Abstract
Objective:
To disrupt cycles of health inequity, traceable to dietary inequities in the earliest stages of life, public health interventions should target improving nutritional wellbeing in preconception/pregnancy environments. This requires a deep engagement with pregnant/postpartum people (PPP) and their communities (including their health and social care providers, HSCP). We sought to understand the factors that influence diet during pregnancy from the perspectives of PPP and HSCP, and to outline intervention priorities.
Design:
We carried out thematic network analyses of transcripts from ten focus group discussions (FGD) and one stakeholder engagement meeting with PPP and HSCP in a Canadian city. Identified themes were developed into conceptual maps, highlighting local priorities for pregnancy nutrition and intervention development.
Setting:
FGD and the stakeholder meeting were run in predominantly lower socioeconomic position (SEP) neighbourhoods in the sociodemographically diverse city of Hamilton, Canada.
Participants:
All local, comprising twenty-two lower SEP PPP and forty-three HSCP.
Results:
Salient themes were resilience, resources, relationships and the embodied experience of pregnancy. Both PPP and HSCP underscored that socioeconomic-political forces operating at multiple levels largely determined the availability of individual and relational resources constraining diet during pregnancy. Intervention proposals focused on cultivating individual and community resilience to improve early-life nutritional environments. Participants called for better-integrated services, greater income supports and strengthened support programmes.
Conclusions:
Hamilton stakeholders foregrounded social determinants of inequity as main factors influencing pregnancy diet. They further indicated a need to develop interventions that build resilience and redistribute resources at multiple levels, from the household to the state.
Keywords: Developmental origins, Pregnancy, Dietary inequities, Health inequities, Community engagement
Within and across nation-states, risks of illness and death vary systematically among groups of people(1). Most health inequities relate to socioeconomic position (SEP), with people(s) of higher SEP more likely to be alive and thriving at any given age(1). In 2008, the WHO called for the elimination of such inequities within a generation(2). The largest contributors to inequitable health outcomes are non-communicable diseases (NCDs), including CVD and diabetes(3,4). NCD disparities are widening steadily(3), raising the questions, why are they widening and, in keeping with the WHO’s call-to-action, what can be done to reverse this trend?
Developmental origins of health inequities
One step towards answering these questions is to reflect on how socioeconomic and political inequities interact with biology to affect health(5). The well-supported Developmental Origins of Health and Disease (DOHaD) hypothesis holds that inequities become incorporated into our biology largely during early development(5,6). The DOHaD framework suggests developing embryos/fetuses/infants receive biological signals about their environments from parents, and these signals shape energy investment and growth(7,8). Growth patterns then influence social, educational and labour force performance and, eventually, disease susceptibilities(9). Differential educational and employment performance leads to differential access to income/wealth, knowledge and influence through the reproductive years for parents, perpetuating inequities across generations(10). DOHaD-based research indicates that, because small alterations during early development have outsized effects on downstream health(11), interventions should focus on improving health preconception and through pregnancy/infancy to effectively disrupt cycles of inequity(12,13).
Nutrition constitutes a key modifiable factor influencing equitability of periconceptional/pregnancy environments(8). Pregnant/postpartum people’s (PPP) frequency, nutritional quality and socio-environmental context of meals/snacks vary substantially by SEP(14–16). Disparities in pregnancy diet impact maternal health and fetal/postnatal development, with lower SEP PPP and their children at increased risks of pregnancy complications and subsequent development of NCDs(17–20). Thus, improving pregnancy diets of lower SEP people will reduce NCD inequities.
To date, pregnancy diet intervention strategies have framed eating predominantly as a product of individual motivations and behaviours(21). However, people eat within households, communities and nation-states(22–24). These higher-level factors exert stronger influences on eating patterns than do individual intentions(15). Improving pregnancy diets therefore requires moving beyond approaches focusing primarily on individuals(21,25,26) to multi-level ones that scaffold existing community supports and resources and emphasise building empowerment and resilience(27–29). Such approaches rely on a deep engagement with participants throughout intervention development, with participants invested in improving their communities’ health(30).
Project goal and study aims
We, members of the Mothers to Babies (M2B) Study research team, employed a multi-pronged, deep engagement strategy to support pregnancy nutrition and reduce dietary inequities in the city of Hamilton, Ontario, Canada. Here, we report findings from focus group discussions (FGD) and a stakeholder engagement meeting with Hamiltonians who were pregnant/postpartum at the time of participation (i.e., PPP), as well as with health and social care providers (HSCP) who support PPP. We aimed to answer two questions: (i) What influences diet during pregnancy? and (ii) How can people be supported to improve their diets during pregnancy?
Methods
Setting
M2B Study is based in Hamilton, a Canadian city of approximately 750 000(31). The city is sociodemographically diverse(32) (see online supplementary material, Supplemental Table 1) and characterised by striking inter-neighbourhood economic and health inequities(33).
Most of Hamilton’s inter-neighbourhood health disparities have persisted or worsened over the last decade, despite efforts to remove health barriers for lower SEP residents(33). An exception concerns a reduction in low birth weight inequity, accomplished through deep community engagement. This success and the persistence of the city’s other inequities highlight the need for community-based interventions.
Participants and procedures
We carried out ten FGD and one stakeholder meeting. The core topics for the FGD were identified by the research team, in consultation with local public health administrators, as priority areas for investigation. Four of the FGD were with PPP, held in neighbourhoods characterised by high rates of poverty and NCDs. PPP participants were recruited through locally administered prenatal programmes or M2B Study follow-up. The prenatal programmes combine weekly education, meal-sharing, provision of grocery and prenatal multivitamin gift cards, and social support(34). Sociodemographic characteristics of PPP participants are presented in Table 1.
Table 1.
