Abstract
Objective:
When breast-feeding is not possible, commercially made human milk substitute is recommended. Some consumers would prefer to make their own homemade infant formula (HIF) and may seek information on this practice from internet sources. The purpose of the current study was to investigate the content of blogs posting HIF recipes.
Design:
Blog postings were identified through a comprehensive search conducted using the Google search engine and the following search terms along with the term ‘blog’: ‘Make Your Own Baby Formula’, ‘Homemade Baby Formula’, ‘Do It Yourself (DIY) Baby Formula’, ‘DIY Baby Formula’, ‘Baby Formula Recipe’ and ‘All Natural Baby Formula’. A quantitative content analysis of blogs offering recipes for HIF was completed. Blogs that met the inclusion criteria were reviewed for disclaimers, blogger’s credentials, rationale for HIF use, advertisement or sale of recipe ingredients and recipe ingredients.
Setting:
Worldwide Web.
Results:
Fifty-nine blogs, featuring one hundred forty-four recipes, met inclusion criteria. Among reviewed blogs, 33·9 % did not provide a disclaimer stating breast milk is the preferred option, 25·4 % recommended consulting a healthcare professional before using, and 76·3 % and 20·3 % either advertised or sold ingredients or recipe kits, respectively. Credentials of bloggers varied and only seven bloggers identified themselves as ‘nutritionists’. The three most frequently mentioned recipe ingredients were whole raw cow’s milk (24·3 %), raw goat’s milk (23·6 %) and liver (14·5 %).
Conclusions:
Clinicians should be aware of this trend, discuss source of formula with parents, advocate for appropriate infant feeding practices and monitor for side effects.
Keywords: Home-made infant formula, Infant feeding, Blogs, Human milk substitute, Formula
The Academy of Nutrition and Dietetics, American Academy of Pediatrics (AAP) and the WHO recommend that new mothers breast-feed infants exclusively for the first 6 months of life followed by the addition of complementary foods to breast milk from 6 months to 1 year(1–3). When breast-feeding is not an option, commercially prepared human milk substitute (HMS) (formula) is a safe and nutritious alternative to breast milk(1). As commercially prepared HMS is considered a food, it must be manufactured according to laws and regulations set forth by the Federal Food, Drug, and Cosmetic Act(4). HMS provides sources of protein, carbohydrate and fat and must meet minimum requirements for twenty-nine nutrients and not exceed maximum levels for nine nutrients(4). Ingredients used in making HMS must be generally recognised as safe for use in infant formula. In addition, the manufacturer must provide verification that the formula has been tested and contents comply with the law. The Food and Drug Administration (FDA) monitors both the facilities in which HMS and the ingredients are made(4). To reduce the chance of contamination, the HMS product label provides helpful advice to consumers such as directions for safe preparation, storage and use by dates.
Consumers rely on social media and online platforms including blogs to obtain health information(5). A blog can be defined as a website that contains personal beliefs, photographs, videos, hyperlinks and other content written by the blogger(6). Blogging has become a popular way to provide advice and advertise or sell goods directly. Authorship of blogs in the USA is projected to exceed 31 million in 2020(7). An estimated 32 % and 39 % of millennial mothers in the USA and Canada, respectively, refer to blogs for parenting-related information(8,9). According to the Pew Research Center, 79 % of parents use social media to obtain parenting information(10). In 2015, 19 % of parents worldwide referred to baby blogs to learn about baby food products(11). Information on these platforms may be reliable or questionable depending on the blogger’s qualifications and biases. A recent trend in blogging is to post warnings about the use of commercially made HMS and provide recipes for homemade infant formula (HIF). The FDA does not recommend that consumers make their own HIF(4). An estimate of HIF use by parents has yet to be determined. To date, these websites have not been reviewed. Thus, the aims of the current study were to (i). determine who initiates these blogs and why; (ii). compare the information found within these blogs with the labelling requirements of HMS manufacturers and (iii). determine whether the recipes contain products that may pose a health risk to an infant.
