Abstract
Background and objectives
Carotid-cavernous fistulas (CCFs) represent a group of rare, abnormal arteriovenous communications between the carotid arterial system and the cavernous sinuses (CS). CCFs often produce ophthalmologic symptoms related to increased CS pressures and retrograde venous drainage of the eye. Although endovascular occlusion remains the preferred treatment for symptomatic or high-risk CCFs, most of the data for these lesions is limited to small, single-center series. As such, we performed a systematic review and meta-analysis evaluating endovascular occlusions of CCFs to determine any differences in clinical outcomes based on presentation, fistula type, and treatment paradigm.
Method
A retrospective review of all studies discussing the endovascular treatment of CCFs published through March 2023 was conducted using PubMed, Scopus, Web of Science, and Embase databases. A total of 36 studies were included in the meta-analysis. Data from the selected articles were extracted and analyzed using Stata software version 14.
Results
1494 patients were included. 55.08% were female and the mean age of the cohort was 48.10 years. A total number of 1516 fistulas underwent endovascular treatment, 48.05% of which were direct and 51.95% of which were indirect. 87.17% of CCFs were secondary to a known trauma while 10.18% were spontaneous. The most common presenting symptoms were 89% exophthalmos (95% CI: 78.0–100.0; I2 = 75.7%), 84% chemosis (95% CI: 79.0–88.0; I2 = 91.6%), 79% proptosis (95% CI: 72.0–86.0; I2 = 91.8%), 75.0% bruits (95% CI: 67.0–82.0; I2 = 90.7%), 56% diplopia (95% CI: 42.0–71.0; I2 = 92.3%), 49% cranial nerve palsy (95% CI: 32.0–66.0; I2 = 95.1%), 39% visual decline (95% CI: 32.0–45.0; I2 = 71.4%), 32% tinnitus (95% CI: 6.0–58.0; I2 = 96.7%), 29% elevated intraocular pain (95% CI: 22.0–36.0; I2 = 0.0%), 31% orbital or pre-orbital pain (95% CI: 14.0–48.0; I2 = 89.9%) and 24% headache (95% CI: 13.0–34.0; I2 = 74.98%). Coils, balloons, and stents were the three most used embolization methods respectively. Immediate complete occlusion of the fistula was seen in 68% of cases and complete remission was seen in 82%. Recurrence of CCF occurred in only 35% of the patients. Cranial nerve paralysis after treatment was observed in 7% of the cases.
Conclusions
Exophthalmos, Chemosis, proptosis, bruits, cranial nerve palsy, diplopia, orbital and periorbital pain, tinnitus, elevated intraocular pressure, visual decline and headache are the most common clinical manifestations of CCFs. The majority of endovascular treatments involved coiling, balloons and onyx and a high percentage of CCF patients experienced complete remission with the improvement of their clinical symptoms.
Keywords: CCF, Exophthalmos, Trauma, Diplopia, Proptosis, Visual decline, Chemosis
1. Introduction
Carotid-Cavernous Fistulas (CCFs) are abnormal arteriovenous shunts between the carotid system and the cavernous sinuses (CS) resulting in arterialized blood within the venous structures draining the orbit.1 This frequently causes abnormally increased venous pressures within the CS and orbital veins and can lead to various symptoms such as proptosis, glaucoma, elevated intraocular pressure, cranial neuropathies, and visual decline.2 The decision to treat CCFs remains multifactorial and is often based on the severity of clinical symptoms and the angiographic characteristics of the fistula.3 Although other treatment modalities exist, in recent years endovascular occlusion has become the treatment of choice for the majority of CCFs.4 However, most of the data for these lesions remains limited to small, single-center series. As such, we performed a systematic review and meta-analysis evaluating endovascular occlusions of CCFs to determine any differences in clinical outcomes based on presentation, fistula type, and treatment paradigm.
2. Methods
2.1. Study selection
The systematic review and meta-analysis were performed by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The following electronic databases were queried: PubMed, Scopus, Web of Science (WoS), and Embase. Relevant articles were identified using the following search syntax ((Endovascular treatment) OR (Interventional Neuroradiology)) AND ((Carotid-Cavernous Fistula Embolization) OR (CCF)). After primary database searching was complete, the reference list of each paper was screened and related citations were extracted.
2.2. Inclusion and exclusion criteria
Only peer-reviewed articles evaluating the clinical efficacy of endovascular treatment in CCFs before March 2023 were included. Exclusion criteria were non-English publications, narrative or systematic reviews and meta-analyses, and studies with a lack of sufficient data to determine CCF etiology or type, pre-treatment symptoms, post-treatment outcomes, or endovascular approaches utilized.
2.3. Data extraction
Two of the authors independently assessed the full text of the included studies and extracted the data. All data regarding demographics of patients (e.g., gender, age, country, and duration of follow-up), etiology of CCF (e.g., trauma or spontaneous), surgical approach to the CCF (e.g., application of coil, balloon, and stent), clinical symptoms of the patients (e.g., proptosis, chemosis, diplopia, bruits, visual decline, orbital or pre-orbital pain and headache) and outcomes following endovascular treatment/observation (e.g. complete or partial remission) were extracted.
2.4. Statistical analysis
Categorical variables were reported as a percentage and continuous variables were reported as means and standard deviation (SD). Regarding demographics data we have reported the percentage of males/females, mean age of patients, mean duration of follow-up, and the number of patients in each country. In terms of etiology, we have reported the percentage of patients with an underlying cause of trauma and patients with spontaneous CCF. In surgical approaches, we have calculated the percentage of the application of each endovascular device including coils, balloons, and stents. We have also assessed the prevalence of clinical symptoms in CCF patients including proptosis, chemosis, diplopia, bruits, visual decline, orbital or pre-orbital pain, and headache. At last, we have evaluated the outcomes of endovascular treatment in CCF by reporting the percentage of patients with either partial or complete remission. Following a Binominal distribution, the variance along with a 95% confidence interval was reported. Thereafter, we combined the incidence of each study using average weight and an inverse association between each study variance and its weight was observed. Heterogeneity was assessed using the I2 index and Q statistic (α significance level of 10%), and the Freeman-Tukey Double Arcsine Transformation5 was used to stabilize the variance. If the I2 indices were more than 50%, the studies included in the meta-analysis were considered as heterogenous. To perform the meta-analysis, a random effect model was applied using STATA software (version 14.2). If p was near to 1 or 0, the Metaprop command was exerted. The ethical competence of this research was approved by the ethics committee of our institution Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1400.477).
