Abstract
Objective:
To explore associations of whole grain and cereal fibre intake to CVD risk factors in Australian adults.
Design:
Cross-sectional analysis. Intakes of whole grain and cereal fibre were examined in association to BMI, waist circumference (WC), blood pressure (BP), serum lipid concentrations, C-reactive protein, systolic BP, fasting glucose and HbA1c.
Setting:
Australian Health Survey 2011–2013.
Participants:
A population-representative sample of 7665 participants over 18 years old.
Results:
Highest whole grain consumers (T3) had lower BMI (T0 26·8 kg/m2, T3 26·0 kg/m2, P < 0·0001) and WC (T0 92·2 cm, T3 90·0 cm, P = 0·0005) compared with non-consumers (T0), although only WC remained significant after adjusting for dietary and lifestyle factors, including cereal fibre intake (P = 0·03). Whole grain intake was marginally inversely associated with fasting glucose (P = 0·048) and HbA1c (P = 0·03) after adjusting for dietary and lifestyle factors, including cereal fibre intake. Cereal fibre intake was inversely associated with BMI (P < 0·0001) and WC (P < 0·0008) and tended to be inversely associated with total cholesterol, LDL-cholesterol and apo-B concentrations, although associations were attenuated after further adjusting for BMI and lipid-lowering medication use.
Conclusions:
The extent to which cereal fibre is responsible for the CVD-protective associations of whole grains may vary depending on the mediators involved. Longer-term intervention studies directly comparing whole grain and non-whole grain diets of similar cereal fibre contents (such as through the use of bran or added-fibre refined grain products) are needed to confirm independent effects.
Keywords: Whole grain, Cereal fibre, CVD, Australia, Cross-sectional
CVD is a major public health burden for Australia, with one in six (approximately 4 million) Australians affected by the disease(1). As well as lifestyle factors such as smoking and physical inactivity, a higher burden of related conditions, such as high blood pressure (BP), high blood cholesterol, diabetes, overweight or obesity, increases the risk of CVD and is associated with a higher lifetime risk of death from the disease(2). Diet can play a role in managing and reducing the risk of developing these CVD-related conditions. For example, a high intake of whole grains and whole grain foods has been consistently found to be associated with reduced risk of CVD and related conditions, such as diabetes(3), obesity(4), metabolic syndrome(5) and hyperlipidaemia(6).
While the precise protective mechanisms of whole grains are not yet fully understood, bioactive components in the grain, such as vitamins, minerals and phenolic acids, may each play a role in different pathways(7). Importantly, whole grains are also very high in dietary fibre, known to be protective against many chronic diseases, including CVD(8). In fact, cereal fibre, in particular, is often found to have the strongest association with favourable cardiovascular health compared with other sources of fibre such as fruit or vegetables(9).
The strong cardio-protective associations found for cereal fibre intake may suggest that the fibre component of whole grain is specifically responsible. However, it is also possible that a high cereal fibre intake simply reflects high whole grain intake, as whole grains are a primary source of cereal fibre, and it is difficult to separate the two exposures. To date, there is limited research directly comparing the health associations of cereal fibre and whole grain intakes accurately. Comparisons have been made between whole grain and refined grain intakes, but these have not been matched on fibre contents(10,11). Alternatively, studies may have compared whole grain and cereal fibre intakes, but included added bran as a form of whole grain(12), confounding associations for true whole grains, which are defined as containing the bran, endosperm and germ components. Previous studies also often tended to estimate intakes using food-level analysis, by categorising food items as either entirely whole grain or non-whole grain and estimating intake based on serves of these foods(13). This method fails to account for the mixed composition of many grain foods, which may contain both whole grain and non-whole grain ingredients. To accurately compare associations of whole grain and cereal fibre intakes, food sources need to be examined at an ingredient level such as using food composition databases, allowing whole grain and cereal fibre intake to be reported in terms of total grams per day rather than food serves.
The aim of the current study was to examine associations between whole grain and cereal fibre intakes, quantified systematically using food composition databases and accepted definitions, and risk factors for CVD among adult participants of the 2011–2013 Australian Health Survey (AHS).
Methods
Study population
The current study used data from the 2011–2012 National Nutrition and Physical Activity Survey (NNPAS) and the 2011–2013 National Health Measures Survey (NHMS), located within the ABS Expanded Confidentialised Unit Record Files (CURF). NNPAS is a subcomponent of the 2011–2013 AHS, the most recent nationally representative survey within Australia. The core content of AHS includes household and demographic information from 32 000 Australians. NNPAS was then conducted on 12 153 of these participants, aged between 2 and 85 years, to obtain data on nutritional intake and physical activity. All participants within NNPAS aged ≥5 years were also invited to participate in NHMS, which involved the collection of blood and/or urine to examine nutrient biomarkers and biomarkers of chronic disease. Further details of the survey have been published elsewhere(14).
Within the current study, we excluded participants of AHS who were under 18 years. Additionally, we excluded under-reporters of daily energy intake, based on the Goldberg cut-off of 0·9 for 1 d of intake(15). Of the initial 12 153 participants, 7665 remained in the analysis. However, the available sample size varied between outcomes due to further missing data. The available sample size for each outcome was as follows: BMI (n 6206), waist circumference (n 6214), systolic and diastolic BP (n 6381), total cholesterol and HDL-cholesterol (n 3116), LDL-cholesterol (n 2614), TAG (n 2640), apo-B (n 3115), C-reactive protein (CRP; n 3114), fasting glucose (n 2641) and HbA1c (n 3107).
Food and nutrient intake assessment
Dietary intake data were collected within the survey via one 24-h recall dietary assessment using an adapted version of the Automated Multiple Pass Method(16), a questionnaire designed to maximise food recall and minimise memory bias. The questionnaire includes five phases to develop greater layers of detail and accuracy in answers given. Details on the specific phases and tools used within the questionnaire are provided in the AHS survey user guide(14).
Participant nutrient intake was estimated from the recalled intake data using the customised nutrient composition database developed by Food Standards Australia New Zealand (FSANZ), the AUSNUT 2011–2013 food nutrient database(17). In 2016, free sugars were added to the existing food nutrient database to allow an estimation of free sugar intakes within AHS. Free sugars were defined as per the WHO, including added forms of dextrose, fructose, sucrose, lactose, sugar syrups, fruit syrups, the sugar component of honey, fruit juice and fruit juice concentrates(18). In comparison to total sugars, free sugars do not include natural sugars from intact fruits, vegetables and milk.
Cereal fibre and whole grain intake estimation
Whole grain and cereal fibre contents of each food item reported within the survey were estimated using published food composition databases previously developed from the AUSNUT 2011–2013 food nutrient database(19,20).
In brief, whole grain was defined as per the FSANZ definition as:
‘the intact grain or the dehulled, ground, milled, cracked or flaked grain where the constituents—endosperm, germ and bran—are present in such proportions that represent the typical ratio of those fractions occurring in the whole cereal, and includes wholemeal.’(21)
Whole grain ingredients within the database included BarleyMax™, millet, oats, rice (brown, wild, black and red), rye and wholemeal rye flour, sorghum and wholemeal sorghum flour, triticale, wheat (all varieties) and wholemeal wheat flour, and sprouted whole grains. Pseudo-cereal grains, including amaranth, buckwheat and quinoa, were also considered whole grain due to their similar nutritional composition, preparation and use. The whole grain content of foods was calculated in grams per 100 g of a product on a DW basis, and no limits were set on the minimum whole grain content to be contained in a product.
