Abstract
Introduction
Lorlatinib is a potent, third-generation inhibitor of ALK. In the planned interim analysis of the ongoing, phase 3, randomized, global CROWN trial (NCT03052608), lorlatinib resulted in significantly longer progression-free survival than crizotinib in patients with previously untreated, advanced, ALK-positive NSCLC. Here, we present a subgroup analysis of Asian patients in the CROWN study.
Methods
Patients received lorlatinib 100 mg once daily or crizotinib 250 mg twice daily. The primary end point was progression-free survival assessed by blinded independent central review. Objective response rate (ORR), intracranial ORR, safety, and select biomarkers were secondary end points.
Results
At data cutoff (September 20, 2021), 120 patients were included in the Asian intention-to-treat subgroup (lorlatinib n = 59; crizotinib n = 61). At 36 months, 61% (95% confidence interval [CI]: 47–72) and 25% (95% CI: 12–41) of patients in the lorlatinib and crizotinib groups, respectively, were alive without disease progression (hazard ratio for disease progression by blinded independent central review or death: 0.40; 95% CI: 0.23–0.71). ORR was 78% (95% CI: 65–88) versus 57% (95% CI: 44–70) for patients treated with lorlatinib and crizotinib, respectively. In patients with measurable, nonmeasurable, or both measurable and nonmeasurable brain metastases at baseline, intracranial ORR was 73% (95% CI: 39–94) versus 20% (95% CI: 4–48) for patients treated with lorlatinib and crizotinib, respectively. The definition of nonmeasurable brain metastases is: a brain lesion less than 10 mm in MRI scan is defined as nonmeasurable brain metastasi based on RECIST criteria (Clinical trial evaluation criteria). Hypercholesterolemia, hypertriglyceridemia, and edema were the most frequently reported adverse events with lorlatinib.
Conclusions
Lorlatinib efficacy and safety in the Asian subgroup of CROWN were consistent with those in the overall population.
Keywords: Anaplastic lymphoma kinase, Lorlatinib, Non–small cell lung cancer, Phase 3, Progression-free survival
Introduction
Rearrangements of the ALK receptor tyrosine kinase gene are found in 2% to 5% of NSCLCs; are more frequent in nonsmokers, younger patients, and women; and define a subset of patients who are sensitive to small-molecule ALK tyrosine kinase inhibitors (TKIs).1, 2, 3 As such, ALK TKIs are now standard for first line and later lines of therapy of ALK-positive NSCLC.4,5 Nevertheless, progression of central nervous system (CNS) lesions, development of new intracranial lesions, and development of resistance mechanisms remain as challenges in the treatment of ALK-positive NSCLC.6, 7, 8, 9, 10, 11, 12
Lorlatinib is a potent, third-generation ALK inhibitor. In a phase 1/2 trial including patients with ALK-positive NSCLC, lorlatinib had systemic and intracranial antitumor activity in both treatment-naive and pretreated patients.13,14 In the more recent phase 3 CROWN study, lorlatinib significantly prolonged progression-free survival (PFS) versus crizotinib in patients with previously untreated ALK-positive NSCLC (hazard ratio [HR] = 0.28, 95% confidence interval [CI]: 0.19–0.41, p < 0.001).15 Overall and intracranial response rates were also greater with lorlatinib than crizotinib.15 Moreover, the safety profile of lorlatinib in the CROWN study was consistent with that found in the previous phase 1/2 study in treatment-naive or pretreated patients with ALK-positive NSCLC.14,15 Adverse events (AEs) that were more common with lorlatinib than crizotinib (with ≥10% difference) included hypercholesterolemia, hypertriglyceridemia, edema, increased weight, peripheral neuropathy, cognitive effects, anemia, hypertension, mood effects, and hyperlipidemia.15 Despite a higher incidence of grade 3 or 4 AEs with lorlatinib than crizotinib (72% versus 56%), discontinuations owing to AEs were similar between the two treatment groups (7% with lorlatinib; 9% with crizotinib).15 AEs associated with lorlatinib have been found to be effectively managed by dose modification, standard supportive care, or both dose modification and standard supportive care.16
Although lorlatinib was found to have promising efficacy in the global CROWN study, there are differences in the use of ALK TKIs across geographic regions17,18 and potential differences in efficacy and safety across ethnic groups due in part to differences in drug metabolism.19,20 Therefore, it is important to understand the efficacy and safety of lorlatinib in different geographic regions and ethnic groups. A subgroup analysis of the phase 1/2 lorlatinib trial found that previously treated Japanese patients with ALK-positive NSCLC achieved clinically meaningful responses, including intracranial responses, and that pharmacokinetic (PK) profiles were similar between Japanese and non-Japanese patients.21 In this post hoc subgroup analysis, we report efficacy, biomarker, PK, and safety results in the Asian subgroup of the CROWN study.
