Abstract
The pancreatic hormone glucagon activates the glucagon receptor (GCGR), a class B seven-transmembrane G protein-coupled receptor that couples to the stimulatory heterotrimeric G protein and provokes PKA-dependent signaling cascades vital to hepatic glucose metabolism and islet insulin secretion. Glucagon-stimulation also initiates recruitment of the endocytic adaptors, βarrestin1 and βarrestin2, which regulate desensitization and internalization of the GCGR. Unlike many other G protein-coupled receptors, the GCGR expressed at the plasma membrane is constitutively ubiquitinated and upon agonist-activation, internalized GCGRs are deubiquitinated at early endosomes and recycled via Rab4-containing vesicles. Herein we report a novel link between the ubiquitination status and signal transduction mechanism of the GCGR. In the deubiquitinated state, coupling of the GCGR to Gs is diminished, while binding to βarrestin is enhanced with signaling biased to a βarrestin1–dependent p38 mitogen activated protein kinase (MAPK) pathway. This ubiquitin-dependent signaling bias arises through the modification of lysine333 (K333) on the cytoplasmic face of transmembrane helix V. Compared with the GCGR-WT, the mutant GCGR-K333R has impaired ubiquitination, diminished G protein coupling, and PKA signaling but unimpaired potentiation of glucose-stimulated–insulin secretion in response to agonist-stimulation, which involves p38 MAPK signaling. Both WT and GCGR-K333R promote the formation of glucagon-induced βarrestin1–dependent p38 signaling scaffold that requires canonical upstream MAPK-Kinase3, but is independent of Gs, Gi, and βarrestin2. Thus, ubiquitination/deubiquitination at K333 in the GCGR defines the activation of distinct transducers with the potential to influence various facets of glucagon signaling in health and disease.
Keywords: beta-cell, insulin, MAPK, beta-arrestin, scaffold, adaptor protein, G protein, luciferase
The peptide hormone glucagon secreted by the pancreatic α-cells and the class B seven-transmembrane G protein-coupled receptors (GPCRs) that are activated by glucagon play a fundamental role in regulating blood glucose levels. Glucagon activates two class B GPCRs, namely, the glucagon receptor (GCGR) and the glucagon-like peptide 1 receptor (GLP-1R) (1). These two receptors are expressed in pancreatic beta-cells and can promote insulin secretion in response to glucagon stimulation, although the GLP-1R is activated by glucagon with a lower potency than the GCGR (2, 3, 4, 5). The ability of these receptors to regulate insulin release have established both GLP-1R and GCGR as major targets for developing new treatments for type II diabetes. Additionally, secreted glucagon acts on the GCGR expressed in hepatocytes and not only enhances glucose production but also regulates amino acid and lipid metabolism (6, 7, 8).
Agonist-stimulation of the GCGR provokes coupling of stimulatory heterotrimeric G protein (Gs) and subsequent increase in cellular cAMP leading to the activation of PKA-dependent signaling cascades that have been linked to glycogenolysis and gluconeogenesis in liver hepatocytes and insulin secretion in pancreatic islets (2, 9). In addition to inducing G protein signaling, activated GCGR engages additional versatile transducer proteins, namely, (i) GPCR kinases (GRKs) that phosphorylate seryl/threonyl residues in the GCGR and (ii) βarrestins (βarrs) that serve as multifunctional endocytic adaptors (10, 11, 12, 13, 14). Two highly homologous isoforms of βarr (βarr1 and βarr2) bind to agonist-activated GPCRs to block G protein coupling and attenuate G protein signaling (10, 14, 15). βArrs also promote internalization and trafficking of activated GPCRs and serve as signal transducers to promote endosomal signaling (14, 16).
