Abstract
Objective:
In the current meta-analysis, we aimed to systematically review and summarize eligible studies for the association between dietary inflammatory index (DII) and blood pressure, hypertension (HTN) and glucose homeostasis biomarkers.
Design/Setting:
In a systematic search of PubMed, Scopus and Google Scholar electronic databases up to February 2019, relevant studies were included in the literature review. Observational studies evaluating the association between DII and HTN, hyperglycaemia, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose (FBG), insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and glycated Hb (HbA1c) were included.
Participants:
Not applicable.
Results:
Total numbers of studies were as follows: OR for DII and HTN (n 12), OR for DII and hyperglycaemia (n 9), HTN prevalence (n 9), mean (sd) of SBP and DII (n 12), mean (sd) of DBP and DII (n 10), mean (sd) of FBS and DII (n 13), mean (sd) of HbA1c and DII (n 3), mean (sd) of insulin and DII (n 6), mean (sd) of HOMA-IR and DII (n 7). Higher DII scores were associated with higher odds of HTN (OR = 1·13; 95 % CI 1·01, 1·27; P < 0·001), SBP (weighted mean difference (WMD) = 1·230; 95 % CI 0·283, 2·177; P = 0·011), FBS (WMD = 1·083; 95 % CI 0·099, 2·068; P = 0·031), insulin (WMD = 0·829; 95 % CI 0·172, 1·486; P = 0·013), HbA1c (WMD = 0·615; 95 % CI 0·268, 0·961; P = 0·001) and HOMA-IR (WMD = 0·192; 95 % CI 0·023, 0·361; P = 0·026) values compared with lowest DII categories.
Conclusions:
Lower inflammatory content of diets for prevention of cardiovascular risk factors is recommended.
Keywords: Dietary inflammatory index, Hypertension, Glycaemic markers, Blood pressure
Inflammation characterized by the presence of pro-inflammatory cytokines in the body is a protective bodily response against injury for removing the damaged cells and neutralizing the harmful agents(1). However, chronic and continuous inflammation is associated with numerous chronic diseases including cardiovascular events, diabetes, stroke, metabolic syndrome and cancers(2–4). Diet-induced inflammation is a chronic low-grade inflammatory status caused by overnutrition and inappropriate dietary habits leading to obesity, insulin resistance, diabetes and cardiovascular events(5). Overnutrition induces lipid accumulation in the body, especially in the adipocytes, which activates inflammatory pathways and increases the production of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6 and high-sensitivity C-reactive protein(6,7). It has been demonstrated that inflammatory biomarkers are potent mediators of hyperglycaemia independent of insulin resistance and infusion of pro-inflammatory cytokines like TNF-α is associated with a sustainable increase in fasting blood glucose(8). Accordingly, inflammatory cytokines are potent inducers of hypertension (HTN) via disturbance of the renin–angiotensin system, vascular inflammation and reduced NO production(9). The dietary inflammatory index (DII), first identified by Shivappa et al., is a valid and reliable tool for assessing the pro-inflammatory and anti-inflammatory potential of diets. It was developed according to the review and scoring of 1943 papers; the final scoring algorithm was based on forty-five food parameters and their effects on several inflammatory parameters including high-sensitivity C-reactive protein, IL-1, IL-4, IL-6, IL-8, IL-10 and TNF-α(10,11). Numerous studies have reported the association between DII and inflammatory cytokines; in the study by Shivappa et al.(12), higher DII scores were associated with increased levels of various inflammatory markers including TNF-α, IL-1β, IL-2, interferon-γ and vascular cell adhesion molecule; in another study by Phillips et al.(13), higher energy-adjusted DII scores were positively correlated with higher complement component C3, C-reactive protein (both P < 0·05), IL-6 and TNF-α concentrations, higher leucocyte counts and ratio of neutrophils to lymphocytes, and lower adiponectin levels (all P < 0·001). Since its development, numerous studies have examined the effects of DII on several chronic diseases including cardiovascular events(11), several types of cancer(14–18), metabolic syndrome, pre-diabetes and diabetes(19–22), memory function(23) and cataract(24). In addition, numerous reports are available evaluating the association between DII and cardiometabolic risk factors including HTN(25), blood pressure(22,26,27), insulin resistance(19) and blood glucose(26,28). Moreover, several reviews have been developed evaluating the association between DII and numerous chronic diseases including cancers(29–32), CVD(33–35), obesity(36) and depressive outcomes(37). However, it would be worthwhile to summarize and evaluate the association of DII with biomarkers of these chronic diseases including blood pressure and markers of glycaemic status. In the current systematic review and meta-analysis, we systematically reviewed all of the studies investigating the association between DII and blood pressure, HTN and markers of glucose homeostasis; then, relevant studies were included into meta-analyses to estimate the effect sizes of the mentioned associations including the association of DII with HTN, systolic and diastolic blood pressure (SBP, DBP), insulin, fasting blood sugar (FBS), insulin resistance markers and glycated Hb (HbA1c) concentration.
Methods
Search strategy
A systematic search was performed in PubMed, Scopus and Google Scholar electronic databases for studies that evaluated the association between DII, glycaemic status and blood pressure up to February 2019. No language restriction was applied. Additionally, hand-searching from reference lists of all relevant papers, previous reviews and meta-analyses was performed to cover all relevant publications. For creating a strategy search, a combination of the MeSH (Medical Subject Headings) terms from the PubMed database and free text words was used. For each electronic database, the search strategy was adapted. The PICO (patients, intervention, comparator and outcome) strategy for studies’ selection is presented in Table 1. The PICO model used in the current study is one of the most widely used models for formulating clinical questions and one of the frequently used tools for structuring clinical research questions in connection with evidence syntheses. The Cochrane Handbook for Systematic Reviews of Interventions specifies using PICO as a model for developing a review question, thus ensuring that the relevant components of the question are well defined(38,39). The protocol of the current meta-analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42019122269).
Table 1.
PICO criteria used for the current systematic review
| PICO criteria | Description |
|---|---|
| Participants | General adult populations |
| Interventions (exposure) | Highest category of DII |
| Comparisons | Lowest category of DII |
| Outcome | HTN, SBP, DBP, FBS, insulin, HOMA-IR |
| Study design | Observational studies with the design of cross-sectional, case–control or cohort |
DII, dietary inflammatory index; HTN, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment of insulin resistance.
Selection and characteristics of the included studies
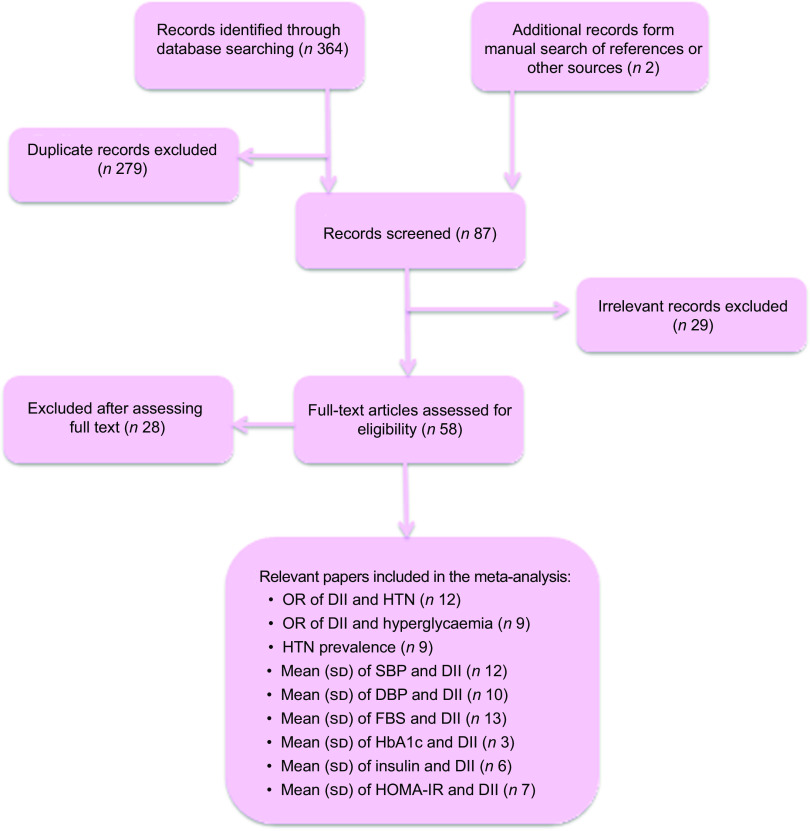
Our search obtained 119 manuscripts from PubMed, 137 from Scopus and 110 from Google Scholar databases. After removal of duplicates, a total of eighty-seven manuscripts remained. From the remaining manuscripts, twenty-nine manuscripts were excluded according to their title and abstract. Thereafter, fifty-eight manuscripts remained and were subjected to full-text screening. Finally, twenty-eight manuscripts were excluded because of their irrelevant design (being reviews or conference/seminar presentations), not relevant age groups, not evaluating the association of the studied parameters (DII, HTN, blood pressure and markers of glycaemia) or not measuring the routine DII. Accordingly, thirty manuscripts were included in the systematic review (Fig. 1).
Fig. 1.
Flow diagram of the study screening and selection process for the current systematic review and meta-analysis (DII, dietary inflammatory index; HTN, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; HbA1c, glycated Hb; HOMA-IR, homeostatic model assessment of insulin resistance)
Inclusion criteria
In the current systematic review and meta-analysis, observational studies with the design of cross-sectional, case–control or cohort evaluating the association of DII with HTN, SBP, DBP, serum or plasma glucose, insulin, HbA1c, homeostatic model of insulin resistance (HOMA-IR), homeostatic model of pancreatic β-cell function (HOMA-B) or quantitative insulin sensitivity check index (QUICKI) were included. According to our set of parameters, we conducted numerous meta-analyses. The DII–HTN or DII–hyperglycaemia meta-analysis included studies that evaluated the odds ratio, relative risk or prevalence of HTN or hyperglycaemia in the highest v. lowest DII categories. Accordingly, in DII–blood pressure or DII–glycaemic markers meta-analysis, the study must have reported the mean and standard deviation of SBP or DBP or glycaemic markers (including fasting serum or plasma glucose (FBS) or insulin, HbA1c, HOMA-IR, HOMA-B or QUICKI) in participants in the highest DII v. lowest DII category as the reference group. The reviewed literatures were inserted into the EndNote software version X8 for Windows (Thomson Reuters, Philadelphia, PA, USA). Consequently, retrieved citations were merged, duplications were eliminated, and the review process facilitated. Titles and abstracts of all articles were evaluated independently by three reviewers (M.A.F., L.N. and Z.N.). Articles not meeting the eligibility criteria were excluded. Moreover, the reference lists of relevant review articles were also evaluated to include additional studies. Full texts of relevant articles meeting the eligibility criteria were retrieved and evaluated. Any disagreements were discussed and resolved by consensus.
