Abstract
The therapeutic efficacy of radioimmunotherapy against triple negative breast cancer (TNBC) is largely limited by the complicated tumor microenvironment (TME) and its immunosuppressive state. Thus developing a strategy to reshape TME is expected to achieve highly efficient radioimmunotherapy. Therefore, we designed and synthesized a tellurium (Te)-driven maple leaf manganese carbonate nanotherapeutics (MnCO3@Te) by gas diffusion method, but also provided a chemical catalytic strategy in situ to augment ROS level and activate immune cells for improving cancer radioimmunotherapy. As expected, with the help of H2O2 in TEM, MnCO3@Te heterostructure with reversible Mn3+/Mn2+ transition could catalyze the intracellular ROS overproduction to amplify radiotherapy. In addition, by virtue of the ability to scavenge H+ in TME by carbonate group, MnCO3@Te directly promote the maturation of dendritic cells and macrophage M1 repolarization by stimulator of interferon genes (STING) pathway activation, resulting in remodeling immuno-microenvironment. As a result, MnCO3@Te synergized with radiotherapy and immune checkpoint blockade therapy effectively inhibited the breast cancer growth and lung metastasis in vivo. Collectively, these findings indicate that MnCO3@Te as an agonist, successfully overcome radioresistance and awaken immune systems, showing promising potential for solid tumor radioimmunotherapy.
Keywords: Radioimmunotherapy, Triple negative breast cancer, Tellurium, Manganese carbonate, Tumor microenvironment
Highlights
-
•
Driven by Te, maple leaf-shaped MnCO3@Te were fabricated.
-
•
MnCO3@Te as an agonist to enhanced radioimmunotherapy.
1. Introduction
Due to the risk factors related to modern lifestyles, the incidence rate of cancer has increased rapidly worldwide [1]. Breast cancer is the cancer with the highest incidence rate among women [2]. And then triple negative breast cancer (TNBC), which accounts for 10–20% of breast cancers relative to other breast cancers, lacks estrogen, progesterone, and human epidermal growth factor receptor 2 (HER-2), leading to its highly malignant, aggressive nature and tendency to relapse after chemotherapy [3]. Therefore, there is still no effective and specific therapy in clinical practice, which encourages scientists to discover and develop novel therapies for TNBC targeted therapy.
In recent years, cancer immunotherapy aims to remodel the patient's own immune system and realize the recognition and destruction of cancer cells, has made a major breakthrough in the field of oncology, especially immune checkpoint blockade therapy (ICB) [4,5]. Although the application of CTLA-4 and anti-PD-1 therapy have produced impressive clinical effects, the effectiveness of these methods still depends on the presence of tumor inflammation and the activation of the patient's immune system [6,7]. Unfortunately, TNBC is considered to be a typical immune "cold" tumor, in which the suppressed tumor microenvironment (TME) makes it impossible for cytotoxic T cells to be effectively activated, thus unable to trigger a strong anti-tumor immune response [8,9]. So it is of great significance to develop an strategies to reshape TME from "cold tumor" to “hot tumor, so as to restore the anti-tumor T cell response [10,11].
Meanwhile, radiotherapy is currently the mainstream clinical strategy for 70% of cancer patients including TNBC patients, that is utilize high intensity ionizing radiation to induce DNA damage or generate reactive oxygen species (ROS) to induce tumor inhibition [[12], [13], [14], [15]]. However, radiation resistance and toxic side effects are the important culprit in reducing efficiency of radiotherapy [[16], [17], [18]]. Of note, recent studies indicated that the combination strategy of RT and ICB provides an opportunity to augment immune responses against tumors [19,20]. Sun et al. designed a stabilized theranostic NIR-II nanoprobe (QD-Cat-RGD) and found that under the action of QD-Cat-RGD probe, the synergistic effect of radiotherapy and immunotherapy can improve the inhibition of immunogenic radiotherapy and inhibit cancer metastasis [21]. Impressively, this combination strategy has also been implemented in many clinical trials and was exhibits superior therapeutic effects [[22], [23], [24]]. Unfortunately, even under this strategy, the stimulation efficiency of cytotoxic T cells still limited by suppressive tumor immune microenvironment (TIME) that is caused by the acidity of tumor [[25], [26], [27], [28]]. Specifically, the acidity in tumor tissue can lead to the immune tolerance of cytotoxic T lymphocytes (CTLs) and induce the massive infiltration of immunosuppressive cells including myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) etc [[29], [30], [31]]. Therefore, relieving acidic microenvironment of tumor plays an important role in enhancing tumor immune response by recovering of functions of antitumor T cells [32].
Nowadays, with the rapid development of nanotechnology, scientists have developed many nanosystems to enhance radiosensitivity of tumor cells or achieve antiacid therapy for tumors [33]. Tellurium (Te) belongs to the oxygen group, which was found to be similar to the element selenium (Se), has antioxidant and antitumor properties [[34], [35], [36]]. For example, Wu et al. found Te nanowires (TeNWs) triggered by hydrogen peroxide (H2O2) in TME to produce toxic TeO66− that could be used as prodrug for highly selective cancer chemotherapy [37]. Moreover, Te semiconductor including Te nanorods (TeNDs), nanostars, nanosheets and nanodots have been successively applied in photothermal therapy, photodynamic therapy, radiotherapy and immunotherapy [[38], [39], [40]]. Nevertheless, these Te-based nanosystems remain plagued by short blood circulation time, weak biocompatibility and rapid clearance by reticuloendothelial system (RES).
Manganese carbonate (MnCO3) semiconductor is widely used in ultrasound/magnetic resonance imaging (MRI)-mediated tumor ultrasound therapy/photodynamic therapy due to its widely bandgap, excellent biocompatibility and pH responsiveness etc [[41], [42], [43]]. More excitingly, it is recently showed that Mn element is an innate immune activation adjuvant by activating stimulator of interferon genes (STING) pathway [44,45]. Besides, it is worth noting that like calcium carbonate and calcium phosphate, MnCO3 nanosystem may possess unique advantages in neutralizing tumor acidity in theory [[46], [47], [48]]. However, the complex synthesis method and the large size after synthesis greatly limit its wide application in the field of biomedicine.
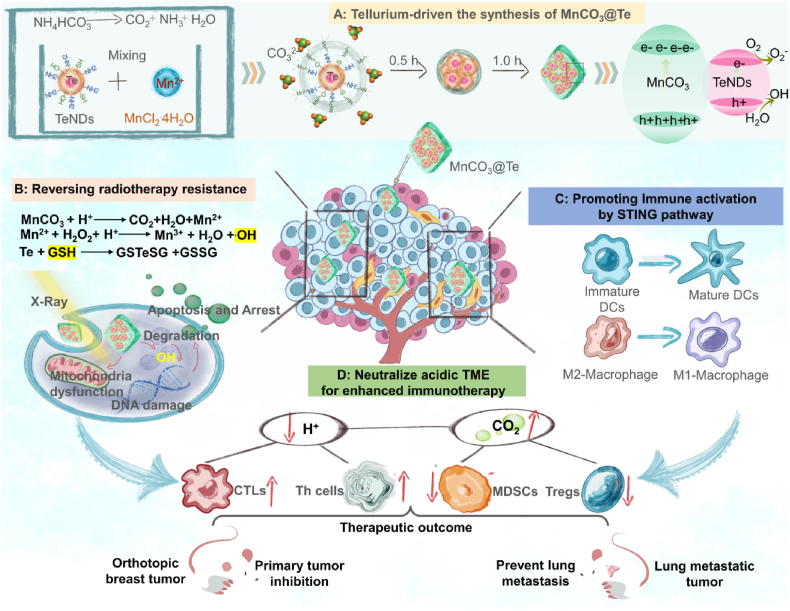
Therefore, considering the above research background, we not only introduce a gas diffusion method to synthesize a Te-driven maple leaf-shaped manganese carbonate nanotherapeutics (MnCO3@Te), but also provide a catalytic strategy in situ to remodel the TME and enhance ROS overproduction for achieving simultaneous radioimmunotherapy. As illustrated in Scheme 1, the nanosystem has several important features: (i) the reasonable design of MnCO3-coated Te has greatly changed the diameter and morphology of MnCO3 crystal and realized the controllable transformation of morphology. (ii) in the TME, MnCO3@Te realized the burst release of Mn2+ and then triggered the intracellular ROS generation by Fenton-like reaction, thereby realizing precision radiotherapy with invisible side-effects. (iii) MnCO3@Te scavenged H+ in the TME, allowing the maturation of dendritic cells and macrophage M1 repolarization by STING pathway activation, thereby relieving suppressive TIME. (iv) with the assistance of the anti-PD-L1 checkpoint blockade, MnCO3@Te synergized with radiotherapy effectively inhibited the breast cancer growth and lung metastasis in vivo. Collectively, this study provides a valid tactic for facile synthesis of shape-controllable MnCO3 nanosystems for solid tumor radioimmunotherapy.
Scheme 1.
Schematic illustration of Te-driven MnCO3 nanotherapeutics synthetic procedure and its mechanism for anticancer and antimetastasis activity in radioimmunotherapy via reshaping tumor microenvironment.
2. Result and discussion
2.1. Design, synthesis, and characterization of MnCO3@Te nanotherapeutics
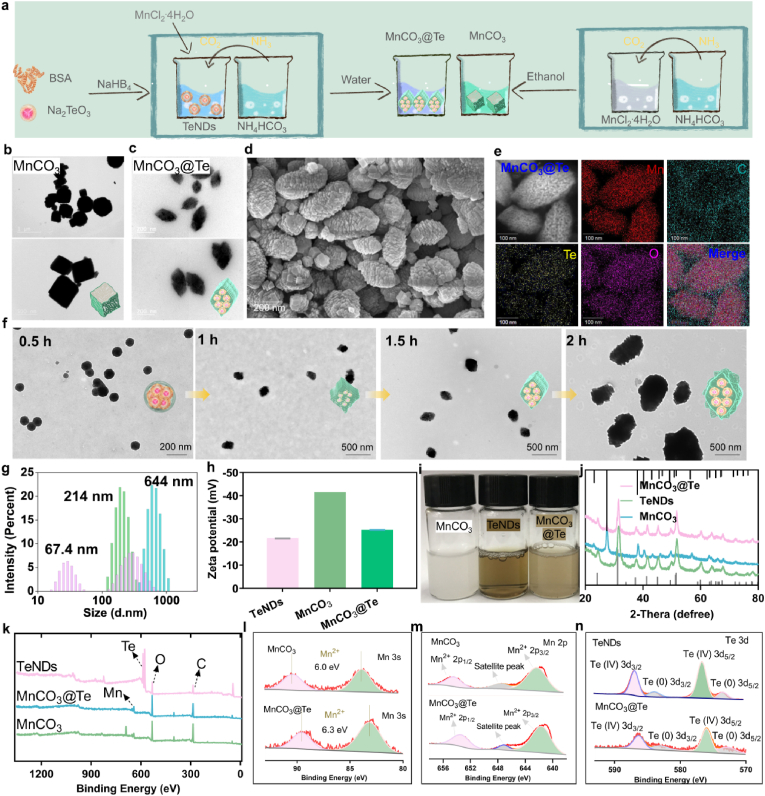
Here as illustrated in Scheme 1, we provide a simple one-pot strategy for the synthesis of maple leaf-shaped Te-loading manganese carbonate nanotherapeutics that can be serve as radiosensitizers and immunotherapeutic boosters to simultaneous enhance radioimmunotherapy by remodeling TME. Firstly, TeNDs with bovine serum albumin (BSA) surface decoration were synthesized as previously described [38]. Transmission electronic microscope (TEM) images in Fig. S1 showed the size of TeNDs was between 10 and 30 nm. Then, the acquired TeNDs was added to MnCl2 solution for mixed for 30 min. Next, the mixture was put into enclosed chamber containing NH4HCO3 solution for 1.5 h to obtain MnCO3@Te composite nanosystem. As shown in Fig. 1a–d, unlike bare MnCO3 nanosystem synthesized in ethanol phase, which exhibited a tridimensional rhomboid morphology with an average diameter of about 600 nm, the obtained MnCO3@Te in the aqueous phase presented a shape similar to a maple leaf, with a diameter of about 220 nm under observation by TEM and scanning electron microscopy (SEM). As analyzed by elemental mapping and EDX (Fig. 1e and Fig. S2), Mn, Te, N, C and O elements were found to be homogeneously distributed in the MnCO3@Te nanosystems, demonstrating TeNDs were successfully wrapped in MnCO3 nanosystems.
