Abstract
Objective:
To examine the gestational weight gain (GWG) trajectory and its possible association with pregnancy outcomes.
Design:
GWG trajectories were identified using the latent class growth model. Binary logistic regression was performed to examine the associations between adverse pregnancy outcomes and these trajectories.
Setting:
Negeri Sembilan, Malaysia.
Participants:
Two thousand one hundred ninety-three pregnant women.
Results:
Three GWG trajectories were identified: ‘Group 1 – slow initial GWG but followed by drastic GWG’, ‘Group 2 – maintaining rate of GWG at 0·58 kg/week’ and ‘Group 3 – maintaining rate of GWG at 0·38 kg/week’. Group 1 had higher risk of postpartum weight retention (PWR) (adjusted OR (AOR) 1·02, 95 % CI 1·01, 1·04), caesarean delivery (AOR 1·03, 95 % CI 1·01, 1·04) and having low birth weight (AOR 1·04, 95 % CI 1·02, 1·05) compared with group 3. Group 2 was at higher risk of PWR (AOR 1·18, 95 % CI 1·16, 1·21), preterm delivery (AOR 1·03, 95 % CI 1·01, 1·05) and caesarean delivery (AOR 1·02, 95 % CI 1·01, 1·03), but at lower risk of having small-for-gestational-age infants (AOR 0·97, 95 % CI 0·96, 0·99) compared with group 3. The significant associations between group 1 and PWR were observed among non-overweight/obese women; between group 1 and caesarean delivery among overweight/obese women; group 2 with preterm delivery and caesarean delivery were only found among overweight/obese women.
Conclusions:
Higher GWG as well as increasing GWG trajectories was associated with higher risk of adverse pregnancy outcomes. Promoting GWG within the recommended range should be emphasised in antenatal care to prevent the risk of adverse pregnancy outcomes.
Keywords: Gestational weight gain trajectory, Caesarean delivery, Postpartum weight retention, Preterm delivery, Low birth weight
Worldwide, about two-thirds of pregnant women had excessive rate of gestational weight gain (GWG), while about one-fifth had inadequate rate of GWG, but the numbers may differ by region and country(1). Studies in USA reported that 47–62 % of pregnant women had excessive rate of GWG, while 17–21 % with inadequate rate of GWG(2–5). Similarly, data from the Maternal and Newborn’s Health Monitoring System in China also showed that about 57·9 % of pregnant women had excessive GWG, while 12·5 % had inadequate GWG(6) and the reported mean rate of GWG in second and third trimesters amounted to 0·56 ± 0·19 kg/week. However, a cross-sectional study of pregnant women attending the Maternal and Child Health clinics for routine antenatal check-up in Malaysia found that pregnant women had an overall higher rate of GWG in second trimester (0·48 kg/week) than in the third trimester (0·40 kg/week), in underweight and normal-weight women. Overweight women had similar GWG in both trimesters, whereas obese women had lower rate of GWG in second trimester than in third trimester(7).
Inappropriate GWG may have profound short- and long-term consequences on health of both mother and infant. Inadequate GWG is associated with an increased risk of preterm delivery, fetal growth restriction, whereas excessive GWG may result in macrocosmic infants, caesarean delivery, maternal postpartum weight retention (PWR) and an increased risk for childhood obesity(6,8–13). A retrospective study on the association between the rate of GWG over different pregnancy stages (early, mid and late) and pregnancy outcomes among Korean pregnant women showed that higher or lower GWG in early and late pregnancy, but not in mid pregnancy, was associated with a risk to develop gestational diabetes mellitus, pregnancy-induced hypertension, large-for-gestational-age (LGA) infants, macrosomia and caesarean delivery(14).
Previous research on GWG mainly focused on either the total GWG or the rate of GWG in a specific trimester(6,9–11,15), since total GWG (kg) and rate of GWG (kg/week) are common indices of GWG, assuming that all women follow the same basic pattern of weight gain over pregnancy. However, there might be subgroups within the population having different patterns of weight gain(16), resulting in a comparable total GWG but through different GWG trajectories. Trajectory modelling can estimate individual trajectories, identify groups of individuals following similar progression over time, estimate group memberships (i.e. within each group, there may be variation in trajectories), as well as identify high-risk groups for targeted intervention(16–18). To date, however, there is limited research on the rate of GWG and GWG trajectories(19) and possible associations with birth outcomes(20,21). Understanding the GWG trajectories and their impact on pregnancy outcomes may contribute to the development of preventive support strategies that would benefit the health of both mother and child. Thus, the current study aims to identify GWG trajectory groups and their characteristics and determine the association with pregnancy outcomes.
Methods
Study design and population
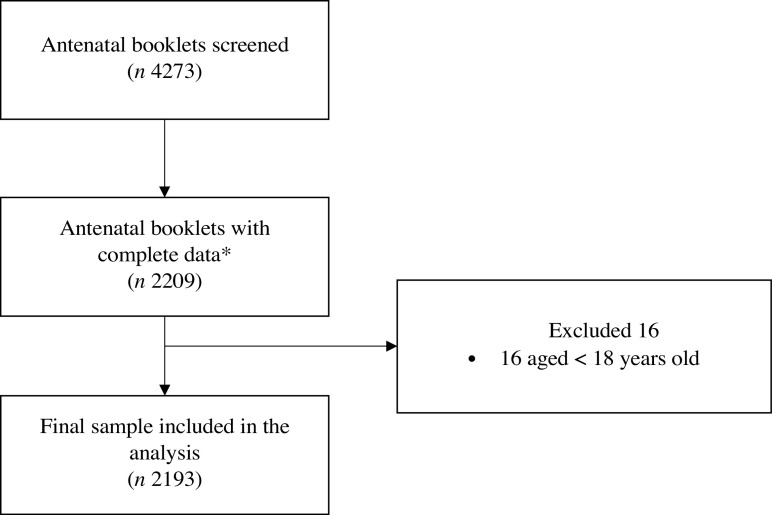
This was a retrospective cohort study of healthy, non-diabetic pregnant woman with a singleton gestation having delivered at government hospitals in Seremban district, Negeri Sembilan, Malaysia between January 2010 and December 2012. A total of 4273 antenatal clinic cards were screened, and the data of 2193 pregnant women were included in this analysis (Fig. 1).
Fig. 1.
Sampling procedure. *Complete data–complete all antenatal care visits
Data source
The source of data was antenatal clinic cards of pregnant women, which contained information on patient’s background, antenatal care, demographic characteristics, obstetric history and infant birth (e.g. gender, gestational age, length, head circumference and birth weight). Data were extracted from the antenatal clinic cards by trained enumerators.