3. Results
3.1. Literature search and study selection
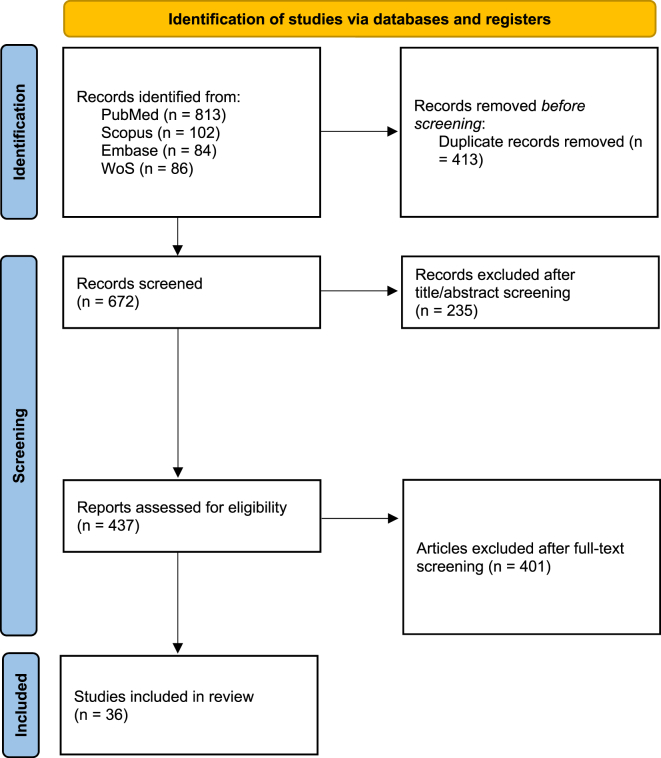
This study has been performed based on the PRISMA checklist.6 After an initial search of four electronic databases, 1085 papers (813 from PubMed, 102 from Scopus, 84 from Embase, and 86 from Web of Science) were obtained employing the search criteria. After an initial evaluation by the authors, 413 studies were removed due to duplication. Reasons such as unavailable abstracts, case reports, review articles, or non-English studies, excluded 235 further articles. The authors then carefully assessed the full text of each study, and papers were excluded due to lack of sufficient data (n = 176), ineligible sample size (n = 63), inapplicable approaches (n = 95), unavailable full text (n = 13), or not reporting the necessary information (n = 54). After applying the exclusion and inclusion criteria, a total of 36 studies from 2012 to 2023 were included in this systematic review and meta-analysis (Fig. 1, Table 1).
Fig. 1.
Flow diagram of studies identified in the systematic review and meta-analysis.
Table 1.
Overview of included studies.
| Author | The nimber of patients | Female | Mean age | Trans arterial route | Transvenous route | Both arterial and venous routes | Etiology |
Symptoms |
Method of embolization |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trauma | Spontaneous | Chemosis | Proptosis | Exophthalmos | Bruits | Cranial nerve palsy | Diplopia | Orbital and pre-orbital pain | Tinnitus | Elevated IOP | Visual decline | Headache | Coil | Onyx | Stent | Balloons | nBCA | Covered stent | Glue | Multiple | |||||||
| Y. Yu, 20127 | 23 | 8 | 36.3 | 23 | 0 | 0 | 0 | 23 | – | – | – | – | – | – | – | – | – | – | – | – | 23 | – | – | – | – | – | – |
| Ying Yu, 20148 | 18 | 4 | 37.8 | 18 | 0 | 0 | 16 | 2 | 9 | 7 | – | 11 | – | – | 6 | – | – | 8 | – | – | – | – | – | – | – | – | 18 |
| Qinglin Liu, 20219 | 10 | 3 | 28 | 10 | 0 | 0 | 10 | 0 | 10 | – | 6 | 5 | 3 | – | – | – | – | – | – | – | – | – | 1 | – | 9 | – | – |
| C. M. Wendl, 201710 | 14 | 11 | 59 | – | – | – | 5 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 11 |
| Tiago Rodrigues, 201411 | 38 | 29 | 63 | 5 | 22 | 1 | – | – | 34 | 32 | – | 5 | 20 | 20 | 6 | 5 | 14 | 19 | 10 | 24 | – | – | – | – | – | 3 | 1 |
| Zihuan Zhang, 202112 | 18 | 9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 14 | – | – | – | – | – | – | 5 |
| Muhamad Thohar Arifin, 202013 | 31 | – | – | 13 | 5 | 0 | 21 | 0 | 21 | 21 | – | – | – | – | – | – | – | – | – | 8 | 2 | – | 10 | – | – | – | – |
| Luı's Henrique de Castro-, 2018Afonso | 63 | – | 62.7 | 2 | 60 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | 33 | 5 | – | – | 3 | – | – | 22 |
| CUONG TRAN CHI, 201414 | 172 | – | – | 171 | 1 | 0 | 172 | 0 | – | 139 | – | 170 | 95 | – | – | – | – | 67 | – | – | – | 138 | – | – | – | 31 | – |
| 15alioscia derenzis, 2013 | 13 | 7 | 53.7 | 10 | 3 | 0 | 6 | 7 | 9 | 10 | – | 4 | – | – | – | – | – | – | – | 1 | – | 12 | – | – | 12 | 12 | – |
| Hui Guo, 201716 | 45 | 28 | 53.4 | 0 | 45 | 0 | – | – | 41 | 37 | – | 36 | – | 26 | – | – | – | 19 | – | – | – | – | – | – | – | – | – |
| Yin Niu, 201917 | 24 | – | – | – | – | – | 24 | 0 | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 21 | – | – | – | 2 |
| Chao-Bao Luo, 201218 | 24 | 9 | 39 | 25 | 0 | 0 | – | – | 24 | 24 | – | 24 | – | – | – | – | – | – | 3 | 25 | – | – | – | – | – | – | – |
| Xiao-Quan Xu, 201219 | 58 | 15 | – | 58 | 0 | 0 | 58 | 0 | 49 | 48 | – | 52 | – | – | – | – | – | 21 | – | – | – | – | 58 | – | – | – | – |
| S.Stepha, 201520 | 60 | 40 | 59 | – | – | – | – | – | 39 | 46 | – | 23 | 36 | 32 | 19 | – | 15 | 19 | – | – | – | – | – | – | – | – | – |