Fig. 1.
Schematic, synthesis and characterization of MnCO3@Te. (a) Synthetic scheme including TeNDs, MnCO3 and MnCO3@Te. TEM images of MnCO3 (b) and MnCO3@Te (c) nanosystems under different scales. (d) SEM image of MnCO3@Te. (e) Elemental mapping analysis of MnCO3@Te. (f) TEM images of MnCO3@Te obtained at different reaction times to show the reaction progress. (g–h) Hydrodynamic diameters and zeta potentials of MnCO3, TeNDs and MnCO3@Te. (i) Digital photograph of MnCO3, TeNDs and MnCO3@Te in water solution. (j) XRD analysis of MnCO3, TeNDs and MnCO3@Te. (k) Whole XPS spectrum of MnCO3, TeNDs and MnCO3@Te. (l–n) High-resolution XPS spectrum of Mn3s, Mn 2p and Te3d in MnCO3 and MnCO3@Te nanomaterials.
To demonstrate the key role of TeNDs on the synthesis of MnCO3, we allowed the growth of TeNDs within MnCO3 for various reaction times, followed by TEM imaging. As shown in Fig. 1f, after 0.5 h of reaction, most of the products appear in the form of nanospheres and TeNDs could be clearly observed in the middle. 1 h later, the edge of the ball grew a "rhombus", similar to a tridimensional rhomboid. At 1.5 h, the morphology evolved into maple leaf in shape, and finally at 2 h, the crystal continued to grow longitudinally to form “nanorod”. Meanwhile, we investigated the influence of BSA on the morphology of MnCO3 by using BSA to replace TeNDs. From Fig. S3, we observed similar phenomenon to MnCO3@Te reaction for 2 h, but the size of MnCO3@BSA was more than 1000 nm. As previous studies showed, BSA can participate in the synthesis of nanomaterials to achieve the goal of modifying and stabilizing nanoparticles [49]. Therefore, based on these results we acquired, we conclude that BSA modified on the surface of TeNDs has key influence on the morphology and diameter of MnCO3@Te nanosystems. This may be due to the preferential coordination between the chemical groups on the BSA and Mn2+, which affects the crystal formation. Moreover, dynamic light scattering (DLS) results revealed the average hydrodynamic sizes of TeNDs, MnCO3 and MnCO3@Te were about 67.4, 644 and 214 nm, respectively and the corresponding zeta-potentials were −21.6, −41.5 and −25.3 mV, respectively (Fig. 1g and h). This phenomenon was due to changes in reactive solvents affecting the particle size of MnCO3 nanomaterials. On the other hand, in order to prove the universality of this synthesis strategy, we attempted to prepare CaCO3@Te nanosystem by using the same preparation method as MnCO3@Te nanosystem. As shown in Fig. S4, TeNDs were highly homogeneous and monodispersed in CaCO3 nanomaterials, which revealed the method was suitable for the synthesis of various carbonate nanomaterials.
Moreover, we could observe that after loading TeNDs, the color of MnCO3@Te solution changes from milky white to light brown (Fig. 1i). Besides, according to the powder X-ray diffraction (XRD) pattern analysis, the diffraction peak of MnCO3@Te was well matched with that of bare MnCO3 and TeNDs (Fig. 1j). Furthermore, the X-ray photoelectron spectroscopy (XPS) was introduced to analysis the chemical composition and valence. As shown in Fig. 1k, characteristic peaks of Te 3d, Mn2p, C1s and O1s were clearly observed. Then, the high-resolution spectra of Mn3s, Mn2p and Te3d were further analyzed as presented in Fig. 1l-n. In general, the valence state of manganese in nanosystem can be determined by the multiple splitting of Mn3s [50]. The separation of peak energies (ΔE) in MnCO3 and MnCO3@Te Mn 3s spectra were 6.0 eV and 6.3 eV, which indicated that the valences of Mn in MnCO3 and MnCO3@Te nanosystems mainly belong to Mn2+. Besides, the peaks at 641.7, 647.2, and 653.5 eV were attributed to Mn2+ 2p3/2, satellite peak, and Mn2+ 2p1/2, respectively. Moreover, Te 3d peaks could be subdivided into four peaks located at 572.9, 576.0, 583 and 586.2 eV, which attributed to Te0 3d5/2, Ten+ 3d5/2, Te0 3d3/2 and Ten+ 3d3/2. Overall, these experiments commonly indicate that the MnCO3@Te formulation with changeable morphology was conducted successfully.
2.2. Response performance of MnCO3@Te in TME
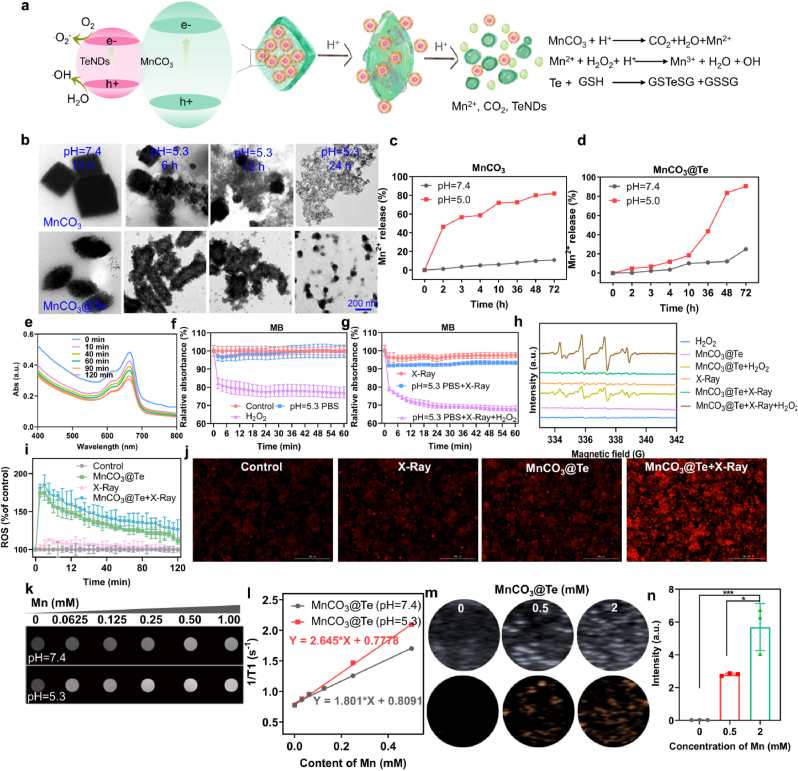
It is known that MnCO3 is stable at neutral and alkaline pH environment, but it can be decomposed into Mn2+ and CO2 gas in acid buffer (s 2a) [51]. Therefore, we recorded the morphology changes and the release of Mn2+ after incubation of MnCO3 and MnCO3@Te in PBS with different pH values (7.4 and 5.3) in different treatment time via TEM and inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. 2b–d, although the morphology of MnCO3 and MnCO3@Te exhibited no obvious change in pH 7.4 solution, we found MnCO3 and MnCO3@Te could rapidly be degraded by time-dependent manner. Meanwhile, quantitative analysis also proved that weak acidic environment promoted the release of Mn2+ rapidly. The results suggested that the MnCO3@Te is excellent acidity-responsive nanocarriers.
Fig. 2.
Responsive behaviors of MnCO3@Te improve ROS generation through fenton-like reaction and realizes efficient MR imaging and ultrasonic imaging in vitro. (a) Schematic illustration reflecting the MnCO3@Te heteronanostructures and its bioresponsibility in TME. (b) TEM image of MnCO3 and MnCO3@Te after incubation in PBS solution with different pH values for different times. (c–d) Release behaviors of Mn2+ from MnCO3@Te in pH 7.4 and 5.0 environment within 72 h. (e) The absorbance spectra of MB solutions containing MnCO3@Te at different time periods under X-Ray radiation for detection of •OH. (f–g) With/without X-Ray irradiation, the degradation curves of MB probes at 655 nm after incubation under various treatments. (h) ESR spectra of detection of •OH of MnCO3@Te immersed in H2O2 solution under X-Ray irradiation. (i) Intracellular ROS levels in MDA-MB-M231 cells, determined by DHE probe. (j) Representative fluorescence images of ROS level in MDA-MB-M231 cells after different treatments. T1-weighted MR imaging (k) and T1 relaxation rates (l) of MnCO3@Te with different concentrations after incubation with different conditions. (m–n) Ultrasound imaging and statistical data of MnCO3@Te in acid buffer.
