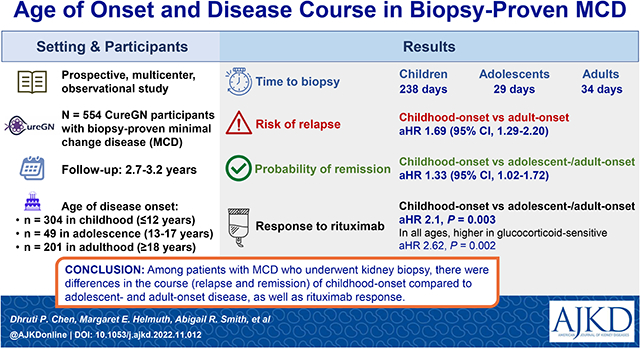
Abstract
Rationale and Objective:
Adolescent- and adult-onset disease minimal change disease (MCD) may have a clinical course distinct from childhood-onset disease. We characterized the course of children and adults with MCD in the Cure Glomerulopathy Network (CureGN) and assessed predictors of rituximab response.
Study Design:
Prospective, multicenter, observational study.
Study Participants:
CureGN participants with proven MCD on biopsy.
Exposures:
Age at disease onset. Initiation of RAAS blockade and immunosuppression including rituximab during the study period.
Outcomes:
Relapse and remission, change in eGFR and kidney failure.
Analytical Approach:
Remission and relapse probabilities are estimated using Kaplan-Meier curves and gap time recurrent event models. Linear regression models were used for the outcome of change in estimated glomerular filtration rate (eGFR). Cox proportional hazards models were used to estimate the association between rituximab administration and remission.
Results:
304 childhood (≤12 years old), 49 adolescent (13-17 years old), and 201 adult onset (≥18 years) participants were included with 2.7-3.2 years of follow-up after enrollment. Children had longer time to biopsy (238 days vs. 23 in adolescents, and 36 in adults, p<0.001) and were more likely to have received therapy prior to biopsy. Children were more likely to be treated with immunosuppression but not RAAS blockade. The rate of relapse was higher in childhood-onset versus adult-onset participants (hazard ratio (HR) 1.69 (95% confidence interval (CI) 1.29-2.21)). The probability of remission was also higher in childhood-onset disease (HR 1.33 (95%CI 1.02-1.72)). eGFR loss in all groups was minimal. Children were more likely to remit after rituximab than adolescents and adults (adjusted HR 2.1, p=0.003). Across all groups, glucocorticoid-sensitivity was associated with a greater likelihood of achieving complete remission after rituximab (adjusted HR 2.62, p=0.002).
Limitations:
CureGN was limited to biopsy-proven disease. Comparisons of childhood to non-childhood cases of MCD may be subject to selection bias, given that childhood cases who are biopsied may be limited to those patients who are least responsive to initial therapy.
Conclusions:
Among patients with MCD who underwent kidney biopsy, there were differences in the course (relapse and remission) of childhood-onset compared to adolescent and adult-onset disease, as well as rituximab response.
Keywords: minimal change disease (MCD), proteinuria, relapse, rituximab, nephrotic syndrome
Grapical Abstract
Plain Language Summary:
Minimal change disease is a biopsy diagnosis for nephrotic syndrome. MCD is diagnosed in childhood, adolescence, or adulthood. Patients and clinicians often have questions about what to expect in the disease course and how to plan therapies. We analyzed a group of patients followed longitudinally as part of the Cure Glomerulopathy Network (CureGN) and describe differences in disease (relapse and remission) based on the age of onset. We also analyzed rituximab response. We found that those with childhood-onset disease had a higher rate of relapse but also have a higher probability of reaching remission when compared to adolescent or adult-onset disease. Children and all steroid-responsive patients are more likely to achieve remission after rituximab.
Introduction
Minimal change disease (MCD) is classically defined by biopsy findings of normal glomeruli on light microscopy accompanied by diffuse podocyte foot process effacement on electron microscopy but the underlying pathophysiology is not yet well understood.1 While many with childhood onset nephrotic syndrome are diagnosed and treated clinically without biopsy, MCD is the biopsy pathologic correlate for steroid sensitive nephrotic syndrome.2 A biopsy provides clarity regarding diagnosis and pathology details such as global glomerulosclerosis and interstitial fibrosis that help with prognostic assessment.3. In contrast, adults with idiopathic nephrotic syndrome typically undergo biopsy prior to immunosuppressive therapy and MCD is identified in approximately 5-15% of biopsied adult.4,5 There is some evidence that adolescent- and adult-onset disease may have a distinct course that has not been differentiated clinically and pathophysiologically from childhood-onset disease.6 In our study, we align a cohort of children, adolescents, and adults with biopsy proven minimal change disease and describe their subsequent disease course (Figure 1).
Figure 1.
Visual summary figure of study design and outcomes.
Until the Cure Glomerulopathy (CureGN) Study7, we lacked adequate sample sizeswith MCD to determine whether there are distinct entities. Few studies have prospectively gathered clinical, treatment, and outcomes data to compare MCD across age groups.8 Adult treatment approaches have been mostly borrowed from studies on childhood-onset MCD but response and long term relapsing patterns have not been well documented.9 Studies suggest adults may have slower response to glucocorticoids or are more likely to be non-responsive.8,10-12 Proteinuria remission is indicative of favorable long-term GFR preservation in adult- and childhood-onset disease.13 Many prior studies in children include patients without biopsy proven disease. There are no direct comparisons between different age groups describing clinical characteristics, hypertension, or renal insufficiency associated with biopsy proven MCD.14,15,16 Differentiating the clinical characteristics based on age of onset can provide insight that may support future research and management of MCD.
We describe differences in childhood-onset, adolescent-onset, and adult-onset biopsy-proven MCD using CureGN data. To our knowledge, this is the largest prospective, multi-center longitudinal study of its kind. We report features at disease onset and therapy response. Longitudinal analyses provide insight into the clinical course of this complex relapsing/remitting disease and long-term outcomes (eGFR and kidney failure). In addition, given the growing use of rituximab as a glucocorticoid-sparing agent in MCD17,18,19-29, we tested the hypothesis that patients who are glucocorticoid-sensitive have a better response to rituximab therapy compared to those who are not glucocorticoid sensitive. While our analyses cannot provide biological evidence of disease differences, the descriptive comparisons between age groups and predictors of successive rituximab therapy set a stage for future studies. For example, before clinical trials can be carried out, it is important to understand whether adults, adolescents, and children should be expected to have similar outcomes. Additionally, evidence from this observational study could serve as a basis to design a randomized clinical trial to test the efficacy of rituximab as a steroid sparing agent.
