Abstract
Objective:
To explore the relationships of serum 25-hydroxyvitamin D (25(OH)D) with obesity and metabolic parameters in US children.
Design:
Cross-sectional analysis. We evaluated the associations between serum 25(OH)D and multiple measurements of adiposity, serum lipid concentrations, fasting glucose and insulin resistance in children aged 6–18 years with adjustments for multiple covariates.
Setting:
The National Health and Nutrition Examination Survey, 2001–2006.
Participants:
A nationally representative sample of 6311 children and adolescents aged 6–18 years.
Results:
Among US children and adolescents, the prevalence of vitamin D deficiency has been especially high in older children, girls and the non-Hispanic Black population. Higher odds of obesity were found at a 25(OH)D concentration of <30 nmol/l (deficiency) than at >50 nmol/l under both criteria for obesity in children (OR = 3·27, P trend ≤ 0.001). Moreover, increased odds of having abnormal HDL-cholesterol (OR = 1·71, P trend ≤ 0.001) and impaired insulin resistance (OR = 4·15, P trend ≤ 0·001) were found for children deficient in 25(OH)D compared with those with normal 25(OH)D concentrations. When the children and adolescents were stratified by gender, we found stronger associations between serum 25(OH)D concentration and both HDL-cholesterol and insulin resistance in girls. No association of 25(OH)D with any other metabolic parameter was found.
Conclusions:
Our results suggest a potential harmful association between low serum 25(OH)D concentration and the risk of obesity among children. However, the underlying mechanisms require further investigation.
Keywords: 25-Hydroxyvitamin D, National Health and Nutrition Examination Survey, Children, Obesity, Metabolic parameters
Vitamin D, a family of fat-soluble secosteroids, has crucial roles in the intestinal absorption of calcium, iron, magnesium, phosphate and zinc, and is responsible for regulating calcium and phosphate homeostasis in man. Vitamin D additionally regulates insulin secretion and glucose tolerance(1).
Vitamin D deficiency is highly prevalent in the general population worldwide. In particular, vitamin D deficiency is common in the USA. Overall, 5 % of the general population has serum 25-hydroxyvitamin D (25(OH)D) concentration below the traditional cut-off of 27·5 nmol/l (11 ng/ml)(2). In adults, the Black and Hispanic populations present an especially high prevalence(3). Among children and adolescents, approximately 9 % of the population is 25(OH)D deficient(4). Vitamin D deficiency is reported to be associated with several disorders. Kunutsor et al. found that high serum vitamin D concentration reduced the risk ratio for hypertension(5). Inverse associations between vitamin D concentration and metabolic syndrome(6), dyslipidaemia(7) and CVD(8) have been reported in adults. In contrast, 25(OH)D is positively correlated with insulin sensitivity(9), as well as reductions in colon, prostate and breast cancer risk(10). Supplementation with vitamin D and its analogues can effectively remediate such disorders, as shown in several randomized controlled trials(11,12).
Many studies have focused on vitamin D deficiency in relation to obesity and metabolic syndrome in adults and suggested its significance in that population(13,14). Because of the differences between adults and children, three main manifestations have been observed: (i) children consume less vitamin D than do adults; (ii) children and adults have different criteria for obesity outcomes; and (iii) some mechanisms affecting vitamin D and obesity are different, such as inflammatory markers, intestinal absorption function and sun exposure. These factors are considered to be the possible causes of low vitamin D and obesity. Additionally, due to gender-specific differences in glucose homeostasis and energy balance(15) and vitamin D status(16), gender may contribute to the associations between vitamin D deficiency and obesity and metabolic indicators. In children, Moore and Liu found that low serum 25(OH)D concentration was associated with elevated systolic blood pressure in the National Health and Nutrition Examination Survey (NHANES) 2007–2010(17). Total vitamin D intake among boys was higher than that among girls in the NHANES 2007–2010(18). The higher vitamin D-intake population had a lower prevalence of overweight or obesity among children in the NHANES 2005–2008(19). In the USA, the obesity prevalence among children and adolescents is 18.4 and 20.6 %, respectively(20). Moore et al. observed that 5 % of US adolescents suffer from metabolic syndrome(21). However, evidence indicating whether circulating vitamin D concentration has the same protective effects in children and adolescents as in adults is insufficient. Therefore, a large cross-sectional study was conducted to investigate whether vitamin D deficiency was associated with obesity and metabolic indicators in children, providing clues for future randomized clinical and animal studies.
In the present study, we aimed to investigate the association of serum 25(OH)D with BMI, waist circumference (WC), waist-to-height ratio (WHtR) and metabolism-related parameters, such as serum lipids, fasting glucose concentration and homeostasis model assessment of insulin resistance (HOMA-IR), in a nationally representative sample of US children and adolescents from the NHANES. These results may be the first to provide evidence supporting relationships between serum 25(OH)D and obesity and associations between serum 25(OH)D and HDL-cholesterol (HDL-C) and insulin resistance in girls.
Methods
Study population
NHANES is a cross-sectional study that uses a stratified, multistage sample representative of non-institutionalized civilians in the USA. The data from the NHANES 2001–2006 were selected because of the availability of simultaneous measurements of serum 25(OH)D and metabolism-related parameters. The population studied in the present work consisted of children aged 6–18 years who participated between 2001 and 2006 and had available data on serum 25(OH)D concentrations, body measurements, total cholesterol (TC), HDL-C, LDL-cholesterol (LDL-C) and TAG. A subgroup of adolescents aged 12–18 years who had fasting glucose and insulin measurements were included. Those who had diabetes or took insulin or antidiabetic pills to lower blood sugar (n 36) were excluded, as were those who were pregnant (n 106).
