Highlights
-
•
In Angola, measles is considered an endemic disease.
-
•
To the authors’ knowledge, this is the first study on the epidemiological pattern of the measles virus in Angola.
-
•
This study evaluated the measles situation in Angola using data analysed by enzyme-linked immunosorbent assay.
-
•
The number of cases of measles increased between 2015 and 2021.
-
•
Surveillance systems and vaccination need to be strengthened in Angola.
Keywords: Vaccination, Re-emergent diseases, Measles, Infectious disease, Angola
SUMMARY
Background
Measles, an acute infectious disease of extremely contagious viral aetiology, has been eliminated in some parts of the world. To the best of the authors’ knowledge, this is the first study on the epidemiological pattern of the measles virus in Angola, and it was carried out through a review of 7 years of observational retrospective data from the national measles laboratory surveillance programme.
Methods
A retrospective study using national databases on the laboratory surveillance of measles was performed. Patients of all ages with suspected measles from all provinces of Angola were included. Serum samples were used to detect IgM-type measles-virus-specific antibodies by enzyme-linked immunosorbent assay.
Findings
In total, 3690 suspected measles samples were sent to the Instituto Nacional de Investigação em Saúde. There were 962 (26.1%) laboratory-confirmed cases, and the most affected age group was children aged 1–4 years. The highest incidence rate per 100,000 population was found in Benguela (17.9%), followed by Huambo (16.7%) and Cuanza Sul (13.6%). Of the study years, the incidence rate per 1,000,000 population was highest in 2020 (11.9%). The most common complication was diarrhoea (n=406, 42.2%). Of the confirmed cases, 209 (21.7%) were vaccinated, 633 (65.8%) were unvaccinated, and 120 (12.5%) had unknown vaccination status. For all study years, vaccination coverage was <70%.
Interpretation
Measles continues to be a serious problem in Angola, and more efforts are needed to increase measles surveillance and achieve a high percentage of vaccination coverage.
Introduction
Measles is an acute infectious disease that is extremely contagious and potentially fatal. This vaccine-preventable disease is caused by the measles virus [1]. Measles has been eliminated in some parts of the world due to the implementation of vaccination programmes. However, outbreaks can occur if vaccination coverage does not reach 95%, causing a high burden of morbidity and mortality [2]. Clinical manifestations include fever, maculopapular rash, and at least one of the signs of cough, coryza or conjunctivitis [1]. This can progress to more serious conditions such as pneumonia, encephalitis, otitis media, laryngitis, laryngotracheobronchitis, diarrhoea and even death [3]. Measles affects both sexes and various age groups, with a higher incidence in children [4]. The Immunization Agenda 2030 sets an ambitious, overarching global vision and strategy for vaccines and immunization for the decade 2021–2030 [5]. A safe and effective injectable measles vaccine has been widely available since 1963, and intensified efforts between 2000 and 2010 reduced measles-related deaths by 74% [6]. Nevertheless, this disease still represents a global threat to public health, and major outbreaks continue, particularly in resource-poor countries that lack sustainable investment in their healthcare system and the health service infrastructure. According to the World Health Organization (WHO), more than 140,000 people died from measles in 2018, and at least one-third of deaths were in Africa [7].
In the Implementation Framework of the WHO 2030 Agenda for Immunization in the African Region, the elimination of measles in all its member countries is incorporated as the main objective, with a view to providing all the necessary support for elimination of this disease. This initiative is part of efforts to strengthen health systems, develop political and community leadership, optimize service delivery to care for unvaccinated or under-vaccinated children, and improve data systems for government decision-making [8].
