Abstract
Objectives:
Determine associations of hearing loss (HL) and hearing aid (HA) use with cognition, health-related quality of life (HRQoL), and depressive symptoms.
Methods:
Participants were from the Epidemiology of Hearing Loss Study or Beaver Dam Offspring Study. HL was defined as pure-tone average (0.5–4.0 kHz) >25 dB. A principal component analysis of 5 cognitive tasks measured cognition. The SF-12 measured mental and physical HRQoL. The Centers for Epidemiological Studies Depression Scale measured depressive symptoms (score ≥16). Regression models returned beta (B) coefficients or odds ratios (OR) with 95% confidence intervals.
Results:
This study included 3574 participants. HL (vs none) was associated with poorer cognition (B −0.12 [−0.18, −0.06]), mental (B −0.99 [−1.65, −0.33]) and physical (B −0.76 [−1.50, −0.03]) HRQoL and increased odds of depressive symptoms (OR 1.49 [1.16, 1.91]). HA users had better cognition than non-users.
Discussion:
HL likely impacts cognition and well-being. HA use may have cognitive benefits.
Keywords: epidemiology, rehabilitation, cognition, sensory / sensorimotor processes
INTRODUCTION
Hearing loss is a highly prevalent chronic condition in middle-aged to older adults and is an important public health concern (Cruickshanks et al., 1998; Nash et al., 2011). Hearing loss has been associated with poorer cognitive function, a worsened health-related quality of life, and a higher risk of depressive symptoms as compared to individuals without hearing loss (Dalton et al., 2003; Gopinath et al., 2003; Schubert et al., 2017, 2019). Although hearing loss often begins around middle age and continues to progress with age, epidemiological studies on hearing loss impact have generally focused only on older adults, thus limiting generalizability of study findings to the desired target population (Chia et al., 2007; Dalton et al., 2003; Gopinath et al., 2009; Nash et al., 2011). Furthermore, few studies have thoroughly investigated the extent to which the potential impacts of hearing loss are modified by the severity of the hearing loss and/or its treatment with hearing aids (Chia et al., 2007; Dalton et al., 2003; Shukla et al., 2021). These data could have valuable implications for clinical practice and policy.
Although hearing aids are the most common form of treatment for hearing loss, epidemiological evidence of their benefit is inconsistent. In part, this may be due to the low prevalence and incidence of hearing aid use in the general population (Dillard et al., 2021; Popelka et al., 1998; Weycker et al., 2021). Among aging individuals eligible for hearing aids, population-based prevalence and 10-year incidence of hearing aid use have been estimated at 15% and 36%, respectively (Popelka et al., 1998; Fischer et al., 2011). The low prevalence of hearing aid use in the general population may limit the statistical power to comprehensively evaluate associations of interest. Studies have consistently indicated that hearing aid use improves hearing handicap and hearing-related quality of life (Ferguson et al., 2019; Mulrow et al., 1990; Tesch-Römer, 1997). However, there is sparse and inconsistent evidence that hearing aid use reduces other burdens of hearing loss, including on outcomes related to cognition, general health-related quality of life, and/or depressive symptoms (Acar et al., 2011; Appollonio et al., 1996; Dalton et al., 2003; Dawes et al., 2015; Humes et al., 2003; Lawrence et al., 2020; Mener et al., 2013; Schubert et al., 2017, 2019; West, 2017). Research that evaluates the potential benefits of hearing aid use is needed given the United States Preventative Services Task Force’s recent conclusion that there is inadequate evidence showing hearing aids as an effective treatment for age-related hearing loss (USPSTS, 2019).
To advance hearing-related public health initiatives, it is necessary to understand hearing loss burden and potential benefits of hearing aids in individuals across the life span. The purpose of this study was to determine associations of hearing loss, its severity, and hearing aid use, with cognitive function, mental and physical health-related quality of life, and depressive symptoms in a sample of middle-aged to older adults in the general population.
MATERIALS & METHODS
Participants were part of the Epidemiology of Hearing Loss Study (EHLS; 1993–2020) or the Beaver Dam Offspring Study (BOSS; 2005-current) which are prospective longitudinal cohort studies of aging and sensory disorders. Participants of the Beaver Dam Eye Study (1987–1988) were eligible to participate in the EHLS (Linton et al., 1991; Klein et al., 1996). The population-based baseline of the EHLS examination (n=3753; age range 48–92 years) was conducted in 1993–1995. EHLS follow-up examinations occurred every 5 years with >80% response rates. The adult offspring of EHLS participants were invited to enroll in BOSS. The baseline examination of BOSS (n=3,296; age range 21–84 years) was conducted in 2005–2008. BOSS follow-up examinations occurred every 5 years with >80% response rates. Details of the EHLS and BOSS, including those related to recruitment and retention, are detailed in previous publications (Cruickshanks et al., 1998, 2003, 2010; Nash et al., 2011).
This study included cross-sectional data from the fourth cycle of EHLS (2009–10; n=1669 with examination) and the third cycle of BOSS (2015–17; n=1964 with examination), both of which had high response rates among eligible individuals (approximately 81% and 86%, respectively). Seventy-five individuals participated in both studies and the cycle with participants’ most complete data was utilized. Across both cohorts, 59 participants (EHLS, n=51; BOSS, n=8) did not have audiometric data so were not included in this study. Previous studies have pooled samples from EHLS and BOSS cohorts (Fischer et al., 2017; Paulsen et al., 2021).
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The University of Wisconsin Health Sciences Institutional Review Board approved these studies and written consent was obtained from participants before each examination.
Measurements
Participants underwent a series of standardized examinations and interviews conducted by examiners trained and certified in all study protocols. All testing was conducted in English and all participants spoke English. Nearly identical standardized data collection and quality assurance methods were used in EHLS and BOSS.
Hearing and hearing aid-related data
Audiometric testing was performed in accordance with American Speech-Language-Hearing Association guidelines (2005) and in compliance with the American National Standards Institute standards (1999, 2010). Testing was performed in sound-treated booths with clinical audiometers (calibrated every 6 months) with use of TDH-50P earphones or ER-3A insert earphones (in cases of probable ear canal collapse). Pure-tone air-conduction thresholds were obtained in both ears at 0.5, 1, 2, 3, 4, 6, and 8 kHz, and bone-conduction thresholds were obtained at 0.5, 2, and 4 kHz. Masking was used as necessary (Cruickshanks et al., 1998; 2003). A four-frequency pure tone average (PTA) was calculated in each ear from 0.5, 1, 2, 4 kHz. PTA in the better ear defined presence (PTA > 25 dB HL) and severity of hearing loss (mild: PTA >25–40 dB HL; moderate or worse: PTA ≥40 dB HL). The category of moderate or worse hearing loss severity included participants with moderate (PTA ≥40–55 dB HL), moderately severe (PTA ≥55–70 dB HL), severe (PTA ≥70–90 dB HL), and profound (PTA ≥90 dB HL) hearing loss to ensure an adequate sample size for analyses stratified by hearing loss severity (mild, moderate or worse). A medical history questionnaire including questions about hearing aid use was administered. Hearing aid use was defined as answering ‘yes’ to the question “Do you use your hearing aid now?”.
