Abstract
Introduction
Extensor mechanism disruption (EMD) following total knee arthroplasty (TKA) is a devastating problem commonly treated with allograft or synthetic reconstruction. Understanding of reconstruction success rates and patient recorded outcomes is lacking.
Methods
Patients who have an EMD after TKA undergoing mesh or whole-extensor allograft reconstruction between 2011 to 2019 with minimum 2-year follow-up were reviewed from two tertiary care centers. Functional failure was defined as extensor lag >30 degrees, amputation, or fusion, as well as revision EMR. Survivorship was assessed using Kaplan-Meier curves and factors for success were determined with logistic regressions.
Results
Of fifty-six EMRs (49 patients), 50.0% (28/56) were functionally successful at 3.2-years mean follow-up (range, 0.2 to 7.4). In situ survivorship of the reconstructions at 36-months was 75.0% (42 of 58). There were 50.0% (14 of 28) of functionally failed EMRs that retained their reconstruction at last follow-up. Mean extensor lag among successes and failures was 5.4 and 71.0° (P=0.01). Mean KOOS JR scores were 67.1 and 48.8 among successes and failures (P=0.01). There were 64.0% (16 of 25) of successes and 1 of 19 failures that obtained a KOOS JR score above the minimum patient-acceptable symptom state for TKA. Survivorship and success rates were similar between reconstruction methods (P=0.86; P=0.76). All-cause mortality was 8.2% (4 of 49), each with EMR failure prior to death. All-cause reoperation rate was 42.9% (24 of 56), with a 14.3% (8 of 56) rate of revision EMR and 10.7% (6 of 56) rate of above-knee-amputation or modular fusion.
Conclusions
This multi-center investigation of mesh or allograft EMR demonstrated modest functional success at 3.2-years. Complication and reoperation rates were high, regardless of EMR technique. Therefore, EMD after TKA remains problematic.
Keywords: Extensor mechanism reconstruction, extensor mechanism disruption, mesh, allograft, total knee arthroplasty complications, KOOS JR
INTRODUCTION:
Extensor mechanism disruption (EMD) after total knee arthroplasty (TKA) creates major functional limitations for the patient and is challenging to treat. The incidence of EMD is low, with reported rates ranging from 0.1 of 1.4%.1–5 Primary repair has unacceptably high failure rates, which led to the development of reconstructive options.1,3,5–11 Contemporary extensor mechanism reconstruction (EMR) includes whole-extensor mechanism or achilles tendon allograft and synthetic polypropylene mesh. A recent meta-analysis demonstrated similar results and a 25% failure rate between the two methods.12 A consensus does not exist on which is superior.1,12
Concern exists for deteriorating results over time with allograft reconstruction, with 69% survivorship at mid-term follow-up and a 56% success rate at 10 years.13,14 Allograft tissue is expensive, difficult to obtain, has disease transmission potential, may not incorporate, and may fracture or degrade over time. The use of synthetic polypropylene mesh has been popularized.15 The developers of this technique have shown good short-term results, with a mean extensor lag of 9° and two-year survivorship of 85%.16 Results from other institutions have not been as successful, with a 2-year success rate of 58% and survivorship of 73%.17
It should be noted that EMR is associated with major morbidity and high complication and reoperation rates, especially in patients who have previous prosthetic joint infection (PJI).1,2,13,14,16,18 Given the expected growth in primary and revision TKA volume, it is important to expand the literature on contemporary EMR outcomes.19 The aims of our study were to examine the results of allograft and mesh EMRs from two tertiary referral centers, to determine factors associated with successful reconstruction, to determine patient recorded outcomes (PROs), as measured by Knee Injury and Osteoarthritis Outcome Score, Joint Replacement (KOOS JR), and to determine rates at which patients achieved the minimal patient acceptable symptom state (PASS) for TKA using KOOS JR scores.
MATERIALS AND METHODS:
Institutional review board approval was obtained for this retrospective chart review of patients treated at two tertiary referral medical centers. There were seventy-three EMRs in 63 patients were identified from 2011 to 2021: 40 EMRs from a mid-South academic center and 33 from a mountain West academic center. There were seven fellowship-trained arthroplasty surgeons who performed the EMRs. Patients who had an EMD in the setting of a TKA and those undergoing an EMR procedure were identified and crossed-referenced with implant logs identifying the use of mesh or whole EM allograft. There were seventeen EMRs in 14 patients excluded due to insufficient follow-up (9), reconstruction with something other than mesh or whole-EM allograft (6), or death prior to EMR failure (2). Included patients had minimum two-year follow-up or documented failure prior to two years. The final study population consisted of 56 EMRs in 49 patients who had 41 mesh and 15 allograft reconstructions. Mean follow-up was longer in the allograft group than the mesh group (4.3 versus 2.8 years, P<0.01), which reflected an overall trend for both centers to switch to mesh reconstructions over time. The EMRs that failed and went on to revision EMR were treated as a separate reconstruction. One patient underwent staged bilateral EMRs following separate EMDs. The remaining difference between the numbers of included EMRs and patients were revision reconstructions.
No significant demographic or medical differences were seen between the allograft and mesh groups (Table 1). Mechanism of EMD and the site of failure were identified. Total number of prior knee surgeries, prior extensor repairs, prior EMR attempts, and history of PJI were recorded. Surgical details including type of reconstruction, method of reconstruction fixation, extent of concurrent arthroplasty revision, and level of TKA constraint were collected. The surgical technique for mesh reconstructions was similar to that described by Browne and Hanssen and updated by Abdel et al.15,16 There were twenty-four knees that had their mesh fixed into an anterior trough in the proximal tibia, while 17 others had them inserted and cemented in the tibial canal and exiting under the tibial baseplate during arthroplasty revision. The allograft reconstruction technique utilized was similar to that published by Burnett et al.8 In all allograft reconstructions, screws were used to fix the tibial tubercle bone block. Patients were immobilized postoperatively, most commonly for 12 weeks, but variability existed. There were eighteen EMRs placed in a brace, 37 in a cast, and 1 in an external fixator. Length of post-operative immobilization was at the discretion of the treating surgeon (mean 57 days (Standard Devaiation (SD) 25.4; 95% confidence interval (CI) 49.6-63.3)).