In Angola, measles is an endemic disease with epidemiological peaks that mainly affects children aged <5 years. Due to the increased number of cases, all children aged ≤5 years should be vaccinated. Measles was considered hyperendemic in Angola before the national vaccination campaign in 2023. The first dose of measles-containing vaccine (MCV1) is administrated at 9 months of age, and the second dose (MCV2) is administrated at 15 months of age. A coverage target for two MCV doses of 95% is recommended in the WHO Africa region. Between 2015 and 2021, Angola had coverage rates of 64.9–74% for MCV1 and 24.7–40% for MCV2. However, because of the economic crisis in Angola, the vaccination programme has not been fully implemented and sustained. Angola showed sustained economic growth after the civil war in 2002, but prolonged diminution of oil prices since 2014 led to an economic crisis in the country, with growing inflation and reduced expenditure in the social and health sectors, particularly affecting children and the most vulnerable people. This situation was exacerbated by the coronavirus disease 2019 (COVID-19) pandemic.
This study aimed to evaluate the epidemiology of measles in Angola between 2015 and 2021, and to assess public health responses in terms of limiting the spread of measles.
Materials and methods
Study design
This observational retrospective study was undertaken using national databases on laboratory surveillance of measles, over a 7-year period (2015–2021), at the national referral laboratory of the Instituto Nacional de Investigação em Saúde, Luanda, the capital city of Angola. Blood samples of suspected measles cases (n=3690) with epidemiological notification sheets were sent to the National Institute for Health Research. Sociodemographic characteristics and clinical information of samples that met the criteria of the study were recorded. Human serum samples were collected and analysed in the serology laboratory to confirm disease based on the presence of immunoglobulin M (IgM) antibodies.
Case-based surveillance
The national health surveillance system of Angola applies the case definition recommended by WHO [9]. A suspected case is defined as ‘anyone suspected by a physician of having measles, or anyone with fever and maculopapular (non-vesicular) rash and cough, coryza (runny nose) or conjunctivitis (red eyes)’. A laboratory-confirmed case is defined as any person who has tested positive for measles-specific IgM antibodies. Subjects who had received one or two doses of MCV were considered to be vaccinated, and subjects who had not received any doses of MCV were considered to be unvaccinated.
Specimen testing
For this serological study, a total of 3690 serum samples were received from health facilities of the 18 provinces of Angola. IgM-type measles-virus-specific antibodies were detected by enzyme-linked immunosorbent assay (Euroimmun kits, Medizinische Labordiagnostika AG, Germany), in accordance with the manufacturer's instructions. Sociodemographic and epidemiological data of the patients were recorded and analysed.
Data analysis
Data were analysed using SPSS Version 29 (IBM Corp., Armonk, NY, USA). Statistical analyses were performed using absolute and relative frequency. Linearity Chi-squared test and slope test were performed. The number of categories minus 2 degrees of freedom was used for the linearity Chi-squared test. In this case, the number of categories was 5, so the results were interpreted with 3 degrees of freedom. Whenever the calculated Chi-squared value was greater than the tabulated value, the null hypothesis was rejected.
Results
Sociodemographic characteristics of the study population
For this seroepidemiological study, 3690 serum samples of suspected measles cases were received from health facilities of the 18 provinces of Angola, and analysed by the National Institute for Health Research between 2015 and 2021. The median age of subjects was 7.3 years, 1881 (51.0%) were male and 1809 (49.0%) were female. The age group with the highest percentage of suspected cases was children aged 1–4 years (41.4%) (Table 1). The highest percentages of samples sent to the national reference diagnostic laboratory were from Cuanza Sul (n=655, 17.8%), Huambo (n=511, 13.8%) and Uíge (n=405, 11.8%).
Table 1.