Cereal fibre was interpreted as fibre that is sourced from cereal grains and pseudo-cereal grains, whether intact or processed within food products, and included cereal fibres that are both intrinsic and extrinsic to the food source. Food sources containing >0·1 g cereal fibre per 100 g of food product were considered sources of cereal fibre, based on the limitations of analytical tests that measure fibre.
Using the databases, whole grain and cereal fibre intakes for the day of the survey were calculated as the summed amount of each whole grain or cereal fibre containing food reported (g) by the participant, multiplied by the amount of whole grain or cereal fibre (g/100 g) within each food.
Outcome assessment
Anthropometric measures of height (cm), weight (kg) and waist circumference (cm) of willing participants were measured in the initial AHS interviews using digital scales, stadiometer and a tape measure, respectively. Waist circumference was measured by placing the tape measure across the top of the umbilicus. BMI was obtained through calculation. BP was measured using an automated BP monitor by trained interviewers. Participants were invited to sit down and extend and relax their left arm (in the case of a prohibitive injury, the right arm was used instead), with their palm facing upwards. Two BP readings were taken, and if either the two systolic or diastolic pressure readings differed by >10 mmHg, a third reading was taken. For participants who needed only two readings, the second reading was used for the measures of systolic and diastolic pressure. When a third reading was needed, the second and third readings were averaged.
Blood samples were collected at Sonic Healthcare collection clinics and analysed at Douglass Hanly Moir (DHM) Pathology (Sydney, Australia). As required for the LDL-cholesterol, TAG and fasting plasma glucose tests, participants providing blood samples were asked to fast for 8 h prior to their test. Total cholesterol and TAG were measured by enzymatic method, and HDL-cholesterol concentrations were measured by enzymatic method with an accelerator-selective detergent (Architect C16200). LDL-cholesterol concentrations were estimated using the Friedewald equation (Architect Ci16200), and apo-B levels were measured by immunoturbidimetry (Integra 800). CRP concentrations were measured by ultrasensitive immunoturbimetric assay (Architect Ci16200); fasting plasma glucose levels were measured by hexokinase method (Integra 800); and HbA1c was measured using cation-exchange HPLC (Bio-Rad Variant™ II TURBO Haemoglobin Testing System). Adult participants who provided lipid profile blood samples in the survey were also asked if they were currently taking lipid-lowering medication as one means of determining dyslipidaemia status. More information on the collection of all outcome measures has been previously described(14).
Lifestyle factors assessment
Sample lifestyle characteristics, including smoking status, physical activity levels (minutes of physical activity for fitness, recreation, sport or transport in the last week), alcohol intake, equivalised weekly income (household income accounting for household size and composition), education level and employment status, were determined within the survey via interview by trained interviewers through computer-assisted personal interview (CAPI)(14).
Data and statistical analysis
Sampling weights were allocated to each participant within the current study to infer results for the total in-scope population. To account for the survey design and sampling process, replica weighting was also applied using jack-knife resampling. All statistical analyses were performed using Stata software, version 14.0 (StataCorp LP).
Participants were divided into quantiles based on energy-adjusted (g/10 MJ per d) whole grain and cereal fibre intake on the day of survey to be analysed separately. Due to varying sample sizes available for each outcome, quantiles were created separately for each outcome analysed. For cereal fibre analyses, participants were categorised into quartiles, with lowest intakes in quartile 1 and highest intakes in quartile 4. As there were a significant number of participants who did not consume any whole grain on the day of survey, participants were divided into tertiles of energy-adjusted whole grain intake (T1 lowest intake; T3 highest intake) or non-whole grain consumers (0 g/d) for whole grain analyses. As quantiles are reflective of weighted participant distribution, the number of unweighted participants (n) in each quantile is not precisely equal.
Sample descriptive characteristics and dietary intakes on the day of survey were first examined between the quantiles of whole grain and cereal fibre intakes. Associations between intakes and continuous variables (e.g., age) were tested with linear regression analysis and a test for trend. Associations with categorical variables (e.g., sex) were tested with a chi-square analysis. Separate linear regression models were then used to determine associations between intakes (in quantiles) and each CVD risk factor examined. TAG concentrations, which were not normally distributed, were log-transformed prior to analysis. Tobit regression was used for CRP analysis, as the data were left-censored. Covariate-adjusted means for each CVD risk factor were predicted for each quantile of whole grain and cereal fibre intake. A test for linear trend was then used to determine statistically significant associations between quantiles of intakes. Statistical significance was set at P < 0·05.
The simple regression model adjusted for age and sex. The multivariate models were further adjusted for energy intake (kJ/d), physical activity (minutes of moderate to intense activity per day), alcohol intake (g/d), smoking status (current smoker, ex-smoker, never smoked) and usual fruit and vegetable intake (serves per day). Within the dietary adjustment model, BP outcomes analysed were further adjusted for Na intake (mg/d); lipid constituent analyses were adjusted for saturated and trans-fat intake and polyunsaturated fat intake (% energy per day). TAG analysis, as well as fasting blood glucose and HbA1c analyses, were further adjusted for free sugars intake (% energy per day). All outcome analyses, except for BMI and waist circumference, were further adjusted for BMI, and all lipid constituent analyses were adjusted for current lipid-lowering medication use, within a lifestyle adjustment model. Lastly, the final models (dietary adjustment models for BMI and waist circumference; lifestyle adjustment models for all other outcomes) used in whole grain analyses were further adjusted for cereal fibre intake (g/d).
Results
Descriptive characteristics and dietary intakes of participants on the day of survey, by quantile of whole grain and cereal fibre intakes, are shown in Tables 1 and 2, respectively. Age was positively associated with both whole grain and cereal fibre intake (both P < 0·0001). Participants within the highest tertile of energy-adjusted whole grain intake (T3) reported a higher intake of cereal fibre, total fibre, carbohydrates and polyunsaturated fat, as well as a higher estimated daily fruit and vegetable servings (all P < 0·0001). These participants reported significantly lower intakes of total fat, free sugars, saturated and trans-fat, monounsaturated fat, Na and alcohol intake (all P < 0·0001), reflecting an overall higher-diet quality in higher whole grain consumers. Participants who did not report consuming any whole grains on the day of survey were more likely to smoke (P < 0·0001), less likely to have a university degree (P = 0·0019), less likely to be currently employed or studying (P = 0·008) and less likely to be meeting current Australian physical activity recommendation for >150 min of exercise per week (P < 0·0001).
Table 1.