Quality assessment
The methodological quality assessment of the included papers was performed by the 9-star Newcastle–Ottawa scale (NOS) for quality assessment of the cross-sectional, case–control and cohort studies. The 9-point NOS has scoring range from 0 to 9, and is categorized into selection, comparability and ascertaining of outcome. Studies with ≥7 stars were categorized as high quality(40).
Data collection and extraction
Data were collected using a standard data extraction form that gathered information about the study characteristics including first author’s name, publication year, geographical area and study design; information about the population including participants’ age range, mean age of case and control groups, number of cases and controls, dietary assessment tool, setting, gender and sample size; and study results including the main findings, estimates of associations and information about adjusting for possible confounders.
Data synthesis and analysis
In the current meta-analysis, three meta-analysis approaches were used. (i) When the association between HTN or hyperglycaemia and DII was analysed by estimating the OR and 95 % CI, the logarithm of the OR and its standard error were calculated as the effect size of the meta-analysis. The pooled OR (and 95 % CI) was estimated using a weighted random-effect model (the DerSimonian–Laird approach)(41). (ii) When the comparison of continuous variables including SBP, DBP, FBS, insulin and HOMA-IR between the highest v. the lowest DII category as reference group was performed, the unstandardized mean difference was measured as the effect size and the pooled estimate was calculated as the weighted mean difference (WMD) with 95 % CI using fixed-effects and random-effects models. (iii) The prevalence of HTN and hyperglycaemia in the highest v. the lowest DII category as reference group was performed by recalculating the proportions of interest from the relevant numerator and denominator. The overall proportions of interest were derived using meta-analysis techniques with the metaprop command in Stata and presented along with the 95 % CI calculated using a normal approximation.
Cochran’s Q test and the I 2 test were used to identify between-study heterogeneity: I 2 < 25 % indicates no heterogeneity; I 2 = 25–50 % indicates moderate heterogeneity; and I 2 > 50 % indicates large heterogeneity(42). The heterogeneity was considered significant if either the Q statistic had P < 0·1 or I 2 > 50 %. Sensitivity analysis was used to explore the extent to which inferences might depend on a particular study or a number of publications. Subgroup analysis was performed to identify possible sources of heterogeneity, if required. Begg’s funnel plots were assessed to evaluate the publication bias followed by Egger’s regression asymmetry test and Begg’s adjusted rank correlation for formal statistical assessment of funnel plot asymmetry. The data were analysed using the statistical software package Stata version 13 and P values less than 0·05 were considered statistically significant
Results
Description of studies reporting associations of dietary inflammatory index with hypertension or blood pressure
From all of the relevant papers included in the systematic review (Table 2), a total of twenty-four manuscripts reported the association of DII with HTN or blood pressure. The findings of all of these reports could be categorized in three dimensions: (i) those that reported a positive association between DII and odds of HTN or a higher prevalence of HTN or higher SBP or DBP values in the highest DII category(21,22,43–49); (ii) those that reported an inverse association between DII and odds of HTN or a lower prevalence of HTN or lower SBP or DBP values in the highest DII category(20,26,50–53); and (iii) those studies that reported no significant association between the mentioned parameters(11,26,27,54–59). Three studies reported the association between DII and blood pressure by gender, providing separate results for men and women(20,46,48). Bodén et al.(46) reported the association between DII and risk of first myocardial infarction in a prospective population-based study. In the separate analysis of baseline parameters, significantly higher SBP in the highest quartile of DII compared with the lowest was observed only among men (P = 0·005) and not in women (P = 0·673). In Mazidi et al.’s study(43) analysing results from the National Health and Nutrition Examination Survey (NHANES) with 15 693 participants, in a model fully adjusted for confounders, women in the fourth quartile of DII were 1·25 times more likely to develop high blood pressure compared with women in the first quartile (OR = 1·25; 95 % CI 1·07, 1·45; P < 0·001), while this association was not significant among men. Sokol et al.(20) evaluated data from the Polish-Norwegian (PONS) Study involving more than 1290 men and 2572 women from the general population and reported the inverse association between DII and odds of HTN among women, while no association among men was observed. In another study, Neufcourt et al.(49) evaluated the prospective association between DII and odds of metabolic syndrome among the general population of the Supplémentation en Vitamines et Minéraux AntioXydants (SU.VI.MAX) cohort with 3726 participants. DII was associated with higher SBP values at baseline and with higher SBP and DBP values after 13-year follow-up. Park et al. also reported lower DBP values in the highest DII tertile among a metabolically unhealthy obese population while no association was observed among metabolically healthy obese individuals(26).
Table 2.
Characteristics of studies included in the current systematic review owing to reporting the association between dietary inflammatory index (DII) and central obesity
| First author, reference | Year | Country | Study design | Sex | Age range (years) | Sample size/population | Number of cases/controls | Dietary assessment/index | Results | Adjusted variables | Quality of the study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shivappa(50) | 2018 | Italy | Cross-sectional | Both | ≥35 | 20 823/general population | 4164/4164 | FFQ/DII | Individuals in highest DII quintile had lower prevalence of HTN, lower SBP and lower FBS compared with lowest. No difference in DBP was observed | Age, sex | 9 |
| Ren(21) | 2018 | China | Cross-sectional | Both | 18–75 | 1712/general population | 579/566 | 24 h recall record/DII | Individuals in highest tertile were 1·40 (95 % CI 1·03, 1·89) times more likely to have HTN compared with lowest tertile. No association with DII and high blood glucose was observed. In gender-stratified results, the DII–HTN association was observed only among women (1·17; 95 % CI 0·80, 1·70) | Age, gender, city, education, family monthly expenditure on food, smoking, BMI | 8 |
| Phillips(25) | 2018 | Ireland | Cross-sectional | Both | 50–69 | 2047/adult general population | 664/664 | Self-completed FFQ/DII | No significant association between DII and HTN was observed | – | 7 |
| Phillips(22) | 2018 | Ireland | Cross-sectional | Both | 50–69 | 1992/general population | 996/996 | FFQ/E-DII | Individuals in higher than median E-DII had higher FPG and SBP values compared with lowest. No significant difference in other glycaemic markers and DBP by DII was reported | – | 7 |
| Park(26) | 2018 | USA | Cross-sectional | Both | 20–90 | 1815/metabolically healthy overweight and obese adults | 634/570 | 24 h dietary recalls/DII | Individuals in highest tertile had higher HOMA-IR compared with lowest. Other parameters were not significantly different | – | 7 |
| Park(26) | 2018 | USA | Cross-sectional | Both | 20–90 | 1918/metabolically unhealthy overweight and obese adults | 610/674 | 24 h dietary recalls/DII | Individuals in highest tertile had lower DBP compared with lowest. Other parameters were not significantly different | – | 7 |
| Farhangi(11) | 2018 | Iran | Cross-sectional | Both | 35–80 | 454/patients, candidates for CABG | 113/113 | FFQ/DII | No significant difference between the prevalence of HTN and serum values of HbA1c in different quartiles | Age, gender, BMI, educational attainment, diabetes, MI | 9 |
| Denova-Gutiérrez(60) | 2018 | USA | Cross-sectional | Both | 20–69 | 1174/general population | 234/235 | FFQ/DII | Individuals in the top quintile of DII had significantly higher FBS and HbA1c compared with the lowest | – | 7 |
| Abdurahman(60) | 2018 | Iran | Cross-sectional | Both | 19–59 | 300/individuals with obesity | 75/75 | FFQ/DII | Non-significant elevation of FPG, SBP and DBP among highest v. lowest quartile of DII. No significant association between DII and HTN or hyperglycaemia in logistic model | Age, sex, PA, BMI, history of chronic diseases | 8 |
| Vissers(44) | 2017 | Australia | Cross-sectional | Women | 52 | 7169/general population | 1664/5505 | FFQ/DII | A pro-inflammatory diet was significantly associated with a higher risk of incident HTN in comparison to the anti-inflammatory diet, with a 24 (95 % CI 6, 46) % higher risk. The prevalence of HTN was also higher among women with more pro-inflammatory diet | Energy intake, age, diabetes, smoking, education, menopausal PA, BMI | 7 |
| Vahid(19) | 2017 | Iran | Case–control | Both | Mean = 47 | 414/pre-diabetics and healthy matched controls | 138/138 | FFQ/DII | DII was associated with higher FPG and HbA1c concentrations | Age, BMI, education, smoking, alcohol, diabetes, LDL-C, TAG | 7 |
| Shivappa(45) | 2017 | USA | Cross-sectional | Both | ≥19 | 12 438/general population | 4119/4183 | 24 h dietary recall/DII | Prevalence of HTN in highest tertile of DII was significantly higher than in the lowest (35·0 v. 32·3 %) | – | 8 |
| Nikniaz(54) | 2018 | Iran | Cross-sectional | Both | 18–64 | 606/general population | 151/151 | FFQ/DII | No significant difference in FBS, SBP, DBP in different DII quartiles; in multivariate logistic model, OR of high FBS was 2·56 times higher in 4th quartile compared with 1st | Smoking, PA, sex, age, BMI | 8 |
| Mirmajidi(28) | 2019 | Iran | Cross-sectional | Both | 18–60 | 171/abdominal obese | 85/86 | FFQ/DII | FBS was significantly higher among individuals with higher than median DII. In regression model, DII was positively associated with FBS | Age, sex, PA, energy intake | 8 |