Cognitive function, health-related quality of life and depressive symptoms
Cognitive tests included the auditory verbal learning test (AVLT), digit symbol substitution test (DSST), trail making test (TMT) parts A and B, and the verbal fluency test (VFT) (Reitan, 1992; Strauss et al., 2006). All test scores were standardized (mean 0, standard deviation [SD] 1) and TMT A and B scores were reversed so lower scores represented worse function. Cognitive function was defined using a principal component analysis (PCA) to construct a composite measure, with a mean of 0 and SD of 1. One eigenvalue was >1 and the first component was retained. The proportion of variance explained by the first component was 62.5%. The PCA score was calculated as a linear combination of the standardized observed variables, using factor loadings from the study cycles included. A higher PCA score indicated better cognitive function. Additional cognitive methods are in supplementary text S1.
Health-related quality of life was measured with the SF-12 short form survey (Ware et al., 1996). The SF-12 physical component score (PCS) and mental component score (MCS) measured self-perceived quality of physical and mental health, respectively (range 0–100). Depressive symptoms were defined as a score ≥16 on the Centers for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977).
Covariates
Covariates were chosen based on the existing literature that highlighted the associations of interest. Demographic factors were age, sex, and education. Education was categorized as high school or less (0–12 years), some college (13–15 years) or college graduate or beyond (16+ years). Education and income were significantly correlated in this sample. Education was included in final models (described below) to increase sample size, as there were substantially more missing data for income. Participants reported their marital status (married, single, divorced, widowed, other).
Participants reported exercise (at least once a week, long enough to sweat), smoking status (past/current or never), and history of heavy alcohol consumption (ever drinking 4+ drinks per day). Positive history of head injury was defined as self-report of concussion, broken nose, skull fracture, or loss of consciousness due to head injury (Schubert et al., 2019). Body mass index (BMI) was calculated as measured weight in kilograms (kg) divided by measured height in meters (m) squared. History of cardiovascular disease was defined as self-report of a physician diagnosed stroke, angina, or myocardial infarction (Cruickshanks et al., 2003; Zhan et al., 2011; Fischer et al., 2019). Contrast sensitivity was measured in each eye separately using Pelli-Robson letter charts. Visual impairment was defined as contrast sensitivity <1.55 log units in the better eye (Paulsen et al., 2018; Pelli et al., 1988). Number of chronic diseases was defined as the sum of the following self-reported physician-diagnosed conditions: cancer (excluding skin cancer), epilepsy, emphysema, arthritis, asthma, osteoporosis, lupus, multiple sclerosis, kidney disease, Crohn’s disease or Parkinson’s disease. Number of chronic disease(s) was categorized as 0, 1, 2, 3, 4+. Current and past noise exposure was defined as self-report of military noise exposure, driving a tractor on a farm, or ever having a full-time noisy job during which it was necessary to speak in a raised voice or louder to be heard by someone two feet away, at the study cycle or in the past, respectively (Cruickshanks et al., 2010).
Hemoglobin A1C (HbA1C) levels were measured using the Tosoh HPLC G7 Glycohemoglobin Analyzer (Tosoh Medics, Inc., San Francisco CA). Diabetes was defined as self-report of physician diagnosis or HbAIC ≥6.5 (Nuttall, 1988; Cruickshanks et al., 2015). High resolution B-mode carotid artery ultrasound images (MyLab 25, Esaote North America, Inc., Indianapolis, IN) evaluated carotid artery plaque in the common carotid, bifurcation and internal carotid arteries on right and left sides (Fischer et al., 2015; Zhong et al., 2011, 2012). The number of sites with plaque were categorized as 0, 1–3 or 4–6.
Statistical methods
All statistical analyses were completed using SAS version 9.4 software (SAS Institute, Inc., Cary, NC). Hearing status was analyzed as an indicator variable categorizing participants into three categories, a) normal hearing: PTA ≤25 dB HL, b) hearing aid “non-users:” PTA >25 dB HL with no hearing aid use, c) “hearing aid users:” PTA >25 dB HL with hearing aid use. Hearing loss was defined as PTA >25 dB HL (b + c). The normal hearing group (a) was used as the referent group for all analyses. Estimated effects of hearing status (vs normal hearing) were evaluated after stratification by hearing loss severity (mild, moderate or worse). Hearing loss severity was treated as an indicator variable (referent = normal hearing). Differences in estimated effect sizes in hearing aid users versus non-users were tested and are presented as p-values.
General linear regression models evaluated associations between hearing status and cognitive function, each individual cognitive task (AVLT, DSST, TMT A & B, VFT), and SF-12 MCS and PCS. Results are presented as beta (B) coefficients with corresponding 95% confidence intervals (95% CI). Logistic regression models evaluated associations between hearing status and depressive symptoms and results are presented as odds ratios (OR) with corresponding 95% CI.
The following covariates (chosen based on existing literature) were evaluated for confounding for each model, separately, by evaluating associations with hearing loss, hearing aid use, and the outcomes: BMI, education, marital status, smoking, history of heavy alcohol consumption, diabetes, exercise, history of head injury, history of cardiovascular disease, visual impairment, number of sites with plaque, and number of chronic diseases. The model selection procedure is as follows. First, separate base models were built that included age, sex, hearing status, and each covariate, separately. Next, covariates with a p-value <0.05 were included in multivariable models. Final multivariable models included only covariates significant at p<0.05 after full adjustment. Results from multivariable-adjusted models are presented.
RESULTS
This study included 3574 participants (56.1% female) with a mean age of 66.2 (SD 12.1; range 27–89+) years. Of these participants, 1618 were EHLS participants and 1956 were BOSS participants. Of the 1163 participants with hearing loss, 320 (27.5%) used hearing aids and 843 (72.5%) did not. In the hearing aid user group, 88 (27.5%) and 232 (72.5%) participants had mild and moderate or worse hearing loss, respectively, and had an overall mean PTA of 49.0 (SD 13.4) dB HL. In the non-user group, 632 (75.0%) and 211 (25.0%) participants had mild and moderate or worse hearing loss, respectively, and had an overall mean PTA of 35.7 (SD 8.5) dB HL. Study sample characteristics are in Table 1.
Table 1.