Sociodemographic characteristics of participants from four focus group discussions (FGD) with pregnant and newly postpartum people (PPP)
| Characteristics | FGD ID | Total count | ||||
|---|---|---|---|---|---|---|
| PPP-FG1 | PPP-FG2 | PPP-FG3 | PPP-FG4 | n | % | |
| Number of participants | 4 | 6 | 7 | 5 | 22 | 100 |
| Participants’ age range (years) | ||||||
| 21–29 | 0 | 3 | 3 | 2 | 8 | 36 |
| 30–44 | 4 | 3 | 4 | 3 | 14 | 64 |
| Participants’ reproductive status at the time of FGD | ||||||
| Pregnant | 1 | 3 | 4 | 1 | 9 | 41 |
| Postpartum | 3 | 3 | 3 | 4 | 13 | 59 |
| Parity | ||||||
| Pregnant/postpartum with first child | 4 | 3 | 2 | 2 | 11 | 50 |
| Pregnant/postpartum with second child | 0 | 2 | 4 | 2 | 6 | 27 |
| Pregnant/postpartum with third+ child | 0 | 1 | 1 | 1 | 3 | 14 |
| Participants’ facility in English | ||||||
| Native English speakers | 2 | 3 | 3 | 2 | 10 | 45 |
| Non-native English speakers | 2 | 3 | 4 | 3 | 12 | 56 |
The remaining six FGD were with HSCP: two with public health nurses and registered dietitians who run the prenatal nutrition programmes, two with midwives, and two with early-childhood educators who staff new-parent/young child neighbourhood drop-in centres. See Table 2 for the sociodemographic characteristics of HSCP participants.
Table 2.
Sociodemographic characteristics of participants from six focus group discussions (FGD) with health and social care providers (HSCP) who support pregnant and newly postpartum people. Prenatal nutrition group leaders include public health nurses (n 8) and registered dietitians (n 6)
| Characteristics | Prenatal nutrition group leaders | Midwives | Early-childhood educators | Total count | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | |||||||
| FGD ID | HSCP-FG1 | HSCP-FG2 | HSCP-FG3* | HSCP-FG4 | HSCP-FG5 | HSCP-FG6 | – | |
| Number of participants | 7 | 7 | 7 | 9 | 8 | 5 | 43 | 100 |
| Participants’ age range | ||||||||
| 21–29 | 0 | 1 | 1 | 1 | 0 | 1 | 4 | 9 |
| 30–44 | 4 | 3 | 5 | 8 | 6 | 3 | 28 | 65 |
| 45–54 | 3 | 3 | 1 | 0 | 2 | 1 | 10 | 23 |
| Participants’ years of professional experience | ||||||||
| 0–4·9 | 0 | 1 | 4 | 3 | 2 | 3 | 13 | 31 |
| 5–9·9 | 1 | 4 | 1 | 4 | 3 | 1 | 14 | 33 |
| 10+ | 6 | 2 | 1 | 2 | 3 | 1 | 15 | 36 |
One participant did not report number of years of experience on the demographic profile form.
Non-research team participants in the stakeholder meeting were five PPP experiencing vulnerabilities, four public health administrators and staff, one midwife, one early-childhood educator, one doctor and one community food centre director.
Focus group discussions
All FGD were facilitated by the lead author, assisted by the co-authors. Further information about the authors’ roles/responsibilities/qualifications with respect to data collection and analysis are available in online Supplemental Text 2, along with notes on the study’s main methodological and conceptual limitations. FGD lasted from 60 to 120 min and followed the interview guides developed by our team (see online supplementary material, Supplemental Appendix 1).
FGDs were audio-recorded and transcribed verbatim. Transcripts were coded using NVivo 12 qualitative analysis software(35). We then carried out a thematic network analysis, following Attride-Stirling(36). We incorporated questions/subheadings from the interview guides into our coding framework, which included five first-order nodes relating to pregnancy diet challenges. After four team members independently coded the same transcript to evaluate inter-coder agreement (ICA >90 %), transcripts were coded to second-order nodes within the first-order nodes. Then, through a series of brainstorming sessions, we developed a network of ‘global’, ‘organising’ and ‘basic’ themes extended in a non-hierarchical web(36). Themes were distilled and organised into two conceptual maps pertaining to our two research questions. This was accomplished through iterative discussions among investigators, in which we asked: Does this basic theme reflect/overlap/nest within other basic or organising themes? If so, can it be discarded or subsumed into another theme? We repeated this process until we agreed that the resulting maps reflected the relationships among the transcripts’ primary themes. Map contours are presented and discussed below, illustrated via quotes selected from the transcripts.
Stakeholder meeting
The 4-h stakeholder meeting was facilitated by a trained group facilitator with expertise in community engagement. The meeting began with a presentation of FGD findings. Next, we held break-out discussions in four groups, each comprising stakeholders from multiple sectors, to identify major issues relating to pregnancy nutrition in Hamilton. Groups then brought the identified issues to another full-group, wrap-up discussion about priority issues and ways to address them. Proceedings of the meeting were recorded.
Two M2B team members reviewed the meeting notes/transcripts and identified stakeholders’ solutions for addressing pregnancy nutrition challenges. We organised proposed solutions under the themes identified through the FGDs as influences on pregnancy diet/nutrition (see online supplementary material, Supplemental Text 3 and Supplemental Table 2).
Results and discussion
Question 1: What influences diets during pregnancy?
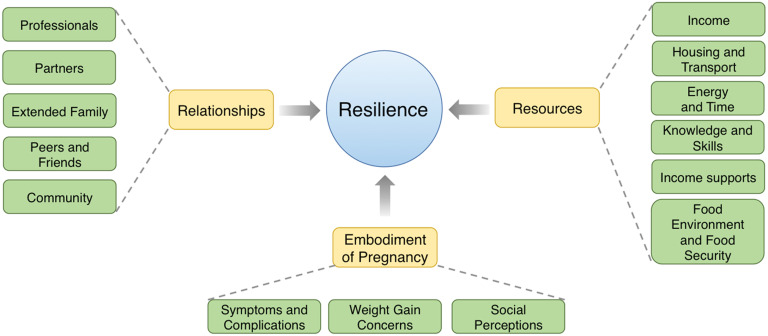
Four themes emerged from our analysis of the FGD (Fig. 1; see also online supplementary material, Supplemental Table 2): Resilience, Resources, Relationships and Embodiment of Pregnancy.
Fig. 1.