Table 1.
Demographics of Mesh and Allograft Cohorts
| Demographic | Study Cohort (%) | Mesh Cohort | Allograft Cohort | P-value |
|---|---|---|---|---|
| No. of kneesα | 56 | 41 | 15 | |
| Age, mean (range, years) | 67 (36 to 93) | 69 (45 to 93) | 64 (36 to 83) | 0.20 |
| Years follow-up, mean (range, years) | 3.2 (0.2 to 7.4) | 2.8 (0.2 to 7.4) | 4.3 (1.6 to 6.0) | < 0.01 |
| Sex | ||||
| Men | 33.9 (n = 19) | 31.7 (n = 13) | 40.0 (n = 6) | 0.56 |
| Women | 66.1 (n = 37) | 68.3 (n = 28) | 60.0 (n = 9) | |
| Race | ||||
| White | 91.1 (n = 51) | 92.7 (n = 38) | 86.7 (n = 13) | 0.47 |
| Black | 7.1 (n = 4) | 4.9 (n = 2) | 13.3 (n = 2) | |
| Hispanic | 1.8 (n = 1) | 2.4 (n = 1) | 0.0 (n = 0) | |
| BMI (range) | 31.9 (18.6 to 47.9) | 31.6 (18.6 to 47.9) | 32.7 (24.1 to 46.6) | 0.57 |
| ASA score (range) | 2.6 (1.0 to 3.0) | 2.7 (2.0 to 3.0) | 2.5 (1.0 to 3.0) | 0.17 |
| Tobacco use | ||||
| Current | 12.5 (n = 7) | 9.8 (n = 4) | 20.0 (n = 3) | 0.13 |
| Former | 32.1 (n = 18) | 26.8 (n = 11) | 46.7 (n = 7) | |
| Never | 55.4 (n = 31) | 63.4 (n = 26) | 33.3 (n = 5) | |
| Diabetes | 21.4 (n = 12) | 22.0 (n = 9) | 20.0 (n = 3) | 0.88 |
| Inflammatory disease | 14.3 (n = 8) | 16.6 (n = 6) | 13.3 (n = 2) | 0.90 |
| Chronic kidney disease | 5.4 (n = 3) | 4.9 (n = 2) | 6.7 (n = 1) | 0.79 |
| PJI history | 30.4 (n = 17) | 39.0 (n = 16) | 6.7 (n = 1) | 0.02 |
| Prior failed EMR | 16.1 (n = 9) | 14.6 (n = 6) | 20.0 (n = 3) | 0.63 |
| >2 prior knee surgeries | 58.2 (n = 32) | 61.0 (n = 25) | 50.0 (n = 7) | 0.47 |
| Prior primary repair | 46.4 (n = 26) | 39.0 (n = 16) | 66.7 (n = 10) | 0.07 |
| Traumatic EMDβ | 53.6 (n = 30) | 51.2 (n = 21) | 60.0 (n = 9) | 0.56 |
| Iatrogenic EMDγ | 5.5 (n = 3) | 5.0 (n = 2) | 6.7 (n = 1) | 0.81 |
| EMD related to PJIδ | 23.2 (n = 13) | 29.3 (n = 12) | 6.7 (n = 1) | 0.08 |
| Location of EMD | ||||
| Patellaε | 26.8 (n = 15) | 26.8 (n = 11) | 26.7 (n = 4) | 0.15 |
| Patellar tendon | 44.6 (n = 25) | 51.2 (n = 21) | 26.7 (n = 4) | |
| Quadriceps tendon | 28.6 (n = 16 | 22.0 (n = 9) | 46.7 (n = 7) |
BMI = body mass index; ASA = American Society of Anesthesiology; PJI = prosthetic joint infection; EMR = extensor mechanism reconstruction; EMD = extensor mechanism disruption
Number of knees/lower extremities included in each cohort.
EMD resulted from a traumatic incident, e.g., a mechanical fall.
EMD as a complication of concurrent or prior surgery.
EMD as a complication of PJI. Uncertain specific single incident resulting in failure.
Either acute fracture or chronic failure through patella.
Success was defined as an in situ EMR with an extensor lag less than 30° at most recent follow-up. This was chosen based on prior published studies8,13 which demonstrated that having a functional quadriceps muscle is important to both prevent falls and perform activities of daily living.20,21 Failure was defined as extensor lag greater than 30°, subsequent above-knee amputation (AKA), modular fusion, or revision EMR. The degree of extensor lag was collected from clinical follow-up reports. Additional secondary outcomes included rates of immobilization ulcers, need for ambulatory aids, reoperations, 90-day emergency department visits, readmissions, and all-cause mortalities. Also, post-EMR KOOS JR scores were collected. The KOOS JR is a clinically validated patient-reported outcome score with a minimal clinically important differences (MCID) of 14 to 20.22,23 The KOOS JR PASS threshold for primary TKA of 63.7 was used.24 Surgical and postoperative care details were examined as factors for success or failure. Logistic regression analysis was used to determine factors that predicted EMR success.
Patients who did not have a recent in-person encounter were contacted via telephone or electronic questionnaire to determine the functional status of their EMR and aforementioned secondary outcomes. A clinically-validated tool, the Copenhagen Knee Range of Motion scale was used to assess extensor lag in patients who were not available for in person follow-up.25 This is a scale which utilizes pictures of a knee at various degrees of motion and has patients rate how far they are able to flex and extend.
