Abstract
Dietary modifications often have a profound impact on the penetrance and expressivity of neurological phenotypes that are caused by genetic defects. Our previous studies in Drosophila melanogaster revealed that seizure-like phenotypes of gain-of-function voltage-gated sodium (Nav) channel mutants (paraShu, parabss1, and paraGEFS+), as well as other seizure-prone “bang-sensitive” mutants (eas and sda), were drastically suppressed by supplementation of a standard diet with milk whey. In the current study we sought to determine which components of milk whey are responsible for the diet-dependent suppression of their hyperexcitable phenotypes. Our systematic analysis reveals that supplementing the diet with a modest amount of milk lipids (0.26% w/v) mimics the effects of milk whey. We further found that a minor milk lipid component, α-linolenic acid, contributed to the diet-dependent suppression of adult paraShu phenotypes. Given that lipid supplementation during the larval stages effectively suppressed adult paraShu phenotypes, dietary lipids likely modify neural development to compensate for the defects caused by the mutations. Consistent with this notion, lipid feeding fully rescued abnormal dendrite development of class IV sensory neurons in paraShu larvae. Overall, our findings demonstrate that milk lipids are sufficient to ameliorate hyperexcitable phenotypes in Drosophila mutants, providing a foundation for future investigation of the molecular and cellular mechanisms by which dietary lipids modify genetically induced abnormalities in neural development, physiology, and behavior.
Keywords: Voltage-gated sodium channel, seizure, neuronal development, RNA-sequencing, α-linolenic acid, gene-environment interaction
INTRODUCTION
Recent advances in genetic and genomic research have led to the identification of an increasing number of mutations and polymorphisms that cause a variety of heritable traits. However, both the statistical occurrence of phenotypes in a group of known genotypes (penetrance) and the degree to which trait expression differs among individuals of such phenotypes (expressivity) (Miko, 2008) are dramatically altered by non-genetic environmental factors (Hunter, 2005; Weeland et al., 2015; Pietropaolo et al., 2017). This is particularly true for neurological phenotypes because the development and function of the nervous system is intrinsically plastic and highly vulnerable to changes in both the internal and external environments. Among various environmental factors that have been implicated, diet is universal and one of the most influential. Diet-based interventions are therefore considered a promising approach for the treatment of neurological and psychiatric disorders that are refractory to conventional therapies. A notable example is the high-fat, low-carbohydrate ketogenic diet (KD), which has been widely used to attenuate symptoms of drug-resistant epilepsies, particularly in children (Vining et al., 1998; Neal et al., 2008). The KD is increasingly being used to treat patients with other disorders of the nervous system (Stafstrom and Rho, 2012), including autism (Herbert and Buckley, 2013; Ruskin et al., 2013; Smith et al., 2016) and Parkinson’s disease (Cheng et al., 2009; Paoli et al., 2014). Although recent studies have suggested mechanisms that might be responsible for the beneficial effects of the KD (Rogawski et al., 2016; Youngson et al., 2017; Olson et al., 2018; Koh et al., 2020; Rudy et al., 2020), it still remains incompletely understood how the KD and other modified diets lead to developmental and functional changes in the nervous system and, ultimately, affect behavioral phenotypes originating from genetic defects.
The fruit fly Drosophila melanogaster is a valuable model organism for gaining fundamental insights into mechanisms underlying gene-environment interactions that affect development, physiology, and behavior. This model provides the distinct advantage that multidisciplinary experimental approaches can be used under strictly controlled genetic and environmental conditions (Hales et al., 2015; Ugur et al., 2016). An example is the study of paraShu, a dominant, gain-of-function mutant allele of paralytic (para), which is the sole voltage-gated sodium (Nav) channel gene in Drosophila (Williamson, 1982; Kaas et al., 2016). Adult paraShu mutants display robust seizure-like phenotypes, including down-turned wing posture (indicative of neuromuscular hyperexcitability) (Ganetzky and Wu, 1983; Stern et al., 1990), spontaneous tremors or shuddering, ether-induced leg shaking, heat-induced convulsions, and increased susceptibility to electroconvulsive seizure discharges (Williamson, 1982; Kaas et al., 2016; Kasuya et al., 2019; Chen et al., 2020). In previous studies, we discovered that paraShu phenotypes were significantly suppressed when a standard diet was supplemented with milk whey (Kasuya et al., 2019). Furthermore, we demonstrated that the severity of adult paraShu phenotypes was reduced when larvae were fed a milk-whey diet, suggesting that the genetic defects in adult paraShu mutants are compensated by diet-induced alterations in neural development. The milk-whey diet was also effective in suppressing seizure-like phenotypes in two other hyperexcitable Nav channel mutants, paraGEFS+ and parabss1, as well as those in seizure-prone mutants of the easily shocked (eas) and slamdance (sda)/julius seizure (jus) genes, which encode ethanolamine kinase (Pavlidis et al., 1994) and a novel transmembrane domain protein (Horne et al., 2017), respectively. These results suggest that the milk whey-diet acts on neurobiological processes that are shared by these hyperexcitable mutants carrying distinct genetic defects.
To elucidate the mechanisms underying the whey diet-dependent suppression of hyperexcitable phenotypes observed in Drosophila mutants, identification of the responsible dietary components is necessary. Here we reveal that the lipid fraction is critical for the effects of milk whey on hyperexcitable mutant phenotypes. We also show that paraShu mutants display abnormal neuronal development and that these defects can be rescued by a diet supplemented with a modest amount of milk lipids (0.26% w/v). Furthermore, α-linolenic acid (ALA), an ω-3 polyunsaturated fatty acid (PUFA), was found to be an important lipid component that contributes to the seizure-suppressing effects. Overall, our study demonstrates that dietary lipids control hyperexcitable phenotypes of Drosophila mutants. This opens up a new avenue for investigating the mechanisms that underlie diet-dependent modulation of neurodevelopmental and neurophysiological phenotypes in Drosophila.
EXPERIMENTAL PROCEDURES
Fly stocks and culture conditions
The Canton-S (CS) strain was used as the wild-type control. paraShu, originally referred to as Shudderer (Shu) (Williamson, 1982), was obtained from Mr. Rodney Williamson (Beckman Research Institute of the Hope, CA). bang senseless (parabss1), easily shocked (eas2), and slamdance (sda) mutants were obtained from Dr. Chun-Fang Wu (University of Iowa, IA). Flies were reared at 25°C, 65% humidity in a 12 hr light/dark cycle on the diet originating from a recipe developed by Edward Lewis (Lewis, 1960) and modified by Rodney Williamson (Beckman Research Institute of the City of Hope, Duarte, CA): a cornmeal/glucose/yeast/agar medium supplemented with the mold inhibitor methyl 4-hydroxybenzoate (0.05 %), propionic acid, and phosphoric acid. The exact composition of the fly food used in this study was described in Kasuya et al. (2019).
Fractionation of milk whey solution
Milk whey powder (Sigma-Aldrich, St. Louis, MO) was dissolved in water (0.84%, w/v) and subjected to filtration using Amicon Ultra-15 centrifugal filter units (MilliporeSigma, Burlington, MA) with molecular weight cutoffs of 3, 30, or 100 kDa. Fractions that did and did not pass through each filter were referred to as the “passing” (PS) and “retained” (RT) fractions, respectively. The RT fractions were reconstituted in a volume of water equal to that of the starting milk whey solution. Equal volumes of the RT fraction, PS fraction, water, or milk whey solution were added separately to fly food and the effects of each on mutant phenotypes were assessed.
Preparation and analysis of milk lipid fraction
Non-homogenized bovine milk (Kalona Supernatural, Kalona, IA, USA) was centrifuged at 3,000 rpm for 15 min. The floating lipid layer was separated from the lower aqueous phase (AQ fraction), transferred to a new tube, and centrifuged again at 3,000 rpm for 15 min. After the aqueous phase was removed, the lipid layer that contains milk fat globules (MFG fraction) was vigorously vortexed and then stored at −80°C overnight. This churned MFG fraction was thawed by incubation at 37°C and centrifuged at 3,000 rpm for 30 min to separate it into a yellow upper phase, which contains the inner core of the MFGs and is composed mainly of TAGs (CORE fraction), and a white lower phase, which contains the milk fat globule membrane (MFGM fraction). The CORE fraction was used as the “milk lipid fraction” throughout the experiments presented herein. In most experiments, milk lipid was added to the fly food at 0.26 % (w/v). The lipid composition of the CORE fraction was determined using gas chromatographic analysis followed by Folch’s extraction at the Vanderbilt University Lipid Core Facility (https://www.vumc.org/hormone/lipid-core).
Behavioral assays
Locomotion assay:
Newly eclosed paraShu females (paraShu/+) were collected and kept in new food vials (<10 flies/vial). Five to seven-day-old flies were individually transferred into a plastic well (15 mm diameter × 3 mm depth) and their locomotion was recorded at 30 frames per sec using a web camera, at a resolution of 320 × 240 pixels for 10 min. The last 5 min of movies were analyzed using pySolo, a multi-platform software for the analysis of sleep and locomotion in Drosophila melanogaster and for computation of the x and y coordinates of individual flies during every frame (Gilestro and Cirelli, 2009). Previous studies had shown that wild-type flies placed into a circular chamber spend most of their time walking along the periphery (Besson and Martin, 2005) and produce circular tracking patterns, whereas paraShu flies placed into such a chamber spend more time in the center because of the uncoordinated movements caused by spontaneous tremor or jerking (Kaas et al., 2016). Tremor severity was therefore assessed indirectly by determining the percentage of time that a fly stayed inside a circle whose radius was 74.3 % of that of the entire chamber. The distance between the position of the fly and the center of the chamber was calculated using the formula , where and are the coordinates of the fly, and and are the coordinates of the chamber center (13 mm is 74.3 % of the chamber radius) (Kaas et al., 2016).
Heat-induced seizure assay:
Newly eclosed flies were collected into groups of 20 and aged for 3 to 5 days before the heat-induced seizure assay was performed as previously described (Sun et al., 2012). Briefly, a single fly was put into a 15 × 45 mm glass vial (Thermo Fisher Scientific, MA) and allowed to acclimate to this environment for 2 to 10 min at room temperature. Subsequently, the glass vials were submerged in a water bath at the specified temperature for 2 min, during which the flies were video-taped and assessed every 5 sec for the presence of seizure behavior. Seizure behavior was defined as loss of standing posture followed by leg shaking.
Bang sensitivity assay:
This assay was performed following a previously described protocol (Zhang et al., 2002). Briefly, flies were raised on control or lipid-containing food until eclosion. Flies were transferred within 24 hours after eclosion (at <10 flies per vial) to vials with control food and maintained in this state for 2–3 days post-eclosion. Prior to testing, individual flies were transferred into a clean glass vial and acclimated for 30 min. Flies were then vortexed at maximum speed, for 10 sec in the cases of eas2 and sda, and for 5 sec in the case of parabss1, and their time to recovery was scored. Recovery was defined as the ability of flies to stand upright following paralysis.
Analysis of dendritic morphology of the larval C4da neurons
The ppkCD4tdGFP transgene (Han et al., 2011) is a cell-specific marker derived from enhancer elements of the Drosophila DEG/ENaC gene pickpocket1, ppk1 (Adams et al., 1998), expressing membrane-localized GFP in class IV multiple-dendritic (C4da) neurons. Third instar female larvae of the designated genotypes carrying the ppkCD4tdGFP transgene (i.e., paraShu/+; ppkCD4tdGFP/+ and +/+; ppkCD4tdGFP/+) were dissected and pin-fileted to remove internal tissues prior to fixation in 4% paraformaldehyde/1X PBS (60 min, RT). Fixed body walls were labeled with anti-GFP monoclonal antibody (12A6, Developmental Studies Hybridoma Bank, Iowa City, IA) in 1X PBT (1X PBS, 0.25% Triton X-100) overnight at 4°C. Repeated 1X PBT washes (4X, 10 min) were followed by incubation with secondary antibody (Alex Fluor 488 goat anti-mouse, A11001, Invitrogen) overnight at 4°C. After extensive washing in 1X PBT, labeled body walls were mounted in Vectashield (Vector Labs). Confocal images were obtained using a Zeiss 510 confocal microscope, and these were used to generate 50–60 μm Z stacks (5 μm/slice) encompassing the entire dendritic arbor of a single dorsal ddaC C4da neuron. Maximum intensity projections of Z stacks served as templates for generating dendritic traces using Simple Neurite Tracer (Fiji). Images were subjected to Sholl analysis (Sholl, 1953) and scoring of skeletonized traces for the numbers of branches and junctions (Fiji). Prism software was used for statistical analysis of data sets, to generate mean values and SEM bars. Statistical significance was determined using one-way ANOVA with Tukey’s posttest in Prism.