Anthropometric and laboratory measures
The weights of the children and adolescents were measured mostly on a Toledo digital scale. Standing height was measured using a fixed stadiometer with a vertical floor and movable headboard(22). BMI was calculated as weight divided by height squared (kg/m2). WC was measured by professionals according to a standardized protocol. First, a skeletal landmark, the lateral boundary of the iliac bone, was located and marked. Then, while standing on the right side of the children and adolescents, the operator placed a measuring belt around the trunk on the horizontal plane marked on the right side of the trunk(22). Serum TC and HDL-C were measured with a Hitachi 704 analyser in 2003–2004 and with a Hitachi 717 or Hitachi 912 analyser in 2005–2006. Specifically, TC was measured enzymatically in serum or plasma in a series of coupled reactions that hydrolyse cholesteryl esters and oxidize the 3-OH group of cholesterol(23). Absorbance was measured at 500 nm, with the colour intensity proportional to the cholesterol concentration. HDL-C(24) and glucose(25) were directly determined in serum using reagents purchased from Roche Diagnostics by detection at absorption wavelengths of 600 and 340 nm, respectively. TAG concentrations were detected by a timed end-point method with the Beckman Synchron LX20. The system monitors variation of the absorption wavelength at 520 nm within a fixed time interval(26). The Friedewald equation was used to calculate the LDL-C concentration based on the concentrations of TC, TAG and HDL-C. Serum insulin concentrations were determined by RIA in 2001–2002(27), by two-site immunoenzymometric assay in 2003–2004(28) and by ELISA with a Mercodia insulin assay in 2005–2006(29).
The data on serum 25(OH)D concentrations from the NHANES 2001–2006 were originally measured at the National Center for Environmental Health with the DiaSorin RIA kit. The excessive methodological bias and imprecision in the methods to measure 25(OH)D have been discussed in many publications in the field of vitamin D research, as have quality control issues for the RIA data from the NHANES. The detailed procedure is presented on the NHANES website. Until October 2015, portions of sera from children and adolescents with available 25(OH)D RIA data from the NHANES 2001–2006 were reanalysed using LC–MS/MS for a bridging (crossover) study to develop regression equations to predict a NHANES participant’s LC–MS/MS-equivalent concentration from his/her previously measured unharmonized (original) RIA value(30). Thus, a model based on RIA quality-control pooled data was selected because the results should be independent of any empirical trend in the sample participant data. As a result, the 25(OH)D data from the NHANES 2003–2004 were generally adjusted to lower values, and the 2005–2006 data were generally adjusted to higher values.
According to the cut-off points in the report issued by the Institute of Medicine(31), we classified the children and adolescents into the following three categories: (i) those who had adequate vitamin D concentration (serum 25(OH)D ≥ 50 nmol/l); (ii) those who had inadequate vitamin D concentration (serum 25(OH)D ≥ 30 nmol/l but < 50 nmol/l); and (iii) those who were deficient in vitamin D (serum 25(OH)D < 30 nmol/l).
Definitions of outcomes
Children and adolescents were classified as obese according to cut-off points based on sex and age(32). In addition, the 85th and 95th percentiles of BMI adjusted for age and gender were calculated based on the 2000 Centers for Disease Control and Prevention growth curves(33). We defined two tiers of abnormal WC according to the 75th and 90th percentiles based on children’s age and gender(34). The WHtR, calculated as WC divided by height, was used to assess central adiposity; when the value was equal to or greater than 0.5, the individual was considered to have a high level of central adiposity. According to National Cholesterol Education Program guidelines(35), abnormal serum TC, serum HDL-C, serum LDL-C, fasting TAG and fasting plasma glucose concentrations, and abnormal HOMA-IR score, were defined as follows: TC ≥ 200 mg/dl, HDL-C ≤ 35 mg/dl, LDL-C ≥ 130 mg/dl, TAG ≥ 150 mg/dl, glucose ≥ 100 mg/dl and HOMA-IR ≥ 4.39(36).
Covariates
Serum cotinine was divided into zero, low and high exposure to reflect the severity of exposure to environmental cigarette smoke. Drinking status was defined by whether an individual consumed alcohol. The poverty:income ratio (PIR), the ratio of family income to the poverty threshold after adjusting for inflation and family size, was used as an index to evaluate socio-economic status.
Statistical analysis
We used multinomial logistic regression models to estimate adjusted OR and 95 % CI for the associations of obesity status and WC as distinct outcomes with categories of vitamin D status. The adequate condition (>50 nmol/l) was used as the reference value. We controlled the unadjusted models and adjusted the models testing the associations of 25(OH)D concentration with BMI and WC for age, race/ethnicity, gender, alcohol use, PIR and serum cotinine concentration. We also used these models to explore the relationships between 25(OH)D concentration and serum metabolites. As gender is an extremely important variable, we conducted separate analyses stratified by gender. All statistical analyses were performed using the statistical software package SAS version 9.2. A P value of 0.05 was designated as the cut-off for statistical significance. The study was approved by the National Center for Health Statistics Research Ethics Review Board.
Results
The participants in the present study included 6311 children and adolescents aged 6–18 years. Table 1 presents the baseline characteristics of the groups stratified by serum 25(OH)D concentration category. Children and adolescents who had diabetes or were taking insulin or antidiabetic pills to lower blood sugar (n 36) were excluded, as were those pregnant (n 106). Among the children and adolescents with 25(OH)D deficiency, the incidence rates were 35.9 % in boys and 64.1 % in girls. Girls were more susceptible than boys to serum 25(OH)D deficiency (64.1 v. 35.9 %, P < 0.001). In addition, the proportions with low 25(OH)D concentration in the non-Hispanic Black (76.2 %) and relatively poor (54.9 %) populations were significantly elevated (P < 0.001). Notably, the subset of individuals with low serum 25(OH)D was older than the overall population (P < 0.001).
Table 1.