| Jacob F Baranoski, 201921 | 5 | 2 | 47 | 1 | 4 | 0 | 3 | 2 | 4 | 4 | – | – | 2 | 4 | – | – | – | – | – | – | – | – | – | – | – | – | 5 |
| Luís Henrique de Castro-Afonso, 201722 | 62 | 38 | 62.7 | 2 | 58 | 1 | – | – | – | – | – | – | 27 | – | – | – | – | – | – | 32 | 6 | – | – | 3 | – | – | 21 |
| Bu-Lang Gao, 201723 | 188 | 50 | 31 | – | – | – | 188 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 188 | – | – | – | – |
| Edgar A Samaniego, 201524 | 7 | – | – | 7 | 0 | 0 | 5 | 2 | 2 | – | 6 | 4 | 1 | – | – | 2 | – | 3 | 2 | 1 | – | – | – | – | 6 | ||
| Xiang Zhang, 201625 | 16 | 4 | 35.7 | 17 | 0 | 0 | 17 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 17 |
| André Beer-Furlan1, 202026 | 7 | 5 | – | 0 | 7 | 0 | – | – | 3 | 5 | – | – | 6 | – | – | 1 | – | – | – | – | 7 | – | – | – | – | – | – |
| Xiaojian Lu, 201427 | 32 | 11 | 32.3 | 32 | 0 | 0 | 30 | 2 | 28 | – | 28 | 31 | 26 | – | – | – | – | 20 | – | 8 | – | – | 21 | – | 2 | – | 1 |
| Bekir Sanal, 201828 | 23 | 16 | 61 | 5 | 18 | 1 | – | – | 17 | 18 | – | – | – | 17 | 10 | – | – | 9 | – | 18 | – | – | 1 | – | 1 | 1 | 3 |
| Jong Kook Rhim, 201829 | 17 | 13 | 64.9 | 0 | 34 | 0 | – | – | – | – | – | – | – | – | – | 4 | – | – | 3 | – | – | – | – | – | – | – | – |
| Francesco Briganti, 201330 | 30 | 22 | 51 | 0 | 30 | 0 | – | – | 16 | – | 30 | – | – | 14 | – | – | – | – | – | 30 | – | – | – | – | – | – | – |
| ALI PASHAPOUR, 201431 | 46 | – | 36.83 | 26 | 10 | 10 | – | – | 46 | 23 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Marcus Ohlsson, 201632 | 9 | 4 | 35 | – | – | – | 5 | 0 | – | – | 3 | 4 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 9 | – |
| Arvinda Hanumanthapura, 2013Ramalin33gaia | 21 | 3 | 31 | 21 | 0 | 0 | 21 | 0 | 20 | 19 | – | 21 | 16 | 18 | – | 20 | – | – | 10 | – | – | – | – | – | – | – | 21 |
| L. Fernando Gonzalez, 201234 | 5 | 4 | – | 2 | 3 | 0 | 2 | 3 | – | – | – | 2 | 5 | – | – | – | – | – | 2 | – | 1 | – | – | – | – | – | 4 |
| Matthew D. Alexander, 201835 | 267 | 209 | 60.9 | 73 | 199 | 7 | – | – | 227 | 221 | – | – | – | 197 | – | – | – | 106 | – | 196 | 7 | – | – | 4 | – | – | 20 |
| Fadi Al Saiegh, 202036 | 42 | 24 | 63.4 | 8 | 32 | 2 | 7 | 0 | 37 | 31 | – | – | 6 | 14 | – | 3 | – | 8 | 6 | 1 | 38 | – | – | – | – | – | 3 |
| Lee J.Holland, 201737 | 39 | 29 | 59 | – | – | – | 13 | 21 | – | 30 | – | – | – | 23 | 24 | 16 | – | – | 18 | 17 | – | – | – | – | – | – | 5 |
| Lorenz Ertl, 202038 | 33 | 23 | 61.9 | 4 | 27 | 2 | – | – | 28 | – | 29 | – | 5 | 4 | 2 | 14 | 9 | 22 | 2 | 17 | 2 | – | – | – | – | 1 | 13 |
| Andres R. Plasencia, 201239 | 24 | 10 | 37.5 | 18 | 5 | 1 | 14 | 8 | 8 | – | – | – | – | – | – | – | 7 | 6 | – | 6 | – | – | 12 | 2 | – | – | 2 |
| Lun-Xin Liu, 201840 | 10 | 4 | 35.6 | 10 | 0 | 0 | 8 | 2 | 10 | 10 | – | 10 | – | – | – | 2 | – | 1 | – | – | – | 10 | – | – | 10 | – | – |
| Total | 1494 | 634 (55.08%) | 48.10 (mean) | 561 | 563 | 26 | 625 | 73 | 682 | 725 | 99 | 402 | 249 | 369 | 67 | 67 | 45 | 328 | 56 | 432 (33.03%) | 91 (6.96%) | 160 (12.23%) | 312 (23.85%) | 12 (0.92%) | 34 (2.60%) | 57 (4.36%) | 180 (13.76%) |
3.2. Demographics
Our meta-analysis included a total of 1494 patients (634females (55.08%), 6 studies have not mentioned the gender of the patients) who underwent endovascular treatment of a CCF. The mean age of patients in this meta-analysis was 48.10 years. 9 of these 36 studies were conducted in China, 5 in the U.S., 2 in Germany, 2 in Italy, 2 in France, 2 in Brazil, 1 in India, 1 in Iran, 1 in Indonesia, 1 in Portugal, 1 in Peru, 1 in Australia, 1 in Turkey, 1 in Vietnam, 1 in Korea, 1 in Taiwan and 1 in Ecuador.
3.3. Etiology
We found that 87.17% of CCFs are caused by trauma, where 10.2% can be considered spontaneous (Table 2).
Table 2.