Study sample characteristics (n=3574 participants).
| Characteristic | Mean or n | SD or % |
|---|---|---|
| Age | 66.2 | 12.1 |
| Female Sex | 2005 | 56.1% |
| Hearing Status | ||
| Hearing loss (hearing aid users + non-users)a | 1163 | 32.5% |
| Hearing aid users | 320 | 9.0% |
| Non-users | 843 | 23.6% |
| Education | ||
| 0–12 years | 1552 | 43.6% |
| 13–15 years | 966 | 27.2% |
| 16+ | 1039 | 29.2% |
| Marital Status | ||
| Married | 2362 | 66.1% |
| Single (never married) | 291 | 8.2% |
| Divorced/Widowed/Other | 919 | 25.7% |
| Smoking Status | ||
| Never smoker | 1823 | 51.3% |
| Past or Current Smoker | 1731 | 48.7% |
| Body mass index (kg/m2) | 30.9 | 6.5 |
| Diabetes | 615 | 17.3% |
| Exercise (at least 1/wk) | 1979 | 55.9% |
| History of heavy alcohol | 776 | 22.2% |
| History of head injury | 1281 | 36.0% |
| History of cardiovascular disease | 737 | 20.9% |
| History of noise exposure | 1908 | 54.1% |
| Visual impairment | 721 | 21.7% |
| Number of sites with plaque (atherosclerosis) | ||
| 0 | 1289 | 38.3% |
| 1–3 | 1633 | 48.5% |
| 4–6 | 443 | 13.2% |
| Number of chronic diseases | ||
| 0 | 607 | 17.8% |
| 1 | 948 | 27.8% |
| 2 | 909 | 26.6% |
| 3 | 527 | 15.4% |
| 4+ | 424 | 12.5% |
Note. SD = standard deviation.
Pure-tone average (PTA; 0.5, 1.0, 2.0, 4.0 kHz) >25 dB HL, better ear.
Cognitive Function
Table 2 shows associations of hearing status (vs normal hearing) with cognitive function. Hearing loss (vs normal hearing) was associated with worse cognitive function (B: −0.12 [95% CI: −0.18, −0.06]). Negative associations of hearing loss with cognitive function were observed in both non-user (B: −0.13 [95% CI: −0.19, −0.06]) and hearing aid user (B: −0.10 [95% CI: −0.19, −0.004]) groups, which did not have significantly different estimated effect sizes (p=0.51).
Table 2.
Multivariable-adjusted associations of hearing status (referent=normal hearing) and cognitive function (measured by principal component analysis), overall and stratified by hearing loss severity. Results are presented as standardized linear regression coefficients and 95% confidence intervals.
| Cognitive Function | ||||||
|---|---|---|---|---|---|---|
| Hearing loss (mild + moderate+) n=1163 | Mild hearing loss n=720 | Moderate or worse hearing loss n=443 | ||||
| B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | |
| Normal hearing n=2411 | REFERENT | |||||
| Hearing loss (hearing aid users + non-users) n=1163 | −0.12 | [−0.18, −0.06] | −0.07 | [−0.13, −0.01] | −0.28 | [−0.37, −0.20] |
| Hearing aid users n=320 | −0.10 | [−0.19, −0.004] | 0.07 | [−0.08, 0.22] | −0.21 | [−0.32, −0.10] |
| Non-users n=843 | −0.13 | [−0.19, −0.06] | −0.09 | [−0.16, −0.02] | −0.39 | [−0.51, −0.26] |
Note. Adjusted for age, sex, education, marital status, visual impairment, n of sites with plaque (atherosclerosis) and diabetes. Cell sizes may vary slightly due to missing data. CI = confidence interval; LL = lower limit; UL = upper limit.
The presence and strength of associations varied by hearing loss severity (Figure 1). Mild hearing loss (vs normal hearing) was associated with poorer cognitive function (B: −0.07 [95% CI: −0.13, −0.01]). A statistically significant negative association between mild hearing loss and cognitive function was observed in non-users (B: −0.09 [95% CI: −0.16, −0.02]) but not in hearing aid users (B: 0.07 [95% CI: −0.08, 0.22]). The non-users showed significantly worse cognitive function than hearing aid users (p=0.04).
Figure 1.
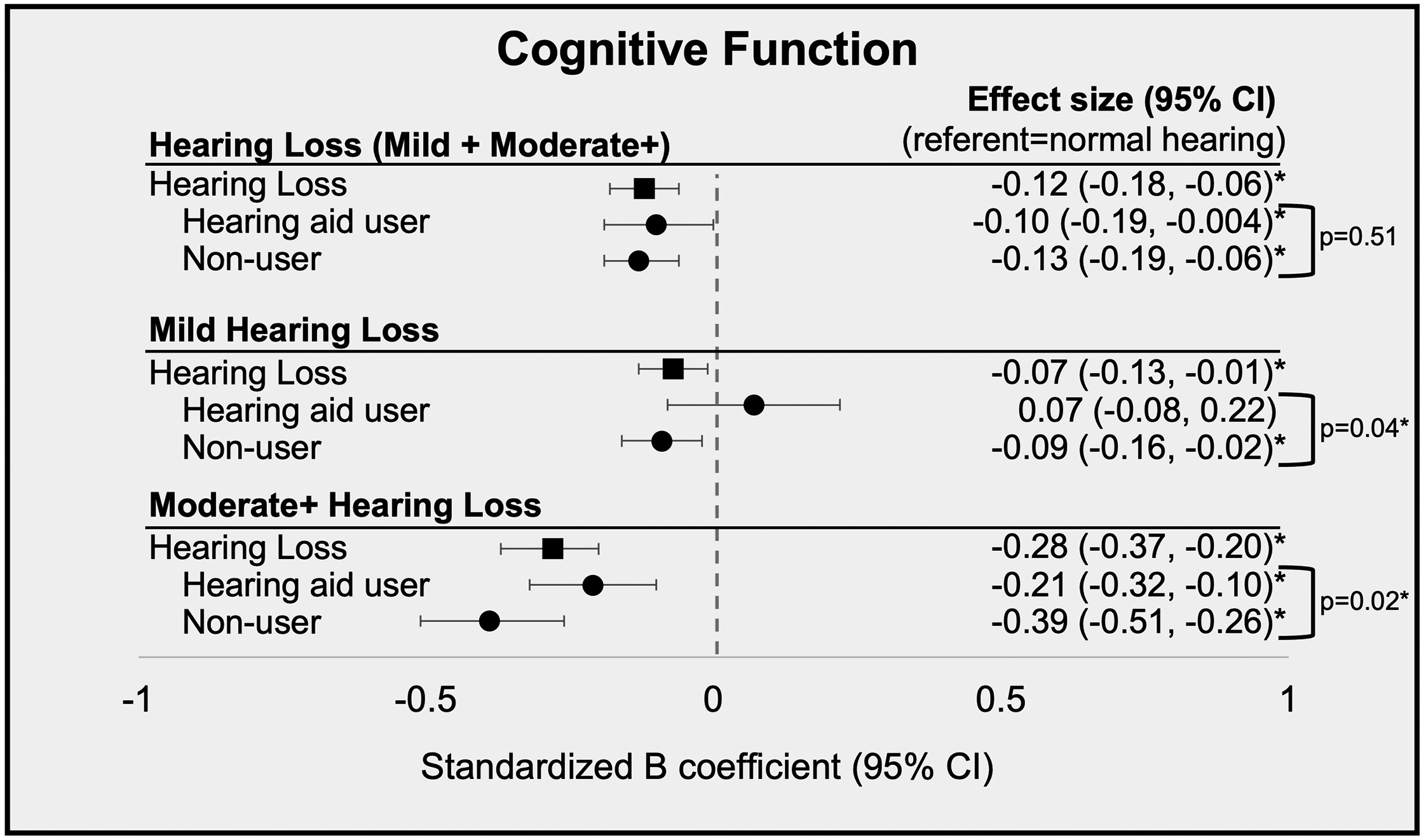
Standardized B coefficients and 95% confidence intervals (95% CI) of multivariate-adjusted associations of hearing status and cognitive function. Models are adjusted for age, sex, education, marital status, visual impairment, n of sites with plaque and diabetes.