Previous studies have shown that the Mn2+/Fe3+ would be promote genernation of highly cytotoxic hydroxyl radical (•OH) by triggering Fenton/Fenton-like reactions in tumor cells [52,53]. Hence, to investigate the effect of MnCO3@Te on generate •OH, we used methylene blue (MB) to examine the generation of •OH [54]. Meanwhile, to simulate the actual catalytic situation in tumor cells, MnCO3@Te was incubated in pH 5.3 PBS solution containing 10 mM H2O2. As shown in Fig. 2e, from the absorption spectra of MB, under X-Ray irradiation, the characteristic absorption peak gradually decreased with time in the presence of H2O2 at pH 5.5, indicating •OH production. It was worth noting that without X-Ray irradiation, MnCO3@Te + H2O2 generated the signal intensity of •OH was obvious lower than that of MnCO3@Te + H2O2+X-Ray, indicating X-Ray irradiation can promote the MnCO3@Te to generate •OH (Fig. 2f and g). Electron spin resonance (ESR) further demonstrated that the characteristic peak intensity of •OH was highest for MnCO3@Te + X-Ray + H2O2 groups, which was consistent with MB analysis (Fig. 2h). Meanwhile, we employed 1,3-diphenyl-isobenzofuran (DPBF) probe to monitor the overproduction of 1O2 radicals induced by the combination of MnCO3@Te and X-Ray irradiation in different mediums. As shown in Fig. S5, with the increase of time, the absorption of DPBF at 410 nm gradually decreased, which revealed that under the X-Ray stimulation, MnCO3@Te could generate 1O2 radicals. We also found that in the environment containing H2O2, MnCO3@Te exhibited a much higher oxidation rate compared with pH5.3 PBS solution. Above results commonly indicated that MnCO3@Te was mainly stimulated by the Fenton-like reaction to produce free radicals. Subsequently, cellular ROS level was examined qualitatively and quantitatively with dihydroethidium (DHE) as a probe. As expected, for the four groups (Control, X-Ray, MnCO3@Te, MnCO3@Te + X-Ray), the gradual increase in red fluorescence reveals more generation of ROS (Fig. 2i and j). Besides, increased evidence showed semiconductor heterostructures can promote the separation of h+-e- pairs and then improve the catalysis ability to produce ROS. According to literature reported [38,41], the valence band (VB) and conduction band (CB) of MnCO3 semiconductor were 2.43 eV and −0.82 eV, which could oxidize H2O to form •OH (EH2O/•OH = 1.99 eV) and stimulate the transition of O2 to •O2− (EO2/•O2- = −0.33 eV). The VB and CB of TeNDs with narrow band gap semiconductor were 0.72 eV and −0.23 eV. The above results proved the between MnCO3 and TeNDs is easy to form heterojunction to promote ROS production (Fig. 2a). All these results showed that MnCO3@Te have good ability and probably used to sensitized tumor radiotherapy by mediating the excessive generation of ROS.
The metabolism and transformation of Te nanomedicine in the body directly affect its toxicity, which is a scientific issue worthy of in-depth exploration. Hence we introduced high-resolution mass spectrometry (HR-MS) to detect the potential metabolites of TeNDs in the medium of GSH in vitro. As shown in Fig. S6, after mixing TeNDs and GSH, the GSSG peak increased obviously and a new GSTeSG peak appeared. The results indicated that Te nanomaterials may be metabolized as metabolic intermediates of GSTeSG in the body, which was consistent with the previous results [55].
Moreover, it is widely known that Mn2+ with five unpaired electrons is an effective T1 contrast agent in MR imaging [56]. To confirm the MR contrast capabilities, we used 3.0-T clinical MR scanner to capture the MR imaging of MnCO3@Te incubated in buffer solutions with different pHs. As presented in Fig. 2k-l, from the T1 MR imaging, the significant concentration-dependent brightening effect of MnCO3@Te were observed. Importantly, the relaxation rate of MnCO3@Te at pH7.4 buffer was 1.801 mM−1s−1, while the relaxation rate was measured to be 2.645 mM−1s−1 after incubation in pH 5.3 solution. Meanwhile, after the injection of MnCO3@Te into the tumor of mice, decreased T1 signal intensity was observed in the tumor tissue, which also confirmed the feasibility of MRI in vivo (Fig. S7). Furthermore, the generation CO2 gas induced by MnCO3@Te can be used as ultrasound imaging agent [57]. As expected, the MnCO3@Te showed obvious imaging signal in the pH = 5.3 solution (Fig. 2m-n). These results proved that MnCO3@Te can act as a good MRI and ultrasound contrast agent to guide tumor treatment at specific tumoral acidic pH.
2.3. Synergistic radiosensitization effects between MnCO3@Te and X-Ray in vitro
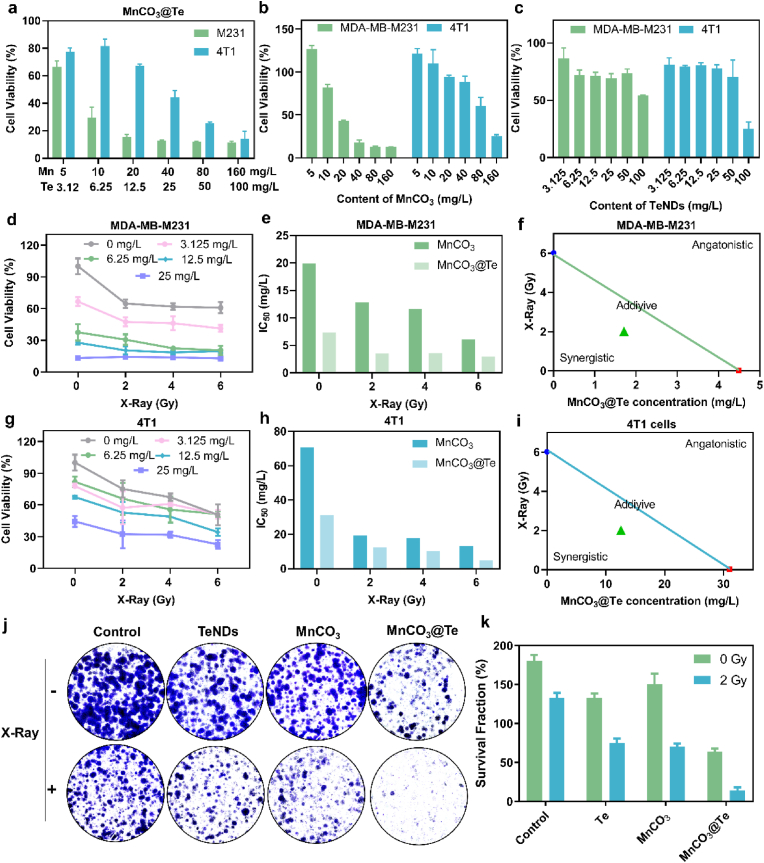
Encouraged by excellent ROS generation ability of MnCO3@Te, we next evaluate the anticancer efficacy and radiosensitization effects of MnCO3@Te in breast cancer cells (4T1 and MDA-MB-M231) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Firstly, without X-Ray irradiation, the viabilities of MnCO3@Te, MnCO3 and TeNDs with different concentrations were showed in Fig. 3a–c. As a results, TeNDs did not exhibit notable toxicity to 4T1 and MDA-MB-M231 cells even at the concentration of 50 mg/L, but MnCO3 and MnCO3@Te displayed concentration dependent cytotoxicity toward 4T1 and MDA-MB-M231 cells, reflecting the high antitumor activity of Mn-based nanosystems. Notably, the result of isobologram analysis showed the anticancer efficacy between TeNDs and MnCO3 on 4T1 and MDA-MB-M231 cells was synergetic effect, as evidenced by the location of the data points far below the line defining additive effect (Fig. S8). Moreover, it was found that the cell viabilities and IC50 value of 4T1 and MDA-MB-M231 cells further decreased after incubation with MnCO3@Te followed by X-Ray irradiation (Fig. 3d and e and 3g-h). In specific, the IC50 values of the combination treatment of MnCO3@Te and X-Ray (2 Gy, 4 Gy and 6 Gy) toward 4T1 cells were 12.583, 10.332, and 4.842 mg/L, respectively, obviously superior to bare MnCO3@Te (31.023 mg/L). In addition, we also carried out isobologram analysis to examine the connection between MnCO3@Te and X-rays. As expected, the synergistic effect could be found in both 4T1 cells and MDA-MB-M231 cells (Fig. 3f and i). Finally, MnCO3@Te also inhibited the colony formation of MDA-MB-M231 cells after X-ray radiation at a significantly higher level than TeNDs and MnCO3 (Fig. 3j and k). Taken together, MnCO3@Te mediated good radiosensitization effect and hold a good application prospect in reversing radioresistance at the cellular level.
Fig. 3.
Synergistic effects of MnCO3@Te and X-Ray on killing breast cancer cells. Without X-Ray irradiation, cell viabilities of MDA-MB-M231 and 4T1 cells treated with different concentrations of MnCO3@Te (a), MnCO3 (b) and TeNDs (c). With different dosage of X-Ray irradiation, cell viabilities of MDA-MB-M231 (d) and 4T1 cells (g) treated with different concentrations of MnCO3@Te (calculated by Te). IC50 value of MnCO3 and MnCO3@Te (calculated by Mn) with or without X-Ray on MDA-MB-M231 cells (e) and 4T1 cells (f) after 72 h-incubation. Isobologram analysis of the synergistic antitumor effects of MnCO3@Te combined with X-Ray in MDA-MB-M231 (f) and 4T1 (i) cells. (j–k) Representative photographs of stained colonies of MDA-MB-M231 cells treated with PBS, PBS + X-Ray, TeNDs, TeNDs + X-Ray, MnCO3, MnCO3+X-Ray, MnCO3@Te and MnCO3@Te + X-Ray after 7 days.