The posttranslational modification known as ubiquitination has been shown to regulate the intracellular trafficking and signaling of a growing list of GPCRs, by modifying either the GPCR itself, GRK2, βarr2, or other associated proteins (17, 18, 19). Although ubiquitination of mammalian GPCRs and its functional role was reported 2 decades ago (20, 21), ubiquitination of the GCGR was uncovered only recently (22). GCGR localized at the plasma membrane is ubiquitinated in quiescent cells and agonist-stimulation provokes rapid deubiquitination of internalized GCGRs by two distinct enzymes: (1) ubiquitin-specific peptidase 33 (USP33) and (2) signal transducing adaptor molecule–binding protein (STAMBP) (22). For a handful of GPCRs, ubiquitination of either the receptor or of βarr2 has been shown to promote mitogen-activated protein kinase (MAPK) signaling through canonical or noncanonical activation mechanisms (23, 24, 25, 26). We therefore evaluated signaling properties of the GCGR constructs impaired in ubiquitination and found a novel link between the ubiquitination profile and signal transduction competency of the GCGR: when basally ubiquitinated, the GCGR signals through Gs coupling as well as via βarr1 signaling, whereas when locked in a deubiquitinated state, the GCGR couples poorly to Gs and the signaling is biased toward a βarr1-dependent mechanism.
Results
GCGR-5KR provokes diminished G protein-mediated signaling but enhanced βarrestin association and p38 MAPK activation
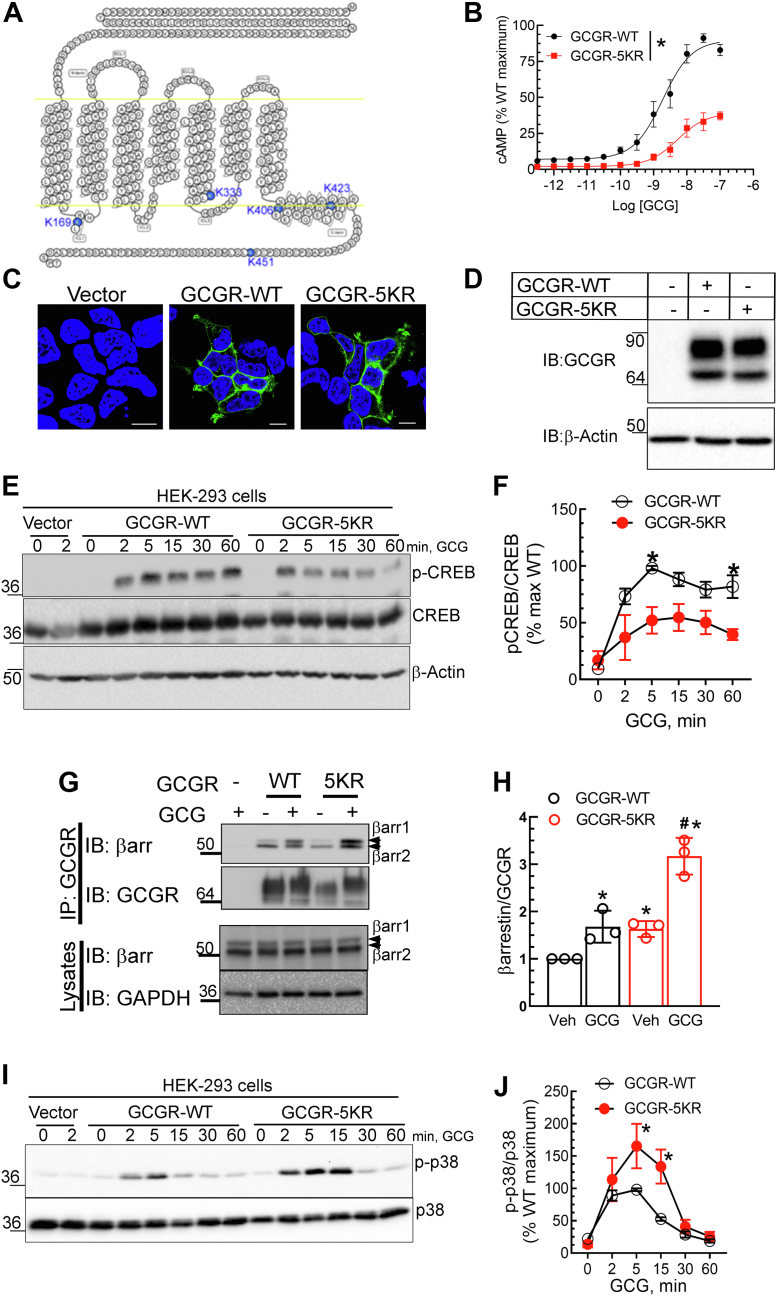
To evaluate whether the ubiquitination status of the GCGR affects its signaling properties, we compared GCGR-5KR (Fig. 1A) with GCGR-WT for its ability to activate the heterotrimeric G protein Gs and generate cAMP after agonist-stimulation. We observed a rightward shift of the cAMP concentration-response curve (5-fold change in EC50) for GCGR-5KR relative to GCGR-WT, in addition to a 3-fold reduction in the maximal response (E-max) (Fig. 1B). This substantial decrease in G protein activation by GCGR-5KR is not attributed to differences in receptor expression as revealed by confocal microscopy (Fig. 1C) and Western blotting (Fig. 1D). Additionally, we observed a corresponding reduction of glucagon-induced phosphorylation of cAMP response element-binding protein (CREB) at Ser133, by GCGR-5KR compared with GCGR-WT (Fig. 1, E and F). Ser133 in CREB is predominantly phosphorylated via the cAMP/PKA pathway (27, 28). Taken together, GCGR-5KR displays deficiency in ubiquitination (22), cAMP generation, and PKA activity (Fig. 1, A–F). Despite these deficiencies in Gs activation, we detected an augmentation in the association of endogenous βarr with the GCGR-5KR compared to GCGR-WT as assessed