Summary of meta-analysis results.
| Number of studies | Pooled Percentage (95% CI) | I2 (%) | |
|---|---|---|---|
| Etiology | |||
| Spontaneous | 21 | 10.2% | |
| Trauma | 21 | 87.17% | |
| Others | 21 | 2.65% | |
| Surgical method | |||
| Coil | 17 | 33.03% | |
| Balloons | 8 | 23.85% | |
| Stent | 3 | 12.23% | |
| Gluebran | 6 | 4.36% | |
| Onyx | 9 | 6.96% | |
| nBCA | 4 | 0.92% | |
| Covered stent | 5 | 2.60% | |
| Multiple | 19 | 13.76% | |
| Other | 3 | 2.29% | |
| Clinical symptoms | |||
| Chemosis | 22 | 84% (79.0%–88.0%) | 91.6% |
| Proptosis | 18 | 79% (72.0%–86.0%) | 91.84% |
| Exophthalmos | 5 | 89% (78.0%–100.0%) | 75.7% |
| Bruits | 15 | 75% (67.0%–82.0%) | 90.7% |
| Cranial nerve palsy | 14 | 49% (32.0%–66.0%) | 95.1% |
| Diplopia | 11 | 56% (42.0%–71.0%) | 92.3% |
| Orbital and pre-orbital pain | 6 | 31% (14.0%–48.0%) | 89.9% |
| Tinnitus | 9 | 32% (6.0%–58.0%) | 96.7% |
| Elevated intraocular pressure | 4 | 29% (22.0%–36.0%) | 0.0% |
| Visual decline | 14 | 39% (32.0%–45.0%) | 71.4% |
| Headache | 9 | 24% (13.0%–34.0%) | 74.98% |
| Surgical approaches | |||
| Direct fistulas | 28 | 48.05% | |
| Indirect fistulas | 28 | 51.95% | |
| Trans arterial route | 28 | 56% (39.0%–73.0%) | 99.93% |
| Trans venous route | 28 | 45% (25.0%–65.0%) | 99.95% |
| Both Trans arterial and Transvenous routes | 28 | 0% (0%-1%) | 100.0% |
| Treatment outcome | |||
| Immediate complete occlusion of fistula | 20 | 75% (67.0%–83.0%) | 99.3% |
| Complete remission | 17 | 82% (71%–94%) | 99.0% |
| Recurrence | 13 | 35% (5.0%–66.0%) | 99.5% |
| Cranial nerve paralysis | 6 | 5% (1.0%–9.0%) | 1.3% |
3.4. Surgical approaches
Endovascular treatment was performed on a total number of 1516 fistulas. In some studies, the type of these fistulas was mentioned. Based on the data available, 54.76% of fistulas were indirect and 45.24% were direct fistulas. A wide range of embolization devices and liquids were used in different studies. Some endovascular treatments consisted of more than one device or liquid. Coils (33.03%) and balloons (23.85%) were the two mostly used devices. The most used liquid embolization method was embolization with Onyx (6.96%). In some cases, these methods were used in combination with each other. In 13.76% of fistulas, several methods were used.
3.5. Clinical symptoms
The outcomes of this analysis show that the most prevalent symptoms of pre-operative CCF treatment were 89% exophthalmos (95% CI: 78.0–100.0; I2 = 75.7%) (Table 2), 84% chemosis (95% CI: 79.0–88.0; I2 = 91.6%) (Table 2), 79% proptosis (95% CI: 72.0–86.0; I2 = 91.84%) (Table 2), 75.0% bruits (95% CI: 67.0–82.0; I2 = 90.7%) (Table 2), 56% diplopia (95% CI: 42.0–71.0; I2 = 92.3%) (Table 2), 49% cranial nerve palsy (95% CI: 32.0–66.0; I2 = 95.1%), 39% visual decline (95% CI: 32.0–45.0; I2 = 71.4%) (Table 2), 32% tinnitus (95% CI: 6.0–58.0; I2 = 96.7%) (Table 2), 29% elevated intraocular pain (95% CI: 22.0–36.0; I2 = 0.0%) (Table 2), 31% orbital or pre-orbital pain (95% CI: 14.0–48.0; I2 = 89.9%) (Table 2), and 24% headache (95% CI: 13.0–34.0; I2 = 74.98%) (Table 2) (Fig. 1s.)
3.6. Outcomes following endovascular treatment/observation
Most papers reported that the closure of the embolization process was successful. Immediate complete occlusion of fistula after treatment was observed in 75.0% (95% CI: 67.0–0.83.0; I2 = 94.91%) of cases. The complete remission rate was 90.0% (95% CI: 86.0–94.0; I2 = 90.15%). Recurrence of CCF was observed in 35% (95% CI: 5.0–66.0; I2 = 99.75%) of patients. Cranial nerve paralysis after treatment occurred in 5% (95% CI: 1.0–9.0; I2 = 43.08%) of patients (Figs. 2s and 3s, Table 2).
3.7. Publication bias
The Begg's funnel plot of included papers is shown in Fig. 4s where no sign of publication bias was detected (P = 0.161). Hence, we can conclude that both negative and positive results have been reported (Fig. 4s).
4. Discussion
CCFs are a type of arteriovenous malformation, leads to direct or indirect arteriovenous shunts from the internal or external carotid arteries into the cavernous sinus.41 This can result in a variety of clinical presentations however most have some ocular involvement at the time of diagnosis.42 Classification systems have been introduced to categorize CCFs according to their etiology (traumatic or spontaneous), their hemodynamic status (high or low flow), and their angiographic arterial supply (direct or indirect).43, 44, 45 Based on an angiographic classification established by Barrow et al,2 CCFs are classified into four types: type A is characterized as high-flow, direct shunts between the CS and the internal carotid artery (ICA). This type of fistula can rarely be a complication of head trauma or be caused by a carotid-cavernous aneurysm rupture. Trauma to the craniofacial region, whether direct or indirect, can weaken the muscle wall of the ICA or result in a laceration that causes a vascular shunt from a high-flow artery system into a low-flow venous sinus, which causes CCF. Type B CCFs are low-flow, indirect, or Dural arteriovenous fistulas (DAVFs) between the meningeal branches of the ICA and the CS. Type C CCFs are shunts between the CS and the external carotid artery (ECA) and, finally, type Ds represent a communication between the CS with both the ICA and ECA. Most direct CCFs will present with a combination of chemosis (90%), proptosis (90%), diplopia (50%), pain (25%), bruit (25%) increased ocular pressure, visual loss, and dysfunction of the trigeminal nerve (up to 50%). Direct CCFs are usually unilateral however bilateral lesions can occur and are often associated with higher morbidity.46 Indirect CCFs more frequently occur in post-menopausal women.4 Some factors including pregnancy, hypertension, diabetes, sinusitis, atherosclerotic disease, and thrombosis of CS predispose patients to develop indirect CCFs.1,2,47 Thrombosis of other veins distant from fistulous communication, large varix of the CS, pseudoaneurysm, and venous drainage into cortical veins are great risk factors of morbidity and mortality in CCF patients. Elevated intracranial pressure, progressive proptosis, declined visual acuity, transient ischemic attacks, and hemorrhage are considered clinical signs and symptoms associated with poorer prognosis.48
In this meta-analysis, data extracted from the 36 included studies were analyzed and four major results were obtained. Regarding the etiology of fistula, 87.24% of CCFs were caused by trauma while 10.1% were spontaneous. In the method of intervention, 36.49% of CCF patients were treated by applying a coil as an endovascular device, 25.04% by balloon, and 12.94% by an onyx (Table 2.). The third significant finding of our study was about pre-operative symptoms of CCF patients, which from the most common to the least were identified as exophthalmos, chemosis, proptosis, bruits, diplopia, cranial nerve palsy, visual decline, tinnitus, elevated intraocular pressure, orbital and periorbital pain, and headache with a prevalence of respectively 89%, 84%, 79%, 76%, 52%, 47%, 39%, 32%, 29%, 24% and 19%. Another important finding was that 68% of patients who underwent endovascular treatment-experienced complete occlusion of the fistula immediately after treatment and 82% of patients experienced complete remission with total improvement of clinical symptoms.