Methods
Participant cohort
CureGN is an on-going, prospective, multicenter, observational cohort study of individuals with biopsy-proven glomerular diseases.7 CureGN excludes patients with diabetes mellitus, systemic lupus erythematosus, HIV-1 infection, active malignancy, hepatitis B or C, kidney failure, and kidney transplant. Participants were enrolled from 70 academic medical centers in the United States, Canada, Italy, and Poland within five years of the diagnostic kidney biopsy and could be enrolled at any stage of disease activity (relapse or remission). This study includes participants enrolled between December 2014 and December 2020 with a pathologic diagnosis of MCD (referred to here as the MCD cohort). Demographics, clinical characteristics, and treatment were collected at enrollment and prospectively. Limited clinical information and treatment history from the time of disease onset and biopsy were collected retrospectively. Participants self-identified race and ethnicity from provided categories. CIinical data were collected approximately every four months after enrollment at in-person or remote study visits. Data collected included non-immunosuppressive treatments, immunosuppressive treatment, clinical laboratory results, and other co-existing conditions. Institutional Review Boards at each center approved the study protocol. All adult participants and legal guardians of minor participants provided written informed consent and, where applicable, minors provided assent.
A subset of the cohort who had not received rituximab (“rituximab-naïve”) prior to enrollment but went on to receive rituximab therapy during the follow up period were identified for sub analysis. For this analysis, only participants who received rituximab after enrollment were included.
Definitions
Outcomes
Urine protein to creatinine ratio (UPCR) was calculated from a 24-hour urine collection, first morning void, or random spot urine using methods previously described.7 Minimal change disease activity was defined with the following criteria. Relapse was defined as UPCR ≥3 g/g on a spot collection, ≥3 g/d protein by timed collection, urine dipstick protein >3+, or study team reported relapse event by Case Report Form (CRF). Complete remission was defined as normal urine protein, including urinalysis dipstick protein negative or trace, UPCR <0.3 g/g, <300 mg protein/d, or reported remission on the CRF. Relapse and remission were considered recurring events, with the first designation (post-enrollment) determined based on above definitions, and subsequent relapse and remission were defined as occurring after complete remission or relapse, respectively. To be considered at risk for subsequent relapse (remission), participants must have returned to remission (relapse) based on the above definition. As data were unavailable from the time of disease onset or biopsy, only post-enrollment events are modeled, with event-specific follow-up time resetting to zero when the participant becomes at risk for the next event, and overall follow-up time censored at the last available study visit with available disease activity data, End Stage Kidney Disease (ESKD) or death.
eGFR was calculated using Chronic Kidney Disease Epidemiology Collaboration formula for participants aged ≥26 years,30 and the bedside Schwartz formula for participants age <18 years.31 For ages 18 to 26, eGFR values from both equations were averaged.32 To account for decreased precision of estimating formulas at low serum creatinine values and reduce any undue influence of high eGFR values that may reflect transient episodes of hyperfiltration, eGFR was winsorized at 120 mL/min per 1.73m2, in multivariable analyses.33 Kidney failure (after enrollment) was defined by transplantation, chronic dialysis, or 2 or more consecutive eGFR measurements < 15 separated by 1 to 6 months. Kidney failure and death were not considered competing events given the low incidence post-enrollment in this cohort.
Exposures
The exposure used for the primary analysis of the MCD cohort was age at disease onset. Childhood-onset was defined as onset at 12 years of age or before, adolescent-onset was defined as onset between 13-17 years of age, and adult-onset was defined as onset at ≥18 years of age. Trained study coordinators enter in date of disease onset based on patient response and chart review (for proteinuria onset, nephrotic syndrome onset or physician diagnosis). Disease onset is defined as “Date of onset of first signs/symptoms (e.g., edema, gross hematuriaetc.)”.
For the rituximab-naive cohort, the primary exposure was treatment response classification in the MCD cohort. At enrollment, local investigators classified participants with MCD as Infrequently Relapsing, Frequently Relapsing/Glucocorticoid-Dependent, Glucocorticoid-Resistant, Multi-Drug Resistant, or Untreated/Unknown (Table S1). Glucocorticoid-resistant and multi-drug resistant were combined and referred to as “steroid-resistant” in multivariable analyses. Many participants did not have disease classification at enrollment (41% adolescents and 48% adults were unknown or untreated). Adjudication of disease classification at the time of rituximab initiation for the rituximab-naïve cohort was completed by study authors (D.P.C., D.S.G., K.T., T.S.) using the previous definitions (Table S1) as this analysis was dependent on the disease classification.
Statistical Methods
Continuous measures are described as mean and standard deviation (SD) or median and interquartile range (IQR), and categorical data are reported as frequencies and percentages. Differences among the MCD onset age groups were assessed using Wilcoxon rank sum tests for continuous variables and chi square or Fisher exact tests for categorical data. Kaplan–Meier curves were used to descriptively display the probability of relapse and remission for each event from the start of the most recent at-risk period and censored at the last study visit with available data, separately for children, adolescents, and adults.
Prentice, Williams, and Peterson (PWP) gap time recurrent event models were fitted to assess the probability of relapse and remission. These models were stratified by event (relapse or remission) number, allowing for a comparison between probabilities of each relapse or remission event between children, adolescents, and adults through testing of interactions between covariates and event number. Due to infrequent occurrence, events beyond the third relapse or remission were collapsed. A mixed effects linear regression model with random intercepts and slopes was used to assess differences in the change in eGFR over time between groups. Models were adjusted for sex, race/ethnicity, steroid use prior to biopsy, and years between disease onset and biopsy.
In a sub-analysis, a Cox proportional hazards model was fitted to assess differences by disease classification (glucocorticoid resistant vs. glucocorticoid sensitive) and rituximab response in the rituximab-naive cohort. Response was defined as time to first complete remission after initiation of rituximab; models were adjusted for MCD onset age groups, sex, race/ethnicity, glucocorticoid use prior to kidney biopsy, and urine protein/creatinine (UPCR) value at the time of rituximab initiation.
Results
Participant Characteristics of CureGN Minimal Change Disease Cohort
There were 554 subjects with biopsy-proven MCD in the MCD cohort: 304 childhood-onset disease, 49 adolescent-onset disease, and 201 adult-onset disease (Table 1). Median ages at disease onset were 4, 15, and 43 years for childhood-, adolescent-, and adult-onset disease, respectively. Median follow-up time was longer in the childhood-onset group (3.2 years) than the adolescent-onset and adult-onset groups (2.7 years for both, p=0.008). There were more females in the adult group (60%) than the childhood- and adolescent-onset groups (39% and 43%, respectively, p<0.001). There were no differences in ethnicity distribution, with the majority identifying as White (range 63%-69%, p=0.27). Participants with adult-onset disease were more likely to have private insurance (71%) than childhood- or adolescent-onset disease (38% and 49%, respectively, p<0.001).
