Abstract
Two c-type cytochromes from the soluble fraction of a deep-sea moderately piezophilic bacterium, Shewanella violacea, were purified and characterized, and the genes coding for these cytochromes were cloned and sequenced. One of the cytochromes, designated cytochrome cA, was found to have a molecular mass of approximately 8.3 kDa, and it contained one heme c per molecule. The other, designated cytochrome cB, was found to have a molecular mass of approximately 23 kDa, and it contained two heme c molecules per protein molecule. The amount of cytochrome cB expressed in cells grown at high hydrostatic pressure (50 MPa) was less than that in cells grown at atmospheric pressure, whereas cytochrome cA was constitutively expressed under all pressure conditions examined. The results of Northern blotting analysis were consistent with the above-mentioned observations and suggested that the pressure regulation of cytochrome cB gene expression occurred at the transcriptional level. These results suggest that the components of the respiratory chain of moderately piezophilic S. violacea could be exchanged according to the growth pressure conditions.
The deep sea is known as an extreme environment with a stable low temperature and very high hydrostatic pressure. Microorganisms living at the sea bottom may have special mechanisms for adaptation to such extreme conditions. Piezophilic bacteria, formerly called barophilic bacteria, are defined as bacteria which show better growth at high pressure than at atmospheric pressure (32), and they were first isolated in 1979 (33). Still, the mechanisms involved in bacterial adaptation to high-pressure environments are not well known. We have isolated a large variety of bacteria from samples of deep-sea sediments obtained by means of manned and unmanned submersibles operated by the Japan Marine Science and Technology Center to investigate the molecular mechanisms of bacterial adaptation to high hydrostatic pressure (13, 14, 16).
Bartlett and his coworkers have studied pressure-sensing mechanisms, focusing on the expression of high-pressure marker outer membrane proteins, OmpH and OmpL, whose gene expressions are controlled by pressure, positively and negatively, respectively, in the moderately piezophilic bacterium Photobacterium profundum SS9 (2–4, 11, 30). Their results suggested that several factors are involved in gene expression controlled by pressure conditions and that biological components of the cell surface may be very important in these mechanisms (1, 15).
Recently, a pressure-regulated promoter was found in a piezophilic bacterium, strain DB6705 (17), and in another moderately piezophilic bacterium, strain DSS12 (12), subsequently identified as Shewanella benthica and Shewanella violacea, respectively (23). Near the promoter in the genomic DNA of S. violacea, an open reading frame homologous to the cydD gene of Escherichia coli was found, and the significance of this gene in bacterial growth under high hydrostatic pressure conditions was suggested (19). The cydD gene product is thought to be required for the assembly of respiratory components in E. coli (24–26). These findings suggested that the expression of the respiratory system may be regulated by hydrostatic pressure, and this regulation may play some role in adaptation to high hydrostatic pressure. Indeed, expression of cytochromes is regulated by pressure in S. benthica DB172F (27, 28) and S. violacea DSS12 (29).
S. violacea is one of the bacteria isolated in our laboratory from deep-sea sediment from the Ryukyu Trench (5,110-m depth) collected by means of the manned submersible SHINKAI 6500 (16), and this organism has been taxonomically characterized (23). It is a moderately piezophilic and psychrophilic bacterium, which shows optimal growth at a pressure of 30 MPa and at a temperature of 8°C, and it exhibits almost the same growth at pressures of 0.1 and 50 MPa. We have previously reported the spectrophotometric analysis of S. violacea membrane fractions from the cells cultivated at both high- and atmospheric-pressure conditions (29). The results suggested that the expression of a membrane-bound d-type cytochrome and the expression of a soluble c-type cytochrome(s) were regulated by hydrostatic pressure. Therefore, these cytochromes may play some role in the piezoadaptibility of this bacterium. But the molecular mechanisms of gene expression regulated by pressure in these cytochromes are still unknown.
In the present study, we purified two soluble c-type cytochromes to an electrophoretically homogeneous state from the moderately piezophilic bacterium S. violacea grown at atmospheric pressure, and the genes coding for these cytochromes were cloned and sequenced. Further, by Western and Northern blotting analyses, we investigated the expression of soluble c-type cytochromes in cells grown under different pressure conditions.
MATERIALS AND METHODS
Microorganism and culture conditions.
S. violacea DSS12 (JCM10179), isolated from deep-sea sediment obtained from the Ryukyu Trench (24°15.23′N, 126°47.30′E) at a depth of 5,110 m by means of the SHINKAI 6500 system (16, 23), was used in this study. Cultivation of S. violacea was performed at 8°C as described previously (16) under various pressure conditions. Marine Broth 2216 (Difco Laboratories, Detroit, Mich.) autoclaved and filtered through a 0.22-μm-pore-size membrane filter was used as the culture medium. Two kinds of cultivation methods were used for cells grown under atmospheric pressure conditions: cells were grown aerobically with shaking, and cells were grown microaerobically in a sterilized package without shaking. On the other hand, some of these packages were placed in the pressure vessel and pressurized as described previously (16, 29). Under both microaerobic cultivations, the only variable factor was the applied pressure, whereas the oxygen concentrations in the medium might be the same. Cultivated cells were collected by centrifugation (10,000 × g for 15 min), washed in 10 mM Tris-HCl buffer (pH 8.0) containing 0.3 M NaCl, and stored at −80°C until use.