As discussed earlier, we extracted data regarding the etiology of CCF and found that the majority of cases were caused by trauma (87.24%) and spontaneous CCF constituted (10.1%) of cases. This finding confirms the previous literature as follows. Different theories explain the post-traumatic CCF mechanism. Trauma that might be accompanied by increased shear force and bone fracture can lead to a rupture in the carotid artery. However, Helmke et al49 reported no fractures in 42 post-traumatic direct CCF cases; thus, the mentioned theory was substituted by another theory that trauma leads to an abrupt rise in the internal carotid artery (ICA) pressure and simultaneous compaction of the distal artery, leading to vessel wall tear and CCF. In addition to penetrating or blunt traumas, iatrogenic damage can also result in CCF, for instance, trans-sphenoidal surgery, carotid angioplasty, etc.47 Spontaneous direct CCFs are formed through a cavernous aneurysm rupture or atherosclerotic artery leading to a weakened ICA wall subsequent to predisposing factors including pseudoxanthoma elasticum and Ehlers-Danlos syndrome. Iatrogenic causes such as previous contralateral ICA occlusion through altering the flow dynamics and pressure can play a role in spontaneous aneurysmal tears.1,2,46,50, 51, 52 The causes of Indirect CCFs are still unknown but there is some evidence supporting congenital origins.1,46,53 Factors such as trauma, pregnancy, diabetes, and hypertension are predisposing factors for dural CCF, while the association of trauma with indirect CCF is less prevalent.1,2,47 Among the included studies, Liu LX et al and Zeineddine HA et al40,54 reported trauma as cause of 80% of CCF cases, which is also confirmed by our study. However, the Sanal B et al study44 demonstrated a higher prevalence of spontaneous causes, which perhaps was due to a higher number of indirect CCF cases than direct ones in that study.
Endovascular intervention technology, which has recently evolved enormously, has provided various options for the treatment of CCFs. Thus, endovascular methods are now counted as the principal therapy in CCFs, or after conservative treatment failure. Regarding direct CCF, the target of therapy is the closure of the rupture between the cavernous sinus and ICA, during which the ICA patency is preserved. This can be achieved by using a detachable balloon to eliminate the fistula trans-arterially, applying coils for the abolition of the cavernous sinus of the ipsilateral site trans-venously or trans-arterially, or positioning a covered stent through the fistula.1 Embolization may be either trans-arterial or transvenous. If trans-arterial, coils are the optimal choice as embolization agent. Liquid agents such as n-BCA and onyx can also be used. In combination with this agent a balloon might be used to keep the parent vessel safe and prevent embolization agent from migrating to cerebral hemispheres. Both coils and liquid agents can be used for transvenous embolization. Based upon our meta-analysis, the prevalence of patients treated by coils, balloons, and stent were, respectively, 36.49%, 25.08%, and 12.94%. Coils are also used in combination with other embolization agents such as ethylene-vinyl alcohol copolymer (EVOH) (onyx), stent, balloons and n-butyl cyanoacrylate (n-BCA) as shown in Table 1. Detachable platinum coils are preferred because apart from simple utilization, whenever they are not placed optimally, they can be removed or adjusted later.55 A downside of using balloons is the potential risk of displacement or deflation which may lead to a recurrence of symptoms and rehabilitation of the malformation. In addition, they can result in constant or temporary paralysis of cranial nerves by inducing a mass effect in the cavernous sinus.56 Onyx, the most used liquid embolization agent, is used both trans-venously and trans-arterially. Stents have some advantages over coils or balloons, including rapid positioning, no risk of coil decampment or herniation, decreased local compaction and mass effects, and not being associated with pseudo-aneurysm formation.57 A major disadvantage of stents is that they cannot be applied in acute stages post trauma.58 In the studies assessed, transvenous approach was used more than trans-arterial route.
According to the results of our study, the most prevalent pre-operative symptom of CCF was exophthalmos (89%) followed by chemosis, proptosis, bruits, diplopia, cranial nerve palsy, visual decline, tinnitus, elevated intraocular pressure, orbital and periorbital pain, and headache respectively, which is in line with findings from previous studies. Drainage of the anterior part of the orbit gives rise to congestion of the orbital vein and subsequent fluids transudation, the elevation of intraocular pressure, laceration of dilated veins, and impairment in perfusion of the retina.4,41,42 Proptosis results from elevated orbital pressure, diplopia from paralysis of cranial nerves, a visual decline from ischemia in the retina or optic nerve, pain from aqueous return decline, and elevation in intraocular pressure. Headache is caused by venous hypertension, hemorrhage, and trigeminal nerve impairment. Direct CCFs generally manifest acutely and progress quickly, which requires immediate action. Perhaps these visual manifestations stem from ischemia in the retina, however, indirect CCFs usually present insidiously.41 We also extracted data regarding the outcome of endovascular treatments and found that 68% of patients who underwent this treatment achieved immediate occlusion of the fistula after treatment, and 82% reached complete remission with no clinical symptoms remained. This supports the finding of the Phan et al study59 which indicated that the success of the endovascular orbital approach in fistula embolization was 89.9% and demonstrated that those few patients who did not improve after the operation, had not received a proper fistula embolization. It is difficult to predict visual outcomes after CCF treatment, but minor disorders are associated with better outcomes. Cases with thrombosis in the superior ophthalmic vein or occlusion of the central retinal vein at the time of diagnosis showed poorer visual recovery. On the other hand, direct CCF patients manifest poorer vision and benefit from endovascular therapy with greater vision recovery.60,61 Most of the included studies’ findings were consistent with our results.1,44,49,40,54,56,37, 62, 63, 64
In summary, in this meta-analysis, we gathered all available relevant evidence about the etiology, endovascular treatment devices, symptoms, and endovascular treatment outcomes. However, there were some potential limitations. Methods used in eligible studies were not similar and we included studies with the most valid methods. In addition, as there was no meta-analysis related to this topic since 2012, we could not compare our findings with previous ones. Further research is required to achieve more definite findings and compare these results to yield a better knowledge regarding different aspects of CCF.