Kaplan-Meier survivorship curves were utilized to determine the in situ survivorship of an EMR, regardless of functional status. Log rank tests were used to determine differences in survivorship. Multivariate logistic regression analyses were used to determine factors associated with success. Stata Version 15.1 (StataCorp LLC; College Station, Texas) was used for all analyses. P≤0.05 was considered statistically significant. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
RESULTS:
The overall rate of a functionally successful EMR was 50.0% (28 of 56) at a mean follow-up of 3.2 years (range, 0.2 to 7.4). Extensor lag among successes was 5.4° (SD 6.8; CI 2.7-8.0), compared to 71.0° (SD 24.7; CI 61.0-81.0), among failures (P<0.01). There were 50.0% (14 of 28) of the failures that had their EMR in situ at their most recent follow-up, resulting in 75.0% (42 of 56) overall in situ survivorship (Figure 1). The mesh and allograft groups experienced a 51.2% (21 of 41) and 7 of 15 rate of successful EMR, respectively, with similar in situ survivorship (P=0.76; P0.86).
Figure 1.
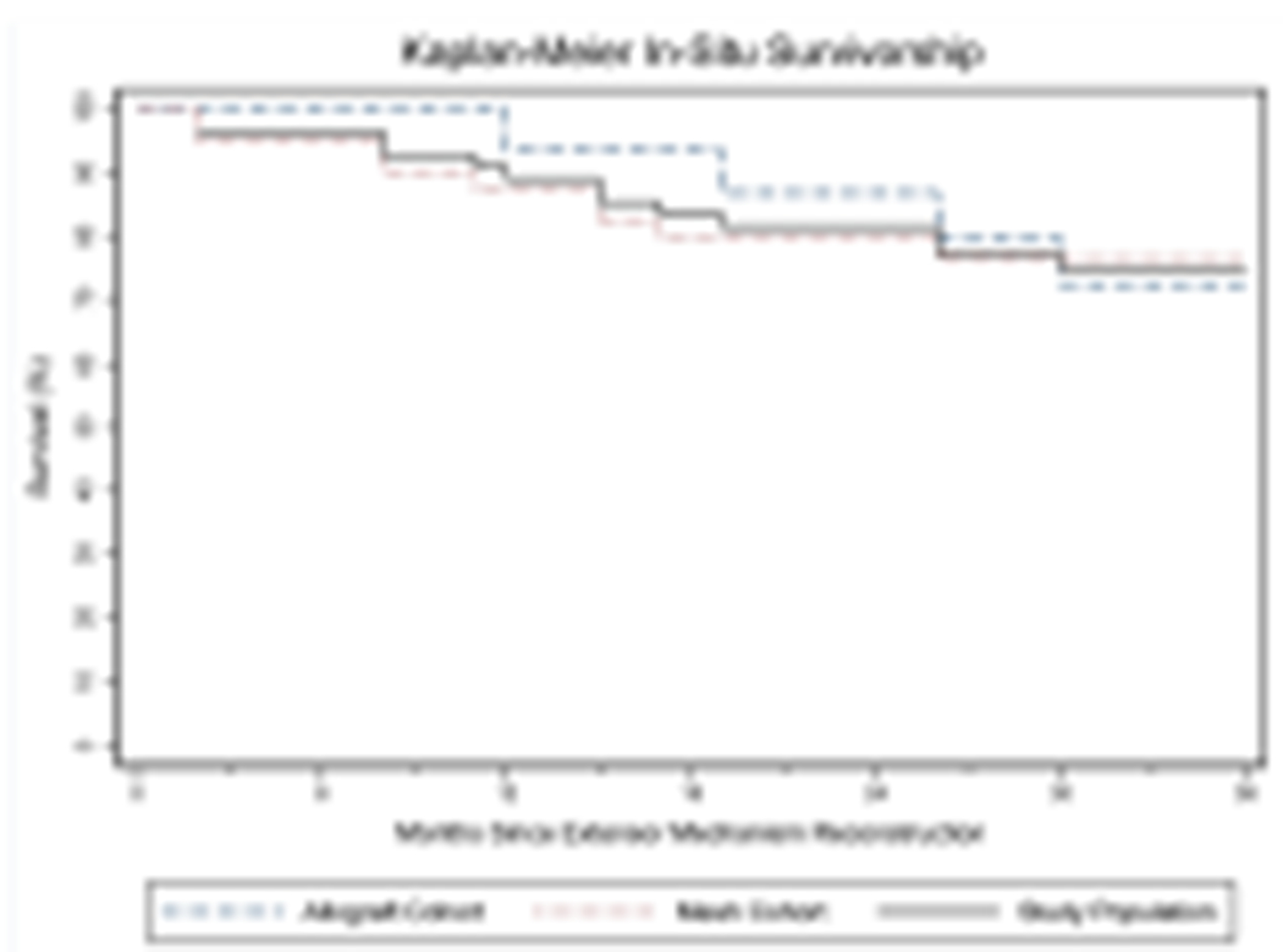
Kaplan-Meier survivorship curves for the in-situ survival of the extensor mechanism reconstruction for the entire study population as well as the mesh and allograft groups.
Significant differences were not seen in demographic variables between successes and failures (Table 2). There were 3 of 15 and 14.6% (6 of 41) of the allograft and mesh groups, respectively, that had a prior failed EMR (P=0.58) with an overall revision EMR success rate of 4 of 9. There were 39.0% (16 of 41) of mesh and 1 of 15 of allograft EMRs that had a prior PJI (P=0.02). Success was not related to PJI history. 58.2% (32 of 56) of knees had more than two prior surgeries and 46.4% (26 of 56) had a history of prior primary repair.
Table 2.