Materials and methods
A quantitative content analysis of blogs offering recipes for HIF was completed using procedures established by Kim and Kuljis(12) and the content analysis design components described by Krippendorff(13). Quantitative content analysis follows similar procedures as other quantitative research methods including selecting a research question, defining the sample of units to assess (unitising), narrowing the original sample to a smaller group based on eligibility criteria (sampling), operationalising categories or units of measurement (recording/coding) and quantifying percentage of units within a designated category (reducing). It differs from other quantitative procedures in that the researcher must develop a coding scheme and detailed definitions for the classification of units for analysis within a sample of communications(12,13).
Unitising
To define a comprehensive sample of units (blogs), in January 2018, six Google searches were conducted using the term ‘blog’ and each of the following terms: ‘Homemade Baby Formula’, ‘Do It Yourself Baby Formula’, ‘DIY Baby Formula’, ‘Make Your Own Baby Formula’, ‘All Natural Baby Formula’ and ‘Baby Formula Recipe’.
Sampling
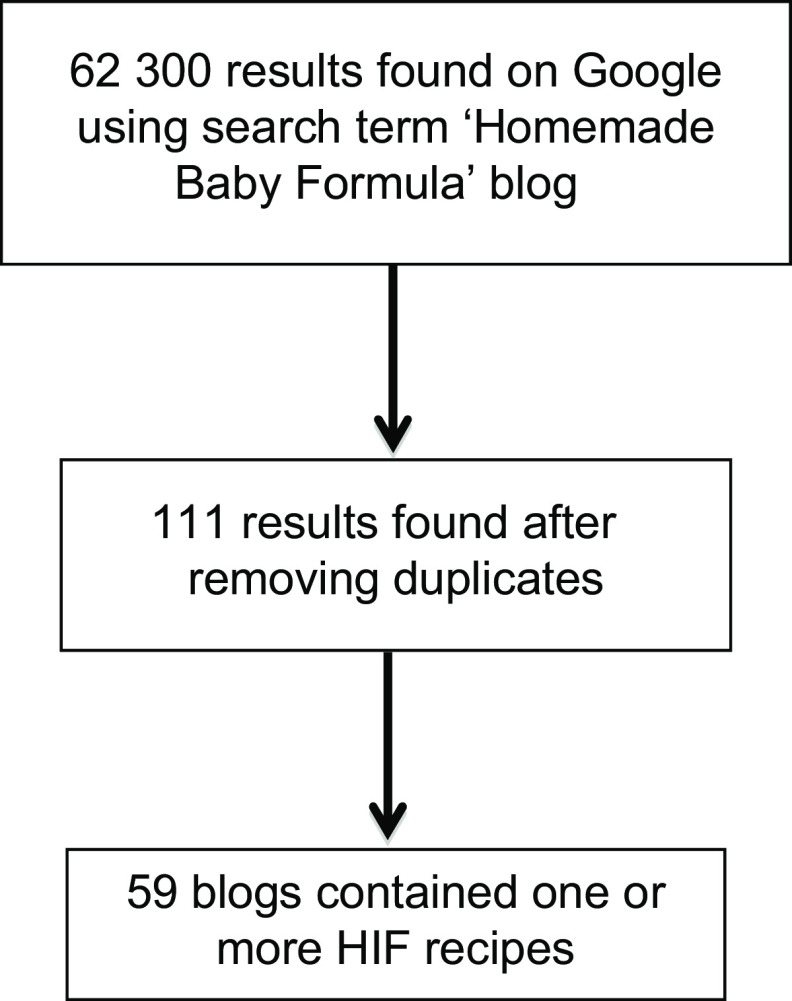
To reduce duplicates, only the Google search engine was used leading to a list of 62 300 initial search results (Fig. 1). Google platform identifies duplicate entries that appear in search results by highlighting previously reviewed results. As the researchers reviewed each link listed in search results, this feature was used to identify duplicate results among the six searches. This step yielded 111 unique blogs. Each blog was reviewed for content. Only blogs (n 59) offering at least one HIF recipe were included in the current analysis. Other reasons for exclusions included: dead links, private accounts, referral to a HIF cookbook and video rather than a written recipe. To provide a snapshot of the content for a given time period, statements and recipes were ‘frozen in time’ by copying and pasting contents of the blog into a text document for analysis.
Fig. 1.