Selected characteristics of the study sample by serum 25-hydroxyvitamin D (25(OH)D) category: children and adolescents aged 6–18 years (n 6311), National Health and Nutrition Examination Survey, 2001–2006
| Serum 25(OH)D | |||||||
|---|---|---|---|---|---|---|---|
| Adequate (>50 nmol/l) | Inadequate (30–50 nmol/l) | Deficient (<30 nmol/l) | |||||
| Characteristic | Mean or % | sd or n | Mean or % | sd or n | Mean or % | sd or n | P value |
| n | 5107 | 2434 | 554 | ||||
| Age (years), mean and sd | 12·2 | 3·7 | 13·6 | 3·2 | 15·1 | 2·5 | <0·001 |
| Abnormal levels, % and n | |||||||
| BMI ≥ 85th percentile | 30·4 | 1551 | 41·7 | 1015 | 44·9 | 249 | <0·001 |
| BMI ≥ 95th percentile | 15·1 | 769 | 23·6 | 575 | 29·6 | 164 | <0·001 |
| Obesity | 13·4 | 683 | 21·9 | 435 | 30·0 | 166 | <0·001 |
| WC ≥ 75th percentile | 33·9 | 1733 | 43·0 | 1047 | 43·1 | 540 | <0·001 |
| WC ≥ 90th percentile | 16·4 | 840 | 22·8 | 554 | 26·4 | 146 | <0·001 |
| WHtR ≥ 0·5 | 29·9 | 1527 | 40·2 | 979 | 43·3 | 240 | <0·001 |
| TC ≥ 200 mg/dl | 8·7 | 442 | 10·7 | 261 | 7·9 | 44 | 0·01 |
| HDL-C ≤ 35 mg/dl | 5·4 | 277 | 5·6 | 137 | 4·9 | 27 | 0·804 |
| LDL-C ≤ 130 mg/dl | 2·3 | 116 | 3·5 | 85 | 3·2 | 18 | 0·011 |
| Fasting TAG ≥ 150 mg/dl | 4·1 | 207 | 3·2 | 79 | 2·2 | 12 | 0·004 |
| Fasting glucose ≥ 100 mg/dl | 3·7 | 188 | 4·8 | 117 | 4·5 | 25 | 0·43 |
| HOMA-IR score ≥ 4·39 | 3·8 | 193 | 7·9 | 193 | 12·1 | 67 | <0·001 |
| Gender, % and n | <0·001 | ||||||
| Male | 54·1 | 2761 | 46·1 | 1122 | 35·9 | 199 | |
| Female | 45·9 | 2346 | 53·9 | 1312 | 64·1 | 355 | |
| Ethnicity, % and n | <0·001 | ||||||
| Mexican American | 32·4 | 1657 | 33·2 | 807 | 18·2 | 101 | |
| Other Hispanic | 3·9 | 201 | 3·1 | 75 | 1·3 | 7 | |
| Non-Hispanic White | 39·4 | 2011 | 8·0 | 194 | 2·3 | 13 | |
| Non-Hispanic Black | 19·6 | 1000 | 51·2 | 1245 | 76·2 | 422 | |
| Other race | 4·7 | 238 | 4·6 | 113 | 2·0 | 11 | |
| Serum cotinine, % and n* | <0·001 | ||||||
| <0·01 ng/ml (LOD) | 19·3 | 985 | 13·8 | 337 | 7·9 | 44 | |
| 0·01–10 ng/ml | 73·2 | 3736 | 77·6 | 1889 | 82·5 | 457 | |
| >10 ng/ml | 6·7 | 343 | 7·9 | 192 | 9·0 | 50 | |
| Alcohol, % and n* | 0·765 | ||||||
| Non-drinker | 91·4 | 4667 | 91·6 | 2229 | 90·1 | 499 | |
| Drinker | 4·4 | 225 | 4·3 | 104 | 4·9 | 27 | |
| PIR, % and n* | <0·001 | ||||||
| ≤1 | 25·6 | 1306 | 35·3 | 858 | 41·5 | 230 | |
| >1 | 70·3 | 3592 | 59·4 | 1447 | 54·9 | 304 | |
WC, waist circumference; WHtR, waist-to-height ratio; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LOD, limit of detection; PIR, poverty:income ratio.
Weighted percentage.
Comparing the variables indicating obesity and metabolic status between categories of 25(OH)D deficiency, we found that the proportions of children and adolescents with high BMI, WC, WHtR and HOMA-IR score were all markedly increased (P < 0.001) in the low 25(OH)D group, while the percentage of individuals with high fasting TAG concentration was decreased (P = 0.004). When we compared other metabolism parameters among these three groups, no statistically significant differences in HDL-C (P = 0.804) or fasting glucose (P = 0.43) were found.
The overall unadjusted OR for serum 25(OH)D and the OR after adjustment for either the factors of age, race/ethnicity and gender, or the factors of age, race/ethnicity, gender, alcohol intake, PIR and serum cotinine concentration from the multinomial logistic regression models, are shown in Table 2. Strong inverse associations between serum 25(OH)D and obesity or WC were found in children and adolescents. Remarkably, serum 25(OH)D deficiency was associated with obesity status (when defined by BMI ≥ 85th percentile: OR = 3.25; 95 % CI 2.54, 4.16; P trend ≤ 0.001; when defined by BMI ≥ 95th percentile: OR = 3.27; 95 % CI 2.55, 4.20; P trend ≤ 0.001), as well as WC (≥90th percentile: OR = 3.38; 95 % CI 2.60, 4.39; P trend ≤ 0.001) and WHtR (OR = 2.97; 95 % CI 2.33, 3.78; P trend ≤ 0.001).
Table 2.