| Mazidi(43) | 2018 | USA | Cross-sectional | Both | ≥18 | 21 874/general population | 5504/5473 | FFQ/DII | Higher FBS, SBP, DBP, HOMA-IR, insulin, HbA1c and 2 h glucose in highest DII quartile compared with lowest. Being in top quartile of DII made individuals 1·21 times more likely to have HTN | Age, race, sex, income/poverty ratio, education, marriage, BMI | 9 |
| Mazidi(63) | 2018 | USA | Cross-sectional | Both | ≥18 | 21 649 | 5128/5153 | FFQ/E-DII | Prevalence of HTN in 4th DII quartile significantly higher than in lowest (34·1 v. 28·1 %). FBS, SBP and DBP in highest DII quartile were significantly higher | – | 8 |
| Kim(55) | 2018 | Korea | Cross-sectional | Men | 19–65 | 3682 | 921/920 | FFQ/DII | In multivariate logistic regression, being in 4th quartile of DII made men 1·30 times more likely to develop hyperglycaemia | Age, BMI, education, alcohol, smoking, PA, energy intake | 8 |
| Kim(55) | 2018 | Korea | Cross-sectional | Women | 19–65 | 5609 | 1402/1403 | FFQ/DII | No significant association between DII, HTN and hyperglycaemia | Age, BMI, education, alcohol, smoking, PA, energy intake | 8 |
| Sokol(20) | 2016 | Poland | Cross-sectional | Men | 45–65 | 1290/general population | 458/213 | FFQ/DII | No significant association between higher DII and HTN or hyperglycaemia was observed | Age, BMI | 7 |
| Sokol(20) | 2016 | Poland | Cross-sectional | Women | 45–65 | 2572/general population | 507/751 | FFQ/DII | More pro-inflammatory diet was associated with decreased prevalence of HTN and hyperglycaemia was observed | Age, BMI | 7 |
| Moslehi(51) | 2016 | Iran | Cross-sectional | Both | 19–75 | 12 523/general population | 744/743 | FFQ/DII | Prevalence of HTN among top quartile of DII was significantly lower than in the lowest. No significant association between markers of glycaemic status and DII was observed | Sex, age, smoking, PAL, family history of diabetes, HTN, glucose- and lipid-lowering medication use, BMI | 8 |
| Ramallal(47 ) | 2015 | Spain | Cohort | Both | Mean = 38 (sd 12) | 18 794/general population | 4698/4699 | FFQ/DII | Baseline prevalence of HTN in different DII quartiles was not different. Individuals in highest DII quartile had higher OR of HTN compared with lowest | Baseline and family history of diabetes, HTN, CVD, hypercholesterolaemia, special diets, smoking, energy, PA, BMI, education, alcohol, snacking, sitting time, time watching TV | 8 |
| Neufcourt(49); baseline analysis | 2015 | France | Cohort | Both | 35–60 (women), 45–60 (men) | 3726/general population | 932/930 | 24 h dietary records/DII | Significantly higher serum glucose and SBP values in highest v. lowest DII quartile. No significant difference in DBP values | – | 8 |
| Neufcourt(49); after follow-up analysis | 2015 | France | Cohort | Both | 35–60 (women), 45–60 (men) | 3726/general population | 932/930 | 24 h dietary records/DII | Significantly higher SBP and DBP values after 13-year follow-up in highest v. lowest DII quartile. No significant difference in FBS | Age gender, supplementation group, energy, number of 24 h records, education, smoking, PA, baseline values | 8 |
| Alkerwi(59) | 2015 | Luxembourg | Cross-sectional | Both | 18–69 | 1352/general population | 338/338 | FFQ/DII | No significant association between DII, SBP, DBP and glycaemic biomarkers was reported | Age, sex, education level, smoking status, PA, energy intake | 9 |
| Wirth(61) | 2014 | USA | Cross-sectional | Both | Mean = 42·4 (sd 8·5) | 447/police officers | 112/111 | FFQ/DII | No significant difference in insulin and FBG between different DII quartiles was observed; however, odds of hyperglycaemia among individuals in 4th quartile was 2·03 times more than in 1st quartile | Age, sex, alcoholic drinks per week | 5 |
| Alkerwi(52) | 2014 | Luxembourg | Cross-sectional | Both | 18–69 | 1352/general population | 450/450 | FFQ/DII | Prevalence of HTN in highest DII tertile was significantly lower than in lowest. SBP in highest tertile was significantly lower than in lowest. No significant in difference in DBP, glucose, insulin and HOMA-IR was observed. Also, no significant association between DII and HTN or hyperglycaemia was observed | Age, sex, education, income, smoking, PA | 8 |
| Woudenbergh(62) | 2013 | Netherlands | Cross-sectional | Both | Mean = 64 (sd 9) | 1024/general population | 341/341 | FFQ/DII-ADII | ADII was adversely associated with HOMA-IR, fasting glucose and post-load glucose but not with HbA1c | Age, sex, cohort, PA, smoking, family history of diabetes, use of lipid-lowering medication, HTN, energy intake | 8 |
| Sánchez-Villegas(53) | 2015 | Spain | Cross-sectional | Both | Mean ≈ 38 | 15 093/university graduates | 3018/3019 | FFQ/DII | HTN prevalence in the highest quintile was significantly lower than in the lowest | – | 6 |
| Naja(27) | 2017 | Lebanon | Cross-sectional | Both | >18 | 331/general population | 66/67 | FFQ/DII | No significant difference in mean DII of individuals with HTN or hyperglycaemia compared with healthy individuals was reported. No significant association was observed between DII and hyperglycaemia or HTN in logistic regression | Age, sex, marital status, education, crowding index, PA, smoking | 9 |
| Hayden(57) | 2017 | USA | Cross-sectional | Women | 65–79 | 7085/older women | 1467/2694 | FFQ/DII | No significant difference in the prevalence of hypertension between lowest v. highest quartile was observed | – | 6 |
| Camargo-Ramos(58) | 2017 | Colombia | Cross-sectional | Both | Mean = 39·7 (sd 6.9) | 90/overweight and sedentary adults | 77/13 | 24 h dietary record/DII | Elevated HbA1c in individuals with pro-inflammatory v. anti-inflammatory diet. Glucose, SBP and DBP were non-significantly higher | Age, sex | 7 |
| Bodén(46) | 2017 | Sweden | Case–control | Men | Mean = 50 | 5284/general population | 1321/1321 | FFQ/DII | Significantly higher SBP values in highest v. lowest DII quartile | – | 7 |
| Bodén(46) | 2017 | Sweden | Case–control | Women | Mean = 50 | 1600/general population | 400/400 | FFQ/DII | No significant difference in SBP between different DII quartiles | – | 7 |
| Wirth(48) | 2016 | USA | Cross-sectional | Men | >20 | 7566/general population | 2097/1586 | 24 h recall/DII | No significant association between DII and HTN was observed | Family member smoking, age, BMI | 8 |
| Wirth(48) | 2016 | USA | Cross-sectional | Women | >20 | 8047/general population | 1818/2326 | 24 h recall/DII | Significant association between DII and HTN was observed | Family member smoking, age, BMI | 8 |
CABG, coronary artery bypass grafting; E-DII, energy-adjusted dietary inflammatory index; ADII, adapted dietary inflammatory index; HTN, hypertension; SBP, systolic blood pressure; FBS, fasting bold sugar; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; HbA1c, glycated Hb; MI, myocardial infarction; PA, physical activity; LDL-C, LDL-cholesterol; PAL, physical activity level; TV, television.
Description of studies reporting associations of dietary inflammatory index with hyperglycaemia or markers of glycaemic status
In total twenty-one manuscripts reported the association between DII and hyperglycaemia or markers of glycaemic status(11,19–22,26–28,43,49–52,54–56,58–63). Among them, fourteen studies reported a positive association between DII and hyperglycaemia or a higher prevalence of hyperglycaemia among individuals in the highest category of DII, or higher insulin, FBS, HOMA-IR or HOMA-B values in the highest DII category v. the lowest category as the reference group(19–22,26,43,49,54,55,58,60–62). Six studies reported no significant association between DII and markers of glycaemic status or the prevalence of hyperglycaemia in different DII categories(11,27,51,52,56,59) and only one study observed an inverse association between DII and glycaemic status, reporting lower FBS concentrations in individuals of the highest DII category v. the lowest(50). Ren et al.(21) reported 17 % higher odds of hyperglycaemia in women of the highest v. the lowest DII category, while no significant association was reported among men or in combined analysis of both genders. The study by Park et al. reported higher HOMA-IR values in the highest tertile of DII only among metabolically healthy overweight and obese adults and not in metabolically unhealthy adults(26). Kim et al.(55) also reported 1·30 times greater chance of developing hyperglycaemia among 3682 men aged 19–65 years of the highest DII quartile, while no association was observed among 5609 women. Neufcourt et al.(49) reported higher FBS concentrations in the highest v. lowest DII category among 3726 participants of the SU.VI.MAX cohort while no difference was observed after 13 years of follow-up.
Findings from meta-analysis of OR and proportions for association of dietary inflammatory index with hypertension
The forest plot of the meta-analysis of OR of DII and HTN is presented in Fig. 2. Totally twelve studies were included in the meta-analysis(20,21,27,44,47,48,52,54–56,61,63). Among these twelve studies reporting the OR of HTN in the highest v. the lowest DII category, a positive association was observed between HTN and DII in the random-effects model (OR = 1·13; 95 % CI 1·01, 1·27; P < 0·001). In other words, being in the highest category of DII increased the chance of HTN by 13 %. A minimum between-study heterogeneity was observed (heterogeneity χ 2 = 31·51, df = 14, P = 0·005; I 2 = 55·6 %; estimate of between-study variance τ 2 = 0·0224). A subgroup analysis was performed to obtain the source of heterogeneity (see online supplementary material, Supplemental Table S1) and accordingly continent, dietary assessment tool and gender were the sources of heterogeneity. Totally, nine studies reported the prevalence of HTN in the highest v. lowest DII category(11,25,26,44,47,50,53,57,63). The forest plot of the prevalence of HTN by subgroups of lowest and highest DII categories is presented in Fig. 3. Accordingly, the prevalence of HTN was 16 (95 % CI 0·16, 0·16) % in the highest v. 18 (95 % CI 0·17, 0·18) % in the lowest category of DII. No heterogeneity was observed in the meta-analysis.
Fig. 2.