Analysis of gene expression by RNA-sequencing
RNA was purified from one-day-old female flies using Trizol solution (Ambion, Carlsbad, CA) and RNasy columns (Qiagen, Valencia, CA). Four different groups of flies were tested: 1) wild-type flies fed a control diet, 2) paraShu flies fed a control diet, 3) wild-type flies fed a lipid-supplemented diet, and 4) paraShu flies a lipid-supplemented diet. For flies under each condition (four biological replicates each), RNA-sequencing (RNA-seq) was performed at the Iowa Institute of Human Genetics (IIHG) Genomics Division (University of Iowa, Iowa). DNase I-treated RNA samples (500 ng) were enriched for poly-A containing transcripts using beads coated with oligo(dT) primers. RNA in this enriched pool was then fragmented, converted to cDNA, and ligated to sequencing adaptors containing indexes, using the Illumina TruSeq stranded mRNA sample preparation kit (Cat. #RS-122-2101, Illumina, Inc., San Diego, CA). The molar concentrations of the indexed libraries were measured using the 2100 Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), and the libraries were combined equally into pools for sequencing. The concentrations of the pools were measured using the Illumina Library Quantification Kit (KAPA Biosystems, Wilmington, MA) and the samples were sequenced on the Illumina HiSeq 4000 genome sequencer using 150 bp paired-end SBS chemistry.
Sequences were provided in FASTQ format and analyzed on the Galaxy platform (https://usegalaxy.org/) (Afgan et al., 2018). The FASTQ files were first evaluated using a quality control tool, FastQC (see “FastQC: A Quality Control tool for High Throughput Sequence Data”. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The sequenced reads were then filtered using a minimum-length cutoff (>20 bp) and a quality score cutoff (>20). Following these assessments, the reads were mapped to Release 6 of the Drosophila melanogaster reference genome assembly (dm6) using the STAR tool (Dobin et al., 2013). The number of reads per annotated gene was counted by running the featureCounts tool (Liao et al., 2014). Analyses of differential gene expression were performed using the DESeq2 tool (Love et al., 2014), which uses the median of ratios method to normalize counts. The P-value was adjusted (Padj) for multiple testing, and the Benjamini-Hochberg procedure was used to control the false discovery rate (FDR).
Statistical analysis
Statistical tests were performed using Sigma Plot (Systat Software, San Jose, CA) or Prizm (GraphPad Software, San Diego, CA). Statistical significance was set at P < 0.05. For data exhibiting non-normal distributions, statistical comparisons between two groups were made using the Mann-Whitney U test. For multiple groups displaying a non-normal distribution, statistical significance was determined using the Kruskal-Wallis one-way ANOVA on ranks test. Comparisons among groups, or among groups and a control, were made using Dunn’s method post hoc. Data not conforming to a normal distribution are presented as box-and-whisker plots showing values of the first, second, and third quartiles (box), as well as the 10th and 90th percentiles (whisker), unless otherwise stated. Temperature-induced behavioral phenotypes were analyzed using two-way repeated measures ANOVA and Holm-Sidak multiple comparisons. Fisher’s exact test was used to analyze the wing posture phenotype of paraShu mutants. For multiple comparison, the P-values were adjusted using the Bonferroni adjusted type I error rate. Statistical significance was set at Padj<0.05. The statistical analyses used in evaluating the significance of RNA-seq experiments are described in the previous section “Gene expression analysis by RNA-sequencing”. The corresponding author (TK) can provide access to the original data upon request.
RESULTS
Dietary factors that contribute to the suppression of paraShu hyperexcitable phenotypes are present in large molecular complexes in a milk whey solution.
Milk whey is a complex mixture of chemical compounds, including carbohydrates, proteins, lipids, vitamins, and minerals. To obtain insights into the physical nature of the milk whey components that are responsible for suppressing paraShu phenotypes, we fractionated a milk whey solution by size using Amicon Centrifugal Filters with different molecular weight cut-offs. Molecular complexes with an apparent molecular mass of less than 3,000, 30,000, and 100,000 are expected to pass through the Amicon Ultracel-3, 30, and 100 filters, respectively. For each filter type, the milk whey components that did and did not pass through were referred to as “passing” (PS) and “retained” (RT) fractions, respectively (Fig. 1A). Each fraction was collected separately and added to the regular fly food, and its effects on paraShu adult phenotypes were assessed. When paraShu mutants were raised on a diet supplemented with a PS fraction, regardless of which filter type had been used for fractionation, more than 70% of adult female mutants (paraShu/+) displayed down-turned wings (Fig. 1B, 3K, 75.0%; 30K, 73.7%; 100K, 83.6%, P > 0.05, Fisher’s exact test with Bonferroni correction) and the mutants exhibited severe spontaneous tremors (Fig. 1C, 3K, Z = 0.19; 30K, Z = 1.45; 100K, Z = 0.76, P > 0.05, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc), like their counterparts raised on the control diet. In contrast, when mutants were raised on a diet supplemented with an RT fraction, regardless of which filter type was used, both the down-turned wing phenotype (Fig. 1B, 3K, 12.9%; 30K, 10.5%; 100K, 40.9%, P < 0.05, Chi-Square test with Bonferroni correction) and tremor severity (Fig. 1C, 3K, Z = 9.09, P < 0.0001; 30K, Z = 4.16, P < 0.001, 100K, Z = 3.61, P < 0.01, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc) were suppressed (comparison was to paraShu mutants raised on the control diet). The phenotypic suppression associated with the RT fractions was comparable to that produced by milk whey for down-turned wing (Fig. 1B, P > 0.05, Fisher’s exact test with Bonferroni correction) and for tremor severity (Fig. 1C, 3K, Z = 2.54, 30K, Z = 0.30, 100K, Z = 1.49, P > 0.05, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc) (comparison was to paraShu mutants raised on the diet with milk whey). These results suggest that small, water-soluble molecules in milk whey solution, such as vitamins and low-molecular weight hydrophilic proteins, do not play an essential role in the diet-dependent suppression of hyperexcitable phenotypes. Rather, molecular complexes larger than 100 kD contribute to the phenotypic suppression by milk whey.
Figure 1. A biological activity in milk whey that can suppress paraShu hyperexcitable phenotypes is associated with large molecular complexes.
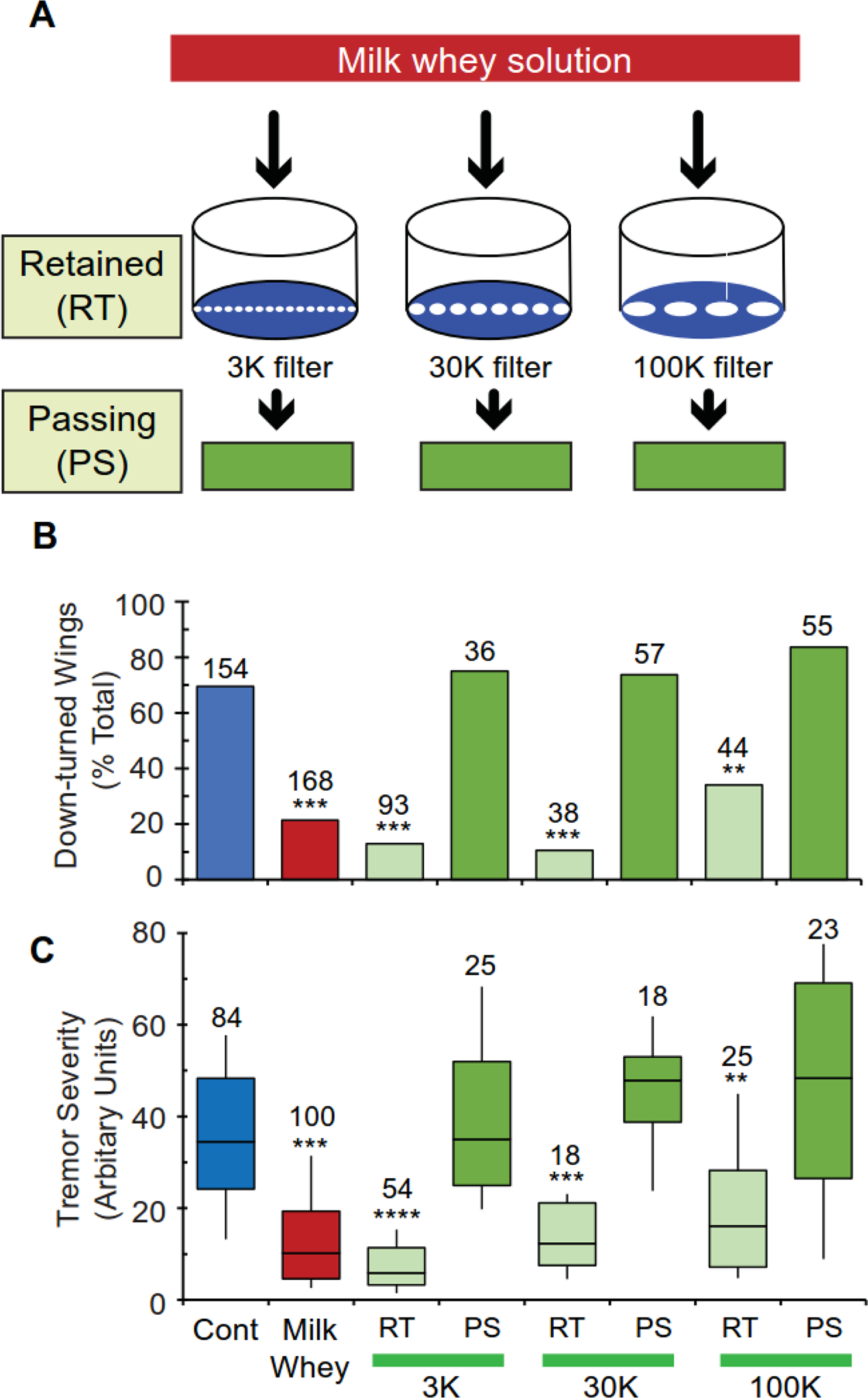
(A) Schematic showing how milk whey components were separated into fractions that pass (passing, PS) and do not pass (retained, RT) through Amicon Centrifugal Filters, Ultracel-3, 30, and 100. (B and C) The effects of diets supplemented with different PS and RT fractions on paraShu adult phenotypes: (B) down-turned wings and (C) severity of spontaneous tremors indirectly accessed by abnormal locomotor activity. For more detail on the assay system, refer to Experimental Procedures. Numbers of flies scored under each condition are indicated above each bar. Statistical analyses were performed using Fisher’s exact test with Bonferroni correction (B) and the Kruskal-Wallis one-way ANOVA on ranks with Dunn’s method (C). Statistically significant differences between the control (regular diet) and experimental groups (supplemented diets) are shown. ***P < 0.001; **P < 0.01.
Milk lipid fractions contain the biological activity required for reducing the severity of paraShu phenotypes.
Next the chemical nature of the milk whey components that contribute to suppression of the paraShu phenotype was examined by separating milk whey solutions into polar and non-polar fractions using Folch’s method (Folch et al., 1957). Each fraction was added to the control diet and tested for its ability to suppress paraShu-associated behavioral abnormalities (see Experimental Procedures). Compared with the control diet, the one supplemented with the non-polar fraction strongly suppressed both the wing posture phenotype (Fig. 2A, down-turned wings, control, 94.4%; non-polar fraction, 30.0%; P < 0.001, Fisher’s exact test with Bonferroni correction) and tremor severity (Fig. 2B, Z = 4.88, P < 0.001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc) of paraShu mutants, and. In contrast, the polar fraction had no significant effect on the tremor phenotype (Fig. 2B, Z = 1.56, P > 0.05, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). Although the polar fraction had a statistically significant effect on the wing posture phenotype compared to the control (Fig. 2A, down-turned wings, polar fraction, 71.7%, P < 0.05, Fisher’s exact test with Bonferroni correction), this effect was considerably weaker than that of the non-polar fraction (Fig. 2A, polar vs. no-polar, down-turned wings, P < 0.001, Fisher’s exact test with Bonferroni correction; spontaneous tremor, Z = 3.66, P < 0.01). This weak suppressing effect of the polar fraction could be due to distributions of small amounts of active non-polar components in the polar fraction or to unidentified water-soluble components with a phenotype-suppressing activity.
Figure 2. Multiple paraShu adult phenotypes are suppressed by diets supplemented with milk lipid-containing fractions.
