywords: Mediterranean dietary pattern, Western dietary pattern, Breast cancer, SUN Project, Cohort study
Worldwide, the number of newly diagnosed female breast cancer (BC) cases in 2018 was estimated to be 2·1 million, accounting for almost 1 in 4 cancer cases among women. BC is the most frequently diagnosed cancer in the vast majority of the countries (154 of 185), and it is also the leading cause of cancer death in over 100 countries(1). Global incidence of BC has raised in the last 10 years, mainly due to population growth and aging and statistics predict that one in every 14 women will develop BC before they turn 80 years according to estimates from 2010 to 2015(2). Similarly, in Spain in 2015, BC was the fourth most common cancer overall (27 747 incident cases) and the first among women(3). Regarding the impact of this disease on public health, preventive strategies may help address this concern and diminish the disease burden.
Several well-established non-modifiable and modifiable risk factors have been identified for BC. Among the latter, the only confirmed associations with higher BC incidence are oral contraceptives(4), menopausal hormone replacement therapy(5), adult attained height(6), greater birth weight (or its consequences)(6), nulli- and late parity(7), alcohol consumption(6), not breast-feeding(6), excess body fat for postmenopausal BC(6) and lack of physical exercise(6).
The role of diet, and particularly individual nutrients or foods, in BC has been broadly explored during decades. Nevertheless, and except for alcohol consumption, the relationships between diet and BC are still conflicting and inconsistent(8). This may be partly due to the fact that people do not consume nutrients or foods in isolation, but foods are consumed within a dietary pattern (DP) that reflects the nature of true dietary exposures (micro- and macronutrients interactions) in a community. Considering the potential effects of diet in a holistic approach to health, there is a growing interest on the evaluation of commonly adopted DPs among the population in relation to incident BC.
A priori and a posteriori DPs represent the amounts, frequency and combinations of the consumption of different foods and nutrients. Interestingly, DPs consider their interactions, which could be masked when analysed separately. In particular, a ‘Western DP’ (WDP) has been associated with increased BC risk(9–11), both among premenopausal(10,12) and postmenopausal women(9,12,13) and, specifically, with the ER+/PR+ BC subtype(9,10). On the other hand, when some studies assessed a ‘Mediterranean DP’ (MDP), they reported an inverse association between this DP and overall BC incidence(10,12,14,15), both for postmenopausal BC(10,12,15–17) and premenopausal BC(18) and, specifically, for certain BC subtypes (ER-/PR-(15), TNBC(10,15)]. Four observational studies – three case–control studies(10,12,16) and one prospective cohort study(17,19) – have evaluated the association between adherence to the MDP and BC, in a Mediterranean country. Moreover, most of those studies considered postmenopausal BC as outcome.
Thus, we aimed to assess if a posteriori identified DPs in a relatively young Mediterranean cohort of university graduates (‘Seguimiento Universidad de Navarra’ [SUN] Project) were associated with BC risk.
Methods
Study population
This work was conducted within the ‘Seguimiento Universidad de Navarra’ [SUN] Project, which is a Spanish ongoing, multipurpose and prospective cohort. The participants are all university graduates, and the enrollment is continuously open. Study design, methods and cohort profile have been published in detail elsewhere(20).
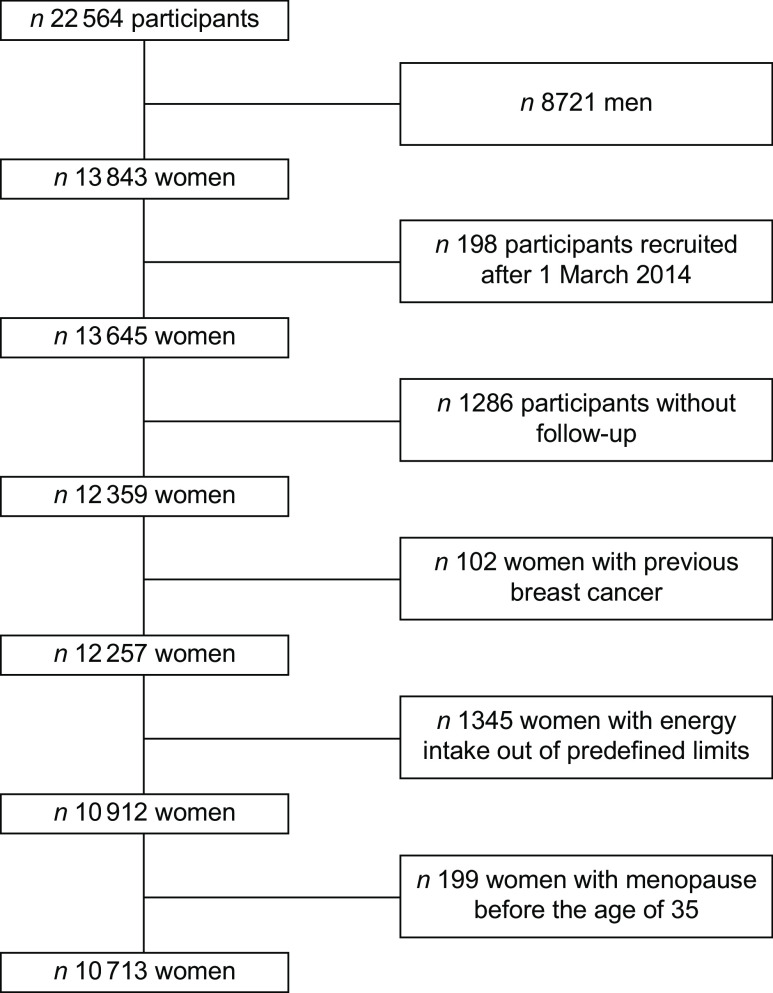
Up to December 2016, the SUN cohort included the data of 22 546 participants, and 13 843 of them were female participants. To guarantee a minimum follow-up time of 2 years, we included in the analysis women recruited before 1 March 2014 (n 13 645). Additionally, women were excluded if they were lost to follow-up (n 1286, retention rate 91 %), were prevalent BC cases at baseline (n 102), presented implausible energy intakes (below 2092 or beyond 14 644 kJ/d) (n 1345) or referred menopause before 35 years of age (n 199). Therefore, our sample size included 10 713 women (Fig. 1).
Fig. 1.
Flow chart of participants recruited in the SUN Project, 1999–2016
Dietary patterns
Information on diet was collected from a previously validated 136-item semi-quantitative food-frequency questionnaire at baseline (21–23). For each food item, there were 9 possible answers on frequencies of consumption (never/seldom, 1–3 servings/month, 1 serving/week, 2–4 servings/week, 5–6 servings/week, 1 serving/d, 2–3 servings/d, 4–6 servings/d and >6 serving/d). We multiplied typical portion sizes by consumption frequency for each food item to calculate daily food consumption in grams and grouped these consumptions into 31 predefined categories(24). To identify the main DPs in the SUN Project, we performed a principal component analysis on these 31 food groups. The number of components to be extracted was determined by a screen plot test. Component loadings >0·25 were considered relevant for the DPs. Each participant score was obtained by summing the consumption of each food group weighted by the component specific loading. Quartiles were obtained from the quantitative score. In addition, Spanish food composition tables were used to derive the nutrient composition of dietary intake(25,26).
