Abstract
Background
Over half of the South African adults aged 45 years and older have hypertension but its effective management along the treatment cascade (awareness, treatment, and control) remains poorly understood.
Methods
We compared the prevalence of all stages of the hypertension treatment cascade in the rural HAALSI cohort of older adults at baseline and after four years of follow-up using household surveys and blood pressure data. Hypertension was a mean systolic blood pressure >140 mm Hg or diastolic pressure >90 mm Hg, or current use of anti-hypertension medication. Control was a mean blood pressure <140/90 mm Hg. The effects of sex and age on the treatment cascade at follow-up were assessed. Multivariate Poisson regression models were used to estimate prevalence ratios along the treatment cascade at follow-up.
Results
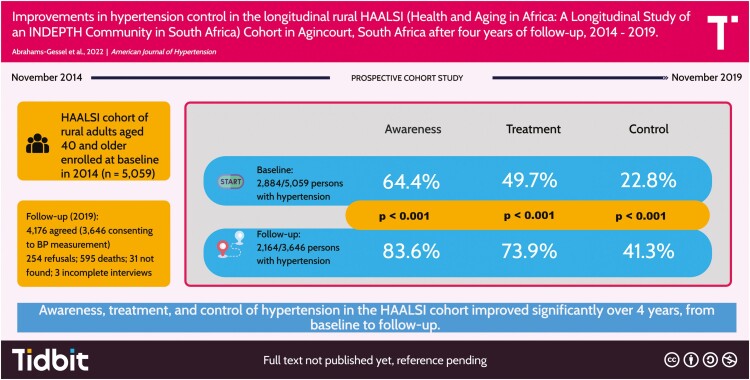
Prevalence along the treatment cascade increased from baseline (B) to follow-up (F): awareness (64.4% vs. 83.6%), treatment (49.7% vs. 73.9%), and control (22.8% vs. 41.3%). At both time points, women had higher levels of awareness (B: 70.5% vs. 56.3%; F: 88.1% vs. 76.7%), treatment (B: 55.9% vs. 41.55; F: 79.9% vs. 64.7%), and control (B: 26.5% vs. 17.9%; F: 44.8% vs. 35.7%). Prevalence along the cascade increased linearly with age for everyone. Predictors of awareness included being female, elderly, or visiting a primary health clinic three times in the previous 3 months, and the latter two also predicted hypertension control.
Conclusions
There were significant improvements in awareness, treatment, and control of hypertension from baseline to follow-up and women fared better at all stages, at both time points.
Keywords: adult, aged, blood pressure, hypertension/therapy, rural population, South Africa
Graphical Abstract
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/improvements-in-hypertension-control-in-the-longitudinal-rural-haalsi-cohort-in-south-africa-after-four-years-of-follow-up-2014-2018.
Cardiovascular disease is a leading cause of mortality in South Africa, accounting for approximately 16% of all-cause mortality.1 Hypertension (HTN) is a leading risk factor for cardiovascular disease2 and its management remains poor,3 despite its increasing prevalence in low- and middle-income countries over the past two decades.4 Effective management of HTN along the treatment cascade (awareness, treated, and controlled) can lead to improved health and decreased mortality. Yet, it is poorly understood in the aging population in South Africa, which increasingly includes persons living with HIV (PLHIV).
The HAALSI (Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) longitudinal cohort aims to understand aging in a rural population of adults aged 40 and older living in the Agincourt sub-district, Mpumalanga Province, South Africa. The cohort focuses on understanding the impact of the changing socioeconomic conditions and biological risk factors on chronic disease, including cardiovascular disease, HIV, cognitive functioning, and dementia.5
The prevalence of HTN in the HAALSI cohort at baseline was 58%,6 while the management of leading cardiovascular disease risk factors, including blood pressure (BP), was poor with less than half of the cohort having three or more risk factors under control.7 Among those with HTN, 64% were aware of their condition, 49% were treated, and 22% had controlled BP.8 The downstream effects of poor control resulted in over 30% of participants with cardiac imaging in the cohort showing left ventricular hypertrophy.9 This level of end-organ damage is consistent with national trends observed within clinical cohorts in South Africa10,11 and reflects the long-term outcomes of poor HTN control.
We expected to see an increase in the prevalence of HTN in the aging cohort, as well as improvements along the treatment cascade, due to the national government’s provision of free healthcare services and medications for chronic conditions at primary health clinics (PHCs). Our primary analysis compared the changes along the treatment cascade from baseline to four years of follow-up and stratified by sex. We modeled the predicted prevalence of awareness, treated, and controlled HTN as explained by predictors of interest.