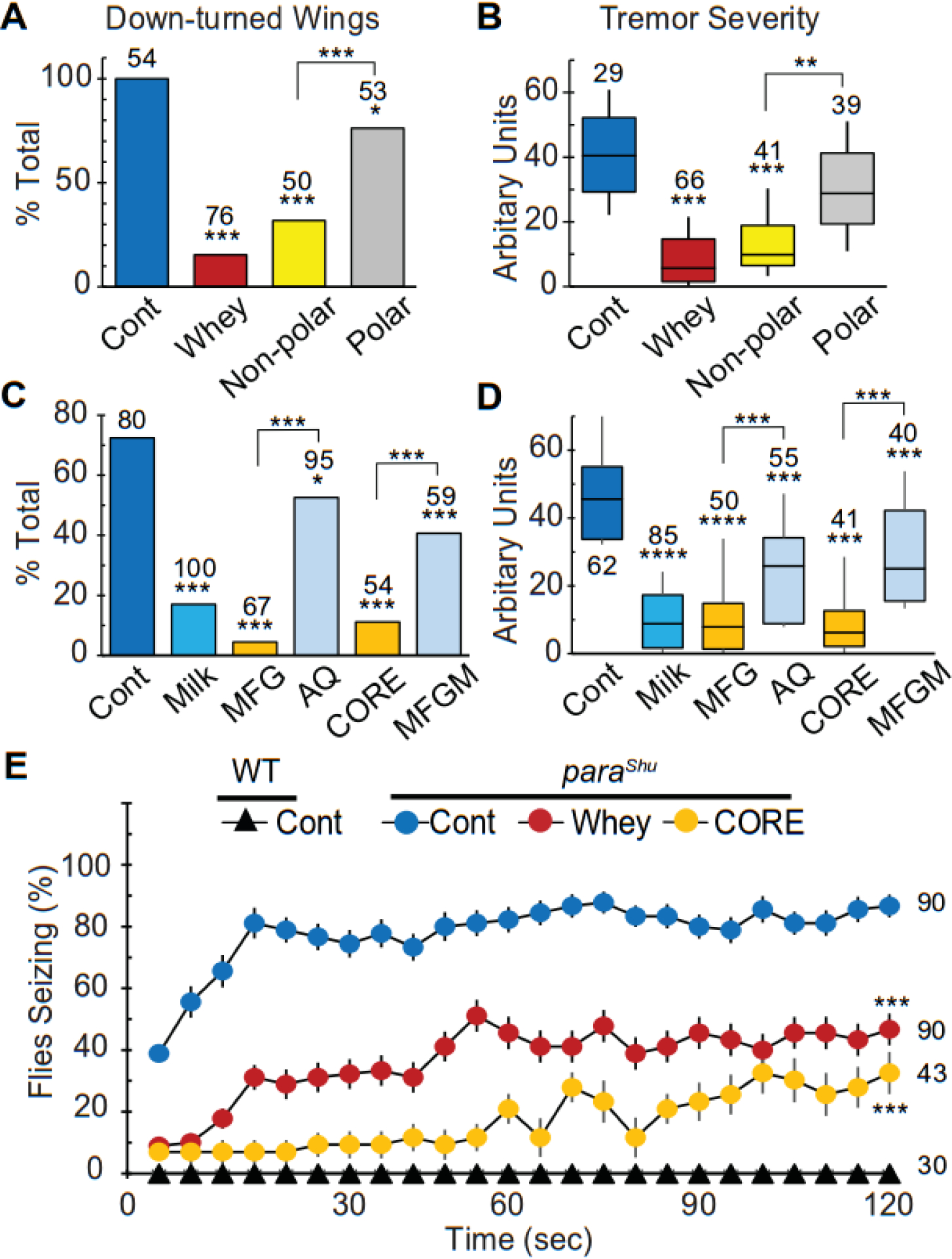
(A and B) Effects of polar and non-polar fractions separated from milk whey solution on (A) down-turned wings and (B) tremor severity in paraShu mutants. (C and D) Effects of different fractions isolated from bovine milk on (C) down-turned wings and (D) tremor severity in paraShu mutants. Fractions include: aqueous (AQ); enriched for milk fat globules (MFG); enriched for milk fat globule membranes (MFGM); and enriched for the triacylglycerol-rich core of the MFGs (CORE). (E) Effects of the whey and CORE fractions on heat-induced seizures in paraShu mutants and control flies. Averages are shown with SEM. Numbers of flies scored under each condition are indicated above each bar. Statistical analyses were performed using Fisher’s exact test with Bonferroni correction (A and C), the Kruskal-Wallis one-way ANOVA on ranks with Dunn’s method (B and D), and two-way repeated measures ANOVA and Holm-Sidak multiple comparisons (E). Statistically significant differences between the controls (regular diet) and experimental groups (supplemented diets) are shown. ****P < 0.0001; ***P < 0.001; *P < 0.05.
Since the non-polar fraction was found to be critical for the milk whey-dependent suppression of paraShu phenotypes, we focused on our analysis on milk lipids. More than 95% of milk lipids are in the form of complex fat-containing particles referred to as milk fat globules (MFGs) (Argov et al., 2008). MFGs are composed of a triacylglycerol (TAG)-rich core and a phospholipid trilayer membrane known as a milk fat globule membrane (MFGM); the latter with the associated proteins surrounds the TAG-rich core. MFGs are large in size; they range in diameter from 0.1 to 15 μm and average 4 μm (Walstra, 1995). Considering that the phenotype-suppressing activity in milk whey was associated with large molecular complexes (Fig. 1B and 1C) and that the non-polar fraction showed significant rescue effects (Fig. 2A and 2B), we sought to determine whether MFG-containing fractions could reduce the severity of paraShu phenotypes. Milk was first centrifuged at 3,000 rpm for 30 min, and a floating layer that is enriched for MFGs (MFG fraction) was separated from a lower aqueous phase (AQ fraction). The MFG fraction was further separated into two fractions by vigorous vortexing and repetitive freeze/thaw cycles followed by centrifugation; the upper lipid layer contained the TAG-rich inner core of the MFGs (CORE fraction) and the lower phase contained the MFGM (MFGM fraction).
The resulting four fractions (MFG, AQ, CORE, and MFGM) were separately added to the control food and their effects on paraShu phenotypes were examined. The amount of each fraction added to the food was adjusted so that the concentration of each component was comparable to that in the original milk (see Experimental Procedures). As shown in Fig. 2, in paraShu mutants raised on a diet supplemented with the MFG fraction, the phenotypes were significantly suppressed (Fig. 2C, abnormal wing posture, control, 72.5%; MFG, 4.5%, P < 0.001, Fisher’s exact test with Bonferroni correction and Fig. 2D, spontaneous tremors, Z = 8.40, P < 0.0001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc, comparison was to mutants fed the control diet). Whereas the AQ fraction likewise led to improvement in the phenotypes (Fig. 2C, abnormal wing posture, 52.6%, P < 0.05, Fisher’s exact test with Bonferroni correction, and Fig. 2D, spontaneous tremors, Z = 4.21, P < 0.001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc), the effect was much milder than that of the MFG fraction (AQ vs MFG, Fig. 2C, abnormal wing posture, P < 0.001, Fisher’s exact test with Bonferroni correction and Fig. 2D, spontaneous tremors, Z = 4.19, P < 0.001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). When the CORE and MFGM fractions (both of which are derived from the MFG fraction) were compared, the former had a stronger phenotype-suppressing activity than the latter (CORE vs MFGM, Fig. 2C, abnormal wing posture, 11.1% vs 40.7%, P < 0.001, Fisher’s exact test with Bonferroni correction and tremor index, Z = 4.92, P < 0.001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). The effects of the MFG and CORE fractions on paraShu phenotypes were comparable to that of milk or milk whey.
The CORE fraction also significantly reduced the sensitivity to heat-induced seizures that is displayed by paraShu mutants; levels were similar to that seen when milk whey was applied. Specifically, although 70–80% of paraShu mutants raised on a regular diet exhibited seizures within 2 min of exposure to 37°C, only 30% of the mutants raised on a diet supplemented with the CORE fraction or milk whey showed the phenotype under the same conditions (Fig. 2E, F = 76.6, P < 0.001, two-way repeated measures ANOVA and Holm-Sidak multiple comparisons). Taken together, these results demonstrate that the lipidic CORE fraction plays a critical role in the milk whey-dependent suppression of paraShu phenotypes that was previously reported by Kasuya et al. (2019).
A diet supplemented with the lipid fraction reduces the severity of the bang-sensitive phenotype of parabss, eas, and sda mutants.
On severe mechanical agitation, the Drosophila “bang-sensitive” mutants, bang senseless (parabss1), easily shocked (eas), and slamdance (sda, also known as julius seizure or jus), exhibit a similar stereotyped sequence of behaviors. These consist of initial uncoordinated movements followed by paralysis, delayed spasm, and recovery. parabss1 is a gain-of-function allele of para (Parker et al., 2011), while eas and sda encode ethanolamine kinase and a novel transmembrane-domain protein, respectively (Pavlidis et al., 1994; Horne et al., 2017). Our previous study demonstrated that a diet supplemented with milk whey reduces the severity of the bang-sensitive phenotype displayed by parabss1, eas, and sda mutants (Kasuya et al., 2019). Here, we examined whether the lipidic CORE fraction had a similar effect as milk whey on the bang-sensitivity of these mutants. In the case of parabss1, hemizygous males (parabss1/Y) were tested for recovery from paralysis induced by agitation (i.e., a 5-sec vortex). The recovery time for parabss1 mutants was significantly reduced when the control diet was supplemented with the CORE fraction (Fig. 3A, U statistic = 925.5, P < 0.001, Mann-Whitney U Test). This dietary treatment also suppressed the bang-sensitive phenotype of eas and sda mutants. Whereas eas hemizygous male mutants (eas2/Y) raised on the control diet required 80 sec to recover from paralysis induced by mechanical agitation, the recovery time for those fed a diet supplemented with the CORE fraction was 27 sec (Fig. 3B, U Statistic = 524.5, P < 0.001, Mann-Whitney U Test). Similarly, the median recovery time for sda homozygous mutants was significantly reduced, from 58 sec to 19.5 sec, when the mutants were fed a diet supplemented with the CORE fraction (Fig. 3C, U Statistic = 599.5, P < 0.001, Mann-Whitney U Test).
Figure 3. A diet supplemented with the CORE fraction reduces the severity of bang-sensitive phenotypes caused by different genetic defects.
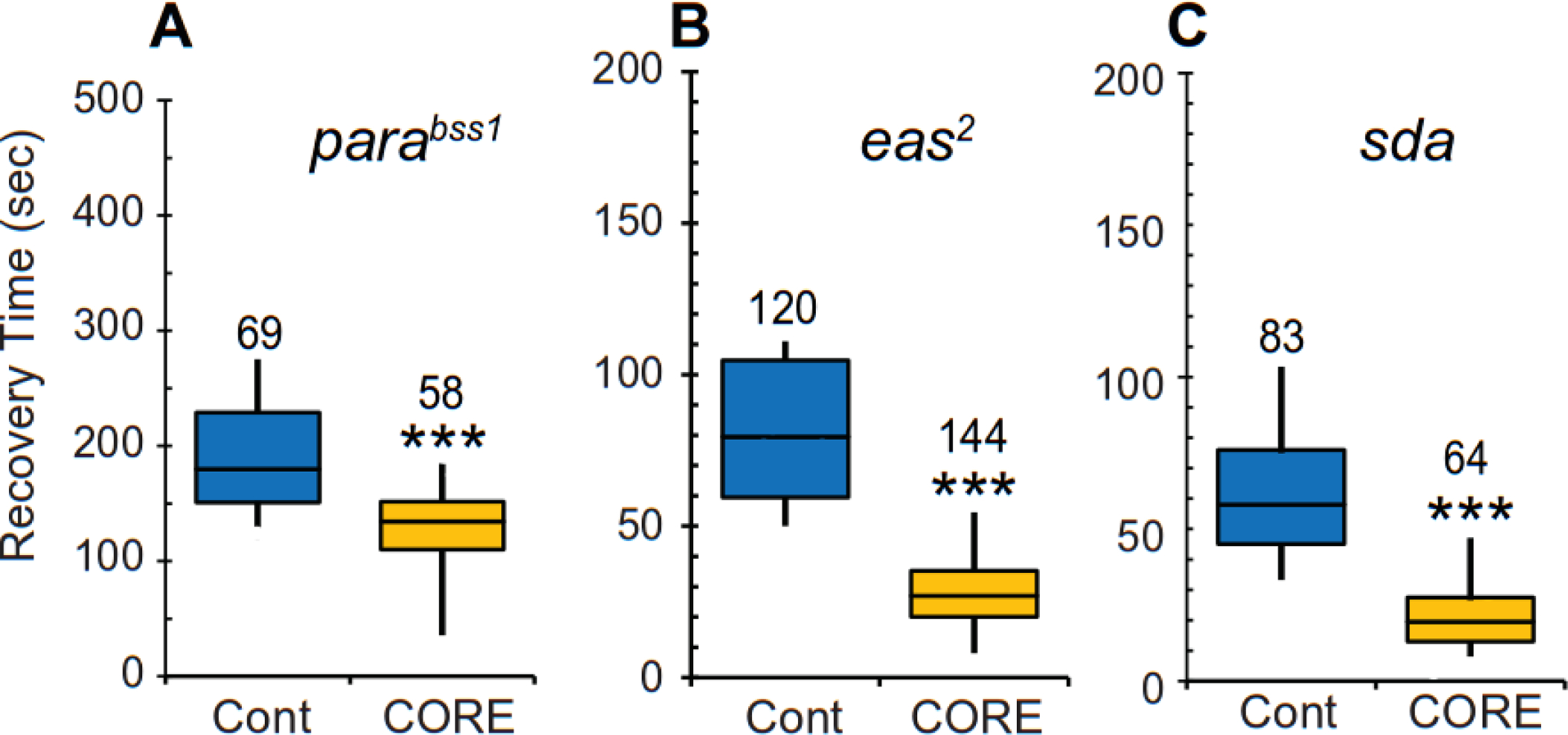
Recovery time for three distinct bang-sensitive mutants (A) bang senseless (bss or parabss1), (B) easily shocked (eas2), and (C) slamdance (sda) when raised on a control diet (blue) or a diet supplemented with the CORE fraction (yellow). Time required for the mutants to recover from paralysis induced by mechanical shock is shown as boxplots. Numbers of flies scored under each condition are indicated above each bar. Statistical analyses were performed using the Mann-Whitney U test. Statistically significant differences are shown. ***P < 0.001.