Gestational weight gain
Height and weight at the first prenatal visit, first, second and third trimesters as well as weight at 6-week postpartum were obtained from the antenatal clinic cards. All women in the analysis had completed weight data. Height and body weight at the first prenatal visit were used to calculate early pregnancy BMI, with early pregnancy weight (kg) divided by the square of height (m2) and were further categorised into four groups: underweight (<18·50 kg/m2), normal weight (18·50–24·99 kg/m2), overweight (25·00–29·99 kg/m2) and obese (≥30·00 kg/m2)(22). Total GWG was defined as the difference between the weight measured at last prenatal visit and the weight at booking. The GWG was then classified, according to the 2009 US Institute of Medicine guidelines, as ‘inadequate’, ‘adequate’ and ‘excessive’(23). Total GWG (kg) was transformed into z-score using the published formula and GWG z-score chart(24,25). Rate of weight gain in second and third trimesters was defined as the average weekly weight gain in that trimester.
Pregnancy outcomes
Evaluated maternal outcomes were including 6-week PWR, preterm delivery and caesarean delivery. The 6-week PWR was calculated by subtracting the weight at early pregnancy from the weight at 6-week postpartum. Preterm delivery was defined as delivery before 37 weeks of gestation(26).
Infant outcomes evaluated in the current study were low birth weight (LBW), small-for-gestational-age (SGA) and LGA. LBW was defined as birth weight <2500 g(27). As there are differences in birth weight between Malaysian and European infants, the fetal growth charts for Malaysian female and male infants were used as a reference for infant’s birth weight percentile by gestational age(28). Infants with a birth weight below the 10th percentile for gestational age were considered as SGA, while those with birth weight more than the 90th percentile for gestational age were considered as LGA(27).
Statistical analysis
The statistical package STATA/SE version 15.0(29) was used to analyse the data obtained. Descriptive statistics are shown as the mean and sd for continuous variables, while frequency and percentage were used for categorical variables. Continuous data were tested for normal distribution using Kolmogorov–Smirnov tests.
Latent class trajectory analysis was used to identify the rate of GWG trajectory patterns(16). A censored normal model was used to identify the patterns in the rate of GWG trajectory(30). A two-stage model selection process was used to determine the trajectory models in relation to the number of groups and trajectory shapes (e.g. linear, quadratic, cubic)(16). The number of groups was determined with the consideration of the average of Bayesian information criteria, and the proportion of estimated trajectory groups (the smallest group includes at least 5 % of patients), as model fit statistics(31). Four models (two trajectory groups, three trajectory groups, four trajectory groups and five trajectory groups) were tested for linear, quadratic and cubic specifications for trajectory shape until the best fitting model was established. After determination of the number of groups and trajectory shapes, women were classified into rate of GWG trajectory groups based on the maximum estimated probability of belonging to each group. An average posterior probability of ≥0·70 for each group is considered as good discrimination in classifying individuals into distinctive groups(16). All groups showed sufficiently high average posterior probability of individuals belonging to each of the groups (0·80–0·90). Three trajectory groups were identified and labelled as having slow initial GWG but followed by drastic GWG (group 1), maintaining the rate of GWG at an average of 0·58 kg/week (group 2) and maintaining the rate of GWG at an average of 0·38 kg/week (group 3).
Binary logistic regression was performed to determine the associations between the rate of GWG trajectory groups and pregnancy outcomes. As maternal characteristics such as age, occupation status, gravidity and total GWG showed significant differences among the trajectory groups, these factors were entered as covariates in the multiple logistic regression. Given the possibility of an interaction effect between age, occupation status, gravidity, BMI at first prenatal visit with trajectory groups, models incorporating interaction terms were also performed. Only BMI at first prenatal visit showed significant interaction effect. The full model, which included all covariates and significant interaction term, was presented. A stratified analysis was further performed for any significant interaction term. Crude and adjusted OR with 95 % CI were presented. The significant level for all statistical analyses was set at P < 0·05.
Results
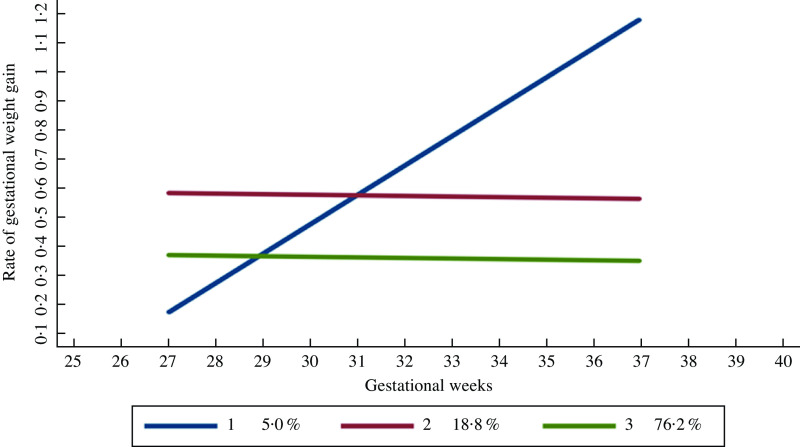
As the interval between the first prenatal visit (mean of gestational weeks of 9·26, sd 1·09 weeks) and visit 1 in the first trimester (mean of gestational weeks of 13·12, sd 1·43 weeks) was short, therefore, the rate of GWG in the first trimester was not included in the trajectory analysis. Figure 2 presents the three distinct trajectories of GWG. Group 1 showed a slow initial GWG but followed by a drastic GWG, which was identified in only 5·0 % of the total sample. Women in this trajectory group gained about 0·17 kg/week in the second trimester and had increased the rate to more than 1·00 kg/week in the third trimester. Group 2 maintained an average of 0·58 kg/week, and it presented 18·8 % of the total sample. Group 3 maintained an average rate of GWG of 0·38 kg/week and comprised 76·2 % of the total sample.
Fig. 2.