Purification of soluble cytochromes c.
Frozen S. violacea cells (about 10 g [wet weight]) which had been cultivated at atmospheric pressure were thawed, and NaCl was added to a final concentration of 1.0 M. The cell suspension was treated with a sonic oscillator (20 kHz, 200 W; model UD-201; Tomy Seiko Co., Tokyo, Japan) for a total period of 45 min. Unbroken cells were removed by centrifugation (10,000 × g for 15 min). The cell-free extract obtained was centrifuged at 143,000 × g for 1 h. The supernatant obtained by ultracentrifugation was dialyzed for 12 h against 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM sodium l-(+)-ascorbate at 4°C. The dialyzed solution was applied to a diethylaminoethyl (DEAE)-Toyopearl column (TSK-gel 650M; 2.6 by 14 cm; Tosoh Co., Tokyo, Japan) equilibrated with the same buffer as used for dialysis. After washing the column with the same buffer, the cytochromes c adsorbed on the column were eluted with a 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM sodium l-(+)-ascorbate and 0.15 M NaCl. The eluted fraction was dialyzed again for 3 h against 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM sodium l-(+)-ascorbate. The fraction obtained was subjected to chromatography on a DEAE-Toyopearl column (TSK-gel 650M; 2.6 by 6 cm) equilibrated with the same buffer used for dialysis. Two types of cytochromes c (named cytochrome cA and cytochrome cB) adsorbed on the column were eluted with a linear gradient produced from 245 ml each of 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM sodium l-(+)-ascorbate and the buffer containing 0.3 M NaCl as shown in Fig. 1. Cytochrome cA and cytochrome cB were eluted at approximately 65 and 85 mM NaCl, respectively. A 1/10 volume of 1 M Tris-HCl buffer (pH 8.0) was added to each fraction. Subsequently, ammonium sulfate was added to each fraction to yield 80% saturation, and after stirring for 15 h, the resulting solution was centrifuged at 10,000 × g for 15 min. Cytochrome cA in the supernatant was further purified as follows. The solution was applied to an EXPRESS-ION EXCHANGER D column (1 by 2 cm; Whatmann International Ltd., Kent, United Kingdom) equilibrated with 10 mM Tris-HCl buffer (pH 8.0) and 80% saturated with ammonium sulfate. The cytochrome cA adsorbed on the column was eluted with a small volume of 10 mM Tris-HCl buffer (pH 8.0). Cytochrome cB was obtained as a precipitate in the above-mentioned centrifugation step and was dissolved in 1 ml of 10 mM Tris-HCl buffer (pH 8.0) containing 0.3 M NaCl. Each of these protein solutions obtained was subjected to gel filtration on a HiLoad Superdex 75 prep grade column (1.6 by 60 cm; Pharmacia Biotech. Co., Uppsala, Sweden) equilibrated with 10 mM Tris-HCl buffer (pH 8.0) containing 0.3 M NaCl. The fractionated cytochromes c were used as the purified cytochrome cA and cytochrome cB preparations. Purification of these cytochromes is summarized in Table 1.
FIG. 1.
Elution profiles of the soluble fraction prepared from S. violacea cells during the second DEAE-Toyopearl chromatography step of the purification procedure. The elution of heme c was monitored at 410 nm (circles). The dashed line denotes the NaCl concentration. Cytochrome cA and cytochrome cB eluted at about 65 and 85 mM NaCl, respectively.
TABLE 1.
Purification of 10 g (wet weight) of soluble cytochromes c from strain DSS12
| Experimental step | Total protein (mg) | Amt of heme c (μmol) | Content of cytochrome cA
|
Content of cytochrome cB
|
||||
|---|---|---|---|---|---|---|---|---|
| Amt of protein (mg) | Amt of heme c (μmol) | Concn of heme c (μmol/mg of protein) | Amt of protein (mg) | Amt of heme c (μmol) | Concn of heme c (μmol/mg of protein) | |||
| Soluble fraction | 377 | 173 | ||||||
| First anion exchange | 99.1 | 55.2 | ||||||
| Second anion exchange | 3.07 | 32.5 | 10.59 | 1.78 | 12.3 | 6.91 | ||
| Ammonium sulfate precipitation (80%) | 0.475 | 22.5 | 47.37 | 0.177 | 4.42 | 24.98 | ||
| Gel filtration (Superdex, 75 pg) | 0.223 | 16.2 | 72.65 | 0.085 | 2.61 | 30.70 | ||
Physical and chemical measurements.