Sociodemographic characteristics and incidence rates of suspected and confirmed measles.
| Suspected cases (n, %) | Confirmed cases (%) | ||
|---|---|---|---|
| Total number (n) | 3690 | 962 | |
| Median age (years) | 7.3 | 6.4 | |
| Age group (years) | |||
| <1 | 262 (7.1) | 97 (10.1) | |
| 1–4 | 1527 (41.4) | 460 (47.8) | |
| 5–9 | 1039 (28.1) | 219 (22.8) | |
| 10–14 | 419 (11.4) | 73 (7.6) | |
| 15–19 | 156 (4.2) | 33 (3,4) | |
| ≥20 | 287 (7.8) | 80 (8.3) | |
| Sex | |||
| Female | 1809 (49.0) | 488 (50.7) | |
| Male | 1881 (51.0) | 474 (49.3) | |
| Place of residence | Incidence per 100,000 | ||
| Uíge | 405 (11.0) | 168 (17.5) | 8.8 |
| Luanda | 362 (9.8) | 148 (15.4) | 5.7 |
| Lunda Sul | 60 (1.6) | 44 (4.6) | 8.6 |
| Benguela | 159 (4.3) | 69 (7.2) | 17.9 |
| Namibe | 38 (1.0) | 11 (1.1) | 0.5 |
| Lunda Norte | 135 (3,7) | 52 (5.4) | 3.5 |
| Huíla | 167 (4.5) | 14 (1.5) | 1.9 |
| Cuanza Sul | 655 (17.8) | 75 (7.8) | 13.6 |
| Bié | 197 (5.3) | 82 (8.5) | 8.0 |
| Cabinda | 113 (3.1) | 40 (4.2) | 4.5 |
| Huambo | 511 (13.8) | 93 (9.7) | 16.7 |
| Zaire | 65 (1.8) | 18 (1.9) | 0.2 |
| Cuanza Norte | 330 (8.9) | 56 (5.8) | 5.5 |
| Bengo | 40 (1.1) | 18 (1.9) | 2.3 |
| Malanje | 86 (2.3) | 32 (3.3) | 2.1 |
| Moxico | 192 (5.2) | 30 (3.1) | 6.5 |
| Cunene | 141 (3.8) | 8 (0.8) | 0.4 |
| Cuando Cubango | 34 (0.9) | 4 (0.4) | 0.6 |
Epidemiological aspects of laboratory-confirmed cases
IgM antibodies to measles virus were detected in 962 (26.1%) samples. The median age of all patients with confirmed measles was 6.4 years, 474 (49.3%) were male and 488 (50.7%) were female. The age group most affected by measles was children aged 1–4 years (47.8%), followed by children aged 5–9 years (22.8%). The incidence rate in infants aged <1 year was 10.1%. The provinces with the most laboratory-confirmed cases were Uíge (n=168, 17.5%) and Luanda (n=148, 15.4%) (Table 1).
In order to ascertain the cumulative incidence rate by province, the number of cases per 100,000 population was calculated using the estimated population at the beginning of the study period. The highest cumulative incidence rate was found in Benguela (17.9%), followed by Huambo (16.7%) and Cuanza Sul (13.6%). The other provinces had incidence rates <10%, and the lowest incidence rates were reported in Cuando Cubango (0.6%), Namibe (0.5%) and Cunene (0.4%) (Table 1).
Between 2015 and 2018, the incidence rate of laboratory-confirmed measles was <1 per 1,000,000 population. However, between 2019 to 2021, the incidence rate was >1 per 1,000,000 population, with an increasing number of laboratory-confirmed cases (n=901, 93.7%). The highest incidence rate was reported in 2020 [11.9%, 95% confidence interval (CI) 11.7–12.2], followed by 2021 (9.5%, 95% CI 9.0–10.0) (Table 2). According to WHO, the adequacy of surveillance should be measured by the number of non-measles febrile rash illness cases reported per 100,000 population, and this should be >2/100,000. More than two discarded cases per 100,000 population are required to achieve adequate surveillance. Using this indicator, Angola only reached the required goal in 2019.
Table 2.