Nutrient intakes and demographic characteristics of participants in Australian Health Survey 2011–2013 by tertile of energy-adjusted whole grain intake
| Non-consumers (n 2043)* | T1 (n 1794) | T2 (n 1874) | T3 (n 1954) | P-value† | |||||
|---|---|---|---|---|---|---|---|---|---|
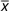 or %
or % |
se |
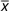 or %
or % |
se |
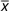 or %
or % |
se |
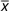 or %
or % |
se | ||
| Age (years)‡ | 42·0 | 0·5 | 44·1 | 0·4 | 48·1 | 0·5 | 49·8 | 0·6 | <0·0001 |
| Sex (% female) | 48·7 | – | 50·3 | – | 51·5 | 49·6 | – | 0·65 | |
| Energy (kJ/d)§ | 9163·4 | 90·8 | 10 086·2 | 94·5 | 9878·9 | 90·1 | 9103·9 | 90·3 | 0·33 |
| Cereal fibre (g/d)‖ | 5·8 | 0·1 | 7·1 | 0·1 | 10·2 | 0·1 | 14·6 | 0·2 | <0·0001 |
| Total fibre (g/d)‖ | 19·4 | 0·3 | 22·3 | 0·3 | 26·3 | 0·3 | 31·6 | 0·4 | <0·0001 |
| Carbohydrate (% kJ)‖ | 42·0 | 0·4 | 42·0 | 0·3 | 42·8 | 0·3 | 46·0 | 0·3 | <0·0001 |
| Protein (% kJ)‖ | 18·0 | 0·2 | 17·5 | 0·2 | 18·3 | 0·1 | 18·0 | 0·1 | 0·13 |
| Total fat (% kJ)‖ | 32·0 | 0·3 | 32·3 | 0·3 | 31·5 | 0·3 | 29·6 | 0·2 | <0·0001 |
| Free sugars (% kJ)‖ | 11·9 | 0·3 | 11·2 | 0·2 | 10·1 | 0·2 | 8·7 | 0·2 | <0·0001 |
| Saturated and trans-fat (% kJ)‖ | 13·0 | 0·2 | 12·7 | 0·2 | 12·5 | 0·1 | 11·2 | 0·1 | <0·0001 |
| MUFA (% kJ)‖ | 12·4 | 0·1 | 12·5 | 0·1 | 11·9 | 0·1 | 11·1 | 0·1 | <0·0001 |
| PUFA (% kJ)‖ | 4·5 | 0·1 | 4·8 | 0·1 | 4·9 | 0·1 | 5·2 | 0·1 | <0·0001 |
| Na (mg/d)‖ | 2769·0 | 44·9 | 2692·1 | 34·3 | 2627·3 | 31·8 | 2479·9 | 33·0 | <0·0001 |
| Fruit (serves/d) | 1·4 | 0·04 | 1·5 | 0·03 | 1·7 | 0·30 | 1·8 | 0·04 | <0·0001 |
| Vegetable (serves per day) | 2·2 | 0·05 | 2·4 | 0·05 | 2·4 | 0·04 | 2·5 | 0·04 | <0·0001 |
| Daily alcohol intake (standard drinks per day)§ ¶ | 1·9 | 0·1 | 2·1 | 0·1 | 1·5 | 0·1 | 1·0 | 0·1 | <0·0001 |
| Equivalised weekly income (AUD)§ | 946·3 | 25·2 | 1104·4 | 28·6 | 1076·4 | 28·0 | 996·5 | 21·4 | 0·28 |
| University graduate (%) | 22·0 | – | 29·6 | – | 26·1 | – | 27·0 | – | 0·0019 |
| Unemployed, not studying (%) | 3·0 | – | 2·1 | – | 1·0 | – | 1·4 | – | 0·0078 |
| Current smoker (%) | 26·2 | – | 16·2 | – | 14·4 | – | 10·5 | – | <0·0001 |
| Physical activity (% meeting recommendation)** | 42·7 | – | 53·1 | – | 53·0 | – | 54·4 | – | <0·0001 |
| Lipid-lowering medication use†† | 8·9 | – | 11·4 | – | 13·1 | – | 19·4 | – | 0·0007 |
Median whole grain intakes (g/d): non-consumers 0; T1 14·0; T2 44·9; T3 95·1.
Associations with continuous variables were tested with linear regression analysis and a test for trend; associations with categorical variables were tested with a chi-square analysis.
Adjusted for sex.
Adjusted for age and sex.
Adjusted for age, sex and total energy intake (kJ/d).
One standard drink is equivalent to 10 g of alcohol.
Australian physical activity recommendation of 150 min of exercise per week.
Based on the data available from smaller sample of participants (n 2608).
Table 2.
Nutrient intakes and demographic characteristics of participants in Australian Health Survey 2011–2013 by quartile of energy-adjusted cereal fibre intake
| Q1 (n 1940)* | Q2 (n 1941) | Q3 (n 1801) | Q4 (n 1983) | P-value† | |||||
|---|---|---|---|---|---|---|---|---|---|
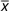 or %
or % |
se |
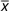 or %
or % |
se |
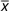 or %
or % |
se |
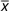 or %
or % |
se | ||
| Age (years)‡ | 44·0 | 0·4 | 44·5 | 0·5 | 46·6 | 0·5 | 48·4 | 0·6 | <0·0001 |
| Sex (% female) | 51·0 | – | 50·0 | – | 50·6 | – | 48·2 | – | 0·60 |
| Energy (kJ/d)§ | 9858·9 | 107·5 | 9840·7 | 111·1 | 9360·4 | 110·0 | 9117·6 | 74·1 | <0·0001 |
| Whole grain (g/d)‖ | 6·0 | 0·5 | 20·8 | 0·7 | 41·8 | 0·9 | 79·2 | 1·7 | <0·0001 |
| Total fibre (g/d)‖ | 18·8 | 0·3 | 22·6 | 0·3 | 25·3 | 0·3 | 32·2 | 0·4 | <0·0001 |
| Carbohydrate (% kJ)‖ | 37·3 | 0·3 | 42·5 | 0·3 | 44·9 | 0·3 | 48·0 | 0·3 | <0·0001 |
| Protein (% kJ)‖ | 18·6 | 0·2 | 18·1 | 0·2 | 17·7 | 0·2 | 17·4 | 0·1 | <0·0001 |
| Total fat (% kJ)‖ | 33·6 | 0·3 | 31·9 | 0·3 | 30·9 | 0·3 | 28·9 | 0·2 | <0·0001 |
| Free sugars (% kJ)‖ | 12·3 | 0·3 | 11·3 | 0·3 | 10·0 | 0·2 | 8·5 | 0·2 | <0·0001 |
| Saturated and trans-fat (% kJ)‖ | 13·2 | 0·2 | 12·8 | 0·2 | 12·3 | 0·1 | 11·2 | 0·1 | <0·0001 |
| MUFA (% kJ)‖ | 13·3 | 0·1 | 12·2 | 0·1 | 11·6 | 0·1 | 10·8 | 0·1 | <0·0001 |
| PUFA (% kJ)‖ | 4·8 | 0·1 | 4·8 | 0·1 | 4·9 | 0·1 | 4·9 | 0·1 | 0·35 |
| Na (mg/d)‖ | 2516·9 | 47·1 | 2778·1 | 35·8 | 2688·8 | 33·7 | 2601·6 | 32·9 | 0·41 |
| Fruit (serves per day)‖ | 1·4 | 0·05 | 1·5 | 0·04 | 1·7 | 0·04 | 1·7 | 0·04 | <0·0001 |
| Vegetables (serves per day)‖ | 2·3 | 0·1 | 2·3 | 0·04 | 2·4 | 0·04 | 2·4 | 0·05 | 0·30 |
| Daily alcohol intake (standard drinks per day)§ ¶ | 3·0 | 0·2 | 1·7 | 0·1 | 1·2 | 0·1 | 0·7 | 0·1 | <0·0001 |
| Equivalised weekly income (AUD)§ | 1068·4 | 24·9 | 1044·0 | 27·8 | 990·9 | 23·2 | 1014·8 | 25·1 | 0·07 |
| University graduate (%) | 25·4 | – | 24·9 | – | 27·3 | – | 26·6 | – | 0·57 |
| Unemployed, not studying (%) | 2·7 | – | 2·4 | – | 1·6 | – | 0·9 | – | 0·0277 |
| Current smoker (%) | 25·2 | – | 17·9 | – | 13·9 | – | 11·6 | – | <0·0001 |
| Physical activity (% meeting recommendation)** | 47·1 | – | 50·0 | – | 53·7 | – | 51·4 | – | 0·08 |
| Lipid-lowering medication use†† | 11·2 | – | 12·4 | – | 12·8 | – | 16·8 | – | 0·18 |
Median cereal fibre intake (g/d): Q1 3·9; Q2 7·4; Q3 10·7; Q4 16·3.