Ascertainment of incident breast cancer cases
For the current analysis, incident BC was the primary endpoint. Participants who self-reported a newly diagnosed BC in any of the biennial follow-up questionnaires were asked to provide a medical record validating the diagnosis. Thereupon, these documents were evaluated by a trained oncologist, completely blinded to dietary exposures of participants, which confirmed the diagnoses and these cases were classified as confirmed cases for the analyses. Other BC cases for which clinical data could not be obtained were considered for the analyses as probable incident cases. Subjects’ next of kin and work associates reported fatal cases to our research team, while postal authorities also provided information about recent deaths. For participants lost to follow-up or with unknown causes of death, the National Death Index was consulted at least once a year to identify deceased cohort members and confirm the cause of death.
We stratified our analyses by pre- or postmenopausal BC. Information on age at menopause was collected in the baseline questionnaire and updated in the 16-year follow-up questionnaire. For women lacking of information on age at menopause, we defined postmenopausal status according to the 75th percentile of age at menopause in the study population (52 years of age)(27). When assessing premenopausal BC as outcome, we excluded those women who reported having had menopause before recruitment.
Evaluation of covariates
The baseline questionnaire also included information about participants’ lifestyles, anthropometric measures, medical history and socio-demographic characteristics. Physical activity was assessed with a previously validated questionnaire(28). We estimated metabolic equivalents (METs) for each participant to obtain METs-h/week scores(29). We had previously validated the accuracy of self-reported weight and height for BMI in a subsample of this cohort(30).
Statistical analysis
We described baseline characteristics of the participants according to quartiles of the two a posteriori identified DPs. We used means and sd for continuous variables and percentages for categorical variables.
Person-years of follow-up were calculated for each participant from the date of completion of the baseline questionnaire to the date of BC diagnosis, the date of death or the date of return of the last follow-up questionnaire, whichever occurred first. Hazard ratios (HRs) and 95 % CI were estimated using Cox proportional hazard models considering the lowest quartile of each DP as the reference category and with age as underlying time-variable. In an attempt to control for potential confounding factors, we used successive degrees of adjustment: model 1) adjustment for age as underlying time variable and age (in decades) and calendar year of recruitment in the cohort (3 categories) as stratification variables; model 2) additional adjustment for height (cm), years at university (years), relatives with history of BC (none, at least one aged up to 45 years or at least one but after the age of 45 years for mother, grandmothers and sisters), smoking status (never smoker; former smoker; current smoker), physical activity (METs-h/week), alcohol intake (g/d), BMI (kg/m2), age of menarche (years), age of menopause (years, for postmenopausal women only), number of pregnancies of more than 6 months (0–13), pregnancy before the age of 30 years (yes; no), lifetime breastfeeding (months), use of hormone replacement therapy (yes; no) and its duration (years); and 3) additional adjustment for diabetes (yes; no) and total energy intake (kJ/d). When we assessed postmenopausal BC risk, we additionally adjusted for the time since recruitment and for age at menopause. Finally, given the number of events in our cohort, we estimated an alternative model adjusting for tertiles of propensity scores of adherence to the quartiles of the DPs calculated with the same potential confounders that were described for the fully adjusted model. Odds of adherence to the quartiles of the DPs were estimated with multinomial logistic regression models with quartiles of adherence to the DP as dependent variable and the potential confounders considered in model 3 as predictors.
Tests of linear trend were conducted assigning to each category of the DPs its quartile-specific median and using the resulting variable as continuous in the above-mentioned models.
In order to assess the incidence of pre- or postmenopausal BC as outcome, we split the time at risk of our participants into the time during which they were premenopausal and the time during which they were postmenopausal. A question on age at menopause was included in the baseline questionnaire and after 16 years of follow-up. When assessing premenopausal BC as the outcome, we excluded those women who were postmenopausal at study onset and censored follow-up time at the age of 52 years or at the age of menopause – for those women with menopause during follow-up at an age earlier than 52 years. For postmenopausal BC, we considered to be at risk only those women who had turned 52 years old or after their menopause – for those women with menopause during follow-up at an age older than 52 years. In the analysis with postmenopausal BC as the outcome, we additionally adjusted for time since recruitment until the beginning of the time at risk and age at menopause (<50 years, ≥50 years).
The assumption of the proportionality of the hazards was assessed with the Schoenfeld residuals test.
All P values were two-tailed, and a P value < 0·05 was deemed as statistically significant. All analyses were performed with Stata/SE 15.0.
Results
The principal component analysis yielded two main a posteriori DPs (Table 1), which explained 14 % of the total food consumption variability. Factor loadings for the two identified DPs are presented in Table 1. The first vector was characterised by a high consumption of whole-fat dairy products, commercial bakery, sauces, processed meals, fast food, processed and unprocessed red meat and potatoes and by a low consumption of vegetables, fruits, whole-grains, low-fat dairy products and fish and we labeled it as a ‘Western Dietary Pattern’ (WDP). The second vector was characterised by a high consumption of vegetables, fruits, legumes, nuts, eggs, fish, natural fruit juices, processed and unprocessed red meat, poultry, olive oil, potatoes and other fruits. We labelled this second pattern as a ‘Mediterranean Dietary Pattern’ (MDP).
Table 1.
Component loadings for the Western and Mediterranean dietary patterns identified with principal component analyses in the Seguimiento Universidad de Navarra Project (1999–2014)
| Food group | WDP | MDP |
|---|---|---|
| Vegetables | –0·41 | 0·51 |
| Fruits | –0·47 | 0·38 |
| Legumes | 0·30 | |
| Whole grains | –0·33 | |
| Whole-fat dairy products | 0·42 | |
| Low-fat dairy products | –0·37 | |
| Nuts | 0·28 | |
| Eggs | 0·26 | |
| Fish | –0·28 | 0·46 |
| Natural fruit juices | 0·27 | |
| Commercial bakery | 0·41 | |
| Sauces | 0·35 | |
| Processed meals | 0·33 | |
| Fast food | 0·45 | |
| Processed meats | 0·32 | 0·39 |
| Unprocessed red meat | 0·40 | 0·28 |
| Poultry | 0·36 | |
| Olive oil | 0·25 | |
| Potatoes | 0·34 | 0·30 |
| Other fruits* | 0·36 |
WDP, Western dietary pattern; MDP: Mediterranean dietary pattern.
Only component loadings >0·25 are displayed in the table.
Including canned fruit, dried fruit, avocado and olives.
Table 2 shows the main characteristics of the 10 713 women included in our cohort according to extreme quartiles of the two a posteriori DPs identified in our cohort (the WDP and the MDP). The mean (sd) age of the participants at recruitment was 34·5 years (10·8).
Table 2.