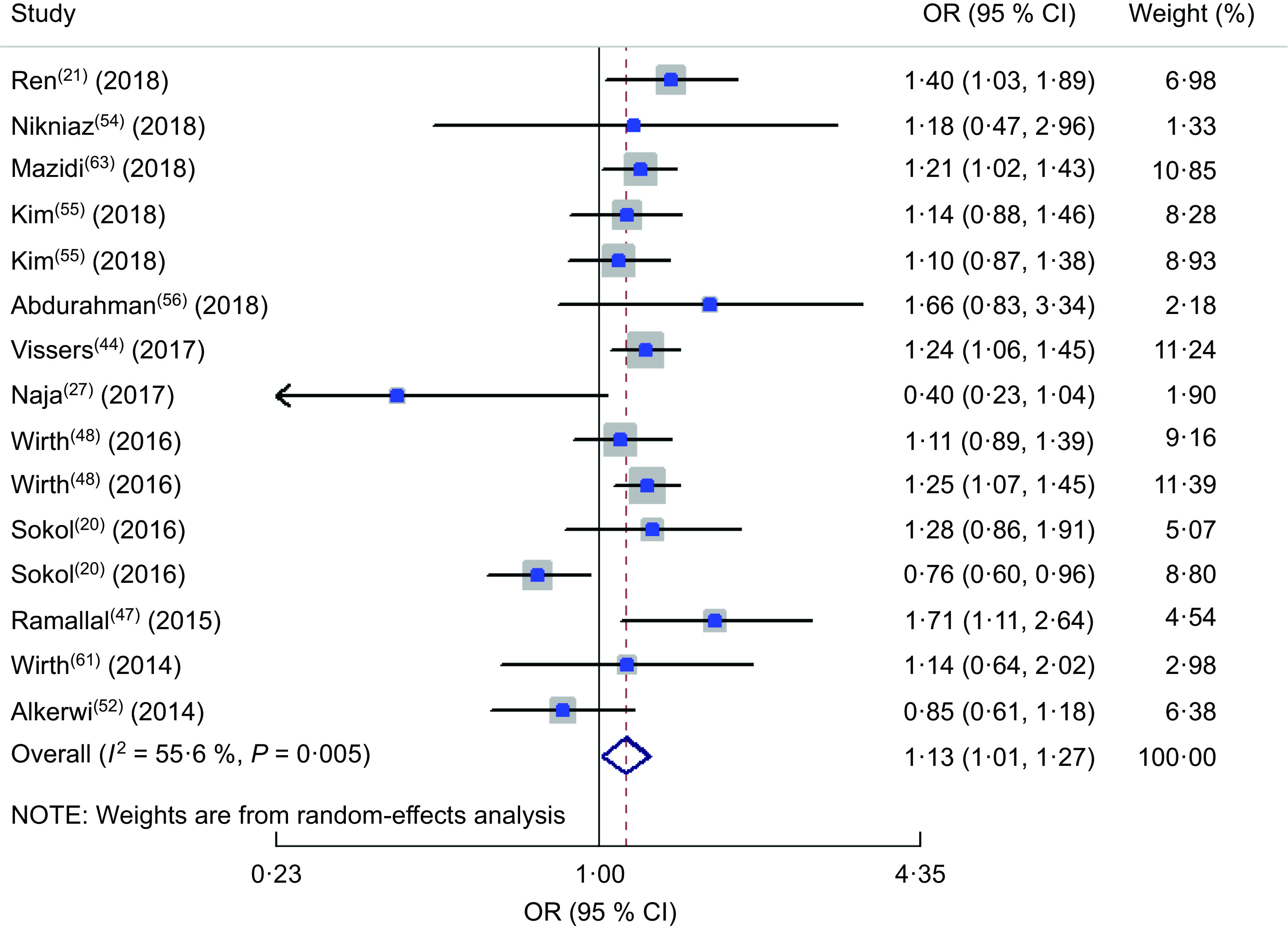
Forest plot illustrating OR for hypertension in the highest v. the lowest category of dietary inflammatory index. The study-specific OR and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled OR, and the width of the open diamond represents the pooled 95 % CI
Fig. 3.
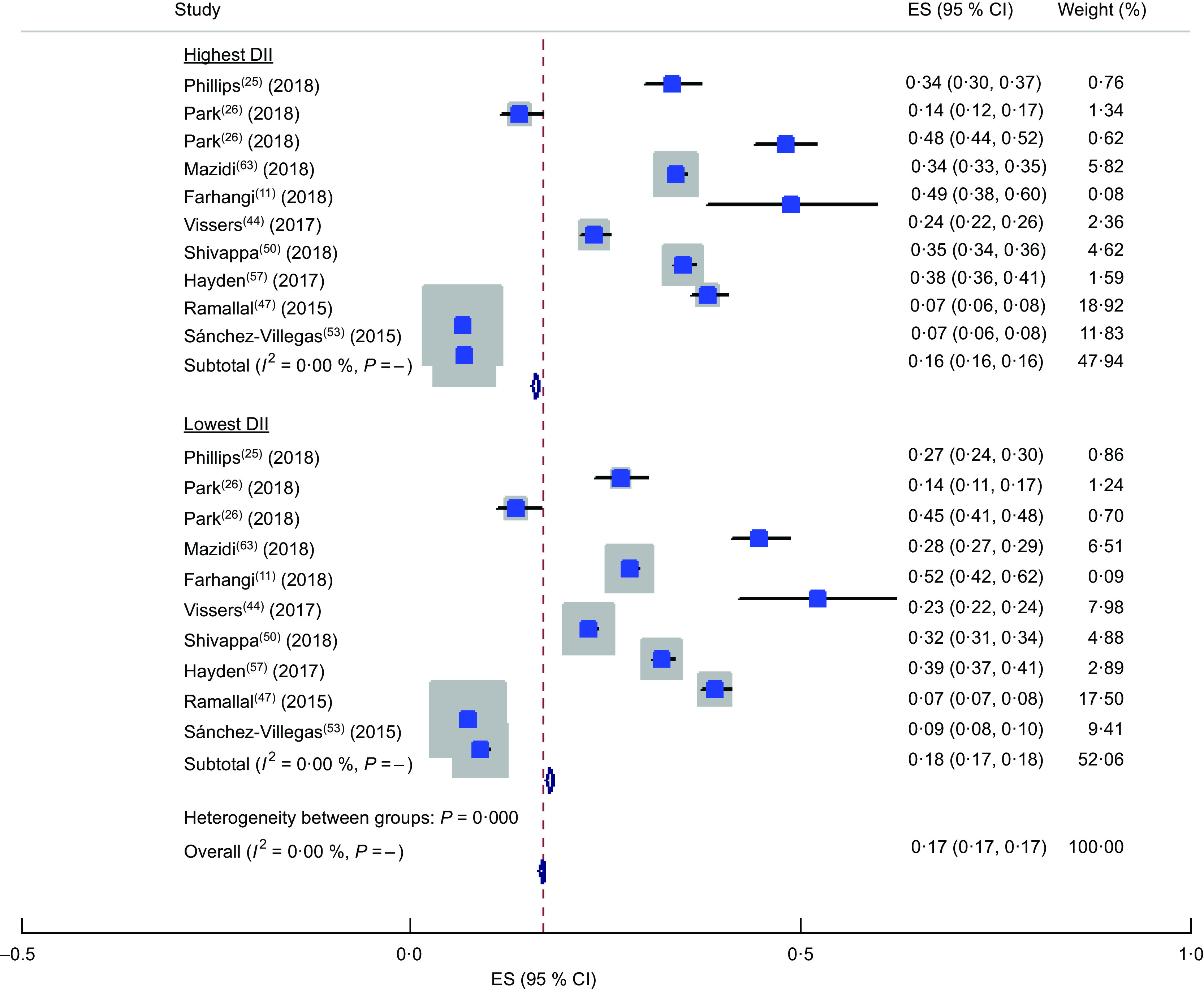
Forest plot illustrating proportion of hypertension in the highest and the lowest categories of dietary inflammatory index (DII). The study-specific effect size (ES) and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled ES, and the width of the open diamond represents the pooled 95 % CI
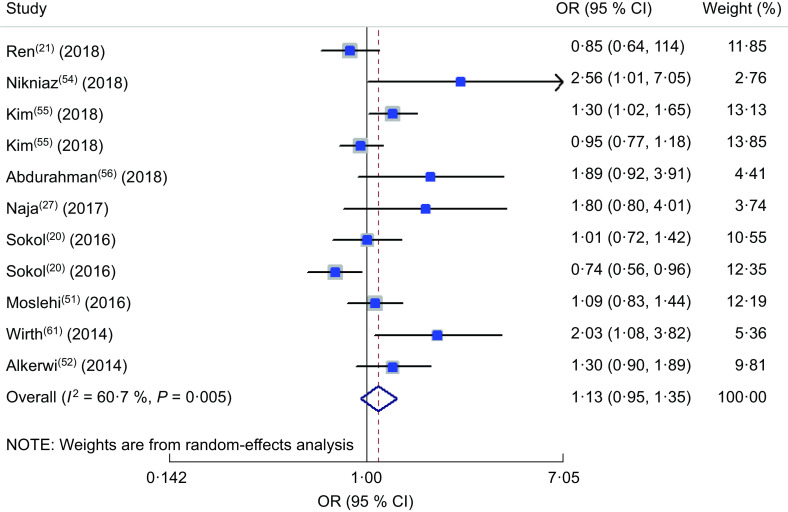
Findings from meta-analysis of OR for association of dietary inflammatory index with hyperglycaemia
The odds of hyperglycaemia in different DII categories were reported in a total of nine studies(20,21,27,51,52,54,55,56,61). The forest plot of the mentioned association is presented in Fig. 4. Accordingly, no significant association was observed between hyperglycaemia and DII in the random-effects model (OR = 1·13; 95 % CI 0·95, 1·35; P = 0·17). A high heterogeneity was observed (heterogeneity χ 2 = 25·47, df = 10, P = 0·005; I 2 = 60·7 %; estimate of between-study variance τ 2 = 0·0463). A subgroup analysis was performed to obtain the source of heterogeneity (see online supplementary material, Supplemental Table S2). Accordingly, none of the studied parameters explained the possible sources of heterogeneity. In sensitivity analysis, when we removed the study by Sokol et al. in women(20), the effect size reached a significant level and the heterogeneity reduced (OR = 1·180; 95 % CI 0·997, 1·395; P = 0·054; I 2 = 49·4 %).