Conceptual thematic map outlining themes regarding influences on diet during pregnancy, highlighting that individual resilience is influenced by individual and structural/structured resources, relationships and embodiment of pregnancy
Resilience
PPP and HSCP raised issues around poor mental and physical health as influencing pregnancy diet, but tempered these points with determination to ‘cope’ (PPP-FG1) or ‘deal with’ (PPP-FG3) life’s challenges. We foregrounded these indicators of what we view as resilience, given recent work highlighting the necessity of positive framing and of focusing on modifiable factors in translational DOHaD research(21,25,26). Resilience here refers to a PPP’s capacity to adapt and feed herself, despite adversity, to support her own health and that of her developing fetus(37,38). We underscore, however, that resilience, while treated here as an individual attribute, is determined predominantly by other themes in our thematic network – resources, relationships, embodiment of pregnancy – that extend beyond the individual (cf. reference (38)). In the words of one HSCP, ‘it’s all mostly social, poverty-related issues [rather than individual ones] … everything is political will and money’ (HSCP-FG1). Moreover, resilience should be conceived as modifiable, through the building of individual capacity and through the (re)allocation of resources at household, community and policy levels(38).
Resilience in the face of challenge featured in all FGD and the stakeholder meeting. A midwife, for example, highlighted that, despite growing rates of anxiety among her clientele, her clients resolve to ‘deal’ with the ‘unpredictability of pregnancy and birth’ (HSCP-FG3).
When we spoke with PPP, they showed determination to overcome poverty-associated barriers so as to take care of their families. One mother explained, ‘I’m worried about … health stuff … money … that’s the reality of being a parent and you just have to do what you can’ (PPP-FG3, emphasis ours).
Another mother shared:
I have so many life challenges. I think as mothers and as women we suck it up and do what we have to do for our children (emphasis ours). And that’s just the reality of life. (PPP-FG3)
These quotes illustrate that managing hardship is central to the experiences shaping pregnancy diet, implying trade-offs between competing priorities in challenging environments. As a nurse put it:
[The] issues [facing PPP living with poverty] are so much more base that I feel like just motivating them to think about nutrition … is challenging … The topic of nutrition isn’t really big on their priority list. (HSCP-FG2)
That is, PPP must prioritise meeting other basic needs before eating nutritious, healthful foods.
Resources
PPP and HSCP identified resource access as a main determinant of pregnancy diet. Resources encompasses individual/household resources (income; time/energy; knowledge/skills) and resources provided by the state (community/municipal health and social programmes shaping the local food/health environment; provincially/federally administered financial resources). Provincial/federal resources discussed in FGD include tax credits for people with children, social assistance for people with disabilities, welfare, employment insurance and paid parental leave.
PPP viewed provincial/federal benefits programmes as essential but underfunded. As has been reported in other populations(39,40), PPP perceived income transfers as insufficient to meet the needs of growing families as housing, transport and other living costs rise. One participant, whose income was provincially supplemented, highlighted challenges around housing specifically, saying:
Housing in Hamilton has skyrocketed to the point where 90 % of your income … feels like it’s going to [your] home. And that takes away from your ability to provide food for your family. (PPP-FG1)
HSCP echoed the sentiments of PPP, especially regarding the insufficiency of income supports. One midwife stated:
The most important thing [for PPP] is money. The more money we have, the healthier we are … So, the [money allotted as a prenatal bonus] for people on [welfare], I don’t think that’s sufficient. (HSCP-FG3)
Taken together, these discussions indicate that PPP and HSCP deem government cash transfers insufficient to allow PPP to prioritise their own nutritional needs over competing needs for housing, childcare and feeding older children. This view was further reflected in the stakeholder meeting (see online supplementary material, Supplemental Table 2).
Regarding municipal resources, participants in all FGD highlighted Hamilton’s richness in maternal–child health services and programmes. The prenatal nutrition programme was seen as particularly valuable in connecting PPP with HSCP, peers, and information about other services and resources. One PPP explained that she ‘found out about [the city’s other maternal–child health resources]’ (PPP-FG4) from the programme. Nonetheless, both PPP and HSCP raised criticisms of community-level services. They were viewed as poorly integrated with one another and with government/healthcare services. A dietitian noted that connections with doctors and with the welfare office were nearly non-existent. She noted that HSCP at the prenatal nutrition programme ‘talk [with clients] about the pregnancy nutritional allowance [for welfare recipients] but … don’t always know what their [welfare] worker is telling them’ (HSCP-FG1). Although the prenatal nutrition programme directs vulnerable people to many of the city’s resources, staff – frustrated by ongoing cuts to their programme – indicated that they sometimes lack the time or knowledge to do this effectively. Furthermore, many PPP in the city are unaware of the programme. One PPP, after learning that her FGD peer had missed the opportunity to enrol in the programme, said wryly: ‘My advice would be marketing this program’ (PPP-FG4). A public health nurse expressed frustration that doctors do not refer PPP to the prenatal nutrition programme or similar maternal–child health services, because they are not indexed in their electronic medical checklist system (HSCP-FG1).
In terms of individual and family resources, PPP and HSCP generally agreed that household finances, time and energy constrained pregnancy diet. Regarding time/energy, an early-childhood educator, when discussing meal preparation, noted: ‘you never feel like you have time to do anything … [of] quality’ (HSCP-FG6). A PPP related how, after a long day of work or infant care, she ‘would just grab whatever [she] could to throw in a microwave … So, [she] didn’t really [eat] any meals’ (PPP-FG1).
There were also important differences in perceptions of individual-level influences on pregnancy diet between PPP and HSCP. PPP raised few concerns about lack of knowledge about how to eat healthfully, prepare food, budget, look after their bodies or navigate the health system. Rather, most PPP appeared empowered by their resourcefulness under tight constraints. One lower SEP mother said proudly, ‘My biggest thing is: I budget!’ (PPP-FG1).
In contrast, HSCP were likely to discuss their clients’ need for, in one dietitian’s words, ‘financial literacy … health literacy … nutrition literacy, every form of literacy’ (HSCP-FG1). A midwife argued that ‘fresh products are often cheaper than highly-processed foods, if you know where to shop’ (HSCP-FG3), implying her clients lacked this knowledge. So, HSCP but not PPP viewed health literacy as determining the capacity to eat healthfully during pregnancy. Although numerous explanations could account for this discrepancy in views, we suggest a plausible one is that PPP who volunteer for FGD represent a self-selected sample of more resilient, already-health-literate PPP facing challenge. Another plausible explanation is that most PPP are pregnancy health-literate, but live with so many other constraints that they cannot foreground – let alone apply – their knowledge(41).