Spectrophotometric measurements were performed using a Shimadzu UV-2400PC spectrophotometer (Shimadzu Co., Kyoto, Japan) with 1-cm light path cuvettes at room temperature. The heme c content was determined on the basis of the millimolar extinction coefficient (ɛmM) of pyridine ferrohemochrome c having a value of 29.1 mM−1 cm−1 (8).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (20). The samples containing 1% (wt/vol) SDS and 1% (vol/vol) β-mercaptoethanol were treated by heating at 98°C for 10 min. SDS-PAGE Standards, Broad range (Bio-Rad Co., Hercules, Calif.), were used as molecular weight markers. The presence of heme in the gel was detected by means of a heme-staining reagent (5). The protein content was determined by using the Bio-Rad Protein Assay kit with bovine serum albumin as a standard.
The midpoint redox potential of cytochrome c was determined spectrophotometrically with the ferro/ferricyanide system (7). Horse heart cytochrome c (Em, 7 = 255 mV; Sigma-Aldrich Co., St. Louis, Mo.) was used as a standard.
The N-terminal amino acid sequence of each of the purified proteins was determined by Edman degradation (9) by using a model G1005A protein sequencing system (Hewlett-Packard Co., Palo Alto, Calif.).
Construction of a λ phage library of the S. violacea chromosome and screening for the cytochrome c genes.
Chromosomal DNA isolated from S. violacea was partially digested with Sau3AI. DNA fragments 15 to 23 kb in size, fractionated by electrophoresis on a 0.7% agarose gel, were inserted into the BamHI site of λEMBL3 (Stratagene Co., La Jolla, Calif.). The ligated DNA was employed for in vitro packaging by using GIGAPACK III XL packaging extracts (Stratagene Co.) according to the manufacturer's instructions. The λ phage library was screened by using the digoxigenin (DIG) detection system (Boehringer Mannheim Co., Mannheim, Germany). Based on the determined N-terminal amino acid sequence of cytochrome cA (YDKAZHIZHSMG) and that of cytochrome cB (EGNAEVGKTKAIVZS), two degenerate oligonucleotides were designed. The nucleotide sequences of these probes were 5′-TAY GAY AAR GCI TGY CAY ATH TGY CAY AGY ATG GG-3′ (35-mer) specific for the cytochrome cA gene and 5′-GAR GGN AAY GCN GAR GTN GGN AAR CAN AAR GCN ATH GTN TGY TC-3′ (45-mer) specific for the cytochrome cB gene. These oligonucleotides were labeled with digoxigenin at the 5′ end for use as hybridization probes. The library was screened with both probes, and then positive plaques were purified by four serial plaque hybridization steps. The inserts in λ phage amplified by long and accurate PCR were sonicated, and a shotgun library was constructed in the pUC18 vector (10). For sequencing of these fragments, the random shotgun sequencing method was used with a DNA sequencer (model 373S; Perkin-Elmer/Applied Biosystems Co., Foster City, Calif.). Assembling and editing of the determined DNA sequences were performed with AutoAssembler version 2.0 (Perkin-Elmer/Applied Biosystems Co.), and GENETYX-MAC version 10 from Software Development (Tokyo, Japan) was used for sequence analysis.
Western blot analysis.
Frozen cells of S. violacea DSS12, which had been cultivated at atmospheric pressure or at 50 MPa, were thawed separately, and NaCl was added to a final concentration of 1 M. The cell suspension was treated with a sonic oscillator (20 kHz, 200 W) for a total period of 30 min. Unbroken cells were removed by centrifugation (10,000 × g for 15 min). The cell-free extract obtained was centrifuged at 143,000 × g for 1 h. The supernatant was dialyzed for 16 h against 10 mM Tris-HCl buffer (pH 8.0) containing 1 mM EDTA, and the resulting solution was used as the soluble fraction for Western blotting analyses. Western blotting after SDS-PAGE was carried out by using the NovaBlot system (Pharmacia Biotech. Co.), with 1/500 dilutions of anti-serum (rabbit) against the purified cytochromes, which was prepared by Sawady Technology Corporation (Tokyo, Japan). The antibody-cytochrome complexes were detected by means of an anti-rabbit immunoglobulin G secondary antibody coupled to alkaline phosphatase, using the Immuno-Blotting Kit according to the manufacturer's instructions (Bio-Rad Co.).
Northern blotting and primer extension.
RNA was prepared from S. violacea as described previously (17). The RNA pellet was dissolved in diethyl pyrocarbonate-treated water, quantified by spectrophotometry, and stored at −80°C. Northern blotting analysis was performed by the method reported previously (17). Probes for Northern blotting analysis were constructed by the PCR DIG Probe Synthesis Kit (Boehringer Mannheim Co.) with the following synthesized primers. Primer set for the cytochrome cA gene was as follows: forward, 5′-GTTAGCAATGACTGCAGTCG-3′, and reverse, 5′-CTGTAAAACATACCACCTGG-3′. Primer set for the cytochrome cB gene was as follows: forward, 5′-GTTAGCTCTTGCACTATCAG-3′, and reverse, 5′-TAATGCTTCGATATCGTCGC-3′.