Lipid feeding solely during larval stages leads to suppression of adult paraShu phenotypes.
The adult phenotypes of the paraShu flies were effectively suppressed when milk whey was fed only during larval stages (Kasuya et al., 2019). We found that feeding larvae lipids (CORE fraction) had a similar effect on the adult paraShu phenotypes. When paraShu mutants were fed a diet supplemented with the CORE fraction during larval stages and then switched to regular diet after eclosion, the mutants displayed significant phenotypic suppression compared with the mutants fed only a control diet (Fig. 4, Z = 9.57, P < 0.0001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). The effect of CORE feeding only during the larval stages was comparable to that observed in their counterparts maintained on the CORE-supplemented diet throughout development and adulthood (Fig. 4, Z = 0.429, P > 0.05, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). Although the adult paraShu phenotype was also suppressed by feeding the CORE-containing diet for 5–7 days only during adulthood (Fig. 4, Z = 5.78, P < 0.0001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc), feeding during larval stages was more effective with respect to reducing the severity of the adult phenotype (Fig. 4, Z = 4.28, P < 0.001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc).
Figure 4. Administration of a diet supplemented with the CORE fraction during larval stages leads to suppression of the adult paraShu phenotypes.
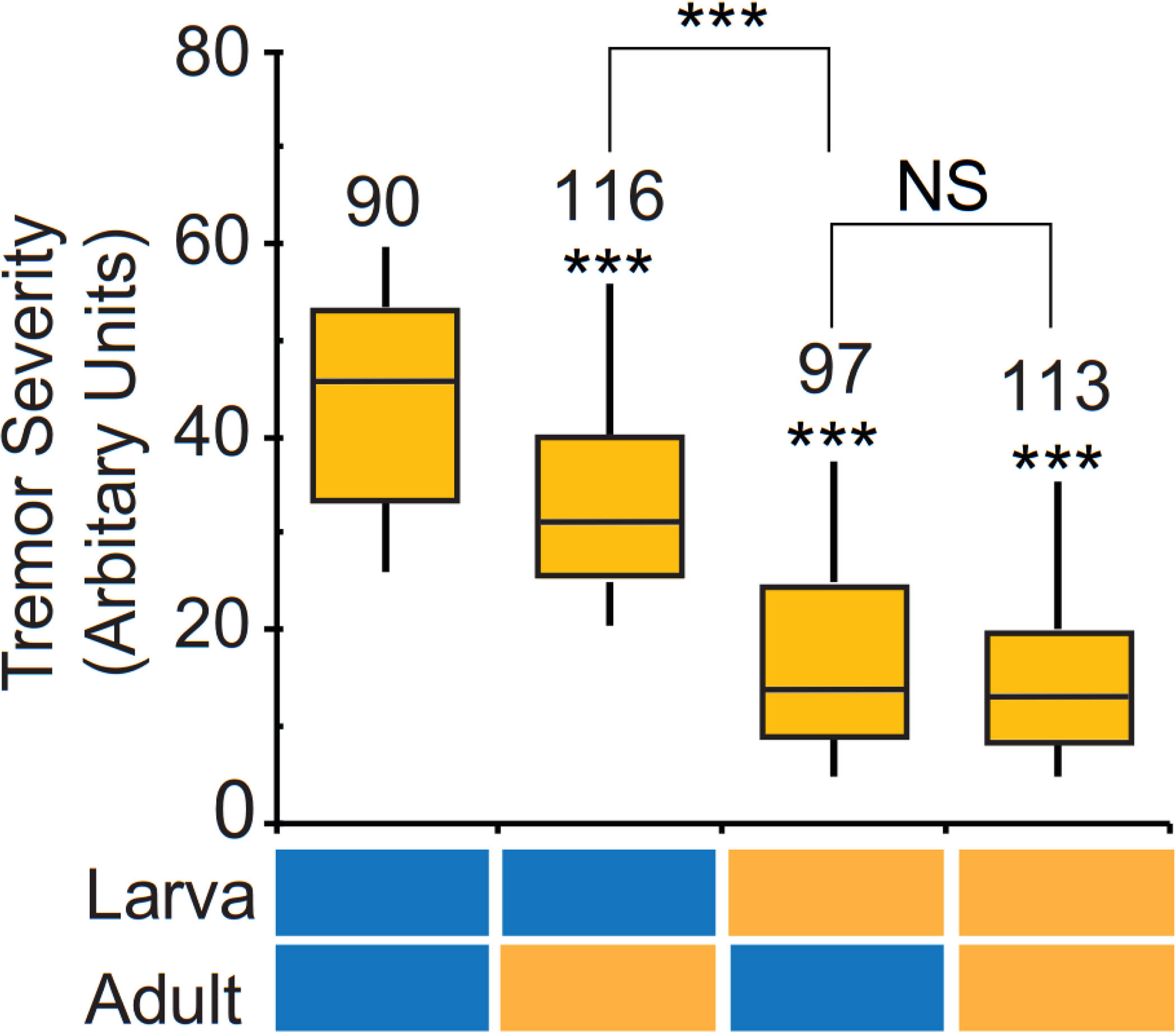
Tremor severity in paraShu mutants fed the control diet or a diet supplemented with the CORE fraction during the larval stage and/or adulthood. Feeding paradigms are indicated below the plot (control, blue; CORE-supplemented, yellow). Numbers of flies scored under each condition are indicated above each box. Statistical analyses were performed using the Kruskal-Wallis one-way ANOVA on ranks with Dunn’s method. Statistical significance of differences between the control group (fed the regular diet during both larval and adult stages) and experimental groups, and between CORE-treated groups are shown. ****P < 0.0001, ***P < 0.001.
α-linolenic acid is a dietary lipid species critical for the suppression of paraShu hyperexcitable phenotypes.
The lipid composition of the CORE fraction was examined by separating it into five different lipid species using thin-layer chromatography. These are triacylglycerols (TAGs), diacylglycerols (DAGs), phospholipids (PLs), cholesteryl esters (CEs), and free fatty acids (FFAs). The proportions of these components were 95.7, 0.8, 0.18, 0.21, and 3.1 % (w/w), respectively. The fatty-acid moieties were enzymatically released from each lipid species and analyzed by gas chromatography. Fatty acid compositions were comparable across lipid species (Table 1). Palmitic acid (PA, C16:0) was the most abundant (38–54%), whereas stearic acid (SA, C18:0), myristic acid (MA, C14:0), and monounsaturated oleic acid (OA, C18:1) represented 12–17%, 10–22%, and 15–24% of total FA, respectively. In addition to these major FAs, lauric acid (C12:0), monounsaturated palmitoleic acid (C16:1), and odd-chain FAs, pentadecylic acid (C15:0), and margaric acid (C17:0), were detected in some lipid species (Table 1). Two C18 polyunsaturated fatty acids (PUFAs), linoleic acid (LA, C18:2w6) and α-linolenic acid (ALA, C18:3w3), were identified as minor components. PUFAs longer than C18 were not detected. Overall, these results were consistent with previously reported bovine milk lipid profiles (Gresti et al., 1993; Hullar and Brand, 1993; van Valenberg et al., 2013).
Table 1.
Fatty acid composition of the CORE fraction.
| FA | FA (% of total) | ||||
|---|---|---|---|---|---|
| TG | FFA | PL | DG | CE | |
| 12:0 | 3.03 | 3.28 | 0.00 | 0.68 | 0.00 |
| 14:0 | 12.84 | 13.68 | 12.03 | 10.41 | 22.74 |
| 15:0 | 1.55 | 0.00 | 0.00 | 1.89 | 0.00 |
| 16:0 | 39.82 | 46.03 | 54.94 | 46.96 | 38.45 |
| 16:1 | 1.43 | 1.63 | 0.00 | 1.70 | 10.25 |
| 17:0 | 0.82 | 1.09 | 0.00 | 0.00 | 0.00 |
| 18:0 | 13.13 | 12.45 | 17.33 | 15.29 | 14.80 |
| 18:1w9 | 23.75 | 18.49 | 15.70 | 20.96 | 13.78 |
| 18:1w7 | 0.58 | 0.00 | 0.00 | 0.00 | 0.00 |
| 18:2 | 2.13 | 2.09 | 0.00 | 2.11 | 0.00 |
| 18:3w6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 18:3w3 | 0.94 | 1.25 | 0.00 | 0.00 | 0.00 |
| 20:3w6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20:4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20:5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22:4w6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22:5w6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22:5w3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
To identify lipid components that significantly contribute to the observed diet-dependent suppression of paraShu phenotypes, we examined the effects of FAs detected in the CORE fraction (Table 1: BA, DA, MA, PA, SA, OA, LA, or ALA). Two short carbon chain-fatty acids, butyric acid (BA, C4:0) and decanoic acid (DA, C10:0), were also examined as references. The control diet was supplemented with different FAs at a final concentration of 1 mM, and the effects on adult paraShu phenotypes were assessed. SA, OA, and ALA were found to reduce the severity of the wing phenotype with ALA producing the strongest suppression (Fig. 5A, control, 85.9%; SA, 64.6%; OA, 62.5%; ALA, 35.1%, P < 0.001, Fisher’s exact test with Bonferroni correction). In the case of the spontenaous tremor phenotype, PA, SA, and ALA were the FA that resulted in significant suppression (Fig. 5B, PA, Z = 4.10, P < 0.01; SA, Z = 3.41, P < 0.05, ALA, Z = 7.83, P < 0.0001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). ALA feeding was also effective in suppressing seizures induced by high ambient temperature (Fig. 5C, F = 80.1, P < 0.0001, two-way repeated measures ANOVA and Holm-Sidak multiple comparisons). Given that approximately 95% of lipids in the CORE fraction are present as a form of TAG, we examined the effects of trilinolenin (TLN), a TAG molecule composed of a glycerol unit and three ALA units. Consistent with the phenotype-suppressing effects of ALA as a free acid form, a diet supplemented with TLN reduced the severity of the wing-down phenotype (Fig. 5A, 27.2%, P < 0.001, Fisher’s exact test with Bonferroni correction), spontenaous tremors (Fig. 5B, Z = 4.17, P < 0.01), and heat-induced seizures (Fig. 5C, P < 0.001, two-way repeated measures ANOVA and Holm-Sidak multiple comparisons). We also demonstrated that ALA feeding during larval stages is critical for suppressing the adult paraShu phenotypes (Fig. 5D, Z = 5.06, P < 0.0001, Kruskal-Wallis one-way ANOVA with Dunn’s method post hoc). This temporal requirement of ALA recapitulated previous findings that the effective suppression of adult paraShu phenotypes requires a diet supplemented with milk whey (Kasuya et al., 2019) or the milk lipid (CORE) fraction (Fig. 4) during the larval stages.
Figure 5. α-linolenic acid contributes to diet-dependent suppression of paraShu phenotypes.