Despite varying perspectives on the relative importance of different kinds of resources – individual/family, community and state – our findings accord with those from other populations in which nutritional inequities during pregnancy are viewed as a products of resource inequities(24,42).
Relationships
FGD participants discussed PPP’s interpersonal relational supports (spouses/partners, parents, siblings, friends/peers/communities, and HSCP) as sometimes supporting and sometimes undermining pregnancy diet quality, depending on relational power dynamics(43).
Both social isolation and embeddedness in familial relationships were identified by PPP as barriers to control over food and thus to well-rounded pregnancy diets. Lacking support and/or autonomy at home leaves PPP, in one mother’s words, ‘eating whatever you can, whenever you can’ (PPP-FG1). Others indicated their partners either already held meal preparation responsibilities or took these on during their pregnancies. For those who received partner support with food work, this offered relief from discomfort, tiredness and nausea. However, support came at the cost of poorer adherence to pregnancy dietary recommendations. One participant described her husband’s cooking as ‘delicious, but [with] lots of fat’ (PPP-FG2). Another mother-to-be described her situation living with her husband’s family like this:
We don’t have any control over what we’re eating. I try and cook but it’s … impossible. If you can’t control what you’re buying or preparing, you just eat what’s available. (PPP-FG2)
These findings regarding partners and in-laws were not surprising, as one recent study on fathers’ contributions to food work in North America showed that fathers tend to undermine mothers’ diet and health aspirations(44).
HSCP echoed these ideas that support people, particularly partners and grandmothers-to-be, could improve or inhibit pregnancy wellbeing. From the ‘support people are beneficial’ perspective, one nurse suggested that grandmothers-to-be were pillars for many newcomers-to-Canada participants in her prenatal nutrition group: ‘It’s mainly mothers and mothers-in-law … You can tell how important they are to our clients’ (HSCP-FG2).
From the other perspective, a midwife told a story in which a husband angled to get her ‘endorsement that [his pregnant wife] was eating too much sugar’, in a way that was ‘hurtful or even controlling for the woman’ (HSCP-FG4). Along the same lines, a dietitian related that:
it’s the men who are doing the shopping … So maybe another barrier for the women is that they don’t have … much control of their groceries. (HSCP-FG2)
Relationships between PPP and HSCP were generally highly valued. HSCP emphasised that they viewed their roles as sources of information, but also as psychosocial/emotional supports. In the words of an early-childhood educator, HSCP ‘initiate conversations with families about health … as a whole … only … once [they]’ve built a rapport’ (HSCP-FG5).
PPP mostly expressed gratitude for the support offered by HSCP. Nonetheless, a few mothers hinted at resisting HSCP’s authority. One mother said, ‘There are a lot of rules [outlined by staff] that we as parents might say we follow but we don’t’ (PPP-FG3). Her friend then chimed in:
A lot of these [prenatal nutrition] classes are great, but the guidelines are just so by the book … and the policies. It’s like if you try to tell [the HSCP] any different [than what they advise], then it’s like ‘no, no, no’ … So you don’t say anything. You just keep it to yourself, and while you’re at [the programme] you do what you’re supposed to do until you get home. (PPP-FG3)
These examples illustrate that well-intentioned advice/support from professionals does not translate directly into better health for PPP or babies. Rather, a person’s sense of power and control over her own life drives what becomes embodied and practiced during pregnancy(45).
In contrast to some of the complexities of other relationships, friends/peers were viewed as positively affecting pregnancy nutrition and wellbeing. A new mother said that her friend who had introduced her to a prenatal nutrition programme became ‘a really good friend’ (PPP-FG1) who supported her through health challenges during pregnancy. An early-childhood educator described the dynamics among the participants at her family drop-in centre as profoundly supportive, saying that participants, as peers, ‘really build each other up’ (HSCP-FG5). This aligns with the sense of empowerment reportedly gained through the establishment of peer support groups/participatory women’s groups in other contexts(29,46).
Embodiment of pregnancy
Participants in all FGD recognised pregnancy as a time of bodily transformation, with a unique role in shaping diet. Thus, embodiment of pregnancy describes the physical, psychological and social factors affecting diet, channelled through the body. These factors were similar to those found in other populations. They include physiological changes that affect appetite, energy levels, weight gain and complications(17,47,48) as well as psychosocial concerns around weight gain, body image and behavioural surveillance/policing of the pregnant body(49).
PPP focused on physiological changes affecting appetite (nausea, vomiting, aversions, cravings) and dealing with complications as impacting their pregnancy eating patterns/diets. At least three participants in three different FGD (PPP-FG1, -FG2 and -FG3) were hyperemetic, which may have skewed discussions towards the centrality of vomiting and nausea in determining diet during pregnancy. One of the people who lived with ‘very serious morning sickness’ described her pregnancy diet like this:
Since I [became] pregnant, especially in the first four months, I cannot smell oil, or cooking smell[s, without vomiting]. I just [eat] fruit and lots of very soft food. So, my husband [is] always cutting … food for me before he goes to work. (PPP-FG1)
Another participant talked about how experiences of discomfort impacted her diet: ‘My heartburn was so bad, and I was nauseous … I … wasn’t able to eat as healthy as I wanted to’ (PPP-FG2). These conversations were contrasted by discussions around pregnancy cravings. One mother recalled, ‘When you’re having a craving and you’re pregnant, you just follow your craving … because your baby obviously is wanting something from you’ (PPP-FG1).
PPP also related that concerns around pregnancy complications, particularly gestational diabetes, played a central role in shaping their diets. One participant said of her visit to an obstetrics clinic:
There’s been this … epidemic of gestational diabetes, and so [the staff] basically scared me into just being really focused on ‘if you’re gonna have sugar make it a natural sugar, like a fruit. And, if you’re gonna have fruit, make sure it’s during the day. And I ended up being a little obsessive about it. (PPP-FG2)
Another participant felt prejudged regarding her risk of developing complications, saying: ‘Most people are like … “You’re a big person. You’re going to have gestational diabetes”’. Her friend noted that PPP who ‘have diabetes or have some … health problems … look [at] every single thing that [they] put in [their] mouth[s]’ (PPP-FG1). These quotes suggest that, from the perspectives of PPP, complications like gestational diabetes narrow the windows of acceptable pregnancy eating behaviours.