Incidence rates of non-measles febrile rash illness and confirmed cases of measles between 2015 and 2021.
| Year | Population | Suspected measles cases, n=3690 | Confirmed measles cases, n= 962 | Non-measles febrile rash rate per 100,000 | Laboratory-confirmed measles case rate per 1,000,000 (95% CI) |
|---|---|---|---|---|---|
| 2015 | 26,681,590 | 335 | 18 | 1.2 | 0.7 (0.66–0.72) |
| 2016 | 27,503,526 | 251 | 16 | 0.9 | 0.6 (0.58–0.63) |
| 2017 | 28,359,634 | 314 | 7 | 1.1 | 0.2 (0.18–0.22) |
| 2018 | 29,250,009 | 394 | 20 | 1.3 | 0.7 (0.67–0.73) |
| 2019 | 30,175,553 | 821 | 225 | 2.7 | 7.5 (7.1–7.9) |
| 2020 | 31,127,674 | 728 | 371 | 1.4 | 11.9 (11.7–12.2) |
| 2021 | 32,097,671 | 847 | 305 | 1.7 | 9.5 (9.0–10.0) |
CI, confidence interval.
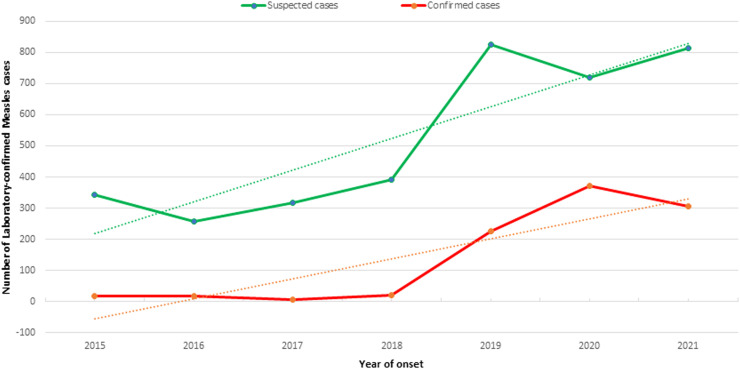
Epidemiological trends of measles are shown in Figure 1, where the trend lines of suspected and confirmed measles cases for the study period (2015–2021) show an increase over the study years. The highest numbers of suspected and confirmed cases of measles were found in 2019, and the highest number of IgM-confirmed measles cases was found in 2020.
Figure 1.
Trends in the numbers of suspected and confirmed measles cases reported by the National Health Surveillance System between 2015 and 2021 in Angola.
Complications of measles cases according to age
Measles-related complications were observed in 480/962 (49.9%) patients. These complications included diarrhoea (406/962, 42.2%), pneumonia (178/962, 18.5%) and otitis (57/962, 5.9%) (Table 3). Infants aged <1 year presented the highest number of complications (73/97, 75.3%). In total, 49/97 (50.5%) cases among infants aged <1 year reported diarrhoea as a complication. Also, this group presented the highest percentage of hospitalized patients (76.3%) (Table 3).
Table 3.
Measles cases, presentation of complications, and hospitalization by age group.