by co-immunoprecipitation (Fig. 1, G and H). βarr 1 and βarr2 are not only involved in the desensitization and internalization of GPCR proteins but are also known to scaffold MAPKs leading to βarr–mediated signaling (10, 16, 29, 30, 31, 32). Our assays revealed a more robust activation of p38 MAPK by GCGR-5KR than by GCGR-WT upon agonist-stimulation (Fig. 1, I and J), while agonist-induced p42/p44 ERK activation was only minimally augmented by GCGR-5KR compared to GCGR-WT (Fig. S1). Accordingly, differential ubiquitination status of the GCGR promotes differences at transducer coupling of the GCGR. A deubiquitinated state of the GCGR facilitates binding of βarrs and activation of p38 MAPK while concomitantly reducing G protein coupling.
Figure 1.
GCGR-5KR is significantly impaired in cAMP generation and G protein-dependent signaling but enhanced in βarrestin association as well as p38 MAP Kinase activation compared with WT GCGR.A, snake-plot (gpcrdb.org) of glucagon receptor (GCGR) highlighting intracellular lysines in blue that are mutated to arginine in GCGR-5KR. B, cAMP production of WT and 5KR plotted as a percent normalized to maximal level of cAMP generated by GCGR-WT. Data are comprised of means ± SEM from six independent experiments. Representative confocal images (C) and immunoblots (D) that reveal comparable receptor expression levels of WT and 5KR. The scale bar represents 10 μm. E, PKA phosphorylation of CREB (Ser133) by WT and 5KR. F, quantification for phospho-CREB, normalized to total CREB shown are means ± SEM of three independent experiments. ∗p < 0.05 WT versus 5KR, two-way ANOVA, Holm-Šídák's multiple comparisons test. G, GCGR-WT or GCGR-5KR were immunoprecipitated using M2 anti-Flag affinity agarose (Sigma-Aldrich) and eluted samples as well as lysate inputs were immunoblotted for the indicated proteins. GCGR was detected using a MYC IgG (Cell Signaling Technology). H, the scatter plot with bar represents the quantification for the binding of βarr1/2 to WT or mutant 5KR receptor from three independent experiments. ∗p < 0.05 versus Veh, WT; #p < 0.05 versus all other samples, two-way ANOVA, and Holm-Šídák's multiple comparisons test. I, GCGR-WT or GCGR-5KR expressing HEK-293 cells were stimulated with 100 nM glucagon for the indicated times after serum starvation and whole cell extracts were analyzed by immunoblotting. J, line graphs summarize data for phospho-p38 normalized to cognate total p38 from three independent experiments. ∗p < 0.05 versus WT, two-way ANOVA, and Holm-Šídák's multiple comparisons test. The mobility of molecular weight markers (kDa) is shown beside each blot panel. CREB, cAMP response element-binding protein; MAPK, mitogen-activated protein kinase.