Group 1 – slow initial gestational weight gain (GWG) but followed by drastic GWG. Group 2 – maintaining at an average rate of GWG of 0·58 kg/week. Group 3 – maintaining at an average rate of GWG of 0·38 kg/week
Table 1 summarises the characteristics of the cohort. Socio-demographic, obstetrical information, anthropometric measurements and pregnancy outcomes of the women were comparable between the trajectory groups. Overall, most women were Malay (83·7 %), had completed at least secondary education (60·1 %) and were employed (60·7 %). Women in group 3 were significantly older (29·34 (sd 4·54) years) and higher gravidity (2·77 (sd 1·57)) than those in group 2 (age 28·52 (sd 3·94) years; gravidity 2·38 (sd 1·26)). Women in group 2 were slightly taller but had a lower weight at the first prenatal visit (gestational weeks of 9·09 (sd 1·94)) and thus had a lower BMI than the two other groups. Women in group 1 had the highest weight at the first prenatal visit (65·12 (sd 17·59) kg) with a mean BMI at the first prenatal visit within the overweight range (25·00–29·99 kg/m2). Women in group 2 had the highest total GWG, with about 45·2 % had excessive GWG, and the highest proportion of women with substantial weight retention of more than 5 kg after 6-week postpartum (94·9 %) (45·53 (sd 1·61) weeks). For pregnancy outcomes, there were significant differences between groups for all outcome variables except for infant’s birth length, head circumference and birth weight percentile. Women in group 1 had significantly higher rates of preterm delivery (15·5 %) compared with the other groups. Women in group 2 had infants with significantly higher mean birth weight (3·08 (sd 0·47) kg) than women in group 1 (2·87 (sd 0·53) kg) and group 3 (3·03 (sd 0·45) kg). Women in group 1 had the highest percentage of SGA (37·3 %) and LGA infants (9·1 %) compared with those in group 2 (SGA = 32·5 %; LGA = 8·7 %) and group 3 (SGA = 33·2 %; LGA = 7·7 %).
Table 1.
Characteristics of women by trajectory groups (n = 2193)
| Characteristics | Total | Trajectory groups† | P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (5·0 %) | Group 2 (18·8 %) | Group 3 (76·2 %) | |||||||
| n | % | n | % | n | % | n | % | ||
| Age at study entry (years) | 0·001**c | ||||||||
| Mean | 29·15 | 28·55 | 28·52 | 29·34 | |||||
| sd | 4·45 | 4·57 | 3·94 | 4·54 | |||||
| <30 | 1329 | 60·6 | 78 | 70·9 | 274 | 66·5 | 977 | 58·5 | 0·001** |
| ≥30 | 864 | 39·4 | 32 | 29·1 | 138 | 33·5 | 694 | 41·5 | |
| Ethnicity | |||||||||
| Malay | 1835 | 83·7 | 93 | 84·5 | 345 | 83·7 | 1397 | 83·6 | 0·92 |
| Chinese | 102 | 4·7 | 4 | 3·6 | 22 | 5·3 | 76 | 4·5 | |
| Indian and others | 256 | 11·6 | 13 | 11·9 | 45 | 11·0 | 198 | 11·9 | |
| Education level | |||||||||
| Secondary and lower | 1318 | 60·1 | 73 | 66·4 | 230 | 55·8 | 1015 | 60·7 | 0·20 |
| STPM/matric/diploma/certificate | 580 | 26·4 | 24 | 21·8 | 122 | 29·6 | 434 | 26·0 | |
| Tertiary and above | 295 | 13·5 | 13 | 11·8 | 60 | 14·6 | 222 | 13·3 | |
| Occupation | |||||||||
| Housewife | 861 | 39·3 | 47 | 42·7 | 135 | 32·8 | 679 | 40·6 | 0·01* |
| Working | 1332 | 60·7 | 63 | 57·3 | 277 | 67·2 | 992 | 59·4 | |
| Obstetrical information | |||||||||
| Gravidity | 0·001**bc | ||||||||
| Mean | 2·67 | 2·31 | 2·38 | 2·77 | |||||
| sd | 1·52 | 1·33 | 1·26 | 1·57 | |||||
| 1 | 546 | 24·9 | 35 | 31·8 | 128 | 31·1 | 383 | 22·9 | 0·001** |
| 2 | 531 | 24·2 | 34 | 30·9 | 104 | 25·2 | 393 | 23·5 | |
| ≥3 | 1116 | 50·9 | 41 | 37·3 | 180 | 43·7 | 895 | 53·6 | |
| Anthropometric measurements | |||||||||
| Height (m) | 0·02*ac | ||||||||
| Mean | 1·56 | 1·56 | 1·57 | 1·56 | |||||
| sd | 0·06 | 0·06 | 0·05 | 0·06 | |||||
| Weight at first prenatal visit (kg) | 0·001**c | ||||||||
| Mean | 60·69 | 65·12 | 57·84 | 61·11 | |||||
| sd | 14·12 | 17·59 | 13·02 | 14·02 | |||||
| BMI at first prenatal visit (kg/m2) | 0·001**abc | ||||||||
| Mean | 24·91 | 26·53 | 23·54 | 25·15 | |||||
| sd | 5·54 | 6·56 | 5·16 | 5·51 | |||||
| Underweight (<18·5) | 222 | 10·2 | 7 | 6·4 | 62 | 15·0 | 153 | 9·2 | 0·001**abc |
| Normal (18·5–24·9) | 995 | 45·4 | 50 | 45·5 | 219 | 53·2 | 726 | 43·4 | |
| Overweight (25·0–29·9) | 629 | 28·7 | 27 | 24·5 | 94 | 22·8 | 508 | 30·4 | |
| Obese (≥30·0) | 347 | 15·8 | 26 | 23·6 | 37 | 9·0 | 284 | 17·0 | |
| Total GWG‡ | 0·001**abc | ||||||||
| Mean | 9·96 | 11·00 | 14·59 | 8·75 | |||||
| sd | 4·50 | 6·11 | 4·08 | 3·63 | |||||
| Inadequate | 998 | 45·5 | 40 | 36·4 | 54 | 13·1 | 904 | 54·1 | 0·001** |
| Adequate | 787 | 35·9 | 38 | 34·5 | 172 | 41·7 | 577 | 34·5 | |
| Excessive | 408 | 18·6 | 32 | 29·1 | 186 | 45·2 | 190 | 11·4 | |
| Weight at 6-week postpartum (kg) | 64·42 | 13·93 | 69·06 | 16·80 | 66·65 | 13·27 | 63·67 | 13·76 | 0·001**bc |
| Postpartum weight retention (kg) | 3·73 | 0·10 | 3·94 | 0·48 | 8·81 | 0·16 | 2·46 | 4·30 | 0·001**abc |
| <5 | 1308 | 59·6 | 59 | 53·6 | 21 | 5·1 | 1228 | 73·5 | 0·001** |