The transcriptional start points were determined by primer extension analysis with biotinylated oligonucleotides, 5′-AGACAAAGTTAAGACAGCGACTGC-3′ for the cytochrome cA gene and 5′-GCGAAGAGATACAGGCTAACACTG-3′ for the cytochrome cB gene, synthesized on an Applied Biosystems Model 392 DNA/RNA Synthesizer (Perkin-Elmer/Applied Biosystems Co.). The sequences of these primers are complementary to nucleotides 194 to 217 of the cytochrome cA gene (see Fig. 4A) and nucleotides 197 to 220 of the cytochrome cB gene (see Fig. 4B). The transcripts expressed by these strains at several pressures were detected by chemiluminescence as described previously (17).
FIG. 4.
Nucleotide and deduced amino acid sequences of the cloned genes coding for cytochromes cA (A) and cB (B). The underlined amino acids were identified by automated Edman sequencing of the purified proteins. The possible signal sequences are shown in italic characters. A heme c binding motif (CXXCH) is shaded. The putative −35 and −10 sequences (underlined) are located upstream from the transcriptional start point, as determined by primer extension (+1).
Nucleotide sequence accession numbers.
The DNA sequences reported in this paper have been deposited in the DDBJ (Mishima, Japan), EMBL (Heidelberg, Germany), and GenBank (Mountain View, Calif.) nucleotide sequence databases. The accession numbers of the DNA sequences containing the genes coding for cytochromes cA and cB of S. violacea DSS12 are AB032404 and AB032405, respectively.
RESULTS
Purification of soluble cytochromes c from S. violacea.
Two kinds of soluble cytochromes c were purified by several steps of column chromatography (Table 1); the one that was eluted first from the DEAE-Toyopearl column was designated cytochrome cA and the one that was eluted later from the same column was designated cytochrome cB. When these cytochromes c were subjected to SDS-PAGE, each showed one major band in the gel stained with Coomassie brilliant blue and a heme-staining reagent, as shown in Fig. 2. Both of the cytochromes c were purified to an electrophoretically homogeneous state. The molecular masses of the purified cytochromes as determined by SDS-PAGE were 8.3 kDa for cytochrome cA and 23 kDa for cytochrome cB, and both proteins had heme iron.
FIG. 2.
SDS-PAGE gel of purified soluble cytochromes c from S. violacea cells. After electrophoresis (15% acrylamide gel), the gel was stained with Coomassie brilliant blue (B) and a heme-staining reagent (A). Contents of the purified proteins are about 5 μg in each lane.
Molecular properties of the cytochromes c.
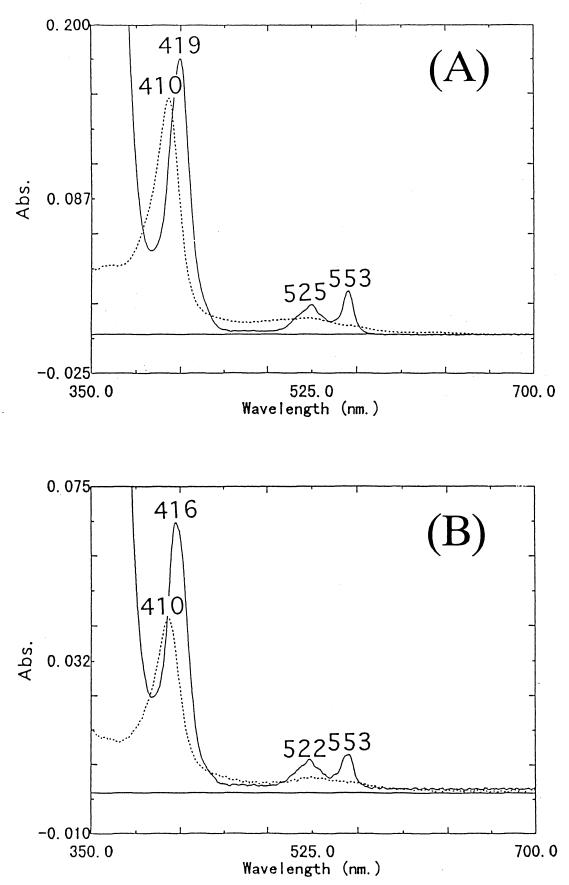
Figure 3 shows the absorption spectra of the purified cytochrome cA (Fig. 3A) and cytochrome cB (Fig. 3B). Cytochrome cA showed an absorption peak at 410 nm in the oxidized form and peaks at 419, 525, and 553 nm in the reduced form (Fig. 3A). The pyridine ferrohemochrome of cytochrome cA showed peaks at 521 and 550 nm (data not shown). This result suggested that cytochrome cA has heme c as a prosthetic group. The millimolar extinction coefficient of the reduced cytochrome cA at 553 nm was determined to be 19.5 mM−1 cm−1, based on that of the α-peak of the pyridine ferrohemochrome c. The midpoint redox potential was determined to be 0.301 mV. The results of SDS-PAGE indicated that this cytochrome consists of one polypeptide with a molecular mass of 8.3 kDa. The mass of the cytochrome per heme c was estimated to be 9,300 Da, based on the protein content and the heme c content. Thus, cytochrome cA of S. violacea has one heme c per molecule.