Fig. 4.
Forest plot illustrating OR for hyperglycaemia in the highest v. the lowest category of dietary inflammatory index. The study-specific OR and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled OR, and the width of the open diamond represents the pooled 95 % CI
Findings from meta-analysis of mean systolic and diastolic blood pressure across different dietary inflammatory index categories
In the comparison of SBP values in different classifications of DII, totally twelve studies were included(22,26,43,46,49,50,52,54,56,58,61,63). The forest plot is presented in Fig. 5. Accordingly, being in the highest category of DII was accompanied with a 1·2 mmHg significant increase in SBP (WMD = 1·230; 95 % CI 0·283, 2·177; P = 0·011), although a great heterogeneity was observed between included studies (heterogeneity χ 2 = 165·10, df = 14, P < 0·001; I 2 = 91·5 %; estimate of between-study variance τ 2 = 2·4277). Sensitivity analysis showed no significant change in the findings. In subgroup analysis (see online supplementary material, Supplemental Table S3), partly gender-specified results could explain the source of heterogeneity. Other parameters were not considered a source of heterogeneity. The forest plot of the comparison of DBP in different DII categories is presented in Fig. 6 including ten studies(22,26,43,49,50,52,54,56,58,63). No significant difference in the DBP values in DII categories was identified (WMD = 0·008; 95 % CI −0·686, 0·703; P = 0·98). Although a great heterogeneity was observed between included studies (heterogeneity χ 2 = 132·19, df = 11, P < 0·001; I 2 = 91·7 %; estimate of between-study variance τ 2 = 1·0409), the results of subgroup analysis showed that dietary assessment tool and design of the study could partly explain the source of heterogeneity (Supplemental Table S4).
Fig. 5.
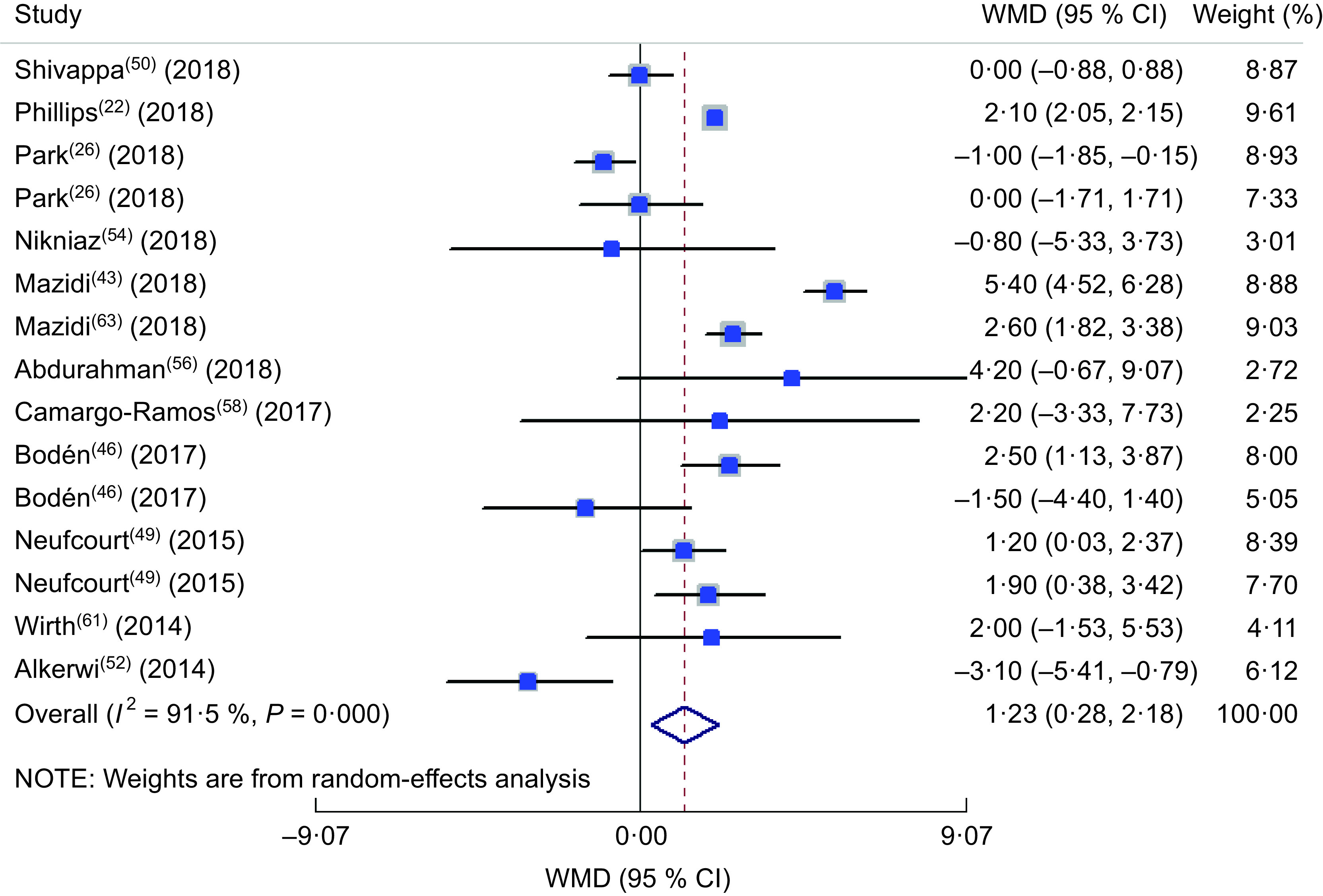
Forest plot illustrating the weighted mean difference (WMD) in systolic blood pressure among participants in the highest v. the lowest category of dietary inflammatory index. The study-specific WMD and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled WMD, and the width of the open diamond represents the pooled 95 % CI
Fig. 6.
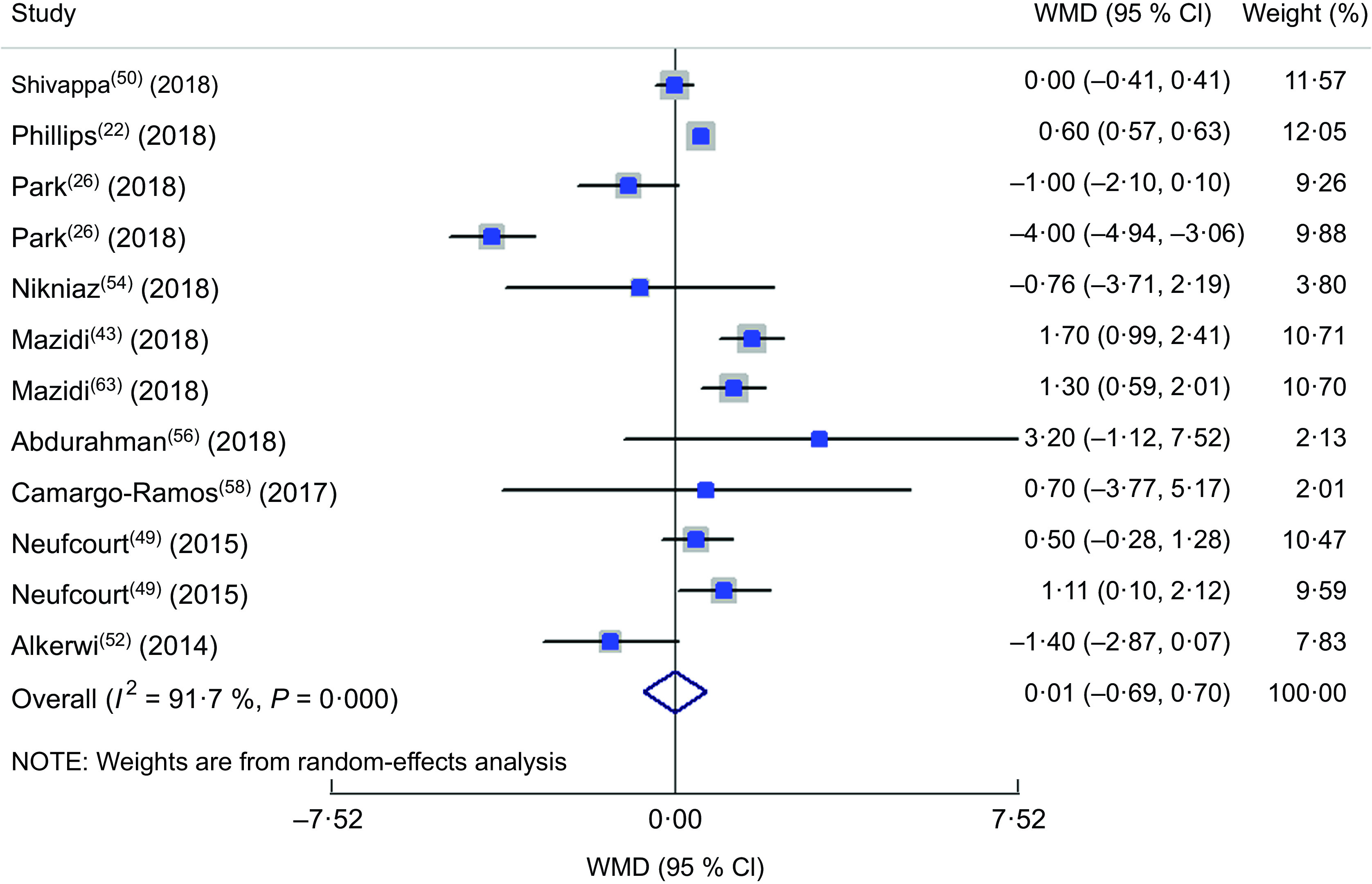
Forest plot illustrating the weighted mean difference (WMD) in diastolic blood pressure among participants in the highest v. the lowest category of dietary inflammatory index. The study-specific WMD and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond represents the pooled WMD, and the width of the open diamond represents the pooled 95 % CI
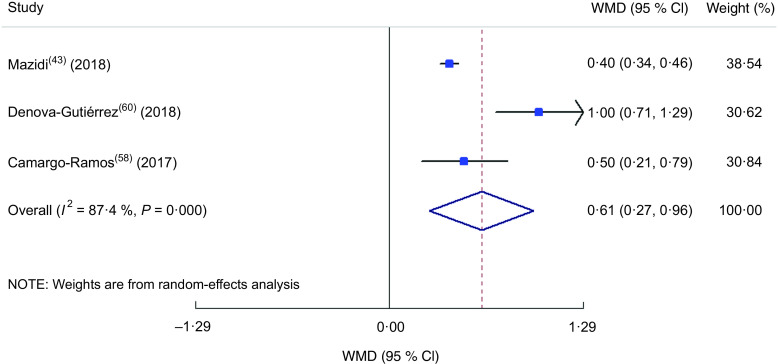
Findings from meta-analysis of mean fasting blood sugar and glycated Hb across different dietary inflammatory index categories
Totally, thirteen studies(22,26,28,43,49–52,54,56,58,60,63) were included in the meta-analysis of the comparison of FBS between different DII categories (Fig. 7). The highest DII category was associated with a 1·08 mg increase in serum or plasma FBS in comparison to the lowest DII category (WMD = 1·083; 95 % CI 0·099, 2·068; P = 0·031). A great heterogeneity was also identified in the included studies (heterogeneity χ 2 = 127·17, df = 14, P < 0·001; I 2 = 89·0 %; estimate of between-study variance τ 2 = 2·32). By subgrouping (see online supplementary material, Supplemental Table S5), dietary assessment tool could partly explain the heterogeneity of studies. In meta-analysis of the association between HbA1c and DII, only three studies were eligible to be included(43,58,60) and the results showed a 0·62 % increase in HbA1c in the highest v. the lowest DII category (WMD = 0·615; 95 % CI 0·268, 0·961; P = 0·001; Fig. 8) with a great heterogeneity (heterogeneity χ 2 = 15·89, df = 2, P < 0·001; I 2 = 87·4 %; estimate of between-study variance τ 2 = 0·0803).