Moderate or worse hearing loss was also associated with poorer cognitive function (vs normal hearing; B: −0.28 [95% CI: −0.37, −0.20]), and the estimated effect size was approximately 4 times larger than that for mild hearing loss (Figure 1; Table 2). A negative association of moderate or worse hearing loss with cognitive function was observed in the non-users (B:−0.39 [95% CI: −0.51, −0.26]) and hearing aid users (B: −0.21 [95% CI: −0.32, −0.10]) groups and the non-users showed significantly worse cognitive function than hearing aid users (p=0.02). Negative associations for non-users were stronger in participants with moderate or worse hearing loss as compared to those with mild hearing loss.
Table 3 shows associations of hearing status with each individual cognitive test that comprised the PCA. Hearing loss (vs normal hearing) was associated with poorer performance on the VFT (B: −0.11 [95% CI: −0.19, −0.03]), TMT A (B: −0.13 [95% CI: −0.18, −0.07]), TMT B (B: −0.14 [95% CI: −0.21, −0.07]), and DSST (B: −0.10 [95% CI: −0.16, −0.04]), but not the AVLT (B: −0.04 [95% CI: −0.11, 0.04]). For the VFT, TMT B, and DSST, negative associations with hearing loss (vs normal hearing) were observed in non-users but not in hearing aid users. On the TMT A, both non-users and hearing aid users performed significantly worse than normal hearing individuals. Estimated effect sizes were not significantly different between hearing aid users versus non-users for each cognitive test.
Table 3.
Multivariable-adjusted associations of hearing status (referent=normal hearing) with performance on each cognitive test. Results are presented as standardized linear regression coefficients and 95% confidence intervals.
| Cognitive Test Performance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Auditory Verbal Learning Test (AVLT) | Verbal Fluency Test (VFT) | Trail Making Test A (TMT A)a | Trail Making Test B (TMT B)a | Digit Symbol Substitution Test (DSST) | ||||||
| B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | |
| Normal hearing n=2411 | REFERENT | |||||||||
| Hearing loss (hearing aid users + non-users) n=1163 | −0.04 | [−0.11, 0.04] | −0.11 | [−0.19, −0.03] | −0.13 | [−0.18, −0.07] | −0.14 | [−0.21, −0.07] | −0.10 | [−0.16, −0.04 |
| Hearing aid users n=320 | −0.03 | [−0.15, 0.08] | −0.10 | [−0.22, 0.02] | −0.12 | [−0.21, −0.03] | −0.07 | [−0.17, 0.04] | −0.07 | [−0.16, 0.03] |
| Non-users n=843 | −0.04 | [−0.12, 0.04] | −0.12 | [−0.20, −0.03] | −0.13 | [−0.19, −0.06] | −0.17 | [−0.24, −0.09] | −0.11 | [−0.18, −0.05] |
Note. Models adjusted for age, sex, education, marital status, visual impairment, n of site with plaque, and diabetes. Cell sizes may vary slightly due to missing data.
Trail making test scores were reversed so that lower scores represented worse function.
Health-Related Quality of Life & Depressive Symptoms
Associations between hearing status and self-perceived mental (MCS) and physical (PCS) health-related quality of life and depressive symptoms are in Table 4.
Table 4:
Multivariable-adjusted associations of hearing status (referent=normal hearing) with health-related quality of life (mental component score; physical component score) and depressive symptoms. Results for health-related quality of life are presented as linear regression coefficients and 95% confidence intervals. Results for depressive symptoms are presented as odds ratios (OR) and 95% confidence intervals.
| Health-Related Quality of Life: Mental Component Scorea | ||||||
|---|---|---|---|---|---|---|
| Hearing loss (mild + moderate+) n=1163 | Mild hearing loss n=720 | Moderate or worse hearing loss n=443 | ||||
| B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | |
| Normal hearing n=2411 | REFERENT | |||||
| Hearing loss (hearing aid users + non-users) n=1163 | −0.99 | [−1.65, −0.33] | −0.90 | [−1.61, −0.19] | −1.82 | [−2.78, −0.86] |
| Hearing aid users n=320 | −0.91 | [−1.93, 0.10] | −0.52 | [−2.16, 1.13] | −1.42 | [−2.59, −0.26] |
| Non-users n=843 | −1.00 | [−1.72, −0.29] | −0.96 | [−1.70, −0.21] | −2.33 | [−3.63, −1.04] |
| Health-Related Quality of Life: Physical Component Scoreb | ||||||
| Hearing loss (mild + moderate+) n=1163 | Mild hearing loss n=720 | Moderate or worse hearing loss n=443 | ||||
| B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | B | 95% CI [LL, UL] | |
| Normal hearing n=2411 | REFERENT | |||||
| Hearing loss (hearing aid users + non-users) n=1163 | −0.76 | [−1.50, −0.03] | −0.65 | [−1.45, 0.15] | −2.13 | [−3.18, −1.08] |
| Hearing aid users n=320 | −0.95 | [−2.08, 0.17] | −1.21 | [−3.04, 0.61] | −1.70 | [−3.00, −0.40] |
| Non-users n=843 | −0.69 | [−1.49, 0.11] | −0.57 | [−1.41, 0.27] | −2.65 | [−4.04, −1.25] |
| Depressive Symptomsc | ||||||
| Hearing loss (mild + moderate+) n=1163 | Mild hearing loss n=720 | Moderate or worse hearing loss n=443 | ||||
| OR | 95% CI [LL, UL] | OR | 95% CI [LL, UL] | OR | 95% CI [LL, UL] | |
| Normal hearing n=2411 | REFERENT | |||||
| Hearing loss (hearing aid users + non-users) n=1163 | 1.49 | [1.16, 1.91] | 1.39 | [1.05, 1.84] | 1.96 | [1.38, 2.75] |
| Hearing aid users n=320 | 1.33 | [0.91, 1.94] | 0.88 | [0.39, 1.77] | 1.66 | [1.07, 2.53] |
| Non-users n=843 | 1.55 | [1.19, 2.02] | 1.47 | [1.09, 1.96] | 2.31 | [1.51, 3.51] |
Note. Cell sizes may vary slightly due to missing data.