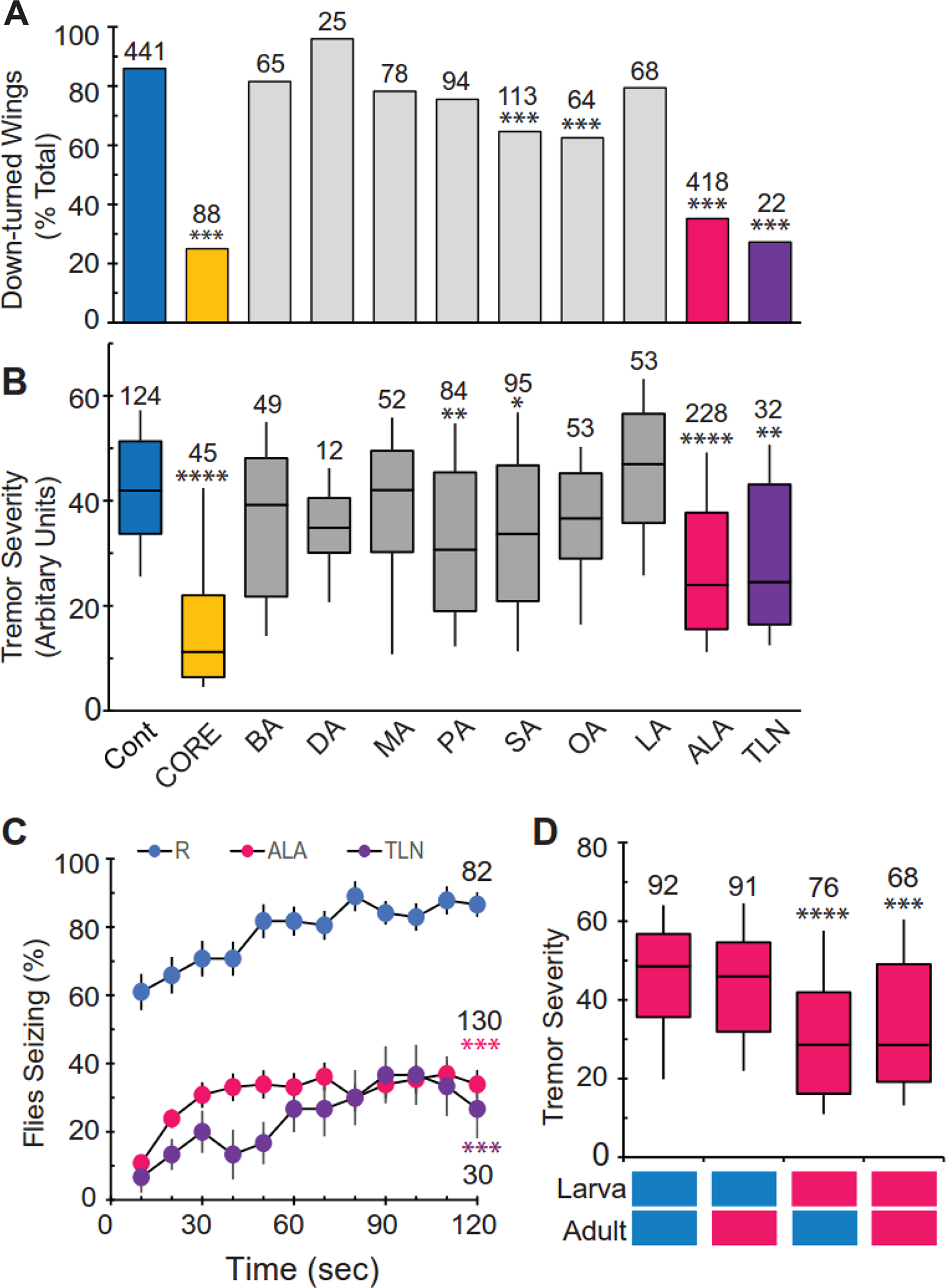
(A and B) Effects of modified diets on (A) down-turned wings and (B) tremor severity in paraShu mutants. Diets include: regular diet (Cont), diet supplemented with the CORE fraction (CORE), and diets supplemented with specific fatty acids or TAG (1 mM). These include butyric acid (BA, C4:0), decanoic acid (DA, C10:0), myristic acid (MA, C14:0), palmitic acid (PA, C16:0), stearic acid (SA, C18:0), oleic acid (OA, C18:1), linoleic acid (LA, C18:2), α-linolenic acid (ALA, C18:3), and trilinolenin (TLN). (C) Effects of diets supplemented with ALA or TLN on heat-induced seizures in paraShu mutants. (D) Effects of timing of ALA feeding on suppression of paraShu adult tremor phenotype. Tremor severity in paraShu mutants fed the control diet or a diet supplemented with ALA during the larval stage and/or adulthood. Feeding paradigms are indicated below the plot (control, blue; ALA-supplemented, red). Numbers of flies scored under each condition are indicated. Statistical analyses were performed using Fisher’s exact test with Bonferroni correction (A), the Kruskal-Wallis one-way ANOVA on ranks with Dunn’s method (B and D), and two-way repeated measures ANOVA and Holm-Sidak multiple comparisons (C). Statistically significant differences between controls (regular diet) and experimental groups (supplemented diets) are shown. ****P < 0.0001, ***P < 0.001; **P < 0.01; *P < 0.05.
Lipid feeding rescues abnormal dendrite growth in paraShu larval sensory neurons.
Adult paraShu phenotypes can be suppressed by a diet supplemented with the CORE fraction or ALA during larval stages (Fig. 4 and Fig. 5). This fact suggested that their rescue effects are mediated, at least in part, by lipid-induced alterations in neural development. We thus sought to determine whether and how the lipid diet has a generalized effect on the morphology of developing neurons. For this purpose, we took advantage of the class IV dendritic arborization (C4da) neurons, a class of sensory neurons in the larval peripheral nervous system. C4da neurons extend a complex dendritic arbor that tiles the inner surface of the larval body wall. They have been extensively characterized and used as a model for studying developmental regulation of dendritic arbor formation and modulation (Dong et al., 2015; Kanamori et al., 2015). Although C4da neurons are not likely to play a significant role in the adult seizure phenotypes in paraShu mutants, their highly consistent dendritic morphology can be reproducibly imaged and quantitated, making them a useful model for a detailed analysis of the potential effects of dietary modifications on various aspects of neuronal development in hyperexcitable mutants.
Using a ppkCD4tdGFP marker transposon from which membrane-localized GFP is expressed in C4da neurons (Han et al., 2011), we examined their dendritic morphology in wild-type controls and paraShu mutants. In wild-type larvae, three bilaterally symmetrical C4da neurons per larval segment (ddaC, v’ada and vdab) extend complex non-overlapping dendritic arbors to tile the inner surface of the larval body wall limited by segmental boundaries. In our analysis, we focused on the dorsal ddaC neurons because they display the most consistent symmetrical arbors. Primary branches proximal to the neuronal soma generated a limited number of secondary branches, with increased complexity in distal regions of the arbor. In wild-type larvae, the primary branches normally display only a few terminal dendrites (Fig. 6A). Neurons in paraShu larvae displayed significantly greater dendritic complexity in the regions of the dendritic arbor proximal to the soma (Fig. 6C). To quantify relative dendritic complexity in wild-type and paraShu larvae, we employed Sholl analysis, a representation of dendritic intersections with increasing concentric radii extending out from the soma (Sholl, 1953) (Fig. 6D). Analysis of area under the curve (AUC) on the Sholl plot revealed that whereas the regions >150 μm from the soma did not differ significantly with respect to dendritic complexity between control and paraShu neurons (Fig. 6D, average ± SEM, +/+, no lipid, 9226 ± 688.1; paraShu/+, no lipid, 9052 ± 572.2, one-way ANOVA with Tukey post-test, P > 0.05), those 10–150 μm from the soma were significantly more complex in the paraShu neurons (Fig. 6E, +/+, no lipid, 2726±111.4; paraShu/+, no lipid, 3699±108.4, one-way ANOVA with Tukey post-test, F(1.95, 21.4) = 19.5, P < 0.0001). Analysis of digital dendritic traces also demonstrated that paraShu neurons had significantly more branches (Fig. 6G, +/+, no lipid, 72.1 ± 3.6; paraShu/+, no lipid, 127.2 ± 5.5, one-way ANOVA with Tukey post-test, F(1.93, 21.2) = 32.4, P < 0.0001) and junctions (Fig. 6H, +/+, no lipid, 28.6 ± 1.3; paraShu/+, no lipid, 55.0 ± 2.9, one-way ANOVA with Tukey post-test, F(1.83, 29.1) = 38.9, P < 0.0001) in the somatodendritic region within a radius of <100 μm from the soma (most proximal region; see Fig. 6A and 6C, red circles). In paraShu mutants whose diets were supplemented with dietary lipids during the larval stages, these effects of paraShu were suppressed to near wild-type levels (Fig. 6H, paraShu/+, with lipid, AUC 10–150 μm, 2977 ± 83.2, Fig. 6E; branches, 90.3 ± 5.3, Fig. 6G; junctions, 38.5 ± 2.8). Our results suggest that the effects of dietary lipids on neuronal development and morphology may be widespread, potentially impacting generalized mechanisms that modulate neuronal activity, morphology, and function in coordinated fashion.
Figure 6. Increased proximal dendritic branching of C4da neurons associated with paraShu hyperexcitability is suppressed by dietary lipid supplementation.
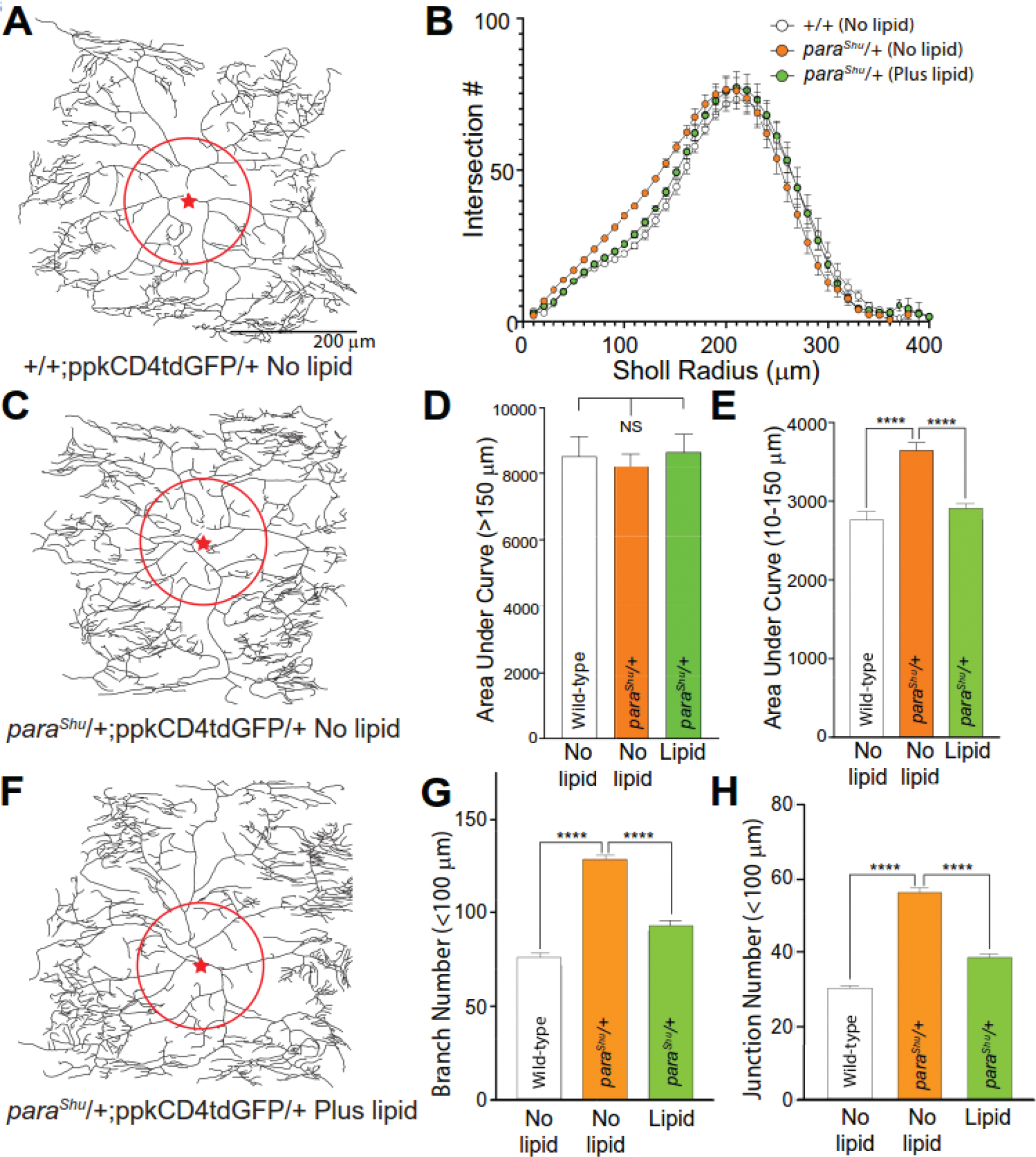
(A, C, and F) Representative dendritic traces of larval class IV dendritic arborization (C4da) neurons expressing ppkCD4tdGFP in the presence or absence of dietary lipid supplementation. Proximal somatodendritic regions within a 100 μm radius and soma position are indicated by a red circle and red star respectively. (A) +/+; ppkCD4tdGFP/+ (No lipid); (C) paraShu/+; ppkCD4tdGFP/+ (No lipid); (F) paraShu/+; ppkCD4tdGFP/+ (Plus lipid). (B) Sholl analysis of C4da dendritic branching +/− dietary lipid supplementation indicates increased dendritic complexity in regions proximal to the soma. (D) Dendritic complexity represented by area under the Sholl curve at radii >150 μm is not significantly different between control (+/+) and paraShu neurons +/− dietary lipids (AUC, panel D). (E) Dendritic complexity (AUC) is increased in the proximal somatodendritic regions of paraShu neurons between 10–150 μm radii relative to wild-type controls. This increase in AUC (10–150 μm) is suppressed in paraShu neurons from larvae receiving dietary lipid supplementation. (G and H) Increased dendritic branch formation in proximal regions (<100 μm radius) of paraShu mutant neurons is also reflected as significantly increased branch numbers (G) and junction number (H). Both values representative of increased branch formation are suppressed by dietary lipid supplementation. n=12 for all genotypes; ****P < 0.0001, one-way ANOVA with Tukey post-test.