Lysine333 is a critical site in the GCGR for engendering ubiquitin-dependent signal bias between cAMP production and p38 MAPK activation
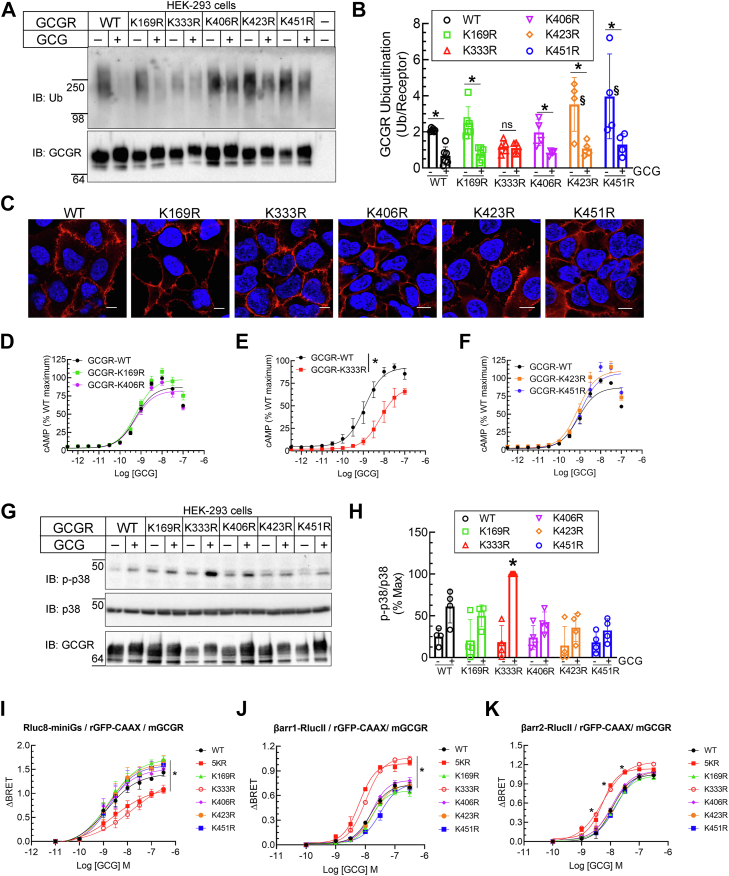
To evaluate the contribution of each of the five lysine residues that are putative target sites for ubiquitination, we generated and tested five separate GCGR mutants, each with one of the lysines (K169, K333, K406, K423, or K451) mutated to arginine. In our ubiquitination assays, all of these mutants with only a single lysine changed to arginine, recapitulated the pattern of glucagon-induced deubiquitination possessed by the WT, except the mutant GCGR-K333R, which showed markedly less ubiquitination at baseline and no further decrease after glucagon stimulation (Fig. 2, A and B). The GCGR mutants, K423R and K451R presented greater basal ubiquitination than GCGR-WT but nonetheless were rapidly deubiquitinated with agonist stimulation (Fig. 2, A and B). Each of the GCGR single lysine mutant showed normal expression at the cell membrane, which was equivalent to the pattern obtained for GCGR-WT as assessed by immunostaining and confocal microscopy (Fig. 2C). As observed with the GCGR-5KR, glucagon-induced cAMP accumulation was also significantly reduced in cells expressing GCGR-K333R compared with GCGR-WT and the other four lysine mutants as shown by both a reduced potency (6-fold increase in EC50 for GCGR-K333R) and efficacy (30% decrease in E-max), suggesting a direct correlation between GCGR ubiquitination status and G protein activation (Fig. 2, D–F). In stark contrast to the blunted effect on cAMP response, GCGR-K333R promoted a much more robust p38 MAPK activation than GCGR-WT and all other single GCGR single lysine mutants (Fig. 2, G and H). In essence, the signal bias between G protein coupling and p38 MAPK activation invoked by the GCGR-5KR was replicated by the GCGR-K333R, suggesting that Lys333 is the site targeted for GCGR ubiquitination at the basal state and for deubiquitination after agonist activation. Our results suggest that ubiquitin tag appended at Lys333 in the GCGR can define its potency for G protein coupling. Our data also suggest that in a deubiquitinated state (as mimicked by the ubiquitin-impaired GCGR-K333R), the GCGR is poised for promoting robust p38 MAPK signaling.