OR for the associations between serum 25-hydroxyvitamin D (25(OH)D) concentration and measures of adiposity: children and adolescents aged 6–18 years (n 6311), National Health and Nutrition Examination Survey, 2001–2006
| Serum 25(OH)D | |||||||
|---|---|---|---|---|---|---|---|
| Adequate (>50 nmol/l) | Inadequate (30–50 nmol/l) | Deficient (<30 nmol/l) | |||||
| Measure of adiposity | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | P trend |
| BMI ≥ 85th percentile | |||||||
| Sample size | 4054 | 1813 | 444 | ||||
| Unadjusted | 1·00 | Reference | 1·66 | 1·50, 1·84 | 1·93 | 1·62, 2·31 | <0·001 |
| Adjusted | 1·00 | Reference | 2·11 | 1·81, 2·45 | 3·25 | 2·54, 4·16 | <0·001 |
| BMI ≥ 95th percentile | |||||||
| Sample size | 4054 | 1813 | 444 | ||||
| Unadjusted | 1·00 | Reference | 1·76 | 1·56, 1·99 | 2·43 | 1·99, 2·96 | <0·001 |
| Adjusted | 1·00 | Reference | 2·18 | 1·86, 2·55 | 3·27 | 2·55, 4·20 | <0·001 |
| BMI* | |||||||
| Sample size | 4054 | 1813 | 444 | ||||
| Unadjusted | 1·00 | Reference | 2·06 | 1·81, 2·35 | 2·95 | 2·39, 3·63 | <0·001 |
| Adjusted | 1·00 | Reference | 2·14 | 1·83, 2·49 | 3·28 | 2·56, 4·19 | <0·001 |
| WC ≥ 75th percentile | |||||||
| Sample size | 4023 | 1798 | 439 | ||||
| Unadjusted | 1·00 | Reference | 1·78 | 1·57, 2·00 | 2·29 | 1·87, 2·81 | <0·001 |
| Adjusted | 1·00 | Reference | 2·09 | 1·81, 2·42 | 3·22 | 2·53, 4·10 | <0·001 |
| WC ≥ 90th percentile | |||||||
| Sample size | 4023 | 1798 | 439 | ||||
| Unadjusted | 1·00 | Reference | 1·97 | 1·72, 2·26 | 2·55 | 2·05, 3·18 | <0·001 |
| Adjusted | 1·00 | Reference | 2·28 | 1·93, 2·68 | 3·38 | 2·60, 4·39 | <0·001 |
| WHtR ≥ 0.5 | |||||||
| Sample size | 4023 | 1798 | 439 | ||||
| Unadjusted | 1·00 | Reference | 1·94 | 1·71, 2·19 | 2·57 | 2·09, 3·15 | <0·001 |
| Adjusted | 1·00 | Reference | 2·00 | 1·72, 2·31 | 2·97 | 2·33, 3·78 | <0·001 |
WC, waist circumference; WHtR, waist-to-height ratio; LOD, limit of detection.
Adjusted for age (continuous), gender (male, female), race and ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, Other), poverty:income ratio (<1, ≥1), alcohol use (yes or no) and cotinine concentration (<0.01 ng/ml (LOD), 0.01–10 ng/ml, >10 ng/ml).
≥30 kg/m2 v. <30 kg/m2.
In exploring the relationships between serum metabolism parameters and 25(OH)D deficiency, we found higher odds of HDL-C ≤ 35 mg/dl with inadequate serum 25(OH)D concentration than with adequate concentration (OR = 1.64; 95 % CI 1.22, 2.19) and higher odds with deficiency than with adequate concentration (OR = 1.71; 95 % CI 1.01, 2.90; Table 3). In addition, we found increased odds of HOMA-IR score ≥ 4.39 with inadequate serum 25(OH)D v. adequate concentrations (OR = 2.55; 95 % CI 1.89, 3.44) and with deficient v. adequate concentrations (OR = 4.15; 95 % CI 2.66, 6.47). No association was found between 25(OH)D deficiency and the other parameters, including TC, LDL-C, fasting TAG and fasting glucose.
Table 3.
OR for the associations between serum 25-hydroxyvitamin D (25(OH)D) concentration and serum metabolic disorder risk factors: children and adolescents aged 6–18 years (n 6311), National Health and Nutrition Examination Survey, 2001–2006
| Serum 25(OH)D | |||||||
|---|---|---|---|---|---|---|---|
| Adequate (>50 nmol/l) | Inadequate (30–50 nmol/l) | Deficient (<30 nmol/l) | |||||
| Risk factor | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | P trend |
| TC ≥ 200 mg/dl | |||||||
| Sample size | 4025 | 1803 | 441 | ||||
| Unadjusted | 1·00 | Reference | 1·38 | 1·14, 1·66 | 1·04 | 0·73, 1·48 | 0·471 |
| Adjusted | 1·00 | Reference | 1·37 | 1·10, 1·71 | 0·99 | 0·66, 1·48 | 0·919 |
| HDL-C ≤ 35 mg/dl | |||||||
| Sample size | 4025 | 1803 | 441 | ||||
| Unadjusted | 1·00 | Reference | 1·20 | 0·94, 1·54 | 1·09 | 0·70, 1·70 | 0·032 |
| Adjusted | 1·00 | Reference | 1·64 | 1·22, 2·19 | 1·71 | 1·01, 2·90 | <0·001 |
| LDL-C ≥ 130 mg/dl | |||||||
| Sample size | 1605 | 741 | 189 | ||||
| Unadjusted | 1·00 | Reference | 1·64 | 1·16, 2·31 | 1·49 | 0·83, 2·68 | 0·280 |
| Adjusted | 1·00 | Reference | 1·80 | 1·19, 2·73 | 1·64 | 0·82, 3·28 | 0·523 |
| Fasting TAG ≥ 150 mg/dl | |||||||
| Sample size | 1633 | 772 | 199 | ||||
| Unadjusted | 1·00 | Reference | 0·82 | 0·60, 1·13 | 0·54 | 0·28, 1·03 | 0·159 |
| Adjusted | 1·00 | Reference | 1·20 | 0·82, 1·74 | 1·10 | 0·51, 2·35 | 0·112 |
| Fasting glucose ≥ 100 mg/dl | |||||||
| Sample size | 1171 | 650 | 190 | ||||
| Unadjusted | 1·00 | Reference | 1·05 | 0·79, 1·40 | 0·91 | 0·56, 1·46 | 0·403 |
| Adjusted | 1·00 | Reference | 1·40 | 1·00, 1.97 | 1·88 | 1·07, 3·28 | 0·19 |
| HOMA-IR score ≥ 4.39 | |||||||
| Sample size | 1159 | 645 | 188 | ||||
| Unadjusted | 1·00 | Reference | 2·28 | 1·77, 2·94 | 3·04 | 2·12, 4·35 | <0·001 |
| Adjusted | 1·00 | Reference | 2·55 | 1·89, 3·44 | 4·15 | 2·66, 6·47 | <0·001 |
TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LOD, limit of detection.