Materials and Methods
Study Design
Full details of the CROWN study (ClinicalTrials.gov identifier: NCT03052608) have been reported previously.15 Briefly, CROWN is an ongoing, global, randomized, phase 3 study comparing lorlatinib with crizotinib in previously untreated patients (N = 296) with advanced ALK-positive NSCLC. Patients were randomized (1:1) to receive oral lorlatinib 100 mg once daily or oral crizotinib 250 mg twice daily. Randomization was stratified by the presence of brain metastases (yes or no) and ethnicity (Asian or non-Asian). Treatment continued until independently assessed Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1)–defined disease progression, death, withdrawal of consent, or unacceptable toxic effects. Patients were able to continue treatment beyond RECIST 1.1–defined disease progression at the investigator’s discretion.
Tumor assessments were performed at screening and then every 8 weeks (±1 wk) from randomization to disease progression. Imaging included computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis. Magnetic resonance imaging of the CNS was performed at baseline and each tumor assessment.
The study protocol and amendments were approved by the institutional review board or independent ethics committee at each site and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, Declaration of Helsinki, and local laws.
Patients
This study included patients of Asian ethnicity (identified by self-reported race) from sites in Japan, South Korea, People’s Republic of China, Taiwan, Singapore, and Hong Kong. Eligible patients had ALK-positive histologically or cytologically confirmed locally advanced or metastatic NSCLC. ALK status was determined with the Ventana ALK (D5F3) CDx immunohistochemical assay. In addition, patients had at least one extracranial measurable target lesion (according to RECIST 1.1) that had not been previously irradiated; an Eastern Cooperative Oncology Group performance status of 0 to 2; and adequate bone marrow, liver, pancreatic, and renal function. Patients with asymptomatic treated or untreated CNS metastases were eligible for inclusion; however, no prior systemic treatment for metastatic disease was permitted. All patients provided written informed consent.
End Points and Assessments
The primary end point was PFS by blinded independent central review (BICR). Secondary end points included overall survival, PFS by investigator assessment, objective response, objective intracranial response, intracranial time to progression by BICR, biomarkers, and safety. PK parameters assessed after multiple dosing were exploratory end points.
Molecular Profiling
Molecular profiling of biomarkers, including both plasma circulating tumor DNA (ctDNA) and tumor tissue, were performed as described previously.22 A central, customized, next-generation sequencing assay on the Ion Torrent PGM platform at MolecularMD (Portland, OR, and Cambridge, MA) was used to profile formalin-fixed, paraffin-embedded tumor tissue. DNA extraction and analysis of ctDNA were performed in a central laboratory using a validated, commercially available, 74-gene ctDNA next-generation sequencing assay (Guardant360, panel version 2.11, bioinformatics pipeline version 3.5.3; Guardant Health, Inc., Redwood City, CA).
Statistical Analysis
Full details of the statistical methods for this study have been described previously.15 The primary and key secondary end point analyses were conducted in the Asian subgroup using a data cutoff of March 20, 2020, consistent with the formal interim analysis for PFS in the global study. September 20, 2021, was the data cutoff for this updated analysis. Analyses were conducted per the overall population within the Asian subgroup, with an emphasis on estimating the treatment effect, rather than hypothesis testing. No adjustments for multiplicity were performed on the analyses presented here. Given the limited sample size, unstratified analyses were conducted in the Asian subgroup. PK assessments were performed for all patients treated with lorlatinib who had at least one measurable plasma concentration of lorlatinib or its most abundant circulating metabolite, PF-0689571.
Results
Patients
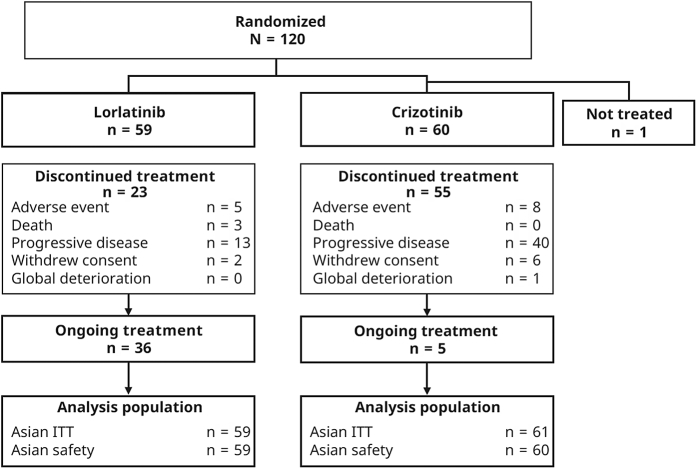
Overall, 120 Asian patients were randomized to receive treatment with lorlatinib (n = 59) or crizotinib (n = 61 [one patient was not treated]) (Fig. 1). Patients were located in Japan (n = 48), South Korea (n = 21), mainland China (n = 20), Taiwan (n = 16), Singapore (n = 8), and Hong Kong (n = 7). At data cutoff, 36 (61%) and five patients (8%) in the lorlatinib and crizotinib groups, respectively, were still receiving study treatment. Patient demographics and disease characteristics were generally well balanced between the treatment groups, with the exception of a greater proportion of female patients in the crizotinib group (61%) than the lorlatinib group (46%) (Table 1).
Figure 1.
Participant disposition (Asian population). ITT, intention to treat.
Table 1.