Fig. 7.
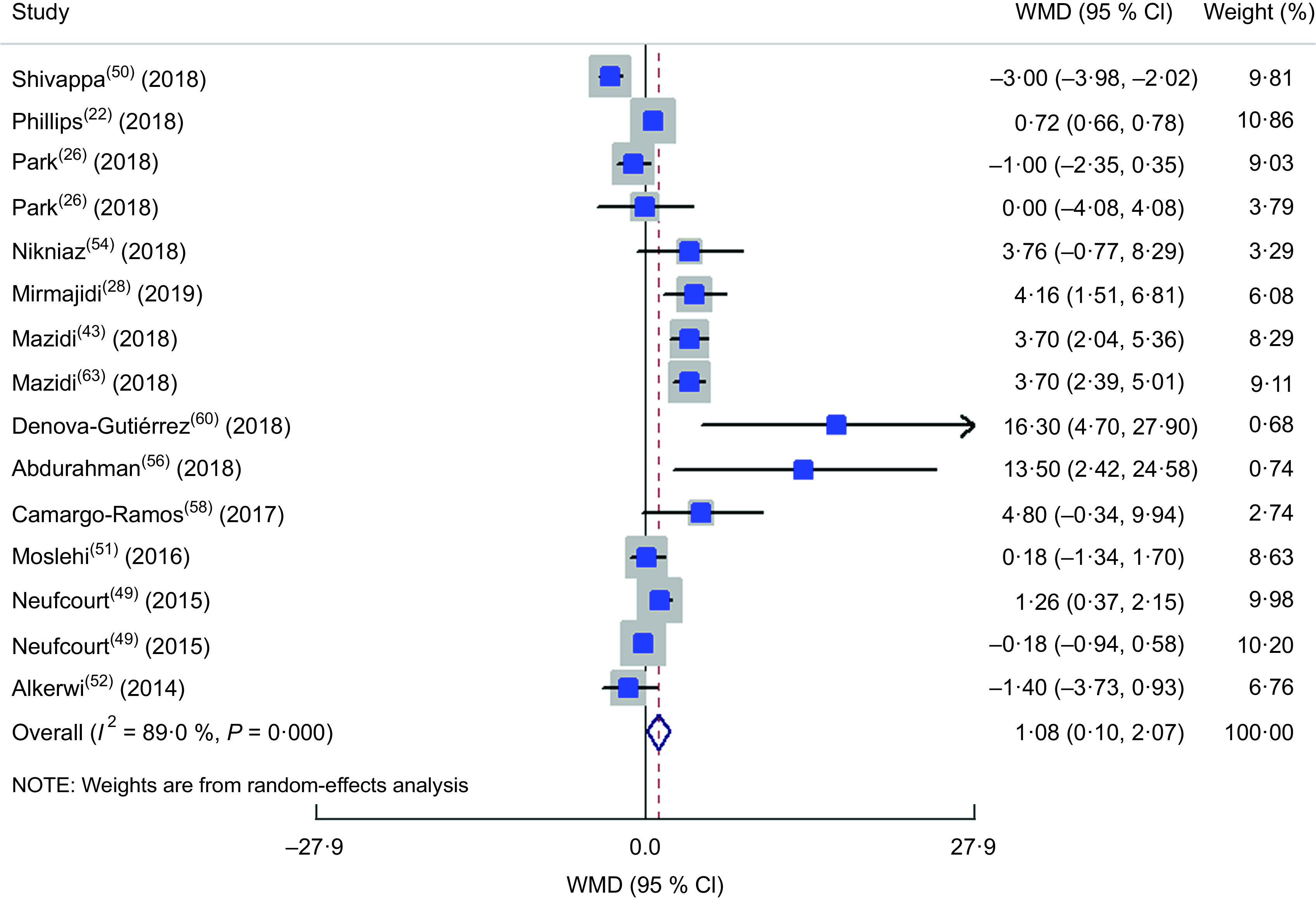
Forest plot illustrating weighted mean difference (WMD) in fasting blood sugar among participants in the highest v. the lowest category of dietary inflammatory index. The study-specific WMD and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled WMD the pooled WMD, and the width of the open diamond represents the pooled 95 % CI
Fig. 8.
Forest plot illustrating the weighted mean difference (WMD) in glycated Hb among participants in the highest v. the lowest category of dietary inflammatory index. The study-specific WMD and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled WMD the pooled WMD, and the width of the open diamond represents the pooled 95 % CI
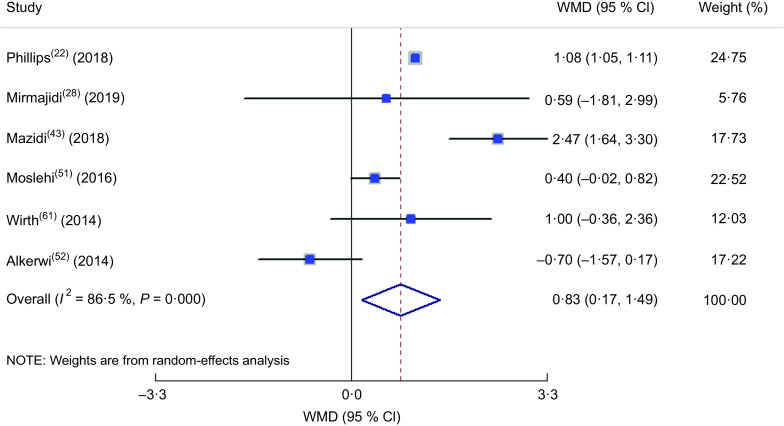
Findings from meta-analysis of mean insulin and homeostatic model assessment of insulin resistance across different dietary inflammatory index categories
The meta-analysis of the studies included in the model of insulin comparison between different DII categories(22,28,43,51,52,61) is presented in Fig. 9, revealing a 0·83 µIU/ml increase in insulin concentration in the highest v. lowest DII group (WMD = 0·829; 95 % CI 0·172, 1·486; P = 0·013) while a great between-study heterogeneity was also observed (heterogeneity χ 2 = 37·02, df = 5, P < 0·001; I 2 = 86·5 %; estimate of between-study variance τ 2 = 0·4540). In subgroup analysis (see online supplementary material, Supplemental Table S6), all of the studies were cross-sectional, used FFQ as dietary assessment tool, evaluated both genders and participants were from the general population; therefore, they were placed into subgroups according to continent and sample size, and both parameters could be considered as the sources of heterogeneity according to the findings. The meta-analysis of the eligible studies(22,26,28,43,51,59) showed that being in the highest DII category increased HOMA-IR value by 0·19 (WMD = 0·192; 95 % CI 0·023, 0·361; P = 0·026; Fig. 10). Again, a great heterogeneity was observed (heterogeneity χ 2 = 89·51, df = 6, P < 0·001; I 2 = 93·3 %; estimate of between-study variance τ 2 = 0·0429). Subgrouping (Supplemental Table S7) also could not explain the heterogeneity, except for continent, which reduced the heterogeneity.
Fig. 9.
Forest plot illustrating the weighted mean difference (WMD) in insulin among participants in the highest v. the lowest category of dietary inflammatory index. The study-specific WMD and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled WMD the pooled WMD, and the width of the open diamond represents the pooled 95 % CI
Fig. 10.