Demographics of Successes and Failures
| Demographic | Successes (%) | Failures | P-value |
|---|---|---|---|
| No. of kneesα | 50.0 (n = 28) | 50.0 (n = 28) | |
| Age (range, years) | 68 (45 to 93) | 66 (36 to 84) | 0.46 |
| Years follow-up (range, years) | 3.6 (2.2 to 6.0) | 2.8 (0.2 to 7.4) | 0.02 |
| Sex | |||
| Male | 32.1 (n = 9) | 35.7 (n = 10) | 0.78 |
| Female | 67.9 (n = 19) | 64.3 (n = 18) | |
| Race | |||
| White | 92.9 (n = 26) | 89.3 (n = 25) | 0.36 |
| Black | 3.6 (n = 1) | 10.7 (n = 3) | |
| Hispanic | 3.6 (n = 1) | 0.0 (n = 0) | |
| BMI (range) | 32.1 (18.6 to 47.9) | 31.7 (21.0 to 46.6) | 0.81 |
| ASA score (range) | 2.7 (2.0 to 3.0) | 2.6 (1.0 to 3.0) | 0.45 |
| Tobacco use | |||
| Current | 7.1 (n = 2) | 17.9 (n = 5) | 0.31 |
| Former | 28.6 (n = 8) | 35.7 (n = 10) | |
| Never | 64.3 (n = 18) | 46.4 (n = 13) | |
| Diabetes | 17.9 (n = 5) | 25.0 (n = 7) | 0.52 |
| Inflammatory disease | 14.3 (n = 4) | 14.3 (n = 4) | 1.00 |
| Chronic kidney disease | 7.1 (n = 2) | 3.6 (n = 1) | 0.55 |
| PJI history | 32.1 (n = 9) | 28.6 (n = 8) | 0.77 |
| Prior failed EMR | 14.3 (n = 4) | 17.9 (n = 5) | 0.72 |
| >2 prior knee surgeries | 60.7 (n = 17) | 55.6 (n = 15) | 0.70 |
| Prior primary repair | 42.9 (n = 12) | 50.0 (n = 14) | 0.59 |
| Traumatic EMDβ | 64.3 (n = 18) | 42.9 (n = 12) | 0.11 |
| Iatrogenic EMDγ | 0.0 (n = 0) | 11.1 (n = 3) | 0.07 |
| EMD related to PJIδ | 32.1 (n = 9) | 28.6 (n = 8) | 0.77 |
| Location of EMD | |||
| Patellaε | 39.3 (n = 10) | 14.3 (n = 4) | 0.11 |
| Patellar tendon | 35.7 (n = 10) | 53.6 (n = 15) | |
| Quadriceps tendon | 25.0 (n = 7) | 32.1 (n = 9) |
BMI = body mass index; ASA = American Society of Anesthesiology; PJI = prosthetic joint infection; EMR = extensor mechanism reconstruction; EMD = extensor mechanism disruption
Number of knees/lower extremities included in each cohort.
EMD resulted from a traumatic incident, e.g., a mechanical fall.
EMD as a complication of concurrent or prior surgery.
EMD as a complication of PJI. Uncertain specific single incident resulting in failure.
Either acute fracture or chronic failure through patella.
Success rates were not statistically different between mesh patients where the mesh fixation was through a tibial trough compared to intramedullary fixation (P=0.85) (Table 1). The extent of concurrent revision TKA and the level of constraint were similar between successes and failures (P=0.50; P=0.86). The method and duration of immobilization were statistically similar between successes and failures (P=0.60; P=0.47). Mean duration of full immobilization among successes was 59 days (SD 24.0; CI 49.6-68.2), compared to 53.9 (SD 27.0; CI 43.2-64.6) among failures (P=0.47). Patients immobilized for more than 6 weeks had a success rate of 56.8% (21 of 37), compared to 7 of 18 among those with a shorter duration of immobilization. However, this was not statistically significant (P=0.21). Success rates were also similar between the two medical centers (P=0.88) and the treating surgeons (P=0.77).
Complications rates were high in the study group (Table 4). Immobilization ulcers occurred in 21.4% (12 of 56) of reconstructions. The all-cause reoperation rate was 42.9% (24 of 56), with 10.7% (6 of 56) of knees going on to above knee amputation or modular fusion. Reoperation for infection occurred in 16.1% (9 of 56) of knees, 3 of 9 of which had a PJI history. The 90-day ED visit and readmission rates were not different between success and failures (P=0.72; P=0.45). The all-cause mortality rate within the study population was 8.2% (4 of 49), each patient who had a documented EMR failure prior to death. Mean days to death was 1,071 (range, 505 to 1,533). The 1- and 2-year all-cause mortality rates were 0 and 2.0%, respectively. 69.1% (38 of 56) of the population required an ambulatory aid at last follow-up. Successes had a 31.6% lower rate of using such aids than failures (53.6% (15 of 28) vs 85.2% (23 of 28); P<0.01).
Table 4.