2.4. Action mechanism of MnCO3@Te cooperating with radiotherapy for killing cancer cells
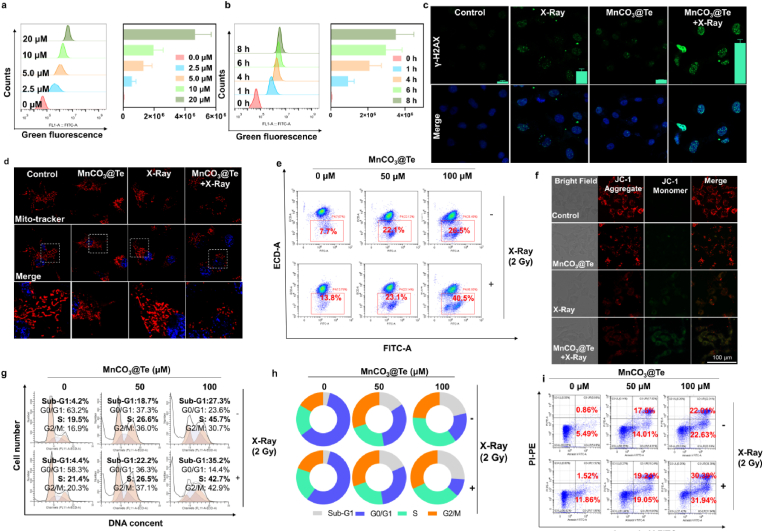
The intracellular mechanism of synergistic effect of MnCO3@Te and radiotherapy on killing tumor cells was evaluated by confocal fluorescence imaging and flow cytometry. Generally, anticancer effect of the nanosystem prerequisites its effective cellular uptake, thus, we analyzed the cellular uptake of MnCO3@Te with different concentrations in different incubation times. The results in Fig. 4a and b showed MnCO3@Te could rapidly enter tumor cells and continuous increased with incubation time prolonged. Since the damage of mitochondria and DNA caused by ROS produced in cancer cells is the main reason for X-rays to kill cancer cells, thus we further carried out γ-H2AX detection and mitochondrial determination to explain the mechanism of MnCO3@Te sensitizing X-ray [58]. We could observe from Fig. 4c that MnCO3@Te + X-ray group induced largest number of fluorescent spots of γ-H2AX, which reflected the most serious DNA damage. Consistently, western blotting results (Fig. S9) also showed MnCO3@Te + X-ray group induced the strongest DNA damage. In addition, Mito-tracter and Hoechst 33342 probes were used to label mitochondria and nucleus of MDA-MB-231 cells. For cells treated with X-Ray/MnCO3@Te alone, only slight fragmentation could be observed. In contrast, when X-ray was used simultaneously, MnCO3@Te caused scattered visual spots throughout cytoplasm (Fig. 4d). Moreover, JC-1 fluorescent probe was used to measure mitochondrial membrane potential (ΔΨm). Consistent with the above results (Fig. 4d), compared with X-Ray group, the percentage and intensity of ΔΨm with J-aggregate was significantly increased in the MnCO3@Te + X-Ray group (Fig. 4e and f). Furthermore, to explore the death mode of MDA-MB-231 cells induced by MnCO3@Te and X-Ray, PI staining and Annexin V-FITC/PI double staining assay were performed. As shown in Fig. 4g–i, apoptosis and S-phase arrest were the main mode of cell death caused by MnCO3@Te and X-Ray in combination. For example, when concentration of MnCO3@Te was 100 μM, under X-Ray radiation, the total apoptosis rate of MDA-MB-231 cells was 62.33%, higher than that of bare MnCO3@Te (44.64%) and X-Ray (13.48%). PARP are vital characteristic hallmarks of apoptosis of cell apoptosis. The results (Fig. S9) showed treatment with MnCO3@Te combined with X-Ray activated the proteolytic cleavage of PARP, further confirming the important role of apoptotic in MnCO3@Te-induced cell death. Overall, the mechanism of cell death revealed that MnCO3@Te cooperating with radiotherapy triggered ROS overproduction in cancer cells and then caused DNA damage and mitochondrial dysfunction, thereby inducing cancer cell cycle arrest and apoptosis to kill cancer cells.
Fig. 4.
Anticancer action mechanism of combination treatment of MnCO3@Te and X-Ray to MDA-MB-231 cells. (a–b) Cellular uptake of different concentrations MnCO3@Te for different time points, determined by flow cytometry. (c) Representative fluorescence images of DNA fragmentation of MDA-MB-231 cells treated with MnCO3@Te and X-ray radiation. (d) Representative images of mitochondrial dysfunction caused by MnCO3@Te and X-ray radiation. (e–f) JC-1 assay for illustrating the depletion of mitochondrial membrane potential in MDA-MB-231 cells treated with MnCO3@Te and then X-Ray irradiation. (g–h) Flow cytometric analysis of the cell cycle of MDA-MB-231 cells after introduction MnCO3@Te with or without X-rays (2 Gy). (i) Annexin V-FITC/PI staining kit detected the apoptosis of MDA-MB-231 cells treated with MnCO3@Te followed by X-Ray exposure.
2.5. Immune activation effect of MnCO3@Te nanosystem based on STING pathway
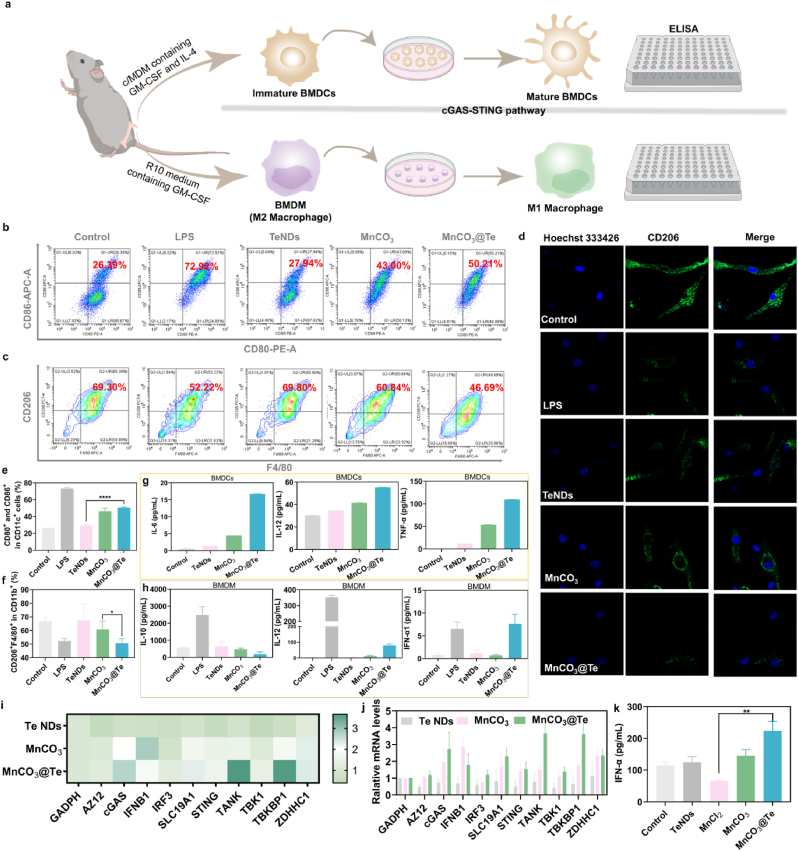
The latest results have indicated that Mn2+ play an key role in immune activation, which can serve as a potent STING agonist to effectively promote the maturation of DCs and the polarization of M1 macrophages [59,60]. Hence, we evaluated the effects of the MnCO3@Te nanosystem on the immune activation by introducing a series of experiments in vitro (Fig. 5a). Firstly, we extracted bone-marrow-derived macrophages (BMDMs) and bone marrow-derived dendritic cells (BMDCs) from BALB/c mice. Then, the abilities of MnCO3@Te nanosystem to activate DC maturation and induce macrophage repolarization were investigated by flow cytometry. As shown in Fig. 5b and c and 5e-f, compared with PBS group, there were no obvious changes in the percentage of matured DCs (CD11c + CD80+CD86+) and M2-type macrophage (CD11b + F4/80+CD206+) in TeNDs group, indicating that the TeNDs had little effect on immune activation. Notably, the treatment of MnCO3@Te significantly increased the level of matured DCs from 28.3% to 50.21% and decreased the percentage of M2 macrophage from 69.3% to 46.69%, both of which were better than the single MnCO3 nanosystem. Meanwhile, the results of CD206 immunofluorescence in BMDMs further proved that MnCO3@Te could promote the polarization of macrophages (Fig. 5d). Moreover, relevant cytokines secreted by BMDCs and BMDMs have also been changed to a certain level (Fig. 5g and h).
Fig. 5.
MnCO3@Te as immunomodulator to promote immune activation effect via STING pathway. (a) Schematic illustration of experimental design for detection of DC maturation and macrophage M1 repolarization. (b, e) Representative flow cytometry images and its corresponding statistical data of mature DCs (CD11c + CD86+CD80+, gated on CD11c + cell) after different treatment for 12 h. (c, f) Representative images and statistical data to show the M2-type tumor-associated macrophage (CD11b + F4/80+CD206+, gated on CD11b+). (d) Confocal fluorescence images of CD206 stained-DC2.4 cells after treatment with PBS, LPS, TeNDs, MnCO3 and MnCO3@OVA for 12 h. Related cytokines expression secreted by BMDCs (g) and BMDMs (h) in the medium after treatment with different conditions for 12 h. (i–j) qPCR analysis to examine the relative gene expression of cGAS-STING in BMDCs with different treatments for 24 h. (k) IFN-α level in the culture supernatant of BMDCs after different treatments.
Furthermore, we would like to investigate the ability of MnCO3@Te to activate the cGAS-STING pathway by real-time quantitative PCR (RT-qPCR) assay. As shown in Fig. 5i and j, MnCO3@Te group trigger the expression of cGAS-STING axis genes in BMDCs, which was much stronger than TeNDs and MnCO3 groups. Additionally, Enzyme-linked immunosorbent assay (ELISA) assay revealed MnCO3@Te nanovaccine potently enhanced the expression levels of cytokines of IFN-α in the culture supernatant of treated BMDCs. Taken together, these findings argued that MnCO3@Te could promote DCs maturation through the activation of cGAS-STING pathway, and induce the secretion of type I IFN, which may be indispensable for the activation of adaptive immunity against tumors.
2.6. In vivo anticancer effects of MnCO3@Te evaluation by remodeling immuno-microenvironment
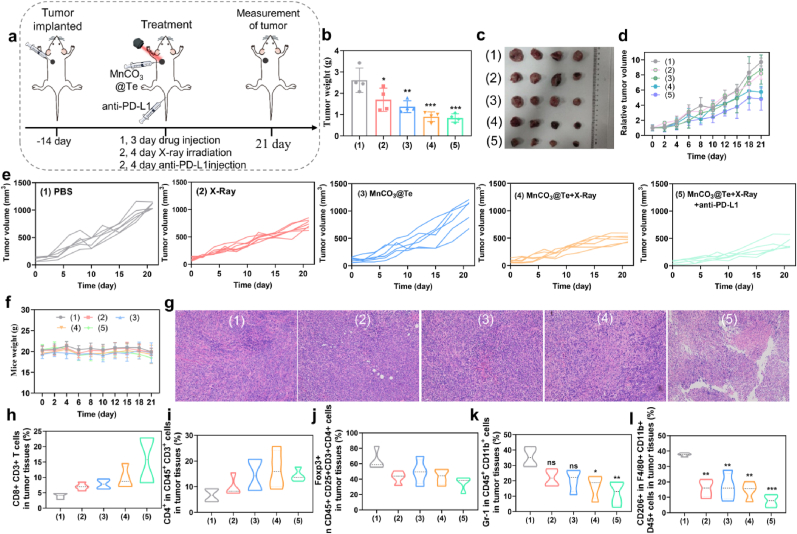
After confirming the immunostimulating efficacy in vitro, we next evaluated the antitumor effect of the MnCO3@Te-based radiotherapy combined with anti-PD-L1 checkpoint blockade in vivo. Here, we established BALB/c orthotopic breast tumor model with 4T1 cells. According to the regime in Fig. 6a, the mice were randomized into five groups: (1) PBS, (2) X-Ray, (3) MnCO3@Te, (4) MnCO3@Te + X-Ray and (5) MnCO3@Te + X-Ray + anti-PD-L1. Then, on the first and third days of treatment, mice were injected with MnCO3@Te intratumorally. Then, on the second and the fourth days, the mice received radiotherapy (2 Gy) and immunotherapy (anti-PD-L1). Firstly, during the treatment, the body weight of mice did not change significantly, suggesting biosafely of MnCO3@Te in tumor treatment (Fig. 6b). The therapeutic efficacy was presented in Fig. 6c–g. As expected, these results of tumor weight, tumor volume and of tumor growth curves commonly demonstrated that the tumor growth in MnCO3@Te + X-Ray + anti-PD-L1 group was most significantly inhibited, followed by MnCO3@Te + X-Ray group. For example, tumor weight after dissection in MnCO3@Te + X-Ray group at 21 days of treatments was 0.90 g, much lower than that of X-ray treatment (1.69 g) and MnCO3@Te (1.37 g), reflecting that the excellent radiosensitization ability of MnCO3@Te in vivo (Fig. 6c). Correspondingly, no obvious necrosis could be observed in the hematoxylin and eosin (H&E) staining of tumor slices from the mice treated with PBS or X-Ray. However, combined treatment of radiotherapy and immunotherapy based on MnCO3@Te caused serious cell necrosis and hemorrhagic inflammation (Fig. 6g). Moreover, survival rate of mice was assessed over the course of 30 days. As shown in Fig. S10, the group of control and free MnCO3@Te exhibited 33.3% and 35.5% of survival, while the (4) and (5) groups remains 81.8% and 77.9% within 30 days. The results showed our developed “MnCO3@Te + X-Ray” and “MnCO3@Te + X-Ray + anti-PD-L1” combination strategy could enhance the survival of tumor-bearing mice than single modality. Taken together, MnCO3@Te helps to enhance the sensitivity of tumor cells to X-rays and achieve the synergistic effect of radiotherapy and immunotherapy.