Adjusted for age (continuous), gender (male, female), race and ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, Other), poverty:income ratio (<1, ≥1), alcohol use (yes or no), and cotinine level (<0.01 ng/ml (LOD), 0.01–10 ng/ml, >10 ng/ml).
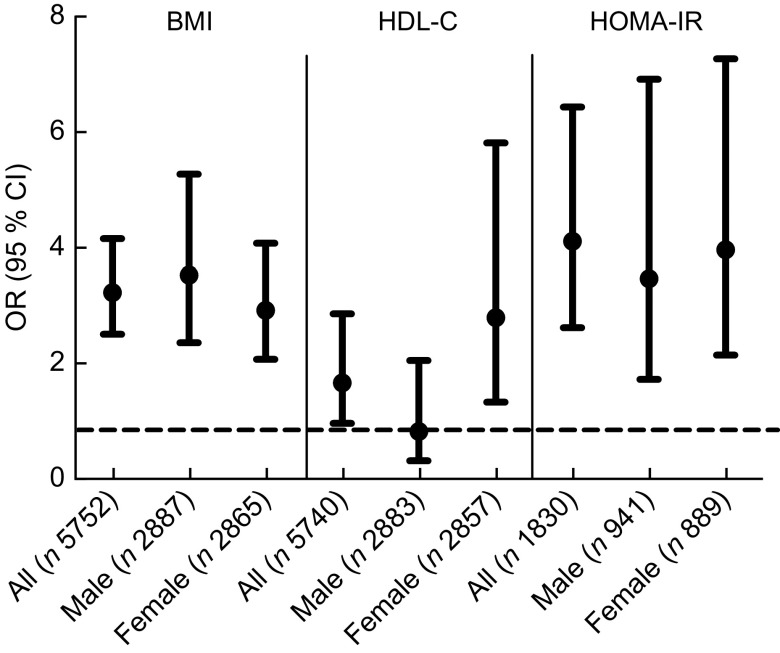
Boys and girls differ in physical composition, hormonal biology, weight-gain patterns and sensitivity to certain genetic, environmental, social and racial factors(37). Additionally, previous research has shown gender differences in vitamin D intake(18). Our analysis was further performed after stratification by gender. We found that low concentration of 25(OH)D was related to an elevated risk of obesity in boys (OR = 3.57; 95 % CI 2.40, 5.31), as well as with low HDL-C (OR = 2.84; 95 % CI 1.38, 5.85) and high HOMA-IR score (OR = 4.00; 95 % CI 2.19, 7.30) in girls (Fig. 1).
Fig. 1.
Odds of obesity (BMI ≥ 95th percentile for age and sex), low HDL-cholesterol (HDLC ≤ 35 mg/dl) and insulin resistance (homeostasis model assessment of insulin resistance (HOMA-IR) score ≥ 4.39) for the vitamin D deficient group (serum 25-hydroxyvitamin D (25(OH)D) < 30 nmol/l) compared with the adequate group (25(OH)D > 50 nmol/l): children and adolescents aged 6–18 years (n 6311), National Health and Nutrition Examination Survey, 2001–2006. Results are presented as OR (•), with their 95 % CI represented by vertical bars
Discussion
In the present study, we used data on serum 25(OH)D concentrations from the National Center for Environmental Health released recently in October 2015. The results provided the first evidence of associations between low serum 25(OH)D concentration and obesity (indicated by BMI), WC and WHtR in a large-scale, nationally representative sample of US children and adolescents. Furthermore, we found inverse associations between serum 25(OH)D and serum HDL-C concentrations as well as with HOMA-IR. Interestingly, among children and adolescents with serum 25(OH)D deficiency, boys were prone to be obese, while girls had increased risks of abnormal HDL-C concentration and insulin resistance.
Several covariates may mediate mechanisms affecting the relationship between serum 25(OH)D and obesity, and we adjusted for them in the logistic regression model. For example, smoke exposure may induce inflammation and oxidative stress(38), alcohol intake likely adds energy to a meal and also stimulates food intake(39) and socio-economic status seems to be associated with an increased appetite(40). These variables and molecular mechanisms may contribute to a greater BMI among children.
We found that vitamin D deficiency was highly prevalent in older children, girls and the non-Hispanic Black population. Concentration of 25(OH)D is widely known to decline with age(41). Here, we showed similar changes in both children and adolescents. The gender difference may be due to reduced intake of vitamin D in girls(42). Girls may stay in indoor settings such as school or the library longer than boys, and insufficient sun exposure can result in 25(OH)D deficiency(43). Genetic predisposition may be responsible for low 25(OH)D in the non-Hispanic Black population(44).