Outcomes and Complications
| Outcome | Study cohort | Successes | Failures | P-value |
|---|---|---|---|---|
| No. of kneesα | 56 (%) | 28 | 28 | - |
| Last extensor lag (range) | 36.9° (0.0 to 90.0) | 5.4° (0.0 to 20.0) | 71.0° (10.0 to 90.0) | < 0.01 |
| KOOS JR score (range) | 59.2 (8.0 to 100.0) | 67.1 (42.3 to 100.0) | 48.8 (8.0 to 73.3) | < 0.01 |
| KOOS JR meeting TKA PASS thresholdβ | 38.6 (n = 17/44) | 64.0 (n = 16/25) | 5.3 (n = 1/19) | < 0.01 |
| EMR in-situ at final follow-up | 75.0 (n = 42) | 100.0 (n = 28) | 50.0 (n = 14) | < 0.01 |
| Immobilization ulcer | 21.4 (n = 12) | 25.0 (n = 7) | 17.9 (n = 5) | 0.52 |
| Requiring ambulatory assist | 69.1 (n = 38) | 53.6 (n = 15) | 85.2 (n = 23) | < 0.01 |
| Any reoperation | 42.9 (n = 24) | 28.6 (n = 8) | 57.1 (n = 16) | 0.03 |
| Any I&Dδ | 16.1 (n = 9) | 14.3 (n = 4) | 17.9 (n = 5) | 0.72 |
| No. of reoperations (range) | 0.7 (0.0 to 3.0) | 0.5 (0.0 to 3.0) | 1.1 (0.0 to 3.0) | 0.06 |
| Above knee amputation | 7.1 (n = 4) | - | 14.3 (n = 4) | 0.04 |
| Modular fusion | 3.6 (n = 2) | - | 7.1 (n = 2) | 0.15 |
| Days to amputation or fusion (range) | - | 489.7 (21.0 to 936.0) | ||
| Requiring revision EMR | 14.3 (n = 8) | - | 28.6 (n = 8) | < 0.01 |
| Days to EMR failure (range) | - | 363.6 (64.0 to 801.0) | ||
| 90-day ED visit rate | 16.1 (n = 9) | 17.9 (n = 5) | 14.3 (n = 4) | 0.72 |
| Number of 90-day ED visits (range) | 0.1 (0.0 to 2.0) | 0.1 (0.0 to 1.0) | 0.2 (0.0 to 2.0) | 0.66 |
| 90-day readmission rate | 14.3 (n = 8) | 10.7 (n = 3) | 17.9 (n = 5) | 0.45 |
| Number of 90-day readmissions (range) | 0.2 (0.0 to 2.0) | 0.2 (0.0 to 2.0) | 0.2 (0.0 to 2.0) | 0.95 |
| All-cause mortality | 8.2 (n = 4/49) | 0.0 (n = 0/25) | 16.7 (n = 4/24) | 0.04 |
EMR = extensor mechanism reconstruction; I&D = irrigation and debridement; ED = emergency department; KOOS JR = Short form of knee injury and osteoarthritis outcome score; PASS = patient acceptable symptom state
Number of knees/lower extremities included in each cohort.
Rate at which the most recent KOOS JR score was at or above the minimum patient acceptable symptom state (PASS) for TKA. KOOS JR scores were not reported in 100 of study patients.
Of those undergoing I&D, 3 of the 9 had a prior history of prosthetic joint infection
The EMD through the patellar tendon was negatively associated with success when compared to failure of the quadriceps tendon or failure through the patella itself (Odds Ratio (OR) 0.24; P=0.05). No other factors in univariate modeling were found to be statistically significant. When the patellar tendon was the site of disruption, the associated EMR success rate was 40.0% (n=10 of 25). In situ survivorship stratified by site of failure was significantly different (P=0.05). The 3-year survivorship of EMRs when the EMD was through the patellar tendon was 40.0% (n=10 of 25), compared to 14 of 15 and 12 of 16 among failures through the patella and quadriceps tendon, respectively.
The KOOS JR score was 18.3 points higher among successes than failures (67.1 vs 48.8; P<0.01). The rate of obtaining a KOOS JR score over the PASS threshold for TKA was 38.6% (17 of 44), 64.0% (16/25) among success and only 1 of 19 among failures (P<0.01, Figure 2). Mean extensor lag for patients whose KOOR JR met the PASS threshold was 8.8° (SD 21.8, CI −2.4-20.0), compared to 46.0° (SD 37.3, CI 30.9-61.0) among those who did not (P<0.01). There were 25.0% (n=14 of 56) of knees that had a 0° lag at most recent follow-up.
Figure 2.

Scatter plot of KOOS JR scores stratified by extensor lag. Reference lines representing the minimum TKA Patient Acceptable Symptom State for KOOS JR scores and the success/failure extensor lag threshold are included.
DISCUSSION:
Extensor mechanism disruption in the setting of TKA is a challenging complication associated with major morbidity and modest outcomes.8,13,14,16–18,26 In this consecutive series of 56 mesh and allograft EMRs from two tertiary referral centers, we demonstrated a success rate of 50.0%. In situ survival was consistent with previously published studies at 75.0%.16,17 Half of the reconstructions in the failure group had their EMR in situ at last follow-up, highlighting the limitations of evaluating EMR outcomes with in situ survivorship only.
We did not demonstrate significant differences in clinical outcomes, success rates, or survivorships between mesh and allograft reconstructions. This is consistent with a 2018 systematic review and meta-analysis.12 However, our study had a mean follow-up of 3.2 years and allograft reconstructions have been associated with late failures.27,28 Additionally, the number of mesh and allograft reconstructions were unevenly weighted. Given this and the relatively small numbers in this study, statistical comparisons between the two techniques may have suffered from being underpowered. Of note, a total of 6 gastro-achilles EMRs were performed during the study period. Only four of these would have met study inclusion criteria. Two of the four were successful out to two years, resulting in the same success rate seen with the mesh and allograft reconstructions. The gastro-achilles was sometimes used as a salvage option after prior failed attempts at an EMR or if a soft tissue defect was present over the knee. Including these four EMRs would have potentially introduced additional heterogeneity into the population.
Immobilization is thought to be a key component to a successful EMR with contemporary techniques. We also did not find differences in success rates with type and duration of postoperative immobilization. Buller et al. did not find an association between immobilization type and mesh success between casting and immobilizer use.17 Given the retrospective nature of this study, duration of immobilization was not standardized between surgeons or medical centers. The mean duration was lower than in other reported series.16,29 This created heterogeneity which may have affected outcomes. Subjects immobilized for more than 6 weeks had a higher rate of success (56.7 vs 38.9%) that was not statistically significant. Nearly a quarter of EMRs developed immobilization ulcers, indicating that vigilance is needed in clinical follow-up.