Baseline characteristics of the Seguimiento Universidad de Navarra Project participants according to quartiles of adherence to the Western or the Mediterranean dietary pattern, 1999–2016
| Dietary pattern quartiles | ||||||||
|---|---|---|---|---|---|---|---|---|
| Western dietary pattern | Mediterranean dietary pattern | |||||||
| Q1 | Q4 | Q1 | Q4 | |||||
| Variable | Mean | sd | Mean | sd | Mean | sd | Mean | sd |
| N | 2679 | 2678 | 2679 | 2678 | ||||
| Age (years) | 39·2 | 11·7 | 31·1 | 8·8 | 34·7 | 10·3 | 35·2 | 10·9 |
| Body mass index (kg/m2) | 22·6 | 3·2 | 21·6 | 2·9 | 22·2 | 3·1 | 22·3 | 3·1 |
| Physical activity (METs-h/week) | 28·4 | 22·7 | 20·9 | 17·3 | 22·5 | 19·7 | 26·7 | 22·5 |
| Total energy intake (kJ/d)* | 9075 | 2452 | 10 857 | 2017 | 7046 | 1665 | 11 987 | 1523 |
| Alcohol intake (g/d) | 4·2 | 6·5 | 3·7 | 5·2 | 3·2 | 4·8 | 4·9 | 6·8 |
| Years at university | 4·9 | 1·4 | 4·8 | 1·3 | 4·9 | 1·4 | 4·8 | 1·3 |
| Height (cm) | 163 | 6 | 164 | 6 | 163 | 6 | 164 | 6 |
| Number of pregnancies of more than 6 months | 0·8 | 1·2 | 0·6 | 1·2 | 0·6 | 1·1 | 0·7 | 1·1 |
| Lifetime breastfeeding (months) | 2·6 | 5·1 | 2·1 | 5·3 | 2·0 | 4·6 | 2·6 | 5·3 |
| Diabetes (%) | 1·7 | 0·6 | 1·0 | 1·5 | ||||
| Pregnancy before age of 30 years (%) | 23·1 | 16·1 | 17·1 | 20·3 | ||||
| Tobacco (%) | ||||||||
| Never smoker | 48·8 | 50·4 | 48·0 | 52·8 | ||||
| Former smoker | 20·6 | 21·1 | 28·4 | 14·8 | ||||
| Current smoker | 27·7 | 26·1 | 20·9 | 30·0 | ||||
| Family history of BC (%)† | ||||||||
| No | 88·7 | 88·8 | 89·2 | 88·8 | ||||
| Yes, after the age of 45 years | 9·2 | 8·9 | 8·6 | 9·1 | ||||
| Yes, up to the age of 45 years | 2·1 | 2·3 | 2·2 | 2·1 | ||||
| Age of menarche (%) | ||||||||
| <10 years | 1·8 | 0·8 | 1·2 | 1·2 | ||||
| 10–11 years | 21·2 | 16·3 | 18·6 | 19·6 | ||||
| 12–13 years | 54·6 | 55·1 | 54·6 | 53·8 | ||||
| 14–15 years | 20·0 | 24·5 | 22·9 | 23·6 | ||||
| >=16 years | 2·4 | 3·3 | 2·6 | 2·8 | ||||
| Menopausal status (%) | ||||||||
| Premenopausal (%) | 77·8 | 95·5 | 89·3 | 87·8 | ||||
| Postmenopausal (%) | 20·7 | 4·2 | 10·7 | 12·2 | ||||
| Postmenopausal <50 years (%)‡ | 65·6 | 71·1 | 67·5 | 68·2 | ||||
| Postmenopausal >=50 years (%)‡ | 34·4 | 28·9 | 32·5 | 31·8 | ||||
| Use of hormone replacement therapy (%)‡ | 38·0 | 33·9 | 38·1 | 33·3 | ||||
| Time of use of hormone replacement therapy (years)† | 1·3 | 2·4 | 1·3 | 2·5 | 1·3 | 2·4 | 1·1 | 2·2 |
BC: breast cancer.
To convert kcal to kJ, multiply by 4·184.
Information from mother, sister and both grandmothers was collected.
Only for postmenopausal women.
Dietary patterns and risk of BC
During a median follow-up time of 10·3 years and the observation of 105 847 person-years at risk, we registered 168 probable or confirmed incident cases of BC. Among them, 100 cases were definitively confirmed by medical records among 106 189 person-years of follow-up (probable cases without available medical records were 68). For the main analyses, we only used confirmed cases.
Analyses for WDP and MDP among the confirmed cases are shown in Table 3 for total BC and in Table 4 according to menopausal status. We found a significant association between a higher adherence to a WDP and overall BC risk (multivariable-adjusted HR Q4 vs Q1 1·70; 95 % CI 0·93, 3·12; P for trend = 0·045). Contrarily, we observed an inverse association between a moderate (multivariable-adjusted HR for confirmed premenopausal BC Q2 vs Q1 0·30; 95 % CI 0·13, 0·72), high (multivariable-adjusted HR Q3 vs Q1 0·34; 95 % CI 0·14, 0·84) or very high (multivariable-adjusted HR Q4 vs Q1 0·33; 95 % CI 0·12, 0·91) adherence to the MDP and premenopausal BC. No significant associations were observed for postmenopausal BC. Similarly, results for probable BC cases are shown in Tables 5 and 6.
Table 3.
Hazard ratio (HR) (95 % CI) of confirmed breast cancer for each quartile of Western and Mediterranean dietary pattern in the Seguimiento Universidad de Navarra Project
| Variable | Dietary pattern quartiles | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Western dietary pattern | |||||
| Cases | 25 | 21 | 28 | 26 | |
| Person-years of follow-up | 25 031 | 25 957 | 26 814 | 28 386 | |
| Incidence rate/10 000 person year | 9·99 | 8·09 | 10·44 | 9·16 | |
| Model 1 | 0·079 | ||||
| HR | 1 | 1·00 | 1·51 | 1·59 | |
| 95 % CI | ref | 0·56, 1·79 | 0·87, 2·63 | 0·90, 2·83 | |
| Model 2 | 0·115 | ||||
| HR | 1 | 0·98 | 1·54 | 1·52 | |
| 95 % CI | ref | 0·54, 1·76 | 0·88, 2·70 | 0·84, 2·74 | |
| Model 3 | 0·132 | ||||
| HR | 1 | 0·98 | 1·54 | 1·53 | |
| 95 % CI | ref | 0·54, 1·76 | 0·87, 2·71 | 0·82, 2·84 | |
| Model 4 | 0·045* | ||||
| HR | 1 | 1·06 | 1·65 | 1·70 | |
| 95 % CI | ref | 0·59, 1·91 | 0·94, 2·88 | 0·93, 3·12 | |
| Mediterranean dietary pattern | |||||
| Cases | 28 | 25 | 21 | 26 | |
| Person-years of follow-up | 26 690 | 26 821 | 36 799 | 25 880 | |
| Incidence rate/10 000 person year | 10·49 | 9·32 | 7·84 | 10·05 | |
| Model 1 | 0·652 | ||||
| HR | 1 | 0·89 | 0·72 | 0·92 | |
| 95 % CI | ref | 0·52, 1·53 | 0·41, 1·28 | 0·54, 1·58 | |
| Model 2 | 0·734 | ||||
| HR | 1 | 0·92 | 0·74 | 0·95 | |
| 95 % CI | ref | 0·53, 1·59 | 0·42, 1·31 | 0·55, 1·64 | |
| Model 3 | 0·261 | ||||
| HR | 1 | 0·78 | 0·57 | 0·63 | |
| 95 % CI | ref | 0·43, 1·43 | 0·28, 1·16 | 0·28, 1·45 | |
| Model 4 | 0·335 | ||||
| HR | 1 | 0·65 | 0·46 | 0·64 | |
| 95 % CI | ref | 0·35, 1·23 | 0·22, 0·94 | 0·30, 1·37 | |
Model 1: age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period 3 categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 2: adjusted for height (continuous), smoking habit (3 categories), leisure-time physical activity (tertiles), alcohol intake (continuous), BMI (3 categories), age of menarche (4 categories), pregnancies of at least 6 months (continuous), pregnancies before the age of 30 years (yes/no), lifetime breastfeeding (continuous), use of hormone replacement therapy (yes/no), time of use of hormone replacement therapy (continuous), years of university studies (4 categories), family history of breast cancer (3 categories) and age at menopause (3 categories), with age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (three categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 3: additionally adjusted for total energy intake (continuous) and diabetes (yes/no).