Baseline Characteristics (Asian ITT Population)
| Characteristics | Lorlatinib (n = 59) | Crizotinib (n = 61) |
|---|---|---|
| Age, median (range), y | 61 (30–83) | 55 (26–84) |
| Female, n (%) | 27 (46) | 37 (61) |
| ECOG performance status, n (%) | ||
| 0 | 21 (36) | 22 (36) |
| 1 | 37 (63) | 37 (61) |
| 2 | 1 (2) | 2 (3) |
| Use of previous anticancer drug therapy, n (%)a | 7 (12) | 6 (10) |
| Brain metastasis at baseline, n (%)b | 11 (19) | 15 (25) |
ECOG, Eastern Cooperative Oncology Group; ITT, intention to treat.
According to the protocol, previous adjuvant or neoadjuvant anticancer therapy was allowed if it had been completed more than 12 months before randomization.
Per independent central neuroradiological review.
Efficacy
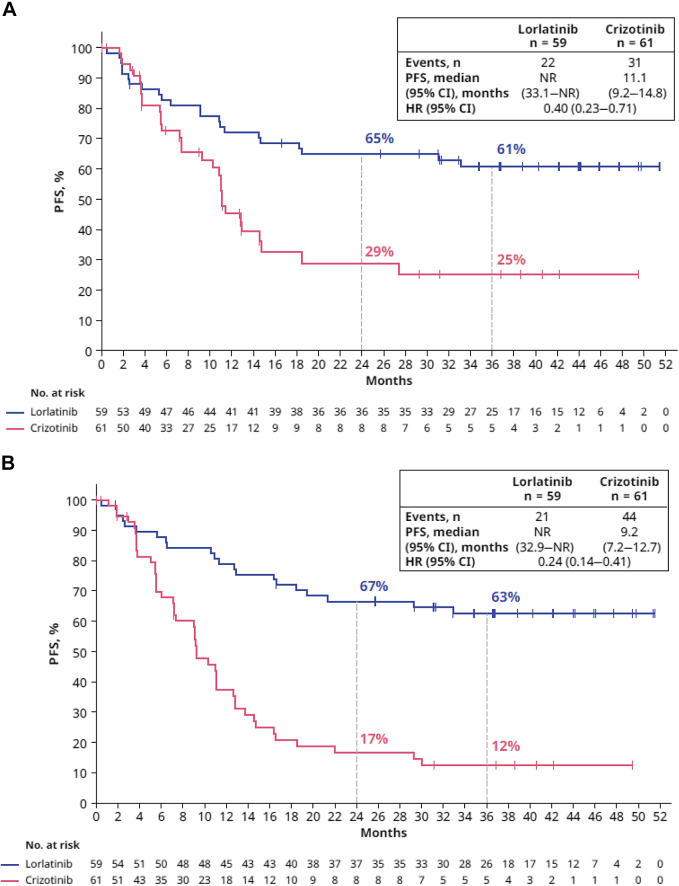
Of the 120 Asian patients in the intention-to-treat population, 22 of 59 (37%) in the lorlatinib group and 31 of 61 (51%) in the crizotinib group had PFS events (disease progression or death) by the data cutoff on September 20, 2021 (Fig. 2A). The HR for disease progression by BICR or death was 0.40 (95% CI: 0.23–0.71). The percentage of patients alive without disease progression at 24 and 36 months was 65% (95% CI: 51–76) and 61% (95% CI: 47–72), respectively, in the lorlatinib group and 29% (95% CI: 15–44) and 25% (95% CI: 12–41) in the crizotinib group (Fig. 2A). The median PFS by BICR was not reached (NR) (95% CI: 33.1–NR) in the lorlatinib group and 11.1 months (95% CI: 9.2–14.8) in the crizotinib group (Fig. 2A). A clinically meaningful improvement in investigator-assessed PFS was also observed in the lorlatinib group compared with the crizotinib group (HR = 0.24, 95% CI: 0.14–0.41) (Fig. 2B).
Figure 2.
PFSa by (A) BICR and (B) investigator assessment (Asian ITT population). aDefined as the time from randomization to RECIST-defined progression as assessed by the independent radiologist (A) or investigator (B) or death due to any cause, whichever occurred first. BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; ITT, intention to treat; NR, not reached; PFS, progression-free survival.
A clinically meaningful improvement in objective response rate (ORR; assessed by BICR) was observed in the lorlatinib group compared with the crizotinib group (78% [95% CI: 65–88] versus 57% [95% CI: 44–70], respectively) (Table 2). Moreover, similar responses were determined by investigator assessment (81% [95% CI: 69–90] versus 59% [95% CI: 46–71], respectively) (Supplementary Table 1). Overall, 80% of patients with an objective response in the lorlatinib group and 29% in the crizotinib group had a duration of response (DOR) of at least 12 months.
Table 2.