Figure 2.
Lysine 333 in the GCGR is a critical site for engendering ubiquitin-dependent signal bias between G protein recruitment/cAMP production and βarrestin association/p38 MAPK activation.A, GCGR ubiquitination for WT and constructs with single lysine mutation was detected using the anti-ubiquitin antibody, FK1 (Enzo Life Sciences). The blot was then reprobed with an antibody that detects the MYC tag (Cell Signaling Technology). B, ubiquitinated smear was quantitated and normalized to cognate receptor bands and plotted as ratio. The scatter graph with bars presents receptor:ubiquitin ratio from six (WT, K169R), five (K333R, K406R), or four (K423R, K451R) independent experiments. ∗p ≤ 0.05 versus respective nonstimulated, §p < 0.05 versus WT nonstimulated, two-way ANOVA, Holm-Šídák's multiple comparisons test. C, confocal images of immunostaining of single lysine mutant expressing stable HEK-293 cells with anti-MYC IgG followed by secondary IgG conjugated with Alexa594 (Red channel). 4′,6-diamidino-2-phenylindole (DAPI) staining was used to label nuclei. The scale bar represents 10 μm. D–F, HEK-293 cells were transiently transfected with the GCGR-WT or the indicated GCGR lysine mutant and cAMP generation was determined as in Figure 1B. Concentration-response curves are comprised of means ± SEM from three independent experiments. ∗p < 0.05, WT versus K333R, two-way ANOVA, Holm-Šídák's multiple comparisons test. G, HEK-293 cells stably expressing WT or indicated single lysine mutants were stimulated with 100 nM glucagon for 15 min and solubilized cell extracts were immunoblotted as indicated. H, the scatter plot with bar summarizes the quantification of phospho p38 normalized to total p38, from four independent experiments plotted as percent maximal response ∗p < 0.05 versus all the other samples, two-way ANOVA, Holm-Šídák's multiple comparisons test. I, enhanced bystander BRET measured between Rluc8-miniGs and the plasma membrane marker rGFP-CAAX to monitor mini-Gs recruitment to the active GCGR WT, single lysine mutants or 5KR mutant after 10 min of GCG stimulation at indicated doses. Data are shown as means ± SEM (N = 3). ∗p < 0.01 for WT versus 5KR, K333R, and K169R, two-way ANOVA, Holm-Šídák's multiple comparisons test. Enhanced bystander BRET between (J) βarrestin1-RlucII or (K) βarrestin2-RlucII and the plasma membrane marker rGFP-CAAX to monitor βarrestin recruitment to the active GCGR-WT, single lysine mutants or 5KR mutant after 10 min of GCG at indicated doses. Data are represented as the means ± SEM (N = 3). ∗p < 0.01 for 5KR, K333R, versus WT in panel J and ∗p < 0.01 for WT versus 5KR and K333R as indicated in panel K; two-way ANOVA, Holm-Šídák's multiple comparisons test. The mobility of molecular weight markers (kDa) is shown beside each blot panel. GCGR, glucagon receptor; MAPK, mitogen-activated protein kinase.