Model 4: adjusted for propensity scores (tertiles) of adherence to the quartiles of the dietary patterns calculated with the potential confounders included in model 2.
Table 4.
Hazard ratio (95 % CI) of confirmed breast cancer (BC) for each quartile of Western and Mediterranean dietary pattern in the SUN Project for pre- and postmenopausal BC
| Dietary pattern quartiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal BC | Postmenopausal BC | |||||||||
| Q1 | Q2 | Q3 | Q4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend | |
| Western dietary pattern | ||||||||||
| N | 2354 | 2354 | 2354 | 2354 | 712 | 711 | 711 | 711 | ||
| Cases | 15 | 12 | 12 | 18 | 5 | 12 | 7 | 10 | ||
| Person-years of follow-up | 19 259 | 20 696 | 21 859 | 23 626 | 4931 | 4587 | 4156 | 4024 | ||
| Incidence rate/10 000 person year | 7·79 | 5·80 | 5·49 | 7·62 | 10·14 | 26·16 | 16·84 | 2·49 | ||
| Model 1 | 0·169 | 0·262 | ||||||||
| HR | 1 | 0·87 | 0·95 | 1·50 | 1 | 2·51 | 1·55 | 2·15 | ||
| 95 % CI | ref | 0·41, 1·86 | 0·44, 2·04 | 0·74, 3·06 | ref | 0·88, 7·13 | 0·49, 4·94 | 0·72, 6·40 | ||
| Model 2 | 0·179 | 0·571 | ||||||||
| HR | 1 | 0·88 | 0·97 | 1·46 | 1 | 2·13 | 1·47 | 1·65 | ||
| 95 % CI | ref | 0·41, 1·90 | 0·45, 2·12 | 0·70, 3·05 | ref | 0·73, 6·22 | 0·45, 4·75 | 0·53, 5·11 | ||
| Model 3 | 0·113 | 0·713 | ||||||||
| HR | 1 | 0·86 | 0·98 | 1·67 | 1 | 2·11 | 1·45 | 1·48 | ||
| 95 % CI | ref | 0·40, 1·85 | 0·45, 2·16 | 0·77, 3·63 | ref | 0·72, 6·18 | 0·45, 4·70 | 0·46, 4·76 | ||
| Model 4 | 0·209 | 0·548 | ||||||||
| HR | 1 | 0·94 | 1·04 | 1·64 | 1 | 2·49 | 1·51 | 1·77 | ||
| 95 % CI | ref | 0·44, 2·02 | 0·48, 2·27 | 0·77, 3·51 | ref | 0·86, 7·18 | 0·47, 4·87 | 0·57, 5·56 | ||
| Mediterranean dietary pattern | ||||||||||
| N | 2354 | 2354 | 2354 | 2354 | 712 | 711 | 711 | 711 | ||
| Cases | 22 | 9 | 13 | 13 | 6 | 11 | 8 | 9 | ||
| Person-years of follow-up | 21 531 | 21 561 | 21 506 | 20 841 | 4390 | 4511 | 4461 | 4336 | ||
| Incidence rate/10 000 person year | 10·22 | 4·17 | 6·05 | 6·34 | 8·43 | 15·47 | 11·25 | 12·66 | ||
| Model 1 | 0·171 | 0·649 | ||||||||
| HR | 1 | 0·43 | 0·58 | 0·60 | 1 | 1·69 | 1·28 | 1·53 | ||
| 95 % CI | ref | 0·20, 0·93 | 0·29, 1·16 | 0·30, 1·19 | ref | 0·62, 4·57 | 0·44, 3·69 | 0·54, 4·32 | ||
| Model 2 | 0·192 | 0·448 | ||||||||
| HR | 1 | 0·41 | 0·58 | 0·60 | 1 | 2·06 | 1·36 | 1·70 | ||
| 95 % CI | ref | 0·19, 0·90 | 0·29, 1·17 | 0·30, 1·20 | ref | 0·74, 5·73 | 0·46, 4·00 | 0·58, 4·97 | ||
| Model 3 | 0·081 | 0·419 | ||||||||
| HR | 1 | 0·32 | 0·40 | 0·33 | 1 | 1·96 | 1·24 | 1·52 | ||
| 95 % CI | ref | 0·13, 0·74 | 0·16, 0·99 | 0·11–0·99 | ref | 0·64, 6·00 | 0·34, 4·51 | 0·33, 6·98 | ||
| Model 4 | 0·055 | 0·686 | ||||||||
| HR | 1 | 0·30 | 0·34 | 0·33 | 1 | 1·07 | 0·67 | 0·86 | ||
| 95 % CI | ref | 0·13, 0·72 | 0·14, 0·84 | 0·12, 0·91 | ref | 0·32, 3·59 | 0·18, 2·54 | 0·21, 3·47 | ||
Model 1: age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (3 categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 2: adjusted for height (continuous), smoking habit (3 categories), leisure-time physical activity (tertiles), alcohol intake (continuous), BMI (3 categories), age of menarche (4 categories), pregnancies of at least 6 months (continuous), pregnancies before the age of 30 years (yes/no), lifetime breastfeeding (continuous), use of hormone replacement therapy (yes/no) (postmenopausal BC), time of use of hormone replacement therapy (continuous) (postmenopausal BC), years of university studies (4 categories), family history of BC (3 categories) and age at menopause (3 categories), and time on study between recruitment and menopause (continuous) (for postmenopausal BC), with age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (three categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 3: additionally adjusted for total energy intake (continuous) and diabetes (yes/no).
Model 4: adjusted for propensity scores (tertiles) of adherence to the quartiles of the dietary patterns calculated with the potential confounders included in model 2.
Table 5.