Table 1:
Baseline characteristics of 554 participants with minimal change disease, including those with childhood-onset disease (age ≤12), adolescent-onset disease (age 13-17) and adult-onset disease (age ≥18 years).
| Child at Disease Onset (n=304) |
Adolescent at Disease Onset (n=49) |
Adult at Disease Onset (n=201) |
P-value | |
|---|---|---|---|---|
| Female, n(%) | 118 (39%) | 21 (43%) | 120 (60%) | <0.001 |
| Age at Disease Onset (years) | 4.0 (2.0-6.0) | 15.0 (14.0-16.0) | 43.0 (28.0-57.0) | <0.001 |
| Age at Biopsy (years) | 5.0 (3.0-9.0) | 15.0 (14.0-16.0) | 44.0 (30.0-58.0) | <0.001 |
| Incident Cohort, n(%) | 101 (33%) | 21 (43%) | 65 (32%) | 0.4 |
| Race, n(%)a | 0.40 | |||
| White/Caucasian | 193 (63%) | 30 (61%) | 138 (69%) | |
| Black/African American | 58 (19%) | 12 (24%) | 27 (13%) | |
| Asian | 21 (7%) | 5 (10%) | 23 (11%) | |
| Other | 20 (7%) | 1 (2%) | 5 (2%) | |
| Ethnicity: Hispanic/Latino n(%) | 38 (13%) | 4 (8%) | 19 (10%) | 0.4 |
| Insurance, n(%) | <0.001 | |||
| Private Insurance | 117 (38%) | 24 (49%) | 143 (71%) | |
| Public Insurance (US) | 120 (39%) | 14 (29%) | 30 (15%) | |
| Public Insurance (non-US) | 37 (12%) | 7 (14%) | 16 (8%) | |
| Other Insurance | 15 (5%) | 1 (2%) | 7 (3%) | |
| No Insurance | 3 (1%) | 1 (2%) | 3 (1%) | |
| Overweight/Obese, n (%) | 166 (55%) | 23 (47)% | 133 (66%) | 0.02 |
| Country, n (%) | 0.002 | |||
| United States | 264 (87%) | 41 (84%) | 173 (86%) | |
| Canada | 15 (5%) | 4 (8%) | 20 (10%) | |
| Italy | 21 (7%) | 4 (8%) | 1 (1%) | |
| Poland | 4 (1%) | 0 (0%) | 7 (3%) | |
| Median Follow up time (years, IQR) | 3.2 (2.1,4.4) | 2.7 (1.8,4.1) | 2.7 (1.6,3.9) | 0.008 |
Race Unknown, total n = 21
N(%) or mean (standard deviation) or median (IQR).
Differences at time of biopsy between groups
Date of disease onset was based on self-report by participants and confirmed by coordinators and/or physician with medical chart review (when available). Children had longer time to biopsy after disease onset (238 days) than those with adolescent-onset (23 days) or adult-onset disease (36 days, p<0.001, Table 2). UPCR at biopsy increased with age at onset (3.0 g/g vs. 5.6 g/g vs. 6.9 g/g) for childhood-, adolescent- and adult- onset disease, respectively (p<0.001), and eGFR decreased with age at onset (139 vs. 95 vs. 88 mL/min/1.73m2, respectively, p<0.001). Children had higher serum albumin at biopsy than those with adolescent- and adult-onset disease (2.6 vs. 1.9 and 2.2 g/dL, respectively, p=0.002). Hypertension was more common among adults (47%) than children (33%) or adolescents (14%, p<0.002).
Table 2.
Clinical characteristics and therapy
| Child at Disease Onset (n=304) |
Adolescent at Disease Onset (n=49) |
Adult at Disease Onset (n=201) |
P-value | |
|---|---|---|---|---|
| Days between onset and biopsy | 238 (85,663) | 23 (11,62) | 36 (16,93) | <0.001 |
| UPCR at Biopsy * | 3.0 (0.4-8.9) | 5.6 (1.0-8.3) | 6.9 (3.6-10.0) | <0.001 |
| UPCR at Disease Onset * | 7.5 (2.3-12.2) | 4.6 (1.0-10.7) | 6.5 (2.8-10.0) | 0.2 |
| eGFR (mL/min/1.73m^2) at Biopsy** | 139 (110-173) | 95 (78-114) | 88 (63-108) | <0.001 |
| eGFR (mL/min/1.73m^2) at Disease onset** | 127 (101-165) | 100 (67-114) | 89 (64-109) | <0.001 |
| Serum albumin (g/dL) at Biopsy*** | 2.6 (1.8-3.3) | 1.9 (1.6-2.4) | 2.2 (1.7-2.8) | 0.002 |
| Serum albumin (g/dL) at Disease Onset*** | 1.8 (1.3-2.4) | 1.9 (1.7-2.3) | 2.2 (1.7-2.8) | 0.002 |
| Hypertension, n(%) |
99 (33%) | 7 (14%) | 94 (47%) | 0.001 |
| Steroid Response Classification at Enrollment | <0.001 | |||
| Infrequently Relapsing | 24 (8%) | 6 (12%) | 30 (15%) | |
| FRNS/SDNS | 173 (57%) | 9 (18%) | 56 (29%) | |
| Steroid Resistant | 50 (16%) | 11 (22%) | 22 (11%) | |
| Multi-drug resistant | 6 (2%) | 0 (0%) | 8 (4%) | |
| Untreated | 14 (5%) | 6 (12%) | 15 (8%) | |
| Unknown | 38 (12%) | 18 (36%) | 65 (33%) | |
| Immunosuppression prior to biopsy | ||||
| Any Immunosuppression | 245 (81%) | 16 (33%) | 51 (26%) | <0.001 |
| Corticosteroids | 231 (76%) | 16 (33%) | 45 (23%) | <0.001 |
| CNIa | 44 (15%) | 1 (2%) | 4 (2%) | <0.001 |
| Rituximaba | 8 (3%) | 0 (0%) | 5 (5%) | 0.5 |
| Mycophenolatea | 20 (7%) | 1 (2%) | 2 (1%) | 0.007 |
| RAAS Blockade prior to biopsy | 23 (8%) | 4 (8%) | 39 (20%) | <0.001 |
missing at biopsy: 115 (38%) pediatric, 13 (26%) adolescent, 69 (36%) adult, missing at disease onset: 193 (66%) pediatric 28 (56%) adolescent and 87 (46%) adult-onset participants
missing at biopsy: 74 (24%) pediatric, 6 (12%) adolescent, 50 (26%) adult, missing at disease onset: 186 (61%) pediatric, 23 (46%) Adolescent-, 89 (47%) adult-onset participants
missing at biopsy: 90 (30%) pediatric, 8 (16%) adolescent, 3 (31%) adult, missing at disease onset: 189 (62%) pediatric 24 (48%), 87 (46%) adult-onset participants
FRNS/SDNS – frequently relapsing nephrotic syndrome/steroid dependent nephrotic syndrome
Fisher’s exact test.
The most common disease classification at enrollment in the childhood-onset group was frequent relapsing/glucocorticoid-dependent (57%); among adolescents and adults, the most common classification was “Untreated/Unknown” (36% and 33%, respectively). Children were more likely to receive immunosuppressive medication pre- biopsy compared to adolescents and adults (81% vs. 33% and 26%, respectively, p<0.001). In children, pre-biopsy therapy was mainly prednisone (76%), but calcineurin inhibitors were also more commonly used than in adolescents or adults (15% vs. 2% and 2%, respectively, p<0.001) (Table 2). Prior to biopsy, 20% of adults were treated with renin angiotensin-aldosterone system (RAAS) blockade, compared to 8% of adolescents and children (p<0.001). Since the details of many patients at disease onset were unknown, we started clinical analysis at time of enrollment when most data were available. After enrollment, we had evidence to determine relapse or remission (corroborated by proteinuria or medication changes) in 93% of relapse events and 95% of remission events.