5. Conclusion
CCF, a type of arteriovenous malformation, leads to direct or indirect arteriovenous shunts. Generally, post-traumatic CCFs constitute a significantly higher percentage of CCF cases than spontaneous ones. Exophthalmos, chemosis, proptosis, bruits, diplopia, cranial nerve palsy, visual decline, tinnitus, elevated intraocular pressure, orbital and periorbital pain, and headache are the most common clinical manifestations of CCFs. The majority of endovascular treatments involved coiling, balloons and stent and a high percentage of CCF patients experienced complete remission with the improvement of their clinical symptoms.
Credit author statement
Aryoobarzan Rahmatian: Conceptualization, Methodology, Writing – original draft. Mobina Fathi: Conceptualization, Writing – original draft, Software, Supervision and editing. Shirin Yaghoobpoor: Writing – original draft. Robert M. Starke: Writing – original draft, reviewing. Evan M. Luther: Writing – original draft, reviewing. reviewing Fatemeh Sodeifian: Writing – original draft Tara Fazel: Writing – original draft. Mina Dehghani: Writing – original draft, investigating and edition. Reza Ramezan: Writing – original draft. Masood Zangi: Writing – original draft, reviewing. Reza Goharani: Conceptualization, Writing – original draft, Software, Supervision, and editing.
Funding statement
The authors received no financial support for the preparation of this article for publication.
Declarations of competing interest
None.
Acknowledgments
The authors of this study are thankful to the Clinical Research Development Center (CRDC) and Skull Base Research Center of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation, and assistance throughout the period of study.
Footnotes
Approved by ethics committee (IR.SBMU.RETECH.REC.1399.083).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wnsx.2023.100189.
Contributor Information
Aryoobarzan Rahmatian, Email: rahmatian-a@medilam.ac.ir.
Shirin Yaghoobpoor, Email: sh.yaghoobpoor98@gmail.com.
Arian Tavasol, Email: ariantavasol1378@gmail.com.
Komeil Aghazadeh-Habashi, Email: komeil.aghazade@gmail.com.
Zahra Hasanabadi, Email: z.hasanabadi42@gmail.com.
Matin Bidares, Email: matin.bidares@gmail.com.
Borna Safari-kish, Email: borna.safari.93@gmail.com.
Robert M. Starke, Email: RStarke@med.miami.edu.
Evan M. Luther, Email: evan.luther@jhsmiami.org.
Mohammadreza Hajiesmaeili, Email: mrhajiesmaeili@sbmu.ac.ir.
Fatemeh Sodeifian, Email: Ftmhsodeifian@yahoo.com.
Tara Fazel, Email: tarafazel7899@gmail.com.
Mina Dehghani, Email: minadehghani2001@gmail.com.
Reza Ramezan, Email: rramezan@uwaterloo.ca.
Masood Zangi, Email: Masood_zangi@yahoo.com.
Niloofar Deravi, Email: niloofar.deravi@gmail.com.
Reza Goharani, Email: r.goharani@sbmu.ac.ir.
Mobina Fathi, Email: Mobina.fathi78@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Gemmete J.J., Ansari S.A., Gandhi D.M. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuro Ophthalmol. 2009;29(1):62–71. doi: 10.1097/WNO.0b013e3181989fc0. [DOI] [PubMed] [Google Scholar]
- 2.Barrow D.L., et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(2):248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 3.Brzozowski K., et al. Superior ophthalmic vein and ophthalmic artery in immediate evaluation after endovascular treatment of carotid-cavernous fistulas. Pol J Radiol. 2019;84:e32. doi: 10.5114/pjr.2019.82807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korkmazer B., et al. Endovascular treatment of carotid cavernous sinus fistula: a systematic review. World J Radiol. 2013;5(4):143. doi: 10.4329/wjr.v5.i4.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman M.F., Tukey J.W. The Annals of Mathematical Statistics; 1950. Transformations Related to the Angular and the Square Root; pp. 607–611. [Google Scholar]
- 6.Liberati A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4) doi: 10.7326/0003-4819-151-4-200908180-00136. W-65-W-94. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y., et al. Use of onyx for transarterial balloon-assisted embolization of traumatic carotid cavernous fistulas: a report of 23 cases. AJNR Am J Neuroradiol. 2012;33(7):1305–1309. doi: 10.3174/ajnr.A2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y., et al. Embolization of direct carotid cavernous fistula with Onyx and coils under transarterial balloon protection. Cardiovasc Intervent Radiol. 2014;37(3):679–685. doi: 10.1007/s00270-013-0732-x. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q., et al. Treatment of direct carotid-cavernous fistula with Willis covered stent with midterm follow-up. Chin Neurosurg J. 2021;7(1):41. doi: 10.1186/s41016-021-00256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wendl C.M., et al. Direct carotid cavernous sinus fistulae: vessel reconstruction using flow-diverting implants. Clin Neuroradiol. 2017;27(4):493–501. doi: 10.1007/s00062-016-0511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues T., et al. Management of dural carotid cavernous fistulas: a single-centre experience. Eur Radiol. 2014;24(12):3051–3058. doi: 10.1007/s00330-014-3339-y. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z., et al. A modified treatment through point-to-point coil embolization for direct carotid cavernous to fistula: a single-center result. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.639552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thohar Arifin M., et al. Neuro-endovascular intervention in traumatic carotico-cavernous fistulae: a single-center experience. Int J Gen Med. 2020;13:917–925. doi: 10.2147/IJGM.S273603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi C.T., et al. Direct traumatic carotid cavernous fistula: angiographic classification and treatment strategies. Study of 172 cases. Intervent Neuroradiol. 2014;20(4):461–475. doi: 10.15274/INR-2014-10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Renzis A., et al. Balloon-assisted coiling of the cavernous sinus to treat direct carotid cavernous fistula. A single center experience of 13 consecutive patients. Intervent Neuroradiol. 