| ≥5 | 885 | 40·4 | 51 | 46·4 | 391 | 94·9 | 443 | 26·5 | |
| Pregnancy outcomes | |||||||||
| Gestational age at delivery (weeks) | 0·001**ab | ||||||||
| Mean | 38·50 | 38·01 | 38·56 | 38·50 | |||||
| sd | 1·63 | 1·81 | 1·63 | 1·63 | |||||
| Preterm (<37) | 172 | 7·8 | 17 | 15·5 | 33 | 8·0 | 122 | 7·3 | 0·01** |
| Full-term (≥37) | 2021 | 92·2 | 93 | 84·5 | 379 | 92·0 | 1549 | 92·7 | |
| Mode of delivery | |||||||||
| Others | 1760 | 80·3 | 78 | 70·9 | 319 | 77·4 | 1363 | 81·6 | 0·01** |
| Caesarean section | 433 | 19·7 | 32 | 29·1 | 93 | 22·6 | 308 | 18·4 | |
| Infant’s length (cm) | 0·10 | ||||||||
| Mean | 49·10 | 48·57 | 49·43 | 49·05 | |||||
| sd | 2·53 | 2·80 | 2·38 | 2·55 | |||||
| Infant’s head circumference (cm) | 0·56 | ||||||||
| Mean | 32·90 | 32·87 | 33·00 | 32·88 | |||||
| sd | 1·97 | 2·37 | 1·83 | 1·97 | |||||
| Infant’s birth weight (kg) | 0·001**ab | ||||||||
| Mean | 3·03 | 2·87 | 3·08 | 3·03 | |||||
| sd | 0·46 | 0·53 | 0·47 | 0·45 | |||||
| <2·5 (low birth weight) | 216 | 9·8 | 24 | 21·8 | 35 | 8·5 | 157 | 9·4 | 0·01** |
| ≥2·5 | 1977 | 90·2 | 86 | 78·2 | 377 | 91·5 | 1514 | 90·6 | |
| Birth weight percentile§ | |||||||||
| SGA (<10) | 729 | 33·2 | 41 | 37·3 | 134 | 32·5 | 554 | 33·2 | 0·80 |
| AGA (10–90) | 1288 | 58·7 | 59 | 53·6 | 242 | 58·8 | 987 | 59·1 | |
| LGA (>90) | 176 | 8·1 | 10 | 9·1 | 36 | 8·7 | 130 | 7·7 | |
STPM, Malaysian Higher School Certificate; GWG, gestational weight gain; SGA, small-for-gestational-age; AGA, appropriate for gestational-age; LGA, large-for-gestational-age.
Mean values with unlike superscript letters were significantly different from each other: aGroup 1 v. group 2; bGroup 1 v. group 3; cGroup 2 v. group 3.
*P < <p 3. chP < 0·001.
Group 1 – slow initial gestational weight gain (GWG) but followed by drastic GWG; Group 2 – maintaining the rate of GWG at 0.58 kg/week; Group 3 – maintaining the rate of GWG at 0.38 kg/week.
Institute of Medicine, 2009.
Fisher’s exact test.
Table 2 shows the OR of having adverse pregnancy outcomes for each group with group 3, that is, ‘maintained at a lower rate of GWG’ used as the reference group. Women in group 1 were at significantly higher risk for 6-week PWR of more than 5 kg (AOR 1·02, 95 % CI 1·01, 1·04), caesarean delivery (AOR 1·03, 95 % CI 1·01, 1·04) and LBW infants (AOR 1·04, 95 % CI 1·02, 1·06) than women in group 3. Women in group 2 had significantly higher risk for 6-week PWR of more than 5 kg (AOR 1·18, 95 % CI 1·16, 1·21), preterm delivery (AOR 1·03, 95 % CI 1·02, 1·05) and caesarean delivery (AOR 1·02, 95 % CI 1·01, 1·03) but had a lower risk for SGA (AOR 0·97, 95 % CI 0·96, 0·99) than women in group 3. Only BMI at first prenatal showed significant interaction effect between the trajectory groups with pregnancy outcomes.
Table 2.
Crude and adjusted OR and 95 % CI for associations between trajectory groups and pregnancy outcomes
| Trajectory groups† | Maternal outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-week PWR ≥ 5 kg | Preterm delivery (<37 weeks) | Caesarean delivery | ||||||||||
| Crude OR | 95 % CI | AOR | 95 % CI | Crude OR | 95 % CI | AOR | 95 % CI | Crude OR | 95 % CI | AOR | 95 % CI | |
| Group 1 | 1·68 | 1·14, 2·48* | 1·02 | 1·01, 1·04** | 1·58 | 1·00, 2·52 | 1·01 | 0·98, 1·02 | 1·79 | 1·17, 2·75** | 1·03 | 1·01, 1·04** |
| Group 2 | 18·81 | 11·88, 29·78** | 1·18 | 1·16, 1·21** | 2·41 | 1·40, 4·17** | 1·03 | 1·02, 1·05* | 1·42 | 1·03, 1·97* | 1·02 | 1·01, 1·03** |
| Group 3 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | ||||||
PWR, postpartum weight retention; SGA, small-for-gestational-age; LGA, large-for-gestational-age; AOR, adjusted OR; GWG, gestational weight gain.
*P < 0·05, **P < 0·001.
Group 1 – slow initial gestational weight gain (GWG) but followed by drastic GWG; Group 2 – maintaining the rate of GWG at 0.58 kg/week; Group 3 – maintaining the rate of GWG at 0.38 kg/week.
Full model, which included covariates (maternal age, occupation status, gravidity and total GWG z-score) and significant interaction term (pre-pregnancy BMI).
Table 3 shows the associations between trajectory groups and pregnancy outcomes stratified by BMI categories. Non-overweight/obese women with increasing rate of GWG (group 1) had higher risk for 6-week PWR of more than 5 kg (AOR 3·52, 95 % CI 2·01, 6·16). Meanwhile, overweight/obese women who maintained their rate of GWG at 0·58 kg/week were at significantly higher risk for preterm delivery (AOR 2·87, 95 % CI 1·36, 6·07) and caesarean delivery (AOR 2·04, 95 % CI 1·14, 3·68). For women in group 2, overweight/obese women had higher risk for 6-week PWR of more than 5 kg (AOR 42·39, 95 % CI 23·85, 75·34) than non-overweight/obese women (AOR 59·92, 95 % CI 28·68, 70·21). Similarly, in group 1, overweight/obese women had higher risk for having LBW infants (AOR 3·24, 95 % CI 1·64, 6·40) as compared with non-overweight/obese women (AOR 2·14, 95 % CI 1·06, 4·31).
