Abstract
Introduction:
To reduce air pollution exposure, the United States asthma guidelines recommend children check the Air Quality Index (AQI) prior to outdoor activity. Whether adding the AQI and recommendations to asthma action plans (AAP) reduces exacerbations and improves control and quality of life in children with asthma is unknown.
Methods:
A pilot, unblinded, randomized clinical trial of 40 children with persistent asthma, stratified by age and randomized 1:1, recruited from University of Pittsburgh Medical Center (UPMC) Children’s Hospital of Pittsburgh (Pittsburgh, PA) was conducted. All participants received an AAP and AQI education. The intervention group received printed AQI information and demonstrated the ability to use AirNow. Asthma exacerbations were assessed via questionnaire, asthma control with the Asthma Control Test (ACT) and Childhood-ACT (CACT), and quality of life with the Pediatric Asthma Quality of Life Questionnaire (PAQLQ). After randomization (July – October 2020), participants were followed monthly for 6 months (exit January – March 2021). Outcome differences between groups were evaluated at the exit visit and over-time (analysis 2021).
Results:
At randomization there were no significant differences in age, sex, race, or asthma severity. At exit, more intervention participants checked the AQI (63% vs. 15%) with no differences in proportion of asthma exacerbations or mean CACT or PAQLQ scores. The mean △ACT score was higher in the intervention group (△CT=2.00 vs 0.15 control), which was modified by time (β = 1.85 (CL 0.09–3.61)). Physical activity was decreased overall and demonstrated modification by treatment and time.
Conclusions:
Addition of the AQI to AAP led to improved asthma control by ACT score but may decrease outdoor activity.
INTRODUCTION
In the United States (US), both the National Asthma Education and Prevention Program Guidelines and the American Academy of Pediatrics recommend that healthcare providers advise children with asthma to check the Air Quality Index (AQI) prior to outdoor activity to prevent harms from exposure to high air pollution.1–3 While such advice is prudent, there are no clinical trials examining the effect of adding the AQI to childhood asthma action plans. Thus, clinicians have scant evidence of both the benefits and harms of adhering to the US government-sponsored AQI. To test the hypothesis that adding the AQI to asthma action plans (hereinafter “plan”) reduces asthma exacerbations and improves asthma control and quality of life, a pilot, unblinded, randomized clinical trial in children with persistent asthma was conducted.
METHODS
Forty children with physician-diagnosed persistent asthma (defined by receiving Step 2 or greater therapy), ages 8–17 years, with either smartphone or home internet access were recruited from UPMC Children’s Hospital of Pittsburgh from pulmonology clinics and an asthma registry (NCT:04454125).1 Older ages were chosen to promote independent checking of the AQI and self-regulation of activity. Inclusion of smartphone and/or internet access was to ensure ability to access the AQI. Children with other chronic respiratory diseases, immunodeficiency, neuromuscular disease, disability affecting ambulation, cyanotic congenital heart disease, or plans to move out of the area were excluded.
Participants were unblinded and randomized to either the control or intervention group 1:1, stratified by age group: 8–12 and 13–17 years (Figure 1). Assignments, by age strata, were sealed in envelopes prior to enrollment and opened at the randomization visit (July–October 2020). All participants and their caregivers received an asthma action plan (Appendix Figure 1) and basic AQI education (Appendix Figure 2); the intervention group additionally received AQI information printed on their plan (Appendix Figure 3) and demonstrated the ability to check the AQI on AirNow (either app or website). Following the in-person randomization visit, participants were followed monthly for 24 weeks, with 5 telephone visits and an in-person exit visit (January-March 2021) (Appendix Figure 4). The study protocol was approved by University of Pittsburgh Institutional Review Board (STUDY19120083). Both written parental consent and child assent were obtained.