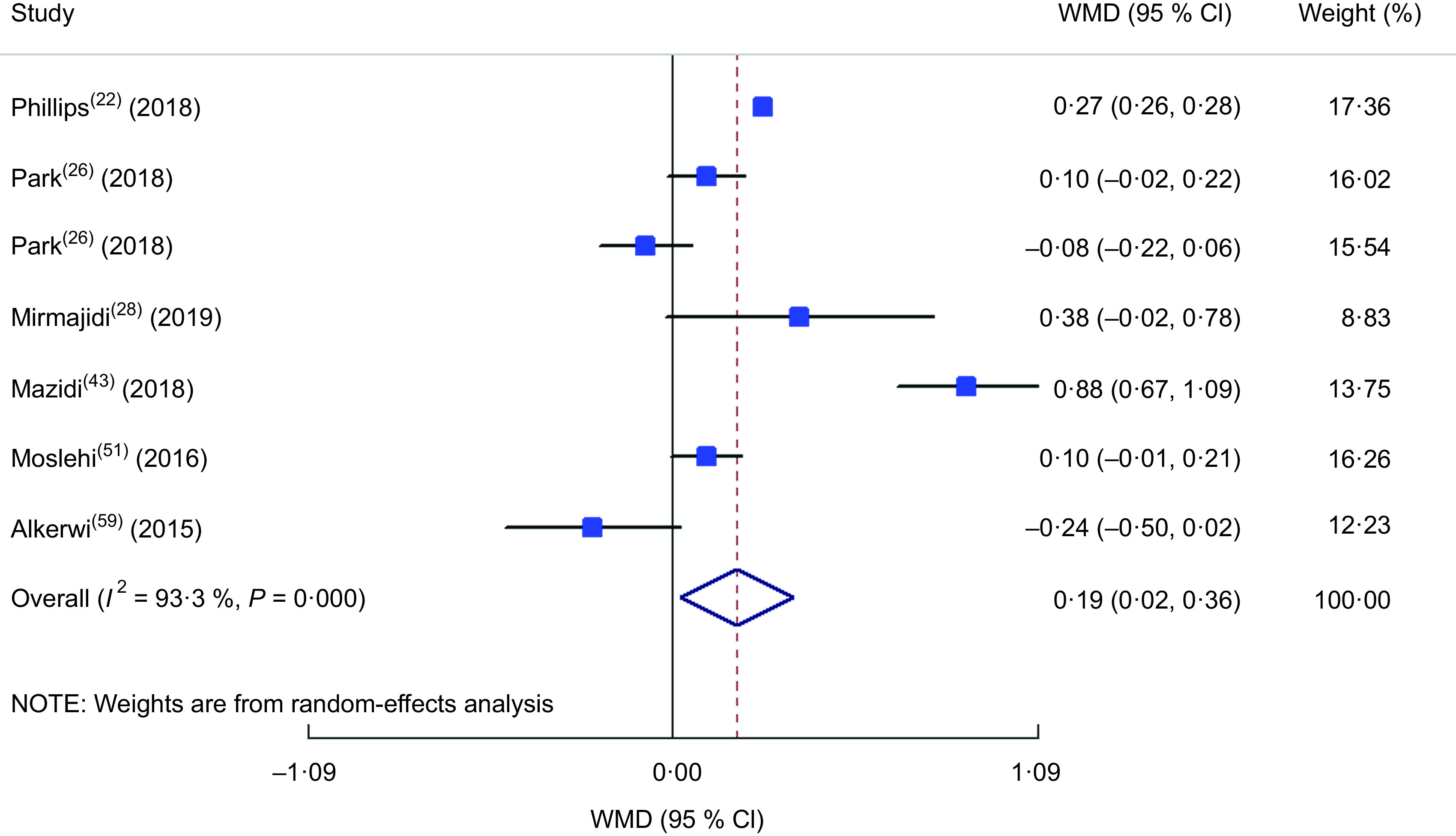
Forest plot illustrating the weighted mean difference (WMD) in homeostatic model assessment of insulin resistance among participants in the highest v. the lowest category of dietary inflammatory index. The study-specific WMD and 95 % CI are represented by the square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled WMD, and the width of the open diamond represents the pooled 95 % CI
Publication bias
The funnel plots revealed moderate asymmetry (see online supplementary material, Supplemental Figs S1–S8). However, the Begg’s and Egger’s tests provided no evidence of substantial publication bias for all of the variables, as follows: HTN (OR), Egger’s test (P = 0·123) and Begg’s test (P = 0·533); hyperglycaemia (OR), Egger’s test (P = 0·06) and Begg’s test (P = 0·161); SBP, Egger’s test (P = 0·293) and Begg’s test (P = 0·833); DBP, Egger’s test (P = 0·325) and Begg’s test (P = 0·493); FBS, Egger’s test (P = 0·671) and Begg’s test (P = 0·151); HOMA-IR, Egger’s test (P = 0·444) and Begg’s test (P = 0·881); insulin, Egger’s test (P = 0·508) and Begg’s test (P = 0·188); HbA1c, Egger’s test (P = 0·405) and Begg’s test (P = 0·117).
Discussion
In the current systematic review and meta-analysis of 194 959 participants from the general population, we found that the highest DII category was associated with higher SBP, FBS, insulin, HbA1c and HOMA-IR. Moreover, the chance of HTN occurrence was increased in the highest DII category v. the lowest. No association between DII and DBP and odds of hyperglycaemia was observed. The populations of included studies were general apparently healthy populations or overweight and obese individuals with no serious inflammation-related disease; the studies evaluating the association between DII and cancers, chronic kidney disease and multiple sclerosis were excluded. Moreover, the age range was more than 18 years, and studies among children and adolescents as well as pregnant women or gestational diabetes mellitus were not included in the current meta-analysis. No previous systematic review and meta-analysis is available evaluating the association between DII and blood pressure, HTN, hyperglycaemia and biomarkers of glucose homeostasis.
The heterogeneity of studies included in the current systematic review and meta-analysis should be discussed here. The Western dietary pattern, with high dietary inflammatory potential, is a potent inducer of central obesity and metabolic syndrome; several studies have revealed significant relationships between the Western dietary pattern and increased risk of metabolic syndrome, higher serum cholesterol, and increased waist circumference and BMI. Accordingly, the Western dietary pattern with high content of red meat, eggs and refined grains is associated with increased risk of obesity and increased levels of blood sugar, SBP and TAG, and reduced HDL-cholesterol(64–66). As mentioned in the ‘Results’ section, gender, dietary assessment tool and continent could be a source of heterogeneity among observed associations. In the current meta-analysis, DII was calculated from self-reported data gathered by the 24 h recall method, 24 h record method or FFQ, which may be a potential source of bias. Moreover, differences in the items of the studies’ FFQ might be a source of heterogeneity (the FFQ used ranged from sixty-three to 168 items) and local foods in the FFQ could also affect the heterogeneity as described previously(67), although almost all of the included studies used a valid and reliable FFQ. FFQ cover a wide range of dietary ingredients and are more accurate than the 24 h recall method reflecting usual dietary intake in a short period of time; it has been confirmed that FFQ could be more helpful in evaluating diet–disease relationships(68). Another source of heterogeneity, the continent (i.e. study location), presents the possible role of geographical distribution and cultural factors influencing the association between DII and the studied parameters(69). In the current meta-analysis, the baseline characteristics of participants were also different and therefore subgrouping was performed according to obesity status; however, findings were more relevant and stronger among studies conducted among general populations as participants rather than obese individuals. This finding might be explained by the fact that obese individuals have greater under-reporting and tend to underestimate usual dietary intakes of total energy and sugar(70,71) which might affect the results; for example, in the association of DII with DBP in which the WMD shows an inverse association between DII and DBP in obese individuals. The meta-analysis of proportions of HTN in different DII categories in the current study found higher prevalence of HTN in the lowest v. highest DII category, while in meta-analysis of OR of HTN, the odds of HTN were highest in the highest DII category. This inconsistency in finding might be due to the fact that most of the studies reporting the prevalence of HTN and included in the current meta-analysis were baseline self-reported information about HTN without blood pressure measurement(11,45,47,54,57,63), which might be a source of error.
Conclusion
In conclusion, in the current study, after a systematic extensive literature review, we carried out several meta-analyses to summarize the findings of eligible studies about the possible relationship between DII and cardiometabolic risk factors. We found a positive association between DII and SBP, FBS, HTN, insulin, HbA1c and HOMA-IR values. Previously conducted meta-analyses evaluated the association between DII and the risk of CVD and mortality(35,72), and our study was the first one evaluating the association of DII with each of the cardiovascular risk factors separately in an independent meta-analysis model.
According to our findings, diets with less inflammatory potential – containing higher amounts of fruits, vegetables, fish or fish oil, walnuts, brown rice and bulgur wheat, and with avoidance of red meat, refined or processed foods and high-fat dairy products – are recommended for prevention of cardiovascular events.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare that there is no conflict of interest. Authorship: M.A.F. was the main researcher, designed the hypothesis, extracted the data, wrote the manuscript, revised the manuscript and supervised the project. L.N. and Z.N. were involved in data extraction, data analysis and manuscript reading. P.D. was involved in manuscript writing. All authors have read and approved the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and the protocol of the study has been approved by the ethics committee of the Tabriz University of Medical Sciences.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019003070.