Objective Response by BICR in All Patients and Intracranial Objective Response by BICR in Patients With Brain Metastases at Baseline (Asian Population)
| Outcome | Asian Population |
|
|---|---|---|
| Lorlatinib (n = 59) | Crizotinib (n = 61) | |
| Best overall response, n (%) | ||
| Complete response | 1 (2) | 0 |
| Partial response | 45 (76) | 35 (57) |
| Stable disease | 6 (10) | 15 (25) |
| Neither complete response nor progressive disease | 1 (2) | 2 (3) |
| Progressive disease | 5 (9) | 3 (5) |
| Not evaluable | 1 (2) | 6 (10) |
| Confirmed objective response rate, n (% [95% CI])a | 46 (78 [65–88]) | 35 (57 [44–70]) |
| OR (95% CI) | 2.63 (1.11–6.38) | |
| Median duration of response (95% CI), mob | NR (NR–NR) | 12.8 (9.3–NR) |
| Median time to tumor response (IQR), mo | 1.8 (1.7–1.9) | 1.8 (1.7–1.9) |
| Patients with measurable or nonmeasurable brain metastases at baseline | ||
| n | 11 | 15 |
| Confirmed CNS response, % (95% CI)a,c | 73 (39–94) | 20 (4–48) |
| CNS complete response, n (%) | 8 (73) | 2 (13) |
| Median duration of response (95% CI), mob | NR (NR–NR) | 17 (11–NR) |
BICR, blinded independent central review; CI, confidence interval; CNS, central nervous system; IQR, interquartile range; NR, not reached; OR, odds ratio.
Clopper-Pearson method.
Brookmeyer and Crowley method.
Intracranial assessment was performed by independent central neuroradiological review.
The HR for intracranial progression by BICR was 0.03 (95% CI: 0.004–0.200). The percentage of patients alive without intracranial progression at 24 and 36 months was 98% (95% CI: 86–100) and 98% (95% CI: 86–100) in the lorlatinib group and 48% (95% CI: 30–64) and 42% (95% CI: 24–60) in the crizotinib group, respectively (Supplementary Fig. 1). The median time to intracranial progression by BICR was not reached (NR) in the lorlatinib group and 16.6 months (95% CI: 11.0–NR) in the crizotinib group.
In patients with measurable, nonmeasurable, or both measurable and nonmeasurable brain metastases at baseline (11 and 15 patients in the lorlatinib and crizotinib groups, respectively), a consistent improvement in ORR (assessed by BICR) was observed in the lorlatinib group (73% [95% CI: 39–94]) compared with the crizotinib group (20% [95% CI: 4–48]); 73% versus 13% of patients, respectively, had a complete intracranial response (Table 2). Overall, 88% of patients in the lorlatinib group and no patients in the crizotinib group had a duration of intracranial response of at least 12 months. At the previous data cutoff of March 20, 2020, overall survival data were immature, with deaths having occurred in 10 patients (17%) in the lorlatinib group and nine patients (15%) in the crizotinib group (HR = 0.99 [95% CI: 0.40–2.45]).
Biomarkers
Plasma samples from most Asian patients were available for analysis, and ctDNA was detectable in 44 patients (75%) in the lorlatinib arm and 48 (79%) in the crizotinib arm. ALK fusions were detected in the ctDNA of 26 (59%) and 31 (65%) of these patients in the lorlatinib and crizotinib arm, respectively (Table 3). Nevertheless, very few ALK kinase domain mutations were detected in this treatment-naive patient population, as expected.
Table 3.
Frequency of EML4::ALK Fusion Variants and TP53 Mutations and Response to Lorlatinib and Crizotinib (Asian Population)
| Biomarker | Lorlatinib (n = 59) | Crizotinib (n = 61) | ||||||
|---|---|---|---|---|---|---|---|---|
| ctDNA, n (%) | 44 (75) | 48 (79) | ||||||
| ALK fusion, n (%) | 26 (59) | 31 (65) | ||||||
| Lorlatinib | Crizotinib | |||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | ORR, % (95% CI)a | PFS, median, mo (95% CI)b | DOR, median, mo (95% CI)b | n (%) | ORR, % (95% CI)a | PFS, median, mo (95% CI)b | DOR, median, mo (95% CI)b | |
| EML4::ALK variant 1 | 6 (14) | 100 (54–100) | NR (NR–NR) | NR (NR–NR) | 11 (23) | 36 (11–69) | 7.4 (5.5–12.9) | NR (5.5–NR) |
| PFS HR (95% CI) | 0.13 (0.015–1.051) | – | ||||||
| EML4::ALK variant 3 | 11 (25) | 82 (48–98) | NR (5.3–NR) | NR (12.8–NR) | 13 (27) | 77 (46–95) | 10.3 (5.4–12.8) | 9.6 (4.6–12.8) |
| PFS HR (95% CI) | 0.25 (0.064–0.944) | – | ||||||
| TP53 mutation positive | 16 (36) | 63 (35–85) | NR (5.3–NR) | NR (NR–NR) | 18 (38) | 56 (31–79) | 5.6 (3.7–12.9) | 8.5 (4.6–NR) |
| TP53 mutation negative | 28 (64) | 79 (59–92) | NR (18.4–NR) | NR (16.5–NR) | 30 (62) | 57 (37–75) | 11.4 (10.9–14.8) | 12.8 (9.4–NR) |
CI, confidence interval; ctDNA, circulating tumor DNA; DOR, duration of response; HR, hazard ratio; NR, not reached; ORR, objective response rate; PFS, progression-free survival.