FIG. 3.
Absorption spectra of cytochromes cA (A) and cB (B) from S. violacea cells. Each of the cytochromes c was solubilized in 10 mM Tris-HCl (pH 8.0) containing 0.3 M NaCl. The protein concentration in the case of cytochrome cA was 1.43 μM and that in the case of cytochrome cB was 0.81 μM. The reduced form of each (solid line) was prepared by the addition of a small amount of Na2S2O4, and the oxidized form of each (dashed line) was prepared by adding a small amount of (NH4)2S2O8.
On the other hand, cytochrome cB showed an absorption peak at 410 nm in the oxidized form and peaks at 416, 522, and 553 nm in the reduced form, and a little shoulder was evident at around 550 nm (Fig. 3B). The pyridine ferrohemochrome of cytochrome cB showed peaks at 521 and 550 nm (data not shown). The result of this spectrophotometric analysis showed that cytochrome cB also has heme c as a prosthetic group. The millimolar extinction coefficient at 553 nm of reduced cytochrome cB was 12.4 mM−1 cm−1, based on that of the α-peak of the pyridine ferrohemochrome. The results of SDS-PAGE indicated that this cytochrome also consists of one polypeptide, with a molecular mass of 23 kDa. The mass of the cytochrome per heme c was estimated to be 14,000 Da from the protein content and the heme c content. Therefore, the cytochrome cB of S. violacea has two heme c molecules per protein molecule.
Cloning and sequencing of the genes coding for the soluble cytochromes cA and cB and primer extension analyses.
The N-terminal amino acid sequences of cytochromes cA and cB were determined to be QEGKAVYDKAZHIZHSMGVAGA and EGNAEVGKTKAIVZSAZHGVDG, respectively. These sequences were used to design degenerate oligonucleotide probes for screening the phage library of S. violacea genomic DNA. The nucleotide sequences of the probes synthesized were as follows: cytochrome cA, 5′-TAYGAYAARGCITGYCAYATHTGYCAYAGYATGGG-3′, and cytochrome cB, 5′- GARGGNAAYGCNGARGTNGGNAARACNAARGC NATHGTNTGYTC-3′. A few positive plaques were obtained, and the phages in these plaques were purified by a repeated plaque hybridization procedure. Finally, we identified a single positive plaque for each of the above cytochromes c gene sequences, and we determined the DNA sequence of the region including the open reading frame coding for each of these proteins.
As shown in Fig. 4, the structural gene encoding cytochrome cA was comprised of 258 bp and that of cytochrome cB was comprised of 621 bp. The cytochrome cA appears to consist of 85 amino acid residues, including a 21-residue signal peptide sequence (MKKLLAMTAVAVLTLSANVSA), as predicted from the deduced amino acid sequence. The cytochrome cA polypeptide contains one heme c binding motif (CXXCH) (21), consistent with our finding that there was one heme c per molecule (Fig. 4A). The calculated molecular mass of the processed cA apocytochrome is 6,801 Da, while the holocytochrome which includes one heme (molecular weight, 616.5) is predicted to have a mass of 7,417 Da, which is similar to the estimate of the protein mass obtained by SDS-PAGE (Fig. 2). On the other hand, cytochrome cB appears to consist of 206 amino acid residues, including a 20-residue signal peptide (MKKLALALSVLACISSPAMA) predicted from the deduced amino acid sequence. The cytochrome cB polypeptide contains two heme c binding motifs, consistent with our finding that there were two heme c molecules per protein molecule (Fig. 4B). The calculated molecular mass of the processed cB apocytochrome is 19,811 Da, while the holocytochrome which includes two heme molecules is predicted to have a mass of 21,044 Da, similar to the estimate of the protein mass obtained by SDS-PAGE (Fig. 2).
The 5′ ends of the mRNAs produced from the cytochrome cA and cB genes were identified as shown in Fig. 5A and B, respectively. The promoter consensus sequence found in each of these genes was somewhat similar to the recognition site for E. coli ς70 (6), as shown in Fig. 4; however, these sequences did not show a high level of homology.
FIG. 5.
Primer extension analysis to determine the transcriptional start points of the genes coding for cytochromes cA (A) and cB (B). The DNA sequence ladders of these genes were obtained by the dideoxy termination method employing the same primer. The nucleotide sequence corresponding to the ladder is shown on the left. The transcriptional start points are indicated by arrows and asterisks, and the 5′ end of the mRNA is also shown.