Hazard ratio (95 % CI) including the probable breast cancer cases for each quartile of Western and Mediterranean dietary pattern in the Seguimiento Universidad de Navarra Project
| Dietary pattern | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Western dietary pattern | |||||
| Cases | 43 | 37 | 45 | 43 | |
| Person-years of follow-up | 24 952 | 25 864 | 26 744 | 28 288 | |
| Incidence rate/10 000 person year | 17·23 | 14·31 | 16·83 | 15·20 | |
| Model 1 | 0·073 | ||||
| HR | 1 | 0·99 | 1·35 | 1·41 | |
| 95 % CI | ref | 0·64, 1·54 | 0·88, 2·06 | 0·90, 2·19 | |
| Model 2 | 0·088 | ||||
| HR | 1 | 1·01 | 1·38 | 1·39 | |
| 95 % CI | ref | 0·65, 1·58 | 0·89, 2·13 | 0·88, 2·19 | |
| Model 3 | 0·127 | ||||
| HR | 1 | 1·01 | 1·36 | 1·36 | |
| 95 % CI | ref | 0·64, 1·57 | 0·88, 2·11 | 0·85, 2·19 | |
| Model 4 | 0·099 | ||||
| HR | 1 | 1·04 | 1·40 | 1·39 | |
| 95 % CI | ref | 0·67, 1·62 | 0·91, 2·17 | 0·87, 2·21 | |
| Mediterranean dietary pattern | |||||
| Cases | 40 | 45 | 36 | 47 | |
| Person-years of follow-up | 26 613 | 26 743 | 26 719 | 25 772 | |
| Incidence rate/10 000 person year | 15·03 | 16·83 | 13·47 | 18·24 | |
| Model 1 | 0·615 | ||||
| HR | 1 | 1·12 | 0·89 | 1·18 | |
| 95 % CI | ref | 0·73, 1·72 | 0·56, 1·39 | 0·77, 1·81 | |
| Model 2 | 0·650 | ||||
| HR | 1 | 1·12 | 0·89 | 1·17 | |
| 95 % CI | ref | 0·73, 1·73 | 0·56, 1·40 | 0·76, 1·80 | |
| Model 3 | 0·760 | ||||
| HR | 1 | 1·03 | 0·78 | 0·95 | |
| 95 % CI | ref | 0·64, 1·66 | 0·44, 1·36 | 0·50, 1·82 | |
| Model 4 | 0·717 | ||||
| HR | 1 | 0·94 | 0·67 | 0·92 | |
| 95 % CI | ref | 0·57, 1·55 | 0·38, 1·20 | 0·50, 1·69 | |
Model 1: age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (3 categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 2: adjusted for height (continuous), smoking habit (3 categories), leisure-time physical activity (tertiles), alcohol intake (continuous), BMI (3 categories), age of menarche (4 categories), pregnancies of at least 6 months (continuous), pregnancies before the age of 30 years (yes/no), lifetime breastfeeding (continuous), use of hormone replacement therapy (yes/no), time of use of hormone replacement therapy (continuous), years of university studies (4 categories), family history of BC (3 categories) and age at menopause (3 categories), with age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (three categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 3: additionally adjusted for total energy intake (continuous) and diabetes (yes/no).
Model 4: adjusted for propensity scores (tertiles) of adherence to the quartiles of the dietary patterns calculated with the potential confounders included in model 2.
Table 6.
Hazard ratio (95 % CI) including the probable breast cancer (BC) cases for each quartile of Western and Mediterranean dietary pattern in the Seguimiento Universidad de Navarra Project for pre- and postmenopausal women
| Dietary pattern quartiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal BC | Postmenopausal BC | |||||||||
| Variable | Q1 | Q2 | Q3 | Q4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend |
| Western dietary pattern | ||||||||||
| N | 2354 | 2354 | 2354 | 2354 | 708 | 707 | 707 | 707 | ||
| Cases | 29 | 22 | 25 | 30 | 10 | 16 | 9 | 14 | ||
| Person-years of follow-up | 19 212 | 20 650 | 21 800 | 23 566 | 4895 | 4544 | 4139 | 4012 | ||
| Incidence rate/10 000 person year | 15·10 | 10·65 | 11·47 | 12·73 | 14·12 | 22·63 | 12·73 | 19·80 | ||
| Model 1 | 0·293 | 0·226 | ||||||||
| HR | 1 | 0·80 | 0·97 | 1·21 | 1 | 1·65 | 1·05 | 1·60 | ||
| 95 % CI | ref | 0·46, 1·40 | 0·57, 1·67 | 0·72, 2·06 | ref | 0·75, 3·64 | 0·42, 2·59 | 0·70, 3·65 | ||
| Model 2 | 0·365 | 0·411 | ||||||||
| HR | 1 | 0·81 | 0·97 | 1·17 | 1 | 1·47 | 0·98 | 1·34 | ||
| 95 % CI | ref | 0·46, 1·41 | 0·56, 1·67 | 0·68, 2·00 | ref | 0·65, 3·30 | 0·39, 2·48 | 0·57, 3·18 | ||
| Model 3 | 0·327 | 0·548 | ||||||||
| HR | 1 | 0·80 | 0·96 | 1·22 | 1 | 1·47 | 0·97 | 1·23 | ||
| 95 % CI | ref | 0·45, 1·39 | 0·55, 1·67 | 0·69, 2·15 | ref | 0·65, 3·31 | 0·38, 2·44 | 0·51, 3·00 | ||
| Model 4 | 0·486 | 0·642 | ||||||||
| HR | 1 | 0·82 | 0·99 | 1·19 | 1 | 1·69 | 1·04 | 1·41 | ||
| 95 % CI | ref | 0·47, 1·44 | 0·57, 1·71 | 0·68, 2·08 | ref | 0·75, 3·77 | 0·41, 2·60 | 0·59, 3·36 | ||
| Mediterranean dietary pattern | ||||||||||
| N | 2354 | 2354 | 2354 | 2354 | 708 | 707 | 707 | 707 | ||
| Cases | 31 | 24 | 24 | 27 | 8 | 15 | 11 | 15 | ||
| Person-years of follow-up | 21 480 | 21 508 | 21 448 | 20 792 | 4369 | 4488 | 4431 | 4303 | ||
| Incidence rate/10 000 person year | 14·43 | 11·16 | 11·19 | 12·99 | 18·31 | 33·42 | 24·83 | 34·86 | ||
| Model 1 | 0·655 | 0·898 | ||||||||
| HR | 1 | 0·80 | 0·78 | 0·89 | 1 | 1·76 | 1·33 | 1·91 | ||
| 95 % CI | ref | 0·47, 1·37 | 0·46, 1·34 | 0·53, 1·49 | ref | 0·75, 4·15 | 0·53, 3·30 | 0·81, 4·52 | ||
| Model 2 | 0·612 | 0·676 | ||||||||
| HR | 1 | 0·77 | 0·79 | 0·86 | 1 | 2·01 | 1·38 | 1·98 | ||
| 95 % CI | ref | 0·45, 1·32 | 0·46, 1·35 | 0·51, 1·45 | ref | 0·84, 4·80 | 0·55, 3·47 | 0·82, 4·80 | ||
| Model 3 | 0·328 | 0·621 | ||||||||
| HR | 1 | 0·68 | 0·65 | 0·64 | 1 | 2·09 | 1·45 | 2·19 | ||
| 95 % CI | ref | 0·38, 1·23 | 0·33, 1·29 | 0·29, 1·42 | ref | 0·80, 5·43 | 0·48, 4·35 | 0·62, 7·75 | ||
| Model 4 | 0·162 | 0·784 | ||||||||
| HR | 1 | 0·64 | 0·53 | 0·56 | 1 | 1·49 | 0·99 | 1·40 | ||
| 95 % CI | ref | 0·35, 1·19 | 0·26, 1·06 | 0·25, 1·19 | ref | 0·51, 4·35 | 0·30, 3·24 | 0·41, 4·75 | ||
Model 1: Age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (3 categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 2: adjusted for height (continuous), smoking habit (3 categories), leisure-time physical activity (tertiles), alcohol intake (continuous), BMI (3 categories), age of menarche (4 categories), pregnancies of at least 6 months (continuous), pregnancies before the age of 30 years (yes/no), lifetime breastfeeding (continuous), use of hormone replacement therapy (yes/no) (postmenopausal BC), time of use of hormone replacement therapy (continuous) (postmenopausal BC), years of university studies (4 categories), family history of BC (3 categories), age at menopause (3 categories) and time on study between recruitment and menopause (continuous) (for postmenopausal BC), with age as underlying time variable in all analyses and all analyses stratified by age (in decades) and recruitment period (three categories: 1999–2003, 2004–2009, 2009-ongoing).