Figure 1:
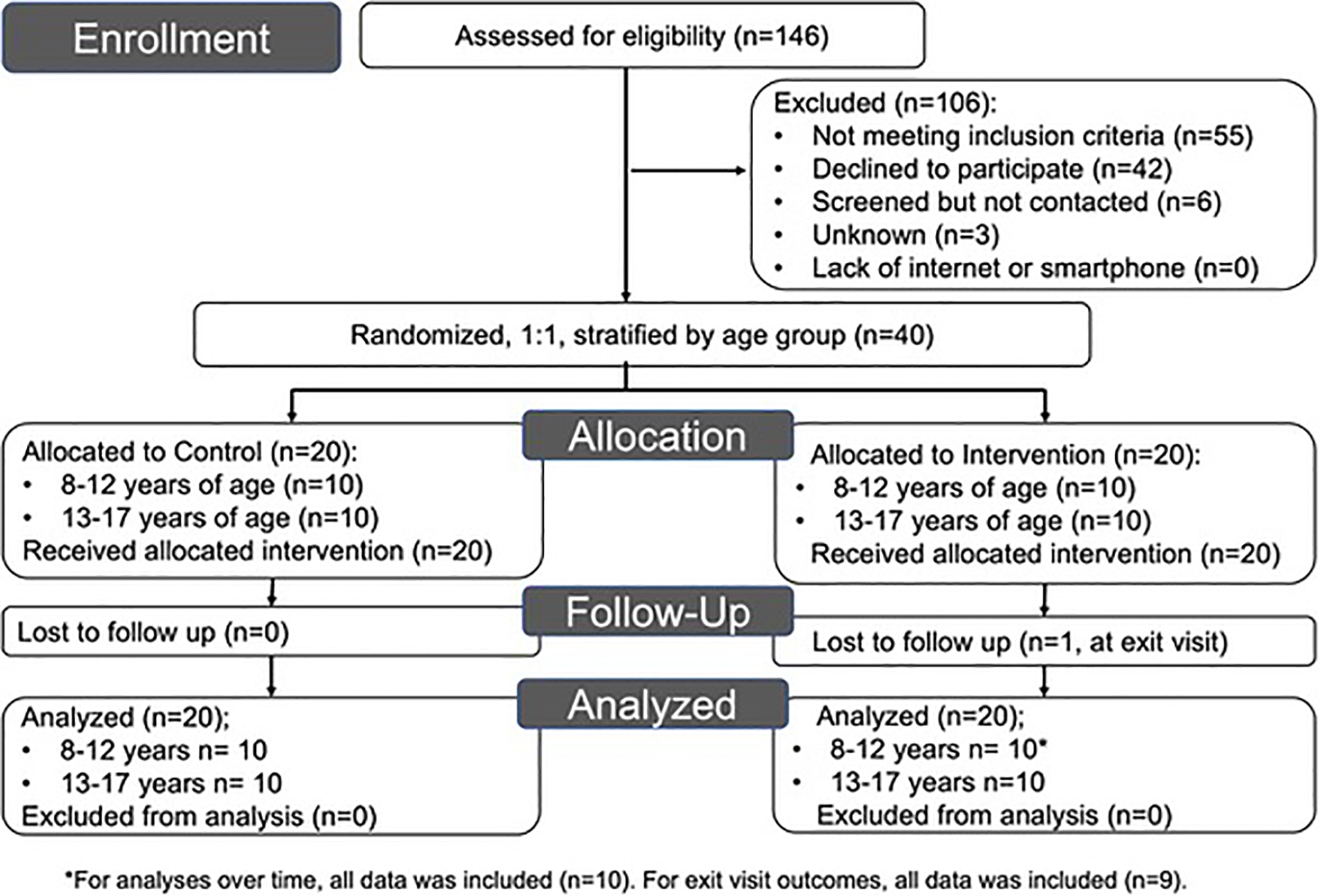
Selection of Study Participants
Asthma exacerbations were assessed monthly after randomization; severe asthma exacerbations were defined as requiring systemic steroids for ≥3 days or having an emergency department visit or hospitalization treated with systemic steroids, and moderate exacerbations as having asthma symptoms requiring albuterol for ≥2 days or an unplanned visit to a healthcare provider for symptoms not treated with systemic steroids.4 Asthma control was assessed at all visits using the Asthma Control Test (ACT) and the Childhood ACT (CACT) for children 12–17 and 8–11 years old, respectively. Data on the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) were collected at the randomization and exit visits. Usage of the AQI and physical activity were accessed via questionnaire at all visits. Race and sex were caregiver-reported.
Primary and secondary outcomes were examined as: mean difference between groups at the exit visit, change from exit-randomization, and changes over time (both within and between groups). Wilcoxon rank sum and χ2 or Fischer Exact test were used for bivariate analyses. Generalized estimating equations were used for repeat, correlated measures and are presented as β or Odds Ratios. A sample size of 20 participants per group provided ≥90% power, with two-tailed t test and alpha 0.05, to detect a standardized mean score difference of 1.05 between groups for asthma exacerbations, ACT and C-CACT, and PAQLQ and for comparison within each treatment group provided ≥80% power to detect a standardized within mean group difference of 0.66. Two-sided P values < .05 were considered significant (One-sided P values in Appendix Table 1). Secondary outcomes, considered exploratory, included checking the AQI prior to outdoor activity, behavioral modification based on the AQI, and physical activity (Appendix Table 2). All analyses were performed using SAS v 9.4 (SAS Institute, Cary NC).