Associations with continuous variables were tested with linear regression analysis and a test for trend; associations with categorical variables were tested with a chi-square analysis.
Adjusted for sex.
Adjusted for age and sex.
Adjusted for age, sex and total energy intake (kJ/d).
One standard drink is equivalent to 10 g of alcohol.
Australian physical activity recommendation of 150 min of moderate exercise per week.
Based on the data available from smaller sample of participants (n 2608).
Participants reporting the highest consumption of energy-adjusted cereal fibre (Q4) on the day of survey had similar diet and lifestyle characteristics to high whole grain consumers. Participants in Q4 of energy-adjusted cereal fibre intake reported higher whole grain, total fibre, carbohydrate and estimated daily fruit intakes (all P < 0·0001) while reporting lower protein, total fat, free sugars, saturated and trans-fat, monounsaturated fat and daily alcohol intakes (P < 0·0001). There were fewer smokers in Q4 (P < 0·0001), and participants were more likely to be currently employed or studying (P = 0·03).
Tables 3 and 4 report associations between whole grain and cereal fibre intakes and CVD risk factors. Whole grain and cereal fibre intake were both inversely associated with BMI and waist circumference within our multivariate analysis. Compared with participants who did not consume any whole grain on the day of survey, those in the highest tertile of intake had lower BMI (non-consumers 26·8 kg/m2; T3 26·0 kg/m2, P < 0·0001) and waist circumference (non-consumers 92·2 cm; T3 89·7 cm, P = 0·0005). After adjusting for cereal fibre intake, the inverse association to BMI was no longer significant (P = 0·11), while the association to waist circumference was attenuated but remained significant (P = 0·03). When cereal fibre intake was analysed separately, comparable inverse associations were found for BMI (Q1 27·1 kg/m2; 26·1 kg/m2, P < 0·0001) and waist circumference (Q1 92·3 cm; Q4 90·1 cm, P = 0·0008).
Table 3.
CVD risk factors according to tertiles of energy-adjusted whole grain intake among participants of Australian Health Survey 2011–2013
| Non-consumers | T1 | T2 | T3 | P for trend | |||||
|---|---|---|---|---|---|---|---|---|---|
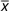
|
se |
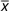
|
se |
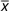
|
se |
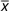
|
se | ||
| BMI (kg/m2) (n 6206) | |||||||||
| Simple adjustment* | 26·9 | 0·2 | 26·9 | 0·2 | 26·7 | 0·1 | 25·9 | 0·1 | <0·0001 |
| Multivariate adjustment† | 26·8 | 0·2 | 26·8 | 0·2 | 26·6 | 0·1 | 26·0 | 0·1 | <0·0001 |
| Cereal fibre adjustment† ‡ | 26·7 | 0·2 | 26·7 | 0·2 | 26·7 | 0·1 | 26·3 | 0·2 | 0·11 |
| Waist circumference (n 6214) | |||||||||
| Simple adjustment* | 92·3 | 0·5 | 91·9 | 0·4 | 91·9 | 0·4 | 89·4 | 0·4 | <0·0001 |
| Multivariate adjustment† | 92·2 | 0·5 | 91·8 | 0·5 | 91·8 | 0·4 | 89·7 | 0·4 | 0·0005 |
| Cereal fibre adjustment† ‡ | 92·0 | 0·6 | 91·7 | 0·5 | 91·8 | 0·4 | 90·0 | 0·4 | 0·03 |
| Systolic BP (mmHg) (n 6381) | |||||||||
| Simple adjustment* | 122·3 | 0·5 | 121·8 | 0·6 | 122·1 | 0·5 | 121·6 | 0·6 | 0·51 |
| Multivariate adjustment† | 122·2 | 0·5 | 121·7 | 0·7 | 122·1 | 0·5 | 121·9 | 0·6 | 0·87 |
| Dietary adjustment† § | 122·2 | 0·5 | 121·6 | 0·7 | 122·1 | 0·5 | 122·0 | 0·6 | 0·97 |
| Lifestyle adjustment† § ‖ | 122·2 | 0·6 | 121·8 | 0·7 | 122·2 | 0·5 | 122·4 | 0·6 | 0·69 |
| Cereal fibre adjustment† ‡ § ‖ | 122·2 | 0·6 | 121·7 | 0·6 | 122·2 | 0·5 | 122·4 | 0·8 | 0·71 |
| Diastolic BP (mmHg) (n 6381) | |||||||||
| Simple adjustment* | 76·9 | 0·4 | 75·9 | 0·4 | 76·0 | 0·3 | 75·2 | 0·4 | 0·004 |
| Multivariate adjustment† | 76·7 | 0·4 | 75·7 | 0·4 | 76·1 | 0·4 | 75·5 | 0·4 | 0·07 |
| Dietary adjustment† § | 76·7 | 0·4 | 75·6 | 0·4 | 76·1 | 0·4 | 75·6 | 0·4 | 0·09 |
| Lifestyle adjustment† § ‖ | 76·6 | 0·4 | 75·4 | 0·4 | 76·0 | 0·3 | 75·8 | 0·4 | 0·33 |
| Cereal fibre adjustment† ‡ § ‖ | 76·4 | 0·4 | 75·3 | 0·4 | 76·1 | 0·3 | 76·1 | 0·5 | 0·91 |
| Total cholesterol (mmol/l) (n 3116) | |||||||||
| Simple adjustment* | 5·10 | 0·06 | 5·09 | 0·06 | 4·95 | 0·06 | 5·02 | 0·05 | 0·15 |
| Multivariate adjustment† | 5·07 | 0·06 | 5·07 | 0·06 | 4·97 | 0·06 | 5·04 | 0·05 | 0·52 |