Pressure regulation of expression of the cytochromes c.
The results of Western blot analysis of the soluble fraction of cells grown aerobically or microaerobically at 0.1 MPa or microaerobically at 50 MPa, using antisera raised against purified cytochrome cA and cytochrome cB, are shown in Fig. 6A. The results showed that cytochrome cB is expressed only in cells grown at atmospheric pressure. In contrast, cytochrome cA is expressed regardless of the pressure conditions during growth. Further, the pattern of expression of the cytochromes c was the same in the case of both cells grown aerobically and cells grown microaerobically at atmospheric pressure.
FIG. 6.
(A) Western blot analysis of soluble fractions from S. violacea cells grown at 0.1 and 50 MPa. Whole protein present in the gel was transferred to a polyvinylidene difluoride membrane and then treated with antiserum raised against either cytochrome cA or cytochrome cB. The protein concentration in each soluble fraction was 1.67 mg/ml. Lanes 1, proteins from cells grown aerobically at 0.1 MPa; lanes 2, proteins from cells grown microaerobically at 0.1 MPa; lanes 3, proteins from cells grown microaerobically at 50 MPa. (B) Northern blot analysis of mRNAs from S. violacea cells grown at 0.1 and 50 MPa. Total RNA (30 μg) was loaded onto an agarose gel containing formaldehyde and transferred to a nylon membrane. PCR products described in the Materials and Methods section were used as probes. Lanes 1, RNA from cells grown aerobically at 0.1 MPa; lanes 2, RNA from cells grown microaerobically at 0.1 MPa; lanes 3, RNA from cells grown microaerobically at 50 MPa. DNA molecular weight marker III digoxigenin-labeled (Boehringer Mannheim Co.) was used as the size marker, and the sizes are indicated at the right side.
The results of Northern blotting analysis (Fig. 6B) showed that the gene for cytochrome cA was constitutively expressed and not regulated under different pressure conditions; however, the level of expression of the transcript of the cytochrome cB gene was clearly diminished under higher pressure conditions (50 MPa). These results, including the results of the Western blotting study, suggested that expression of these cytochromes c was controlled at the level of transcription, and only expression of the cytochrome cB gene was repressed by high pressure.
DISCUSSION
Two soluble c-type cytochromes were purified to an electrophoretically homogeneous state from a deep-sea piezophilic bacterium, S. violacea, grown at a pressure of 0.1 MPa, and the genes coding for these cytochromes c were cloned and sequenced. The deduced amino acid sequence of one of these cytochromes (cytochrome cB) showed homology with those of the cytochromes c4 group, and the other (cytochrome cA) was homologous to the cytochromes c5 group (Table 2). Cytochromes c4- and c5-related cytochromes are found in many bacteria (31) and are considered to be involved in aerobic respiration (21). In the case of Azotobacter vinelandii, it is thought that both cytochromes c transfer electrons to an o-type terminal oxidase in parallel (22).
TABLE 2.
Identity of amino acid sequences between the cloned cytochromes and others
| Organism | Protein | % Identity with S. violacea cytochromes c |
|---|---|---|
| Shewanella violacea | Cytochrome cA | 100 |
| Shewanella putreficians MR-1 | c-Type cytochrome ScyA | 72.2 |
| Chlorobium sp. | Cytochrome c-555 | 46.8 |
| Prosthecochloris aestuarii | Cytochrome c-555 | 44.7 |
| Pseudomonas mendocina | Cytochrome c5 | 41.5 |
| Azotobacter vinelandii | Cytochrome c5 | 40.8 |
| Shewanella violacea | Cytochrome cB | 100 |
| Pseudomonas stutzeri | Cytochrome c4 | 49.3 |
| Paracoccus sp. | Cytochrome c-554 | 45.9 |
| Pseudomonas aeruginosa | Cytochrome c4 | 45.4 |
| Azotobacter vinelandii | Cytochrome c4 | 44.5 |
As shown in Fig. 6, the level of expression of cytochrome cA was found to be the same in cells grown at either 0.1 or 50 MPa, but cytochrome cB was expressed only in cells grown at 0.1 MPa. Further, the pattern of expression of the cytochromes c was same in the case of both cells grown aerobically and cells grown microaerobically at a pressure of 0.1 MPa. These results suggest that the expression of cytochrome cB is regulated by hydrostatic pressure, regardless of the oxygen concentration. These observations are consistent with recent results obtained by spectrophotometric analyses (29). Those results suggested that the respiratory system of this bacterium, when grown at high pressure, is organized in a manner different from that in cells grown at atmospheric pressure. In the case of A. vinelandii, cytochrome c4 seems to act as a bypass for consumption of oxygen to protect the nitrogen fixation system from oxygen (22). Cytochrome cB of S. violacea may act as bypass of the electron transport system to overcome the atmospheric pressure stress, because this bacterium is one of piezophiles that are usually found growing in a high-pressure environment, like deep-sea bottoms. This speculation is similar as in the case of cytochrome c4 of A. vinelandii at oxygen stress; thus, such bypass may have a role to overcome from such stress conditions. But further studies are necessary to explain such phenomena.