Selection of the sample of blogs for analysis
Recording/Coding
The blogs and recipes were assessed separately. Blogs were initially reviewed, and the research team developed an a priori list of common characteristics within the blog posts (Table 1). As blogs were reviewed, the research team made decisions regarding the addition of characteristics and codes. To address the research question, who initiates these blogs and why, a single coder reviewed the selected blogs for the following characteristics: rationale for HIF use, disclaimers, credentials of blogger and advertisement or sale of recipe ingredients. If the blogger provided an argument or rationale for use of HIF, this information was coded as yes/no and the author’s reasons were noted and later coded. Disclaimers were defined as either a reference to breast milk as the preferred option for an infant or a passage that suggested that readers consult with a healthcare professional prior to using HIF. If available, the bloggers credentials were recorded from the biography section. Many of the listed ingredients within the recipes are not common grocery items. However, bloggers would either supply online shopping links or advertise/sell items on their blogs either individually or as a kit. The coder noted whether the blogger advertised the kits and/or ingredients (yes/no).
Table 1.
Categories and codes describing content of blogs (n 59) featuring human infant formula recipes (n 144)
| Category | Calculations based on | Codes/examples |
|---|---|---|
| Author’s rationale for HIF use |
|
|
| Credentials of blogger |
|
Nutritionists, Nutritional Therapy Practitioner, Certified Nutritional Therapist, Certified Nutrition and Body Detox Coach, Registered Acupuncturist, Certified Nutritional Practitioner and Charter Herbalist, Registered Nutritional Consulting Practitioner, Licensed Clinical Psychologist, Naturopathic Physician |
| Advertisements for recipe ingredients |
|
|
| Handling procedures |
|
|
| Storage |
|
|
| Expected shelf life |
|
|
| Protein base |
|
Whole, raw cow’s milk, raw goat’s milk, liver, fortified, commercial human milk substitute (altered by consumer), powdered goat’s milk, coconut milk, rice milk, almond milk, hemp milk, soy milk, hemp seeds, camel’s milk, evaporated cow’s milk, powdered cow’s milk, evaporated goat’s milk, Piima milk or more than one ‘protein’ base |
To answer the second research question, how well does the information found within these blogs compare with the labelling requirements of HMS manufacturers, the recipes were assessed for directions that are common on the labels of HMS. Recipes, ingredients, amounts and preparation were copied and pasted into a text document. The coder assessed each recipe for descriptions of sanitation procedures, proper storage of ingredients and final product and discussion of use by dates.
Lastly, to answer the research question, do the recipes, if prepared as directed, contain products that may pose a health risk to an infant, the coder assessed the ingredient lists within the recipes. Recipes included ingredients (e.g. Bifidobacterium infantis, acerola powder or homemade whey) that could not be entered into a nutrient analysis software. Thus, researchers noted only the base ingredient or largest component within the recipe, which was usually a protein source. Recipe items coded as bases were agreed upon a priori. As recipes were reviewed, if a new base was identified, three members of the research team (dietitian, food chemist and primary coder) made the decision of whether to include the new base within the list of categories.
Reducing
Web address for each blog and extracted data were entered into an Excel (Microsoft; 2013) spreadsheet based on pre-established definitions for each code. Descriptive statistics were computed for selected blog and recipe characteristics. Percentages of blogs with a posted disclaimer, a stated rationale for HIF use and advertisements for ingredients were calculated based on the number of non-duplicate blogs visited (n 59). Many blogs featured multiple recipes. Thus, the frequencies in which a protein source appeared within the recipes and whether the recipe cited proper food handling and storage techniques were calculated based on the total number of recipes (n 144).
Results
Among the fifty-nine blogs selected for the current analysis, 64·4 % included the author’s rationale for choosing HIF. Reasons fell into two categories: personal reasons and safety concerns about commercial HMS ingredients, processing and packaging. Personal reasons included financial reasons, family/friend recommendations and testimonials such as difficulty breast-feeding and/or infant intolerance to commercial formulas or ingredients. Concerns about HMS focused on specific ingredients, high heat processing, ‘bisphenol A (BPA) found in cans’ and lack of ‘organic ingredients’. None of the bloggers were registered dietitians and 11·9 % were self-proclaimed ‘nutritionists’. Additionally, 76·3 % of the reviewed blogs advertised recipe ingredients and 20·3 % advertised kits including all recipe ingredients. Lastly, 33·9 % of blogs did not feature a disclaimer stating that breast milk was the best option, and 74·6 % of blogs did not suggest that the reader consult a healthcare professional before using HIF recipe.