Our results are similar to but not completely consistent with those of previous studies reporting the association between serum 25(OH)D and adiposity in different regions and populations. Cediel et al. reported that serum 25(OH)D was inversely associated with insulin resistance in Chilean children(45). Lee et al. found that low vitamin D concentration was associated with TAG in Korean children(46). Obese Turkish children and adolescents were more commonly diagnosed with vitamin D deficiency(47), suggesting that vitamin D is linked to obesity status. The results of case–control studies indicated that obesity is associated with vitamin D deficiency in Danish(48) and Chinese children and adolescents(49). Obesity plays a critical role in metabolic syndrome parameters. Associations between vitamin D level and components of metabolic syndrome were also found in Korean children and adolescents(50,51), Iranian adolescents(52) and Chinese adolescents(53). Here, we observed elevated risks of decreased HDL-C and impaired insulin resistance in US children and adolescents with vitamin D deficiency, especially in girls. In contrast to previous findings among adults(6), only obesity and a few markers of metabolic syndrome in children and adolescents were observed to be significantly associated with vitamin D deficiency. However, the current study did not examine whether any gender difference was present in adults.
Previous publications have used the NHANES database to investigate the associations between serum 25(OH)D and metabolism disorders(4,6,54,55). With the use of unadjusted data from the NHANES 2001–2004, Reis et al.(54) reported associations between serum 25(OH)D and both hypertension and hyperglycaemia in youths aged 12–19 years. Because of high methodological bias and inaccuracy in the measuring methods, the previous publications using non-updated data may report false-positive results and ignore false-negative results. Thus, portions of the serum samples from participants with available 25(OH)D data from the NHANES 2001–2006 were selected by the National Center for Environmental Health for additional analyses. Significant relationships between obesity, WC, HDL-C and HOMA-IR score and low 25(OH)D concentration in children and adolescents aged 6–18 years were found in the current analysis. Furthermore, girls may be more susceptible to deficiency than boys and may need greater vitamin D intake. However, no relationship between vitamin D status and fasting glucose could be found in our analysis, contradicting significant results from a previous study(54).
We carried out many statistical tests; thus, adjustment for multiple comparisons was necessary in the present study. Methods such as Bonferroni correction or the false discovery rate were not suitable as these methods treat all P values as equivalent. Considerable structure exists in the NHANES data. Therefore, P for trend was tested to examine the trend in 25(OH)D concentration and supporting evidence for a real association was found. If only a test with logistic regression models is performed and P for trend is not tested, significant values may be expected by chance. In the present study, the P values of the logistic regression and the trend test, which were both <0.05, were treated as positive results.
Little evidence is available to support a direct causal relationship between vitamin D and obesity and metabolic parameters. Inflammation may be one of the mechanisms that explain the associations between vitamin D and obesity and metabolic parameters. Vitamin D has been reported to inhibit the concentration of 25(OH)D as an acute-phase reactant in inflammation(56). Obesity is now widely recognized as a chronic, low-grade systemic inflammatory state(57,58). Improvements in 25(OH)D concentration have been reported to enhance the beneficial effects of weight loss, which may be attributed at least in part to the anti-inflammatory effects of vitamin D(59,60). Although a meta-analysis of randomized clinical trials showed no effects of vitamin D supplementation on inflammatory biomarkers among overweight/obese adults(61), more research evidence is needed. Recently, vitamin D supplementation was shown to decrease inflammatory biomarkers (i.e. IL-6 level) in adults with metabolic syndrome(62). More randomized clinical trials of obese children and adolescents are needed to elucidate the mechanism.
The present study has some limitations. First, the different ethnicities and dietary habits of the children and adolescents prevented us from comparing results between the present study and previous ones. Second, some confounders, such as sun exposure level and genetic susceptibility, may influence the associations reported herein. In addition to common covariates, we analysed other covariates that may affect obesity and metabolic indices, such as PIR, drinking status and serum cotinine concentration. Performing this ANCOVA ensured that the present results reflected true circumstances. Because of the cross-sectional nature of the present study, whether 25(OH)D affects obesity or vice versa cannot be determined. However, some evidence from in vivo studies(63) and randomized controlled trials(64) suggests that vitamin D supplementation may prevent obesity by mediating the apoptotic pathway in adipose tissue in mice and may improve insulin resistance and sensitivity in South Asian women.
Conclusions
Our findings suggest the need for longitudinal analyses to elucidate temporal relationships between low 25(OH)D concentration and the development of obesity and serum metabolism markers in children. Although the exact mechanism by which 25(OH)D contributes to obesity, HDL-C and insulin resistance remains unclear, the present results may contribute to a better understanding of obesity from an epidemiological perspective.
Acknowledgements
Financial support: This work was supported by funding from the National Key Research and Development Program of China (grant numbers 2017YFC1308105 and 2016YFC1101001); the Maternal and Child Health Research Project of Jiangsu Province (grant number F201755); the China Postdoctoral Science Foundation (grant number 2018M630585); the Science and Technology Development Project of Nanjing (grant number 210723008); and the Key Project of Science and Technology Development Fund of Nanjing Medical University (grant number 2017NJMUZD060). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None declared. Authorship: Z.F. and C.X. contributed equally to the present study and should be regarded as joint first authors. X.M. and C.X. conceived and designed the study, analysed and interpreted the data, and wrote the manuscript. Z.F. and Y.S. conducted data collection and statistical analyses. Z.X. and C.L. reviewed the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all subjects.