Table 3.
Adjusted OR and 95 % CI for associations between trajectory groups and pregnancy outcomes stratified by overweight/obesity†
| Maternal outcomes | Infant outcomes | |||||||
|---|---|---|---|---|---|---|---|---|
| 6-week PWR ≥5 kg | Preterm delivery (<37 weeks) | Caesarean delivery | Low birth weight (kg) | |||||
| AOR | 95 % CI | AOR | 95 % CI | AOR | 95 % CI | AOR | 95 % CI | |
| Non-overweight/obese (n = 1217) | ||||||||
| Group 1‡ | 3·52 | 2·01, 6·16** | 1·98 | 0·86, 4·59 | 1·61 | 0·80, 3·23 | 2·14 | 1·06, 4·31* |
| Group 2‡ | 42·39 | 23·85, 75·34** | 1·19 | 0·71, 1·98 | 1·36 | 0·95, 1·95 | 0·98 | 0·59, 1·48 |
| Group 3‡ | 1·00 | 1·00 | 1·00 | 1·00 | ||||
| Overweight/obese (n = 976) | ||||||||
| Group 1‡ | 1·56 | 0·83, 2·92 | 1·09 | 0·56, 2·15 | 2·04 | 1·14, 3·68* | 3·24 | 1·64, 6·40** |
| Group 2‡ | 59·92 | 28·68, 70·21** | 2·87 | 1·36, 6·07** | 1·60 | 1·06, 2·42* | 1·23 | 0·36, 1·51 |
| Group 3‡ | 1·00 | 1·00 | 1·00 | 1·00 | ||||
PWR, postpartum weight retention; AOR, adjusted OR.
Non-overweight/obese: BMI < 25·00 kg/m2; Overweight/obese: BMI ≥ 25·00 kg/m2.
Adjusted for maternal age, occupation status, gravidity and total GWG z-score: *P < 0·05, **P < 0·001.
Group 1 – slow initial gestational weight gain (GWG) but followed by drastic GWG; Group 2 – maintaining the rate of GWG at 0.58 kg/week; Group 3 – maintaining the rate of GWG at 0.38 kg/week.
Discussion
In the current study, three distinct trajectories of GWG were identified using the growth mixture modelling approach and these included group 1 – slow initial GWG but followed by drastic GWG (5·0 %), group 2 – maintained the rate of GWG at an average of 0·58 kg/week (18·8 %) and group 3 – maintained the rate of GWG at an average of 0·38 kg/week (76·2 %). Women in group 3 (maintained the rate of GWG at an average of 0·38 kg/week) were considered as having desirable rate of GWG as based on the Institute of Medicine GWG guidelines, and the recommended rate of GWG was 0·23–0·45 kg/week depending on the pre-pregnancy BMI categories. Therefore, this group was used as the reference group throughout the analysis. About 23·8 % had either slow initial GWG but followed by drastic GWG (group 1) or maintained the rate of GWG at an average of 0·58 kg/week (group 2). These women should be the target for intervention as they are at higher risk for adverse outcomes in the subsequent pregnancies.
In the current study, women with a slow initial GWG but a later drastic GWG (group 1) were considered as having excessive GWG, since the mean rate of GWG increased from 0·17 kg/week in the second trimester to 1·00 kg/week in the third trimester. A higher proportion of women in this group were in the age group <30 years old (70·9 %), housewives (42·7 %), first time mothers (31·8 %) and had secondary education level (66·4 %). In addition, these women had a higher mean BMI at first prenatal visit with slightly less than half (48·1 %) were overweight (24·5 %) or obese (23·6 %). Previous studies have also shown that overweight women were at higher risk for excessive GWG(23,32,33). It is plausible that mothers who were less educated have poor knowledge of GWG recommendations, consequences of having inappropriate GWG, as well as strategies to achieve healthy weight gain in pregnancy(34,35). Studies have consistently reported that younger mothers were at greater risk of excessive GWG, whereas older mothers may be more disciplined regarding lifestyle choices and thus tend to gain less weight(36–38). Heery et al.(39) found that women in their first pregnancy were not particularly concerned about weight gain during pregnancy, and most of them perceived that big babies are healthy and that a higher GWG is beneficial for fetal development. The study findings suggest that nutrition education, particularly among high-risk groups (e.g. younger age mothers, housewife, lower education, as well as overweight/obese women), to prevent excessive GWG should start as early as possible ideally before pregnancy as to achieve optimal GWG and subsequent healthy pregnancy outcomes. A close monitoring of GWG over the course of pregnancy is also essential to identify mothers with inadequate or excessive rate of GWG in specific trimesters for further nutrition counselling by nutritionists at the health clinics.
For women in group 2 (maintained a higher rate of GWG (0·58 kg/week)), they had a higher education level (tertiary education) than women in group 1 and group 3. Several studies in the Western countries reported that women with higher education were associated with lower risk for excessive GWG(40,41). The inconsistent finding might be due to the variation in food choices, cooking methods and fast-food v. home cooking between populations of developed and developing countries(42,43). In developed countries, highly educated pregnant women are more likely to comply with healthy lifestyle (e.g. choose low-fat, high-nutrient lean protein sources; use healthier cooking methods, such as grilling and lower-temperature cooking; prefer home-cooked food)(35). However, in the developing countries, generally individuals with higher education level have greater purchasing power and thus could afford processed foods, fast foods and eating outside the home on a more regular basis(44). Consequently, they are at a higher risk for being overweight and obese. Previous studies have also showed that most people with higher education were white-collar workers and tend to have higher sedentariness, whereby spending most of their daily time seated in front of computers(45,46). Women with higher sedentary behaviour tend to have excessive GWG(47). In the current study, as 85·8 % of women with at least tertiary education level were employed, these women could have higher total energy diet as well as higher sedentary behaviour due to occupational sitting.
Women in group 3 (maintained the rate of GWG at an average of 0·38 kg/week) were significantly older and had higher gravidity compared with women in group 2. Ebrahimi et al.(48) reported that older women (30–35 years old) were more likely to have adequate GWG than inadequate GWG. As the majority of women (77·1 %) in this cohort were not first-time mothers, it is plausible that these women had accumulated knowledge and experience of GWG from previous pregnancies and births which could help in achieving the recommended weight gain in next pregnancy(49). Despite being considered as having desirable rate of GWG throughout pregnancy, women in group 3 too had the highest percentage of inadequate total GWG (54 %) compared with group 1 and group 2. This might be due to the actual time of these women gained their weight. GWG trajectory was derived based on the rate of GWG, which was defined as an average weight gain per week. It is possible that women in this group might have gained more weight during the second trimester but less in the third trimester. Thus, although they seemed to maintain the rate of GWG in both trimesters, their total GWG could be less than the recommended range.