Model 3: additionally adjusted for total energy intake (continuous) and diabetes (yes/no).
Model 4: adjusted for propensity scores (tertiles) of adherence to the quartiles of the dietary patterns calculated with the potential confounders included in model 2.
Discussion
In this cohort study, we identified two main a posteriori DPs: a WDP and a MDP. Our results suggest that even small changes towards a higher adherence to the MDP could be associated with a decreased risk of premenopausal BC in the SUN Project, but we found no association for postmenopausal BC. On the other hand, a higher adherence to a WDP might be linked to higher risk of BC.
Several DPs have been associated with the risk of different cancer types, and, particularly, BC. Numerous reviews have shown ‘healthy DPs’ to be protective against different cancer types, mostly in the digestive system (colorectum(31), stomach(32), oesophageal cancer(33) and pancreatic cancer(34)), but also in other locations such as endometrium(35), prostate(36), larynx(37) or lung(38). Otherwise, unhealthy DPs, such as ‘WDP’, are considered to play a deleterious role in the development of colorectal cancer(31), endometrial cancer(35), ‘laryngeal cancer’(37), gastric cancer(32), pancreatic cancer(34) and prostate cancer(39); ‘alcohol dietary pattern’ (characterised by high consumption of alcohol-containing beers, wines and white spirits) with colorectal cancer(31), gastric cancer(32), oesophageal cancer(33) and pancreatic cancer (heavy drinking pattern)(34) and, finally, ‘carbohydrate dietary pattern’ with prostate cancer(39).
In spite of its relevance, epidemiological studies on the association between dietary factors and BC are scarce and heterogeneous. They are focused on different populations and therefore different cultures and DPs, which makes it difficult to obtain strong and homogeneous evidence. In addition, between-study disparities could also be due to the heterogeneity in the composition and labelling of the patterns, even in similar populations and cultures, which probably may lead to the inclusion of somewhat heterogeneous DPs under the same label. However, evidence is available supporting the applicability of data-driven DPs to other populations(40).
We found that a higher adherence to the MDP – characterised by a high consumption of vegetables, fruits, legumes, fish, poultry and olive oil – was associated with a lower risk of premenopausal BC. This result is consistent with the results from the EpiGEICAM study, a case–control study on BC conducted in Spain(10). Nevertheless, in the EpiGEICAM study, as well as in the MCC-Spain case–control study(12), an inverse association was observed for postmenopausal BC, which was not replicated in our cohort study. However, our study may have limitations for studying DPs and postmenopausal BC. It should be born in mind that almost 90 % of our participants were premenopausal. Thus, some of the analyses for postmenopausal BC were based on the diet observed when these women were still premenopausal, and, in some cases, several years before their age at menopause. Also, the differences between these two studies could be explained in part by the fact that, in their studies, food items like meat (specially white and processed meats) which has been related to risk of different cancer types(41) were less represented. Another differential point is the study design: case–control studies tend to overestimate the associations due to a memory bias. In this regard, results of the California Teachers Study prospective cohort, comprised by 96 959 women aged from 22 to 104 years followed for 1 354 947 person-years, have been recently published. They observed that diet indexes mainly characterised by vegetables, whole grains, legumes, fruits, nuts and seeds and with low amounts of red/processed meats and sugar-sweetened beverages [Alternate Mediterranean Diet (aMED), Alternative Healthy Eating Index-2010 (AHEI-2010) and Dietary Approaches to Stop Hypertension (DASH)] were associated with a decrease in postmenopausal BC risk(42). In contrast to the previous two studies and similar to our work, this study included a large cohort of women followed prospectively. Evidence for the association between MDP and BC is more diverse than the one for WDP, which could be due to a lower reproducibility of this pattern because it might be highly sample-specific. This fact may impair the capacity to replicate the associations in different independent studies(40,43).
As abovementioned, there is a large body of evidence that ratified the association of a WDP – usually rich in whole-fat dairy products, sugar-sweetened sodas, commercial bakery, sauces, processed meals and meats, unprocessed red meat and fast food – with a higher risk of overall and pre- and postmenopausal BC. For example, in the MCC-Spain case–control study, a higher adherence to a WDP was associated with a higher risk of BC in both premenopausal and postmenopausal women. In addition, the EpiGEICAM study also found a harmful effect of a WDP on BC risk, especially in premenopausal women(10). Several mechanisms may explain this association. Particularly, a WDP is likely to be related to developing or maintaining a low-grade inflammatory status(44), which has been implicated in BC development(45–48). Recent studies also described a direct association between adherence to a WDP and mammographic density(49), a strong risk factor for BC. A higher adherence to a WDP may also be implicated in increasing serum concentrations of free estradiol(50), which play a role in BC development, especially for hormone receptor positive subtypes(9). In line with the above-mentioned results, we found a detrimental association between a higher adherence to a WDP and BC risk. Even though results pointed in the same direction when we stratified our results by menopausal status, we could not further confirm these results, probably due to the small number of postmenopausal BC cases reported in our young cohort and also to the overall healthy eating habits of our cohort’s participants. The latter together with an overall lower adherence to the WDP among older women might have decreased the between participants’ variability and precluded us from finding statistically significant associations.
The current study has some potential limitations. First, we are aware that the number of incident cases of BC is limited, which is good news for the women in the SUN cohort, but may reduce our statistical power and in turn limit our potential to find statistically significant results. Nevertheless, the age standardised incidence of BC in our cohort was similar to the national estimates from cancer registries(3). This limited number of incident cases could contribute to the lack of evidence linking DPs and postmenopausal BC. Also, our cohort comprised mainly young women and most of them were premenopausal (88·70 %), which reduces the number of incident postmenopausal BC cases. Finally, only some women were postmenopausal at study inception. As such, for some women who were observed after menopause, dietary information had been collected when they were still premenopausal. Second, dietary information was collected only at baseline, which might have led to a non-differential misclassification bias. Nevertheless, this non-differential misclassification would have biased our results towards the null. Third, and due to the small number of incident BC cases, we did not perform a sub-analysis by subtypes of BC cases, because it would be clearly underpowered. Fourth, dietary information was based on self-reported information. This may leave some room for non-differential misclassification. Nevertheless, the food-frequency questionnaire had been repeatedly validated in specific studies conducted in Spain(22,23), and the non-differential misclassification would bias our results towards the null value. Fifth, information on BC incidence was also self-reported. To confirm the accuracy of the information, we asked our participants for a copy of their medical reports. We conducted our main analyses considering only confirmed cases by the medical reports. Only as ancillary analyses we included all the cases – adding also those without available medical reports for confirmation. In spite of this, we might have missed some cases of BC. Accordingly, this might have lowered our sensitivity to detect incident cases of BC. However, the close follow-up of our participants and the periodic consultation of the National Death Index will have constrained the number of missed BC cases. Sixth, a posteriori DPs are data driven. In this line, it is noteworthy that we found similar characteristics between the components of our DPs and previously described DPs in the literature(10,12,19).