Adjusted for age, sex, education, marital status, exercise, and n of sites with plaque.
Adjusted for age, sex, education, marital status, smoking, exercise, diabetes, BMI, chronic disease and history of head injury.
Adjusted for age, sex, education, marital status, noise exposure, exercise, diabetes, chronic disease and history of head injury.
Mental Health-Related Quality of Life (MCS)
Hearing loss (vs normal hearing) was associated with worse mental health-related quality of life (B: −0.99 [95% CI: −1.65, −0.33]). A negative association of hearing loss with mental health-related quality of life was observed in non-users (B: −1.00 [−1.72, −0.29]) but not in hearing aid users (B: −0.91 [95% CI: −1.93, 0.10]). Estimated effect sizes for hearing aid users versus non-users were not significantly different (p=0.86).
Mild hearing loss (vs normal hearing) was associated with poorer mental health-related quality of life (B: −0.90 [95% CI: −1.61, −0.19]). Compared to normal hearing individuals, non-users (B: −0.96 [95% CI: −1.70, −0.21]) with mild hearing loss reported poorer mental health-related quality of life, whereas hearing aid users (B: −0.52 [95% CI: −2.16, 1.13]) with mild hearing loss had similar scores to normal hearing individuals. Estimated effect sizes for hearing aid users versus non-users with mild hearing loss were not significantly different (p=0.62).
The negative association of moderate or worse hearing loss (vs normal hearing) on mental health-related quality of life (B: −1.82 [95% CI: −2.78, −0.86]) was approximately twice as strong as the association for mild hearing loss. Both non-users (B: −2.33 [95% CI: −3.63, −1.04]) and hearing aid users (B: −1.42 [95% CI: −2.59, −0.26]) with moderate or worse hearing loss showed associations with poorer mental health-related quality of life, and estimated effect sizes were not significantly different (p=0.25). Negative associations for the groups of hearing aid users were strongest in participants with moderate or worse hearing loss.
Physical Health-Related Quality of Life (PCS)
Hearing loss (vs normal hearing) was associated with poorer physical health-related quality of life (B: −0.76 [95% CI: −1.50, −0.03]). However, associations of hearing loss with physical health-related quality of life were not observed when stratified to hearing aid users (B: −0.95 [95% CI: −2.08, 0.17]) and non-users (B: −0.69 [95% CI: −1.49, 0.11]).
Mild hearing loss (vs normal hearing) was not associated with physical health-related quality of life (B: −0.65 [95% CI: −1.45, 0.15]). Associations were not observed in non-users (B: −0.57 [95% CI: −1.41, 0.27]) or hearing aid users (B: −1.21 [95% CI: −3.04, 0.61]) with mild hearing loss.
Moderate or worse hearing loss (vs normal hearing) was associated with poorer physical health-related quality of life (B: −2.13 [95% CI: −3.18, −1.08]), and this negative association was observed in both non-users (B: −2.65 [95% CI: −4.04, −1.25]) and hearing aid users (B: −1.70 [95% CI: −3.00, −0.40]). The estimated effect size was not significantly different in hearing aid users versus non-users with moderate or worse hearing loss (p=0.27).
Depressive symptoms
Hearing loss (vs normal hearing) was associated with increased odds of depressive symptoms (OR: 1.49 [95% CI: 1.16, 1.91]) and there were differences in the presence of associations by hearing aid use. Compared to normal hearing individuals, non-users showed increased odds of depressive symptoms (OR: 1.55 [95% CI: 1.19, 2.02]) whereas hearing aid users did not (OR: 1.33 [95% CI: 0.91, 1.94]). Estimated effect sizes for hearing aid users and non-users with hearing loss were not significantly different (p=0.42).
Mild hearing loss was associated with increased odds of depressive symptoms (OR: 1.39 [95% CI: 1.05, 1.84]) as compared to normal hearing individuals. Non-users with mild hearing loss had increased odds of depressive symptoms (OR: 1.47 [95% CI: 1.09, 1.96]) and hearing aid users did not (OR: 0.88 [95% CI: 0.39, 1.77]). The difference in estimated effect sizes for hearing aid users and non-users with mild hearing loss was not significant (p=0.19).
Moderate or worse hearing loss (vs normal hearing) was associated with almost two times higher odds of depressive symptoms (OR: 1.96 [95% CI: 1.38, 2.75]). Associations were observed in both non-users (OR: 2.31 [95% CI: 1.51, 3.51]) and hearing aid users (OR: 1.66 [95% CI: 1.07, 2.53]). Estimates were not significantly different for hearing aid users versus non-users with moderate or worse hearing loss (p=0.19).
Base models
Supplementary tables S1 and S2 show results from base models for each outcome (adjusted for age, sex, hearing status). Estimated effect sizes were attenuated after multivariable adjustment.
DISCUSSION
In this study, hearing loss was associated with poorer cognitive function, poorer mental and physical health-related quality of life, and increased odds of depressive symptoms in middle-aged to older adults in the general population. Associations were strongest in individuals with moderate or worse hearing loss, indicating individuals with greater severity of hearing loss are more likely to experience more pronounced negative impacts of hearing loss. In general, hearing aid users had better scores than non-users for all outcomes in this study, although group comparisons were significant only for global cognitive function.
The associations between hearing loss and the outcomes in this study are consistent with previous epidemiological studies (Amieva et al., 2015; Chia et al., 2007; Dalton et al., 2003; Fischer et al., 2016; Gopinath et al., 2009; Schubert et al., 2017, 2019, Shukla et al., 2021). Using a PCA of cognitive test data (presented via both visual and auditory stimuli) to measure global cognitive function was a strength of this study. The PCA is a composite cognitive measure that overcomes limitations of individual tests. Limitations of individual cognitive tests include that the administration of some tests is reliant on audition, and that individually, these tests capture certain cognitive processes, thus making it more difficult to comment on global cognitive function across several cognitive domains. The use of a PCA provides a more comprehensive view of global cognitive function across several cognitive domains. Secondary cognitive analyses showed hearing loss was associated with tasks focused on language, processing, and executive function, but not memory. These results are consistent with evidence indicating processing and executive function show early age-related changes whereas short-term memory is thought to remain relatively stable in aging (Hedden & Gabrieli, 2004). Possible mechanisms of associations of hearing loss and cognitive function, health-related quality of life and depressive symptoms include co-occurring shared risk factors or negative cascading effects resulting from degraded auditory input (Baltes & Lindenberger, 1997; Lindenberger & Baltes, 1994; Wahl & Heyl, 2003).