Our EMR patients had high rates of reoperations (42.9%), complications, and all-cause mortality (8.2%). The five-year mortality rates of prostate, melanoma, breast, localized colon, and other gynecologic cancers are lower.30 Of note, the majority of these deaths were more than 2-years out from EMR. 16.1% of EMRs were a revision reconstruction with a subsequent success rate of 44%. In a previous report on mesh reconstructions, 20.8% of patients required a revision EMR and 58.3% were considered successes.16 While the success rate among revision EMRs in our series was marginally lower, revision EMR was not statistically associated with lower success rates. There were 21.4% of patients who failed their EMR and went on to amputation or fusion.
Concurrent PJI with an EMD has been reported to portend particularly dismal results with EMR.18 However, patients who had a prior PJI and those who had an EMD related to chronic PJI did not demonstrate higher failure rates in our study. In arthroplasty risk modeling, current smokers have the highest risk of perioperative complications, including infection and a history of tobacco use also elevates risk.31,32 Tobacco use history was not associated with failure in our study. No statistical differences in success rates based on level of arthroplasty constraint at the time of EMR were found. Our study may have been underpowered to detect these differences.
Patients who have an EMD through the patellar tendon had lower rates of functional successful and in-situ survivorship. The reasons behind this are uncertain. Lack of patellar tendon tissue may result in a less robust reconstruction. Contemporary literature on EMRs either do not report17,18 or do not stratify outcomes by site of failure.8,13,15,16,26 Mechanism of failure is also not discussed. Furthermore, MRIs were not routinely obtained preoperatively to evaluate for the integrity of the quadriceps musculature. This may be an avenue for further research, since a quadriceps with major fatty infiltration and atrophy may demonstrate lower rates of reconstruction/repair like large rotator cuff tears.
Limited information is available on PROs following EMR. Our study utilized the KOOS JR score, which is validated in the revision TKA setting.33 Both treating centers routinely collect this PRO during clinical follow-up. Successes had significantly higher KOOS JR scores than failures. The mean difference of 18.3 falls within reported MCID ranges of 14 to 20.22–24 Even in some patients who were deemed successes in this study, the overall function of their knee might be lacking. There were 54% of successes who were using the assistance of a gait aid to ambulate. We examined the effect of EMR on gaining the patient acceptable symptom state for KOOS JR (PASS=63.7).24 No current PASS threshold is available for revision TKAs. The PASS minimum used in this study is from primary TKA literature.24 The overall study population had a mean KOOS JR score of 59.2, indicating that, on average, patients who underwent EMR fell below the published minimum perceived health state acceptable to a patient following primary TKA. Those considered EMR successes had a higher rate of patients achieving PASS (64.0 versus 5.3% among failures). This may indicate only two-thirds of patients who had a successful EMR have a well-functioning knee. One case series of mesh reconstructions reports a mean KOOS JR score of 64.0 among successes, which is similar to our study.17 Several other studies report improvements in Knee Society Scores (KSS) above the MCID.13,14,16
Our definition of a success was an extensor lag <30°, as described by Burnett et al and used by others.8,13,14 Other definitions in the literature include in situ survivorship17, while others report this in addition to clinical outcomes instead of a binary success/failure outcome.16 The ambiguity in the literature on what the definition of successful EMR makes interpretation and generalizability of outcomes more challenging. We utilized extensor lag to try to have a more quantifiable definition of failure. Many of the successful reconstructions did not achieve PASS. Any extensor lag leads to weakness of the quadriceps mechanism and difficulty with gait, and only 25.0% of EMRs had no lag. If our definition of success was changed to a lag of <10°, our success rate would be 41.1% (23 of 56) with a PASS rate of 66.7% (14 of 21).
This study had many potential limitations. Its retrospective nature introduces heterogeneity and bias. There were twelve patients who had 15 EMRs that were excluded due to insufficient follow-up (9) or a reconstruction with something other than mesh or allograft (6), which leads to follow-up bias. The number of different surgeons, medical centers, surgical techniques, and postoperative protocols likely introduced heterogeneity into the study that may impact outcomes. Also, the relatively low numbers of patients in this study may lead to underpowered analyses and type II statistical errors. This and the lack of standardized surgical and postoperative care protocols may limit the generalizability of our results. However, EMD is a rare complication, and this study is one of the largest case series on contemporary EMR techniques. Furthermore, for some patients, their most recent follow-up was a telephone call and/or a validated questionnaire, which may have introduced recall bias. There were 21.4% (12 of 56) of the EMR patients who did not have a KOOS JR score, which may have biased the results. During the study period, both medical centers underwent changes in electronic medical records and were not collecting KOOS JR scores in the early 2010’s. Only 5.4% (n=3 of 56) of EMRs completed their 2-year follow-up remotely, and the tool used to assess extensor lag in these patients has been clinically validated.25 Longer-term follow-up would further improve the generalizability of this study’s results.
CONCLUSIONS:
Contemporary outcomes of mesh and allograft EMRs remain modest at best with a 50.0% rate of a functionally successful reconstruction at a mean of 3.2 years in this multicenter case series. While in situ survival was 75.0%, this did not necessarily correlate to having a successful outcome. No clear differences were seen between the use of allograft and mesh reconstruction techniques. Only two-thirds of patients achieved the minimum PASS KOOS JR score for primary TKA when the reconstruction was otherwise deemed clinically successful. An EMD remains a challenging complication associated with significant morbidity and mortality. Our data will hopefully inform surgeons on the outcomes of allograft and mesh EMRs as well as provide valuable data to temper the expectations of patients who sustain this unfortunate complication. Prevention remains the best treatment for EMD in the setting of TKA.