METHODS
Cohort design and membership
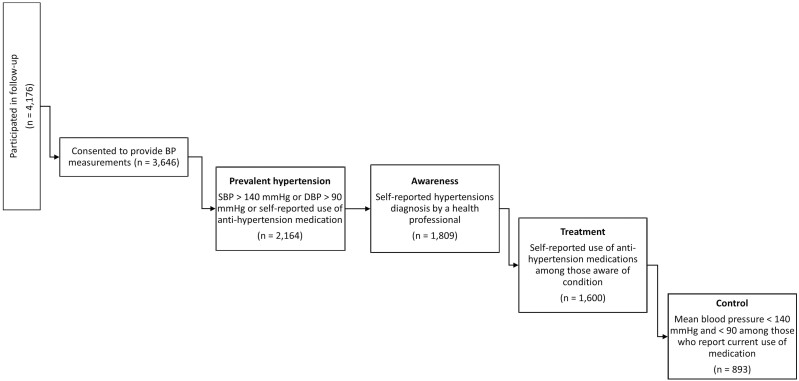
The HAALSI cohort has been extensively described elsewhere.5 It is nested in the Agincourt Health and Socio-demographic Surveillance System (HDSS) in Mpumalanga Province, South Africa. The Agincourt sub-district has a population of approximately 116,000 people who reside in 31 villages covering 450 km.2 There are two health centers, six clinics, three district hospitals, high unemployment rates, and poor basic services infrastructure.5 Cohort members aged 40 years and older as of 1 July 2014 who were permanently living in Agincourt for the 12 months preceding the completion of the 2013 HDSS census were screened. Between November 2014 and November 2015 (baseline), locally trained field workers visited households, enrolled, and obtained written informed consent from willing participants (n = 5,059). At follow-up (October 2018–November 2019), 595 (11.8%) members were determined to have died since baseline. Among the 4,464 living cohort members, 254 (5.7%) refused to participate in the follow-up study interview, 31 (0.7%) members were not found, 3 (0.07%) failed to complete the study interview, and 4,176 (93.5%) completed participation.
Data collection
At baseline and follow-up, field workers administered in-person computer-assisted personal interviews using a laptop computer. The survey comprised two parts: (i) collection of household, socioeconomic, and individual participant data; (ii) anthropometry, BP measurement, physical, clinical, and cognitive functioning assessments; (iii) collection of a blood sample in the form of dried blood spots (DBSs) for point-of-care tests. Prior to BP measurement, participants were seated for at least 5 min and asked to remove all outer layers of clothing. Three BP measurements were then taken by the field worker at two-minute intervals using an Omron M6W automated cuff (Omron, Kyoto, Japan). The mean BP was calculated using the second and third BP readings. Field workers collected blood droplets that were stored DBS on Whatman 903 paper (Whatman, Buckinghamshire, UK) and analyzed for HIV infection status (one to three drops).
HTN was defined as a mean systolic BP (SBP) ≥140 mm Hg, or mean diastolic BP (DBP) ≥90 mm Hg, or self-reported current use of anti-hypertension medication.12 Contradictory responses for self-reported use of anti-HTN medication were recorded as positive at follow-up if the respondent reported use at baseline but denied use at follow-up (n = 51), as we wanted to include all persons who met criteria for HTN at either baseline or follow-up. HIV infection was defined as the detection of the virus by DBS assay and a protocol of sequential assays was run in accordance with the World Health Organization guidelines.5 Physical limitation (PL) was defined as self-reported difficulties with activities of daily living, excluding difficulty dressing. Cohort members aged 60 years or older were defined as elderly. Socioeconomic status (SES) is a constructed variable that incorporates measures of traditional and modern wealth captured in the computerized questionnaire, using methodology created for Demographic Health Surveys13 and categorized as low (1st–3rd quintiles) or high (4th–5th quintiles). Cohort members who self-reported being born outside South Africa were defined as migrants, while those who self-reported an ability to read or write were defined as literate. Awareness of HTN was defined as individuals self-reporting that a health professional diagnosed them with HTN. Treated was defined as self-reported current use of anti-HTN medication and controlled was defined as having a measured mean BP less than 140/90 mm Hg.
Statistical analyses
All analyses were performed using STATA V 17.0 software (Stata Corp., College Station, TX, United States). Descriptive statistics for the cohort were adjusted by applying inverse-probability weights (IPWs) to account for (i) nonresponse due to mortality within the cohort; (ii) non-mortality-related attrition from the HAALSI study; (iii) respondent non-consent to anthropometric measures of weight, height, and BP; (iv) respondent non-consent to providing DBSs to assess HIV status. Logistic regression models were used to predict survival and the IPW is the inverse of this resulting probability. Composite weights were created for mortality, attrition, and non-consent to anthropometric, and DBS measurements by multiplying individual IPWs due to nonresponse (1–4 above), as appropriate for the analysis. Final models and complete documentation for the creation of IPW can be accessed at the Harvard University Dataverse public data repository.14 A detailed overview is provided in the Supplementary Material (Text S1). We report means and standard deviations for continuous variables, and proportions and standard errors for categorical variables. The prevalence of awareness, treatment, and control of HTN among all persons with HTN in the cohort was calculated at both baseline and follow-up. These main results were also stratified by sex, given that the evidence shows women are more likely to use primary health care services.15,16 HTN is known to increase with age and older people are more likely to be screened for HTN in healthcare settings. To explore whether prevalence across the treatment cascade stages changed with increasing age and was modified by sex, we performed a cross-sectional, nonparametric test for trends across increasing age groups, stratified by sex.
We performed a cross-sectional analysis to obtain adjusted prevalence ratios for individual stages of HTN management at follow-up. Poisson regression using the log-link function and robust variance estimation was used to generate models with multiple categorical predictors of interest given that the prevalence of each stage of the treatment cascade was >10%.17–19 The at-risk time for individuals was set to a constant value of 1 with this procedure and the models generated point estimates with 95% confidence intervals. IPW were applied to all models to adjust estimates to account for mortality, attrition, refusal to provide BP measurements, and refusal to provide a DBS sample. Based on prior work,8 multivariate models included sex, migrant status, literacy, PLHIV, elderly, high socioeconomic status, and PL. Additionally, we included the status of viral suppression among PLHIV, and self-reported number of visits (1–3) made to a PHC in the previous three months to explore the impact of linkage with primary health care services.