In this study, hearing aid users generally had better outcomes than non-users. Importantly, hearing aid users had significantly better global cognitive function than non-users after stratification by hearing loss severity. Because these group differences were not observed in analyses of the entire sample (mild + moderate or worse hearing loss), these results demonstrate the importance of considering hearing loss severity when evaluating effects of hearing aid use. Although group differences between hearing aid users and non-users were not significantly different on outcomes of health-related quality of life or depressive symptoms, it is important that hearing aid users in the entire cohort and with mild hearing loss generally had similar scores as compared to normal hearing individuals whereas non-users had worse scores than normal hearing individuals. These findings suggest hearing aid users may have slightly better health-related quality of life and lower likelihood of depressive symptoms than non-users. Statistical significance is likely influenced by the sample sizes of comparison groups, so it is important to consider patterns in study findings in addition to the statistical significance of findings. Importantly, results were robust to adjustment for confounders, suggesting that the observed associations likely reflect effects of hearing aid use rather than differences in comparison groups. Therefore, results from this study suggest hearing aid use may have positive effects on global cognitive function and may have minor positive effects on health-related quality of life and depressive symptoms.
Results from previous studies evaluating hearing aid use on similar outcomes have been inconsistent (Brewster et al., 2021; Dalton et al., 2003; Dawes et al., 2015; Schubert et al., 2019; Shukla et al., 2021). Benefits of hearing aids may result from improved audibility in daily life, which may lead to reduced listening effort or fatigue (Picou et al., 2013). It has also been hypothesized that hearing aid use reduces social isolation and/or loneliness (Bott & Saunders, 2021). While we did not directly measure social isolation or loneliness, this study does not suggest strong evidence towards hearing aids reducing social isolation or loneliness as there were not robust effects of hearing aid use on depression or health-related quality of life. Mechanisms of the benefits of hearing aids on the outcomes presented in this study warrant additional research.
This study suggests that the burden of hearing loss is highest in cases of more severe hearing loss and that hearing aid benefits may be modified by hearing loss severity. Hearing aid users with mild hearing loss generally had similar scores on outcomes as compared to normal hearing individuals. This suggests that treating mild hearing loss with hearing aids may mitigate the burden of hearing loss.
Conversely, individuals with moderate or worse hearing loss consistently had poorer scores on outcomes as compared to normal hearing individuals, regardless of their hearing aid use. Hearing aids do not completely restore sensory input and amplification does not improve audibility when cochlear cells are severely degenerated (as in many cases of more severe hearing losses) (Moore, 2001). Therefore, hearing aids may not be corrective for individuals with more severe hearing losses, and as supported by this study, hearing aid use likely does not eliminate the negative effects of hearing loss. Another potential explanation for these relationships between hearing loss severity and hearing aid use is that, on average, individuals with more severe hearing loss are older and may experience more age-related degeneration and poorer health. However, our models controlled for age and other confounders to minimize those effects. Relationships of hearing loss severity and hearing aid use were consistent across all outcomes except physical health-related quality of life. Study findings suggest associations of hearing loss and hearing aid use with physical health-related quality of life may be absent for mild hearing loss but present in cases of moderate or worse hearing loss.
Although all models were adjusted for confounders, this study did not control for certain psychosocial constructs associated with healthier aging and health care utilization such as locus of control, motivation, or self-efficacy (Bryant et al., 2001; Meyer et al., 2014). There are likely complex relationships among these constructs, aging, hearing aid use, hearing loss severity, and the outcomes in this study. For example, individuals with more internal control, motivation, and self-efficacy may experience healthier aging (Bryant et al., 2001), be more likely to obtain and use hearing aids and also may be most likely to benefit from hearing aids (Garstecki & Erler, 1998; Meyer et al., 2014). In this observational study, it is possible that differences between hearing aid users and non-users (overall and stratified by hearing loss severity) may partially be explained by these, or other unmeasured, factors.
Strengths of this study include its large, well-characterized population of middle-aged to older adults from recent years (2009–2017) and the consideration of multiple outcomes of cognition and psychosocial well-being. Including participants from across the age range enhances generalizability to the target population. This study included a relatively large number of hearing aid users, whereas previous epidemiological studies have been limited by low numbers of hearing aid users in the general population (i.e., given smaller sample sizes in those studies). The cross-sectional design of this study allowed us to evaluate the extent to which hearing loss and hearing aid use may impact concurrent cognitive function, health-related quality of life and depressive symptoms. However, some limitations exist. This study is observational and cross-sectional design and therefore, it is not possible to determine the temporality of effects or comment on causal pathways of hearing loss or hearing aid use. Methods and results from this cross-sectional study can inform future design of longitudinal or experimental studies. Despite models being well-controlled for potential confounders, this observational study may not have accounted for all differences between comparison groups. Data on frequency of hearing aid use or appropriateness of hearing aid fitting were not available. EHLS and BOSS participants are mostly non-Hispanic white individuals so findings may not be generalizable to all populations.
CONCLUSION
Hearing loss was associated with poorer cognitive function, health-related quality of life, and depressive symptoms, and these associations were most pronounced in individuals with more severe hearing loss. Hearing aid users showed significantly better global cognitive function than non-users and appeared to have slightly better health-related quality of life and slightly reduced depressive symptoms. Broadly, study findings suggest that hearing loss may impact cognitive and psychosocial function, and that hearing aid users may perform better on tasks of cognitive function and may have slightly better well-being.
Supplementary Material
Supplementary Material S1. Supplementary cognitive methods
Supplementary Table S1. Age-sex adjusted models of hearing status (referent=normal hearing) and cognitive function (measured by principal component analysis), overall and stratified by hearing loss severity. Results are presented as standardized linear regression coefficients and 95% confidence intervals.
Supplementary Table S2. Age-sex adjusted associations of hearing status (referent=normal hearing) with health-related quality of life (mental component score; physical component score) and depressive symptoms. Results for health-related quality of life are presented as linear regression coefficients and 95% confidence intervals. Results for depressive symptoms are presented as odds ratios (OR) and 95% confidence intervals.
FUNDING:
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging (grant numbers R37AG011099, R01AG021917) and an unrestricted grant from the Research to Prevent Blindness, Inc. to the Department of Ophthalmology and Visual Sciences at the University of Wisconsin-Madison.
Footnotes
DECLARATION OF CONFLICTING INTERESTS: The authors declare that there is no conflict of interest
ETHICAL APPROVAL: Approval for this research was obtained from the Health Sciences Institutional Review Board of the University of Wisconsin. Written informed consent was obtained from all participants prior to each examination.