| All cases, n= 3,690 | Age group (years) |
||||||
|---|---|---|---|---|---|---|---|
| <1 | 1–4 | 5–9 | 10–14 | 15–19 | ≥20 | Total | |
| n (%a) | |||||||
| Measles-negative cases | 165 (4.5) | 1067 (28.9) | 820 (22.2) | 346 (9.4) | 123 (3.3) | 207 (5.6) | 2728 (73.9) |
| Confirmed measles cases | 97 (2.6) | 460 (12.4) | 219 (5.9) | 73 (2.0) | 33 (0.9) | 80 (2.2) | 962 (26.1) |
| n (%b) | |||||||
| Without complications | 24 (24.7) | 208 (45.1) | 125 (57.1) | 53 (72.6) | 25 (75.8) | 47 (58.8) | 482 (50.1) |
| With complications | 73 (75.3) | 252 (54.9) | 94 (42.9) | 20 (27.4) | 8 (24.2) | 33 (41.2) | 480 (49.9) |
| Types of complications (%b) | |||||||
| Pneumonia | 19 (19.6) | 94 (20.4) | 42 (19.2) | 5 (6.8) | 2 (6.1) | 16 (20.0) | 178 (18.5) |
| Diarrhoea | 49 (50.5) | 223 (48.5) | 91 (41.6) | 18 (24.7) | 5 (15.2) | 20 (25.0) | 406 (42.2) |
| Otitis media | 6 (6.2) | 26 (5.7) | 10 (4.6) | 5 (6.8) | 3 (9.1) | 7 (8.8) | 57 (5.9) |
| Hospitalized (%b) | |||||||
| Hospitalized | 74 (76.3) | 253 (55.0) | 100 (45.7) | 20 (27.4) | 8 (24.2) | 16 (20.0) | 471 (49.0) |
| Not hospitalized | 15 (15.5) | 120 (26.1) | 100 (45.7) | 30 (41.1) | 15 (45.5) | 36 (45.0) | 316 (32.8) |
| Without information | 8 (8.2) | 87 (18.9) | 19 (8.7) | 23 (31.5) | 10 (30.3) | 28 (35.0) | 175 (18.2) |
Percentage calculated based on the total number of cases studied.
Percentage calculated based on the number of confirmed measles cases by age group.
Vaccination status
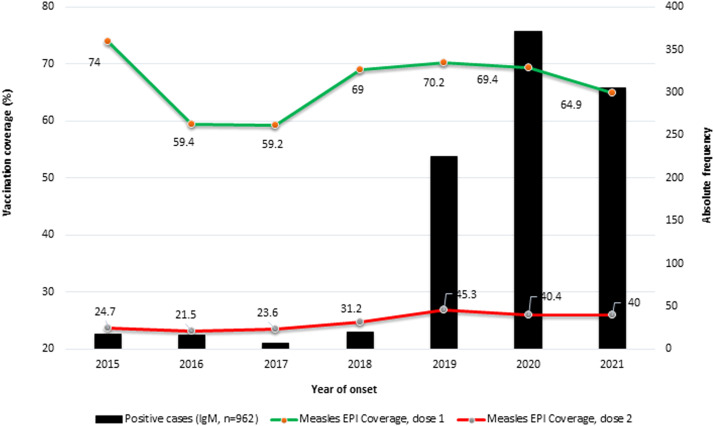
The data showed that 209 (21.7%) laboratory-confirmed cases had been vaccinated against measles, 633 (65.8%) were unvaccinated, and 120 (12.5%) had unknown vaccination status. The highest percentage of unvaccinated subjects was found in 2020 (n=271 (42.8%). The WHO Africa region recommends a coverage target for two doses of MCVs of 95%. Between 2015 and 2021, Angola had coverage rates of 64.9–74% for MCV1 and 24.7–40% for MCV2 (Figure 2).
Figure 2.
Number of laboratory-confirmed measles cases and vaccination coverage rates for measles-containing-vaccine 1 (MCV1) and MCV2 by year, from 2015 to 2021, in Angola. EPI, Expanded Programme on Immunization.
Discussion
To the best of the authors’ knowledge, this is the first study on epidemiological behaviour of the measles virus in Angola, and it was carried out through a review of 7 years of observational retrospective data from the national measles laboratory surveillance programme. Measles surveillance supported by laboratory confirmation through serological analysis of IgM has been implemented in the WHO Africa region since 2002 as part of the strategy to achieve measles elimination [10]. Between 2015 and 2021, a total of 962 (26.1%) measles cases were confirmed by laboratory diagnosis. This number is considered low, and no previous studies in the country have demonstrated the contrary.
This study demonstrated that the age group most affected by measles was children aged <5 years. This result is consistent with previous published studies which alluded to the higher prevalence of measles in children aged <5 years [11], [12], [13].