Table 3.
Surgical and Postoperative Variables
| Surgical variable | Study cohort | Successes | Failures | P-value |
|---|---|---|---|---|
| No. of knees | 56 (%) | 28 | 28 | - |
| Type of EMR | 0.76 | |||
| Mesh | 73.2 (n = 41) | 75.0 (n = 21) | 71.4 (n = 20) | |
| Allograft | 26.8 (n = 15) | 25.0 (n = 7) | 28.6 (n = 8) | |
| Intramedullary mesh fixationα | 41.5 (n = 17/41) | 42.9 (n = 9/21) | 40.0 (n = 8/20) | 0.85 |
| Concurrent revision performed | 0.50 | |||
| Full | 53.6 (n = 30) | 57.1 (n = 16) | 50.0 (n = 14) | |
| Partial | 1.8(n = 1) | 3.6 (n = 1) | 0.0 (n = 0) | |
| Polyethylene componentβ | 25.0 (n = 14) | 17.9 (n = 5) | 32.1 (n = 9) | |
| Noneγ | 19.6 (n = 11) | 21.4(n = 6) | 17.9 (n = 5) | |
| Level of constraint | 0.86 | |||
| Hinge | 33.9 (n = 19) | 35.7 (n = 10) | 32.1 (n = +) | |
| Varus-valgus constraint | 39.3 (n = 22) | 35.7 (n = 10) | 42.9 (n = 12) | |
| Noneδ | 26.8 (n = 15) | 28.6 (n = 8) | 25.0 (n = 7) | |
| Patella management | ||||
| Fully removed | 32.1 (n = 18) | 35.1 (n = 10) | 28.6 (n = 8) | 0.87 |
| Partially removed | 32.1 (n = 18) | 28.6 (n = 8) | 35.7 (n = 10) | |
| Not present | 8.9 (n = 5) | 10.7 (n = 3) | 7.1 (n = 2) | |
| Not removed | 26.8 (n = 15) | 25.0 (n = 7) | 28.6 (n = 8) | |
| Method of immobilization | 0.60 | |||
| Cast | 66.1 (n = 37) | 67.9 (n = 19) | 64.3 (n = 18) | |
| Brace | 32.1 (n = 18) | 32.1 (n = 9) | 32.1 (n = 9) | |
| External fixator | 1.7 (n = 1) | 0.0 (n = 0) | 3.6 (n = 1) | |
| Days immobilized (range) | 57.5 (14 to 97) | 58.9 (15 to 97) | 53.9 (14 to 97) | 0.47 |
| Location | 0.77 | |||
| Medical center 1 | 69.6 (n = 39) | 71.4 (n =20) | 67.9 (n =19) | |
| Medical center 2 | 30.4 (n = 17) | 28.6 (n =8) | 32.1 (n =9) |
EMR = extensor mechanism reconstruction
Mesh cemented into tibial canal underneath tibial baseplate as opposed to cemented into an anterior trough made in the tibia. All allografts were fixed in place with screws.
Of the 14 patients who underwent a polyethylene component exchange, 3 already had a hinge, 3 varus-valgus constraint, and the remaining 8 no constraint.
Of the 11 patients who did not have anything revised, 5 hinges were in place, 1 VVC, and 5 did not constraint.
Primary TKA components were in place at the end of EMR.
HIGHLIGHTS:
Functionally successful rate of extensor mechanism reconstruction out to 3 years was 50.0%.
Neither mesh nor allograft was superior for extensor mechanism reconstruction.
KOOS JR scores were low, even for successes.
In-situ survivorship of the reconstructions, albeit not necessarily functional, was 75.0% at 36 months.
High rates of complications, reoperations, and mortality were noted.
ACKNOWLEDGEMENTS:
The authors would like to acknowledge the following:
Brenna E. Blackburn, MPH, PhD of the University of Utah Department of Orthopaedics for help with data acquisition, IRB support, and help with remote patient clinical follow-up.
Paul K. Edwards, MD of Bowen Hefley Orthopedics of Little Rock, AR for clinical contributions to the study.
The Bill and Betty Petty Research Fund at the University of Arkansas for Medical Sciences who supported this work.