REFERENCES
- Acar B, Yurekli MF, Babademez MA, Karabulut H, & Karasen RM (2011). Effects of hearing aids on cognitive functions and depressive signs in elderly people. Archives of Gerontology and Geriatrics, 52, 250–252. doi: 10.1016/j.archger.2010.04.013. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute (ANSI). 2010. “Specification for Audiometers.” New York, NY: American National Standards Institute. [Google Scholar]
- American Speech-Language-Hearing Association. 2005. Guidelines for Manual Pure-Tone Threshold Audiometry. Available from www.asha.org/policy.
- American National Standards Institute (ANSI). 1999. “Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms.” New York, NY: American National Standards Institute. [Google Scholar]
- Amieva H, Ouvrard C, Giulioli C, Meillon C, Rullier L, & Dartigues JF (2015). Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: A 25-year study. Journal of the American Geriatrics Society, 63(10), 2099–2104. doi: 10.1111/jgs.13649. [DOI] [PubMed] [Google Scholar]
- Appollonio I, Carabellese C, Frattola L, & Trabucchi M (1996). Effects of sensory aids on the quality of life and mortality of elderly people: A multivariate analysis. Age and Ageing, 25(2), 89–96. doi: 10.1093/ageing/25.2.89. [DOI] [PubMed] [Google Scholar]
- Baltes PB, & Lindenberger U (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging?. Psychology and Aging, 12(1), 12. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bott A, & Saunders G (2021). A scoping review of studies investigating hearing loss, social isolation and/or loneliness in adults. International Journal of Audiology, 1–17. doi: 10.1080/14992027.2021.1915506. [DOI] [PubMed] [Google Scholar]
- Brewster K, Choi CJ, He X, Kim AH, Golub JS, Brown PJ, … & Rutherford BR (2021). Hearing rehabilitative treatment for older adults with comorbid hearing loss and depression: effects on depressive symptoms and executive function. American Journal of Geriatric Psychiatry. doi: 10.1016/j.jagp.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant LL, Corbett KK, & Kutner JS (2001). In their own words: a model of healthy aging. Social Science & Medicine, 53(7), 927–941. doi: 10.1016/s0277-9536(00)00392-0 [DOI] [PubMed] [Google Scholar]
- Chia EM, Wang JJ, Rochtchina E, Cumming RR, Newall P, & Mitchell P (2007). Hearing impairment and health-related quality of life: The Blue Mountains Hearing Study. Ear and Hearing, 28(2), 187–195. doi: 10.1097/AUD.0b013e31803126b6. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, et al. , (1998). Prevalence of Hearing Loss in Older Adults in Beaver Dam, Wisconsin: The Epidemiology of Hearing Loss Study. American Journal of Epidemiology, 148(9), 879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Tweed TS, Wiley TL, Klein BE, Klein R, Chappell R, … & Dalton DS (2003). The 5-year incidence and progression of hearing loss: The Epidemiology of Hearing Loss Study. Archives of Otolaryngology–Head & Neck Surgery, 129(10), 1041–1046. doi: 10.1001/archotol.129.10.1041 [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Nondahl DM, Tweed TS, Wiley TL, Klein BE, Klein R, … & Nash SD (2010). Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hearing Research, 264(1–2), 3–9. doi: 10.1016/j.heares.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshanks KJ, Nondahl DM, Dalton DS, Fischer ME, Klein BE, Klein R, Nieto FJ, Schubert CR, & Tweed TS (2015). Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. Journal of the American Geriatrics Society, 63(5), 918–924. 10.1111/jgs.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, & Nondahl DM (2003). The impact of hearing loss on quality of life in older adults. The Gerontologist, 43(5), 661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Dawes P, Cruickshanks KJ, Fischer ME, Klein BEK, Klein R, & Nondahl DM (2015). Hearing-aid use and long-term health outcomes: Hearing handicap, mental health, social engagement, cognitive function, physical health, and mortality. International Journal of Audiology, 54(11), 838–844. doi: 10.3109/14992027.2015.1059503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard LK, Cochran AL, Pinto A, Fowler CG, Fischer ME, Tweed TS, & Cruickshanks KJ (2021). Predicting hearing aid use in adults: the Beaver Dam Offspring Study. International Journal of Audiology, 60(8), 598–606. doi: 10.1080/14992027.2020.1853260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, & Tweed TS (2011). Determinants of hearing aid acquisition in older adults. American Journal of Public Health, 101(8), 1449–1455. doi: 10.2105/AJPH.2010.300078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Schubert CR, Nondahl DM, Dalton DS, Huang GH, Keating BJ, … & Cruickshanks KJ (2015). Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis, 238(2), 344–349. doi: 10.1016/j.atherosclerosis.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BE, … & Tweed TS (2016). Age-related sensory impairments and risk of cognitive impairment. Journal of the American Geriatrics Society, 64(10), 1981–1987. doi: 10.1111/jgs.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Pankow JS, … & Paulsen AJ (2017). Dichotic digits test performance across the ages: results from two large epidemiologic cohort studies. Ear and Hearing, 38(3), 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Dillard LK, Nondahl DM, Klein BE, Klein R, … & Paulsen AJ (2019). An epidemiologic study of the association between free recall dichotic digits test performance and vascular health. Journal of the American Academy of Audiology, 30(04), 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garstecki DC, & Erler SF (1998). Hearing loss, control, and demographic factors influencing hearing aid use among older adults. Journal of Speech, Language, and Hearing Research, 41(3), 527–537. doi: 10.1044/jslhr.4103.527. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Wang JJ, Schneider J, Burlutsky G, Snowdon J, McMahon CM, … & Mitchell P (2009). Depressive symptoms in older adults with hearing impairments: The Blue Mountains Study. Journal of the American Geriatrics Society, 57(7), 1306–1308. doi: 10.1111/j.1532-5415.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JD (2004). Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience, 5(2), 87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Humes LE, & Wilson DL (2003). An examination of changes in hearing-aid performance and benefit in the elderly over a 3-year period of hearing-aid use. Journal of Speech, Language and Hearing Research, 46(1), 137–145. doi: 10.1044/1092-4388(2003/011). [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, & Lee KE (1996). Changes in visual acuity in a population: The Beaver Dam Eye Study. Ophthalmology, 103(8), 1169–1178. [DOI] [PubMed] [Google Scholar]
- Ferguson MA, Kitterick PT, Chong LY, Edmondson-Jones M, Barker F, & Hoare DJ (2017). Hearing aids for mild to moderate hearing loss in adults. Cochrane Database of Systematic Reviews, 9. doi: 10.1002/14651858.CD012023.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BJ, Jayakody DM, Bennett RJ, Eikelboom RH, Gasson N, & Friedland PL (2020). Hearing loss and depression in older adults: a systematic review and meta-analysis. Gerontologist, 60(3), e137–e154. doi: 10.1093/geront/gnz009. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, & Baltes PB (1994). Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging, 9(3), 339. doi: 10.1037//0882-7974.9.3.339 [DOI] [PubMed] [Google Scholar]