Clopper-Pearson method.
Brookmeyer and Crowley method.
EML4::ALK variants 1 and 3 were the most frequent fusions identified; they were detected in six (14%) and 11 (25%) patients in the lorlatinib arm and 11 (23%) and 13 (27%) patients in the crizotinib arm, respectively. ORRs were numerically higher in the lorlatinib arm than the crizotinib arm in patients with EML4::ALK variant 1 (100% [95% CI: 54–100] versus 36% [95% CI: 11–69]) and variant 3 (82% [95% CI: 48–98] versus 77% [95% CI: 46–95]). There were too few patients with the other ALK fusion subtypes to make meaningful observations. Median DOR and PFS in the lorlatinib arm were NR in either variant subgroup 1 or 3; in the crizotinib arm, median DOR was NR and 9.6 months, and median PFS was 7.4 and 10.3 months, in variant subgroups 1 and 3, respectively. The rate of progression or death was lower with lorlatinib than crizotinib, with an HR of 0.13 (95% CI: 0.015–1.051) and 0.25 (95% CI: 0.064–0.944) in patients with variants 1 and 3, respectively (Table 3 and Supplementary Fig. 2).
In patients with detectable ctDNA at baseline, TP53 mutations were the most frequent co-alterations, found in 16 patients (36%) in the lorlatinib arm and 18 (38%) in the crizotinib arm. In general, ORRs were slightly higher in the TP53 mutation-negative groups than in the TP53 mutation-positive groups. Median DOR was NR in both TP53 mutation-positive and -negative subgroups of the lorlatinib arm and 8.5 months (95% CI: 4.6–NR) and 12.8 months (95% CI: 9.4–NR) in TP53 mutation-positive and -negative subgroups of the crizotinib arm, respectively. Median PFS was NR for both TP53 mutation-positive and -negative groups in the lorlatinib arm and 5.6 months (95% CI: 3.7–12.9) and 11.4 months (95% CI: 10.9–14.8) for TP53 mutation-positive and -negative groups, respectively, in the crizotinib arm. ORRs were higher and median DOR and PFS were potentially longer with lorlatinib than crizotinib regardless of TP53 mutation status (Table 3).
PK Analysis
Overall, 59 patients in the Asian population had at least one PK assessment and were included in the PK analysis set. Lorlatinib plasma concentrations reached steady state within the first two cycles of dosing, and steady-state exposure was similar between the Asian and overall populations (Supplementary Fig. 3).
Safety
Treatment-emergent AEs (TEAEs) of any grade occurred in 100% of patients in both treatment arms. TEAEs that occurred more frequently with lorlatinib than crizotinib (with ≥10% difference) included hypertriglyceridemia (73% versus 5%), hypercholesterolemia (71% versus 3%), edema (44% versus 32%), increased weight (49% versus 17%), peripheral neuropathy (34% versus 18%), pyrexia (27% versus 13%), cognitive effects (25% versus 5%), hypertension (25% versus 2%), upper respiratory tract infection (22% versus 12%), hyperlipidemia (20% versus 0%), hyperuricemia (19% versus 7%), and pain in the extremity (17% versus 7%) (Fig. 3 and Supplementary Table 2). TEAEs that were more common with crizotinib than lorlatinib (with ≥10% difference) included diarrhea (58% versus 15%), nausea (58% versus 19%), vomiting (45% versus 10%), vision disorder (43% versus 15%), constipation (43% versus 22%), increased alanine aminotransferase level (42% versus 20%), increased aspartate aminotransferase level (35% versus 19%), decreased appetite (32% versus 3%), decreased neutrophil count (23% versus 5%), increased blood creatinine level (22% versus 3%), fatigue (22% versus 10%), bradycardia (20% versus 3%), decreased white blood cell count (18% versus 2%), dysgeusia (18% versus 9%), sinus bradycardia (17% versus 5%), and increased blood alkaline phosphatase level (12% versus 2%) (Fig. 3 and Supplementary Table 2).
Figure 3.
All-causality AEs with more than or equal to 20% incidence in either group and more than or equal to 10% difference between groups (Asian safety population). aCluster AE term per Medical Dictionary for Regulatory Activities, version 24.1. AE, adverse event.
Grade 3 or 4 TEAEs were reported by more patients receiving lorlatinib (80%) than crizotinib (62%). The most common grade 3 or 4 TEAEs (reported in ≥10% of patients) were hypertriglyceridemia (31%), hypercholesterolemia (20%), and increased weight (17%) with lorlatinib and decreased neutrophil count (15%) with crizotinib (Supplementary Table 2). All-cause serious AEs occurred in 46% and 30% of patients in the lorlatinib and crizotinib groups, respectively. All-cause fatal AEs occurred in four patients (7%) in the lorlatinib group (lower respiratory tract infection, malignant lung neoplasm, respiratory failure, and acute cardiac failure; n = 1 each); no fatal AEs were reported in the crizotinib group. AEs leading to temporary discontinuation and dose reduction were reported in 63% and 24% of patients in the lorlatinib group and 58% and 15% of patients in the crizotinib group, respectively. Fewer patients had AEs leading to permanent treatment discontinuation in the lorlatinib arm (9%) compared with the crizotinib arm (13%).