RESULTS
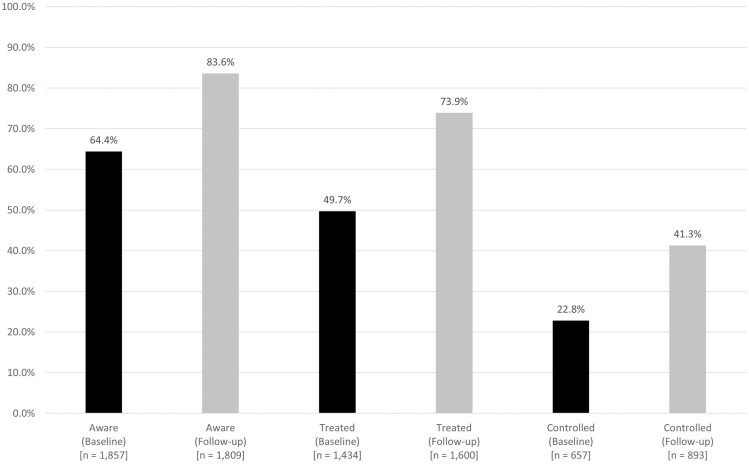
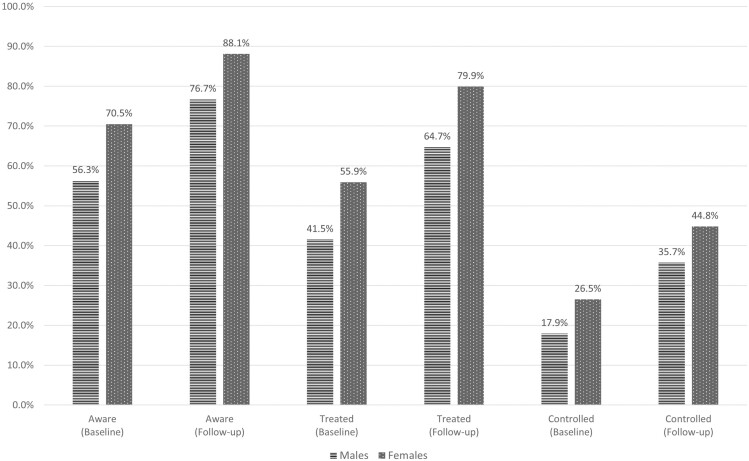
Participation and breakdown of the treatment cascade at follow-up are summarized in Figure 1. At follow-up, the mean SBP and DBP were similar for men and women (Table 1) and women had a higher mean BMI (29.0 kg/m2) compared to men (24.7 kg/m2). The prevalence of HIV infection was 23.9% and approximately 72% of these PLHIV had their viral load suppressed (Table 1). These characteristics were similar to the unweighted results (Table S1, Supplementary Material) and there were reductions in both mean SBP (−9.5 mm Hg) and DBP (−2.5 mm Hg) (Table S2, Supplementary Material) from baseline to follow-up. The proportion of prevalent HTN did not increase appreciably from baseline to follow-up (58.4% vs. 59.8%). Among all persons with HTN, increases in prevalence across the treatment cascade were observed between baseline and follow-up: aware (64.4% to 83.6%), treated (49.7% to 73.9%), and controlled (22.8% to 41.3%) (Figure 2). At both baseline and follow-up, women had the highest prevalence for each stage in the HTN treatment cascade and the prevalence for both men and women improved at each stage over the 4 years of follow-up (Figure 3). Men experienced larger relative increases for each treatment stage, compared to women (aware: 36.2% vs. 25.0%; treated 55.9% vs. 42.9%; controlled 99.4% vs. 69.1%). The proportion of people with prevalent HTN who were aware of their condition at the time of follow-up increased with age (Table 2). Among those with HTN, the proportion of persons treated was similar across age groups and by sex, and all persons aged 45 and older had a treatment prevalence of at least 90%. Men aged 65 and older were 1.5 times more likely to have their HTN controlled (74.9%), compared to men aged 45–64 (approximately 50.0%). Similarly, women in the 65+ age group were also more likely (70.4%) to have their HTN controlled compared to women aged 45–54 (67.3%), and to women aged 55–64 (59.1%).
Figure 1.
Stages of the hypertension treatment cascade at follow-up.
Table 1.