- Linton KL, Klein BE, & Klein R (1991). The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. American Journal of Epidemiology, 134(12), 1438–1446. [DOI] [PubMed] [Google Scholar]
- Mener DJ, Betz J, Genther DJ, Chen D, & Lin FR (2013). Hearing loss and depression in older adults. Journal of the American Geriatrics Society, 61, 1627–1629. doi: 10.1111/jgs.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Hickson L, & Fletcher A (2014). Identifying the barriers and facilitators to optimal hearing aid self-efficacy. International Journal of Audiology, 53(sup1), S28–S37. doi: 10.3109/14992027.2013.832420. [DOI] [PubMed] [Google Scholar]
- Moore BC (2001). Dead regions in the cochlea: Diagnosis, perceptual consequences, and implications for the fitting of hearing aids. Trends in Amplification, 5(1), 1–34. doi: 10.1177/108471380100500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrow CD, Aguilar C, Endicott JE, Tuley MR, Velez R, Charlip WS, … & DeNino LA (1990). Quality-of-life changes and hearing impairment: A randomized trial. Annals of Internal Medicine, 113(3), 188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]
- Nash SD, Cruickshanks KJ, Klein R, Klein BEK, Nieto FJ, Huang GH, … Tweed TS (2011). The prevalence of hearing impairment and associated risk factors: The Beaver Dam Offspring Study. Archives of Otolaryngology - Head and Neck Surgery, 137(5), 432–439. doi: 10.1001/archoto.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall FQ, (1998). Comparison of percent total GHb with percent HbA1c in people with and without known diabetes. Diabetes Care, 21(9), 475–1480. [DOI] [PubMed] [Google Scholar]
- Paulsen AJ, Schubert CR, Johnson LJ, Chen Y, Dalton DS, Klein BE, … & Cruickshanks KJ (2018). Association of cadmium and lead exposure with the incidence of contrast sensitivity impairment among middle-aged adults. JAMA Ophthalmology, 136(12), 1342–1350. doi: 10.1001/jamaophthalmol.2018.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen AJ, Fischer ME, Pinto A, Merten N, Dillard LK, Schubert CR, … & Cruickshanks KJ (2021). Incidence of hearing impairment and changes in pure-tone average across generations. JAMA Otolaryngology–Head & Neck Surgery, 147(2), 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG, & Robson JG (1988). The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Sciences, 2(3), 187–199. [Google Scholar]
- Picou EM, Ricketts TA, & Hornsby BW (2013). How hearing aids, background noise, and visual cues influence objective listening effort. Ear and Hearing, 34(5), e52–e64. doi: 10.1097/AUD.0b013e31827f0431. [DOI] [PubMed] [Google Scholar]
- Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, & Klein R (1998). Low prevalence of hearing aid use among older adults with hearing loss: The Epidemiology of Hearing Loss Study. Journal of the American Geriatrics Society, 46(9), 1075–1078. doi: 10.1111/j.1532-5415.1998.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Reitan RM (1992). Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory. [Google Scholar]
- Ridgway J, Hickson L, & Lind C (2015). Autonomous motivation is associated with hearing aid adoption. International Journal of Audiology, 54(7), 476–484. doi: 10.3109/14992027.2015.1007213 [DOI] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Fischer ME, Chen Y, Klein BEK, Klein R, & Pinto AA (2017). Sensory impairments and cognitive function in middle-aged adults. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 72(8), 1087–1090. doi: 10.1093/gerona/glx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Fischer ME, Pinto AA, Chen Y, Huang GH, … & Dalton DS (2019). Sensorineural impairments, cardiovascular risk factors, and 10-year incidence of cognitive impairment and decline in midlife: The Beaver Dam Offspring Study. Journals of Gerontology: Series A, 74(11), 1786–1792. doi: 10.1093/gerona/glz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Reed NS, Armstrong NM, Lin FR, Deal JA, & Goman AM (2021). Hearing loss, hearing aid use, and depressive symptoms in older adults: Findings from the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Journals of Gerontology, Series B, 76(3), 518–523. doi: 10.1093/geronb/gbz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, & Spreen OA (2006). A compendium of neuropsychological tests: Administration, norms and commentary (3rd edition). Oxford University Press. [Google Scholar]
- Tesch-Römer C (1997). Psychological effects of hearing aid use in older adults. Journals of Gerontology, Series B, 52(3), 127–P138. doi: 10.1093/geronb/52b.3.p127 [DOI] [PubMed] [Google Scholar]
- United States Preventive Services Task Force (2019). Hearing loss in older adults: screening. Rockville, MD. [Google Scholar]
- Wahl HW, & Heyl V (2003). Connections between vision, hearing, and cognitive function in old age. Generations, 27(1), 39–45. [Google Scholar]
- Ware JE Jr, Kosinski M, & Keller SD (1996). A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- West JS (2017). Hearing impairment, social support, and depressive symptoms among US adults: A test of the stress process paradigm. Social Science & Medicine, 192, 94–101. doi: 10.1016/j.socscimed.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Weycker JM, Dillard LK, Pinto A, Fischer ME, Cruickshanks KJ, & Tweed TS (2021). Factors Affecting Hearing Aid Adoption by Adults With High-Frequency Hearing Loss: The Beaver Dam Offspring Study. American Journal of Audiology, 30(4), 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Cruickshanks KJ, Klein BE, Klein R, Huang GH, Pankow JS, … & Tweed TS (2011). Modifiable determinants of hearing impairment in adults. Preventive medicine, 53(4–5), 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Cruickshanks KJ, Huang GH, Klein BE, Klein R, Nieto FJ, … & Schubert CR (2011). Carotid atherosclerosis and cognitive function in midlife: the Beaver Dam Offspring Study. Atherosclerosis, 219(1), 330–333. doi: 10.1016/j.atherosclerosis.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Cruickshanks KJ, Schubert CR, Acher CW, Carlsson CM, Klein BE, … & Chappell RJ (2012). Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis, 224(2), 506–510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1. Supplementary cognitive methods
Supplementary Table S1. Age-sex adjusted models of hearing status (referent=normal hearing) and cognitive function (measured by principal component analysis), overall and stratified by hearing loss severity. Results are presented as standardized linear regression coefficients and 95% confidence intervals.
Supplementary Table S2. Age-sex adjusted associations of hearing status (referent=normal hearing) with health-related quality of life (mental component score; physical component score) and depressive symptoms. Results for health-related quality of life are presented as linear regression coefficients and 95% confidence intervals. Results for depressive symptoms are presented as odds ratios (OR) and 95% confidence intervals.