HSCP noted dealing frequently with many of the same embodied challenges of pregnancy highlighted by PPP, but also discussed a factor largely ignored by PPP – gestational weight gain (GWG). GWG is a public health risk metric, but also a socio-psychologically fraught, complex topic. A dietitian discussed excessive GWG as a risk factor for poor infant outcomes in the contexts of western culture, wherein it is ‘normalise[d]’ that everyone ‘need[s] a [sweet] beverage all the time’, and ‘You need to eat, you’re pregnant, you’re eating for two’ (HSCP-FG1). These excerpts suggest that HSCP, especially those working in public health, are concerned with the environmental and cultural contexts shaping GWG.
While midwives shared these concerns, some were ambivalent about emphasising appropriate GWG with their clients. One commented:
I try not to pathologize … weight and nutrition … I don’t … want anxiety over weight gain … I usually say ‘if you’re gaining nothing or if you’re gaining 100 pounds, then we’re concerned. But, if you’re somewhere in the middle, we’re not too concerned … as long as you’re eating healthy’. (HSCP-FG3)
Her colleague added:
I find it difficult sometimes for … our … large low-income population…, when you’re talking about fresh fruits, vegetables, staying away from processed foods, sometimes they just don’t have the resources … So, you … get to a point where you’re like, you can tell them what to do but they don’t have the means to do it. (HSCP-FG3)
In contrast to the HSCP, PPP did not focus on GWG, although a few PPP mentioned it in passing, dismissively. For example, one PPP felt worried as she was ‘gaining too fast’ despite eating nutritiously and then her GWG trajectory just ‘petered out’ without any dietary changes, so she ‘threw her hands up in the air’ (PPP-FG2). The finding that PPP were uninterested/frustrated with the topic of GWG contrasts with previous work in other contexts showing enthusiasm among PPP for frank conversations about managing GWG(48). We suspect that PPP were more concerned with other embodied features of pregnancy (e.g., hyperemesis, gestational diabetes) and lacked the bandwidth/time to focus on discussions about GWG.
In sum, both PPP and HSCP perceived pregnancy as a time of bodily transition, of increased attention to the body, and thus as an opportunity for thoughtful engagement with health, but varied in which embodied features of pregnancy they emphasised.
Question 2: How can people be supported to improve their diets during pregnancy?
Data to answer this question come from both FGD and from the stakeholder meeting. Proposals for solutions/interventions are summarised in Fig. 2 and discussed below, where we have linked them to the themes of resources, relationships and embodiment of pregnancy. As a successful implementation of these proposed interventions implies an increase in resilience for PPP in Hamilton, the theme of resilience is interwoven throughout our discussion of other themes.
Fig. 2.

Conceptual thematic map outlining possible strategies for intervening to improve women’s diets during pregnancy, highlighting that building individual resilience can be accomplished largely through improving individual and structural/structured resources, relationships and taking account of the embodied-ness of pregnancy. DOHaD, Developmental Origins of Health and Disease
Resources
Participants of FGD and the stakeholder meeting highlighted three resource-related intervention areas: increasing income, introducing/expanding subsidies and improving services. The first group zeroes in on ‘supporting income’, thereby reducing the extent to which food/nutrition competes against other priorities, while expanding the capacity for independent decision-making about nutrition (HSCP-FG1). A midwife noted her support of a Basic Income Pilot Project that, at the time of data collection, was being evaluated by the provincial government. She suspected that the Project, through increasing and stabilising incomes of low-income families, would ‘impact … the nutritional status’ of PPP (HSCP-FG4). Multiple studies demonstrated that income-boosting interventions improve pregnancy diets, health outcomes and individual resilience(50,51). Unfortunately, the Project was cancelled by an incoming, fiscally conservative provincial government, illustrating the political challenges cash transfer programmes present.
Another income-boosting strategy focuses on improving income tax-filing rates among lower-income families. Successful tax-filing entitles all but the wealthiest Canadians with children to substantial government cash transfers(52). Unfortunately, numerous barriers prevent many lower-income families from completing their income taxes(52). HSCP suggested reducing those barriers, allowing families to access their entitlements (HSCP-FG1, -FG2). This suggestion was endorsed by PPP and others during the stakeholder meeting. Notably, this income transfer strategy is politically robust, because it progressively benefits most Canadian tax-paying families, and because survey data show that people in Ontario generally support investing financially in the health/nutrition of developing children(53).
The second group of resource-related interventions concerns subsidies in the domains of housing, childcare and food. Food subsidies and/or subsidies to requisite expenses – like income supplements – would reduce trade-offs between prenatal nutrition/health and other household financial demands. Loosening such constraints can build empowerment and, ultimately, resilience(54). Housing subsidies may be particularly relevant in Hamilton, where housing costs are skyrocketing, requiring many Hamiltonians to allocate unacceptably high proportions of their income to shelter(55). As one mother put it, ‘If [the government] could … make your rent more affordable … that would help’ (PPP-FG1). Similarly, participants called for subsidies aimed directly at improving the accessibility of nutritious food. Food-based subsidies have improved prenatal diet quality along with pregnancy and birth outcomes in lower-income households elsewhere in North America(56,57). So, improving/expanding local nutrition subsidies may be an efficacious, desirable intervention component.
The third kind of intervention proposed under resources concerns developing new and/or improving accessibility and integration of Hamilton’s services supporting PPP/potential parents. Service sectors suggested intervention targets should include transport, language interpretation, early-childhood education, adolescent education and preconception care. Participants suggested expanding interpretive services for newcomers (HSCP-FG1, -FG2, -FG6), providing prenatal grocery buses (HSCP-FG1, -FG4, PPP-FG2) and integrating food literacy into early-childhood/primary education programmes (HSCP-FG3, -FG5). All suggestions warrant consideration, but one deserves special attention, because it has a solid evidence base from other socio-ecological contexts and because it was raised independently in all FGD and in the stakeholder meeting. That is, participants recommended upstream investment in the next generation(s) of prospective parents, through offering universal access to skills training in food/nutrition, health, budgeting and tax-filing, that is ‘every form of literacy’ in secondary school (HSCP-FG1). Efforts along these lines in New Zealand, the UK and Uganda showed promise, often leading to a greater empowerment and greater interest in setting health/nutrition goals(58–60).