In the last 7 years, the highest incidence rates of measles per 100,000 inhabitants were found in the provinces of Benguela, Huambo and Cuanza Sul. These results may be compromised by several factors, such as the quality of the measles surveillance system, the reporting of suspected cases, and the transportation of samples to the national referral laboratory for confirmation. Nevertheless, the incidence rate of confirmed measles cases in Angola was less than one case per 100,000 inhabitants at national level between 2015 and 2018. It is likely that this result does not reflect the actual behaviour of the disease in those years, due to the low unreported number of cases.
In early 2019, the Ministry of Health decided to strengthen the disease surveillance system. In this same year, the incidence rate of non-measles febrile rash was 2.7 per 100,000 population, which is above the recommended WHO limit. This demonstrates the effect of strengthening the surveillance system. Although the decision to strengthen the disease surveillance system was taken, some provinces experienced difficulties implementing this change due to the lack of resources and human capacity, which explains the large differences in the number of measles samples submitted from different provinces.
Nevertheless, from 2019 to 2021, the incidence rate was higher than one per 1,000,000 inhabitants, with the highest rate reported in 2020. The incidence rate in Angola remained below the regional rate (38.4 cases per million inhabitants), but was higher than the incidence rates in South Africa, Algeria, Cape Verde and Guinea-Bissau [8]. This could be due to the COVID-19 pandemic, which was declared in Africa in February 2020. Many health facilities were used to respond to the COVID-19 pandemic, and the fear of contracting COVID-19 in healthcare settings contributed to the interruption of routine immunization services, as well as postponement of the Expanded Programme on Immunization against measles, yellow fever and polio by the Ministry of Health. The vaccination programmes for these diseases were also postponed in at least 15 African countries in 2020 [10,14]. Also, the increased number of cases observed in this study could be due to the fact that neighbouring countries such as the Democratic Republic of Congo (DRC) reported measles outbreaks during this period, and, according to WHO, the largest and most fatal measles outbreak in DRC occurred in 2019. The measles virus generally disrupts epithelial cells and suppresses the immune system, resulting in infection in various organ systems. The respiratory and intestinal tracts are the most affected sites in infected children, and pneumonia and diarrhoea occur in 10–40% of measles cases as complications or as secondary infections [15]. In the present study, children aged <1 and 1–4 years presented the highest percentages of complications related to measles. Diarrhoea was the most common complication, followed by pneumonia and otitis media. These results are in contrast with previous studies which reported that pneumonia was the most common and severe complication in children aged <5 years [16], [17], [18]. However, other studies have also reported that diarrhoea was the most serious complication, especially when combined with malnutrition, immunosuppression and vitamin A deficiency; elements that contribute to deterioration and death of the patient more quickly, and mainly occur in underdeveloped or developing countries [15,19,20]. On the other hand, vaccination against measles can play an important role in reducing infant mortality from complications associated with this disease, particularly diarrhoea, in countries with a high incidence of the disease [15].
Vaccination coverage is an important indicator of population health and reflects routinely collected information on administered vaccines. WHO recommends a coverage target ≥95% with two MCV doses to interrupt endemic disease transmission and eradicate measles [21]. However, between 2015 and 2021, Angola failed to reach this coverage target; since 2015, coverage of MCV1 stagnated at approximately 70%, and coverage of MCV2 was lower. Thus, Angola faces increased risk of an explosive measles outbreak as it has not been able to achieve the vaccination coverage target [22]. This has also been reported in other African countries, including Somalia, Cameroon, Central African Republic, Chad, DRC, Ethiopia, Guinea and Nigeria [23]. It is important to highlight here that, in a study carried out in Angola in 2014, the refusal of some parents to allow vaccination of their children was one of the causes of the increase in measles in children. Many children were not vaccinated for reasons ranging from cultural and economic status, to causes related to religious beliefs, superstitions and myths [24]. This may result in clustering of people who do not routinely vaccinate against measles, which can lead to accumulation of susceptible persons, and create a niche of sustained measles transmission.