Among 144 extracted recipes, 91·0 %, 84·0 % and 18·8 % of recipes included handling precautions when preparing the formula, instructions for storage and shelf-life recommendations, respectively. HIF recipes extracted from blogs featured seventeen bases (Table 2). Three most frequently used bases were whole raw cow’s milk (24·3 %), raw goat’s milk (23·6 %) and liver (14·5 %). Liver-based formulas included raw liver, desiccated liver, pureed liver and liver oil (Table 2).
Table 2.
Characteristics of recipes in homemade infant formula recipes from 59 blog posts found in January 2017
| ‘Protein’ source of recipe | Number (%) | n 144 |
|---|---|---|
| ‘Protein’ Base | ||
| Whole, raw cow’s milk | 35 | 24·3 |
| Raw goat’s milk | 34 | 23·6 |
| Liver | 21 | 14·5 |
| Fortified, commercial human milk substitute (altered by consumer) | 19 | 13·2 |
| Powdered goat’s milk | 9 | 6·3 |
| Coconut milk | 9 | 6·3 |
| Rice milk | 6 | 4·1 |
| Almond milk | 6 | 4·1 |
| Hemp milk | 4 | 2·8 |
| Soy milk | 4 | 2·8 |
| Hemp seeds | 2 | 1·4 |
| Camel’s milk | 2 | 1·4 |
| Evaporated cow’s milk | 2 | 1·4 |
| Powdered cow’s milk | 2 | 1·4 |
| Evaporated goat’s milk | 1 | 0·7 |
| Piima milk | 1 | 0·7 |
| Breast milk | 1 | 0·7 |
| More than one ‘protein’ base | 14 | 10·4 |
Discussion
Short- and long-term effects of HIF use on infant health are unknown. The aims of the current study were to (i). determine who initiates these blogs and why; (ii). compare the information found within these blogs with the labelling requirements of HMS manufacturers and (iii). determine whether the recipes contain products that may pose a health risk to an infant. The current study reveals many safety concerns in blogs promoting the use of HIF provided by untrained individuals. These concerns include misinformation regarding the safety of HMS, use of unpasteurised ingredients, recipes that may not meet nutritional needs of infants and ingredients that may be harmful to infants.
A major concern is the risk of foodborne illness, such as Salmonella, Listeria, Brucellosis, Toxoplasmosis and Escherichia coli 0157:H7, due to the use of raw or unpasteurised cow’s and/or goat’s milk, an ingredient in 47·9 % of recipes(14–16). According to the Centers for Disease Control and Prevention (CDC), unpasteurised milk is 150 times more likely to cause a foodborne illness than pasteurised dairy products, and approximately 59 % of food safety outbreaks involving raw milk are among children under the age of 5 years(17). In contrast, HMS must be tested for harmful pathogens(18).
It is unknown whether these recipes would meet nutritional needs of an infant or support optimal growth. These recipes feature cow, goat and alternative milks as recipe bases which differ from HMS in composition impacting infant nutrient intake(15,19). Cow’s milk contains higher amounts of protein and lower amounts of iron, which may lead to dehydration and iron deficiency anemia(15). Thus, the American Academy of Pediatrics recommends against use of whole cow’s milk for the first 12 months of life(19). When compared to HMS, unmodified goat’s milk is much higher in protein, potassium and chloride, which may lead to metabolic acidosis and electrolyte imbalances(20). Undiluted goat’s milk is high in tyrosine and phenylalanine, which has led to reports of false positives in newborn screening tests for tyrosinaemia type 1(15,20,21). Unmodified goat’s milk is lower in folate than HMS and consumption has been linked to megaloblastic anemia(15,16,22). Additionally, many recipes contained alternative milks (almond, soy, rice and coconut) as the base ingredient; however, these alternative milks may not contain adequate levels of protein, calories, calcium or vitamin D(23–25). Lastly, use of tree nut-based milks may pose harm as tree nuts are a common food allergen(26). Unlike HIF, the nutritional content of HMS must be tested in the final stage of production and at its designated shelf-life. HMS manufacturers must also provide evidence to the FDA that their products promote normal physical growth in infants(18).