These observations were very similar to results that we reported previously about regulation of the expression of two different membrane-bound cytochromes c by pressure in another piezophilic bacterium, Shewanella sp. strain DB-172F (27). In these piezophiles, the respiratory chain seems to be more compact and “short-cut” than that under normal atmospheric pressure conditions. This may be intimately related to the bacterial piezophilic property (15). It is possible that the regulation of expression of the respiratory system by hydrostatic pressure is one of the important mechanisms allowing these bacteria to adapt to an environment in which the hydrostatic pressure is low relative to that in the deep sea.
The putative promoter sequence of the cytochrome cB gene, [−35; CTTACA]-16 bp-[−10; TAAAAT] (Fig. 4B), is similar with that of ompL of another piezophilic bacterium, P. profundum SS9, [−35; CTAACA]-18 bp-[−10; TAAAAA] (30). Expression of ompL was found to be regulated negatively at the transcription level under elevated pressure conditions (30), similar to the regulation of the cytochrome cB gene. We have also reported that the promoter region of the pressure-regulated operon from deep-sea piezophilic strain DB6705, identified as S. benthica (23), is very similar to that of the ompH gene of P. profundum SS9 (3), and both of these genes are positively regulated by elevated pressure (18). Therefore, it seems possible that such deep-sea strains may have similar RNA polymerases which recognize these promoters involved in pressure regulation.
ACKNOWLEDGMENT
We are grateful to W. R. Bellamy for assistance in editing the manuscript.
REFERENCES
- 1.Allen E E, Facciotti D, Bartlett D H. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum SS9 at high pressure and low temperature. Appl Environ Microbiol. 1999;65:1710–1720. doi: 10.1128/aem.65.4.1710-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett D, Wright M, Yayanos A A, Silverman M. Isolation of a gene regulated by hydrostatic pressure in a deep-sea bacterium. Nature. 1989;342:572–574. doi: 10.1038/342572a0. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett D H, Welch T J. ompH gene expression is regulated by multiple environmental cues in addition to high pressure in the deep-sea bacterium Photobacterium species strain SS9. J Bacteriol. 1995;177:1008–1016. doi: 10.1128/jb.177.4.1008-1016.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi E, Bartlett D H. An rpoE-like locus controls outer membrane protein synthesis and growth at cold temperatures and high pressures in the deep-sea bacterium Photobacterium sp. strain SS9. Mol Microbiol. 1995;17:713–726. doi: 10.1111/j.1365-2958.1995.mmi_17040713.x. [DOI] [PubMed] [Google Scholar]
- 5.Connely J L, Morrison M, Stotze E. Hemins of beef heart muscle. J Biol Chem. 1958;233:743–747. [PubMed] [Google Scholar]
- 6.Cowing D W, Bardwell J C A, Craig E A, Woolford C, Hendrix R W, Gross C A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci USA. 1985;82:2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenport H E, Hill R. The preparation and some properties of cytochrome f. Proc R Soc Ser B. 1952;139:327–345. doi: 10.1098/rspb.1952.0016. [DOI] [PubMed] [Google Scholar]
- 8.Drabkin D L. Spectrophotometric studies. Structural interpretation of the spectra of cyanide, pyridine, and carbon monoxide derivatives of cytochrome c and hemoglobin. J Biol Chem. 1942;146:605–617. [Google Scholar]
- 9.Edman P, Henschen A. Sequence determination. In: Needleman S B, editor. Protein sequence determination. 2nd ed. Berlin, Germany: Springer-Verlag; 1975. pp. 232–279. [Google Scholar]
- 10.Fitz-Gibbon S, Choi A J, Miller J H, Stetter K O, Simon M I, Swanson R, Kim U-J. A fosmid-based genomic map and identification of 474 genes of the hyperthermophilic archaeon Pyrobaculum aerophilum. Extremophiles. 1997;1:36–52. doi: 10.1007/s007920050013. [DOI] [PubMed] [Google Scholar]
- 11.Kato C, Bartlett D H. The molecular biology of barophilic bacteria. Extremophiles. 1997;1:111–116. doi: 10.1007/s007920050023. [DOI] [PubMed] [Google Scholar]
- 12.Kato C, Ikegami A, Smorawinska M, Usami R, Horikoshi K. Structure of genes in a pressure-regulated operon and adjacent regions from a barotolerant bacterium strain DSS12. J Mar Biotechnol. 1997;5:210–218. [Google Scholar]