Relationships
Peer support, engagement with partners/spouses and equipping HSCP to better support behavioural change were proposed under the theme relationships. One mother proposed creating peer groups/networks as nexuses for advocacy, which was generally supported by other members of her discussion group (PPP-FG3) as well as by stakeholder meeting attendees. In her own words,
I’d like to see more peer-led stuff … it’s hard when there [are] all these policies and you can’t really talk about the actual things … going on because you’re afraid you’ll get judged … So, peer-led groups are great … in that … we can talk about the real stuff. (PPP-FG3)
Establishing participatory groups for PPP and/or new parents may represent a crucial step towards improving prenatal health for people experiencing vulnerabilities in Hamilton. In other contexts, the establishment of such groups has improved health experiences and birth outcomes(29,61,62). Furthermore, such groups can and do serve as critical jumping-off points for organising and mobilisation(63).
A second set of intervention proposals under relationships emphasised going beyond the mother–child dyad when supporting pregnancy health(21). Developing programmes for main interpersonal supports of PPP was suggested. Although services for partners was not a frequently proposed solution in the FGD, participants of the stakeholder meeting endorsed the organisation of programmes for support people.
The final suggestion under relationships was to provide HSCP with skills to support health behaviour changes, particularly relating to early-life environment (i.e., DOHaD) in PPP, given that PPP and HSCP mutually value and invest in their relationships. While knowledge building does not necessarily translate into healthier behaviours(41), the building of HSCP skills in supporting behaviour change is associated with increased empowerment, improved psychological resilience and perhaps healthier behaviours in those with whom HSCP work(28,64).
Embodiment of pregnancy
Participants offered two main solutions for challenges under this theme. The first was to digitally integrate referrals to prenatal/maternal services into primary care (HSCP-FG1, -FG2, -4). Participants of the stakeholder meeting supported this idea, although both a public health administrator and a family doctor highlighted that financial, regulatory and time factors would constrain its implementation. The second set of ideas involved offering key services supporting family wellbeing under a single roof (HSCP-FG1, -FG4, -FG5, PPP-FG1, -FG3, -FG4). Although Hamilton has one such centre, participants recognised that at least four other neighbourhoods would benefit from similar institutions. Evidence from other contexts suggests that locating services/providers close together would reduce access barriers – crucial given the challenging aspects of pregnancy embodiment – and provides opportunities for service providers to better coordinate with each other(65,66). Moreover, such resilience-building centres would help PPP to overcome multitiered dietary and health constraints. But, while participants of the stakeholder meeting supported creating physically integrated care centres around Hamilton, many also questioned these proposals’ feasibility, because construction and staffing costs would require massive upfront investments. So, developing service integration interventions will require a combination of political will and identification of (less expensive) opportunities to tweak existing programmes supportive of pregnancy wellbeing(22).
Conclusions
Widening inequities in NCD prevalence(3) rooted in early life (as suggested by the DOHaD hypothesis)(8) call for the development of interventions during preconception/pregnancy(11,12). Our data suggest that environmental, social and individual factors impede some PPP and their families from prioritising investment in high-quality food, and this contributes to inequities in pregnancy diet and health. Participants recognised that many of the factors shaping pregnancy diet are systemic, complex and multi-level. They therefore argued that strategies for improving pregnancy health equity must work at multiple levels, from supporting individual resilience to leveraging social relationships, to building up community networks, to taking larger political actions.
The human and economic arguments for long-term investments in maternal–child health equity are undeniable(1,2). Global health policy leaders suggest that multi-level interventions targeting mothers and children can yield 10-fold returns on investment through better educational attainment, workforce participation and social contributions, in addition to improved long-term health(67). Thus, the likely benefits of such a policy focus to a Canadian city grappling with rising levels of social and health inequity are self-evident. The priority now should be to make these investments in ways feasible and sustainable within the local sociopolitical context.
Acknowledgements
Acknowledgements: The study reported here would have been impossible without the support of a number of people and organisations. Next, we express our gratitude for the practical and logistical support we received from our partners in Hamilton’s Public Health Services and in Hamilton’s Ontario Early Years Centres. We would especially like to highlight the integral roles of Michaela Servos, Kathy Pierce, Laura Wilson and their staff and colleagues who collectively run a variety of programmes aimed at improving maternal–child health, wellbeing and nutrition in the city of Hamilton. Much of our study promotion was carried out through and with these organisations, with the support and encouragement of their leaders and staff. We also thank other members of the M2B study team, especially Debbie Kao and Fei Fei Xia, along with members of the Sloboda Lab, for insightful comments on earlier presentations of our data. Finally, our deepest gratitude goes to the twenty-two PPP who took time from their hectic lives to participate in FGD and a stakeholder meeting with members of our team. Financial support: We acknowledge the contributions of our funders – the Canadian Institutes for Health Research (Hugs for Health Team Grant, no. 146333), the Women’s College Hospital (XChange Grant 15k Challenge) and McMaster University (Provost’s Award). Conflict of interest: We have no conflicts of interest to declare. Authorship: All authors contributed to the design of the study, spearheaded by D.M.S. and M.B. Additionally, all authors participated in a minimum of one analytical discussion session, and agreed on data interpretation and data presentation strategy. L.M. and M.B. wrote the manuscript and sketched first drafts of the figures. L.M. and S.O. constructed data tables. S.O. developed the final digital versions of figures. All authors read, edited, discussed and approved the submitted manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Hamilton Integrated Research Ethics Board. Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020001093.
click here to view supplementary material
References
- 1. Marmot M & Bell R (2012) Fair society, healthy lives. Public Health 126, s4–s10. [DOI] [PubMed] [Google Scholar]
- 2. Commission on Social Determinants of Health (2008) Closing the gap in a generation: Health equity through action on the social determinants of health. Final report of the Commission on Social Determinants of Health, p. 256, Geneva.