To ascertain if the ubiquitination status of GCGR influences its interaction with G protein complexes and βarr isoforms, we utilized the recently developed enhanced bystander bioluminescence resonance energy transfer (ebBRET) assay (33, 34, 35). We employed mini-Gs (35) and βarr, which are tagged with Renilla Luciferase (RLuc) and assessed their agonist-induced translocation to rGFP-tagged plasma membrane marker (rGFP-CAAX) in HEK-293 cells expressing WT or mutant GCGR (Fig. 2, I–K). Agonist concentration-dependent recruitment of mini-Gs to the plasma membrane in cells expressing GCGR-K333R or GCGR-5KR was significantly impaired as compared with cells expressing GCGR-WT or all other lysine mutants as reflected by a decrease in both the potency and efficacy of glucagon to promote mini-Gs recruitment (Fig. 2I). Accordingly, we infer that the association of mini-Gs is weakened when the GCGR is in a deubiquitinated state. We next assessed agonist-induced BRET between βarr1-RLucII and rGFP-CAAX (Fig. 2J) as well as βarr2-RLucII and rGFP-CAAX (Fig. 2K) in HEK-293 cells expressing GCGR-WT or each GCGR lysine mutant construct. GCGR-K333R, and GCGR-5KR, that were impaired in coupling to mini-Gs, showed enhanced βarr1 (Fig. 2J) as well as βarr2 recruitment (Fig. 2K) than either GCGR-WT or other GCGR single lysine mutants. This was reflected by an increase in potency and efficacy for βarr1 and potency only for βarr2 for glucagon-promoted recruitment to the plasma membrane. These results support our inference that in a deubiquitinated state, the GCGR is poised for increased βarr association, and decreased Gs coupling than in a ubiquitinated state.
GCGR-induced p38 MAPK activation is dependent on βarrestin1 and canonical upstream kinase MKK3 but not on βarrestin2 or noncanonical upstream kinase TAB1
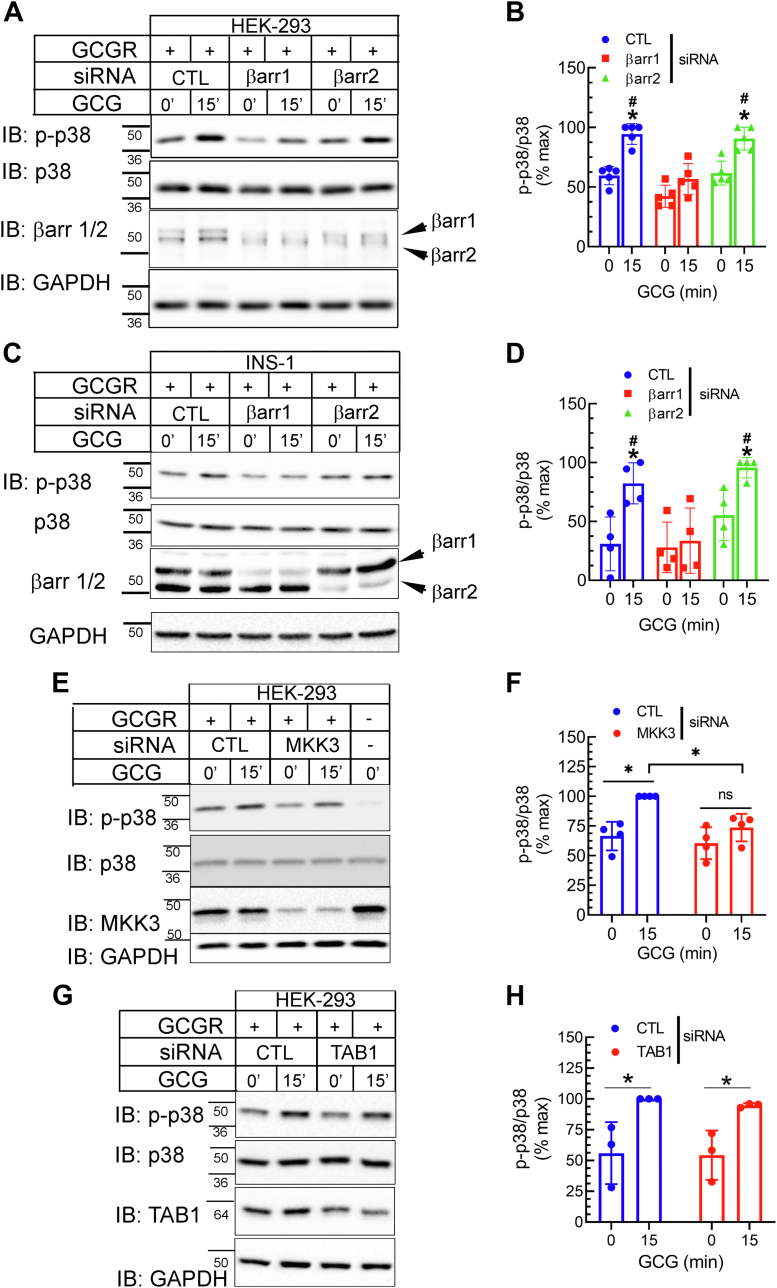
βarrs are multifunctional adaptor proteins, and not only do they block G protein coupling but also promote GPCR endocytosis and act as scaffolds for propagating and localizing MAPK activities (14, 15, 32, 36). Despite sharing 78% amino acid identity and overlapping functions in GPCR desensitization and trafficking, the two βarr isoforms can have nonredundant roles in signal transduction (37, 38, 39). Thus, to delineate the contribution of individual βarr in the activation of p38 MAPK we silenced their gene expression using previously validated siRNA targeting each isoform (16) and analyzed the effect on GCGR-induced phosphorylation of p38 MAPK. In our assays, 48 h after transient transfection with respective siRNA oligonucleotides the abundance of the targeted isoform(s) was reduced by 85% for βarr1 and by 80% for βarr2. Knockdown of βarr1 led to statistically significant reduction