Fig. 6.
Anticancer immunity of MnCO3@Te-mediated radiotherapy in combination of anti-PD-L1 in vivo. (a) Schematic illustration showing the experiment design using MnCO3@Te-based RT and anti-PD-L1 to treat mice bearing 4T1 tumors. (b) Average tumor weight of various groups after treatment. (c) Photographs of tumors excised from Balb-c mice. (d) Relative tumor growth curves in different treatment groups. (e) Growth curves of tumor volume for individual Balb-c mice following various treatments. (f) Mice weight changes within treatment period. (g) H&E-stained images of tumor tissues collected from mice post various treatments. Quantification analysis of the infiltration of T lymphocytes including CD8+CD3+ T cells (h) and CD3+CD4+ T cells (i) after various treatments. Quantification analysis of decline of intratumoral immunosuppressive cells including Treg cells (j) and MDSC cells (k) in tumor tissues. (l) Flow cytometry analysis of the proportion of M2-phenotype tumor associated macrophages within tumor tissues from different immunized mice.
It is well known that TME is immunosuppressive, which may largely offset the impact of anti-tumor immunity [61]. Thus the influence of MnCO3@Te on the tumor immuno-microenvironment was explored to uncover the mechanisms underlying the observed antitumor efficacy. Firstly, we soaked Mn in an acidic environment with pH = 5.3, and found that the pH value of the solution gradually increased, close to that of normal tumor tissue, demonstrating MnCO3@Te has the potential to neutralize acidic TME (Fig. S11) The infiltration of immune cells including CTLs, CD4+ helper T lymphocytes, MDSCs, Tregs and M2-like macrophage in the tumor sites was measured using a subset of the tested mice on day 21. Firstly, the levels of CD3+CD8+ CTL cells and the CD3+CD4+ helper T cells in the MnCO3@Te + X-Ray + anti-PD-L1 group were significantly increased to 15.66% and 14.27% compared with the controls (Fig. 6h–i and Fig. S12). Meanwhile, immunofluorescence staining of tumor slices from different treatment groups was carried out. As shown in Fig. S13, green (CD8+ T cells) fluorescence of MnCO3@Te and X-Ray plus anti-PD-L1 group were significantly stronger than that of other groups, which ultimately in line with the results of flow cytometry. Therefore, the combined radiotherapy and immunotherapy with synthesized MnCO3@Te can trigger infiltration of CTLs in tumor tissues, thus triggering immune responses. In addition to immune-activated cells, M2-phenotype macrophages, MDSCs and Tregs were the typical immunosuppressive cells in TME. The results showed the frequency of M2-phenotype macrophages, M2-MDSCs and Tregs in mice received MnCO3@Te + X-Ray + anti-PD-L1 treatment was significantly lower than control and X-Ray groups (Fig. 6j-l and Fig. S12). Overall, the combination of radiotherapy and immunotherapy based on MnCO3@Te could induce strong antitumor immunity and remodel immuno-microenvironment.
2.7. MnCO3@Te-mediated radiotherapy and anti-PD-L1 combination therapy prevent lung metastasis
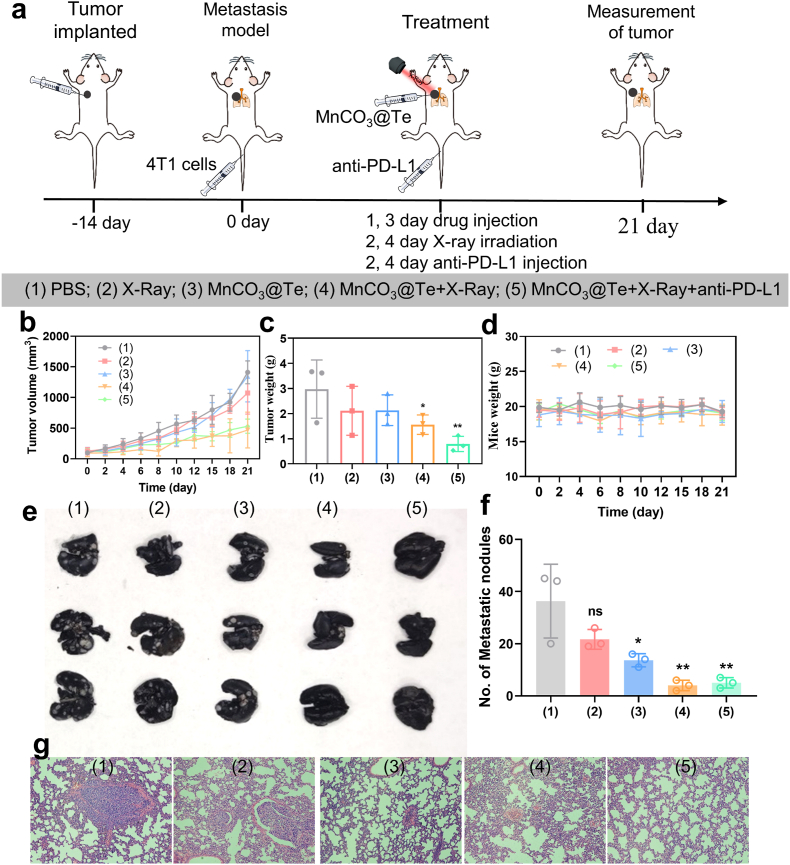
Tumor metastasis is the main cause of high tumor mortality, and it is also a challenge for clinical tumor treatment [62]. As one of the most invasive cancers, breast cancer is most likely to metastasize to lung tissues through circulatory system with epithelial-mesenchymal transformation [63,64]. Hence to explore the ability to prevent lung metastasis, according to the experimental design in Fig. 7a, after primary tumor generation, mice were intravenous injected 4T1 cells to build lung metastatic tumor model. First of all, the therapeutic effect of 4T1 tumor-bearing mice on primary tumors after various treatments was consistent with the above tumor model (Fig. 7b–d). After 21 days of treatment, mice were dissected and their lung tissues were taken out, fixed, stained with ink. Finally, the number of nodules on the surface of lungs were counted. As shown in Fig. 7e–f, a large number of lung metastases were seen in PBS group. Importantly, the most significant reduction in the number of lung lesions was observed in the fifth combined treatment group. Correspondingly, the results of H&E staining of lung slices were consistent with those observed in lung photographs (Fig. 7g). These results revealed that combination treatment with MnCO3@Te + X-Ray + anti-PD-L1 was much more effective in preventing lung metastasis than MnCO3@Te or X-Ray alone. Based on those result, we concluded that the therapeutic strategy using MnCO3@Te combined with X-Ray and anti-PD-L1 has the advantage of preventing tumor metastasis, which probably attributed to the strong systemic immune response.
Fig. 7.
MnCO3@Te-based radiotherapy in combination with aPD-1 for boosting antimetastasis activity. (a) Schematic of the experimental procedure of MnCO3@Te combined with X-Ray and anti-PD-L1 on metastatic melanoma model. (b) Tumor growth curves from different group. (c) Tumor weight profiles of each group at the 21st day post treatment. (d) Mice weight changes within treatment period. (e) Images of metastatic nodules in lungs after different treatments. (f) The quantification of metastatic nodules in lungs after different treatments. (g) H&E-stained images of lung tissues collected from mice post various treatments.
Metabolism and toxic side effects of nanomedicines are another key issue of concern to researchers except its anticancer effect. Firstly, we performed pharmacokinetics analysis of MnCO3@Te to quantify the metabolism behavior. After intravenous injection (i.v.) of the MnCO3@Te, plasma concentration of Te from mice in different group at various times were examined by inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. S14, the plasma concentration of MnCO3@Te gradually decreased with time. In an analysis of pharmacokinetic parameters, we found MnCO3@Te exhibited an elimination half-life (t1/2β) of 36.59 h and area under the curve of 214.32 μg/mL*h, which was higher than that of TeNDs reported in previous literature [38]. The results confirmed that the packaging of MnCO3 nanosystem helps to improve and prolong the blood circulation life of TeNDs. Furthermore, we carried out H&E staining of major organs of mice to analysis their status and then confirm biosafely. As shown in Fig. S15, obvious tumor metastasis could be observed in the liver tissues in the groups treated PBS, X-Ray and MnCO3@Te. However, the slices of major organs including heart, liver, spleen and kidney could hardly detect obvious inflammation or other pathological change induced by MnCO3@Te nanosystems even after the X-Ray irradiation plus anti-PD-L1 treatment, which verified its high safety and low toxicity as agonist.
3. Conclusion
An increasing number of studies found radiotherapy is closely associated with immune system in the treatment of breast cancer. Thus the combination of radiotherapy and immune checkpoint therapies have received considerable attention in the application of various tumor therapies. However, radioresistance and immunosuppression in TME have limited its therapeutic effectiveness across patients. Therefore, it is urgent to develop a nanoplatform to simultaneous overcome radioresistance and reprogram tumor immune microenvironment, so as to realize the efficient combination of radiotherapy and immunotherapy. Hence, in this work, we successfully designed and developed a Te-directed maple leaf MnCO3 nanotherapeutics for the radioimmunotherapy of cancer to inhibit tumor progression and lung metastasis.