Cohort characteristics for HAALSI participants at follow-up (2018–2019)
| Continuous indicatorsa | Total (n = 4,176) |
Male (n = 1,862) |
Female (n = 2,314) |
|||
|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | |
| Age (years) | 66.0 | 13.0 | 66.3 | 12.9 | 65.8 | 13.1 |
| Weight (kg) | 71.9 | 17.5 | 71.0 | 16.6 | 72.8 | 18.1 |
| Height (m) | 1.6 | 0.1 | 1.7 | 0.1 | 1.6 | 0.1 |
| BMI (kg/m2) | 27.0 | 6.5 | 24.7 | 5.3 | 29.0 | 6.8 |
| Average SBP (mm Hg) | 128.4 | 20.4 | 129.5 | 20.9 | 127.5 | 19.9 |
| Average DBP (mm Hg) | 79.4 | 11.7 | 79.5 | 12.1 | 79.4 | 11.4 |
| Categorical indicators | % | Standard error | % | Standard error | % | Standard error |
| Current smokers | 11.8 | 0.5 | 23.4 | 1.0 | 1.7 | 0.3 |
| Elderly (≥60 years) | 64.7 | 0.74 | 66.0 | 1.1 | 63.5 | 1.0 |
| Migrant (born outside of South Africa) | 30.1 | 0.7 | 29.0 | 1.1 | 31.1 | 1.0 |
| High SESb | 39.9 | 0.8 | 39.3 | 1.2 | 40.5 | 1.0 |
| Literatec | 58.5 | 0.8 | 67.6 | 1.1 | 50.7 | 1.1 |
| Physical limitationsd | 11.1 | 0.5 | 11.3 | 0.8 | 11.0 | 0.7 |
| Visits to primary health clinic in last 3 months | ||||||
| 0 | 5.3 | 0.5 | 6.1 | 0.8 | 4.8 | 0.6 |
| 1 | 58.3 | 1.0 | 57.2 | 1.7 | 59.1 | 1.3 |
| 2 | 17.3 | 0.8 | 17.4 | 1.3 | 17.1 | 1.0 |
| 3 | 19.1 | 0.8 | 19.4 | 1.4 | 19.0 | 1.0 |
| HIV+e | 23.9 | 0.8 | 23.4 | 1.2 | 24.4 | 1.0 |
| HIV+ with viral suppression (≥100 copies/ml) | 71.6 | 1.7 | 69.5 | 2.7 | 3 | 2.2 |
| HIV+ without viral suppression (<100 copies/ml) | 28.4 | 1.7 | 30.6 | 2.7 | 26.7 | 2.2 |
| Mean | Inter-quartile Range | Mean | Inter-quartile Range | Mean | Inter-quartile Range | |
| Glucose (mmol/l)f | 6.9 | 2.1 | 6.8 | 2.1 | 7.1 | 2.1 |
Abbreviations: DBS, dried blood spot; DBP, diastolic blood pressure; IPW, inverse probability weight; SES, socioeconomic status; SBP, systolic blood pressure.
aDescriptive statistics have been weighted using IPWs to account for attrition, refusal to provide anthropometric measures, and/or refusal to provide a DBS sample.
bComposite variable of the highest (4th and 5th) quintiles of wealth.
cSelf-reported ability to read or write.
dSelf-reported presence of limitations in activities of daily living (excluding difficulty in dressing as activities of daily living).
eHIV positive as determined by lab assays using DBSs.
fRandom (non-fasting) glucose.
Figure 2.
Hypertension awareness, treatment, and control among persons with hypertension in the HAALSI cohort at baseline (n = 2,884) and follow-up (n = 2,164). Hypertension: systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self-reported use of medication. Awareness: self-reported diagnosis by a health professional among those meeting the definition of hypertension. Treatment: self-reported use of medication among those meeting the definition of hypertension. Control: measured systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg, among those meeting the definition of hypertension.
Figure 3.
Hypertension awareness, treatment, and control among persons with hypertension in the HAALSI cohort at baseline (n = 2,884) and follow-up (n = 2,164): stratified by sex. Hypertension: Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self-reported use of medication. Awareness: Self-reported diagnosis by a health professional among those meeting the definition of hypertension. Treatment: Self-reported use of medication among those meeting the definition of hypertension. Control: Measured systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg, among those meeting the definition of hypertension.
Table 2.
Hypertension awareness, treatment, and control among persons with hypertension at follow-up, by age group
| Total | Awarenessa | Treatmentb | Controlc | |
|---|---|---|---|---|
| N | n (%) | n (%) | n (%) | |
| Hypertension d | 2,164 | 1,809 (83.6) | 1,600 (73.9) | 893 (41.3) |
| Overall trend P-valuee | <0.001 | 0.6415 | <0.001 | |
| Males | ||||
| 40–44 years | 8 | 2 (25.0) | 1(100) | 0 (0) |
| 45–54 years | 97 | 62 (63.9) | 45 (95.7) | 20 (50.0) |
| 55–64 years | 201 | 143 (71.1) | 117 (90.7) | 50 (49.5) |
| 65+ years | 542 | 443 (81.7) | 386 (93.0) | 233 (74.9) |
| P-value for trende | <0.001 | 0.9176 | <0.001 | |
| Females | ||||
| 40–44 years | 7 | 3 (42.9) | 2 (66.7) | 1 (100) |
| 45–54 years | 186 | 152 (81.72) | 127 (96.2) | 76 (67.3) |
| 55–64 years | 386 | 338 (87.6) | 301 (95.3) | 152 (59.1) |
| 65+ years | 737 | 666 (90.4) | 621 (96.7) | 361 (70.4) |
| P-value for trende | <0.001 | 0.2354 | 0.0826 | |
aAwareness: Self-reported diagnosis by a health professional among those meeting the definition of hypertension.
bTreatment: Self-reported use of medication among those meeting the definition of hypertension.
cControl: Measured systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg, among those meeting the definition of hypertension.
dHypertension: Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or self-reported use of medication.
e P-value for linear trend in across age groups from baseline to follow-up, at α = 0.05.