Several limitations of this analysis should be considered. Firstly, the authors did not investigate which genotype of measles virus is circulating in Angola. Secondly, the underlying immunity levels among the Angolans were unknown. Finally, mortality data were incomplete or unavailable for each province during this period.
This evaluation revealed that a small percentage of laboratory-confirmed cases were vaccinated against measles. In this sense, the protective efficacy given by the vaccine is excellent; however, it is not absolute, as there may be primary immunity failures or, in some cases, secondary failures that may explain the appearance of some cases of measles among vaccinated subjects [25]. There are other reasons that could lead to failure of the vaccine, including poor quality of the cold chain in storage, transportation and handling, and an ineffective immunization technique [26]. The low case numbers reported in 2020, after worldwide resurgence of measles between 2017 and 2019, have to be interpreted with caution due to the effect of the COVID-19 pandemic on disease surveillance. Disrupted vaccination activities during the pandemic have increased the potential for the resurgence of measles in the near future, and effective, timely catch-up vaccination campaigns, strong leadership and commitment, and sufficient resources are required to mitigate this threat.
Losing ground on measles prevention through vaccination may lead to the re-emergence of measles into new populations, which may pose new and varied challenges to public health systems [27].
Conclusions
The vaccination of susceptible people, particularly children, is the backbone of prevention for measles. The study data suggest that the vaccination measures that were instituted in Angola may not have been effective in curtailing measles. More efforts are needed to increase measles control, maintain a high percentage of vaccination coverage, and achieve compliance with global health policies in order to ensure a complete vaccination scheme in the most susceptible populations.
Funding
This study was funded by Orçamento Geral do Estado 2022, allocated to the National Institute for Health Research through the Ministry of Health, Angola. The funding agency was not involved in the design of the study; gathering, analysis and interpretation of the data; writing of the manuscript; or the decision to submit the manuscript for publication.
Ethical approval
The Ethics Committee Board of the Ministry of Health (Angola) approved this study (Ref. No. 16/CE/2021). Informed consent was obtained from all subjects and/or their legal guardian(s).
Author contributions
MAR, MTFS, NMF: Conceptualization. MMG, GJ, CG, JP: Formal analysis. HF, JM, NMF: Funding acquisition. MAR, MTFS, MMG, GJ, RRC, CG, EM, CS, CG, JP, AMP, HF, JM: Investigation. MAR, MTFS, RRC, NMF: Data analysis. MAR, MTFS, NMF: Writing – original draft. Writing – review and editing: All authors reviewed the manuscript and approved the submission of the final version.
Conflict of interest statement
None declared.
Acknowledgments
The authors wish to thank the following physicians, technicians and staff members from the National Institute for Health Research for their technical support and assistance: Mr. António Benza, Ms. Marina Terra, Ms. Jael Gonçalo and Mr. Rui Van Dúnem.
References
- 1.Hubschen JM, Gouandjika-Vasilache I, Measles Dina J. Lancet. 2022;399:678–690. doi: 10.1016/S0140-6736(21)02004-3. [DOI] [PubMed] [Google Scholar]
- 2.Gastanaduy PA, Budd J, Fisher N, Redd SB, Fletcher J, Miller J, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med. 2016;375:1343–1354. doi: 10.1056/NEJMoa1602295. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661–1663. doi: 10.1056/NEJMp1408696. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . WHO; Geneva: 2019. Measles, fact sheet. [Google Scholar]
- 5.World Health Organization . WHO; Geneva: 2020. Immunization Agenda 2030: a global strategy to leave no one behind. [DOI] [PubMed] [Google Scholar]