Discussion
The CROWN trial was the first to evaluate the efficacy and safety of a third-generation ALK inhibitor, lorlatinib, compared with crizotinib in patients with untreated ALK-positive NSCLC. The findings from this subgroup analysis of Asian patients located in Japan, South Korea, People’s Republic of China, Taiwan, Singapore, and Hong Kong are consistent with the primary analysis results in the overall population of the CROWN trial.15
Patient demographics and disease characteristics were generally well balanced between treatment groups and were consistent with those of the overall population in the CROWN trial,15,23 with the exception of a slightly greater imbalance of male versus female patients between treatment groups. The efficacy results in this Asian subgroup are also consistent with those in the overall population.15,23 A clinically meaningful improvement in PFS was observed in patients who received first-line lorlatinib, compared with those who received crizotinib, and was evident in both the BICR and investigator-assessed end points. Although the median duration of PFS in the lorlatinib group was NR, the HR for disease progression or death was 0.40 (95% CI: 0.23–0.71), as assessed by BICR, which corresponds to a 60% lower rate of progression or death with lorlatinib versus crizotinib. This is comparable with the HR for disease progression or death in the overall population (0.27).23 Furthermore, after 36 months, 61% of patients in the lorlatinib arm remained alive without progression compared with only 25% of patients in the crizotinib arm. These results are consistent with those in the overall population (64% versus 19%), respectively.23 A consistent and clinically meaningful improvement in the ORR was observed with lorlatinib compared with crizotinib (78% versus 57%). In patients with brain metastases at baseline, the intracranial ORR was numerically higher in the lorlatinib group than in the crizotinib group (73% versus 20%). Results from the other secondary end point analyses also confirmed the favorable efficacy of lorlatinib.15,23
Our biomarker analysis reveals that the subtype of EML4::ALK variants at baseline did not affect the clinical efficacy of lorlatinib in the Asian population. Although no formal statistical analysis was possible, ORRs were higher in the lorlatinib arm than in the crizotinib arm in patients with most variants (EML4::ALK variants 1, 3, and others). Patients with EML4::ALK variant 3, which has been found to more easily develop ALK kinase domain resistance mutations (specifically ALK G1202R), derived benefit from lorlatinib treatment, suggesting that the broader coverage and potency of lorlatinib may improve several efficacy measures for this specific variant compared with crizotinib; furthermore, lorlatinib benefits extended across all ALK fusion subgroups. Outcomes were also improved in the lorlatinib group versus the crizotinib group regardless of TP53 mutation status.
Overall, the safety profile of lorlatinib in this subgroup analysis is consistent with that in the overall population in the CROWN trial. The most common AEs in both the Asian patient group and the overall population were hypercholesterolemia and hypertriglyceridemia. Grade 3 or 4 AEs were more frequent with lorlatinib than crizotinib (80% versus 62%). Discontinuations due to AEs were similar between the Asian subgroup and the overall population in patients treated with lorlatinib (9% versus 7%) and were lower than in the crizotinib group. Cognitive and mood-related AEs are known adverse effects of lorlatinib. Overall, fewer mood-related AEs were reported with lorlatinib in the Asian subgroup compared with the overall population (10% versus 16%), which is similar to previous findings.16 The incidence of cognitive AEs was similar in the lorlatinib arms between the Asian subgroup and the overall population (25% versus 21%, respectively). Recommendations for the management of cognitive AEs have been published; these AEs can largely be managed with dose reduction or interruption, and frequent monitoring should be carried out.16,24,25 Moreover, we did not find any notable differences in lorlatinib PK between the Asian and overall populations.
Prevention and control of brain metastases are of prime importance for the relatively young patient population with ALK-positive NSCLC.26 The data here reveal high intracranial efficacy of lorlatinib for Asian patients with CNS metastases (73% ORR). Previously, the second-generation ALK TKI brigatinib was found to have objective intracranial responses in 60% of patients treated with brigatinib in the Asian subpopulation of the ALTA-1L trial; however, crosstrial comparisons should be made with caution.27
Given the nature of the subgroup analyses, small patient populations make it difficult to draw sound conclusions. Nevertheless, in totality, these data reveal consistent and favorable outcomes with lorlatinib compared with crizotinib.
The findings of this subgroup analysis support the third-generation ALK TKI, lorlatinib, as a highly effective and safe first-line treatment option for Asian patients with ALK-positive NSCLC. Overall, efficacy findings were consistent with those in the overall study population with no new safety signals identified.
CRediT Authorship Contribution Statement
Yi-Long Wu: Data curation, Methodology, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Qing Zhou: Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Ross A. Soo: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Gee-Chen Chang: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Chao-Hua Chiu: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Hidetoshi Hayashi: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Sang-We Kim: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Shunsuke Teraoka: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Yasushi Goto: Data curation, Methodology, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Jianying Zhou: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Victor Ho-Fun Lee: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Dong-Wan Kim: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Baohui Han: Data curation, Investigation, Writing—original draft, Writing—review and editing.