In detail, the conclusions can be summarized as follows. (a) The reasonable design of MnCO3 wrapped Te greatly affects the diameter and morphology of MnCO3 crystal, including the transformation from spherical to maple leaf to rod. (b) MnCO3 nanomaterials can release a large number of Mn2+ and react with H2O2 to generate free radicals such as •OH under the acidic environment of tumor, therapy realizing the anti-tumor activity of CDT through changing the fenton-like reaction of Mn valence. (c) MnCO3@Te exhibits excellent radiosensitization efficacy by inducing cellular apoptosis and mitochondrial fragmentation, which was higher than Te/MnCO3 alone. (d) Increased the pH value of TME to create a favorable environment for immune activation, realizing strong inhibitory efficacy on the tumor growth containing primary tumor and metastatic tumor. Overall, our study not only provides a facile way to synthesize different morphology MnCO3 systems but also highlights the potential of MnCO3@Te as radiosensitizers and immunomodulator for the therapy of breast cancer lung metastases by combining with immunotherapy.
4. Experimental section
4.1. Materials
Manganese chloride tetrahydrate (MnCl2∙4H2O), ammonium bicarbonate (NH4HCO3), sodium tellurite (Na2TeO3), sodium borohydride (NaHB4), BSA, phosphate-buffered saline (PBS), DMEM medium, fetal bovine serum (FBS) and paraformaldehyde were purchased from Sigma. propidium iodide (PI) and Mito-Tracker Red were purchased from Thermo Fisher Scientific. All flow cytometric antibodies were obtained from Biolegend. Anti-PD-L1 monoclonal antibody was purchased from Neobioscience. Ultrapure water used in the experiments was supplied by a Mili-Q water purification instrument from Millipore.
4.2. Preparation of TeNDs
TeNDs was obtained based on previous explorations [38]. In detail, 25 mg/mL BSA (10 mL) was mixed with 20 mM Na2TeO3 (2 mL) under mild stirring. Then, adjust the pH value of the above mixed solution to 10–12 with NaOH solution. Subsequently, NaHB4 with a concentration of 100 mM was quickly added to the above mixed solution for reaction 4 h at 50 °C. Finally, the acquired solution was dialyzed against Milli-Q water for 24 h using a dialysis bag (50000 Da).
4.3. Preparation of MnCO3@Te nanotherapeutics
The solution of 1 mL MnCl2∙4H2O (6.25 mg/mL) and TeNDs (4 mL) was mixed and placed into an airtight container containing ammonium bicarbonate, reacted for 1.5 h until the solution appeared obvious turbidity. The material was obtained after washing three times by centrifugation (12000 rpm) with ultrapure water. Meanwhile, to analysis the formation progress of maple leaf-shaped MnCO3@Te nanotherapeutics, we changed the synthesis time in the reaction system under other unchanged conditions. In addition, we also prepared CaCO3@Te nanotherapeutics with the synthesis strategy of MnCO3@Te by using CaCl2 to replace MnCl2∙4H2O.
4.4. Characterization of MnCO3@Te nanotherapeutics
The structure and chemical composition of the prepared MnCO3@Te were characterized by high resolution transmission electron microscope (HRTEM, JEM-2100) equipped with energy-dispersive X-ray spectroscopy (EDS), field emission scanning electron microscope (FESEM, ULTRA-55), X-ray diffraction (XRD, miniflex 600) and X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha+). The size distribution and zeta potential of nanomaterials were characterized by using Zetasizer Nano ZS particle analyzer (Malvern Instruments Limited). Concentration of the MnCO3@Te were determined by inductively coupled plasma mass spectrometry (ICP-MS, Thermo UltiMate 3000+iCAP RQ). Meanwhile, in order to explore the formation process of the nanosystem, the changes in MnCO3@Te morphology at different reaction times were characterized by HRTEM.
4.5. Response performance of MnCO3@Te nanotherapeutics
To detect the acid sensitivity of nanomaterials, MnCO3 and MnCO3@Te were placed in PBS solution with pH = 7.4 and pH = 5.3, respectively. Then, the morphology changes of MnCO3 and MnCO3@Te in an acidic environment for 6 h, 12 h, and 24 h were obtained from the transmission electron microscopy. Meanwhile, the content of Mn in the supernatant after centrifugation was detected by ICP-MS to understand the decomposition of the MnCO3 and MnCO3@Te.
4.6. MR imaging and ultrasound imaging
To evaluate the MR imaging effect of the MnCO3 and MnCO3@Te, we scanned MR imaging of the MnCO3@Te sample dispersed in pH 7.4 and 5.3 conditions at various concentrations. Meanwhile, in vivo MR imaging preformed on 4T1 tumor-bearing BALB/c mice before and after administration with MnCO3@Te, which were detected on 3.0T clinical MRI scanner. Moreover, ultrasound imaging in vitro of MnCO3@Te at different concentrations was performed after incubation in acid environment (pH = 5.3).
4.7. Generation of reactive oxygen species
Methylene blue (MB) was mixed with MnCO3@Te nanomaterial containing 10 mM H2O2 followed by X-Ray irradiation 2 Gy. Then, the sample was scanned at 500–750 nm with a UV–vis spectrophotometer to prove the •OH generation. Meanwhile, the absorbance value at 670 nm under different conditions was measured to evaluate the yield of •OH. In addition, the •OH induced by MnCO3@Te were detected by ESR technique by using DMPO capture agent (A300, Bruker). The detection of •OH produced by MnCO3@Te combined with X-ray and H2O2 was similar to those measured above. Similarly, DPBF probe was introduced to monitor the overproduction of 1O2 radicals.
The level of intracellular ROS produced by MnCO3@Te and X-Ray was detected by DHE fluorescent probe, and the fluorescence intensity changes were photographed by fluorescence microscope (EVOS), the absorbance (510 nm) of DHE in the culture medium was measured with a plate detector (cytation5, BioTek).
4.8. Metabolite analysis of TeNDs in vitro
Previous studies have reported that telluride could react with glutathione (GSH) in vivo to form trisulfide telluride (GSTeSG), and then converted to hydrogen telluride, eventually excreted from the body through methylation metabolism [55]. For this reason, we speculate whether TeNDs could produce metabolites of GSTeSG. Hence, the TeNDs were dispersed in 1 mL the buffer with GSH (10 mg/mL), followed by HR-MS detection.
4.9. Cell culture and cytotoxicity assays
The MDA-MB-231 cell line and the 4T1 cell line were derived from American Type Culture Collection (ATCC). In short, MDA-MB-231 and 4T1 cells were seeded in 96-well plates with 2000 cells in each well. After cell attachment, a series of concentrations of the material were incubated with the cells, and the cell survival rate was evaluated by MTT assay. At the same time, in order to study the effect of the material to enhance RT, after the material was incubated with cells, different doses of X-rays (2, 4 and 6 Gy) were used to evaluate the effect by MTT assay. In addition, the isobologram method previously described was introduced to analyze the synergy effect between MnCO3@Te and X-ray.
Similarly, to evaluate the synergistic effect of MnCO3@Te and X-ray on cancer cells, MDA-MB-231 cells were seeded in 6-well plates with 2000 cells per well. After cell adherence, the cells were incubated with the material and irradiated with 2 Gy of radiation for 7 days, washed lightly with PBS, and fixed with 4% paraformaldehyde. Then, the spots were stained with crystal violet and analyzed for number of spots.
4.10. Cellular uptake
To evaluate uptake of MnCO3@Te in cells, intracellular uptake assays were performed according to the fluorescence intensity of coumarin-6-labeled MnCO3@Te. MDA-MB-231 cells were incubated with MnCO3@Te (2.5 μM, 5 μM, 10 μM, 20 μM) for 1 h, 4 h, 6 h, 8 h. The relative uptake of MnCO3@Te was then measured by flow cytometry to obtain the cellular uptake relationship of MnCO3@Te with times and concentrations.
4.11. Action mechanism of MnCO3@Te cooperating with radiotherapy
To evaluate the damage of MnCO3@Te on cancer cells, the cell cycle was analyzed by flow cytometry after labeling the cells with propidium iodide (PI) staining, and the protein expression of p-histon which associated DNA damage in the treated cells was analyzed by immunofluorescence staining and western blotting. Besides, to evaluate the apoptosis of the treated group, the apoptosis states of the cells were stained by labeling PI and annexin-V FITC dyes and analyzed by flow cytometry. Moreover, the expression level of PARP protein of MDA-MB-231 cells after different treatments was further determined by western blotting as previously described [65].
To analyze the mitochondrial damage after MnCO3@Te treatment, the turnover of cell membrane potential in the drug-treated groups was evaluated by flow cytometry and fluorescence microscopy analysis of cells labeled with JC-1 fluorescent probe. The intracellular mitochondria were labeled with Mitotrack red, to analyze the mitochondrial morphology changes in the cells after drug treatment were observed by confocal fluorescence microscopy (LSM 700).
4.12. DC maturation, macrophage polarization, and cytokines
Bone Marrow-Derived Dendritic Cells (BMDCs) and bone marrow-derived macrophages (BMDMs) were isolated according to the method reported previously [66]. To measure the maturation of DCs induced by MnCO3@Te, the isolated BMDCs were incubated with MnCO3@Te, MnCO3, TeNDs and LPS for 24 h, respectively, and then the cells were washed by PBS and labeled with flow antibody against CD11c, CD80, CD86, at the same time, the proportion of these antibodies in the cells was measured by flow cytometry. Similarly, the polarization of macrophages was further analyzed by flow cytometry with CD11b, F4/80 and CD206 labeled antibodies.
To further analyze the effect of MnCO3@Te and MnCO3 in immune cells, cytokines (IL-6, IL-12, TNF-α) in the supernatants of different treated group's BMDC were detected with ELISA kits according to vendors' protocols (obtained from Biolegend). Similarly, cytokines (IL-10, IL-12, IFN-α1) in the supernatants of different treated group's mouse BMDM were detected with ELISA kits according to vendors' protocols (obtained from Biolegend).
4.13. Rt-qPCR
The RT-qPCR assay was introduced to detect the gene expression of cGAS-STING in the treated cells. Briefly, the treated cells were each washed three times with cold PBS solution, total RNA was then extracted using TaKaRa MiniBEST Universal RNA Extraction Kit and transcribed to cDNA by Quantscript RT Kit (Tiangen Biotech Co., LTD, China) according to the manufacturer's instructions. Finally, qPCR assay was done using SYBR® Premix Ex Taq™ II (Takara, Japan) performed with CFX Connect™ Real-Time PCR Detection System (Bio-rad, USA) following the manufacturer's instruction. The relative gene expression was determined by the 2-△△Ct method after normalization to the internal control gene of GAPDH.