At enrollment, more children were relapsing (36% vs. 20% for the adolescent and adult groups, p<0.001, Table S2). Overall, children had higher remission and relapse rates (61.2 remission events/100-person-years, 47.1 relapse events/100-person-years) than adolescent-onset (56.0 remission events/100-person-years and 33.3 relapse events/100-person-years, p<0.001) and adult-onset (41.7 remission events/ 100-person-years, 22.7 relapse events/100-person-years) participants. Children remitted faster (median 53 days) than adolescents (62 days) and adults (73 days), p=0.003. Children also relapsed faster (159 days) than adolescents (171 days) and adults (246 days), p = 0.008.
Probability of Relapse and Remission
To understand the nature of this relapsing/remitting disease and to compare the groups, we used post-enrollment data as it is most robust. Event-specific follow-up time was reset to zero when the participant was at risk for the next event. Of those in remission at enrollment, 50% relapsed by 1.3 years (children) and 2.7 years (adolescents). Only 45% of adults ever relapsed during a median observation period of 2.7 years (range 1.6 to 3.9 years). The probability of first relapse at one year was highest in children and lower in both adolescent- and adult-onset disease (44%, 30%, and 25%, respectively, p<0.001, Figure 2A). After remission, the probability of a second relapse at one year was higher among childhood-onset and adolescent-onset disease, and slightly lower in adult-onset disease (52%, 50%, and 40%, respectively, p=0.08, Figure 2B). After three or more remission occurrences, at one year there was a 67%, 37%, and 26% probability of relapse in childhood-, adolescent-, and adult-onset disease, respectively (p<0.001, Figure 2C). Among those who reached the third or subsequent remission, half relapsed again by 0.6, 1.7, and 2.5 years, respectively. Median time to relapse decreased from 1.9 to 1.0 to 0.7 years for the first, second, and third or more relapse event, respectively (p<0.001). Participants were only included in the middle and right panels if they experienced at least one or two events, respectively, as shown by the number at risk in each group along the x-axis.After adjustment for sex, race/ethnicity, glucocorticoid use prior to biopsy, and years from onset to enrollment, those with childhood-onset disease had 69% (hazard ratio [HR]=1.69, 95% CI=1.29-2.21) higher risk of relapse compared to those with adult-onset disease. Risk of relapse was similar between adolescent-onset and adult-onset disease age groups (Table S3a). There was no significant interaction between age at onset and relapse number (first, second, etc.) (p=0.62). The same analysis was done on a subset of patients (n=166) with incident disease (defined as patients with biopsy within 6 months of enrollment). These results (non-significant) show similar direction and magnitude of effect within patients with new or recent onset disease (Table S3b).
Figure 2:
Among CureGN participants, time from complete remission to first initial relapse and subsequent relapses (index date is first remission after enrollment): A) Time to first initial relapse comparing childhood, adolescent and adult-onset disease, B) Time to subsequent second relapse comparing childhood-onset, adolescent-onset, and adult onset disease. C) Time to third or more subsequent relapses. Gray horizontal line shows the number of years from remission when 50% of the population experienced relapse.
Among those in relapse at enrollment, the six-month probability of remission was highest in the childhood-onset (63%) compared to the adolescent-onset (52%) and the adult-onset group (54%, p=0.009, Figure 3A). Subsequently, after the second relapse, the probability of remission by 6 months was 80% for childhood-onset compared to 69% for adolescent-onset, and 61% for adult-onset disease (p=0.003, Figure 3B). The probability of a third or later remission event was similar across the three groups (range 64% to 83%, p=0.17, Figure 3C). After adjustment for sex, race, ethnicity, glucocorticoid use prior to biopsy, and years from onset to enrollment, participants with childhood-onset disease had a 1.33-fold greater chance of achieving remission compared to those with adult-onset disease (HR=1.33, 95% CI 1.02-1.73, p=0.03), while there was no difference between adolescent- versus adult-onset disease (p=0.40) (Table S4a). We performed additional analyses limited to patients classified as steroid sensitive or non-steroid sensitive (excluding unknown or untreated patients) and found similar risks of relapse in children compared to adults or adolescents. An analysis of incident patients (n=166) as above found similar results (non-significant) shown in Table S4b.
Figure 3:
Time from relapse to complete remission is faster in childhood-onset disease compared to adult-onset and adolescent-onset for initial and subsequent second remission and similar for all age groups for subsequent remissions (index date is first relapse after enrollment). A) Time to initial complete remission comparing childhood-, adolescent-, and adult-onset disease. B) Time to subseqent second remission comparing childhood-onset, adolescent-onset and adult-onset disease. C) Time to third or subsequent remissions. Gray horizontal line shows the number of years from relapse when 50% of the population achieved remission.
Kidney Function Outcomes
There were no substantial differences in the frequency of kidney failure (4% in childhood- vs. 6% in adolescent- and 3% in adult-onset disease) or rate of kidney failure (1.40, 2.22 and 1.34 events per 100 patient years for childhood, adolescent, and adult onset respectively) and there were very few death events (1 in childhood onset disease and 5 in adult onset disease) (Table S5). Children and adolescents had a higher eGFR at the time of biopsy (139 and 95, respectively) than the 88mL/min/1.73m2 in adults, p<0.001. Unadjusted mean slopes of eGFR were −0.27 (p=0.38), 0.02 (p=0.93), and −1.11 (p=0.001) mL/min/1.73m2 per year for those with childhood-onset, adolescent-onset, and adult-onset disease, respectively. Differences in the rate of eGFR change did not differ statistically between age-at-onset groups before and after adjustment (p=0.172 and p=0.128, respectively) and are shown in Figure 4. After adjustment for sex, race/ethnicity, and pre-biopsy immunosuppression use, these estimates of average yearly rate of change remained consistent, and the overall average rate of change in eGFR was −0.56 mL/min/1.73m2 (p=0.03, Table S6).
Figure 4:
The means and 95% confidence intervals of the eGFR predicted slopes over follow-up duration in childhood-onset, adolescent-onset and adult-onset MCD are shown (index date is date of disease onset). The intercepts differ substantially, but the slopes are not significantly different. Unadjusted slope (Std Err) - childhood-onset: −0.27 (0.23), adolescent-onset: 0.02 (0.73), adult-onset: −1.11 (0.34), adjusted slope (Std Err) - childhood-onset −0.20 (0.23), adolescent-onset: 0.06 (0.74), adult-onset: −1.13 (0.35). eGFR (mL/min/1.73 m2) was calculated using the Schwartz formula for Pediatric patients <18 years, the CKD-EPI formula for Adult patients >26 years, and the average of pediatric and adult formulas for Adolescent patients 18 to 26 years. Post-ESKD values were excluded.