Some recipes lack food safety precautions such as directions for handling ingredients and storage. Thus, readers may not know how to prepare and store HIF, which may lead to contamination or food poisoning. In contrast, companies that manufacture HMS must comply with good manufacturing practices tailored to the production of HMS and quality control standards(18). These companies provide directions on how to safely prepare HMS and ensure that all ingredients were obtained from authorised sources handling the product according to strict food safety regulations. Ingredients ordered online and sent through the mail likely do not follow these same guidelines.
Our study provides insightful results, but it is not exempt from limitations, namely, that the number of blogs populating the search is constantly changing. A new search completed by a different team may yield different results. In addition, our sample was limited to blogs containing recipes. The scope of the current study was limited to a preliminary examination of blog content providing HIF recipes to bring attention to this practice. Recipe analyses were not completed, nor were the number of individuals visiting these blogs determined. These items could be addressed in future research to strengthen the validity of these preliminary findings. Strengths of the study include that the coder consulted the team to determine a priori units to measure and define categories. As the content analysis evolved, the coder identified new categories and sought advice of the team for triangulation of data. However, a single coder may introduce bias because coding is subjective.
Conclusion
HIF recipes reviewed pose several nutritional and food safety concerns such as unknown nutritional content, lack of safe handling procedures and use of harmful ingredients such as raw unpasteurised milks. Future research should evaluate nutritional content of these recipes and identify the percentage of parents using HIF. Additionally, healthcare professionals who encounter patients fed a HIF diet should publish their findings as case studies to document the health implications of HIF use such as gastrointestinal upset, vitamin/mineral deficiencies, altered growth patterns and food allergies/intolerances. Assessment and continued evaluation of children fed HIF should include anthropometrics, biochemical analyses (e.g. Hb, serum creatinine, urea, albumin, ferritin and serum folate) and history/physical assessments for eczema, food allergies, gastrointestinal illness (bloody stools or reflux) and respiratory illness. Furthermore, clinicians should not assume that formula provided to infants was made commercially, especially if he/she presents with or has a history of metabolic acidosis, megaloblastic anemia, electrolyte imbalances or foodborne illness. Lastly, clinicians can help parents identify sources of health misinformation by suggesting that the parent look for credentials and for sources of financial interest which may be fueling blog posts.
Acknowledgements
Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: This work was completed by S.A.D. while completing her master’s degree in Human Nutrition at The University of Alabama. S.A.D., L.L.K., K.C.W. and L.W.T. formulated the research question, designed the study, collected and analysed the data, wrote the first draft of the manuscript and contributed to all revisions of the manuscript. E.M. is an expert in infant feeding, reviewed and edited the final draft of the manuscript. All authors reviewed and contributed to the final version of the manuscript. Ethics of human subject participation: The current study does not involve human subjects and, therefore, was exempt of IRB review.
References
- 1. Lessen R & Kavanagh K (2015) Position of the academy of nutrition and dietetics: promoting and supporting breastfeeding. J Acad Nutr Diet 115, 444–449. [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics (2017) American Academy of Pediatrics Recommends That Pediatric Practices Improve Breastfeeding Support. https://www.aap.org/en-us/about-the-aap/aap-press-room/pages/American-Academy-of-Pediatrics-Recommends-that-Pediatric-Practices-Improve-Breastfeeding-Support.aspx. Published April 17, 2017 (accessed March 2019).
- 3. World Health Organization (2011) Maternal, Newborn, Child, and Adolescent Health: breastfeeding. http://www.who.int/maternal_child_adolescent/topics/newborn/nutrition/breastfeeding/en/. Published December 14, 2011 (accessed March 2019).
- 4. U S Food and Drug Administration, Center for Food Safety and Applied Nutrition (2006) Guidance Documents & Regulatory Information by Topic – Guidance for Industry:frequently Asked Questions about FDA’s Regulation of Infant Formula. https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm056524.htm. Published March 1, 2006. Revised September 20, 2018 (accessed March 2019).
- 5. Dalmer NK (2017) Questioning reliability assessments of health information on social media. J Med Libr Assoc 105, 61–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blog (2018) Merriam-Webster. https://www.merriam-webster.com/dictionary/blog. Published 2018 (accessed March 2019).
- 7. Statista Research Development (2016) Number of bloggers in the United States from 2004 to 2020 (in millions). https://www.statista.com/statistics/187267/number-of-bloggers-in-usa/ Updated February 29, 2016 (accessed August 2019).