- 13.Kato C, Li L, Nakamura Y, Nogi Y, Tamaoka J, Horikoshi K. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl Environ Microbiol. 1998;64:1510–1513. doi: 10.1128/aem.64.4.1510-1513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato C, Masui N, Horikoshi K. Properties of obligately barophilic bacteria isolated from a sample of deep-sea sediment from the Izu-Bonin Trench. J Mar Biotechnol. 1996;4:96–99. [Google Scholar]
- 15.Kato C, Qureshi M H. Pressure response in deep-sea piezophilic bacteria. J Mol Microbiol Biotechnol. 1999;1:87–92. [PubMed] [Google Scholar]
- 16.Kato C, Sato T, Horikoshi K. Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers Conserv. 1995;4:1–9. [Google Scholar]
- 17.Kato C, Smorawinska M, Sato T, Horikoshi K. Cloning and expression in Escherichia coli of a pressure-regulated promoter region from a barophilic bacterium, strain DB6705. J Mar Biotechnol. 1995;2:125–129. [Google Scholar]
- 18.Kato C, Smorawinska M, Sato T, Horikoshi K. Analysis of a pressure-regulated operon from the barophilic bacterium strain DB6705. Biosci Biotechnol Biochem. 1996;60:166–168. doi: 10.1271/bbb.60.166. [DOI] [PubMed] [Google Scholar]
- 19.Kato C, Tamegai H, Ikegami A, Usami R, Horikoshi K. Open reading frame 3 of the barotolerant bacterium strain DSS12 is complementary with cydD in Escherichia coli: cydD functions are required for cell stability at high pressure. J Biochem. 1996;120:301–305. doi: 10.1093/oxfordjournals.jbchem.a021413. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Moore G R, Pettigrew G W. Amino acid sequence of cytochrome c. In: Moore G R, Pettigrew G W, editors. Cytochrome c—Evolutionary, structural and physicochemical aspects. Berlin, Germany: Springer-Verlag; 1990. pp. 115–155. [Google Scholar]
- 22.Ng T C N, Laheri A, Maier R J. Cloning, sequencing, and mutagenesis of the cytochrome c4 gene from Azotobacter vinelandii; characterization of the mutant strain and proposed new branch in respiratory chain. Biochim Biophys Acta. 1995;1230:119–129. doi: 10.1016/0005-2728(95)00043-i. [DOI] [PubMed] [Google Scholar]
- 23.Nogi Y, Kato C, Horikoshi K. Taxonomic studies of deep-sea barophilic Shewanella species, and Shewanella violacea sp. nov., a new barophilic bacterial species. Arch Microbiol. 1998;170:331–338. doi: 10.1007/s002030050650. [DOI] [PubMed] [Google Scholar]
- 24.Poole R K, Gibson F, Wu G. The cydD gene product, component of a heterodimeric ABC transporter, is required for assembly of periplasmic cytochrome c and of cytochrome bd in Escherichia coli. FEMS Microbiol Lett. 1994;117:217–224. doi: 10.1111/j.1574-6968.1994.tb06768.x. [DOI] [PubMed] [Google Scholar]
- 25.Poole R K, Hatch L, Cleeter M W, Gibson F, Cox G B, Wu G. Cytochrome bd biosynthesis in Escherichia coli: the sequences of the cydC and cydD genes suggest that they encode the components of an ABC membrane transporter. Mol Microbiol. 1993;10:421–430. [PubMed] [Google Scholar]
- 26.Poole R K, Williams H D, Downie A, Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: identification and mapping of a fourth locus, cydD. J Gen Microbiol. 1989;135:1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi M H, Kato C, Horikoshi K. Purification of two pressure-regulated c-type cytochromes from a deep-sea barophilic bacterium, Shewanella sp. strain DB-172F. FEMS Microbiol Lett. 1998;161:301–309. [Google Scholar]
- 28.Qureshi M H, Kato C, Horikoshi K. Purification of a ccb-type quinol oxidase specifically induced in a deep-sea barophilic bacterium, Shewanella sp. strain DB-172F. Extremophiles. 1998;2:93–99. doi: 10.1007/s007920050047. [DOI] [PubMed] [Google Scholar]
- 29.Tamegai H, Kato C, Horikoshi K. Pressure-regulated respiratory system in barotolerant bacterium, Shewanella sp. strain DSS12. J Biochem Mol Biol Biophys. 1998;1:213–220. [Google Scholar]
- 30.Welch T J, Bartlett D H. Isolation and characterization of the structural gene for OmpL, a pressure-regulated porin-like protein from the deep-sea bacterium Photobacterium species strain SS9. J Bacteriol. 1996;178:5027–5031. doi: 10.1128/jb.178.16.5027-5031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanaka T. The biochemistry of bacterial cytochromes. Tokyo, Japan: Japan Scientific Society Press; 1992. [Google Scholar]
- 32.Yayanos A A. Microbiology to 10,500 meters in the deep sea. Annu Rev Microbiol. 1995;49:777–805. doi: 10.1146/annurev.mi.49.100195.004021. [DOI] [PubMed] [Google Scholar]
- 33.Yayanos A A, Dietz A Z, Boxtel R V. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science. 1979;205:808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]