RNA sequencing analysis identified genes that are differentially regulated in response to lipid feeding.
To gain insights into the mechanisms underlying the effects of lipid-supplemented diet on hyperexcitable mutants, we carried out RNA-seq analysis and determined how gene expression in adult flies is affected by feeding lipids (CORE fraction). RNA samples were prepared from the entire body of 0–1 day-old adult females in four groups: 1) wild-type flies fed a control diet, 2) paraShu mutants fed a control diet, 3) wild-type flies fed a lipid-supplemented diet, and 4) paraShu mutants fed a lipid-supplemented diet. Each RNA sample was subjected to RNA-seq analysis and generated at least 21 million sequencing reads. Over 99% of reads passed the threshold of a quality score >20 and a length >20 bp. Moreover, duplicate reads encompassed about 70% of total reads, as expected from properly generated RNA-seq data (Bansal, 2017).
When wild-type and paraShu larvae were fed a diet supplemented with the CORE fraction, changes in whole-body gene expression profiles were modest. For wild-type flies, no differentially expressed gene (DEG) was discovered following lipid feeding when 0.05 was used as an adjusted cut-off P-value for statistical significance. For paraShu mutants, the same feeding conditions were associated with changes in 21 genes; these were classified as paraShu lipid-dependent DEGs (Table 2). Among these DEGs, 7 were up-regulated and 14 were down-regulated in response to lipid feeding (Fig. 7A). According to the RNA-seq-based anatomical expression data available at FlyAtlas 2 (https://motif.mvls.gla.ac.uk/FlyAtlas2) (Leader et al., 2018), 14 out of the 21 DEGs are preferentially expressed in the adult digestive systems, mainly in the midgut (Table 2). For example, three midgut-positive genes, Maltase-A2 (Mal-A2), Maltase-A3 (Mal-A3), and Maltase-A4 (Mal-A4) were down-regulated by lipid feeding. These genes encode isoforms of maltase, which catalyzes the breakdown of maltose to glucose. They are present as a cluster at chromosomal position 44D1 and are likely under common transcriptional control given that the fold changes of their expression were similar (0.74, 0.72, and 0.69 for Mal-A2, A3, and A4, respectively). CG8093, a predicted ortholog of mammalian lipase family member M (LIPM), is expressed specifically in the midgut (Leader et al., 2018). Expression of CG8093 was previously shown to be down-regulated in response to a high-fat diet (20% w/v, food-grade coconut oil) (Stobdan et al., 2019).
Table 2.
Genes differentially expressed in response to a diet supplemented with the CORE fraction in parashu mutants.
| Gene ID | Symbol/Name | Molecular Function | Fold Change | Padj | Biological Pathway Involved | Main Expression in Adults |
|---|---|---|---|---|---|---|
| FBgn0031034 | CG14205 | Transferring acyl groups | 2.51 | 4.60 E-17 | Not known | Midgut |
| FBgn0023541 | Cyp4d14/Cytochrome P450 4d14 | Oxidoreductase activity; Heme binding | 2.16 | 9.89 E-11 | Oxidation-reduction process | Midgut |
| FBgn0039091 | CG10182 | Transferring acyl groups | 1.79 | 1.61 E-05 | Not known | Midgut |
| FBgn0038484 | CG5246 | Serine-type endopeptidase activity | 1.54 | 1.46 E-02 | Proteolysis | Midgut |
| FBgn0038714 | Cpr92A/Cuticular protein 92A | Structural constituent of cuticle | 1.52 | 2.17 E-02 | Chitin-based cuticle development | Testis |
| FBgn0000047 | Act88F/Actin 88F | Structural gene encoding actin III | 1.50 | 2.37 E-02 | Mitotic cytokinesis; Skeletal myofibril assembly; Muscle thin filament assembly | Thoracicoabdomi nal ganglion, Carcass |
| FBgn0051269 | CG31269 | Serine-type endopeptidase activity | 1.49 | 3.19 E-02 | Proteolysis | Midgut |
| FBgn0002569 | Mal-A2/Maltase A2 | Maltose alpha-glucosidase activity | 0.74 | 1.16 E-02 | Carbohydrat <e metabolic process | Midgut |
| FBgn0041579 | AttC/Attacin-C | Antibacterial humoral response | 0.73 | 2.17 E-02 | Response to stimulus; Multiorganism process; Response to bacterium; Response to biotic stimulus; Immune response | Head, eye, Fat body, heart, Spermatheca, carcass |
| FBgn0002571 | Mal-A3/Maltase A3 | Maltose alpha-glucosidase activity | 0.72 | 1.84 E-02 | Carbohydrat e metabolic process | Midgut |
| FBgn0039840 | pHCl-2/pH-sensitive chloride channel 2 | Extracellular ligand-gated ion channel activity; pH-gated chloride channel activity; GABA-A receptor activity | 0.69 | 2.17 E-02 | Ion transmembrane transport; Transport; Cell communicati on; system process; Cellular process | Midgut, Malpighian tubules |
| FBgn0051148 | Gba1a/Glucocerebrosid ase 1a | Glucosylceramid ase activity | 0.69 | 1.46 E-02 | Multicellular organismal process; cognition; Metabolic process; Determination of adult lifespan; adult behavior | Midgut |
| FBgn0033294 | Mal-A4/Maltase A4 | Maltose alpha-glucosidase activity | 0.69 | 1.59 E-04 | Carbohydratemetabolic process | Midgut |
| FBgn0034317 | CG14499 | Carbohydrate binding | 0.67 | 3.19 E-02 | Not known | Midgut |
| FBgn0033999 | CG8093 | Lipase activity | 0.67 | 2.11 E-02 | Multicellular organism reproduction; Lipid metabolic process | Midgut |
| FBgn0263336 | lncRNA:CR4341 7/long noncoding RNA:CR43417 | NA | 0.66 | 1.46 E-02 | Not known | Fat body, Heart, Carcass |
| FBgn0028533 | <CG7953 | NA | 0.65 | 6.14 E-03 | Not known | Midgut |
| FBgn0261989 | CG42807 | NA | 0.65 | 1.46 E-02 | Not known | Midgut, Malpighiantubules, Spermatheca |
| FBgn0001112 | Gld/Glucose dehydrogenase | Oxidoreductase activity | 0.65 | 1.16 E-02 | Oxidation-reduction process; Glucose metabolic process; Sperm storage; Pupal chitin-based cuticle development | Crop, Rectalpad |
| FBgn0033603 | Cpr47Ef/Cuticul ar protein 47Ef | Structural constituent of cuticle | 0.63 | 7.11 E-06 | Chitin-based cuticle development | Carcass |
| FBgn0043578 | PGRP-SB1/Peptidoglycan recognition protein SB1 | Zinc ion binding; Peptidoglycan binding; N-acetylmuramoyl-L-alanine amidase activity | 0.62 | 3.15 E-03 | Peptidoglyca n catabolic process, Innate immune response | Head, Eye, Fat body, Heart, Spermatheca, Carcass |
Figure 7. In paraShu mutants, the expression levels of four genes are altered in the same directions by a GstS1 mutation and supplementation of the diet with milk lipids.
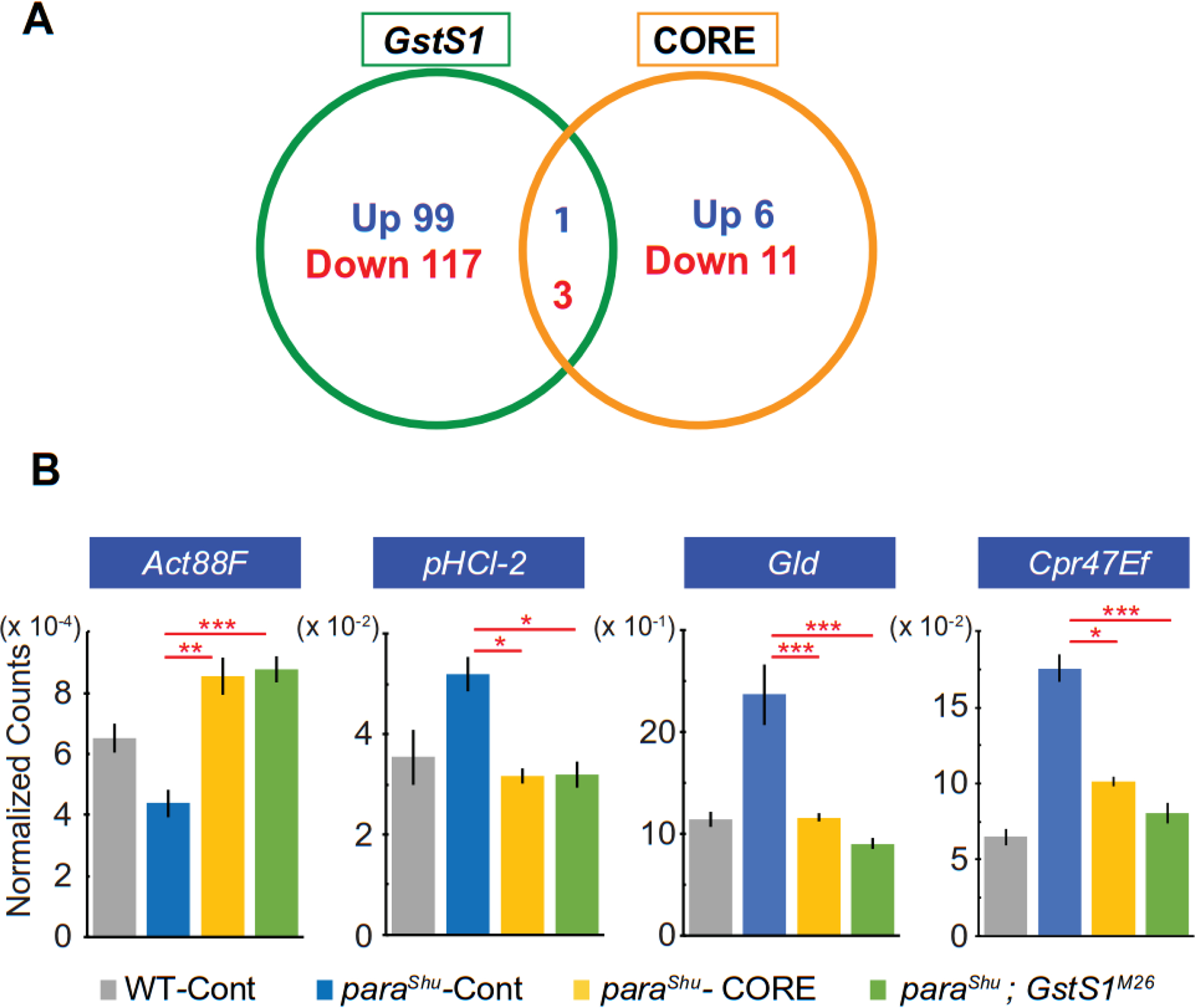
(A) Numbers of genes that are differentially expressed in paraShu mutants in response to the presence of the GstS1M26 mutation and to a diet supplemented with the CORE fraction. (B) Transcript levels of Act88F, pHCl-2, Gld, and Cpr47Ef, as assessed by RNA-seq analysis in four biological replicates of the following treatment groups: Canton-S flies fed a regular diet, paraShu mutants fed a regular diet, paraShu mutants fed a diet supplemented with lipids, and paraShu; GstS1M26 double mutants fed a regular diet. Averages of normalized read counts are shown, with SEM. Statistical analyses were performed using one-way ANOVA with Tukey post-test. Statistically significant differences are shown. ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05.