RESULTS
Table 1 displays the baseline characteristics of study participants. Most children reported a severe asthma exacerbation in the prior year (55% control, 65% intervention). For both groups, the mean baseline ACT and CACT indicated good asthma control and PAQLQ score indicated better quality of life (5.96 control, 6.11 intervention). Few children reported checking the AQI prior to outdoor activity and most reported moderate or vigorous outdoor activity at least once per week.
Table 1:
Baseline demographics
| Variables | Control | Intervention |
|---|---|---|
| N | 20 | 20 |
| Age, years | 12.5 (2.6) | 12.5 (2.9) |
| 8 – 12 | 10 (50%) | 10 (50%) |
| 13 – 17 | 10 (50%) | 10 (50%) |
| Sex, female | 10 (50%) | 10 (50%) |
| Race | ||
| Black | 9 (45%) | 6 (30%) |
| White | 8 (40%) | 10 (50%) |
| More than 1 | 3 (15%) | 4 (20%) |
| Asthma severitya | ||
| Mild | 4 (20%) | 5 (25%) |
| Moderate/Severe | 16 (80%) | 15 (75%) |
| Health insurance, Medicaid | 10 (50%) | 11 (55%) |
| Current second-hand tobacco smoke exposure, yes | 7 (35%) | 4 (20%) |
| Body mass index, z score | 1.51 (1.35) | 1.03 (1.22) |
| Forced expiratory volume in 1 second (FEV1), percent predictedb | 102.2 (14.1) | 97.3 (16.7) |
| FEV1/Forced vital capacity, percent predictedb | 94.6 (7.0) | 88.0 (9.0) |
| Severe asthma exacerbation in last 12 monthsc, yes | 11 (55%) | 13 (65%) |
| Asthma Control Test (n=13 per group) | 20.9 (4.6) | 21.2 (3.0) |
| Childhood Asthma Control Test (n=7 per group) | 22.6 (1.3) | 20.7 (8.3) |
| Pediatric Asthma Quality of Life Questionnaire | 5.96 (1.02) | 6.11 (0.84) |
| Checked Air Quality Index (AQI) prior to outdoor activity, yes | 2 (10%) | 2 (10%) |
| Reported any change in outdoor activity in response to AQI, yes | 5 (25%) | 3 (15%) |
| Moderate or vigorous outdoor physical activity in typical week, yes | 14 (70%) | 18 (90%) |
Notes: Results displayed as N (%) or mean (SD).
Severity was determined by therapy as per asthma action plan: step 2- mild, step 3 & 4- moderate, step 5 & 6- severe. Children receiving a biologic immunomodulator but no oral corticosteroids were categorized as step 5.
Spirometry percent predicted based on GLI2012 prediction equations.
Asthma exacerbation history obtained via parental-report, severe asthma exacerbation defined as reporting systemic steroids or an emergency department/urgent care visit or hospitalization for asthma.
All participants completed six study visits, with one participant lost to follow up at exit (Figure 1). The mean study duration was 24.9 and 24.4 weeks for the control and intervention groups respectively. There was no statistically significant difference in exit BMI z-score, change in BMI z-score, or exit FEV1 between groups. There were 13 severe exacerbations in the control group vs. 5 in the intervention group (p = 1.00); and 44 vs 28 moderate exacerbations, respectively (p=0.81).
Table 2 shows the results of the primary analyses. There were no differences in severe asthma exacerbations within or between the groups over time. Moderate asthma exacerbations decreased over time in both groups, without significant differences. Mean ACT scores were higher for the intervention group than the control group during the study and at study exit, with a mean score difference of 1.85 (95% confidence interval (CI) 0.09–3.61, p=0.04). A similar trend was observed for CACT scores but this was not significant (p=0.13). The mean PAQLQ score change over time for the intervention was 0.54 (95% CI=0.20–0.88, p=0.002), with no difference over time between groups.