in GCGR-stimulated p38 MAPK activation compared to control knockdown conditions, whereas βarr2 knockdown in the continuous presence of βarr1 produced little change compared to samples with control siRNA knockdown (Fig. 3, A and B). We tested the same experimental samples for the levels of GCGR-induced phospho-CREB and found that neither βarr1 or βarr2 knockdown affected glucagon-activated CREB (Fig. S2, A and B). In order to ascertain whether the βarrestin-mediated p38 MAPK activation is applicable to other model systems, we also tested the effect of siRNA-mediated knockdown of each βarr isoform in the widely used incretin-responsive INS-1 β-cell line 832/3 (40). The knockdown efficiency for each βarr isoform in INS-1 cells was comparable to what we obtained in HEK-293 cells. As in HEK-293 cells, GCGR-induced p38 MAPK activity was almost completely abolished in INS-1 cells with βarr1 knockdown as compared with cells transfected with control siRNA or a βarr2 targeting siRNA (Fig. 3, C and D). These data indicate that βarr1 selectively promotes GCGR-induced p38 MAPK activity, while βarr2 appears to have no major role in this signaling pathway.
Figure 3.
GCGR-induced p38 activation is dependent on βarrestin1 and canonical upstream kinase MKK3.A, HEK-293 cells expressing GCGR-WT were transfected with siRNA targeting either no mRNA (CTL), βarr1 or βarr2. Serum-starved cells were stimulated with 100 nM GCG for 15′ and lysates were immunoblotted sequentially for the indicated proteins. B, phospho-p38 bands were normalized to cognate p38 bands and summarized as a percent of experimental maximum from four independent experiments ∗p < 0.05 versus respective 0 min; #p < 0.05 versus βarr1 knockdown samples; two-way ANOVA and Holm-Šídák's multiple comparisons test. C and D, INS-1832/3 cells were transfected with siRNA as in panel A along with GCGR-WT plasmid and stimulation and analyses were as in panels A and B. ∗p < 0.05 versus respective 0 min; #p < 0.05 versus βarr1 knockdown samples; two-way ANOVA and Holm-Šídák's multiple comparisons test. E–H, HEK-293 cells expressing GCGR-WT were transfected with control (CTL) siRNA that has no mRNA target or siRNA targeting MKK3 (E and F) or TAB1 (G and H). Serum-starved cells were stimulated with 100 nM GCG for 15′ and lysates were immunoblotted sequentially as indicated. F and H, phospho-p38 bands were normalized to cognate p38 bands and summarized as a percent of experimental maximum from four (F) or three (H) independent experiments ∗p < 0.05 as indicated; two-way ANOVA and Holm-Šídák's multiple comparisons test. The mobility of molecular weight markers (kDa) is shown beside each blot panel. GCGR, glucagon receptor; TAB1, TGF-β-activated protein kinase binding protein 1.