In paraShu mutants, most of differentially expressed genes in response to lipid feeding are those encoding metabolic enzymes that are primarily expressed in the digestive system, such as the midgut. Two exceptions are Attacin-C (AttC) and Peptidoglycan recognition protein SB1 (PGRP-SB1), which encode an antimicrobial peptide (AMP) and a secreted protein that recognizes peptidoglycan, a bacterial cell wall component, respectively (Table. 2). Both genes play critical roles in innate immune responses; PGRP-SB1 functions upstream of the signaling cascades regulating the production of AMPs, which can have broad activity to directly kill bacteria, fungi, and viruses (Lemaitre and Hoffmann, 2007). Both Attic and PGRP-SB1 are significantly down-regulated by the lipid-supplemented diet (Table 2).
We previously found that a null allele of glutathione S-transferase S1 (GstS1M26) mimicked the effect of the milk-whey diet and dominantly suppressed paraShu adult phenotypes (Chen et al., 2020). Our RNA-seq analysis demonstrated that 220 genes were differentially expressed in response to the introduction of one copy of GstS1M26 (Fig. 7A, 120 up-regulated and 100 down-regulated) (Chen et al, 2020). Four DEGs were common in the GstS1M26 and lipid-diet RNA-seq analyses. The Actin 88F (Act88F) gene was up-regulated in both cases (Fig. 7B, control, 64911 ± 5461, CORE, 85481 ± 7188, P < 0,01; GstS1M26, 87614 ± 5124, P < 0.001, one-way ANOVA with Tukey post-test, F(3, 12) = 12.4), whereas pHCl-2/pH-sensitive chloride channel 2 (pHCl-2) (Fig. 7B, control, 519.6 ± 40.0, CORE, 317.2 ± 17.3, P < 0.05; GstS1M26, 319.1 ± 29.7, P < 0.05, F(3, 12) = 5.52), Glucose dehydrogenase (Gold) (Fig. 7B, control, 236.8 ± 34.0, CORE, 116.2 ± 4.6, P < 0.01; GstS1M26, 90.7 ± 6.3, P < 0.001, F(3, 12) = 13.37), and Cuticular protein 47Ef (Cpr47Ef) (Fig. 7B, control, 1756 ± 101.8, CORE, 1011 ± 37.3, P < 0.0001; GstS1M26, 805.8 ± 74.7, P < 0.0001, F(3, 12) = 44.9) were down-regulated. In paraShu mutants, lipid-induced fold changes in expression of these genes were 1.5, 0.69, 0.65, and 0.63 for Act88F, pHCl-2, Gld, and Cpr47Ef, respectively.
DISCUSSION
This study extends our previous finding that a standard fly diet supplemented with milk whey significantly suppressed the hyperexcitable phenotypes of Drosophila mutants, including the gain-of-function Nav channel mutant paraShu (Kasuya et al., 2019). The current follow-up study reveals that it is primarily milk lipids that are responsible for the diet-induced suppression of the mutant phenotypes. Dietary lipids are known to influence hyperexcitability of the brain in patients with epilepsy. Notably, clinical studies have demonstrated that the high-fat, low-carbohydrate ketogenic diets (KDs) can effectively mitigate symptoms of drug-resistant epilepsy (Vining et al., 1998; Neal et al., 2008). The high fat-to-carbohydrate ratios of the KDs force the body to burn fats rather than carbohydrates (i.e., 70–80% of daily caloric intake in KD-fed patients coming from fat) and, as a result, the KDs induce ketosis, a state of elevated levels of ketone bodies in the blood. Although the exact mechanisms underlying the efficacy of the KDs remain elusive (Hartman et al., 2007; Rho and Stafstrom, 2012), it is generally believed that the antiseizure effects of the KDs are a function of ketosis, which forces the body to shift of the main brain energy source from glucose to ketone bodies (Likhodii et al., 2003; Gasior et al., 2007; Kim et al., 2015). Similar to these findings from humans, the seizure-like activity and paralysis associated with the Drosophila bang-sensitive mutants are suppressed by exposure to a KD (Radlicz et al., 2019). Unlike the conventional KDs, however, the lipid-supplemented diet described in the current study contains a normal level of carbohydrates, and the increase in levels of dietary lipids is small (2.6 mg/ml or 0.26% w/v), maintaining a similar fat-to-carbohydrate ratio to the original diet. Thus, the modified diets would not induce ketosis, and the observed diet-induced suppression of hyperexcitability in Drosophila mutants is not likely due to global changes in the energy usage in the brain. Consistently, our RNA-seq analysis showed that the lipid-supplemented diet used in this study elicits only modest changes in gene expression profiles (Fig. 7). This implies that, unlike the KD, the lipid-supplemented diet used in this study does not cause a major metabolic shift in the body, and that its effects on mutant phenotypes are rather due to specific and localized changes in development and/or physiology of the nervous system.
Although lipids serve primarily as an energy source and as essential structural components of biological membranes, they can also directly modulate protein activities and play vital roles in development and function of the brain and other organ systems. Polyunsaturated fatty acids (PUFAs), which contain more than one double bond in their carbon backbone, are particularly important bioactive lipid molecules (Chalon et al., 2001; Schuchardt et al., 2010; Taha et al., 2010; Bazinet and Laye, 2014; Denis et al., 2015). The two major classes of PUFAs are the ω-3 and ω−6 fatty acids; these contain a carbon-carbon double bond three (ω-3) and six (ω−6) carbons away from the methyl end of the molecule, respectively. Among the fatty acids detected in milk lipids, we found that α-linolenic acid (ALA), an 18-carbon (C18) ω-3 fatty acid, is particularly effective in suppressing hyperexcitable phenotypes: when it was added to the standard diet at a final concentration of 1 mM or 278 μg/ml, the severity of paraShu phenotype was greatly reduced (Fig. 5). ALA is a minor component of milk lipids and naturally occurs in high amounts in certain plant oils, such as flaxseed, canola, and soy oils (van Valenberg et al., 2013). In vertebrates, ALA is the precursor of longer-chain ω-3 fatty acids, such as eicosapentaenoic acid (EPA, C20:5w3) and docosahexaenoic acid (DHA, C22:6w3) (Riediger et al., 2009; Shahidi and Ambigaipalan, 2018). Epidemiologic and clinical studies have indicated that foods enriched with ω-3 fatty acids, particularly EPA and DHA, have a number of health benefits throughout life, including reducing risk of cardiovascular, neurological, and psychiatric disorders (Swanson et al., 2012). In Drosophila, C20 and C22 PUFAs are not detected in larvae, pupae, or adult flies even after they are fed a diet abundant in the precursors C18 ω-3 ALA (C18:3w3) and ω−6 LA (C18:2w6) (Shen et al., 2010). It is thus possible that the phenotype-suppressing effects of ALA in Drosophila mutants are mediated by ALA in its original form or by biologically active ALA metabolites other than EPA or DHA. Alternatively, ALA could suppress hyperexcitable phenotypes indirectly by affecting ω−6 LA-dependent signaling cascades, considering that ω-3 fatty acids often counteract the effects of the corresponding ω−6 fatty acids by competing for the same metabolic enzymes (Maskrey et al., 2013). In this regard, one of the potentially important metabolites of LA is 13-hydroxyoctadecadienoic acid (13-HODE), a C18 bioactive derivative of LA, which exerts potent anti-inflammatory effects in mammals (Shapiro et al., 2016). Panettieri et al. (2019) recently showed that Drosophila can produce 13-HODE and that 13-HODE can regulate inflammation in Drosophila (Panettieri et al., 2019). Despite the potential importance of lipid signaling molecules in the nervous system, their roles and action mechanisms remain understudied in Drosophila. A better understanding of the diet-induced suppression of Drosophila hyperexcitable phenotypes will require knowledge of how dietary supplementation with milk lipids or ALA alters the internal levels of bioactive lipid molecules derived from ALA or LA (e.g., 13-HODE) and how they are involved in modification of neural development and function.
Our previous forward genetic screen revealed that loss-of-function mutations in GstS1 mimicked the effect of the lipid-supplemented diets and significantly reduced the severity of paraShu phenotypes (Chen et al., 2020). This finding was interesting because GstS1 has several unique features that are implicated in lipid metabolism and signaling. Glutathione S-transferases (GSTs) conjugate the reduced form of glutathione to endogenous and xenobiotic electrophiles primarily for detoxification (Hayes et al., 2005; Allocati et al., 2018). Among 36 cytosolic GSTs encoded in the Drosophila genome, GstS1 is the sole member of the sigma class of GSTs; and in spite of having unusually low catalytic activity for typical GST substrates, it efficiently acts on amphipolar substrates such as the lipid peroxidation product 4-hydroxynonenal (4-HNE) (Agianian et al., 2003). Furthermore, combined bioinformatics analyses (Scarpati et al., 2019) identified GstS1 as a fly ortholog of the vertebrate hematopoietic prostaglandin D2 synthases (HPGDSs). These sigma class GST family members catalyze the conversion of PUFA metabolites to prostaglandin D2 (PGD2), an important lipid mediator that regulates various biological processes including inflammation and innate immune responses (Rajakariar et al., 2007; Joo and Sadikot, 2012). In fact, insect GstS1 is known to play a role in innate immunity. In Drosophila, overexpression of GstS1 leads to an increase in the number of hemocytes, which are responsible for cellular immunity (Stofanko et al., 2008). Furthermore, endogenous expression of GstS1 in hemocytes is augmented ~10-fold at the onset of metamorphosis, during which hemocytes play major roles in innate immune processes, such as phagocytosis and antimicrobial peptide (AMP) production (Regan et al., 2013). In a lepidopteran insect Spodoptera exigua, reducing levels of the GstS1 ortholog SePGDS by RNA interference caused immunosuppression in larvae (Sajjadian et al., 2019). These findings raise the interesting possibility that the phenotypic suppression mediated by lipid-supplemented diets and GstS1 loss-of-function mutations may share a common mechanism, i.e., in both cases innate immunity may be influenced by changes in lipid-mediated biological processes. The hypothesis is further supported by our recent finding that GstS1 knockdown in hemocytes mimics the effect of the lipid-supplemented diets on paraShu phenotypes (unpublished observation). This idea is also consistent with our previous microarray analysis, which showed that a paraShu mutation induces significant changes in the expression of genes involved in innate immune responses, such as antimicrobial peptide (AMP) genes (Kaas et al., 2016). Specifically, when gene expression profiles were compared between paraShu adults and genetically matched wild-type flies, 14 genes displayed a significant increase in transcript levels (P < 0.05, a fold change >2). Of the 14 up-regulated genes in paraShu, seven genes are directly involved in the innate immune response (Kaas et al., 2016). These include AttC and PGRP-SB1, which are down-regulated in response to lipid feeding (Table 2). Overall, our previous and current gene expression analyses strongly suggest the importance of immune-related genes for severity of paraShu phenotypes. Future studies will be needed to determine exactly how the innate immune system is involved in dietary lipid- and GstS1-mediated suppression of hyperexcitable phenotypes in paraShu and other mutants.
We hypothesize that the effects of lipid-supplemented diets on the phenotypes of hyperexcitable mutants relate to the induction of changes in neural development and that these changes functionally compensate for neurological defects caused by the mutations. Previous studies in Drosophila demonstrated that inappropriate neural activity during a sensitive period in early development leads to seizure-like phenotype at later developmental stages (Giachello and Baines, 2015). Similarly, elevated neuronal excitability in paraShu mutants during development may have effects on neuronal development and critically contribute to the hyperexcitable phenotypes displayed by adult mutants. By analyzing dendritic structures of larval C4da sensory neurons in paraShu mutants raised on diets with or without lipid supplementation, we could show that neuronal development is indeed affected in paraShu mutants and the defects are rescued by lipid feeding. Larval C4da neurons are not likely to play a significant role in regulating behavioral hyperexcitability in adult mutants. However, because C4da neurons have been extensively used to study the roles of autonomous and nonautonomous regulators of dendritic arborization (Jan and Jan, 2003; Parrish et al., 2007), they serve as a valuable experimental tool to investigate if and how the paraShu mutation causes distinctive defects in neural development and how dietary lipids counteract the effects of the mutation. Two important findings were made concerning paraShu C4da neurons: 1) their dendritic arborization in the somatodendritic region exhibits significantly more branches and junctions than that of wild-type neurons, and 2) this neurodevelopmental phenotype in paraShu mutants is fully suppressed by lipid-supplemented diets (Fig. 6). In Drosophila motoneurons, neural activity has various effects on dendrite growth and branching (Duch et al., 2008; Hartwig et al., 2008; Tripodi et al., 2008), suggesting that elevated neuronal excitability in paraShu mutants during development critically contributes to greater complexity in dendritic arborization of their Cd4a neurons. Similar developmental anomalies may exist in the paraShu brain and could contribute to phenotypic severity in adult mutants, and lipid-supplemented diets could counteract these defects. In future, it will be important to elucidate how second-site genetic mutations (such as GstS1M26) and lipid-supplemented diets influence the central neurons and neuronal circuits directly involved in the hyperexcitable phenotypes. Interestingly, Kumari et al. (2019) demonstrated that exposing zebrafish to ALA during early development reduces chemically induced seizures at later stages (Kumari et al., 2019). Further studies in Drosophila should provide new insights into the mechanisms by which developmental exposure to particular lipid species such as ALA suppresses seizures.