- 3. Niessen S, Mohan D, Akuoku JK et al. (2018) Tackling socioeconomic inequalities and non-communicable diseases in low-income and middle-income countries under the sustainable development agenda. Lancet 391, 2036–2046. [DOI] [PubMed] [Google Scholar]
- 4. Sommer I, Griebler U, Mahlknecht P et al. (2015) Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Public Health 15, 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hertzman C & Boyce T (2010) How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health 31, 329–347. [DOI] [PubMed] [Google Scholar]
- 6. Kuzawa C & Sweet E (2009) Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol 21, 2–15. [DOI] [PubMed] [Google Scholar]
- 7. Belsky J (2019) Early-life adversity accelerates child and adolescent development. Curr Dir Psychol Sci 28, 241–246. [Google Scholar]
- 8. Low F, Gluckman P & Hanson M (2018) A lifecourse approach to public health: Why early life matters. In Oxford Textbook of Nature in Public Health: The Role of Nature in Improving The Health of a Population, pp. 11–25 [van Den Bosch M & Bird W, editors]. Oxford: Oxford University Press. [Google Scholar]
- 9. Heckman J (2011) The economics of inequality: the value of early childhood education. Am Educ 35, 31–37. [Google Scholar]
- 10. Scorza P, Duarte CS, Hipwell P et al. (2019) Research review: intergenerational transmission of disadvantage: epigenetics and parents’ childhoods as the first exposure. J Child Psychol Psychiatry 60, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Godfrey K, Gluckman P & Hanson M (2010) Developmental origins of metabolic disease: lifecourse and intergenerational perspectives. Trends Endocrinol Metab 21, 199–205. [DOI] [PubMed] [Google Scholar]
- 12. Stephenson J, Helsehurst N, Schoenaker D et al. (2018) Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 391, 1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Victora C, Bahl R, Barros AJD et al. (2016) Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490. [DOI] [PubMed] [Google Scholar]
- 14. Doyle I-M, Borrmann B, Grosser A et al. (2017) Determinants of dietary patterns and diet quality during pregnancy: a systematic review with narrative synthesis. Public Health Nutr 20, 1009–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laraia B, Leak TM, Tester JM et al. (2017) Biobehavioral factors that shape nutrition in low-income populations: a narrative review. Am J Prev Med 52, S118–S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brunst KJ, Wright RO, DiGiola K et al. (2014) Racial/ethnic and sociodemographic factors associated with micronutrient intakes and inadequacies among pregnant women in an urban US population. Public Health Nutr 17, 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carpenter M (2007) Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care 30, S246–S250. [DOI] [PubMed] [Google Scholar]
- 18. Laraia B, Siega-Riz A & Gunderson C (2010) Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain and pregnancy complications. J Am Diet Assoc 110, 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monteiro L, Norman JE, Rice GE et al. (2016) Fetal programming and gestational diabetes mellitus. Placenta 30, S54eS60. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed F & Tseng M (2013) Diet and nutritional status during pregnancy. Public Health Nutr 16, 1337–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharp G, Lawler D & Richardson S (2018) It’s the mother!: How assumptions about the causal primacy of maternal effects influence research on the developmental origins of health and disease. Soc Sci Med 213, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baker PHC, Hawkes C, Wingrove K et al. (2018) What drives political commitment for nutrition? A review and framework synthesis to inform the United Nations Decade of Action on Nutrition. BMJ Glob Health 2018, e000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunneram Y & Jeewon R (2015) Healthy diet and nutrition education program among women of reproductive age: a necessity of multilevel strategies or community responsibility. Health Promot Perspect 5, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nisbett N (2019) Understanding the nourishment of bodies at the centre of food and health systems – systemic, bodily and new materialist perspectives on nutritional inequity. Soc Sci Med 228, 9–16. [DOI] [PubMed] [Google Scholar]
- 25. Mckerracher L, Moffat T, Barker ME et al. (2019) Translating the Developmental Origins of Health and Disease concept to improve the nutritional environment for our next generations: a call for a reflexive, positive, multi-level approach. J Dev Orig Health Dis 10, 420–428. [DOI] [PubMed] [Google Scholar]
- 26. Winett L, Wulf A & Wallack L (2016) Framing strategies to avoid mother blame in communicating the origins of chronic disease. Am J Public Health 106, 1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fox E, Davis C, Downs S et al. (2019) Who is the woman in women’s nutrition? A narrative review of evidence and actions to support women’s nutrition throughout life. Curr Dev Nutr 3, nzy076. [Google Scholar]
- 28. Lawrence W, Black C, Tinati T et al. (2016) Making every contact count: evaluation of the impact of an intervention to train health and social practitioners in skills to support behaviour change. J Health Psychol 12, 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prost A, Colbourn T, Seward N et al. (2013) Women’s groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet 381, 1736–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brunton G, Omara-Eves A & Thomas J (2014) The ‘active ingredients’ for successful community engagement with disadvantaged expectant and new mothers: a qualitative comparative analysis. J Adv Nurs 70, 2847–2860. [DOI] [PubMed] [Google Scholar]
- 31. Statistics Canada (2019) Census Profile, 2016 Census. Statistics Canada. [S.l.].
- 32. Harris R, Dunn J & Walkefield S (2015) A City on the Cusp: Neighbourhood Change in Hamilton since 1970, p. 236. Toronto: Neighbourhood Change Research Partnership.
- 33. Buist S (2019) A five-alarm fire: Code Red, ten years later. The Hamilton Spectator, 21 fev. 2019. https://projects.thespec.io/codered10/ (accessed May 2019).
- 34. City of Hamilton (2019) Prenatal Nutrition Programs in Hamilton. [S.l.]. https://www.hamilton.ca/public-health/classes/prenatal-nutrition-programs-in-hamilton (accessed July 2019).
- 35. QSR International Pty Ltd (2018) NVivo 12 Qualitative Data Analysis Software. [S.l.].