| Dietary adjustment† ¶ | 5·07 | 0·06 | 5·07 | 0·06 | 4·96 | 0·06 | 5·04 | 0·05 | 0·49 |
| Lifestyle adjustment† ¶ ** | 5·03 | 0·05 | 5·05 | 0·06 | 4·95 | 0·06 | 5·07 | 0·05 | 0·97 |
| Cereal fibre adjustment† ‡ ¶ ** | 5·00 | 0·05 | 5·03 | 0·06 | 4·96 | 0·06 | 5·11 | 0·06 | 0·40 |
| HDL-cholesterol (mmol/l) (n 3116) | |||||||||
| Simple adjustment* | 1·35 | 0·02 | 1·38 | 0·02 | 1·35 | 0·02 | 1·36 | 0·02 | 0·96 |
| Multivariate adjustment† | 1·35 | 0·02 | 1·37 | 0·02 | 1·35 | 0·02 | 1·36 | 0·02 | 0·86 |
| Dietary adjustment† ¶ | 1·35 | 0·02 | 1·37 | 0·02 | 1·35 | 0·02 | 1·36 | 0·02 | 0·78 |
| Lifestyle adjustment† ¶ ** | 1·35 | 0·02 | 1·36 | 0·02 | 1·35 | 0·02 | 1·36 | 0·02 | 0·97 |
| Cereal fibre adjustment† ‡ ¶ ** | 1·36 | 0·02 | 1·37 | 0·02 | 1·35 | 0·02 | 1·34 | 0·02 | 0·50 |
| LDL-cholesterol (mmol/l) (n 2614) | |||||||||
| Simple adjustment* | 3·17 | 0·06 | 3·13 | 0·06 | 3·08 | 0·05 | 3·09 | 0·05 | 0·25 |
| Multivariate adjustment† | 3·16 | 0·05 | 3·12 | 0·05 | 3·08 | 0·06 | 3·11 | 0·05 | 0·40 |
| Dietary adjustment† ¶ | 3·16 | 0·05 | 3·12 | 0·06 | 3·08 | 0·06 | 3·11 | 0·05 | 0·39 |
| Lifestyle adjustment† ¶ ** | 3·14 | 0·05 | 3·11 | 0·05 | 3·06 | 0·06 | 3·14 | 0·05 | 0·88 |
| Cereal fibre adjustment† ‡ ¶ ** | 3·10 | 0·05 | 3·09 | 0·05 | 3·07 | 0·06 | 3·18 | 0·05 | 0·40 |
| Apo-B (mmol/l) (n 3115) | |||||||||
| Simple adjustment* | 1·00 | 0·02 | 0·99 | 0·02 | 0·97 | 0·02 | 0·97 | 0·01 | 0·15 |
| Multivariate adjustment† | 0·99 | 0·02 | 0·99 | 0·02 | 0·98 | 0·02 | 0·97 | 0·02 | 0·38 |
| Dietary adjustment† ¶ | 0·99 | 0·02 | 0·99 | 0·02 | 0·98 | 0·02 | 0·97 | 0·01 | 0·30 |
| Lifestyle adjustment† ¶ ** | 0·98 | 0·02 | 0·98 | 0·02 | 0·97 | 0·02 | 0·98 | 0·02 | 0·79 |
| Cereal fibre adjustment† ‡ ¶ ** | 0·97 | 0·02 | 0·97 | 0·02 | 0·98 | 0·02 | 0·99 | 0·01 | 0·42 |
| C-reactive protein (mmol/l) (n 3114) | |||||||||
| Simple adjustment* | 3·19 | 0·41 | 2·93 | 0·29 | 2·51 | 0·21 | 2·52 | 0·25 | 0·10 |
| Multivariate adjustment† | 3·17 | 0·39 | 2·88 | 0·29 | 2·52 | 0·21 | 2·59 | 0·25 | 0·14 |
| Dietary adjustment† ¶ | 3·13 | 0·40 | 2·88 | 0·29 | 2·55 | 0·21 | 2·59 | 0·25 | 0·20 |
| Lifestyle adjustment† ¶ ** | 3·04 | 0·43 | 2·71 | 0·27 | 2·52 | 0·21 | 2·59 | 0·28 | 0·36 |
| Cereal fibre adjustment† ‡ ¶ ** | 3·16 | 0·42 | 2·78 | 0·31 | 2·49 | 0·21 | 2·44 | 0·28 | 0·15 |
| TAG (mmol/l) (n 2640) | |||||||||
| Simple adjustment* | 1·16 | 0·02 | 1·10 | 0·03 | 1·07 | 0·03 | 1·10 | 0·03 | 0·14 |
| Multivariate adjustment† | 1·13 | 0·03 | 1·11 | 0·03 | 1·07 | 0·03 | 1·10 | 0·03 | 0·39 |
| Dietary adjustment† †† | 1·12 | 0·03 | 1·11 | 0·03 | 1·07 | 0·03 | 1·11 | 0·03 | 0·60 |
| Lifestyle adjustment† ** †† | 1·11 | 0·02 | 1·11 | 0·03 | 1·07 | 0·03 | 1·11 | 0·03 | 0·88 |
| Cereal fibre adjustment† ‡ ** †† | 1·11 | 0·02 | 1·11 | 0·03 | 1·07 | 0·03 | 1·10 | 0·03 | 0·69 |
| Fasting glucose (mmol/l) (n 2641) | |||||||||
| Simple adjustment* | 5·09 | 0·04 | 5·11 | 0·03 | 5·00 | 0·03 | 5·00 | 0·03 | 0·02 |
| Multivariate adjustment† | 5·09 | 0·04 | 5·11 | 0·04 | 5·01 | 0·03 | 5·01 | 0·03 | 0·07 |
| Dietary adjustment† ‡‡ | 5·09 | 0·04 | 5·11 | 0·04 | 5·01 | 0·03 | 5·01 | 0·03 | 0·07 |
| Lifestyle adjustment† ‖ ‡‡ | 5·09 | 0·04 | 5·11 | 0·03 | 5·00 | 0·03 | 5·02 | 0·03 | 0·06 |
| Cereal fibre adjustment† ‡ ‖ ‡‡ | 5·10 | 0·05 | 5·11 | 0·04 | 5·00 | 0·03 | 5·00 | 0·03 | 0·048 |
| HbA1c (%) (n 3107) | |||||||||
| Simple adjustment* | 5·48 | 0·04 | 5·43 | 0·03 | 5·39 | 0·02 | 5·38 | 0·02 | 0·02 |
| Multivariate adjustment† | 5·47 | 0·04 | 5·44 | 0·03 | 5·39 | 0·02 | 5·38 | 0·02 | 0·03 |
| Dietary adjustment† ‡‡ | 5·47 | 0·04 | 5·44 | 0·03 | 5·39 | 0·02 | 5·38 | 0·02 | 0·03 |
| Lifestyle adjustment† ‖ ‡‡ | 5·47 | 0·04 | 5·44 | 0·03 | 5·40 | 0·02 | 5·38 | 0·02 | 0·05 |
| Cereal fibre adjustment† ‡ ‖ ‡‡ | 5·48 | 0·04 | 5·45 | 0·03 | 5·39 | 0·02 | 5·37 | 0·02 | 0·03 |
BP, blood pressure.
Adjusted for age and sex.
Adjusted for age, sex, total energy intake, alcohol intake, smoking status, physical activity, fruit and vegetable serves.
Adjusted for cereal fibre intake.
Adjusted for Na intake.
Adjusted for BMI.
Adjusted for saturated and trans-fat intake and PUFA intake.
Adjusted for BMI and lipid-lowering medication use.
Adjusted for saturated and trans-fat intake, PUFA intake and free sugar intake.
Adjusted for free sugar intake.
Table 4.