Nonetheless, our study also shows some strengths. First, it is a dynamic cohort with more than 16 years of follow-up, it includes 10 713 women with 91 % retention rate. Second, we have a thorough information about the diet and nutrition of the participants collected by the above-mentioned validated food-frequency questionnaire. Lastly, multiple potential confounders were included in the multivariable analyses, which reduce the room for residual confounding and other potential biases in our analyses.
Conclusions
In this Mediterranean cohort study, we observed a harmful association between adherence to the WDP and BC risk. Moreover, small changes towards higher adherence to the MDP were found to be protective against premenopausal BC. These results need to be further confirmed in longitudinal studies to inform public health recommendations.
Acknowledgements
Acknowledgements: The authors thank other members of the SUN Group: Alonso A, Álvarez-Álvarez I, Balaguer A, Barbagallo M, Barrientos I, Barrio-López MT, Basterra-Gortari FJ, Battezzati A, Bazal P, Benito S, Bertoli S, Bes-Rastrollo M, Beulen Y, Beunza JJ, Buil-Cosiales P, Canales M, Carlos S, Carmona L, Cervantes S, Cristobo C, de Irala J, de la Fuente-Arrillaga C, de la O V, de la Rosa PA, Delgado-Rodríguez M, Díaz-Gutiérrez J, Díez Espino J, Domínguez L, Donat-Vargas C, Donazar M, Eguaras S, Fernández-Montero A, Fresán U, Galbete C, García-Arellano A, García López M, Gea A, GutiérrezBedmar M, Gomes-Domingos AL, Gómez-Donoso C, Gómez-Gracia E, Goñi E, Goñi L, Guillén F, Henríquez P, Hernández A, Hershey MS, Hidalgo-Santamaría M, Hu E, Lahortiga F, Leone A, Llorca J, López del Burgo C, Marí A, Marques I, Martí A, Martín Calvo N, Martín-Moreno JM, Martínez JA, Martínez-Lapiscina EH, Mendonça R, Menéndez C, Molendijk M, Molero P, Murphy K, Muñoz M, NúñezCórdoba JM, Pajares R, Papadaki A, Parletta N, Pérez de Ciriza P, Pérez Cornago A, Pérez de Rojas J, Pimenta AM, Pons J, Ramallal R, Rico-Campà A, Ruano C, Ruiz L, Ruiz-Canela M, Ruiz Zambrana A, Salgado E, San Julián B, Sánchez D, Sánchez-Bayona R, Sánchez-Tainta A, Sánchez-Villegas A, Santiago S, Sayón-Orea C, Schlatter J, Serrano-Martinez M, Toledo J, Tortosa A, Valencia F, Vázquez Z, Zarnowiecki D, Zazpe I. The authors thank very specially all participants in the SUN cohort for their long-standing and enthusiastic collaboration and their advisors from Harvard TH Chan School of Public Health Walter Willett, Alberto Ascherio, Frank B. Hu and Meir J. Stampfer who helped them to design the SUN Project, the PREDIMED study and the PREDIMED-PLUS ongoing trial. Financial support: This work was supported by the Spanish Government-Instituto de Salud Carlos III and the European Regional Development Fund (FEDER) (RD 06/0045, CIBER-OBN, Grants PI10/02658, PI10/02293, PI13/00615, PI14/01668, PI14/01798, PI14/01764, PI17/01795 and G03/140), the Navarra Regional Government (45/2011, 122/2014, 41/2016) and the University of Navarra. Conflict of interest: None. Authorship: I.G. and E.T. wrote the draft of the manuscript; I.G., A.R.N. and E.T. were responsible for data analysis; M.A.M.G. and E.T. were responsible for conception and design, data acquisition and interpretation. All co-authors revised the manuscript critically for important intellectual content and approved the final version to be published. Ethics of human subject participation: All potential candidates were duly informed of their right to refuse to participate in the SUN study or to withdraw their consent to participate at any time without reprisal, according to the assumptions of the Declaration of Helsinki. Particular attention was given to the specific information needs of individual potential candidates as well as to the techniques used to deliver their information and the feedback that may receive in the future from the research team. After ensuring that the candidate had understood the information, we sought their potential freely given informed consent and their voluntary fulfillment of the baseline questionnaire. These methods were accepted by our Institutional Review Board as to imply an appropriately obtained informed consent. The SUN cohort was registered at ClinicalTrials.gov (identifier: NCT02669602).
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Cancer Collaboration C, Fitzmaurice C, Allen C et al. (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 3, 524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galceran J, Ameijide A, Carulla M et al. (2017) Cancer incidence in Spain, 2015. Clin Transl Oncol 19, 799–825. [DOI] [PubMed] [Google Scholar]
- 4. Mørch LS, Skovlund CW, Hannaford PC et al. (2017) Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 377, 2228–2239. [DOI] [PubMed] [Google Scholar]
- 5. Gartlehner G, Patel S V, Feltner C et al. (2017) Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: Evidence report and systematic review for the us preventive services task force. JAMA 318, 2234–2249. [DOI] [PubMed] [Google Scholar]
- 6. World Cancer Research Fund/American Institute for Cancer Research (2018) Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. Available at dietandcancerreport.org.