James Chung Man Ho: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Chia-Chi Lin: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Shun Lu: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Anna Polli: Data curation, Methodology, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Anna Maria Calella: Formal analysis, Writing—original draft, Writing—review and editing.
Jean-François Martini: Data curation, Methodology, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Chew Hooi Wong: Formal analysis, Writing—original draft, Writing—review and editing.
Tony Mok: Data curation, Investigation, Writing—original draft, Writing—review and editing.
Hye Ryun Kim: Data curation, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Acknowledgments
This study was sponsored by Pfizer Inc. Editorial and medical writing support was provided by Annette Smith, PhD, and Laura George, PhD, CMPP, of CMC AFFINITY, McCann Health Medical Communications, and Alana Dorfstatter, PhD, of ClinicalThinking, Inc., and was funded by Pfizer. The authors thank the participating patients and their families, including the research nurses, trial coordinators, and operations staff. The authors also thank Deborah Shepard for support with the biomarker analyses for this study. All authors critically reviewed the manuscript and approved the final version for submission.
Data Sharing Statement
On request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Footnotes
Disclosure: Dr. Wu reports providing advisory services for AstraZeneca, Boehringer Ingelheim, Novartis, and Takeda; receiving personal fees from AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Merck Sharp & Dohme, Pfizer, Roche, and Sanofi; and receiving grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Hengrui, and Roche, outside the submitted work. Dr. Q. Zhou reports receiving honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Merck Sharp & Dohme, Pfizer, Roche, and Sanofi, outside the submitted work. Dr. Soo reports providing consulting for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Pfizer, Roche/Genentech, Taiho Pharmaceutical, Yuhan, Takeda, Amgen, Lilly, and Merck; receiving honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Novartis, Pfizer, Roche/Genentech, Takeda, Yuhan, Amgen, Bayer, and Merck; and receiving research funding from AstraZeneca and Boehringer Ingelheim. Dr. Chiu reports receiving honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharma, Lilly, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, Pfizer, Roche, and Takeda and providing consulting for Lilly, Merck Sharp & Dohme, Novartis, and Roche. Dr. Hayashi reports receiving research grants from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. Dr. Teraoka reports receiving honoraria from Chugai Pharma, Novartis, AstraZeneca, Taiho Pharmaceutical, Ono Pharmaceutical, and Boehringer Ingelheim. Dr. Goto reports receiving grant support, lecture fees, and advisory board fees from Lilly, Taiho Pharmaceutical, Pfizer, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, Kyorin Pharmaceutical, and Bristol Myers Squibb; lecture fees and advisory board fees from Chugai Pharmaceutical, Boehringer Ingelheim, and AstraZeneca; grant support and advisory board fees from Guardant Health and Daiichi Sankyo; and advisory board fees from Illumina. Dr. Lee reports receiving honoraria from Roche, Pfizer, AstraZeneca, Takeda, Novartis, Merck Sharp & Dohme, Lilly, and Amgen and research support grants from AstraZeneca, Pfizer, and Varian Medical Systems. Dr. D-W Kim reports receiving travel support for advisory board meeting attendance from Daiichi Sankyo and Amgen and research funding to affiliated institution from Alpha Biopharma, Amgen, AstraZeneca/MedImmune, Boehringer Ingelheim, Daiichi Sankyo, Hanmi, Janssen, Pfizer, SK Biopharmaceuticals, Takeda, and Yuhan. Dr. Ho reports receiving honoraria from AstraZeneca, Roche, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme, Lilly, Bristol Myers Squibb, Novartis, and Takeda and grants from Roche and AstraZeneca. Dr. Lin reports receiving honoraria from Lilly, Novartis, and Roche; providing consulting for AbbVie, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Merck KGaA, Novartis, and PharmaEngine; and receiving travel grants from BeiGene and Lilly. Dr. Lu reports receiving grants from AstraZeneca, Hutchison MediPharma, Bristol Myers Squibb, Heng Rui, and Roche; speakers’ fees from AstraZeneca, Roche, and Hansoh Pharma; and consultant fees from AstraZeneca, Boehringer Ingelheim, Hutchison MediPharma, Simcere, ZaiLab, GenomiCare, and Roche. Ms. Polli, Dr. Calella, Dr. Martini, and Dr. Wong are employees of Pfizer Inc. and may own stock or stock options in Pfizer. Dr. Mok reports receiving research funding from AstraZeneca, Bristol Myers Squibb, Clovis Oncology, G1 Therapeutics, Merck Sharp & Dohme, Merck Serono, Novartis, Pfizer, Roche, SFJ, Takeda, and Xcovery; receiving speaker fees from ACEA Pharma, Alpha Biopharma Co., Ltd., Amgen, Amoy Diagnostics Co., Ltd., AstraZeneca (before January 2019), Boehringer Ingelheim, Bristol Myers Squibb, Lilly, InMed Medical Communication, Merck Sharp & Dohme, Novartis, Pfizer, prIME Oncology, Roche/Genentech, and Taiho; receiving honoraria from AbbVie, Inc., ACEA Pharma, Alpha Biopharma Co., Ltd., Amgen, Amoy Diagnostics Co., Ltd., AstraZeneca, Bayer, Boehringer Ingelheim, Blueprint Medicines Corporation, Bristol Myers Squibb, Celgene, CStone Pharmaceuticals, Daiichi Sankyo, Lilly, and Fishawack; having shares in Hutchison Chi-Med and Sanomics Ltd.; and providing advisory board service for AbbVie, Inc., ACEA Pharma, Amgen, AstraZeneca, Bayer, Blueprint Medicine Corporation, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cirina, CStone Pharmaceuticals, Daiichi Sankyo, Lilly, Fishawack Facilitate, Ltd., G1 Therapeutics, Inc., and GeneDx. Dr. H. R. Kim reports providing advisory services for AstraZeneca and Merck Sharp & Dohme and receiving grants from AstraZeneca, Bristol Myers Squibb, Ono, and Roche, outside the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Zhou Q, Soo RA, Chang GC, et al. Asian subgroup analysis of the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive NSCLC. JTO Clin Res Rep. 2023;4:100499.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100499.