- 36. Attride-Stirling J (2001) Thematic networks: an analytic tool for qualitative research. Qual Res 1, 385–405. [Google Scholar]
- 37. Fletcher D & Sarkar M (2013) Psychological resilience: a review and critique of definitions, concepts, and theory. Eur Psychol 18, 12–45. [Google Scholar]
- 38. Panter-Brick C (2014) Health, risk, and resilience: interdisciplinary concepts and applications. Annu Rev Anthropol 43, 431–448. [Google Scholar]
- 39. Lucas P, Jessiman T & Cameron A (2015) Healthy start: the use of welfare food vouchers by low income parents in England. Soc Policy Soc 14, 57–469. [Google Scholar]
- 40. Wong MW, Leung CW, Cheung LWY et al. (2014) Public support for policies to improve the nutritional impact of the Supplemental Nutrition Assistance Program (SNAP). Public Health Nutr 17, 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lawrence W, Skinner C, Halsam C et al. (2009) Why women of lower educational attainment struggle to make healthier food choices: the importance of psychological and social factors. Psychol Health 24, 1003–1020. [DOI] [PubMed] [Google Scholar]
- 42. Attree J (2005) Low-income mothers, nutrition and health: a systematic review of qualitative evidence. Matern Child Nutr 1, 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moss N (2002) Gender equity and socioeconomic inequality: a framework for the patterning of women’s health. Soc Sci Med 54, 649–661. [DOI] [PubMed] [Google Scholar]
- 44. Fielding-Singh P (2017) Dining with Dad: fathers’ influences on family food practices. Appetite 117, 98–108. [DOI] [PubMed] [Google Scholar]
- 45. Kendall A, Olson C & Frongillo EJ (2001) Evaluation of psychosocial measures for understanding weight-related behaviors in pregnant women. Ann Behav Med 23, 50–58. [DOI] [PubMed] [Google Scholar]
- 46. Heberlein EC, Frongillo EA, Picklesimer AH et al. (2016) Effects of group prenatal care on food insecurity during late pregnancy and early postpartum. Matern Child Health J 20, 1014–1024. [DOI] [PubMed] [Google Scholar]
- 47. Crozier SR, Inskip HM, Godfrey KM et al. (2017) Nausea and vomiting in early pregnancy: effects on food intake and diet quality. Matern Child Nutr 13, e12389–e12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vanstone D, Kandasamy S, Giacomini M et al. (2017) Pregnant women’s perceptions of gestational weight gain: a systematic review and meta-synthesis of qualitative research. Matern Child Nutr 13, e12374–e12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harper E & Rail G (2012) ‘Gaining the right amount for my baby’: young women’s discursive constructions of health. Health Soc Rev 21, 69–81. [Google Scholar]
- 50. Brownell M, Nickel NC, Chartier M et al. (2018) An unconditional prenatal income supplement reduces population inequities in birth outcomes. Health Aff 37, 447–455. [DOI] [PubMed] [Google Scholar]
- 51. Collins A, Jacob M & Klerman M (2017) Improving nutrition by increasing supplemental nutrition assistance program benefits. Am J Prev Med 52, S179–S185. [DOI] [PubMed] [Google Scholar]
- 52. Li J & Neborak J (2018) Tax, race, and child poverty: the case for improving the Canada Child Benefit Program (Part II). J Law Soc Policy 28, 67–96. [Google Scholar]
- 53. Kirst M, Shankardass K, Singhal S et al. (2017) Addressing health inequities in Ontario, Canada: what solutions do the public support? BMC Public Health 17, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bastagli F, Hagen-Zanker J, Harman L et al. (2016) Cash Transfers: What Does the Evidence Say? A Rigorous Review of Programme Impact and the Role of Design and Implementation Features, pp. 1–300. London: Overseas Development Institute. [Google Scholar]
- 55. Statistics Canada (2016) Census Profile: 2016 Census, Hamilton [Hamilton Metropolitan Area], Ontario. Government of Canada. [S.l.]. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CMACA&Code1=537&Geo2=PR&Code2=35&Data=Count&SearchText=hamilton&SearchType=Begins&SearchPR=01&B1=All&TABID=1 (accessed May 2019).
- 56. Haeck C & Lefebvre P (2016) A simple recipe: the effect of a prenatal nutrition program on child health at birth. Labour Econ 41, 77–89. [Google Scholar]
- 57. Hoynes H, Page M & Stevens A (2011) Can targeted transfers improve birth outcomes? Evidence from the introduction of the WIC program. J Public Econ 95, 813–827. [Google Scholar]
- 58. Bay JL, Vickers MH, Mora HA et al. (2017) Adolescents as agents of healthful change through scientific literacy development: a school-university partnership program in New Zealand. Int J STEM Educ 4, 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Macnab A & Mukisa R (2017) Priorities for African youth engaging in DOHaD. J Dev Orig Health Dis 9, 15–19. [DOI] [PubMed] [Google Scholar]
- 60. Woods-Townsend K, Leat H, Bay J et al. (2018) LifeLab Southampton: a programme to engage adolescents with DOHaD concepts as a tool for increasing health literacy in teenagers – a pilot cluster-randomised control trial. J Dev Orig Health Dis 9, 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bolton M, Moore I, Ferreira A et al. (2015) Community organizing and community health: piloting an innovative approach to community engagement applied to an early intervention project in South London. J Public Health 38, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Houweling TAJ, Looman CWN, Das S et al. (2017) The equity impact of community women’s groups to reduce neonatal mortality: a meta-analysis of four cluster randomized trials. Int J Epidemiol 48, 168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fleming S (1991) Between the household: researching community organisation and networks. IDS Bull 22, 37–43. [Google Scholar]
- 64. Baird J, Jarman M, Lawrence W et al. (2014) The effect of a behaviour change intervention on the diets and physical activity levels of women attending Sure Start Children’s Centres: results from a complex public health intervention. BMJ Open 4, e005290–e005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gibson CDJ (1968) The neighborhood health center: the primary unit of health care. Am J Public Health 58, 1188–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nathoo T, Poole N, Bryans M et al. (2013) Voices from the community: developing effective community programs to support pregnant and early parenting women who use alcohol and other substances. First Peoples Child Fam Rev 8, 93–106. [Google Scholar]
- 67. UN Secretary General’s Office (2015) The Global Strategy for Women’s, Children’s, and Adolescent’s Health (2016–2030). [S.l.], p. 108.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020001093.
click here to view supplementary material