The project described was supported by the Translational Research Institute (TRI), grant UL1 TR003107 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Bates MD, Springer BD. Extensor mechanism disruption after total knee arthroplasty. J Am Acad Orthop Surg. 2015;23(2):95–106. [DOI] [PubMed] [Google Scholar]
- 2.Dobbs RE, Hanssen AD, et al. Quadriceps tendon rupture after total knee arthroplasty. Prevalence, complications, and outcomes. J Bone Joint Surg Am. 2005;87(1):37–45. [DOI] [PubMed] [Google Scholar]
- 3.Lynch AF, Rorabeck CH, et al. Extensor mechanism complications following total knee arthroplasty. J Arthroplasty. 1987;2(2):135–40. [DOI] [PubMed] [Google Scholar]
- 4.Schroer WC, Berend KR, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 Suppl): 116–9. [DOI] [PubMed] [Google Scholar]
- 5.Rand JA, Morrey BF, et al. Patellar tendon rupture after total knee arthroplasty. Clin Orthop Relat Res. 1989(244):233–8. [PubMed] [Google Scholar]
- 6.Nam D, Abdel MP, et al. The management of extensor mechanism complications in total knee arthroplasty. AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(6):e47. [DOI] [PubMed] [Google Scholar]
- 7.Barrack RL, Stanley T, et al. Treating extensor mechanism disruption after total knee arthroplasty. Clin Orthop Relat Res. 2003(416):98–104. [DOI] [PubMed] [Google Scholar]
- 8.Burnett RS, Berger RA, et al. Extensor mechanism allograft reconstruction after total knee arthroplasty. A comparison of two techniques. J Bone Joint Surg Am. 2004;86(12):2694–9. [DOI] [PubMed] [Google Scholar]
- 9.Crossett LS, Sinha RK, et al. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354–61. [DOI] [PubMed] [Google Scholar]
- 10.Emerson RH Jr, Head WC, et al. Reconstruction of patellar tendon rupture after total knee arthroplasty with an extensor mechanism allograft. Clin Orthop Relat Res. 1990(260):154–61. [PubMed] [Google Scholar]
- 11.Nazarian DG, Booth RE Jr. Extensor mechanism allografts in total knee arthroplasty. Clin Orthop Relat Res. 1999(367):123–9. [PubMed] [Google Scholar]
- 12.Shau D, Patton R, et al. Synthetic mesh vs. allograft extensor mechanism reconstruction in total knee arthroplasty - A systematic review of the literature and meta-analysis. Knee. 2018;25(1):2–7. [DOI] [PubMed] [Google Scholar]
- 13.Brown NM, Murray T, et al. Extensor mechanism allograft reconstruction for extensor mechanism failure following total knee arthroplasty. J Bone Joint Surg Am. 2015;97(4):279–83. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardi BF, Oi K, et al. Survivorship of Extensor Mechanism Allograft Reconstruction After Total Knee Arthroplasty. J Arthroplasty. 2017;32(1): 183–8. [DOI] [PubMed] [Google Scholar]
- 15.Browne JA, Hanssen AD. Reconstruction of patellar tendon disruption after total knee arthroplasty: results of a new technique utilizing synthetic mesh. J Bone Joint Surg Am. 2011. ;93(12): 1137–43. [DOI] [PubMed] [Google Scholar]
- 16.Abdel MP, Salib CG, et al. Extensor Mechanism Reconstruction with Use of Marlex Mesh: A Series Study of 77 Total Knee Arthroplasties. J Bone Joint Surg Am. 2018;100(15):1309–18. [DOI] [PubMed] [Google Scholar]
- 17.Buller LT, Warth LC, et al. Extensor Mechanism Reconstruction Using Marlex Mesh: Is Postoperative Casting Mandatory? The Journal of Arthroplasty. 2020. [DOI] [PubMed] [Google Scholar]
- 18.Anderson LA, Culp BM, et al. High Failure Rates of Concomitant Periprosthetic Joint Infection and Extensor Mechanism Disruption. J Arthroplasty. 2018;33(6): 1879–83. [DOI] [PubMed] [Google Scholar]
- 19.Klug A, Gramlich Y, et al. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg Sports Traumatol Arthrosc. 2021;29(10):3287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord SR, Clark RD. Simple physiological and clinical tests for the accurate prediction of falling in older people. Gerontology. 1996;42(4):199–203. [DOI] [PubMed] [Google Scholar]
- 21.Rowe PJ, Myles CM, et al. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12(2):143–55. [DOI] [PubMed] [Google Scholar]
- 22.Lyman S, Lee YY, et al. Validation of the KOOS, JR: A Short-form Knee Arthroplasty Outcomes Survey. Clin Orthop Relat Res. 2016;474(6):1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyman S, Lee YY, et al. What Are the Minimal and Substantial Improvements in the HOOS and KOOS and JR Versions After Total Joint Replacement? Clin Orthop Relat Res. 2018;476(12):2432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunze KN, Fontana MA, et al. Defining the Patient Acceptable Symptom State for the HOOS JR and KOOS JR After Primary Total Joint Arthroplasty. J Bone Joint Surg Am. 2022;104(4):345–52. [DOI] [PubMed] [Google Scholar]
- 25.Morup-Petersen A, Holm PM, et al. Knee Osteoarthritis Patients Can Provide Useful Estimates of Passive Knee Range of Motion: Development and Validation of the Copenhagen Knee ROM Scale. J Arthroplasty. 2018;33(9):2875–83 e3. [DOI] [PubMed] [Google Scholar]
- 26.Wise BT, Erens G, et al. Long-term results of extensor mechanism reconstruction using Achilles tendon allograft after total knee arthroplasty. Int Orthop. 2018;42(10):2367–73. [DOI] [PubMed] [Google Scholar]
- 27.Leopold SS, Greidanus N, et al. High rate of failure of allograft reconstruction of the extensor mechanism after total knee arthroplasty. J Bone Joint Surg Am. 1999;81(11):1574–9. [DOI] [PubMed] [Google Scholar]
- 28.Emerson RH Jr, Head WC, et al. Extensor mechanism reconstruction with an allograft after total knee arthroplasty. Clin Orthop Relat Res. 1994(303):79–85. [PubMed] [Google Scholar]
- 29.Buller LT, Warth LC, et al. Extensor Mechanism Reconstruction Using Marlex Mesh: Is Postoperative Casting Mandatory? J Arthroplasty. 2020;35(12):3747–53. [DOI] [PubMed] [Google Scholar]
- 30.Siegel RL, Miller KD, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 31.Duchman KR, Gao Y, et al. The Effect of Smoking on Short-Term Complications Following Total Hip and Knee Arthroplasty. J Bone Joint Surg Am. 2015;97(13):1049–58. [DOI] [PubMed] [Google Scholar]
- 32.Bedard NA, DeMik DE, et al. Tobacco Use and Risk of Wound Complications and Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis of Primary Total Joint Arthroplasty Procedures. J Arthroplasty. 2019;34(2):385–96 e4. [DOI] [PubMed] [Google Scholar]
- 33.Buller LT, McLawhorn AS, et al. The Short Form KOOS, JR Is Valid for Revision Knee Arthroplasty. J Arthroplasty. 2020;35(9):2543–9. [DOI] [PubMed] [Google Scholar]