The current study found that women in group 1 had higher risk of caesarean delivery and LBW compared with women in group 3. Further analysis of women in group 1 showed that there was no significant association between caesarean delivery with LBW (χ2 = 0·31, P = 0·58), LGA (χ2 = 0·02, P = 0·89) or preterm delivery (χ2 = 0·11, P = 0·74), respectively. Thus, it is believed that the reason for caesarean delivery in the current study was not related to infant’s birth weight or medical condition of mother or infant as compared with other reasons. Women with previous history of caesarean delivery had a 50 % increased risk of caesarean delivery in their subsequent pregnancy(50). Festin et al.(51) also showed that the most common reason for caesarean delivery in the Southeast Asian countries including Malaysia was previous history of caesarean delivery. Besides, women in this group were also at a higher risk of having LBW infants. Previous studies showed that women with inadequate GWG in early pregnancy tended to have infants with LBW(23,52,53). Women in this trajectory group had the lowest rate of GWG at second trimester but the highest rate of GWG at third trimester. The study findings related to group 1, however, should be interpreted with caution due to the relatively small sample (n = 110) and the wide variation in the rate of GWG.
In the current study, women who maintained the rate of GWG of 0·58 kg/week (group 2) had significantly higher risk for 6-week PWR compared with women who maintained the rate of GWG at an average of 0·38 kg/week (group 3). It is possible that women with higher rate of GWG have higher possibility of storing greater fat, and thus higher PWR. This finding is consistent with previous studies that reported women with the rate of GWG above recommendation had significantly higher PWR than women with inadequate or adequate rate of GWG(54,55). Failure to attain early pregnancy weight at 6-week postpartum could be explained by the patterns of weight loss in the early postpartum. Lawrence et al.(56) reported that fat stores in the early postpartum period are lost at the rate of 0·25 kg/week. Thus, the longer estimated time for weight loss may be more typical in the postpartum period(57). The key factor that influences the amount of weight retention at 6-week postpartum is the amount of weight gain during pregnancy. For example, a woman with a normal pre-pregnancy BMI and an optimal gestational weight gain of 13·75 kg is expected to retain about 4·75 kg after delivery due to the products of conception (5 kg) and early fluid loss and tissue reductions (4 kg) during the first 2 weeks of postpartum. The remaining weight (4·75 kg) is mainly attributed to fat stores(58), and it is estimated to take about 19 weeks to lose 4·75 kg based on the rate of 0·25 kg/week of fat loss. Thus, women in group 2 might require more time to get back to their pre-pregnancy weight.
The overweight/obesity-stratified analysis in the current study showed that women in group 1 had higher risk of PWR compared with women in group 3, and this association was only observed among non-overweight/obese women. Previous studies showed that women with normal pre-pregnancy BMI and had excessive weight gain during pregnancy were more likely to retain more weight(59,60). Meanwhile, the significant association between group 1 and caesarean delivery was only found among overweight/obese women. Similarly, the significant associations between group 2 and preterm delivery and caesarean delivery were also found among overweight/obese women. These findings further support the existing findings in that overweight and obese women were at higher risk of caesarean delivery(61) and preterm delivery(62).
The current study observed that while low birth weight in group 2 (8·5 %) and group 3 (9·4 %) are much lower than that of group 1 (21·8 %), and the prevalence of SGA appears to be quite high in all groups. Women in group 1 had lower mean of gestational age at delivery and higher percentage of preterm delivery compared with women in groups 2 and 3. In addition, women in group 1 had higher percentage of LGA (9·1 %) and caesarean delivery (29·1 %) as compared with women in group 2 (8·7 %; 22·6 %) and group 3 (7·7 %; 18·4 %). This finding revealed that the high proportion of infants in group 1 seems to have LBW, but they were not SGA infants, yet possibly LGA infants. As the current study did not assess the reasons for preterm delivery and caesarean delivery, the underlying factors for this observation are not completely understood. Thus, more studies should be carried out to investigate the reason behind this observation.
The total GWG and rate of GWG have been conventionally used to inform the risk of adverse pregnancy outcomes. These indices of GWG assume all women follow the same basic pattern of weight gain over pregnancy. In the current study, three distinct trajectories of GWG were identified using the latent class growth model and women with inappropriate GWG such as group 1 and group 2 were at significantly higher risk for adverse outcomes, such as PWR, caesarean delivery and preterm delivery. This finding supports the findings from previous studies on GWG trajectory in that GWG patterns during pregnancy are important, and poor GWG trajectory was associated with adverse birth outcomes. Thus, it is very important to closely monitor the women’s weight throughout pregnancy as it could enable the early identification of a poor GWG trajectory that could serve as a prompt for medical officers to refer nutritionists/dietitians to counsel on healthy eating to achieve optimal GWG. It is also suggested that the GWG recommendations on a weekly or monthly basis may be more helpful than the total GWG recommendations.
This is the first study to report on the rate of GWG and GWG trajectories in Malaysia among a large sample of pregnant women. However, several limitations are worth to be mentioned. An important limitation is that the current study was retrospective in nature, which may be influenced by the selection bias and unmeasured confounding factors (i.e. dietary intake, physical activity and household income) as well as the possibility of missing data. The generalisability of the study findings may be limited due to the recruitment of study population (83·7 % Malays) and the selection of the study districts. Therefore, the application of these study findings to other ethnic groups should be interpreted with caution. Besides, the study findings (e.g. significant associations) should be interpreted with caution as the statistical significance of the results may be attributed to the large sample size. The postpartum period examined in the current study was relatively short (i.e. 6-week postpartum), and women might have had insufficient time to lose weight following delivery. Furthermore, it should also be noted that latent trajectories are not directly observed clusters, but groups were constructed based on the pattern responses over a fixed number of observation periods. Thus, this method does not predict the development of excessive GWG in an individual, nor produce overall population prevalence data, instead trajectories are derived by assigning each woman a probability of membership based on their weight during pregnancy. The use of the GWG z-score charts from previous studies to estimate GWG z-score might yield overestimated values. Although adjustment for some covariates was performed, some unmeasured variables (i.e. interpregnancy weight gain, history of caesarean delivery and history of preterm delivery) may have influenced the derived trajectories in the current study. Hence, further studies should consider the contribution of these predictors to the derivation of the GWG trajectories. Despite these limitations, the data from the current study were adequate to examine the associations between the rate of GWG trajectories and pregnancy outcomes.