- 8. BabyCenter (2014) Most Commonly Used Sources of Parenting-Related Information According to Millennial Mothers in the United States as of December 2014. https://www.statista.com/statistics/456916/us-millennial-mom-weekly-parenting-information-resources/. Updated February 10, 2015 (accessed August 2019).
- 9. BabyCenter (2014) Most Commonly Used Sources of Parenting-Related Information According to Millennial Mothers in Canada as of December 2014. https://www.statista.com/statistics/472448/sources-parenting-info-millennial-moms-canada/ Updated February 10, 2015 (accessed August 2019).
- 10. Pew Research Center (2014) Share of Parent Social Media Users in the United States Who Give and Receive Support on Social Media as of September 2014. https://www.statista.com/statistics/456787/us-parents-support-on-social-media/ Updated July 16, 2015 (accessed August 2019).
- 11. Nielsen (2015) Information Sources Used to Learn About Baby Food Products Worldwide in 1st Quarter 2015. https://www.statista.com/statistics/691574/baby-food-products-information-sources/ Updated August 25, 2015 (accessed August 2019).
- 12. Kim I & Kuljis J (2010) Applying content analysis to web-based content. J Comput Inform Tech 4, 369–375. [Google Scholar]
- 13. Krippendorff K (2004) Content Analysis: An Introduction to Its Methodology, 2nd ed. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 14. US Food and Drug Administration (2018). The Dangers of Raw Milk:unpasteurized Milk Can Pose a Serious Health Risk. U S Food and Drug Administration Home Page. https://www.fda.gov/Food/ResourcesForYou/Consumers/ucm079516.htm. Revised November 08, 2018 (accessed March 2019).
- 15. Turck D (2013) Issues in infant feeding: cow’s milk and goat’s milk. World Rev Nutr Diet 108, 56–62. [DOI] [PubMed] [Google Scholar]
- 16. Basnet S, Schneider M, Gazit A et al. (2010) Fresh goat’s milk for infants: myths and realities: a review. Pediatr 125, e973–e977. [DOI] [PubMed] [Google Scholar]
- 17. Food Safety (2018) Centers for Disease Control and Prevention. https://www.cdc.gov/foodsafety/rawmilk/rawmilk-outbreaks.html. Revised November 08, 2018 (accessed March 2019).
- 18. US Food and Drug Administration (2018) Commissioner of the Consumer Updates – FDA Takes Final Step on Infant Formula Protections. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048694.htm. Published June 9, 2014. Revised May 9, 2018 (accessed March 2019).
- 19. Committee on Nutrition, American Academy of Pediatrics (1992) The use of whole cow’s milk in infancy. Pediatr 89, 1105–1109. [PubMed] [Google Scholar]
- 20. Maines E, Gugelmo G, Tadiotto E et al. (2017) High-protein goat’s milk diet identified through newborn screening: clinical warning of potentially dangerous dietetic practice. Public Health Nutr 20, 2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendriksa CJ & Walter JH (2004) Feeding infants with undiluted goat’s milk can mimic tyrosinaemia type 1. Acta Paediatr 93, 552–553. [DOI] [PubMed] [Google Scholar]
- 22. Basnet S, Schneider M, Gazit A et al. (2010) Fresh goat’s milk for infants: myths and realities: a review. Pediatr 125, e973–e977. [DOI] [PubMed] [Google Scholar]
- 23. Datta-Mitra A, Vali-Betts E, Green R et al. (2017) Combined megaloblastic and sideroblastic anemia in an infant fed with goat’s milk. J Pediatr Hematol Oncol 39, 319–320. [DOI] [PubMed] [Google Scholar]
- 24. Lee GJ, Birken CS, Parkin PC & Lebovic G (2016) Goat’s milk, plant-based milk, cow’s milk, and serum 25-hydroxyvitamin D levels in early childhood. Epid 27, e29–e31. [DOI] [PubMed] [Google Scholar]
- 25. Kranthi Vanga S & Raghavan V (2018) How well do plant based alternatives fare nutritionally compared to cow’s milk? J Food Sci Technol 55, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nwara BI, Hickstein L, Panesar S et al. (2014) Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy 69, 992–1007. [DOI] [PubMed] [Google Scholar]