click here to view supplementary material
References
- 1. Keibel A, Singh V & Sharma MC (2009) Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des 15, 1949–1955. [DOI] [PubMed] [Google Scholar]
- 2. Pearson TA, Mensah GA, Alexander RW et al. (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511. [DOI] [PubMed] [Google Scholar]
- 3. McCarty M (1999) Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med Hypotheses 52, 465–477. [DOI] [PubMed] [Google Scholar]
- 4. Festa A, D’Agostino R Jr, Howard G et al. (2000) Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102, 42–47. [DOI] [PubMed] [Google Scholar]
- 5. Li C, Chen R, Wang H et al. (2015) Mechanisms linking inflammation to insulin resistance. Int J Endocrinol 2015, 508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shoelson SE, Lee J & Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116, 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramadori P, Kroy D & Streetz KL (2015) Immunoregulation by lipids during the development of non-alcoholic steatohepatitis. Hepatobiliary Surg Nutr 4, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van der Poll T, Romijn JA, Endert E et al. (1991) Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol 261, E457–E465. [DOI] [PubMed] [Google Scholar]
- 9. Miguel CD, Rudemiller NP, Abais JN et al. (2015) Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 17, 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shivappa N, Steck SE, Hurley TG et al. (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 17, 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farhangi MA & Najafi M (2018) Dietary inflammatory index: a potent association with cardiovascular risk factors among patients candidate for coronary artery bypass grafting (CABG) surgery. Nutr J 17, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shivappa N, Hebert JR, Marcos A et al. (2017) Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res 61, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phillips CM, Shivappa N, Hébert JR et al. (2018) Dietary inflammatory index and biomarkers of lipoprotein metabolism, inflammation and glucose homeostasis in adults. Nutrients 10, 1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zucchetto A, Serraino D, Shivappa N et al. (2017) Dietary inflammatory index before diagnosis and survival in an Italian cohort of women with breast cancer. Br J Nutr 117, 1456–1462. [DOI] [PubMed] [Google Scholar]
- 15. Zucchetto A, Gini A, Shivappa N et al. (2016) Dietary inflammatory index and prostate cancer survival. Int J Cancer 139, 2398–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng J, Tabung FK, Zhang J, et al. (2018) Association between post-cancer diagnosis dietary inflammatory potential and mortality among invasive breast cancer survivors in the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev 27, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng J, Merchant AT, Wirth MD et al. (2018) Inflammatory potential of diet and risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Int J Cancer 142, 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zamora-Ros R, Shivappa N, Steck SE et al. (2015) Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case–control study. Genes Nutr 10, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vahid F, Shivappa N, Karamati M et al. (2017) Association between Dietary Inflammatory Index (DII) and risk of prediabetes: a case-control study. Appl Physiol Nutr Metab 42, 399–404. [DOI] [PubMed] [Google Scholar]
- 20. Sokol A, Wirth MD, Manczuk M et al. (2016) Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr Res 36, 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren Z, Zhao A, Wang Y et al. (2018) Association between dietary inflammatory index, C-reactive protein and metabolic syndrome: a cross-sectional study. Nutrients 10, E831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips CM, Shivappa N, Hébert JR et al. (2018) Dietary inflammatory index and biomarkers of lipoprotein metabolism, inflammation and glucose homeostasis in adults. Nutrients 10, E1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frith E, Shivappa N, Mann JR et al. (2018) Dietary inflammatory index and memory function: population-based national sample of elderly Americans. Br J Nutr 119, 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shivappa N, Hebert JR, Rashidkhani B et al. (2017) Inflammatory potential of diet is associated with increased odds of cataract in a case–control study from Iran. Int J Vitam Nutr Res 87, 17–24. [DOI] [PubMed] [Google Scholar]
- 25. Phillips CM, Shivappa N, Hébert JR et al. (2018) Dietary inflammatory index and mental health: a cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin Nutr 37, 1485–1491. [DOI] [PubMed] [Google Scholar]
- 26. Park YMM, Choi MK, Lee S et al. (2018) Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin Nutr 38, 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naja F, Shivappa N, Nasreddine L et al. (2017) Role of inflammation in the association between the western dietary pattern and metabolic syndrome among Lebanese adults. Int J Food Sci Nutr 68, 997–1004. [DOI] [PubMed] [Google Scholar]
- 28. Mirmajidi S, Izadi A, Saghafi-Asl M et al. (2019) Inflammatory potential of diet: association with chemerin, omentin, lipopolysaccharide-binding protein, and insulin resistance in the apparently healthy obese. J Am Coll Nutr 38, 302–310. [DOI] [PubMed] [Google Scholar]
- 29. Shivappa N, Godos J, Hébert JR et al. (2017) Dietary inflammatory index and colorectal cancer risk – a meta-analysis. Nutrients 9, 1043–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zahedi H, Djalalinia S, Sadeghi O et al. (2018) Dietary inflammatory potential score and risk of breast cancer: systematic review and meta-analysis. Clin Breast Cancer 18, e561–e570. [DOI] [PubMed] [Google Scholar]
- 31. Wang L, Liu C, Zhou C et al. (2019) Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. Eur J Clin Nutr 73, 509–517. [DOI] [PubMed] [Google Scholar]
- 32. Steck SE, Guinter M, Zheng J et al. (2015) Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr 6, 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong X, Guo L, Zhang L et al. (2017) Inflammatory potential of diet and risk of cardiovascular disease or mortality: a meta-analysis. Sci Rep 7, 6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shivappa N, Godos J, Hébert JR et al. (2018) Dietary inflammatory index and cardiovascular risk and mortality – a meta-analysis. Nutrients 10, 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Namazi N, Larijani B & Azadbakht L (2018) Dietary inflammatory index and its association with the risk of cardiovascular diseases, metabolic syndrome, and mortality: a systematic review and meta-analysis. Horm Metab Res 50, 345–358. [DOI] [PubMed] [Google Scholar]
- 36. Varkaneh HK, Fatahi S, Tajik S et al. (2018) Dietary inflammatory index in relation to obesity and body mass index: a meta-analysis. Nutr Food Sci 48, 702–721. [Google Scholar]
- 37. Lassale C, Batty GD, Baghdadli A et al. (2019) Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry 24, 965–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller SA & Forrest JL (2001) Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract 1, 136–141. [Google Scholar]
- 39. Higgins JPT, Thomas J, Chandler J et al. (editors) (2019) Cochrane Handbook for Systematic Reviews of Interventions, version 6.0. https://www.training.cochrane.org/handbook (accessed October 2019).
- 40. Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605. [DOI] [PubMed] [Google Scholar]
- 41. Hardy RJ & Thompson SG (1996) A likelihood approach to meta-analysis with random effects. Stat Med 15, 619–629. [DOI] [PubMed] [Google Scholar]
- 42. Higgins JP & Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- 43. Mazidi M, Shivappa N, Wirth MD et al. (2018) Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis 276, 23–27. [DOI] [PubMed] [Google Scholar]
- 44. Vissers LET, Waller M, van der Schouw YT et al. (2017) A pro-inflammatory diet is associated with increased risk of developing hypertension among middle-aged women. Nutr Metab Cardiovasc Dis 27, 564–570. [DOI] [PubMed] [Google Scholar]
- 45. Shivappa N, Steck SE, Hussey JR et al. (2017) Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in National Health and Nutrition Examination Survey III Study. Eur J Nutr 56, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bodén S, Wennberg M, Van Guelpen B et al. (2017) Dietary inflammatory index and risk of first myocardial infarction; a prospective population-based study. Nutr J 16, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramallal R, Toledo E, Martínez-González MA et al. (2015) Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS One 10, e0135221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wirth MD, Shivappa N, Hurley TG et al. (2016) Association between previously diagnosed circulatory conditions and a dietary inflammatory index. Nutr Res 36, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neufcourt L, Assmann KE, Fezeu LK et al. (2015) Prospective association between the dietary inflammatory index and metabolic syndrome: findings from the SU.VI.MAX study. Nutr Metab Cardiovasc Dis 25, 988–996. [DOI] [PubMed] [Google Scholar]
- 50. Shivappa N, Bonaccio M, Hebert JR et al. (2018) Association of proinflammatory diet with low-grade inflammation: results from the Moli-sani study. Nutrition 54, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moslehi N, Ehsani B, Mirmiran P et al. (2016) Inflammatory properties of diet and glucose-insulin homeostasis in a cohort of Iranian adults. Nutrients 8, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alkerwi A, Shivappa N, Crichton G et al. (2014) No significant independent relationships with cardiometabolic biomarkers were detected in the observation of cardiovascular risk factors in Luxembourg study population. Nutr Res 34, 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sánchez-Villegas A, Ruíz-Canela M, De La Fuente-Arrillaga C et al. (2015) Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr 114, 1471–1479. [DOI] [PubMed] [Google Scholar]
- 54. Nikniaz L, Nikniaz Z, Shivappa N et al. (2018) The association between dietary inflammatory index and metabolic syndrome components in Iranian adults. Prim Care Diab 12, 467–472. [DOI] [PubMed] [Google Scholar]
- 55. Kim HY, Lee J & Kim J (2018) Association between dietary inflammatory index and metabolic syndrome in the general Korean population. Nutrients 10, E648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdurahman AA, Azadbakhat L, Rasouli M et al. (2019) Association of dietary inflammatory index with metabolic profile in metabolically healthy and unhealthy obese people. Nutr Diet 76, 192–198. [DOI] [PubMed] [Google Scholar]
- 57. Hayden KM, Beavers DP, Steck SE et al. (2017) The association between an inflammatory diet and global cognitive function and incident dementia in older women: the Women’s Health Initiative Memory Study. Alzheimers Dement 13, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Camargo-Ramos CM, Correa-Bautista JE, Correa-Rodríguez M et al. (2017) Dietary inflammatory index and cardiometabolic risk parameters in overweight and sedentary subjects. Int J Environ Res Public Health 14, 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alkerwi A, Vernier C, Crichton GE et al. (2015) Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr 113, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Denova-Gutiérrez E, Muñoz-Aguirre P, Shivappa N et al. (2018) Dietary inflammatory index and type 2 diabetes mellitus in adults: the Diabetes Mellitus Survey of Mexico City. Nutrients 10, 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wirth MD, Burch J, Shivappa N et al. (2014) Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 56, 986–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A et al. (2013) Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am J Clin Nutr 98, 1533–1542. [DOI] [PubMed] [Google Scholar]
- 63. Mazidi M, Shivappa N, Wirth MD et al. (2018) Greater Dietary Inflammatory Index score is associated with higher likelihood of chronic kidney disease. Br J Nutr 120, 204–209. [DOI] [PubMed] [Google Scholar]
- 64. Ambrosini GL, Huang R-C, Mori TA et al. (2010) Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr Metab Cardiovasc Dis 20, 274–283. [DOI] [PubMed] [Google Scholar]
- 65. McNaughton SA, Ball K, Mishra GD et al. (2008) Dietary patterns of adolescents and risk of obesity and hypertension. J Nutr 138, 364–370. [DOI] [PubMed] [Google Scholar]
- 66. Shang X, Li Y, Liu A et al. (2012) Dietary pattern and its association with the prevalence of obesity and related cardio-metabolic risk factors among Chinese children. PLoS One 7, e43183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mueller M, Hobiger S & Jungbauer A (2010) Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem 122, 987–996. [Google Scholar]
- 68. Shim JS, Oh K & Kim HC (2014) Dietary assessment methods in epidemiologic studies. Epidemiol Health 36, e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Slimani N, Fahey M, Welch A et al. (2002) Diversity of dietary patterns observed in the European Prospective Investigation into Cancer and Nutrition (EPIC) project. Public Health Nutr 5, 1311–1328. [DOI] [PubMed] [Google Scholar]
- 70. Heitmann BL & Lissner L (1995) Dietary underreporting by obese individuals – is it specific or non-specific? BMJ 311, 986–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Health Service (2015) Obese people ‘underestimate how much sugar they eat’. https://www.nhs.uk/news/food-and-diet/obese-people-underestimate-how-much-sugar-they-eat/ (accessed October 2019).
- 72. Ruiz-Canela M, Bes-Rastrollo M & Martínez-González MA (2016) The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci 17, 1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019003070.
click here to view supplementary material