Table 2:
Primary outcomes
| Outcome | Had at least one during study | Within Group Change | Between Group Difference | |||||
|---|---|---|---|---|---|---|---|---|
| At least one during study, N (%) | Risk difference (95% CI) | Risk ratio (95% CI) | P | Estimate (95% CI), ORa | P | Estimate (95% CI), RORb | P | |
| Severe asthma exacerbations | ||||||||
| Control | 5 (25) | 0.05 (−0.21, 0.31) | 1.25 (0.39, 3.99) | 1.00 | 0.61 (0.23, 1.61) | 0.32 | ||
| Intervention | 4 (20) | 0.31 (0.03, 2.76) | 0.29 | 0.51 (0.05, 5.68) | 0.58 | |||
| Moderate asthma exacerbations | ||||||||
| Control | 13 (65) | 0.05 (−0.25, 0.35) | 1.08 (0.67, 1.75) | 0.74 | 0.50 (0.27, 0.91) | 0.02 | ||
| Intervention | 12 (60) | 0.23 (0.06, 0.86) | 0.03 | 0.47 (0.11, 1.95) | 0.30 | |||
| Outcome | Mean (SE) | Within Group Change | Between Group Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exit | Δ Exit-Rand | P | Unadjusted β (SE) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| Asthma Control Test (ACT) | |||||||||
| Control (n=13) | 21.1 (3.8) | 0.15 (2.5) | 0.04 | 0.17 (-1.17, 1.51) | 0.80 | ||||
| Intervention (n=13) | 23.2 (1.6) | 2.00 (2.2) | 1.85 (0.93) | 0.06 | 2.02 (0.88, 3.15) | 0.001 | 1.85 (0.09, 3.61) | 0.04 | |
| Childhood Asthma Control Test (CACT) | |||||||||
| Control (n=7) | 22.9 (4.3) | 0.29 (3.5) | 0.94 | −0.31 (−1.87, 1.25) | 0.70 | ||||
| Intervention (n=7c) | 25.2 (1.9) | 1.67 (4.4) | 1.38 (2.18) | 0.54 | 3.96 (-1.27, 9.20) | 0.14 | 4.27 (-1.19, 9.73) | 0.13 | |
| Pediatric Asthma Quality of Life Questionnaire (PAQLQ) | |||||||||
| Control | 6.20 (0.9) | 0.25 (0.7) | 0.48 | 0.25 (−0.06, 0.55) | 0.11 | ||||
| Intervention | 6.65 (0.4) | 0.42 (0.6) | 0.18 (0.21) | 0.41 | 0.54 (0.20, 0.88) | 0.002 | 0.30 (−0.16, 0.75) | 0.20 | |
Notes: For asthma exacerbations and asthma control outcomes which were obtained at multiple visits, n=39 for exit and Δ Exit-Rand analyses and n=40 for analyses within and between groups. For PAQLQ which was obtained at 2 visits, all analyses n=39. P value of risk difference and risk ratio obtained from Fisher Exact Test or X2, where appropriate. P value for Δ Exit-Rand obtained from two-sided Wilcoxon rank sum. Models were not adjusted for other variables. Within group change obtained from generalized linear models adjusting for correlation between values including interaction term (between group difference). For asthma exacerbations (both severe and moderate), estimates obtained are from visit 2 ˗7 (no randomization values) and are adjusted for visit 2 values. Boldface indicates statistical significance (P<0.05).
Within-group changes for binary outcomes are quantified by odds ratios (OR); within a given group, OR=λ indicates that the odds of outcome at study exit are λ times the odds of outcome at baseline.
Between-group differences are quantified by ratios of odds ratios (ROR); a ROR=γ indicates that the OR for intervention participants is γ times the OR for controls.
N=6 for exit and △ Exit-Rand, n=7 for analyses within and between groups.
Appendix Table 2 displays results of secondary outcome analyses. The intervention group checked the AQI more often over the study period with a between group ratio of odds ratios of 7.31 (95% CI 1.62–32.9, p = 0.01). The risk of changing outdoor activity in response to the AQI at least once during the study was lower in the control than in the comparison group (risk difference: −0.40 (95% CI −0.66, −0.14)), although there were no within or between groups changes over time. Outdoor activity declined in both groups over time, likely due to seasonality, though this was greater for the intervention group (ROR=0.29, 95% CI=0.10–0.87, p = 0.03).
DISCUSSION
The results demonstrate addition of the AQI to asthma action plans is feasible and may improve asthma control at a clinically meaningful level.5 Concerningly, the results suggest such addition may unintendedly reduce outdoor physical activity. This is the first prospective study evaluating the effects of incorporating the AQI in childhood asthma care.
While recommendations to reduce outdoor air pollution exposure remain prudent, clinicians have little evidence for how well the AQI tracks with childhood asthma outcomes, if personal adherence to AQI recommendations mitigates harm, or if there are unintended consequences such as limitation of outdoor activity.6, 7 Given the known association between outdoor air pollution and harmful childhood asthma outcomes8, such knowledge is critically important for guiding preventative medicine and public health recommendations.