2013;19(3):344–352. doi: 10.1177/159101991301900312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H., et al. Focus on the target: angiographic features of the fistulous point and prognosis of transvenous embolization of cavernous sinus dural arteriovenous fistula. Intervent Neuroradiol. 2018;24(2):197–205. doi: 10.1177/1591019917751894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu Y., et al. Detachable balloon embolization as the preferred treatment option for traumatic carotid-cavernous sinus fistula? Intervent Neuroradiol. 2020;26(1):90–98. doi: 10.1177/1591019919871849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo C.B., et al. Transarterial detachable coil embolization of direct carotid-cavernous fistula: immediate and long-term outcomes. J Chin Med Assoc. 2013;76(1):31–36. doi: 10.1016/j.jcma.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Xu X.Q., et al. Follow-up of 58 traumatic carotid-cavernous fistulas after endovascular detachable-balloon embolization at a single center. J Clin Neurol. 2013;9(2):83–90. doi: 10.3988/jcn.2013.9.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stéphan S., et al. Endovascular treatment of carotid-cavernous fistulae: long-term efficacy and prognostic factors. J Fr Ophtalmol. 2016;39(1):74–81. doi: 10.1016/j.jfo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Baranoski J.F., et al. Flow diverters as a scaffold for treating direct carotid cavernous fistulas. J Neurointerventional Surg. 2019;11(11):1129–1134. doi: 10.1136/neurintsurg-2019-014731. [DOI] [PubMed] [Google Scholar]
- 22.de Castro-Afonso L.H., et al. Transvenous embolization of dural carotid cavernous fistulas: the role of liquid embolic agents in association with coils on patient outcomes. J Neurointerventional Surg. 2018;10(5):461–462. doi: 10.1136/neurintsurg-2017-013318. [DOI] [PubMed] [Google Scholar]
- 23.Gao B.L., et al. Recurrence risk factors in detachable balloon embolization of traumatic direct carotid cavernous fistulas in 188 patients. J Neurointerventional Surg. 2018;10(7):704–707. doi: 10.1136/neurintsurg-2017-013384. [DOI] [PubMed] [Google Scholar]
- 24.Samaniego E.A., Martínez-Galdámez M., Abdo G. Treatment of direct carotid-cavernous fistulas with a double lumen balloon. J Neurointerventional Surg. 2016;8(5):531–535. doi: 10.1136/neurintsurg-2015-011695. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X., et al. Combined use of Onyx and coils for transarterial balloon-assisted embolization of traumatic carotid-cavernous fistulas: a report of 16 cases with 17 fistulas. J Neurointerventional Surg. 2016;8(12):1264–1267. doi: 10.1136/neurintsurg-2015-012107. [DOI] [PubMed] [Google Scholar]
- 26.Beer-Furlan A., et al. Transvenous onyx embolization of carotid-cavernous fistulas: mid- and long-term outcomes. J Neurol Surg B Skull Base. 2021;82(Suppl 3):e278–e284. doi: 10.1055/s-0040-1710514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X., et al. A comparison of different transarterial embolization techniques for direct carotid cavernous fistulas: a single center experience in 32 patients. J Vasc Interv Neurol. 2014;7(5):35–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Sanal B., et al. Endovascular treatment in traumatic and spontaneous carotid cavernous fistulas: with different embolization agents and via various vascular routes. J Vasc Interv Neurol. 2018;10(2):18–24. [PMC free article] [PubMed] [Google Scholar]
- 29.Rhim J.K., et al. Endovascular treatment of bilateral cavernous sinus dural arteriovenous fistula: therapeutic strategy and follow-up outcomes. Korean J Radiol. 2018;19(2):334–341. doi: 10.3348/kjr.2018.19.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briganti F., et al. Endovascular occlusion of dural cavernous fistulas through a superior ophthalmic vein approach. NeuroRadiol J. 2013;26(5):565–572. doi: 10.1177/197140091302600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pashapour A., et al. Long-Term endovascular treatment outcome of 46 patients with cavernous sinus dural arteriovenous fistulas presenting with ophthalmic symptoms. A non-controlled trial with clinical and angiographic follow-up. NeuroRadiol J. 2014;27(4):461–470. doi: 10.15274/NRJ-2014-10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohlsson M., Consoli A., Rodesch G. Endovascular treatment of carotico-cavernous fistulas with acrylic glue: a series of nine cases. Neuroradiology. 2016;58(12):1181–1188. doi: 10.1007/s00234-016-1760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramalingaiah A.H., et al. Transarterial treatment of direct carotico-cavernous fistulas with coils and Onyx. Neuroradiology. 2013;55(10):1213–1220. doi: 10.1007/s00234-013-1224-z. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez L.F., et al. Treatment of carotid-cavernous fistulas using intraarterial balloon assistance: case series and technical note. Neurosurg Focus. 2012;32(5):E14. doi: 10.3171/2012.2.FOCUS1213. [DOI] [PubMed] [Google Scholar]
- 35.Alexander M.D., et al. Long-Term outcomes of endovascular treatment of indirect carotid cavernous fistulae: superior efficacy, safety, and durability of transvenous coiling over other techniques. Neurosurgery. 2019;85(1):E94–e100. doi: 10.1093/neuros/nyy486. [DOI] [PubMed] [Google Scholar]
- 36.Al Saiegh F., et al. Onyx embolization of carotid-cavernous fistulas and its impact on intraocular pressure and recurrence: a case series. Oper Neurosurg (Hagerstown) 2021;20(2):174–182. doi: 10.1093/ons/opaa308. [DOI] [PubMed] [Google Scholar]
- 37.Holland L.J., et al. Endovascular treatment of carotid–cavernous sinus fistulas: ophthalmic and visual outcomes. Orbit. 2019;38(4):290–299. doi: 10.1080/01676830.2018.1544261. [DOI] [PubMed] [Google Scholar]