Highlights.
Feeding Drosophila mutants a small amount of milk lipids can suppress seizures.
Part of the seizure-suppressing effects of milk lipids is due to α-linolenic acid.
Feeding lipids during larval stages effectively suppresses adult seizures.
Feeding lipids rescues neurodevelopmental defects in seizure mutants
ACKNOWLEDGEMENTS
We thank Maggie Sodders, Hanxi Tang (University of Iowa), and Pei-Jen Wang (Tungs’ Taichung MetroHarbor Hospital, Taichung City, Taiwan 43503, ROC) for their technical assistance. This work was supported by National Institutes of Health grants to T.K. (R03NS101541, R21NS101545, and R21 NS127364) and by an Iowa Center for Research by Undergraduates fellowships to T.K. and H.T.
Footnotes
DECLARATIONS OF INTEREST
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ (1998) Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol 140:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D (2018) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic acids research 46:W537–W544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agianian B, Tucker PA, Schouten A, Leonard K, Bullard B, Gros P (2003) Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J Mol Biol 326:151–165. [DOI] [PubMed] [Google Scholar]
- Allocati N, Masulli M, Di Ilio C, Federici L (2018) Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argov N, Lemay DG, German JB (2008) Milk Fat Globule structure & function; nanosciece comes to milk production. Trends Food Sci Technol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V (2017) A computational method for estimating the PCR duplication rate in DNA and RNA-seq experiments. BMC Bioinformatics 18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazinet RP, Laye S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15:771–785. [DOI] [PubMed] [Google Scholar]
- Besson M, Martin JR (2005) Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J Neurobiol 62:386–396. [DOI] [PubMed] [Google Scholar]
- Chalon S, Vancassel S, Zimmer L, Guilloteau D, Durand G (2001) Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission. Lipids 36:937–944. [DOI] [PubMed] [Google Scholar]
- Chen HL, Kasuya J, Lansdon P, Kaas G, Tang H, Sodders M, Kitamoto T (2020) Reduced Function of the Glutathione S-Transferase S1 Suppresses Behavioral Hyperexcitability in Drosophila Expressing Mutant Voltage-Gated Sodium Channels. G3 (Bethesda) 10:1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Yang X, An L, Gao B, Liu X, Liu S (2009) Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res 1286:25–31. [DOI] [PubMed] [Google Scholar]
- Denis I, Potier B, Heberden C, Vancassel S (2015) Omega-3 polyunsaturated fatty acids and brain aging. Curr Opin Clin Nutr Metab Care 18:139–146. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Shen K, Bulow HE (2015) Intrinsic and extrinsic mechanisms of dendritic morphogenesis. Annu Rev Physiol 77:271–300. [DOI] [PubMed] [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S (2008) Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J Neurophysiol 100:2525–2536. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509. [PubMed] [Google Scholar]
- Ganetzky B, Wu CF (1983) Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet 1:17–28. [DOI] [PubMed] [Google Scholar]
- Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA (2007) The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia 48:793–800. [DOI] [PubMed] [Google Scholar]
- Giachello CN, Baines RA (2015) Inappropriate Neural Activity during a Sensitive Period in Embryogenesis Results in Persistent Seizure-like Behavior. Curr Biol 25:2964–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Cirelli C (2009) pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics 25:1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresti J, Bugaut M, Maniongui C, Bezard J (1993) Composition of molecular species of triacylglycerols in bovine milk fat. J Dairy Sci 76:1850–1869. [DOI] [PubMed] [Google Scholar]
- Hales KG, Korey CA, Larracuente AM, Roberts DM (2015) Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 201:815–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Jan LY, Jan YN (2011) Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 108:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA (2007) The neuropharmacology of the ketogenic diet. Pediatric neurology 36:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig CL, Worrell J, Levine RB, Ramaswami M, Sanyal S (2008) Normal dendrite growth in Drosophila motor neurons requires the AP-1 transcription factor. Dev Neurobiol 68:1225–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Buckley JA (2013) Autism and dietary therapy: case report and review of the literature. J Child Neurol 28:975–982. [DOI] [PubMed] [Google Scholar]
- Horne M, Krebushevski K, Wells A, Tunio N, Jarvis C, Francisco G, Geiss J, Recknagel A, Deitcher DL (2017) julius seizure, a Drosophila Mutant, Defines a Neuronal Population Underlying Epileptogenesis. Genetics 205:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar I, Brand A (1993) Nutritional factors affecting milk quality, with special regard to milk protein: a review. Acta veterinaria Hungarica 41:11–32. [PubMed] [Google Scholar]
- Hunter DJ (2005) Gene-environment interactions in human diseases. Nat Rev Genet 6:287–298. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY (2003) The control of dendrite development. Neuron 40:229–242. [DOI] [PubMed] [Google Scholar]
- Joo M, Sadikot RT (2012) PGD synthase and PGD2 in immune resposne. Mediators Inflamm 2012:503128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas GA, Kasuya J, Lansdon P, Ueda A, Iyengar A, Wu CF, Kitamoto T (2016) Lithium-Responsive Seizure-Like Hyperexcitability Is Caused by a Mutation in the Drosophila Voltage-Gated Sodium Channel Gene paralytic. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T, Togashi K, Koizumi H, Emoto K (2015) Dendritic Remodeling: Lessons from Invertebrate Model Systems. Int Rev Cell Mol Biol 318:1–25. [DOI] [PubMed] [Google Scholar]
- Kasuya J, Iyengar A, Chen HL, Lansdon P, Wu CF, Kitamoto T (2019) Milk-whey diet substantially suppresses seizure-like phenotypes of para(Shu), a Drosophila voltage-gated sodium channel mutant. J Neurogenet 33:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM (2015) Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol 78:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Dupuis N, Auvin S (2020) Ketogenic diet and Neuroinflammation. Epilepsy Res 167:106454. [DOI] [PubMed] [Google Scholar]
- Kumari S, Mazumder AG, Bhardwaj A, Singh D (2019) Early alpha-linolenic acid exposure to embryo reduces pentylenetetrazol-induced seizures in zebrafish larva. Prostaglandins Leukot Essent Fatty Acids 143:15–20. [DOI] [PubMed] [Google Scholar]
- Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT (2018) FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic acids research 46:D809–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annual review of immunology 25:697–743. [DOI] [PubMed] [Google Scholar]
- Lewis EB (1960) A new standard food medium. Drosophila Information Service 34:117–118. [Google Scholar]
- Liao Y, Smyth GK, Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. [DOI] [PubMed] [Google Scholar]
- Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC, 3rd, Burnham WM (2003) Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann Neurol 54:219–226. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskrey BH, Megson IL, Rossi AG, Whitfield PD (2013) Emerging importance of omega-3 fatty acids in the innate immune response: molecular mechanisms and lipidomic strategies for their analysis. Mol Nutr Food Res 57:1390–1400. [DOI] [PubMed] [Google Scholar]
- Miko I (2008) Penetrance and expressivity. Nature education 1:137. [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH (2008) The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. The Lancet Neurology 7:500–506. [DOI] [PubMed] [Google Scholar]
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY (2018) The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panettieri S, Paddibhatla I, Chou J, Rajwani R, Moore RS, Goncharuk T, John G, Govind S (2019) Discovery of aspirin-triggered eicosanoid-like mediators in a Drosophila metainflammation blood tumor model. J Cell Sci 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli A, Bianco A, Damiani E, Bosco G (2014) Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int 2014:474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Padilla M, Du Y, Dong K, Tanouye MA (2011) Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics 187:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN (2007) Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci 30:399–423. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Ramaswami M, Tanouye MA (1994) The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell 79:23–33. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Crusio WE, Feldon J (2017) Gene-Environment Interactions in Neurodevelopmental Disorders. Neural Plast 2017:9272804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlicz C, Chambers A, Olis E, Kuebler D (2019) The addition of a lipid-rich dietary supplement eliminates seizure-like activity and paralysis in the drosophila bang sensitive mutants. Epilepsy Res 155:106153. [DOI] [PubMed] [Google Scholar]
- Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW (2007) Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proceedings of the National Academy of Sciences of the United States of America 104:20979–20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JC, Brandao AS, Leitao AB, Mantas Dias AR, Sucena E, Jacinto A, Zaidman-Remy A (2013) Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in Drosophila. PLoS pathogens 9:e1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Stafstrom CE (2012) The ketogenic diet: what has science taught us? Epilepsy Res 100:210–217. [DOI] [PubMed] [Google Scholar]
- Riediger ND, Othman RA, Suh M, Moghadasian MH (2009) A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc 109:668–679. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W, Rho JM (2016) Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb Perspect Med 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy L, Carmen R, Daniel R, Artemio R, Moises RO (2020) Anticonvulsant mechanisms of the ketogenic diet and caloric restriction. Epilepsy Res 168:106499. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M Jr., Boison D, Masino SA (2013) Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One 8:e65021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadian SM, Ahmed S, Al Baki MA, Kim Y (2019) Prostaglandin D2 synthase and its functional association with immune and reproductive processes in a lepidopteran insect, Spodoptera exigua. General and comparative endocrinology 287:113352. [DOI] [PubMed] [Google Scholar]
- Scarpati M, Qi Y, Govind S, Singh S (2019) A combined computational strategy of sequence and structural analysis predicts the existence of a functional eicosanoid pathway in Drosophila melanogaster. PLoS One 14:e0211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A (2010) Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr 169:149–164. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P (2018) Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol 9:345–381. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Singer P, Ariel A (2016) Beyond the classic eicosanoids: Peripherally-acting oxygenated metabolites of polyunsaturated fatty acids mediate pain associated with tissue injury and inflammation. Prostaglandins Leukot Essent Fatty Acids 111:45–61. [DOI] [PubMed] [Google Scholar]
- Shen LR, Lai CQ, Feng X, Parnell LD, Wan JB, Wang JD, Li D, Ordovas JM, Kang JX (2010) Drosophila lacks C20 and C22 PUFAs. J Lipid Res 51:2985–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Smith J, Rho JM, Teskey GC (2016) Ketogenic diet restores aberrant cortical motor maps and excitation-to-inhibition imbalance in the BTBR mouse model of autism spectrum disorder. Behav Brain Res 304:67–70. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Rho JM (2012) The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Kreber R, Ganetzky B (1990) Dosage effects of a Drosophila sodium channel gene on behavior and axonal excitability. Genetics 124:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobdan T, Sahoo D, Azad P, Hartley I, Heinrichsen E, Zhou D, Haddad GG (2019) High fat diet induces sex-specific differential gene expression in Drosophila melanogaster. PLoS One 14:e0213474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofanko M, Kwon SY, Badenhorst P (2008) A misexpression screen to identify regulators of Drosophila larval hemocyte development. Genetics 180:253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O’Dowd DK, Reenan R (2012) A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci 32:14145–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha AY, Burnham WM, Auvin S (2010) Polyunsaturated fatty acids and epilepsy. Epilepsia 51:1348–1358. [DOI] [PubMed] [Google Scholar]
- Tripodi M, Evers JF, Mauss A, Bate M, Landgraf M (2008) Structural homeostasis: compensatory adjustments of dendritic arbor geometry in response to variations of synaptic input. PLoS Biol 6:e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur B, Chen K, Bellen HJ (2016) Drosophila tools and assays for the study of human diseases. Disease models & mechanisms 9:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Valenberg HJ, Hettinga KA, Dijkstra J, Bovenhuis H, Feskens EJ (2013) Concentrations of n-3 and n-6 fatty acids in Dutch bovine milk fat and their contribution to human dietary intake. J Dairy Sci 96:4173–4181. [DOI] [PubMed] [Google Scholar]
- Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, Shinnar S, Shuman R, Trevathan E, Wheless JW (1998) A multicenter study of the efficacy of the ketogenic diet. Arch Neurol 55:1433–1437. [DOI] [PubMed] [Google Scholar]
- Walstra P (1995) Physical chemistry of milk fat globules. In: Lipids, 2 Edition (Fox PF, ed), pp 131–178. London: Chapman & Hall. [Google Scholar]
- Weeland J, Overbeek G, de Castro BO, Matthys W (2015) Underlying Mechanisms of Gene-Environment Interactions in Externalizing Behavior: A Systematic Review and Search for Theoretical Mechanisms. Clin Child Fam Psychol Rev 18:413–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RL (1982) Lithium stops hereditary shuddering in Drosophila melanogaster. Psychopharmacology (Berl) 76:265–268. [DOI] [PubMed] [Google Scholar]
- Youngson NA, Morris MJ, Ballard JWO (2017) The mechanisms mediating the antiepileptic effects of the ketogenic diet, and potential opportunities for improvement with metabolism-altering drugs. Seizure 52:15–19. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye M (2002) The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics 162:1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]