Rituximab Response
Most participants did not receive rituximab prior to study entry (n=462, 83%). Among those, 111 participants [59 childhood-onset (25%), 8 adolescent-onset (19%), 44 adult-onset (24%)] received rituximab during follow-up and this cohort was analyzed to understand the rituximab response. The cohort included in the rituximab analysis is described in Table S7. Among the childhood onset group, only 7% were Hispanic/Latino compared to 13% in the broader cohort. Overall, there was a higher proportion of infrequently relapsing and steroid resistant patients in this group. At the time of rituximab initiation, most patients were classified as frequently relapsing or steroid-dependent (n=46, 41%); the rest were classified as infrequently relapsing (n=27, 24%) or glucocorticoid-resistant (n=28, 25%). Ten participants could not be classified with available data and were excluded from analysis. Subjects were further grouped as glucocorticoid-sensitive if they were frequently relapsing, glucocorticoid-dependent, or infrequently relapsing, vs. glucocorticoid-resistant or multidrug resistant. Remission probability one year after rituximab was higher in the glucocorticoid-sensitive group compared to those who were resistant, (83% vs. 31%, p=<0.001, Figure 5). Glucocorticoid-sensitive patients were more likely to enter complete remission after rituximab initiation (adjusted HR 2.62, p=0.0015) than those who were not (Table S8). Those with childhood-onset disease were more likely to enter remission than either adolescents or adults after rituximab initiation (adjusted HR 2.1, p = 0.0028). The median time of follow-up in all groups prior to rituximab was less than 1 year and therefore we were not powered to compare before and after rituximab therapy. We analyzed whether groups were different in terms of steroid response classification and found no major differences.
Figure 5.
Time from rituximab initiation to complete remission is faster among those who were glucocorticoid-sensitive compared to those who were not glucocorticoid sensitive (index date is date of rituximab therapy).
Discussion
CureGN provides an opportunity to investigate differences in the clinical course of childhood-onset, adolescent-onset, and adult-onset biopsy-proven MCD. Our focus was not to compare clinically diagnosed and treated nephrotic syndrome but rather a group with MCD confirmed by biopsy. There are limited comparably large epidemiological studies in nephrotic syndrome. Since childhood onset nephrotic syndrome is often treated clinically rather than based on pathologic diagnosis, biopsied pediatric patients may represent a complex and distinct group. We reviewed prior studies of pediatric steroid sensitive nephrotic syndrome and noted they have similar make up in terms of sex and age at disease onset though the proportion of frequently relapsing disease was slightly higher in our biopsied cohort.34 Despite similarities, it should be noted that this study highlights findings from a cohort of biopsied patients and may not represent the entire spectrum of minimal change disease.
MCD is a chronic relapsing and remitting disease but predicting either of these events presents a clinical challenge. We found that those with childhood-onset disease had more remission and relapse events overall compared to adults and adolescents. Risk of relapse was higher, and time to relapse shorter, in children than adults or adolescents. This suggests that children with biopsy proven MCD have a distinct course with regard to relapse. Those with childhood-onset disease achieved remission at a higher rate by 6 months. This may reflect the differences in therapies used in children vs. adults or adolescents or may be related to divergent mechanisms of disease. Additional research will be needed to clarify.
We also evaluated differences in longitudinal changes in kidney function based on age of onset. The slope of eGFR over time was stable in all groups, with no significant differences. We analyzed by the number of immunosuppressive medications used and found no differences, though additional studies could analyze the duration of specific medications. Finally, ESKD and all-cause mortality across all ages was low (~5%).
Rituximab is increasingly used as a glucocorticoid-sparing therapy. There is evidence that B cell targeted therapy is effective in a subset of patients with glucocorticoid-sensitive disease.35-38 T cells have long been implicated in the immunopathogenesis of MCD, and the effect of anti-CD20 therapy and T cells is still being studied.39 Overall, data are limited on which patients benefit from rituximab therapy. We explored the role of clinical phenotype and rituximab response in a subgroup of patients from our larger cohort. We found that 83% of those who are frequently relapsing, glucocorticoid-dependent, or infrequently relapsing had a robust response to rituximab therapy, compared to only 31% of those who were classified as glucocorticoid-resistant. Those with childhood-onset disease and glucocorticoid-sensitivity were twice as likely to enter remission after rituximab therapy. Even though we lack validated, clinically available biomarkers to select for rituximab responsiveness, the clinical phenotype based on response or resistance to other immunosuppression can guide clinical decisions on using rituximab, especially for patients who would most benefit from glucocorticoid-sparing agents. Additional research is needed to evaluate long term safety and prognostic biomarkers.
Even in such a large cohort of biopsy proven disease, certain considerations should be mentioned. It is not surprising that those with childhood-onset disease had longer time to biopsy (238 days vs. 36 days in adult-onset disease), as most cases of childhood onset nephrotic syndrome are treated presumptively as MCD and only biopsied for complications or poor treatment response. Given the selection bias inherently present in the CureGN cohort, our results should be extrapolated with caution to children with glucocorticoid-sensitive idiopathic nephrotic syndrome who never undergo kidney biopsy. Within our cohort, sensitivity analyses in steroid sensitive vs. non-steroid sensitive groups showed similar results. Clinical characteristics varied based on age at onset and may be confounded by differences in pre-biopsy treatment approaches across the groups. As CureGN is a multi-center study, we acknowledge that treatment approaches are not standardized across centers. Adults and adolescents had more proteinuria at time of biopsy, which may reflect pre-biopsy treatment effects in children. There were higher rates of hypertension and lower eGFR at disease onset in the adult-onset group, possibly due to age-related changes, e.g. arterionephrosclerosis rather than disease specific differences. While childhood-onset disease was more frequently treated with glucocorticoids pre-biopsy, they were less likely to be treated with RAAS blockade. This may reflect clinical practice patterns, prevalence of hypertension, or assessment of risk benefit considerations related to acute kidney injury and may warrant further investigation.
In addition to the limitations of a biopsied cohort, our study focuses on patients who either lived near or were referred to a glomerular disease center and subsequently enrolled in CureGN. We found that among children and adolescents, 91% obtained local care (their kidney biopsy was performed at the enrolling CureGN site), but non-local adult patients were commonly referred to a glomerular disease center. The mixture of local and referred adults required further investigation. We performed statistical tests (Table S9) to understand how adult participants differed in being “local” (first biopsy at enrolling CureGN site) or “referred” (biopsy outside of the enrolling CureGN site). Those more likely to be referred included females, older age at disease onset, prevalent patients (first biopsy >6 months), non-Hispanics, those overweight or obese, and those with a history of hypertension. Compared to local patients, the referred patients contributed diversity in sex, age, race, and comorbidities. The combination of local and referred patients increases the generalizability of the broad spectrum of MCD.
The design of CureGN allowed for recruiting both incident and prevalent cases, and despite efforts to retrospectively obtain clinical data from the time of biopsy and disease onset, there were missing data in all groups. As expected, data after enrollment was more robust. Data missingness at time of biopsy or enrollment is reported in the tables 1 and 2. The adolescent group was relatively small compared to the other groups. Self-reported race and ethnicity used in models has limitations as our analyses do not fully explore the social implications of race in MCD.
To our knowledge, our study represents the largest cohort of MCD across the lifespan, allowing comparisons among childhood-onset, adolescent-onset, and adult-onset MCD in a well-defined study such as CureGN. The clinical trajectory based on age groups was previously only compared in smaller studies.12,40 We show that childhood-onset MCD is distinct in its course of relapse and remission and established therapeutic approaches. Understanding the similarities and differences is key to development of translational and mechanistic studies to elucidate common and distinct underlying pathophysiology so that targeted therapies can be developed. Increasingly there are studies identifying molecular markers such as antibodies, B cells and urinary markers which may be important in understanding the risk of relapse and remission.41-43 Future investigations correlating pathologic findings and molecular markers can shed light on the phenotypic differences reported here.