Supplementary Data
References
- 1.Koivunen J.P., Mermel C., Zejnullahu K., et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong D.W., Leung E.L., So K.K., et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T., Sonobe M., Kobayashi M., et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17:889–897. doi: 10.1245/s10434-009-0808-7. [DOI] [PubMed] [Google Scholar]
- 4.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 5.Hanna N., Johnson D., Temin S., et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update summary. J Oncol Pract. 2017;13:832–837. doi: 10.1200/JOP.2017.026716. [DOI] [PubMed] [Google Scholar]
- 6.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali A., Goffin J.R., Arnold A., Ellis P.M. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20:e300–e306. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer T.M., Shaw A.T., Johnson M.L., et al. Brain penetration of lorlatinib: cumulative incidences of CNS and non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 2020;15:55–65. doi: 10.1007/s11523-020-00702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon B.J., Mok T., Kim D.W., et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 10.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 11.Soria J.C., Tan D.S.W., Chiari R., et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 12.Camidge D.R., Kim H.R., Ahn M.-J., et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 13.Shaw A.T., Felip E., Bauer T.M., et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon B.J., Besse B., Bauer T.M., et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 15.Shaw A.T., Bauer T.M., de Marinis F., et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 16.Bauer T.M., Felip E., Solomon B.J., et al. Clinical management of adverse events associated with lorlatinib. Oncologist. 2019;24:1103–1110. doi: 10.1634/theoncologist.2018-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto Y., Yamamoto N., Masters E.T., et al. Treatment sequencing in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer in Japan: a real-world observational study. Adv Ther. 2020;37:3311–3323. doi: 10.1007/s12325-020-01392-0. [DOI] [PubMed] [Google Scholar]
- 18.Jahanzeb M., Lin H.M., Pan X., et al. Real-world treatment patterns and progression-free survival associated with anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor therapies for ALK+ non-small cell lung cancer. Oncologist. 2020;25:867–877. doi: 10.1634/theoncologist.2020-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo C.W., Lee S.J., Lee S.S., et al. Discovery of a novel allelic variant of CYP2C8, CYP2C8∗11, in Asian populations and its clinical effect on the rosiglitazone disposition in vivo. Drug Metab Dispos. 2011;39:711–716. doi: 10.1124/dmd.110.035899. [DOI] [PubMed] [Google Scholar]
- 20.Murphy S.E., Park S.S., Thompson E.F., et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–2533. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seto T., Hayashi H., Satouchi M., et al. Lorlatinib in previously treated anaplastic lymphoma kinase-rearranged non-small cell lung cancer: Japanese subgroup analysis of a global study. Cancer Sci. 2020;111:3726–3738. doi: 10.1111/cas.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw A.T., Solomon B.J., Besse B., et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon B.J., Bauer T.M., Mok T., et al. Abstract CT233: Updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive non-small-cell lung cancer. Cancer Res. 2022;82(suppl 12):CT223. doi: 10.1016/j.lungcan.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Reed M., Rosales A.S., Chioda M.D., Parker L., Devgan G., Kettle J. Consensus recommendations for management and counseling of adverse events associated with lorlatinib: a guide for healthcare practitioners. Adv Ther. 2020;37:3019–3030. doi: 10.1007/s12325-020-01365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasaka M., Ge Y., Sukari A., Kukreja G., Ou S.I. A user’s guide to lorlatinib. Crit Rev Oncol Hematol. 2020;151 doi: 10.1016/j.critrevonc.2020.102969. [DOI] [PubMed] [Google Scholar]
- 26.Nagasaka M., Ou S.I. Lorlatinib should be considered as the preferred first-line option in patients with advanced ALK-rearranged NSCLC. J Thorac Oncol. 2021;16:532–536. doi: 10.1016/j.jtho.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Ahn M.J., Kim H., Yang J.C.H., et al. Brigatinib (BRG) versus crizotinib (CRZ) in Asian versus non-Asian patients (pts) in the phase III ALTA-1L trial. J Clin Oncol. 2019;37(suppl 15) 9026–9026. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.