- 38.Ertl L., et al. Patient reported long-term outcome after endovascular therapy of indirect dural carotid cavernous fistulas. PLoS One. 2020;15(4):e0231261. doi: 10.1371/journal.pone.0231261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plasencia A.R., Santillan A. Endovascular embolization of carotid-cavernous fistulas: a pioneering experience in Peru. Surg Neurol Int. 2012;3:5. doi: 10.4103/2152-7806.92167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L.X., et al. Application of the willis covered stent in the treatment of carotid-cavernous fistula: a single-center experience. World Neurosurg. 2019;122:e390–e398. doi: 10.1016/j.wneu.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 41.Ellis J.A., et al. Carotid-cavernous fistulas. Neurosurg Focus. 2012;32(5):E9. doi: 10.3171/2012.2.FOCUS1223. [DOI] [PubMed] [Google Scholar]
- 42.Zanaty M., et al. Endovascular treatment of carotid-cavernous fistulas. Neurosurg Clin. 2014;25(3):551–563. doi: 10.1016/j.nec.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Barber S.M., et al. Mid-and long-term outcomes of carotid-cavernous fistula endovascular management with Onyx and n-BCA: experience of a single tertiary center. J Neurointerventional Surg. 2015;7(10):762–769. doi: 10.1136/neurintsurg-2014-011266. [DOI] [PubMed] [Google Scholar]
- 44.Sanal B., et al. Endovascular treatment in traumatic and spontaneous carotid cavernous fistulas: with different embolization agents and via various vascular routes. Journal of vascular and interventional neurology. 2018;10(2):18. [PMC free article] [PubMed] [Google Scholar]
- 45.Morton R.P., et al. Radiographic and clinical outcomes in cavernous carotid fistula with special focus on alternative transvenous access techniques. J Clin Neurosci. 2015;22(5):859–864. doi: 10.1016/j.jocn.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Ringer A.J., Salud L., Tomsick T.A. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurgery Clinics. 2005;16(2):279–295. doi: 10.1016/j.nec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Tjoumakaris S.I., Jabbour P.M., Rosenwasser R.H. Neuroendovascular management of carotid cavernous fistulae. Neurosurg Clin. 2009;20(4):447–452. doi: 10.1016/j.nec.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Halbach V.V., et al. Carotid cavernous fistulae: indications for urgent treatment. Am J Roentgenol. 1987;149(3):587–593. doi: 10.2214/ajr.149.3.587. [DOI] [PubMed] [Google Scholar]
- 49.Helmke K., Krüger O., Laas R. The direct carotid cavernous fistula: a clinical, pathoanatomical, and physical study. Acta Neurochir. 1994;127(1–2):1–5. doi: 10.1007/BF01808537. [DOI] [PubMed] [Google Scholar]
- 50.Wanke I., et al. Carotid cavernous fistula due to a ruptured intracavernous aneurysm of the internal carotid artery: treatment with selective endovascular occlusion of the aneurysm. J Neurol Neurosurg Psychiatr. 2001;71(6):784–787. doi: 10.1136/jnnp.71.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Germain D., HerreraGuzman Y. Vascular ehlers-danlos syndrome. J. Ann. gen. 2004;47:1. doi: 10.1016/j.anngen.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Debrun G.M. Angiographic workup of a carotid cavernous sinus fistula (CCF) or what information does the interventionalist need for treatment? Surg Neurol. 1995;44(1):75–79. doi: 10.1016/0090-3019(95)00162-x. [DOI] [PubMed] [Google Scholar]
- 53.Pang D., et al. External carotid-cavernous fistula in infancy: case report and review of the literature. Neurosurgery. 1981;8(2):212–218. doi: 10.1227/00006123-198102000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Zeineddine H.A., et al. Embolization of carotid-cavernous fistulas: a technical note on simultaneous balloon protection of the internal carotid artery. J Clin Neurosci. 2020;78:389–392. doi: 10.1016/j.jocn.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Halbach V.V., et al. Transarterial platinum coil embolization of carotid-cavernous fistulas. Am J Neuroradiol. 1991;12(3):429–433. [PMC free article] [PubMed] [Google Scholar]
- 56.De Renzis A., et al. Balloon-Assisted coiling of the cavernous sinus to treat direct carotid cavernous fistula: a single center experience of 13 consecutive patients. Intervent Neuroradiol. 2013;19(3):344–352. doi: 10.1177/159101991301900312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., et al. Treatment of post-traumatic carotid-cavernous fistulas with the Willis covered stent: a preliminary prospective study. Intervent Neuroradiol. 2012;18(2):172–177. doi: 10.1177/159101991201800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho K.-C., et al. Cerebral hemorrhage after endovascular treatment of bilateral traumatic carotid cavernous fistulae with covered stents. Journal of Korean Neurosurgical Society. 2011;50(2):126. doi: 10.3340/jkns.2011.50.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phan K., et al. Orbital approaches for treatment of carotid cavernous fistulas: a systematic review. World Neurosurg. 2016;96:243–251. doi: 10.1016/j.wneu.2016.08.087. [DOI] [PubMed] [Google Scholar]
- 60.Yu S.C.H., et al. Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization through the superior ophthalmic vein. Neurosurgery. 2007;60(6):1032–1038. doi: 10.1227/01.NEU.0000255455.05355.31. [DOI] [PubMed] [Google Scholar]
- 61.Zanaty M., et al. Endovascular treatment of carotid-cavernous fistulas. Neurosurg Clin. 2014;25(3):551–563. doi: 10.1016/j.nec.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Stéphan S., et al. Endovascular treatment of carotid-cavernous fistulae: long-term efficacy and prognostic factors. J Fr Ophtalmol. 2016;39(1):74–81. doi: 10.1016/j.jfo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Tao Z., Yu-feng L., Song-sheng S. Multimodal endovascular treatment for traumatic carotid-cavernous fistula. Chin J Traumatol. 2013;16(6):334–338. [PubMed] [Google Scholar]
- 64.Miller N.R., et al. Treatment of carotid-cavernous sinus fistulas using a superior ophthalmic vein approach. J Neurosurg. 1995;83(5):838–842. doi: 10.3171/jns.1995.83.5.0838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.