Supplementary Material
Table S1. Disease classification for the joint processes of glucocorticoid treatment and the frequency and timing of relapse or remission.
Table S2. Summary table of relapse and remission events for childhood-, adolescent- and adult-onset glomerular disease.
Table S3a. Recurrent event model of time from remission to subsequent relapse in children vs. adults and in adolescents vs. adults.
Table S3b. Analysis limited to subset of “incident” patients.
Table S4a. Recurrent event model of time from relapse to subsequent remission in children vs. adults and in adolescents vs. adults.
Table S4b. Analysis limited to subset of “incident” patients.
Table S5. Mean number of years from enrollment to End Stage Kidney Disease (ESKD) or death; number (%) of ESKD events after enrollment based on age at disease onset.
Table S6. Mixed effects linear regression model with covariate predictors of estimated glomerular filtration rate (eGFR).
Table S7. Patient characteristics of subset of rituximab naive cohort.
Table S8. Time to first remission after rituximab initiation among rituximab naive patients at enrollment.
Table S9. Table of differences between ‘local’ and ‘referred’ CureGN adult participants.
Support:
Funding for the CureGN consortium is provided by U24DK100845 (formerly UM1DK100845), U01DK100846 (formerly UM1DK100846), U01DK100876 (formerly UM1DK100876), U01DK100866 (formerly UM1DK100866), and U01DK100867 (formerly UM1DK100867) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Patient recruitment is supported by NephCure Kidney International. Dates of funding for first phase of CureGN was 9/16/2013-5/31/2019. DPC was supported by an NIH, National Institute of Diabetes and Digestive and Kidney Diseases Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32DK007750; PI RJ Falk). JBK is supported by the NIDDK Intramural Research Program. The funding agencies did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Members of the CureGN Consortium:
At the CureGN Participating Clinical Centers (PCC) network through Columbia University: Wooin Ahn, Gerald Appel, Paul Appelbaum, Revekka Babayev, Andrew Bomback, Brenda Chan, Vivette Denise D'Agati, Samitri Dogra, Hilda Fernandez, Ali Gharavi**, William Hines, Syed Ali Husain, Namrata Jain, Krzysztof Kiryluk, Fangming Lin, Maddalena Marasa#, Glen Markowitz, Hila Milo Rasouly, Sumit Mohan, Nicola Mongera, Jordan Nestor, Thomas Nickolas, Jai Radhakrishnan, Maya Rao, Simone Sanna-Cherchi, Shayan Shirazian, Michael Barry Stokes, Natalie Uy, Anthony Valeri, Natalie Vena (Columbia University, New York, NY); Bartosz Foroncewicz, Barbara Moszczuk, Agnieszka Perkowska-Ptasińska (University of Warsaw, Warszawa, Poland); Gian Marco Ghiggeri*, Francesca Lugani (Gaslini Children’s Hospital, Genoa, Italy). At the CureGN PCC network through the Pediatric Nephrology Research Consortium: Josephine Ambruzs, Helen Liapis (Arkana Laboratories, Little Rock, AR); Rossana Baracco, Amrish Jain* (Children’s Hospital of Michigan, Detroit, MI); Isa Ashoor, Diego Aviles* (Children’s Hospital of New Orleans/LSU Health, New Orleans, LA); Sun-Young Ahn* (Children’s National Medical Center, Washington DC); Prasad Devarajan, Elif Erkan*, Donna Claes, Hillarey Stone (Cincinnati Children’s Hospital Cincinnati, OH); Sherene Mason* (Connecticut Children’s Medical Center, Hartford, CT); Liliana Gomez-Mendez* (East Carolina University Brody School of Medicine, Greenville, NC); Chia-shi Wang, Hong (Julie) Yin (Emory University, Atlanta, GA); Yi Cai*, Goebel Jens, Julia Steinke (Helen DeVos Children’s Hospital, Grand Rapids, MI); Donald Weaver* (Levine Children’s Hospital/Atrium Health, Charlotte, NC); Jerome Lane* (Lurie Children’s Hospital, Chicago IL); Carl Cramer* (Mayo Clinic, Rochester, MN); Cindy Pan, Neil Paloian, Rajasree Sreedharan* (Medical College of Wisconsin, Milwaukee, WI); Corinna Bowers#, Mary Dreher#; John Mahan, Samantha Sharpe#, William Smoyer** (Nationwide Children’s Hospital, Columbus, OH); Amira Al-Uzri*, Sandra Iragorri (Oregon Health and Science University, Portland, OR); Myda Khalid* (Riley Children’s Hospital, Indianapolis, IN); Craig Belsha* (Cardinal Glennon Children’s Medical Center/St. Louis University, St. Louis, MO); Joseph Alge*, Michael Braun, AC Gomez, Scott Wenderfer* (Texas Children’s Hospital, Houston, TX); Tetyana Vasylyeva* (Texas Tech Health Sciences Center, Amarillo, TX); Daniel Feig* (Children’s of Alabama, University of Alabama, Birmingham, AL); Gabriel Cara Fuentes, Melisha Hannah* (University of Colorado Children’s Hospital, Colorado, Aurora, CO); Carla Nester* (University of Iowa Children’s Hospital, Iowa City, IA); Jon Klein** (University of Louisville, Louisville, KY); Chryso Katsoufis, Wacharee Seeherunvong* (Holtz Medical Center, University of Miami, Miami, FL); Michelle Rheault* (University of Minnesota Children’s Hospital, Minneapolis, MN); Craig Wong* (University of New Mexico Health Sciences Center, Albuquerque, NM); Nisha Mathews* (University of Oklahoma Health Sciences Center, Oklahoma City, OK); John Barcia*, Agnes Swiatecka-Urban (University of Virginia, Charlottesville, VA); Sharon Bartosh* (University of Wisconsin, Madison, WI); Vikas Dharnidharka*, Joseph Gaut (Washington University in St. Louis, St. Louis, MO). At the CureGN PCC network through the University of North Carolina: Louis-Philippe Laurin*, Virginie Royal (Hôpital Maisonneuve-Rosemont, Montreal, Canada); Anand Achanti, Milos Budisavljevic*, Sally Self (Medical University of South Carolina, Charleston, SC); Cybele Ghossein, Yonatan Peleg, Shikha Wadhwani* (Northwestern University, Chicago, IL); Salem Almaani, Tibor Nadasdy, Samir, Parikh, Brad Rovin* (Ohio State University, Columbus, OH); Anthony Chang (University of Chicago, Chicago, IL); Huma Fatima, Bruce Julian, Jan Novak, Matthew Renfrow, Dana Rizk* (University of Alabama at Birmingham, Birmingham, AL); Vimal Derebail, Ronald Falk**, Keisha Gibson, Dorey Glenn, Susan Hogan, Koyal Jain, J. Charles Jennette, Caroline Poulton#, Manish Kanti Saha (University of North Carolina Kidney Center, Chapel Hill, NC); Agnes Fogo, Neil Sanghani* (Vanderbilt University, Nashville, TN); Selvaraj Muthusamy (Virginia Commonwealth University, Richmond, VA). At the CureGN PCC network through the University of Pennsylvania: Jeffrey Schelling* (MetroHealth Medical Center/Case Western Reserve University, Cleveland, OH); Jean Hou (Cedars-Sinai Health System, Los Angeles, CA); Kevin Lemley*, Warren Mika, Pierre Russo (Children’s Hospital of LA, Los Angeles, LA); Michelle Denburg, Amy Kogon, Kevin Meyers*, Madhura Pradhan (Children’s Hospital of Philadelphia, Philadelphia, PA); Raed Bou Matar*, John O'Toole*, John