Consistent with previous work, we found that among persons with HTN, those who are female, elderly, have higher socioeconomic status, or have PLs, have a slightly higher prevalence of awareness of their HTN at follow-up (Table 3). Additionally, compared to persons who did not visit a primary health clinic (PHC) in the 3 months prior to the follow-up interview, those who visited a PHC 3 times in this period were 1.12 times more likely to be aware of their HTN (95% CI: 0.83; 1.52). None of the predictors included in the multivariate model were associated with a higher prevalence of being on treatment (Table 3). A control among those with HTN was substantively associated with being elderly (PR = 1.22; 95% CI: 0.99; 1.50), having PLs (PR = 1.10; 95% CI: 0.86, 1.39), and having attended 1–3 visits at a PHC in the previous three months.
Table 3.
Assessment of the individual stages of the hypertension treatment cascade among persons with hypertension at follow-up, by covariates of interest
| Awareness | Treatment | Control | ||||
|---|---|---|---|---|---|---|
| Prevalence ratio | (95% CI)a | Prevalence ratio | (95% CI)a | Prevalence ratio | (95% CI)a | |
| Female (reference group: male) |
1.10 | (0.96; 1.26) | 1.00 | (0.88; 1.15) | 1.05 | (0.88; 1.24) |
| HIV+ with viral suppression (<100 copies/ml)b,c (reference group: HIV−) |
0.97 | (0.81; 1.15) | 1.00 | (0.83; 1.20) | 0.88 | (0.71; 1.11) |
| HIV+ without viral suppression (>100 copies/ml)b,c (reference group: HIV−) |
0.83 | (0.59; 1.17) | 1.02 | (0.71; 1.48) | 0.69 | (0.41; 1.12) |
| Elderly (≥60) (reference group: <60) |
1.13 | (0.96; 1.32) | 0.99 | (0.89; 1.17) | 1.22 | (0.99; 1.50) |
| Migrant (reference group: born in South Africa) |
0.99 | (0.86; 1.14) | 0.99 | (0.86; 1.15) | 0.92 | (0.77; 1.11) |
| High SESd (reference group: Low SES) |
1.06 | (0.93; 1.20) | 1.00 | (0.88; 1.15) | 0.98 | (0.83; 1.15) |
| Literatee (reference group: Illiterate) |
1.04 | (0.91; 1.19) | 1.00 | (0.87; 1.15) | 1.00 | (0.84; 1.19) |
| Physical limitationsf (reference group: no physical limitations) |
1.04 | (0.86; 1.25) | 1.01 | (0.84; 1.22) | 1.10 | (0.86; 1.39) |
| Visits to primary health clinic in last 3 months (reference group: 0 visits in last 3 months) |
||||||
| 1 visit | 1.05 | (0.79; 1.42) | 1.01 | (0.74; 1.36) | 1.21 | (0.82; 1.79) |
| 2 visits | 1.08 | (0.79; 1.48) | 1.02 | (0.75; 1.36) | 1.27 | (0.83; 1.93) |
| 3 visits | 1.12 | (0.83; 1.52) | 1.03 | (0.75; 1.42) | 1.21 | (0.81; 1.83) |
Abbreviations: DBS, dried blood spot; SES, socioeconomic status.
a95% CI-confidence interval (lower limit–upper limit).
bHIV positive as determined by lab assays using DBS.
cReference category is HIV negative.
dComposite variable of the highest (4th and 5th) quintiles of wealth.
eSelf-reported ability to read or write.
fSelf-reported presence of limitations in activities of daily living (excluding difficulty in dressing as activities of daily living.
DISCUSSION
The main finding is that among persons with HTN in the cohort, there was substantial improvement at all the stages of the HTN treatment cascade between baseline and follow-up. Women fare better across all stages of the treatment cascade at both time points. Increasing improvements with age were observed for both women and men across all stages of the treatment cascade. Consistent with previous work, we found that women and the elderly were much more likely to be aware of their HTN. Persons who were elderly had PLs, and visited a PHC 1–3 times in the previous three months were much more likely to have their HTN controlled. When compared to persons who were not HIV-infected, PLHIV were less likely to be aware of their HTN, to be treated, and had the worst control of their HTN, regardless of their viral suppression status.
The prevalence of HTN in the cohort at follow-up (59%) is similar to baseline and also within the reported range for adults aged 45 and older in both the 2016 Demographic and Health Survey20 (55%–84%) and the South African National Health and Nutrition Examination Survey (52%–78%).21
Compared to the level of awareness (30%) observed among older adults in the Study of Global Ageing and Adults’ Health,15 awareness of HTN in the HAALSI cohort was higher at both baseline and follow-up. One explanation is field workers identified and referred HAALSI participants with elevated BP to PHCs for evaluation during the baseline assessment, leading to improvements across the treatment cascade. This is in contrast to the high prevalence of undiagnosed HTN—which results in poor awareness—among South African adults as shown in population surveys22 and longitudinal cohort studies.23 Increased opportunities for BP measurement through other ongoing research activities related to screening for chronic diseases in the HDSS24 likely also contributed to the high level of HTN awareness, potentially leading to subsequent improvements that we observed in treatment and control. In this analysis, we found that increased engagement with PHCs, as reflected in the number of visits to PHCs in the last 3 months, led to appreciable increases in HTN awareness (PR ranges from 1.05–1.12).
Treatment is critical to controlling HTN but, despite reported high rates of treatment initiation in South Africa,20 less than half of adults aged 45 or older report current use of medications even when they are aware of their diagnosis.15,20,21 Poor linkage to care is one possible explanation for low treatment rates in rural areas, with only 27% of persons with HTN reporting being linked to HTN care at a PHC two years after being identified through community-based screening in Kwazulu-Natal.25 That study also found that males and employed persons were least likely to link to a PHC after screening. While it makes intuitive sense that the opportunity costs to attend PHC visits would be too high for employed persons, our results show that almost all persons with HTN are treated, regardless of sex. One possible explanation is that the HAALSI cohort is embedded in the HDSS in Agincourt where exposure to ongoing surveillance and research may positively influence the odds of treatment.