4.14. Tumor immunotherapy in vivo
The work performed in BALB/c mice (6–8 weeks of age) was purchased from the Animal Center, Guangdong Province All animal experiments were conducted in strict accordance with national guidelines for the care and use of experimental animals and were approved by the Ethics Committee of Jinan University (Guangzhou, China) for animal experiments. 107 4T1 cells were injected into the breast of each BALB-c mice. After successful establishment of BALB/c orthotopic breast tumor model, the mice were equally divided into five groups: (1) PBS, (2) X-ray, (3) MnCO3@Te, (4) MnCO3@Te + X-Ray, (5) MnCO3@Te + X-Ray + anti-PD-L1. Among them, the mice in group 1 and 2 were injected with PBS solution. The mice in group 3, 4 and 5 administered with MnCO3@Te by intratumoral injection. In addition, the group of 2, 4 and 5 were given radiotherapy on the 2, 4 days after the administration. Anti-PD-L1 (15 μg each mouse) was administered intravenously on 2nd and 4th day. Mice weight was recorded every 2 days. The width and weight were measured with a vernier caliper, calculated by the formula (tumor volume = length × width2/2), and analyzed to obtain daily maintenance detection. The relative tumor volume was calculated according to the tumor volume after treatment (Vday) divided by the tumor volume before treatment (V0). In addition, according to the feeding protocol, the mice were killed when the tumor volume was more than 1000 mm3. After 21 days of treatment, the tumors were dissected, weighed and fixed for H&E staining.
4.15. Flow cytometry was used to analyze the infiltration of tumor immune cells
After the tumor cells were ground, the dispersed tumor cells into a single cell state were labeled with a series of flow antibodies (Tregs (CD45+CD3+CD4+CD25+FOXP3+), MDSCs (CD45+CD11b + Gr-1+), M1-type TAM (CD45+CD11b + F4/80+CD206+), CTLs (CD45+CD3+CD8+), Helper T Lymphocytes (CD45+CD3+CD4+) to detect the proportion of infiltrating immune cells in the tumor cells, and the proportion of infiltrating immune cells was analyzed by flow cytometry (Antibodies were from biolegend). The MnCO3@Te + X-Ray + anti-PD-L1-induced CTLs infiltration in tumor tissues was examined by immunofluorescence assay.
4.16. Antimetastatic activity in 4T1 tumor mice model
To evaluate the antimetastatic ability of MnCO3@Te, the lung metastatic breast tumor model of BALB/c mice was established [67]. In brief, on the 14th day of primary tumor formation, 105 4T1 cells were injected from the tail vein of the mice. Then, Similar to above treatment method, mice were randomly divided into five groups and treated with PBS, PBS + X-Ray, MnCO3@Te, MnCO3@Te + X-Ray and MnCO3@Te + X-Ray + anti-PD-L1. Finally, mice were perfused with India ink 21 days later, and the number of lung nodules on the BALB/c mice was counted. And the degree of lung metastatic from different group was evaluated by H&E staining of lung sections. Similarly, for evaluating the biosafely, H&E staining of major organs was studied.
4.17. In vivo pharmacokinetic assay
Female SD mice (about 200 g) were administered with MnCO3@Te (0.5 mg/kg, calculated by Te) by intravenous (i.v.) injection. Then, at specific times, blood of mice in different group was collected and centrifuged to obtain plasma. The Te concentration dispersed in plasma was determined using ICP-MS.
4.18. Statistical analysis
All tests were conducted at least three times and all data are expressed as mean G standard deviation (SD). Statistical analysis was performed with the SPSS statistical package (SPSS 13.0 for Windows; SPSS, Chicago, IL) and differences of p < 0.05 (*), p < 0.01 (**), and p < 0.01 (***) were considered statistically significant.
CRediT authorship contribution statement
Wei Huang: Conceptualization, Investigation, Methodology, Writing – original draft, Funding acquisition. Sujiang Shi: Investigation, Methodology, Writing – original draft. Haoran Lv: Investigation, Methodology, Writing – review & editing. Zhenyu Ju: Writing – review & editing. Qinghua Liu: Methodology, Supervision, Writing – review & editing. Tianfeng Chen: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Science Fund for Distinguished Young Scholars (82225025), National Natural Science Foundation of China (21877049, 32171296, 32201166, 82172088), Guangdong Natural Science Foundation (2020B1515120043), Guangdong Basic and Applied Basic Research Fund Project (No. 2021A1515111027), and K. C. Wong Education Foundation.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.04.010.
Contributor Information
Qinghua Liu, Email: liuqhua6@mail.sysu.edu.cn.
Tianfeng Chen, Email: tchentf@jnu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023, CA cancer. J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Giaquinto A.N., Sung H., Miller K.D., Kramer J.L., Newman L.A., Minihan A., Jemal A., Siegel R.L. Breast cancer statistics, 2022. CA A Cancer J. Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 3.Marsolier J., Prompsy P., Durand A., Lyne A.-M., Landragin C., Trouchet A., Bento S.T., Eisele A., Foulon S., Baudre L. H3K27me3 conditions chemotolerance in triple-negative breast cancer. Nat. Genet. 2022;54(4):459–468. doi: 10.1038/s41588-022-01047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Feng Z., Liu J., Li H., Su Q., Zhang J., Huang P., Wang W., Liu J. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact. Mater. 2022;16:359–371. doi: 10.1016/j.bioactmat.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., Vonderheide R.H., Pittet M.J., Jain R.K., Zou W., Howcroft T.K., Woodhouse E.C., Weinberg R.A., Krummel M.F. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Chang Y., Luo H., Jiang W., Xu L., Chen T., Zhu X. Designing immunogenic nanotherapeutics for photothermal-triggered immunotherapy involving reprogramming immunosuppression and activating systemic antitumor responses. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120153. [DOI] [PubMed] [Google Scholar]
- 8.Chao Y., Liu Z. Biomaterials tools to modulate the tumour microenvironment in immunotherapy. Nat. Rev. Bioeng. 2023;1:125–138. [Google Scholar]
- 9.Wang T., Zhang H., Qiu W., Han Y., Liu H., Li Z. Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy. Bioact. Mater. 2022;16:418–432. doi: 10.1016/j.bioactmat.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 2021;6(7):1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Z., Liu H., Xue Y., Lin J., Fu Y., Xia Z., Pan D., Zhang J., Qiao K., Zhang Z., Liao Y. Reversing cold tumors to hot: an immunoadjuvant-functionalized metal-organic framework for multimodal imaging-guided synergistic photo-immunotherapy. Bioact. Mater. 2021;6(2):312–325. doi: 10.1016/j.bioactmat.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Q., Wang L., Ren E., Chen H., Gao X., Cheng H., An Y., Chu C., Liu G. Ruthenium‐based metal–organic nanoradiosensitizers enhance radiotherapy by combining ROS generation and CO gas release. Angew. Chem., Int. Ed. 2022 doi: 10.1002/anie.202211674. [DOI] [PubMed] [Google Scholar]
- 13.Chen H.H., Kuo M.T. Improving radiotherapy in cancer treatment: promises and challenges. Oncotarget. 2017;8(37) doi: 10.18632/oncotarget.18409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W., Yan J., Tian H., Li B., Wang G., Sang W., Zhang Z., Zhang X., Dai Y. A platinum@polymer-catechol nanobraker enables radio-immunotherapy for crippling melanoma tumorigenesis, angiogenesis, and radioresistance. Bioact. Mater. 2023;22:34–46. doi: 10.1016/j.bioactmat.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Tang J., Li C., Lu Y., Cheng L., Liu J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021;6(2):472–489. doi: 10.1016/j.bioactmat.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Z., Liu T., Lai H., Meng X., Yang L., Su J., Chen T. A universally EDTA-assisted synthesis of polytypic bismuth telluride nanoplates with a size-dependent enhancement of tumor radiosensitivity and metabolism in vivo. ACS Nano. 2022;16(3):4379–4396. doi: 10.1021/acsnano.1c10663. [DOI] [PubMed] [Google Scholar]
- 17.Chan L., Gao P., Zhou W., Mei C., Huang Y., Yu X.F., Chu P.K., Chen T. Sequentially triggered delivery system of black phosphorus quantum dots with surface charge-switching ability for precise tumor radiosensitization. ACS Nano. 2018;12(12):12401–12415. doi: 10.1021/acsnano.8b06483. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W., Zhang Z., Ye M., Pan S., Huang G., Chen T., Zhu X. Morphology-directed radiosensitization of MoSe2 nanoplatforms for promoting cervical cancer radiotherapy. Nano Today. 2022;46 [Google Scholar]
- 19.Chao Y., Xu L., Liang C., Feng L., Xu J., Dong Z., Tian L., Yi X., Yang K., Liu Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2018;2(8):611–621. doi: 10.1038/s41551-018-0262-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q., Chen J., Yang Z., Xu J., Xu L., Liang C., Han X., Liu Z. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019;31(10) doi: 10.1002/adma.201802228. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Wang M., Huang B., Zhu S.W., Zhou J.J., Chen D.R., Cui R., Zhang M., Sun Z.J. Theranostic near-infrared-IIb emitting nanoprobes for promoting immunogenic radiotherapy and abscopal effects against cancer metastasis. Nat. Commun. 2021;12(1):7149. doi: 10.1038/s41467-021-27485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard F.M., Pearson A.T., Nanda R. Clinical trials of immunotherapy in triple-negative breast cancer. Breast Cancer Res. Treat. 2022;195(1):1–15. doi: 10.1007/s10549-022-06665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L., Zou C., Zhang S., Chu T.S.M., Zhang Y., Chen W., Zhao C., Yang L., Xu Z., Dong S., Yu H., Li B., Guan X., Hou Y., Kong F.M. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J. Hematol. Oncol. 2022;15(1):87. doi: 10.1186/s13045-022-01307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu X., Zhou D., Lu J., Qin D., Zhou J., Liu H.J. Cancer nanomedicine in preoperative therapeutics: nanotechnology-enabled neoadjuvant chemotherapy, radiotherapy, immunotherapy, and phototherapy. Bioact. Mater. 2023;24:136–152. doi: 10.1016/j.bioactmat.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbet C., Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat. Rev. Cancer. 2017;17(10):577–593. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 26.Ling J., Chang Y., Yuan Z., Chen Q., He L., Chen T. Designing lactate dehydrogenase-mimicking SnSe nanosheets to reprogram tumor-associated macrophages for potentiation of photothermal immunotherapy. ACS Appl. Mater. Interfaces. 2022;14(24):27651–27665. doi: 10.1021/acsami.2c05533. [DOI] [PubMed] [Google Scholar]