Sedor* (Cleveland Clinic, Cleveland, OH); Christine Sethna*, Suzanne Vento# (Cohen Children’s Medical Center, New Hyde Park, NY); Mohamed Atta, Serena Bagnasco, Alicia Neu, (Johns Hopkins University, Baltimore, MD); Sharon Adler*, Tiane Dai, Ram Dukkipati (Lundquist Institute at Harbor-UCLA Medical Center, Torrance, CA); Fernando Fervenza*, Sanjeev Sethi (Mayo Clinic, Rochester, MN); Frederick Kaskel, Kaye Brathwaite, (Montefiore Medical Center, The Bronx, New York, NY); Joseph Weisstuch, Ming Wu, Olga Zhdanova (New York University, New York, NY); Jurgen Heymann, Meryl Waldman, Cheryl Winkler (NIDDK, Bethesda, MD); Katherine Tuttle* (Spokane Providence Medical Center, Spokane, WA); Jill Krissberg, Richard Lafayette*, Kamal Fahmeedah, Elizabeth Talley (Stanford University, Palo Alto, CA); Michelle Hladunewich* (Sunnybrook Health Sciences Centre, Toronto, Canada); Carmen Avila-Casado, Daniel Cattran*, Reich Heather, Philip Boll (University Health Network, Toronto, Canada); Yelena Drexler, Alessia Fornoni* (University of Miami, Miami, FL); Patrick Gipson*, Jeffrey Hodgin, Andrea Oliverio (University of Michigan, Ann Arbor, MI); Jon Hogan, Lawrence Holzman**, Matthew Palmer, Gaia Coppock (University of Pennsylvania, Philadelphia, PA); Blaise Abromovitz*, Michael Mortiz* (University of Pittsburgh School of Medicine, Pittsburgh, PA); Charles Alpers, J. Ashley Jefferson* (University of Washington, Seattle, WA); Elizabeth Brown, Kamal Sambandam*, Bethany Roehm (UT Southwestern, Dallas, TX). At the Data Coordinating Center: Bruce Robinson** (Arbor Research Collaborative for Health, Ann Arbor, MI); Cynthia Nast (Cedar Sinai Medical Center, Los Angeles, CA); Laura Barisoni (Duke University, Durham, NC); Matthias Kretzler, Laura Mariani** (University of Michigan, Ann Arbor, MI). Steering Committee Chair: Lisa M. Guay-Woodford, Children’s National Hospital, Washington DC. **CureGN Principal Investigators; *CureGN Site Principal Investigators; #CureGN Lead Coordinators.
Footnotes
Financial Disclosure: TS has received research funding for multi-center clinical trials from Bristol-Myers-Squibb, Apellis Pharmaceuticals, and Travere Therapeutics (previously Retrophin), and has consulted for Alnylam, Magnolia and GLG. RG has received grants from BMS, Travere Therapeutics, Reata Pharm, Goldfinch biotech. LAG has received grants from Reata Pharmaceuticals and Vertex Pharmaceuticals, has consulted for Novartis, Roche, and Aurinia, and serves on a DMC for a study sponsored by Travere. DSG has received grants from Reata, Novartis, Travere, Goldfinch Bio Pharmaceuticals, has consulted through the University of Michigan for Novartis, Vertex, Roche, Genentech, Boehringer Ingelheim, and serves on a DMC for a study sponsored by NIH. AKM receives funding from Alexion, Boehringer Ingelheim, Calliditias and Pfizer, and is a consultant to Bayer. DPC has received grants from Vertex Pharmaceuticals. PAC has consulted for Otsuka and Travere Therapeutics. DTS has consulted for Travere Therapeutics. KET has received grant funding from Kanaka and is a consultant for Horizon. The remaining authors declare that they have no relevant financial interests.
References:
- 1.Farquhar MG, Vernier RL, Good RA. An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. The Journal of experimental medicine. 1957;106(5):649 DOI: 10.1084/jem.106.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet (London, England). 2003;362(9384):629–639 DOI: 10.1016/s0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 3.Zee J, Liu Q, Smith AR, et al. Kidney Biopsy Features Most Predictive of Clinical Outcomes in the Spectrum of Minimal Change Disease and Focal Segmental Glomerulosclerosis. J Am Soc Nephrol. 2022;33(7):1411–1426 DOI: 10.1681/ASN.2021101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Shaughnessy MM, Hogan SL, Poulton CJ, et al. Temporal and Demographic Trends in Glomerular Disease Epidemiology in the Southeastern United States, 1986-2015. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(4):614–623 DOI: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron JS. The nephrotic syndrome and its complications. Am J Kidney Dis. 1987;10(3):157–171 DOI: 10.1016/s0272-6386(87)80170-1. [DOI] [PubMed] [Google Scholar]
- 6.Keskar V, Jamale TE, Kulkarni MJ, Kiggal Jagadish P, Fernandes G, Hase N. Minimal-change disease in adolescents and adults: epidemiology and therapeutic response. Clin Kidney J. 2013;6(5):469–472 DOI: 10.1093/ckj/sft063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariani LH, Bomback AS, Canetta PA, et al. CureGN Study Rationale, Design, and Methods: Establishing a Large Prospective Observational Study of Glomerular Disease. Am J Kidney Dis. 2019;73(2):218–229 DOI: 10.1053/j.ajkd.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(3):445–453 DOI: 10.2215/cjn.03531006. [DOI] [PubMed] [Google Scholar]
- 9.Durkan AM, Hodson EM, Willis NS, Craig JC. Immunosuppressive agents in childhood nephrotic syndrome: a meta-analysis of randomized controlled trials. Kidney Int. 2001;59(5):1919–1927 DOI: 10.1046/j.1523-1755.2001.0590051919.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang JJ, Hsu SC, Chen FF, Sung JM, Tseng CC, Wang MC. Adult-onset minimal change disease among Taiwanese: clinical features, therapeutic response, and prognosis. Am J Nephrol. 2001;21(1):28–34 DOI: 10.1159/000046215. [DOI] [PubMed] [Google Scholar]
- 11.Colattur SN, Korbet SM. Long-term Outcome of Adult Onset Idiopathic Minimal Change Disease. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2000;11(3):334–344. [PubMed] [Google Scholar]
- 12.Shinzawa M, Yamamoto R, Nagasawa Y, et al. Age and prediction of remission and relapse of proteinuria and corticosteroid-related adverse events in adult-onset minimal-change disease: a retrospective cohort study. Clinical and experimental nephrology. 2013;17(6):839–847 DOI: 10.1007/s10157-013-0793-9. [DOI] [PubMed] [Google Scholar]
- 13.Idelson BA, Smithline N, Smith GW, Harrington JT. Prognosis in steroid-treated idiopathic nephrotic syndrome in adults. Analysis of major predictive factors after ten-year follow-up. Archives of internal medicine. 1977;137(7):891–896. [PubMed] [Google Scholar]
- 14.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20(6):765–771 DOI: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 15.Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG. Adult-onset minimal change nephrotic syndrome: a long-term follow-up. Kidney Int. 1986;29(6):1215–1223 DOI: 10.1038/ki.1986.130. [DOI] [PubMed] [Google Scholar]
- 16.Mak SK, Short CD, Mallick NP. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant. 1996;11(11):2192–2201 DOI: 10.1093/oxfordjournals.ndt.a027136. [DOI] [PubMed] [Google Scholar]