Conclusion
Most of the pregnant women in the current study maintained the rate of GWG at an average of 0·38 kg/week for second and third trimesters. Women with a slow initial GWG but followed by a drastic GWG or with maintained rate of GWG at an average of 0·58 kg/week had higher risk of adverse pregnancy outcomes, such as higher risk for caesarean delivery, preterm delivery, LBW and PWR. Women with maintained rate of GWG at an average of 0·58 kg/week had lower risk of having SGA infants. Lack of knowledge on GWG is considered a barrier to improve pregnancy outcomes. To achieve optimal maternal and infant outcomes, women need to be advised as early as possible, even before pregnancy, regarding recommended GWG and potential risks of insufficient and excess GWG.
Acknowledgements
Acknowledgements: The authors would like to thank all nurses and staff in Maternal and Child Health clinics in Seremban district, Negeri Sembilan for their support and assistance. Financial support: The current study was supported by a research grant from Danone Dumex (Malaysia) Shd. Bhd. Conflict of interest: As the nature of the current study is more of exploratory and does not involve any testing of company product, the funder was not involved in the design of the study protocol. J.B. and E.v.d.B. are employees of Danone Nutricia Research and Yvonne Yee Siang Tee of Danone Dumex Malaysia. The authors declare that they have no conflict of interest. Authorship: Z.M.S. and Y.H.Y. conceptualised and designed the study. Y.H.Y. collected the data, analysed the data and drafted the manuscript. Z.M.S., Z.R. and B.N.M.Y. contributed to the development of study protocol, read and approved the manuscript. G.A. contributed to data analysis and data interpretation. E.v.d.B., J.B., G.A. and Y.Y.S.T. read and approved the manuscript. All authors read and approved the final version of the manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Medical Research Ethics Committee, Universiti Putra Malaysia (UPM/FPSK/100–9/2-MJKEtika) and the Medical Research Ethics Committee, Ministry of Health Malaysia (KKM/NIHSEC/08/0804/P12–613). Written informed consent was not required due to the retrospective design of the study and because all participants were anonymised before data analysis.
Reference
- 1.Goldstein RF, Abell SK, Ranasinha S et al. (2018) Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med 16, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durie DE, Thornburg LL & Glantz JC (2011) Effect of second-trimester and third-trimester rate of gestational weight gain on maternal and neonatal outcomes. Obstet Gynecol 118, 569–575. [DOI] [PubMed] [Google Scholar]
- 3.Deputy NP, Sharma AJ & Kim SY (2015) Gestational weight gain: United States, 2012 and 2013. Morb Mortal Wkly Rep 64, 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza Dr De (2016) Pregnancy risk assessment monitoring system (PRAMS). IOSR J Econ Financ 3, 75. [Google Scholar]
- 5.Walter JR, Perng W, Kleinman KP et al. (2015) Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol 212, 499.e1–499.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang A, Ji Z, Zhao W et al. (2016) Rate of gestational weight gain and preterm birth in relation to prepregnancy body mass indices and trimester: a follow-up study in China. Reprod Health 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong HY, Mohd Shariff Z, Koo SJ et al. (2016) Pre-pregnancy body mass index, height and physical activity are associated with rate of gestational weight gain among Malaysian mothers. J Obstet Gynaecol Res 42, 1094–1101. [DOI] [PubMed] [Google Scholar]
- 8.Han Z, Lutsiv O, Mulla S et al. (2011) Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 90, 935–954. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Han F, Gao X et al. (2017) Relationship between gestational weight gain and pregnancy complications or delivery outcome. Sci Rep 7, 12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong K, Yu K, Han X et al. (2015) Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr 18, 2172–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein RFF, Abell SKK, Ranasinha S et al. (2017) Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317, 2207–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramon-Arbues E, Martinez Abadia B & Martin Gomez S (2017) Gestational weight gain and postpartum weight retention in a cohort of women in Aragon, Spain. Nutr Hosp 34, 1138–1145. [DOI] [PubMed] [Google Scholar]
- 13.He X, Hu C, Chen L et al. (2014) The association between gestational weight gain and substantial weight retention 1-year postpartum. Arch Gynecol Obstet 290, 493–499. [DOI] [PubMed] [Google Scholar]
- 14.Cho EH, Hur J & Lee KJ (2015) Early gestational weight gain rate and adverse pregnancy outcomes in Korean women. PLoS One 10, e0140376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Lutsiv O, Mulla S et al. (2011) Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 90, 935–954. [DOI] [PubMed] [Google Scholar]
- 16.Nagin DS, Jones BL, Passos VL et al. (2018) Group-based multi-trajectory modeling. Stat Methods Med Res 27, 2015–2023. [DOI] [PubMed] [Google Scholar]
- 17.Tu YK, Tilling K, Sterne JAC et al. (2013) A critical evaluation of statistical approaches to examining the role of growth trajectories in the developmental origins of health and disease. Int J Epidemiol 42, 1327–1339. [DOI] [PubMed] [Google Scholar]
- 18.Song M (2019) Trajectory analysis in obesity epidemiology: a promising life course approach. Curr Opin Endocr Metab Res 4, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Che M, Kong L, Bell RC et al. (2017) Trajectory modeling of gestational weight: a functional principal component analysis approach. PLoS One 12, e0186761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugh SJ, Albert PS, Kim S et al. (2017) Patterns of gestational weight gain and birthweight outcomes in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies–Singletons: a prospective study. Am J Obstet Gynecol 217, 346.e1–346.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W, Huang W, Zhang Z et al. (2019) Patterns of gestational weight gain in women with overweight or obesity and risk of large for gestational age. Obes Facts 12, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (1995) Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee [World Health Organization, editor]. Geneva: WHO Technical Report Series No. 854. [PubMed] [Google Scholar]
- 23.Institute of Medicine (2009) Committee to reexamine IOM pregnancy weight guidelines. In Weight Gain During Pregnancy: Reexamining the Guidelines [KM Rasmussen and Yaktine AL, editors]. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 24.Hutcheon JA, Platt RW, Abrams B et al. (2013) A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr 97, 1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheon JA, Platt RW, Abrams B et al. (2015) Pregnancy weight gain charts for obese and overweight women. Obesity 23, 532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowe LP, Metzger BE, Dyer AR et al. (2012) Hyperglycemia and adverse pregnancy outcome (HAPO) study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 35, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization (2010) International Statistical Classification of Diseases and Related Health Problems: Instruction Manual, Vol. 2. Geneva: World Health Organization. [Google Scholar]