CVD risk factors according to quartiles of energy-adjusted cereal fibre intake among participants of Australian Health Survey 2011–2013
| Q1 | Q2 | Q3 | Q4 | P for trend | |||||
|---|---|---|---|---|---|---|---|---|---|
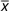
|
se |
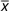
|
se |
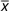
|
se |
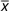
|
se | ||
| BMI (kg/m2) (n 6206) | |||||||||
| Simple adjustment* | 27·1 | 0·2 | 26·9 | 0·2 | 26·3 | 0·2 | 26·0 | 0·2 | <0·0001 |
| Multivariate adjustment† | 27·1 | 0·2 | 26·9 | 0·2 | 26·4 | 0·2 | 26·1 | 0·2 | <0·0001 |
| Waist circumference (n 6214) | |||||||||
| Simple adjustment* | 92·6 | 0·6 | 92·3 | 0·5 | 90·9 | 0·4 | 89·9 | 0·4 | <0·0001 |
| Multivariate adjustment† | 92·3 | 0·5 | 92·1 | 0·5 | 91·0 | 0·4 | 90·1 | 0·4 | 0·0008 |
| Systolic BP (mmHg) (n 6381) | |||||||||
| Simple adjustment* | 122·5 | 0·7 | 121·7 | 0·6 | 122·3 | 0·5 | 121·8 | 0·6 | 0·31 |
| Multivariate adjustment† | 122·1 | 0·4 | 123·2 | 0·4 | 123·6 | 0·4 | 122·7 | 0·4 | 0·86 |
| Dietary adjustment† ‡ | 122·2 | 0·7 | 121·7 | 0·6 | 122·2 | 0·5 | 121·8 | 0·6 | 0·84 |
| Lifestyle adjustment† ‡ § | 122·0 | 0·7 | 121·8 | 0·6 | 122·5 | 0·5 | 122·3 | 0·6 | 0·55 |
| Diastolic BP (mmHg) (n 6381) | |||||||||
| Simple adjustment* | 76·9 | 0·4 | 76·0 | 0·3 | 76·1 | 0·4 | 75·2 | 0·4 | 0·004 |
| Multivariate adjustment† | 76·5 | 0·4 | 75·9 | 0·3 | 76·2 | 0·4 | 75·5 | 0·4 | 0·10 |
| Dietary adjustment† ‡ | 76·5 | 0·4 | 75·9 | 0·3 | 76·2 | 0·4 | 75·5 | 0·4 | 0·10 |
| Lifestyle adjustment† ‡ § | 76·1 | 0·4 | 75·7 | 0·3 | 76·2 | 0·3 | 75·8 | 0·4 | 0·80 |
| Total cholesterol (mmol/l) (n 3116) | |||||||||
| Simple adjustment* | 5·15 | 0·06 | 5·10 | 0·05 | 4·98 | 0·06 | 4·92 | 0·05 | 0·002 |
| Multivariate adjustment† | 5·11 | 0·06 | 5·10 | 0·05 | 4·97 | 0·06 | 4·95 | 0·06 | 0·02 |
| Dietary adjustment† ‖ | 5·11 | 0·06 | 5·10 | 0·05 | 4·97 | 0·06 | 4·95 | 0·05 | 0·02 |
| Lifestyle adjustment† ‖ ¶ | 5·07 | 0·06 | 5·10 | 0·05 | 4·96 | 0·06 | 4·97 | 0·06 | 0·07 |
| HDL-cholesterol (mmol/l) (n 3116) | |||||||||
| Simple adjustment* | 1·37 | 0·02 | 1·37 | 0·02 | 1·35 | 0·02 | 1·35 | 0·02 | 0·26 |
| Multivariate adjustment† | 1·35 | 0·02 | 1·37 | 0·02 | 1·35 | 0·02 | 1·36 | 0·02 | 0·82 |
| Dietary adjustment† ‖ | 1·34 | 0·02 | 1·37 | 0·02 | 1·35 | 0·02 | 1·37 | 0·02 | 0·53 |
| Lifestyle adjustment† ‖ ¶ | 1·35 | 0·02 | 1·37 | 0·02 | 1·34 | 0·02 | 1·36 | 0·02 | 0·92 |
| LDL-cholesterol (mmol/l) (n 2614) | |||||||||
| Simple adjustment* | 3·20 | 0·05 | 3·18 | 0·05 | 3·05 | 0·05 | 3·04 | 0·06 | 0·01 |
| Multivariate adjustment† | 3·20 | 0·05 | 3·18 | 0·05 | 3·03 | 0·05 | 3·05 | 0·06 | 0·02 |
| Dietary adjustment† ‖ | 3·20 | 0·05 | 3·18 | 0·05 | 3·02 | 0·05 | 3·05 | 0·06 | 0·02 |
| Lifestyle adjustment† ‖ ¶ | 3·18 | 0·05 | 3·17 | 0·05 | 3·03 | 0·05 | 3·08 | 0·06 | 0·07 |
| Apo-B (mmol/l) (n 3115) | |||||||||
| Simple adjustment* | 1·02 | 0·02 | 0·99 | 0·02 | 0·97 | 0·02 | 0·95 | 0·02 | 0·006 |
| Multivariate adjustment† | 1·01 | 0·02 | 0·99 | 0·02 | 0·97 | 0·02 | 0·96 | 0·02 | 0·02 |
| Dietary adjustment† ‖ | 1·02 | 0·02 | 0·99 | 0·02 | 0·97 | 0·02 | 0·96 | 0·02 | 0·009 |
| Lifestyle adjustment† ‖ ¶ | 1·00 | 0·02 | 0·99 | 0·02 | 0·97 | 0·02 | 0·96 | 0·02 | 0·06 |
| C-reactive protein (mmol/l) (n 3114) | |||||||||
| Simple adjustment* | 3·10 | 0·35 | 2·63 | 0·18 | 2·56 | 0·24 | 2·83 | 0·26 | 0·51 |
| Multivariate adjustment† | 3·09 | 0·36 | 2·62 | 0·18 | 2·59 | 0·25 | 2·82 | 0·25 | 0·53 |
| Dietary adjustment† ‖ | 3·13 | 0·38 | 2·60 | 0·18 | 2·60 | 0·25 | 2·78 | 0·26 | 0·49 |
| Lifestyle adjustment† ‖ ¶ | 2·90 | 0·43 | 2·46 | 0·15 | 2·62 | 0·24 | 2·83 | 0·27 | 0·97 |
| TAG (mmol/l) (n 2640) | |||||||||
| Simple adjustment* | 1·13 | 0·02 | 1·12 | 0·03 | 1·07 | 0·03 | 1·09 | 0·03 | 0·18 |
| Multivariate adjustment† | 1·12 | 0·02 | 1·12 | 0·03 | 1·06 | 0·03 | 1·10 | 0·03 | 0·35 |
| Dietary adjustment† ** | 1·11 | 0·03 | 1·12 | 0·03 | 1·07 | 0·03 | 1·11 | 0·03 | 0·68 |
| Lifestyle adjustment† ¶ ** | 1·09 | 0·03 | 1·11 | 0·03 | 1·08 | 0·03 | 1·12 | 0·03 | 0·77 |
| Fasting glucose (mmol/l) (n 2641) | |||||||||
| Simple adjustment* | 5·08 | 0·03 | 5·04 | 0·03 | 5·05 | 0·04 | 5·03 | 0·04 | 0·35 |
| Multivariate adjustment† | 5·07 | 0·03 | 5·04 | 0·04 | 5·06 | 0·03 | 5·04 | 0·04 | 0·65 |
| Dietary adjustment† †† | 5·07 | 0·03 | 5·04 | 0·04 | 5·06 | 0·04 | 5·04 | 0·04 | 0·62 |
| Lifestyle adjustment† § †† | 5·06 | 0·03 | 5·03 | 0·04 | 5·07 | 0·04 | 5·05 | 0·04 | 0·99 |
| HbA1c (%) (n 3107) | |||||||||
| Simple adjustment* | 5·43 | 0·03 | 5·43 | 0·03 | 5·42 | 0·03 | 5·41 | 0·02 | 0·51 |
| Multivariate adjustment† | 5·43 | 0·03 | 5·43 | 0·03 | 5·42 | 0·03 | 5·41 | 0·02 | 0·62 |
| Dietary adjustment† †† | 5·43 | 0·03 | 5·43 | 0·03 | 5·42 | 0·03 | 5·41 | 0·02 | 0·47 |
| Lifestyle adjustment† § †† | 5·41 | 0·03 | 5·44 | 0·03 | 5·42 | 0·03 | 5·41 | 0·02 | 0·84 |
BP, blood pressure.
Adjusted for age and sex.
Adjusted for age, sex, total energy, alcohol intake, smoking status, physical activity, fruit and vegetable serves.
Adjusted for sodium intake.
Adjusted for BMI.
Adjusted for saturated and trans-fat intake and PUFA intake.
Adjusted for BMI and lipid-lowering medication use.
Adjusted for saturated and trans-fat intake, PUFA intake and free sugar intake.