- 7. Anderson KN, Schwab RB & Martinez ME (2014) Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 144, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Cancer Research Fund International: Continuous Update Project Summary Report (2007) World Cancer Research Fund. Nutr Food Sci 37, 41–42. [Google Scholar]
- 9. Shin S, Saito E, Inoue M et al. (2016) Dietary pattern and breast cancer risk in Japanese women: the Japan Public Health Center-based Prospective Study (JPHC Study). Br J Nutr 115, 1769–1779. [DOI] [PubMed] [Google Scholar]
- 10. Castelló A, Pollán M, Buijsse B et al. (2014) Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case–control EpiGEICAM study. Br J Cancer 111, 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krusińska B, Hawrysz I, Słowińska MA et al. (2017) Dietary patterns and breast or lung cancer risk: A pooled analysis of 2 case-control studies in north-eastern Poland. Adv Clin Exp Med 26, 1367–1375. [DOI] [PubMed] [Google Scholar]
- 12. Castelló A, Boldo E, Pérez-Gómez B et al. (2017) Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 103, 8–15. [DOI] [PubMed] [Google Scholar]
- 13. Catsburg C, Kim RS, Kirsh VA et al. (2015) Dietary patterns and breast cancer risk: A study in 2 cohorts. Am J Clin Nutr 101, 817–823. [DOI] [PubMed] [Google Scholar]
- 14. Turati F, Carioli G, Bravi F et al. (2018) Mediterranean diet and breast cancer risk. Nutrients 10, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buckland G, Travier N, Cottet V et al. (2013) Adherence to the Mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J cancer 132, 2918–2927. [DOI] [PubMed] [Google Scholar]
- 16. Demetriou CA, Hadjisavvas A, Loizidou MA et al. (2012) The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: A case-control study. BMC Cancer 12, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trichopoulou A, Bamia C, Lagiou P et al. (2010) Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J Clin Nutr 92, 620–625. [DOI] [PubMed] [Google Scholar]
- 18. Cade JE, Taylor EF, Burley VJ et al. (2011) Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr 65, 920–928. [DOI] [PubMed] [Google Scholar]
- 19. Cottet V, Touvier M, Fournier A et al. (2009) Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol 170, 1257–1267. [DOI] [PubMed] [Google Scholar]
- 20. Carlos S, De La Fuente-Arrillaga C, Bes-Rastrollo M et al. (2018) Mediterranean diet and health outcomes in the SUN cohort. Nutrients 10, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin-Moreno JM, Boyle P, Gorgojo L et al. (1993) Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 22, 512–9. [DOI] [PubMed] [Google Scholar]
- 22. Fernández-Ballart JD, Piñol JL, Zazpe I et al. (2010) Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 103, 1808–1816. [DOI] [PubMed] [Google Scholar]
- 23. de la Fuente-Arrillaga C, Vázquez Ruiz Z, Bes-Rastrollo M et al. (2010) Reproducibility of an FFQ validated in Spain. Public Health Nutr 13, 1364–1372. [DOI] [PubMed] [Google Scholar]
- 24. Zazpe I, Sánchez-Tainta A, Toledo E et al. (2014) Dietary patterns and total mortality in a Mediterranean cohort: The SUN project. Eur J Acad Nutr Diet 114, 37–47. [DOI] [PubMed] [Google Scholar]
- 25. Moreiras O, Carbajal A, Cabrera L et al. (2013) Tablas de composición de alimentos: guía de prácticas (Spanish Food Composition Tables: Practice Guide), 16th ed. Madrid: Ediciones Pirámide. [Google Scholar]
- 26. Mataix J (2009) Tablas de Composición de Alimentos (Spanish Food Composition Tables), 5th ed. Granada: University of Granda. [Google Scholar]
- 27. Shivappa N, Sandin S, Löf M et al. (2015) Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer 113, 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martínez-González MA, López-Fontana C, Varo JJ et al. (2005) Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr 8, 920–927. [DOI] [PubMed] [Google Scholar]
- 29. Ainsworth BE, Haskell WL, Whitt MC et al. (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32, S498–504. [DOI] [PubMed] [Google Scholar]
- 30. Bes-Rastrollo M, Pérez Valdivieso JR, Sánchez-Villegas A et al. (2005) Validación del peso e índice de masa corporal auto-declarados de los participantes de una cohorte de graduados universitarios. Rev Esp Obes 3, 352–358. [Google Scholar]
- 31. Feng YL, Shu L, Zheng PF et al. (2017) Dietary patterns and colorectal cancer risk: A meta-analysis. Eur J Cancer Prev 26, 201–211. [DOI] [PubMed] [Google Scholar]
- 32. Shu L, Wang X-Q, Wang S-F et al. (2013) Dietary patterns and stomach cancer: A meta-analysis. Nutr Cancer 65, 1105–1115. [DOI] [PubMed] [Google Scholar]
- 33. Liu X, Wang X, Lin S et al. (2014) Dietary patterns and oesophageal squamous cell carcinoma: A systematic review and meta-analysis. Br J Cancer 110, 2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu P-Y, Shu L, Shen S-S et al. (2017) Dietary patterns and pancreatic cancer risk: A meta-analysis. Nutrients 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Si C-J, Shu L, Zheng P-F et al. (2017) Dietary patterns and endometrial cancer. Eur J Cancer Prev 26, 336–345. [DOI] [PubMed] [Google Scholar]
- 36. Castelló A, Boldo E, Amiano P et al. (2018) Mediterranean dietary pattern is associated with low risk of aggressive prostate cancer: MCC-Spain study. J Urol 199, 430–437. [DOI] [PubMed] [Google Scholar]
- 37. Edefonti V, Bravi F, Garavello W et al. (2010) Nutrient-based dietary patterns and laryngeal cancer: Evidence from an exploratory factor analysis. Cancer Epidemiol Biomarkers Prev 19, 18–27. [DOI] [PubMed] [Google Scholar]
- 38. Sun Y, Li Z, Li J et al. (2016) A healthy dietary pattern reduces lung cancer risk: A systematic review and meta-analysis. Nutrients 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fabiani R, Minelli L, Bertarelli G et al. (2016) A western dietary pattern increases prostate cancer risk: A systematic review and meta-analysis. Nutrients 8, pii: E626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castelló A, Buijsse B, Martín M et al. (2016) Evaluating the applicability of data-driven dietary patterns to independent samples with a focus on measurement tools for pattern similarity. J Acad Nutr Diet 116, 1914–1924.e6. [DOI] [PubMed] [Google Scholar]
- 41. Ferguson LR (2010) Meat and cancer. Meat Sci 84, 308–313. [DOI] [PubMed] [Google Scholar]
- 42. Haridass V, Ziogas A, Neuhausen SL et al. (2018) Diet quality scores inversely associated with postmenopausal breast cancer risk are not associated with premenopausal breast cancer risk in the California teachers study. J Nutr 148, 1830–1837. [DOI] [PubMed] [Google Scholar]
- 43. Castelló A, Lope V, Vioque J et al. (2016) Reproducibility of data-driven dietary patterns in two groups of adult Spanish women from different studies. Br J Nutr 116, 734–42. [DOI] [PubMed] [Google Scholar]
- 44. Christ A, Günther P, Lauterbach MAR et al. (2018) Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coussens LM & Werb Z (2002) Inflammation and cancer. Nature 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Philip M, Rowley DA & Schreiber H (2004) Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol 14, 433–439. [DOI] [PubMed] [Google Scholar]
- 47. Jiang X & Shapiro DJ (2014) The immune system and inflammation in breast cancer. Mol Cell Endocrinol 382, 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhatelia K, Singh K & Singh R (2014) TLRs: Linking inflammation and breast cancer. Cell Signal 26, 2350–2357. [DOI] [PubMed] [Google Scholar]
- 49. Castelló A, Ascunce N, Salas-Trejo D et al. (2016) Association between western and mediterranean dietary patterns and mammographic density. Obstet Gynecol 128, 574–581. [DOI] [PubMed] [Google Scholar]
- 50. Sánchez-Zamorano LM, Flores-Luna L, Angeles-Llerenas A et al. (2016) The Western dietary pattern is associated with increased serum concentrations of free estradiol in postmenopausal women: implications for breast cancer prevention. Nutr Res 36, 845–854. [DOI] [PubMed] [Google Scholar]