- 28.Ministry of Health Malaysia (2013) Perinatal Care Manual, 3rd ed. Putrajaya, Malaysia: Division of Family Health Development, MOH. [Google Scholar]
- 29.StataCorp (2017) Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- 30.Jones BL & Nagin DS (2013) A note on a Stata Plugin for estimating group-based trajectory models. Sociol Methods Res 42, 608–613. [Google Scholar]
- 31.Lemon SC, Roy J, Clark MA et al. (2003) Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med 26, 172–181. [DOI] [PubMed] [Google Scholar]
- 32.Samura T, Steer J, Daniela Michelis L et al. (2016) Factors associated with excessive gestational weight gain: review of current literature. Glob Adv Heal Med 5, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yong HY, Mohd Shariff Z, Koo SJ et al. (2016) Pre-pregnancy body mass index, height and physical activity are associated with rate of gestational weight gain among Malaysian mothers. J Obstet Gynaecol Res 42, 1094–1101. [DOI] [PubMed] [Google Scholar]
- 34.Deputy N, Sharma A, Kim S et al. (2016) Prevalence and characteristics associated with gestational weight gain adequacy. Obs Gynecol 125, 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen AK, Kazi C, Headen I et al. (2016) Educational attainment and gestational weight gain among U.S. mothers. Women’s Heal Issues 26, 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restall A, Taylor RS, Thompson JMDD et al. (2014) Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. J Obes 2014, 148391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaillard R, Durmuş B, Hofman A et al. (2013) Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 21, 1046–1055. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues PL, Costa de Oliveira L, Santos Brito dos A et al. (2010) Determinant factors of insufficient and excessive gestational weight gain and maternal-child adverse outcomes. Nutrition 26, 617–623. [DOI] [PubMed] [Google Scholar]
- 39.Heery E, McConnon Á, Kelleher CC et al. (2013) Perspectives on weight gain and lifestyle practices during pregnancy among women with a history of macrosomia: a qualitative study in the Republic of Ireland. BMC Pregnancy Childbirth 13, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marmitt LP, Gonçalves CV & Cesar JA (2016) Healthy gestational weight gain prevalence and associated risk factors: a population-based study in the far South of Brazil. Rev Nutr 29, 445–455. [Google Scholar]
- 41.Holowko N, Mishra G & Koupil I (2014) Social inequality in excessive gestational weight gain. Int J Obes 38, 91–96. [DOI] [PubMed] [Google Scholar]
- 42.Zienczuk N & Egeland GM (2012) Association between socioeconomic status and overweight and obesity among Inuit adults: International Polar Year Inuit Health Survey, 2007–2008. Int J Circumpolar Health 71, 18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiffin R & Salois M (2012) Inequalities in diet and nutrition. Proc Nutr Soc 71, 105–111. [DOI] [PubMed] [Google Scholar]
- 44.Pingali P (2007) Westernization of Asian diets and the transformation of food systems: implications for research and policy. Food Policy 32, 281–298. [Google Scholar]
- 45.Smith L, McCourt O, Sawyer A et al. (2016) A review of occupational physical activity and sedentary behaviour correlates. Occup Med (Chic. Ill) 66, 185–192. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Hipp JA, Lee JA et al. (2017) Work-related correlates of occupational sitting in a diverse sample of employees in Midwest metropolitan cities. Prev Med Rep 6, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, Qian X, Li M et al. (2012) Can physical activity reduce excessive gestational weight gain? Findings from a Chinese urban pregnant women cohort study. Int J Behav Nutr Phy 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebrahimi F, Shariff ZM, Tabatabaei SZ et al. (2015) Relationship between sociodemographics, dietary intake, and physical activity with gestational weight gain among pregnant women in Rafsanjan city, Iran. J Heal Popul Nutr 33, 168–176. [PMC free article] [PubMed] [Google Scholar]
- 49.Nikolopoulos H, Mayan M, MacIsaac J et al. (2017) Women’s perceptions of discussions about gestational weight gain with health care providers during pregnancy and postpartum: a qualitative study. BMC Pregnancy Childbirth 17, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber EL, Lundsberg LS, Belanger K et al. (2011) Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol 118, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Festin MR, Laopaiboon M, Pattanittum P et al. (2009) Caesarean section in four South East Asian countries: reasons for, rates, associated care practices and health outcomes. BMC Pregnancy Childbirth 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flidel-Rimon O, Rhea DJ, Keith LG et al. (2005) Early adequate maternal weight gain is associated with fewer small for gestational age triplets. J Perinat Med 33, 379–382. [DOI] [PubMed] [Google Scholar]
- 53.DeVader SR, Neeley HL, Myles TD et al. (2007) Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol 110, 745–751. [DOI] [PubMed] [Google Scholar]
- 54.Kac G, Benício MHDAD, Velásquez-Meléndez G et al. (2004) Gestational weight gain and prepregnancy weight influence postpartum weight retention in a cohort of Brazilian women. J Nutr 134, 661–666. [DOI] [PubMed] [Google Scholar]
- 55.Begum F, Colman I, McCargar LJLJ et al. (2012) Gestational weight gain and early postpartum weight retention in a prospective cohort of Alberta women. J Obstet Gynaecol Can 34, 637–647. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence M, Mckillop FM & Durnin JVGA (1991) Women who gain more fat during pregnancy may not have bigger babies: implications for recommended weight gain during pregnancy. BJOG An Int J Obstet Gynaecol 98, 254–259 [DOI] [PubMed] [Google Scholar]
- 57.Walker LO, Sterling BS & Timmerman GM (2005) Retention of pregnancy-related weight in the early postpartum period: implications for women’s health services. J Obstet Gynecol Neonatal Nurs 34, 418–427. [DOI] [PubMed] [Google Scholar]
- 58.Sohlstrom A & Forsum E (1995) Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr 61, 287–295. [DOI] [PubMed] [Google Scholar]
- 59.Endres LK, Straub H, McKinney C et al. (2015) Postpartumweight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 125, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sha T, Cheng G, Li C et al. (2019) Patterns of women’s postpartum weight retention and its associations with maternal obesity-related factors and parity. Int J Environ Res Public Health 16, 4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nkoka O, Ntenda PAM, Senghore T et al. (2019) Maternal overweight and obesity and the risk of caesarean birth in Malawi. Reprod Health 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonald SD, Han Z, Mulla S et al. (2010) Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 341, c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]