Despite the significant increase in control that we observed from baseline to follow-up, the overall level of HTN control in the cohort is only 55%. Long wait times at PHCs, medication stockouts, and frustration with appointment times are some of the systemic barriers that adversely affect compliance with treatment, and negatively affect achieving and sustaining HTN control.26,27 Since 2018, the Central Chronic Medicines Dispensing and Distribution (CCMDD)28 program—designed to mitigate some of these barriers—has enabled patients with control of their HTN to collect medications from convenient pick-up points in the community or at dedicated pick-up queues in clinics. The CCMDD program is available in the HAALSI catchment area29 and likely contributed to the improved control rates that we observed, though no published evidence regarding its impact in the HDSS catchment area is available at this time. Medication shortages continue to be a persistent barrier to effective chronic disease management, especially for HTN, which is the second most widely prescribed medication (5%) in primary care.20 In response to shortages, some providers don’t follow practice guidelines for prescribing treatment in an effort to spread available supplies across larger segments of the patient population over the short term.30 Discontinuation of HTN medications in longitudinal cohorts of adults 40 and older in South Africa has been estimated at 15%–25% over 5–9 years of follow-up and lack of BP control at 36%–54%.23
The government’s integrated chronic disease management (ICDM) program attempts to leverage the success of HIV programs to improve noncommunicable disease management care and associated health outcomes. Results from the implementation of the ICDM in Agincourt showed a small but significant impact on BP control.26,31 The very poor control of HTN that we observed in this analysis could be partly due to the persistent stigma associated with HIV, given that tracing and home visits to monitor PLHIV progress remain significant barriers to effectively managing HTN in the ICDM program. Similar challenges were observed in the Right to Care Clinical HIV Cohort where 24% of members developed incident HTN after initiation on ART. Despite that cohort’s linkage to a PHC for care, only 33% of the incident cases had HTN treatment initiated within 3 months of diagnosis and 43% more than 12 months after diagnosis.32
Given the high prevalence of HIV infection in the HAALSI cohort, the effects of stigmatization could partly explain the poor control among PLHIV observed in our analysis.
Strengths
Strengths of this analysis include the evaluation of the impact of contact with primary healthcare services through the number of visits to PHCs and the evaluation of the impact of viral suppression on all stages of the HTN treatment cascade.
Limitations
The HAALSI cohort is embedded in the HDSS, and the ongoing surveillance of health outcomes may lead to overestimation of the true improvements along the treatment cascade, thereby potentially reducing generalizability to other older and nonrural populations in South Africa. Information on diagnosis, treatment, and medication information for HTN is self-reported and subject to respondent bias.
CONCLUSIONS
We showed that after four years of follow-up, all stages along the HTN treatment cascade improved appreciably in the HAALSI cohort. Men lag behind women at each step of the treatment cascade and would benefit from targeted efforts to improve the management of their HTN. Additionally, control among PLHIV is poor, regardless of viral suppression status, suggesting that stigmatization continues to play a role in the optimal management of HTN. These results highlight important policy implications to consider for the implementation and scaling of the ICDM program.
SUPPLEMENTARY DATA
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
Contributor Information
Shafika Abrahams-Gessel, Center for Health Decision Science, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
F Xavier Gómez-Olivé, Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA; Medical Research Council/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), University of the Witwatersrand, Johannesburg, South Africa; Africa Wits-INDEPTH Partnership for Genomic Studies, University of the Witwatersrand, Johannesburg, South Africa.
Stephen Tollman, Medical Research Council/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), University of the Witwatersrand, Johannesburg, South Africa; Africa Wits-INDEPTH Partnership for Genomic Studies, University of the Witwatersrand, Johannesburg, South Africa; Department of Epidemiology and Global Health, Umeå University, Umeå, Sweden.
Alisha N Wade, Medical Research Council/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), University of the Witwatersrand, Johannesburg, South Africa; Africa Wits-INDEPTH Partnership for Genomic Studies, University of the Witwatersrand, Johannesburg, South Africa.
Jacques D Du Toit, Medical Research Council/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), University of the Witwatersrand, Johannesburg, South Africa.
Enrico G Ferro, Division of Cardiovascular Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Chodziwadziwa W Kabudula, Medical Research Council/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), University of the Witwatersrand, Johannesburg, South Africa; Africa Wits-INDEPTH Partnership for Genomic Studies, University of the Witwatersrand, Johannesburg, South Africa.
Thomas A Gaziano, Center for Health Decision Science, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA; Harvard Center for Population and Development Studies, Harvard University, Cambridge, Massachusetts, USA; Cardiovascular Medicine Division, Brigham & Women’s Hospital, Boston, Massachusetts, USA.
ETHICS APPROVAL
Ethical approvals for the study were obtained from the University of the Witwatersrand Human Research Ethics Committee, the Harvard T.H. Chan School of Public Health, the Office of Human Research Administration, and the Mpumalanga Provincial Research and Ethics Committee. Written informed consent was obtained in xiTsonga, the local language, or in English. Participants unable to read consented with an inked fingerprint as a signature in the presence of a witness.