- 6.Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012;379:2173–2178. doi: 10.1016/S0140-6736(12)60522-4. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . WHO; Geneva: 2019. More than 140,000 die from measles as cases surge worldwide. [Google Scholar]
- 8.World Health Organization Regional Office for Africa . WHO Regional Office for Africa; 2021. Progress towards measles elimination by 2020. [Google Scholar]
- 9.World Health Organization . WHO; Geneva: 1999. WHO-recommended surveillance standard of measles. [Google Scholar]
- 10.Masresha B, Luce R, Katsande R, Dosseh A, Tanifum P, Lebo E, et al. The impact of the COVID-19 pandemic on measles surveillance in the World Health Organization African Region, 2020. Pan Afr Med J. 2021;39:192. doi: 10.11604/pamj.2021.39.192.29491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dia N, Fall A, Ka R, Fall A, Kiori DE, Goudiaby DG, et al. Epidemiology and genetic characterization of measles strains in Senegal, 2004–2013. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutu MA, Bekele A, Seid Y, Woyessa AB. Epidemiology of measles in Oromia region, Ethiopia, 2007–2016. Pan Afr Med J. 2020;37:171. doi: 10.11604/pamj.2020.37.171.23543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodson JL, Masresha BG, Wannemuehler K, Uzicanin A, Cochi S. Changing epidemiology of measles in Africa. J Infect Dis. 2011;204(Suppl. 1):S205–S214. doi: 10.1093/infdis/jir129. [DOI] [PubMed] [Google Scholar]
- 14.Dixon MG, Ferrari M, Antoni S, Li X, Portnoy A, Lambert B, et al. Progress toward regional measles elimination – worldwide, 2000–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1563–1569. doi: 10.15585/mmwr.mm7045a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bawankule R, Singh A, Kumar K, Shetye S. Does measles vaccination reduce the risk of acute respiratory infection (ARI) and diarrhea in children: a multi-country study? PLoS One. 2017;12 doi: 10.1371/journal.pone.0169713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orsoo O, Saw YM, Sereenen E, Yadamsuren B, Byambaa A, Kariya T, et al. Epidemiological characteristics and trends of a nationwide measles outbreak in Mongolia, 2015–2016. BMC Public Health. 2019;19:201. doi: 10.1186/s12889-019-6511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Chetrit E, Oster Y, Jarjou'i A, Megged O, Lachish T, Cohen MJ, et al. Measles-related hospitalizations and associated complications in Jerusalem, 2018–2019. Clin Microbiol Infect. 2020;26:637–642. doi: 10.1016/j.cmi.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Saleh JE. Trends of measles in Nigeria: a systematic review. Sahel Med J. 2016;19:5. [Google Scholar]
- 19.Roberts L. Why measles deaths are surging – and coronavirus could make it worse. Nature. 2020;580:446–447. doi: 10.1038/d41586-020-01011-6. [DOI] [PubMed] [Google Scholar]
- 20.Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(Suppl. 1):S4–16. doi: 10.1086/377712. [DOI] [PubMed] [Google Scholar]
- 21.Yousif M, Hong H, Malfeld S, Smit S, Makhathini L, Motsamai T, et al. Measles incidence in South Africa: a six-year review, 2015–2020. BMC Public Health. 2022;22:1647. doi: 10.1186/s12889-022-14069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagcchi S. Measles immunisation gaps in Africa. Lancet Infect Dis. 2021;21:918. doi: 10.1016/S1473-3099(21)00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutts FT, Ferrari MJ, Krause LK, Tatem AJ, Mosser JF. Vaccination strategies for measles control and elimination: time to strengthen local initiatives. BMC Med. 2021;19:2. doi: 10.1186/s12916-020-01843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira MF, Martinez EZ, Rocha JS. Factors associated with vaccination coverage in children <5 years in Angola. Rev Saude Publ. 2014;48:906–915. doi: 10.1590/S0034-8910.2014048005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol. 2006;4:900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguefack F. Morbidity and mortality from measles in Cameroonian children: implications for measles control. Open Area Stud J. 2011;4:7–13. [Google Scholar]
- 27.Paules CI, Marston HD, Fauci AS. Measles in 2019 – going backward. N Engl J Med. 2019;380:2185–2187. doi: 10.1056/NEJMp1905099. [DOI] [PubMed] [Google Scholar]