- 17.Hogan J, Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol. 2013;24(5):702–711 DOI: 10.1681/ASN.2012070734. [DOI] [PubMed] [Google Scholar]
- 18.Santos JE, Fiel D, Santos R, et al. Rituximab use in adult glomerulopathies and its rationale. J Bras Nefrol. 2020;42(1):77–93 DOI: 10.1590/2175-8239-jbn-2018-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Fresnedo G, Segarra A, Gonzalez E, et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(8):1317–1323 DOI: 10.2215/CJN.00570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong WY, Swaminathan R, Irish A. Our experience with rituximab therapy for adult-onset primary glomerulonephritis and review of literature. Int Urol Nephrol. 2013;45(3):795–802 DOI: 10.1007/s11255-012-0206-0. [DOI] [PubMed] [Google Scholar]
- 21.Brown LC, Jobson MA, Payan Schober F, et al. The Evolving Role of Rituximab in Adult Minimal Change Glomerulopathy. Am J Nephrol. 2017;45(4):365–372 DOI: 10.1159/000464475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munyentwali H, Bouachi K, Audard V, et al. Rituximab is an efficient and safe treatment in adults with steroid-dependent minimal change disease. Kidney Int. 2013;83(3):511–516 DOI: 10.1038/ki.2012.444. [DOI] [PubMed] [Google Scholar]
- 23.Takei T, Itabashi M, Moriyama T, et al. Effect of single-dose rituximab on steroid-dependent minimal-change nephrotic syndrome in adults. Nephrol Dial Transplant. 2013;28(5):1225–1232 DOI: 10.1093/ndt/gfs515. [DOI] [PubMed] [Google Scholar]
- 24.Papakrivopoulou E, Shendi AM, Salama AD, Khosravi M, Connolly JO, Trompeter R. Effective treatment with rituximab for the maintenance of remission in frequently relapsing minimal change disease. Nephrology (Carlton, Vic). 2016;21(10):893–900 DOI: 10.1111/nep.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenoglio R, Sciascia S, Beltrame G, et al. Rituximab as a front-line therapy for adult-onset minimal change disease with nephrotic syndrome. Oncotarget. 2018;9(48):28799–28804 DOI: 10.18632/oncotarget.25612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet (London, England). 2014;384(9950):1273–1281 DOI: 10.1016/S0140-6736(14)60541-9. [DOI] [PubMed] [Google Scholar]
- 27.Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25(4):850–863 DOI: 10.1681/ASN.2013030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(12):2207–2212 DOI: 10.2215/CJN.03470410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravani P, Rossi R, Bonanni A, et al. Rituximab in Children with Steroid-Dependent Nephrotic Syndrome: A Multicenter, Open-Label, Noninferiority, Randomized Controlled Trial. J Am Soc Nephrol. 2015;26(9):2259–2266 DOI: 10.1681/ASN.2014080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612 DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637 DOI: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng DK, Schwartz GJ, Schneider MF, Furth SL, Warady BA. Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int. 2018;94(1):170–177 DOI: 10.1016/j.kint.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delanaye P, Schaeffner E, Ebert N, et al. Normal reference values for glomerular filtration rate: what do we really know? Nephrol Dial Transplant. 2012;27(7):2664–2672 DOI: 10.1093/ndt/gfs265. [DOI] [PubMed] [Google Scholar]
- 34.Banh TH, Hussain-Shamsy N, Patel V, et al. Ethnic Differences in Incidence and Outcomes of Childhood Nephrotic Syndrome. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(10):1760–1768 DOI: 10.2215/CJN.00380116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimbert P, Audard V, Remy P, Lang P, Sahali D. Recent approaches to the pathogenesis of minimal-change nephrotic syndrome. Nephrol Dial Transplant. 2003;18(2):245–248 DOI: 10.1093/ndt/18.2.245. [DOI] [PubMed] [Google Scholar]
- 36.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet (London, England). 1974;2(7880):556–560 DOI: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 37.Shimada M, Araya C, Rivard C, Ishimoto T, Johnson RJ, Garin EH. Minimal change disease: a "two-hit" podocyte immune disorder? Pediatr Nephrol. 2011;26(4):645–649 DOI: 10.1007/s00467-010-1676-x. [DOI] [PubMed] [Google Scholar]
- 38.Chan EY, Webb H, Yu E, et al. Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int. 2020;97(2):393–401 DOI: 10.1016/j.kint.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Colucci M, Carsetti R, Rosado MM, et al. Atypical IgM on T cells predict relapse and steroid dependence in idiopathic nephrotic syndrome. Kidney Int. 2019;96(4):971–982 DOI: 10.1016/j.kint.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Yoo KD, Oh YK, et al. Predictors of Relapse in Adult-Onset Nephrotic Minimal Change Disease. Medicine. 2016;95(12):e3179 DOI: 10.1097/MD.0000000000003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colucci M, Carsetti R, Cascioli S, et al. B Cell Reconstitution after Rituximab Treatment in Idiopathic Nephrotic Syndrome. J Am Soc Nephrol. 2016;27(6):1811–1822 DOI: 10.1681/ASN.2015050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gipson DS, Trachtman H, Waldo A, et al. Urinary Epidermal Growth Factor as a Marker of Disease Progression in Children With Nephrotic Syndrome. Kidney Int Rep. 2020;5(4):414–425 DOI: 10.1016/j.ekir.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watts AJB, Keller KH, Lerner G, et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J Am Soc Nephrol. 2022;33(1):238–252 DOI: 10.1681/ASN.2021060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Disease classification for the joint processes of glucocorticoid treatment and the frequency and timing of relapse or remission.
Table S2. Summary table of relapse and remission events for childhood-, adolescent- and adult-onset glomerular disease.
Table S3a. Recurrent event model of time from remission to subsequent relapse in children vs. adults and in adolescents vs. adults.
Table S3b. Analysis limited to subset of “incident” patients.
Table S4a. Recurrent event model of time from relapse to subsequent remission in children vs. adults and in adolescents vs. adults.
Table S4b. Analysis limited to subset of “incident” patients.
Table S5. Mean number of years from enrollment to End Stage Kidney Disease (ESKD) or death; number (%) of ESKD events after enrollment based on age at disease onset.
Table S6. Mixed effects linear regression model with covariate predictors of estimated glomerular filtration rate (eGFR).
Table S7. Patient characteristics of subset of rituximab naive cohort.
Table S8. Time to first remission after rituximab initiation among rituximab naive patients at enrollment.
Table S9. Table of differences between ‘local’ and ‘referred’ CureGN adult participants.