References
- 1. Garbossa SG & Folli F (2017) Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord 18, 243–258. [DOI] [PubMed] [Google Scholar]
- 2. Yetley EA (2008) Assessing the vitamin D status of the US population. Am J Clin Nutr 88, issue 2, 558S–564S. [DOI] [PubMed] [Google Scholar]
- 3. Forrest KY & Stuhldreher WL (2011) Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31, 48–54. [DOI] [PubMed] [Google Scholar]
- 4. Kumar J, Muntner P, Kaskel FJ et al. (2009) Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124, e362–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kunutsor SK, Apekey TA & Steur M (2013) Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol 28, 205–221. [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, Ajani UA, McGuire LC et al. (2005) Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care 28, 1228–1230. [DOI] [PubMed] [Google Scholar]
- 7. Skaaby T, Husemoen LL, Pisinger C et al. (2012) Vitamin D status and changes in cardiovascular risk factors: a prospective study of a general population. Cardiology 123, 62–70. [DOI] [PubMed] [Google Scholar]
- 8. Grandi NC, Breitling LP & Brenner H (2010) Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med 51, 228–233. [DOI] [PubMed] [Google Scholar]
- 9. Lips P, Eekhoff M, van Schoor N et al. (2017) Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol 173, 280–285. [DOI] [PubMed] [Google Scholar]
- 10. Holick MF (2007) Vitamin D deficiency. N Engl J Med 357, 266–281. [DOI] [PubMed] [Google Scholar]
- 11. Carrillo AE, Flynn MG, Pinkston C et al. (2013) Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr 32, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schleithoff SS, Zittermann A, Tenderich G et al. (2006) Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83, 754–759. [DOI] [PubMed] [Google Scholar]
- 13. Pereira-Santos M, Costa PR, Assis AM et al. (2015) Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev 16, 341–349. [DOI] [PubMed] [Google Scholar]
- 14. Wimalawansa SJ (2018) Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol 175, 177–189. [DOI] [PubMed] [Google Scholar]
- 15. Mauvais-Jarvis F (2017) Epidemiology of gender differences in diabetes and obesity. Adv Exp Med Biol 1043, 3–8. [DOI] [PubMed] [Google Scholar]
- 16. Lucas RM, Ponsonby AL, Dear K et al. (2013) Vitamin D status: multifactorial contribution of environment, genes and other factors in healthy Australian adults across a latitude gradient. J Steroid Biochem Mol Biol 136, 300–308. [DOI] [PubMed] [Google Scholar]
- 17. Moore CE & Liu Y (2017) Elevated systolic blood pressure of children in the United States is associated with low serum 25-hydroxyvitamin D concentrations related to body mass index: National Health and Examination Survey 2007–2010. Nutr Res 38, 64–70. [DOI] [PubMed] [Google Scholar]
- 18. Moore CE, Radcliffe JD & Liu Y (2014) Vitamin D intakes of children differ by race/ethnicity, sex, age, and income in the United States, 2007 to 2010. Nutr Res 34, 499–506. [DOI] [PubMed] [Google Scholar]
- 19. Keast DR, Hill Gallant KM, Albertson AM et al. (2015) Associations between yogurt, dairy, calcium, and vitamin D intake and obesity among US children aged 8–18 years: NHANES, 2005–2008. Nutrients 7, 1577–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hales CM, Carroll MD, Fryar CD et al. (2017) Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief issue 228, 1–8. [PubMed]
- 21. Moore BF, Clark ML, Bachand A et al. (2016) Interactions between diet and exposure to secondhand smoke on metabolic syndrome among children: NHANES 2007–2010. J Clin Endocrinol Metab 101, 52–58. [DOI] [PubMed] [Google Scholar]
- 22. US Department of Health and Human Services, Centers for Disease Control and Prevention (2002) National Health and Examination Survey: Anthropometry Procedures manual. https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/manuals/body_measures_year_3.pdf (accessed November 2018).
- 23. US Department of Health and Human Services, Centers for Disease Control and Prevention (2004) Cholesterol in Refrigerated Serum – NHANES 2001–2002 Collaborative Laboratory Services, LLC. https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l40_b_met_cholesterol.pdf (accessed November 2018).
- 24. US Department of Health and Human Services, Centers for Disease Control and Prevention (2006) Laboratory Procedure Manual. Analyte: Total Cholesterol, HDL-Cholesterol, Triglycerides, and LDL-Cholesterol. https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l13_c_met_lipids.pdf (accessed November 2018).
- 25. US Department of Health and Human Services, Centers for Disease Control and Prevention (2005) Laboratory Procedure Manual. Analyte: Plasma Glucose. https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l10am_b_met_glucose.pdf (accessed November 2018).
- 26. US Department of Health and Human Services, Centers for Disease Control and Prevention (2004) Triglyceride in Refrigerated Serum – NHANES 2001–2002 Collaborative Laboratory Services, LLC. https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l40_b_met_triglycerides.pdf (accessed November 2018).
- 27. US Department of Health and Human Services, Centers for Disease Control and Prevention (2005) Laboratory Procedure Manual. Analyte: Insulin (NHANES 2001–2002). https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l10am_b_met_insulin.pdf (accessed November 2018).
- 28. US Department of Health and Human Services, Centers for Disease Control and Prevention (2006) Laboratory Procedure Manual. Analyte: Insulin (NHANES 2003–2004). https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l10am_c_met_insulin.pdf (accessed November 2018).
- 29. US Department of Health and Human Services, Centers for Disease Control and Prevention (2007) Laboratory Procedure Manual. Analyte: Insulin (NHANES 2005–2006). https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/labmethods/glu_d_met_insulin.pdf (accessed November 2018).