FUNDING
The HAALSI study is funded by the National Institute on Aging (P01 AG041710). The HAALSI study is nested within the Agincourt Health and Socio-Demographic Surveillance System. The MRC/Wits Rural Public Health and Health Transitions Research Unit and Agincourt Health and Socio-Demographic Surveillance System, a node of the South African Population Research Infrastructure Network (SAPRIN), is supported by the Department of Science and Innovation, the University of the Witwatersrand, and the Medical Research Council, South Africa, and previously the Wellcome Trust, UK (grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z). The HAALSI study has been carried out through a collaboration between the Harvard Center for Population and Development Studies at the Harvard T.H. Chan School of Public Health, the MRC/Wits Rural Public Health and Health Transitions Research Unit from the School of Public Health at the University of the Witwatersrand in South Africa, and the INDEPTH Network in Accra, Ghana. Research reported in this publication was also supported by an NIH supplement (3U54HG006938-03S1) to the AWI-Gen Collaborative Centre (1U54HG006938), an H3Africa Consortium member, to enable the integration of HAALSI and AWI-Gen research. Dr. Wade is supported by the Fogarty International Center, National Institutes of Health under award number K43TW010698. The funders did not contribute to design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
S.A.G. and T.A.G. designed the analysis, had full access to the data, and wrote the manuscript with significant input from all the co-authors. S.A.G. performed the statistical analysis. F.X.G.O. supervised the data collection in the field. All authors interpreted the data, revised, and approved the manuscript.
DATA AVAILABILITY
The datasets generated and/or analyzed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) program website: www.haalsi.org. The data supporting the findings of this study are available from the corresponding author upon reasonable request. The datasets generated and/or analyzed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) program website: www.haalsi.org.
DISCLOSURE
The authors declare that they have no conflicts of interest relevant to the research.
REFERENCES
- 1. Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, Nojilana B, Joubert JD, Matzopoulos R, Prinsloo M, Nannan N, Gwebushe N, Vos T, Somdyala N, Sithole N, Neethling I, Nicol E, Rossouw A, Bradshaw D.. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. The Lancet Global Health 2016; 4:e642–e653. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, AlMazroa MA, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT-A, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang Y-H, Khatibzadeh S, Khoo J-P, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Memish ZA, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M.. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012; 380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J.. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. doi: 10.1161/circulationaha.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Bärnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catalá-López F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ.. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017; 317:165–182. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 5. Gómez-Olivé FX, Montana L, Wagner RG, Kabudula CW, Rohr JK, Kahn K, Bärnighausen T, Collinson M, Canning D, Gaziano T, Salomon JA, Payne CF, Wade A, Tollman SM, Berkman L.. Cohort profile: health and ageing in Africa: a longitudinal study of an INDEPTH community in South Africa (HAALSI). International Journal of Epidemiology 2018; 47:689–690j. doi: 10.1093/ije/dyx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, Wade A, Crowther NJ, Alam S, Manne-Goehler J, Kabudula CW, Wagner R, Rohr J, Montana L, Kahn K, Bärnighausen TW, Berkman LF, Tollman S.. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural South Africa: the HAALSI (Health and Aging in Africa: longitudinal studies of INDEPTH communities) study. BMC Public Health 2017; 17:206. doi: 10.1186/s12889-017-4117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jardim TV, Reiger S, Abrahams-Gessel S, Crowther NJ, Wade A, Gómez-Olivé FX, Salomon J, Tollman S, Gaziano TA.. Disparities in management of cardiovascular disease in rural South Africa: data from the HAALSI Study (Health and Aging in Africa: Longitudinal Studies of International Network for the Demographic Evaluation of Populations and Their Health Communities). Circ Cardiovasc Qual Outcomes 2017; 10:e004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jardim TV, Reiger S, Abrahams-Gessel S, Gomez-Olive FX, Wagner RG, Wade A, Bärnighausen TW, Salomon J, Tollman S, Gaziano TA.. Hypertension management in a population of older adults in rural South Africa. J Hypertens 2017; 35:1283–1289. doi: 10.1097/hjh.0000000000001312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferro EG, Abrahams-Gessel S, Jardim TV, Wagner R, Gomez-Olive FX, Wade AN, Peters F, Tollman S, Gaziano TA.. Echocardiographic and electrocardiographic abnormalities among elderly adults with cardiovascular disease in rural South Africa. Circ Cardiovasc Qual Outcomes 2021; 14:e007847. doi: 10.1161/circoutcomes.121.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sliwa K, Wilkinson D, Hansen C, Ntyintyane L, Tibazarwa K, Becker A, Stewart S.. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet 2008; 371:915–922. [DOI] [PubMed] [Google Scholar]
- 11. Sekoba NP, Kruger R, Labuschagne P, Schutte AE.. Left ventricular mass independently associates with masked hypertension in young healthy adults: the African-PREDICT study. J Hypertens 2018; 36:1689–1696. [DOI] [PubMed] [Google Scholar]
- 12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JJL, Jones DW, Materson BJ, Oparil S, Wright JJT, Roccella EJ; the National High Blood Pressure Education Program Coordinating C. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA 2003; 289:2560–2571. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 13. Rutstein SO. Steps to Constructing the New DHS Wealth Index. ICF International. https://preview.dhsprogram.com/programming/wealth%20index/Steps_to_constructing_the_new_DHS_Wealth_Index.pdf. Accessed 30 August 2021. [Google Scholar]
- 14. Harvard Center for Population and Development Studies (Harvard T.H. Chan School of Public Health). Data from: HAALSI Wave 2 Survey: Wave 2 Sample Weights Documentation. 2020;V4. Deposited 2020-11-23. doi: 10.7910/DVN/UELYSV [DOI]
- 15. Peltzer K, Peltzer K, Phaswana-Mafuya N, Phaswana-Mafuya N, Peltzer K.. Hypertension and associated factors in older adults in South Africa: cardiovascular topics. Cardiovasc J Afr 2013; 24:66–71. doi: 10.5830/CVJA-2013-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider M, Bradshaw D, Steyn K, Norman R, Laubscher R.. Poverty and non-communicable diseases in South Africa. Scand J Public Health 2009; 37:176–186. [DOI] [PubMed] [Google Scholar]
- 17. Barros AJ, Hirakata VN.. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bastos LS, Oliveira Rde V, Velasque Lde S.. Obtaining adjusted prevalence ratios from logistic regression models in cross-sectional studies. Cad Saude Publica 2015; 31:487–495. doi: 10.1590/0102-311x00175413 [DOI] [PubMed] [Google Scholar]
- 19. Coutinho LMS, Scazufca M, Menezes PR.. Methods for estimating prevalence ratios in cross-sectional studies. Revista de saúde pública 2008; 42:992–998. [PubMed] [Google Scholar]
- 20. National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC) aI. South Africa Demographic and Health Survey 2016: Key Findings., http://dhsprogram.com/pubs/pdf/FR337/FR337.pdf. Accessed 14 April 2021.