Adjusted for free sugar intake.
Cereal fibre intake, but not whole grain intake, was inversely associated with total cholesterol, LDL-cholesterol and apo-B concentrations within our analysis. Participants in the highest quartile of cereal fibre intake had lower total cholesterol (Q1 5·11 mmol/l; Q4 4·95 mmol/l, P = 0·02), LDL-cholesterol (Q1 3·20 mmol/l; Q4 3·05 mmol/l, P = 0·02) and apo-B concentrations (Q1 1·02 mmol/l; Q4 0·96 mmol/l, P = 0·009) after multivariate and dietary adjustment. After further adjusting for BMI and use of hypercholesterolaemic agents, the significance of all three associations was attenuated to borderline significance (P = 0·07, 0·07 and 0·06, respectively), although the inverse trends were still evident.
In contrast, whole grain intake was slightly associated with fasting plasma glucose and HbA1c, while cereal fibre showed no association. Participants in the highest tertile of whole grain intake had marginally lower fasting plasma glucose compared with non-consumers in the simple model (non-consumers 5·09 mmol/l; T3 5·00 mmol/l, P = 0·02), although statistical significance was lost after adjusting for various relevant dietary and lifestyle factors (P = 0·06). Interestingly, a further adjustment for cereal fibre intake strengthened the association to borderline significance (P = 0·048). A similar trend was found for the association to HbA1c levels, whereby an inverse association was observed after adjustments in the multivariate and dietary adjustment models (non-consumers 5·47 %; T3 5·38 %, P = 0·03), but this was attenuated slightly after further adjusting for BMI (P = 0·05). When the model was additionally adjusted for cereal fibre, the effect became stronger (P = 0·03).
All other risk factors assessed were not significantly associated with cereal fibre or whole grain after multivariate adjustment (P > 0·05 for all).
Discussion
In the current study, whole grain and cereal fibre were both inversely associated with BMI and waist circumference, and the cereal fibre appeared to, at least partially, explain the association. These findings are in line with previous observational studies of similar populations(4,6), and a recent meta-analysis of intervention trials found that higher intakes of both dietary fibre and whole grains reduced bodyweight, with pooled effect estimates showing mean differences of –0·37 (–0·63, –0·11) and –0·62 (–1·19, –0·05), respectively(22). Dietary fibre may play a role in bodyweight regulation through a few related mechanisms, including displacement of available calories and macronutrients in the diet, decreased gastric emptying rate and increased satiation and satiety, therefore reducing energy intake(23). These mechanisms may explain our findings, as energy intake was inversely related with cereal fibre intake (although not whole grain intake) here. Interestingly, while high whole grain and fibre intakes have been shown to increase subjective satiety, few studies demonstrated any impact of this on subsequent energy intake(24), suggesting the mechanisms may not be entirely clear. Additional whole grain characteristics that could also contribute to favourable weight management may include structure(25) and glycaemic index(26), although the findings are inconsistent(27). To some extent, these mechanisms still have some dependency on subsequent reduced energy intake. Longer-term intervention trials, with whole grain and high cereal fibre products provided as part of an otherwise unrestricted diet, are needed to explore the effects of the isolated fibre component on energy intake and subsequent weight management.
Cereal fibre intake was inversely related to total cholesterol, LDL-cholesterol and apo-B, while whole grain intake was not significantly associated to any of these CVD risk factors. This is in contrast to previous studies, whereby either no associations were found for either intake(28), or inverse associations between the two were of similar magnitude(6). Soluble fibre found in grains such as oats and barley may play a role in lowering cholesterol levels by reducing the absorption of cholesterol into the bloodstream, and this is supported by intervention trials(29). Given the relevance of the fibre component, perhaps it is not surprising for the magnitude of association to be stronger for cereal fibre specifically. However, an inverse association for whole grain would still be expected. Notably, the association between cereal fibre intake and lipid constituents here was attenuated when the model was further adjusted for lipid-lowering medication use and BMI. It may be that participants taking lipid-lowering medication were more likely to have made relevant dietary changes, as a significant association was found between whole grain intake and lipid-lowering medication use, and a positive linear trend between cereal fibre intake and lipid-lowering medication use (P = 0·18). However, more likely, BMI may have partially mediated the association between cereal fibre and lipid constituents, as cereal fibre showed a strong inverse association with BMI independently, and hypercholesterolaemia is linked to obesity(30). However, no significant association was seen between whole grains and lipid constituents despite the inverse association present between whole grains and BMI, and the attenuation found may also be partly a result of reduced sample size. The association between cereal fibre and lipid constituents may be explained by a combination of both direct and indirect mechanisms, including weight management.
Whole grain intake was marginally inversely associated with fasting plasma glucose and HbA1c. This is in contrast to similar studies in US cohorts(5,31,32), which showed no relation to blood glucose measures, although these studies tended to show an inverse association to fasting insulin concentrations and insulin resistance. It was interesting that in our study, after adjusting for cereal fibre intake, the inverse associations became stronger. Notably, these unexpected results support the findings of our recent analysis of UK NDNS RP 2008–2014(28), and therefore this area requires further exploration. While it is unlikely that the cereal fibre itself is positively associated with fasting glucose and HbA1c, potentially there is a characteristic of high cereal fibre, low whole grain diets that are affecting these associations. Within the UK study, we found that people consuming high cereal fibre, low whole grain diets tended to consume larger amounts of refined grain products such as white bread and white pasta, therefore still achieving these higher fibre intakes, but not through whole grain choices that would be typically encouraged as part of a healthful diet. Previously, high refined grain intake has been associated with type 2 diabetes mellitus risk, although not consistently(33,34). Certainly, this suggests that the cereal fibre alone may not be the only contributor to any metabolic benefit that exists for whole grains. Beyond fibre, other whole grain constituents such as vitamin E(35), mg(36) and phytoestrogens(37) may also have beneficial effects on glucose metabolism, potentially contributing to associations seen here. Further, these findings suggest that any benefit that cereal fibre may contribute could potentially be outweighed by unhealthful characteristics of particular food choices such as refined grains.
The current study has several limitations. The descriptive, cross-sectional design of AHS poses a limitation in results, as it is impossible to determine causation between whole grain and cereal fibre intakes and the CVD risk factors examined, and the results can only determine associations. While careful adjustment was made for covariates within the analysis, it cannot be ruled out that observed associations may be affected by residual confounding, as higher intakes of whole grain and cereal fibre were strongly associated with other healthy lifestyle and dietary factors, and thus intake may be a proxy of an overall healthy lifestyle. Additionally, as the current study is a secondary analysis of AHS, the extent of analyses possible is limited by the data originally collected by the survey, and there may be other relevant CVD risk factors that we were unable to examine, or relevant confounders that we were unable to adjust for.
The 24-h recall method of gathering dietary information from participants can be limited by recall bias and under-reporting of intake(38). Notably, the multiple pass method used to gather participants’ dietary intake has also been shown to reduce bias in energy reporting(39), and we attempted to further address this through removing under-reporters of energy. Lastly, the 24-h recall method is also limited in its ability to capture an accurate representation of day-to-day variations in diet; however, given the large sample size available within AHS, this method can produce reasonably accurate estimates of dietary intake at a group level(40).