- 27.Khalaf K., Hana D., Chou J.T.-T., Singh C., Mackiewicz A., Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Ye T., Ye C., Wan C., Yuan S., Liu Y., Li T., Jiang F., Lovell J.F., Jin H., Chen J. Secretions from hypochlorous acid-treated tumor cells delivered in a melittin hydrogel potentiate cancer immunotherapy. Bioact. Mater. 2022;9:541–553. doi: 10.1016/j.bioactmat.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D., Wu Y., Shim G., Oh Y.K. Genome-editing-mediated restructuring of tumor immune microenvironment for prevention of metastasis. ACS Nano. 2021;15(11):17635–17656. doi: 10.1021/acsnano.1c05420. [DOI] [PubMed] [Google Scholar]
- 30.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angelin A., Gil-de-Gómez L., Dahiya S., Jiao J., Guo L., Levine M.H., Wang Z., Quinn W.J., Kopinski P.K., Wang L. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metabol. 2017;25(6):1282–1293. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Jia Y., Yang J., Zhang L., Hou S., Niu X., Zhu J., Huang Y., Sun X., Xu Z.P., Liu R. Efficient immunotherapy of drug-free layered double hydroxide nanoparticles via neutralizing excess acid and blocking tumor cell autophagy. ACS Nano. 2022;16(8):12036–12048. doi: 10.1021/acsnano.2c02183. [DOI] [PubMed] [Google Scholar]
- 33.Xie J., Gong L., Zhu S., Yong Y., Gu Z., Zhao Y. Emerging strategies of nanomaterial‐mediated tumor radiosensitization. Adv. Mater. 2019;31(3) doi: 10.1002/adma.201802244. [DOI] [PubMed] [Google Scholar]
- 34.Shi Z., Cao R., Khan K., Tareen A.K., Liu X., Liang W., Zhang Y., Ma C., Guo Z., Luo X., Zhang H. Two-dimensional tellurium: progress, challenges, and prospects. Nano-Micro Lett. 2020;12(1):99. doi: 10.1007/s40820-020-00427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Y., Li T., Zhang Z., Tan Y., Pan S., Zhang L., Xu H. Oxidative polymerization in living cells. J. Am. Chem. Soc. 2021;143(28):10709–10717. doi: 10.1021/jacs.1c04821. [DOI] [PubMed] [Google Scholar]
- 36.Chen M., Cao W., Wang J., Cai F., Zhu L., Ma L., Chen T. Selenium atom-polarization effect determines TrxR-specific recognition of metallodrugs. J. Am. Chem. Soc. 2022;144(45):20825–20833. doi: 10.1021/jacs.2c08802. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y., Guo T., Qiu Y., Lin Y., Yao Y., Lian W., Lin L., Song J., Yang H. An inorganic prodrug, tellurium nanowires with enhanced ROS generation and GSH depletion for selective cancer therapy. Chem. Sci. 2019;10(29):7068–7075. doi: 10.1039/c9sc01070j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang T., Ke H., Wang Q., Tang Y., Deng Y., Yang H., Yang X., Yang P., Ling D., Chen C., Zhao Y., Wu H., Chen H. Bifunctional tellurium nanodots for photo-induced synergistic cancer therapy. ACS Nano. 2017;11(10):10012–10024. doi: 10.1021/acsnano.7b04230. [DOI] [PubMed] [Google Scholar]
- 39.Huang W., Wu H., Li X., Chen T. Facile one-pot synthesis of tellurium nanorods as antioxidant and anticancer agents. Chem. Asian J. 2016;11(16):2301–2311. doi: 10.1002/asia.201600757. [DOI] [PubMed] [Google Scholar]
- 40.Zhao F., Huang W., He L., Nie S., Sun Z., Chen T., Yin H., Zhao J. Reversing lung cancer radioresistance by hyperpermeable tellurium nanotherapeutics via remodeling tumor microenvironment. Nano Today. 2023;50 [Google Scholar]
- 41.Zhang H., Pan X., Wu Q., Guo J., Wang C., Liu H. Manganese carbonate nanoparticles‐mediated mitochondrial dysfunction for enhanced sonodynamic therapy. Explorations. 2021;1(2):1–12. doi: 10.1002/EXP.20210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y., Zhang S., Kang N., Huang J., Lv X., Wen K., Ye S., Chen Z., Zhou X., Ren L. Polydopamine-coated manganese carbonate nanoparticles for amplified magnetic resonance imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces. 2017;9(22):19296–19306. doi: 10.1021/acsami.7b03087. [DOI] [PubMed] [Google Scholar]
- 43.Wang P., Liang C., Zhu J., Yang N., Jiao A., Wang W., Song X., Dong X. Manganese-based nanoplatform as metal ion-enhanced ROS generator for combined chemodynamic/photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11(44):41140–41147. doi: 10.1021/acsami.9b16617. [DOI] [PubMed] [Google Scholar]
- 44.Bao P., Zheng Z.T., Ye J.J., Zhang X.Z. Apoptotic body-mediated intracellular delivery strategy for enhanced STING activation and improved tumor immunogenicity. Nano Lett. 2022;22(6):2217–2227. doi: 10.1021/acs.nanolett.1c03996. [DOI] [PubMed] [Google Scholar]
- 45.Wang C., Guan Y., Lv M., Zhang R., Guo Z., Wei X., Du X., Yang J., Li T., Wan Y., Su X., Huang X., Jiang Z. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48(4):675–687 e677. doi: 10.1016/j.immuni.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Tian L., Yi X., Dong Z., Xu J., Liang C., Chao Y., Wang Y., Yang K., Liu Z. Calcium bisphosphonate nanoparticles with chelator-free radiolabeling to deplete tumor-associated macrophages for enhanced cancer radioisotope therapy. ACS Nano. 2018;12(11):11541–11551. doi: 10.1021/acsnano.8b06699. [DOI] [PubMed] [Google Scholar]
- 47.Tumor microenvironment-activatable Fe-doxorubicin preloaded amorphous CaCO3 nanoformulation triggers ferroptosis in target tumor cells. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aax1346. eaax1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q., Wang C., Zhang X., Chen G., Hu Q., Li H., Wang J., Wen D., Zhang Y., Lu Y., Yang G., Jiang C., Wang J., Dotti G., Gu Z. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019;14(1):89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 49.Cen D., Ge Q., Xie C., Zheng Q., Guo J., Zhang Y., Wang Y., Li X., Gu Z., Cai X. ZnS@BSA nanoclusters potentiate efficacy of cancer immunotherapy. Adv. Mater. 2021;33(49) doi: 10.1002/adma.202104037. [DOI] [PubMed] [Google Scholar]
- 50.Pan S., Huang G., Sun Z., Chen X., Xiang X., Jiang W., Xu Y., Chen T., Zhu X. X‐Ray‐Responsive zeolitic imidazolate framework‐capped nanotherapeutics for cervical cancer‐targeting radiosensitization. Adv. Funct. Mater. 2023 [Google Scholar]
- 51.Xiao J., Zhang G., Xu R., Chen H., Wang H., Tian G., Wang B., Yang C., Bai G., Zhang Z., Yang H., Zhong K., Zou D., Wu Z. A pH-responsive platform combining chemodynamic therapy with limotherapy for simultaneous bioimaging and synergistic cancer therapy. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119254. [DOI] [PubMed] [Google Scholar]
- 52.Yuan Z., Liu X., Ling J., Huang G., Huang J., Zhu X., He L., Chen T. In situ-transition nanozyme triggered by tumor microenvironment boosts synergistic cancer radio-/chemotherapy through disrupting redox homeostasis. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121620. [DOI] [PubMed] [Google Scholar]
- 53.Zou B., Xiong Z., He L., Chen T. Reversing breast cancer bone metastasis by metal organic framework-capped nanotherapeutics via suppressing osteoclastogenesis. Biomaterials. 2022;285 doi: 10.1016/j.biomaterials.2022.121549. [DOI] [PubMed] [Google Scholar]
- 54.Qi C., He J., Fu L.H., He T., Blum N.T., Yao X., Lin J., Huang P. Tumor-specific activatable nanocarriers with gas-generation and signal amplification capabilities for tumor theranostics. ACS Nano. 2021;15(1):1627–1639. doi: 10.1021/acsnano.0c09223. [DOI] [PubMed] [Google Scholar]
- 55.Ba L.A., Doring M., Jamier V., Jacob C. Tellurium: an element with great biological potency and potential. Org. Biomol. Chem. 2010;8(19):4203–4216. doi: 10.1039/c0Ob00086h. [DOI] [PubMed] [Google Scholar]
- 56.Li W., Wang Y., Xue D., Jin L., Liu Y., Lv Z., Cao Y., Niu R., Zhang H., Zhang S., Xu B., Yin N., Zhang S., Zhang H. A novel biodegradable nanoplatform for tumor microenvironments responsive bimodal magnetic resonance imaging and sonodynamic/ion interference cascade therapy. ACS Appl. Mater. Interfaces. 2022;14(45):50616–50625. doi: 10.1021/acsami.2c15806. [DOI] [PubMed] [Google Scholar]
- 57.Min K.H., Min H.S., Lee H.J., Park D.J., Yhee J.Y., Kim K., Kwon I.C., Jeong S.Y., Silvestre O.F., Chen X., Hwang Y.S., Kim E.C., Lee S.C. pH-controlled gas-generating mineralized nanoparticles: a theranostic agent for ultrasound imaging and therapy of cancers. ACS Nano. 2015;9(1):134–145. doi: 10.1021/nn506210a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong X., Cheng R., Zhu S., Liu H., Zhou R., Zhang C., Chen K., Mei L., Wang C., Su C. A heterojunction structured WO2. 9-WSe2 nanoradiosensitizer increases local tumor ablation and checkpoint blockade immunotherapy upon low radiation dose. ACS Nano. 2020;14(5):5400–5416. doi: 10.1021/acsnano.9b08962. [DOI] [PubMed] [Google Scholar]
- 59.Yang Xue, Yang Ying, Bian Jiayi, Wei Jiajia, Wang Zheng, Zhou Zhanwei, Li Zhaoting, Sun M. Converting primary tumor towards an in situ STING activating vaccine via a biomimetic nanoplatform against recurrent and metastatic tumors. Nano Today. 2021 [Google Scholar]
- 60.Hou L., Tian C., Yan Y., Zhang L., Zhang H., Zhang Z. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano. 2020;14(4):3927–3940. doi: 10.1021/acsnano.9b06111. [DOI] [PubMed] [Google Scholar]
- 61.Phuengkham H., Ren L., Shin I.W., Lim Y.T. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv. Mater. 2019;31(34) doi: 10.1002/adma.201803322. [DOI] [PubMed] [Google Scholar]
- 62.Song C., Phuengkham H., Kim Y.S., Dinh V.V., Lee I., Shin I.W., Shin H.S., Jin S.M., Um S.H., Lee H. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat. Commun. 2019;10(1):3745. doi: 10.1038/s41467-019-11730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medeiros B., Allan A.L. Molecular mechanisms of breast cancer metastasis to the lung: clinical and experimental perspectives. Int. J. Mol. Sci. 2019;20(9):2272. doi: 10.3390/ijms20092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao Y., Cong M., Li J., He D., Wu Q., Tian P., Wang Y., Yang S., Liang C., Liang Y. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–437. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 65.He L., Nie T., Xia X., Liu T., Huang Y., Wang X., Chen T. Designing bioinspired 2D MoSe2 nanosheet for efficient photothermal‐triggered cancer immunotherapy with reprogramming tumor‐associated macrophages. Adv. Funct. Mater. 2019;29(30) [Google Scholar]
- 66.Yang G., Xu L., Chao Y., Xu J., Sun X., Wu Y., Peng R., Liu Z. Hollow MnO(2) as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017;8(1):902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang C., Xu L., Song G., Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem. Soc. Rev. 2016;45(22):6250–6269. doi: 10.1039/c6cs00458j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.