- 30. Dalhoff K, Dancey J, Astrup L et al. (2003) A phase II study of the vitamin D analogue Seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer 89, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011) Implications and special concerns: conclusions about vitamin D deficiency in the United States and Canada. In Dietary Reference Intakes for Calcium and Vitamin D [AC Ross, CL Taylor, AL Yaktine et al. , editors]. Washington, DC: National Academies Press; available at https://www.ncbi.nlm.nih.gov/books/NBK56078/#ch8.s2 [PubMed] [Google Scholar]
- 32. Cole TJ, Bellizzi MC, Flegal KM et al. (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320, 1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuczmarski RJ, Ogden CL, Guo SS et al. (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 issue 246, 1–190. [PubMed] [Google Scholar]
- 34. Fernandez JR, Redden DT, Pietrobelli A et al. (2004) Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 145, 439–444. [DOI] [PubMed] [Google Scholar]
- 35. Skinner AC, Perrin EM, Moss LA et al. (2015) Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med 373, 1307–1317. [DOI] [PubMed] [Google Scholar]
- 36. Lee JM, Okumura MJ, Davis MM et al. (2006) Prevalence and determinants of insulin resistance among US adolescents: a population-based study. Diabetes Care 29, 2427–2432. [DOI] [PubMed] [Google Scholar]
- 37. Wisniewski AB & Chernausek SD (2009) Gender in childhood obesity: family environment, hormones, and genes. Gend Med 6, Suppl. 1, 76–85. [DOI] [PubMed] [Google Scholar]
- 38. Makadia LD, Roper PJ, Andrews JO et al. (2017) Tobacco use and smoke exposure in children: new trends, harm, and strategies to improve health outcomes. Curr Allergy Asthma Rep 17, 55. [DOI] [PubMed] [Google Scholar]
- 39. Traversy G & Chaput JP (2015) Alcohol consumption and obesity: an update. Curr Obes Rep 4, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheon BK & Hong YY (2017) Mere experience of low subjective socioeconomic status stimulates appetite and food intake. Proc Natl Acad Sci U S A 114, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Jongh RT, van Schoor NM & Lips P (2017) Changes in vitamin D endocrinology during aging in adults. Mol Cell Endocrinol 453, 144–150. [DOI] [PubMed] [Google Scholar]
- 42. Moore C, Murphy MM, Keast DR et al. (2004) Vitamin D intake in the United States. J Am Diet Assoc 104, 980–983. [DOI] [PubMed] [Google Scholar]
- 43. Schoenmakers I, Goldberg GR & Prentice A (2008) Abundant sunshine and vitamin D deficiency. Br J Nutr 99, 1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christakos S, Dhawan P, Verstuyf A et al. (2016) Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 96, 365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cediel G, Corvalan C, Aguirre C et al. (2015) Serum 25-hydroxyvitamin D associated with indicators of body fat and insulin resistance in prepubertal Chilean children. Int J Obes (Lond) 40, 147–152. [DOI] [PubMed] [Google Scholar]
- 46. Lee SH, Kim SM, Park HS et al. (2013) Serum 25-hydroxyvitamin D levels, obesity and the metabolic syndrome among Korean children. Nutr Metab Cardiovasc Dis 23, 785–791. [DOI] [PubMed] [Google Scholar]
- 47. Gul A, Ozer S, Yilmaz R et al. (2017) Association between vitamin D levels and cardiovascular risk factors in obese children and adolescents. Nutr Hosp 34, 323–329. [DOI] [PubMed] [Google Scholar]
- 48. Plesner JL, Dahl M, Fonvig CE et al. (2018) Obesity is associated with vitamin D deficiency in Danish children and adolescents. J Pediatr Endocrinol Metab 31, 53–61. [DOI] [PubMed] [Google Scholar]
- 49. Liu X, Xian Y, Min M et al. (2016) Association of 25-hydroxyvitamin D status with obesity as well as blood glucose and lipid concentrations in children and adolescents in China. Clin Chim Acta 455, 64–67. [DOI] [PubMed] [Google Scholar]
- 50. Kim YS, Hwang JH & Song MR (2018) The association between vitamin D deficiency and metabolic syndrome in Korean adolescents. J Pediatr Nurs 38, e7–e11. [DOI] [PubMed] [Google Scholar]
- 51. Kwon JH, Lee SE, Lee HA et al. (2015) Relationship of serum 25-hydroxyvitamin D (25[OH]D) levels and components of metabolic syndrome in prepubertal children. Nutrition 31, 1324–1327. [DOI] [PubMed] [Google Scholar]
- 52. Mellati AA, Sharifi F, Faghihzade S et al. (2015) Vitamin D status and its associations with components of metabolic syndrome in healthy children. J Pediatr Endocrinol Metab 28, 641–648. [DOI] [PubMed] [Google Scholar]
- 53. Fu J, Han L, Zhao Y et al. (2018) Vitamin D levels are associated with metabolic syndrome in adolescents and young adults: the BCAMS study. Clin Nutr. Published online: 6 September 2018. doi: . [DOI] [PubMed] [Google Scholar]
- 54. Reis JP, von Muhlen D, Miller ER 3rd et al. (2009) Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 124, e371–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ganji V, Zhang X, Shaikh N et al. (2011) Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am J Clin Nutr 94, 225–233. [DOI] [PubMed] [Google Scholar]
- 56. Waldron JL, Ashby HL, Cornes MP et al. (2013) Vitamin D: a negative acute phase reactant. J Clin Pathol 66, 620–622. [DOI] [PubMed] [Google Scholar]
- 57. Ahima RS & Flier JS (2000) Adipose tissue as an endocrine organ. Trends Endocrinol Metab 11, 327–332. [DOI] [PubMed] [Google Scholar]
- 58. Zhang Y, Proenca R, Maffei M et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- 59. Zittermann A, Frisch S, Berthold HK et al. (2009) Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 89, 1321–1327. [DOI] [PubMed] [Google Scholar]
- 60. Ghashut RA, Talwar D, Kinsella J et al. (2014) The effect of the systemic inflammatory response on plasma vitamin 25(OH)D concentrations adjusted for albumin. PLoS One 9, e92614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zuk A, Fitzpatrick T & Rosella LC (2016) Effect of vitamin D3 supplementation on inflammatory markers and glycemic measures among overweight or obese adults: a systematic review of randomized controlled trials. PLoS One 11, e0154215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salekzamani S, Bavil AS, Mehralizadeh H et al. (2017) The effects of vitamin D supplementation on proatherogenic inflammatory markers and carotid intima media thickness in subjects with metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Endocrine 57, 51–59 9. [DOI] [PubMed] [Google Scholar]
- 63. Sergeev IN & Song Q (2014) High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res 58, 1342–1348. [DOI] [PubMed] [Google Scholar]
- 64. von Hurst PR, Stonehouse W & Coad J (2010) Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomised, placebo-controlled trial. Br J Nutr 103, 549–555. [DOI] [PubMed] [Google Scholar]