- 21. Shisana O LD, Rehle T, Simbayi L, Zuma K, Dhansay A, Reddy P, Parker W, Hoosain E, Naidoo P, Hongoro C, Mchiza Z, Steyn NP, Dwane N, Makoae M, Maluleke T, Ramlagan S, Zungu N, Evans MG, Jacobs L, Faber M, & SANHANES-1 Team. The South African National Health and nutrition examination survey: SANHANES-1. 2013. [Google Scholar]
- 22. Berry KM, Parker WA, McHiza ZJ, Sewpaul R, Labadarios D, Rosen S, Stokes A.. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011-2012. BMJ Glob Health 2017; 2:e000348. doi: 10.1136/bmjgh-2017-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mauer N, Geldsetzer P, Manne-Goehler J, Davies JI, Stokes AC, McConnell M, Ali MK, Winkler V, Sudharsanan N.. Longitudinal evidence on treatment discontinuation, adherence, and loss of hypertension control in four middle-income countries. Sci Transl Med 2022; 14:eabi9522. doi: 10.1126/scitranslmed.abi9522 [DOI] [PubMed] [Google Scholar]
- 24. Kahn K, Collinson MA, Gómez-Olivé FX, Mokoena O, Twine R, Mee P, Afolabi SA, Clark BD, Kabudula CW, Khosa AP.. Agincourt health and socio-demographic surveillance system. Int J Epidemiol 2012; 41:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siedner MJ, Baisley K, Orne-Gliemann J, Pillay D, Koole O, Wong EB, Matthews P, Tanser F, Herbst K, Barnighausen T, Bachmann M.. Linkage to primary care after home-based blood pressure screening in rural KwaZulu-Natal, South Africa: a population-based cohort study. BMJ Open 2018; 8:e023369. doi: 10.1136/bmjopen-2018-023369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ameh S, Klipstein-Grobusch K, D’Ambruoso L, Kahn K, Tollman SM, Gómez-Olivé FX.. Quality of integrated chronic disease care in rural South Africa: user and provider perspectives. Health Policy Plan 2017; 32:257–266. doi: 10.1093/heapol/czw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chikafu H, Chimbari M.. Hypertension care cascade in the Ingwavuma rural community, uMkhanyakude District, KwaZulu-Natal province of South Africa. PeerJ 2021; 9:e12372. doi: 10.7717/peerj.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. South African National Department of Health. The Central Chronic Medicines Dispensing and Distribution (CCMDD) Programme. https://www.health.gov.za/ccmdd/. Accessed 18 February 2022.
- 29. Liu L, Christie S, Munsamy M, Roberts P, Pillay M, Shenoi SV, Desai MM, Linnander EL.. Expansion of a national differentiated service delivery model to support people living with HIV and other chronic conditions in South Africa: a descriptive analysis. BMC Health Serv Res. 2021/05/17 2021; 21:463. doi: 10.1186/s12913-021-06450-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. West RL, Lippman SA, Twine R, Maritze M, Kahn K, Leslie HH.. Providers’ definitions of quality and barriers to providing care: a qualitative study in rural Mpumalanga Province, South Africa. J Glob Health Sci. 2021;3:e1. [DOI] [PMC free article] [PubMed]
- 31. Ameh S. Evaluation of an integrated HIV and hypertension management model in rural South Africa: a mixed methods approach. Global Health Action 2020; 13:1750216. doi: 10.1080/16549716.2020.1750216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brennan AT, Jamieson L, Crowther NJ, Fox MP, George JA, Berry KM, Stokes A, Maskew M, Sanne I, Long L.. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS One 2018; 13:e0204020e0204020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) program website: www.haalsi.org. The data supporting the findings of this study are available from the corresponding author upon reasonable request. The datasets generated and/or analyzed for the follow-up study are available at the Harvard Center for Population and Development Studies (HCPDS) program website: www.haalsi.org.