Limitations
The null findings for asthma exacerbations may be due to limited statistical power from small sample size. The study was limited by a single geographic location with chronically elevated air pollution9 and recruitment from a subspeciality clinic. Further, the study was conducted in a region where the AQI is often in the “moderate” category, with few alert days (Appendix Table 3). Thus, the AQI may have different effects in regions with episodic higher air pollution levels (e.g. wildfire regions).
CONCLUSIONS
Addition of the AQI to asthma action plans improved asthma control but did not reduce asthma exacerbations or improve asthma quality of life. This study is limited by small sample size and larger prospective studies of children with asthma from diverse geographic regions are needed to determine both the benefits and harms of adding the AQI to asthma action plans.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the study participants for their contributions. The research presented in this paper is that of the authors and does not reflect the official policy of the American Thoracic Society Foundation nor the NIH.
This study was funded by an unrestricted grant from the American Thoracic Society Foundation [PI: Rosser, 2019]. The American Thoracic Society Foundation was not involved in study design; collection, analysis, and interpretation of data; writing of the report; nor decision to submit article for publication. Additional support was provided by the U.S. National Institutes of Health [KL2 TR001856 (PI: Rubio, for Rosser) and K08 HL159333 (PI: Rosser) non-concurrent; UL1 TR001857]. Dr. Celedón’s contribution was supported by NIH grants HL117191 and HL152475.
Results of this study have been presented as an abstract at the American Thoracic Society International Conference in 2022, San Francisco, CA.
Drs. Franziska Rosser, Scott Rothenberger, Yueh-Ying Han, and Erick Forno report no financial disclosures. Dr. Celedón has received research materials from Merck (inhaled steroids) to provide medications free of cost to participants in NIH-funded studies, unrelated to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement
Franziska Rosser: Conceptualization, Methodology, Funding Acquisition, Investigation, Project Administration, Data Curation, Formal Analysis, Writing-Original Draft, Writing-Review & Editing. Scott Rothenberger: Data Curation, Formal Analysis, Writing-Review & Editing. Yueh-Ying Han: Data Curation, Formal Analysis, Writing-Review & Editing. Erick Forno: Methodology, Formal Analysis, Writing-Review & Editing. Juan C. Celedón: Conceptualization, Resources, Supervision, Formal Analysis, Writing-Review & Editing.
REFERENCES:
- 1.National Asthma Education and Prevention Program Expert Panel Report 3 Guidelines for Diagnosis and Management of Asthma. In: U.S. Department of Health and Human Services, National Institutes of Health, National Heart Lung and Blood Institute; 2007. [Google Scholar]
- 2.Brumberg HL, Karr CJ, Council On Environmental H. Ambient Air Pollution: Health Hazards to Children. Pediatrics 2021;147(6). doi: 10.1542/peds.2021-051484. [DOI] [PubMed] [Google Scholar]
- 3.US Environmental Protection Agency. Technical Assistance Document for the Reporting of Daily Air Quality-the Air Quality Index (AQI). EPA 454/B-18–007. September 2018. Available via: https://www.airnow.gov/sites/default/files/2020-05/aqi-technical-assistance-document-sept2018.pdf Accessed on 10/17/2021. [Google Scholar]
- 4.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 5.Bonini M, Di Paolo M, Bagnasco D, Baiardini I, Braido F, Caminati M, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev 2020;29(156). doi: 10.1183/16000617.0137-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laumbach RJ, Cromar KR, Adamkiewicz G, Carlsten C, Charpin D, Chan WR, et al. Personal Interventions for Reducing Exposure and Risk for Outdoor Air Pollution: An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 2021;18(9):1435–1443. doi: 10.1513/AnnalsATS.202104-421ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laumbach RJ, Cromar KR. Personal Interventions to Reduce Exposure to Outdoor Air Pollution. Annu Rev Public Health 2022;43:293–309. doi: 10.1146/annurev-publhealth-052120-103607. [DOI] [PubMed] [Google Scholar]
- 8.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet 2014; 383 (9928): 1581–92. doi 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosser F, Han YY, Rothenberger SD, Forno E, Mair C, Celedon JC. Air Quality Index and Emergency Department Visits and Hospitalizations for Childhood Asthma. Ann Am Thorac Soc 2022. Jul;19(7):1139–1148. doi 10.1513/AnnalsATS.202105-539OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