The current study identifies that whole grains and cereal fibre were each favourably associated with various CVD risk factors, although modestly. While both intakes were inversely associated with anthropometric measures within our study, whole grain intake alone tended to be associated with favourable blood glucose measures, while cereal fibre alone was associated with more favourable blood lipid measures, suggesting that there may be a variety of mechanisms and mediators involved in the protective associations of whole grains against CVD, some including and others not including an active role of cereal fibre. In order to gain an insight into the mechanisms involved and the role of cereal fibre in each of them, longer-term intervention studies directly comparing whole grain and non-whole grain diets matched on cereal fibre (such as through the use of bran or added-fibre refined grain products) are needed. While public health guidelines should always place an emphasis on whole foods, a further understanding of the role of specific components within whole grains, such as cereal fibre, may be beneficial for informing individualised dietary advice and counselling, as well as potential food innovation.
Acknowledgements
Acknowledgements: The authors would like to thank the Australian Bureau of Statistics for supplying the AHS data, and all participants of AHS. Financial support: This research has been conducted with the support of the Australian Government Research Training Program Scholarship. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: E.M.B. and E.J.B. designed the research; E.M.B. analysed data; M.J.B. provided statistical support; E.M.B. wrote the paper; all authors reviewed manuscript; E.M.B. had primary responsibility for the final content. Ethics of human subject participation: Ethics approval for the NHMS was granted by the Australian Government Department of Health and Ageing Departmental Ethics Committee (DEC).
References
- 1. Heart Foundation (2018) Heart Disease in Australia. https://www.heartfoundation.org.au/about-us/what-we-do/heart-disease-in-australia (accessed February 2019).
- 2. Berry JD, Dyer A, Cai X et al. (2012) Lifetime risks of cardiovascular disease. N Engl J Med 366, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer KA, Kushi LH, Jacobs DR Jr et al. (2000) Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 71, 921–930. [DOI] [PubMed] [Google Scholar]
- 4. Koh-Banerjee P, Franz M, Sampson L et al. (2004) Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am J Clin Nutr 80, 1237–1245. [DOI] [PubMed] [Google Scholar]
- 5. McKeown NM, Meigs JB, Liu S et al. (2004) Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 27, 538–546. [DOI] [PubMed] [Google Scholar]
- 6. Newby PK, Maras J, Bakun P et al. (2007) Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr 86, 1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slavin J, Jacobs D & Marquart L (1997) Whole-grain consumption and chronic disease: protective mechanisms. Nutr Cancer 27, 14–21. [DOI] [PubMed] [Google Scholar]
- 8. Threapleton DE, Greenwood DC, Evans CEL et al. (2013) Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 347, f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rimm EB, Ascherio A, Giovannucci E et al. (1996) Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA 275, 447–451. [DOI] [PubMed] [Google Scholar]
- 10. Kristensen M, Toubro S, Jensen MG et al. (2012) Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 142, 710–716. [DOI] [PubMed] [Google Scholar]
- 11. Folsom AR, Jacobs DR Jr, Shahar E et al. (2003) Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 78, 383–390. [DOI] [PubMed] [Google Scholar]
- 12. Huang T, Xu M, Lee A et al. (2015) Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med 13, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs DR Jr, Meyer KA, Kushi LH et al. (1999) Is whole grain intake associated with reduced total and cause-specific death rates in older women? The Iowa Women’s Health Study. Am J Public Health 89, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Australian Bureau of Statistics (2013) Australian Health Survey: Users’ Guide, 2011–13. Canberra: Australian Bureau of Statistics. [Google Scholar]
- 15. Goldberg GR, Black AE, Jebb SA et al. (1991) Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nut 45, 569–581. [PubMed] [Google Scholar]
- 16. Blanton CA, Moshfegh AJ, Baer DJ et al. (2006) The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr 136, 2594–2599. [DOI] [PubMed] [Google Scholar]
- 17. Food Standards Australia New Zealand (2014) AUSNUT 2011–13 Australian Food, Supplement and Nutrient Database for Estimation of Population Nutrient Intakes. Canberra, Australia: Food Standards Australia New Zealand. [Google Scholar]
- 18. World Health Organisation & Food and Agriculture Organisation (2003) Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series no. 916. Geneva: WHO. [PubMed] [Google Scholar]
- 19. Galea LM, Dalton SMC, Beck EJ et al. (2016) Update of a database for estimation of whole grain content of foods in Australia. J Food Compost Anal 50, 23–29. [Google Scholar]
- 20. Barrett EM, Probst YC & Beck EJ (2018) Creation of a database for the estimation of cereal fibre content in foods. J Food Compost Anal 66, 1–6. [Google Scholar]
- 21. Food Standards Australia New Zealand (2015) Australia New Zealand Food Standards Code – Standard 2·1.1 – Cereal and Cereal Products. no. F2015L00420. Canberra: FSANZ.
- 22. Reynolds A, Mann J, Cummings J et al. (2019) Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 393, 434–445. [DOI] [PubMed] [Google Scholar]
- 23. Slavin JL (2005) Dietary fiber and body weight. Nutrition 21, 411–418. [DOI] [PubMed] [Google Scholar]
- 24. Thielecke F & Jonnalagadda SS (2014) Can whole grain help in weight management? J Clin Gastroenterol 48, Suppl. 1, S70–S77. [DOI] [PubMed] [Google Scholar]
- 25. Pan A & Hu FB (2011) Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care 14, 385–390. [DOI] [PubMed] [Google Scholar]
- 26. Ludwig DS (2002) The glycemic indexphysiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287, 2414–2423. [DOI] [PubMed] [Google Scholar]
- 27. Vega-Lopez S, Venn BJ & Slavin JL (2018) Relevance of the glycemic index and glycemic load for body weight, diabetes, and cardiovascular disease. Nutrients 10, 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barrett EM, Amoutzopoulos B, Batterham MJ et al. (2020) Whole grain intake compared with cereal fibre intake in association to cardiovascular disease risk factors – a cross sectional analysis of the National Diet and Nutrition Survey (UK). Public Health Nutr, doi: 10.1017/S1368980019004221. [DOI] [PMC free article] [PubMed]
- 29. Thies F, Masson LF, Boffetta P et al. (2014) Oats and CVD risk markers: a systematic literature review. Br J Nutr 112, Suppl. 2, S19–S30. [DOI] [PubMed] [Google Scholar]
- 30. Klop B, Elte JWF & Cabezas MC (2013) Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 5, 1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen MK, Koh-Banerjee P, Franz M et al. (2006) Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation. Am J Clin Nutr 83, 275–283. [DOI] [PubMed] [Google Scholar]
- 32. McKeown NM, Meigs JB, Liu S et al. (2002) Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr 76, 390–398. [DOI] [PubMed] [Google Scholar]
- 33. Hodge AM, English DR, O’Dea K et al. (2004) Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 27, 2701–2706. [DOI] [PubMed] [Google Scholar]
- 34. Hu EA, Pan A, Malik V et al. (2012) White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 344, e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montonen J, Knekt P, Järvinen R et al. (2004) Dietary antioxidant intake and risk of Type 2 diabetes. Diabetes Care 27, 362. [DOI] [PubMed] [Google Scholar]
- 36. Song Y, He K, Levitan EB et al. (2006) Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 23, 1050–1056. [DOI] [PubMed] [Google Scholar]
- 37. Bhathena SJ & Velasquez MT (2002) Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 76, 1191–1201. [DOI] [PubMed] [Google Scholar]
- 38. Macdiarmid J & Blundell J (1998) Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev 11, 231–253. [DOI] [PubMed] [Google Scholar]
- 39. Moshfegh AJ, Rhodes DG, Baer DJ et al. (2008) The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 88, 324–332. [DOI] [PubMed] [Google Scholar]
- 40. Thompson FE & Byers T (1994) Dietary assessment resource manual. J Nutr 124, Suppl. 11, 2245s–2317s. [DOI] [PubMed] [Google Scholar]