p38 MAPK is activated via a cascade of phosphorylation events involving at least two other kinases acting sequentially. The first step is activation of one of ten potential MAP3Ks, which can in turn phosphorylate and activate one of three potential MAP2Ks (41) thus leading p38 MAPK phosphorylation. When activated, MAP2Ks directly phosphorylate the activation loop of p38 on Thr and Tyr residues, leading to a conformational change that results in kinase activation (41). Of the three MAP2Ks, namely, MKK3, MKK4, and MKK6 expressed in mammalian cells, MKK3 is a commonly employed upstream kinase that specifically targets p38 activation. p38 MAPK is also activated through a noncanonical mechanism that is independent of the MAP2Ks and involves autophosphorylation triggered by the binding of TGF-β-activated protein kinase 1 (TAK1) binding protein 1 (TAB1) to p38 MAPK (42). To elucidate the pathways responsible for the phosphorylation of p38 MAPK induced by glucagon, we employed siRNA targeting MKK3 and TAB1 which led to >85% knockdown of target protein in each case and tested their effects on GCGR-induced p38 phosphorylation. GCGR-induced phosphorylation of p38 MAPK was significantly reduced when we knocked down MKK3 in comparison to cells transfected with control siRNA (Fig. 3, E and F). Conversely, knockdown of TAB1 had no effect on glucagon-induced p38 MAPK activation (Fig. 3, G and H). Additionally, MKK3 knockdown had no effect on GCGR-induced CREB activity (Fig. S2, C and D).
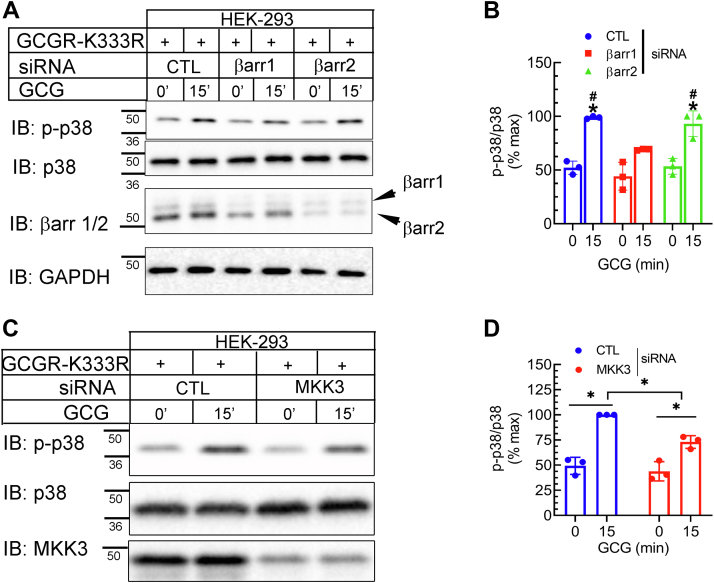
For the GCGR-K333R, βarr1 knockdown caused a significant reduction in p38 MAPK activation compared with control knockdown conditions, whereas βarr2 knockdown had no discernable effect on agonist-induced p38 MAPK activity (Fig. 4, A and B). Our experiments revealed substantial reduction in not only MKK3 expression but also p38 phosphorylation with MKK3 siRNA transfection, compared with control siRNA transfections (Fig. 4, C and D). These data indicate that GCGR-induced p38 MAPK activity proceeds through canonical mechanisms involving MKK3 and βarr1 and that CREB activation stimulated by glucagon, which is unaffected by βarr1 knockdown proceeds through mechanisms independent of MKK3.
Figure 4.
βarrestin1 is required for p38 activation induced by GCGR-K333R. HEK-293 cells expressing GCGR-K333R were transfected with siRNA targeting either no mRNA (control, CTL), βarr1 or βarr2 (A and B); or siRNA targeting CTL, or MKK3 (C and D) and analyzed by Western blotting as indicated. B, ∗p < 0.05 versus respective 0 min samples; #p < 0.05 versus βarr1, 15 min GCG samples. D, ∗p < 0.05 as indicated, two-way ANOVA, and Holm-Šídák's multiple comparisons test. The mobility of molecular weight markers (kDa) is shown beside each blot panel. GCGR, glucagon receptor.
GCGR-induced p38 MAPK activation is independent of PKA and inhibitory heterotrimeric G protein, Gi
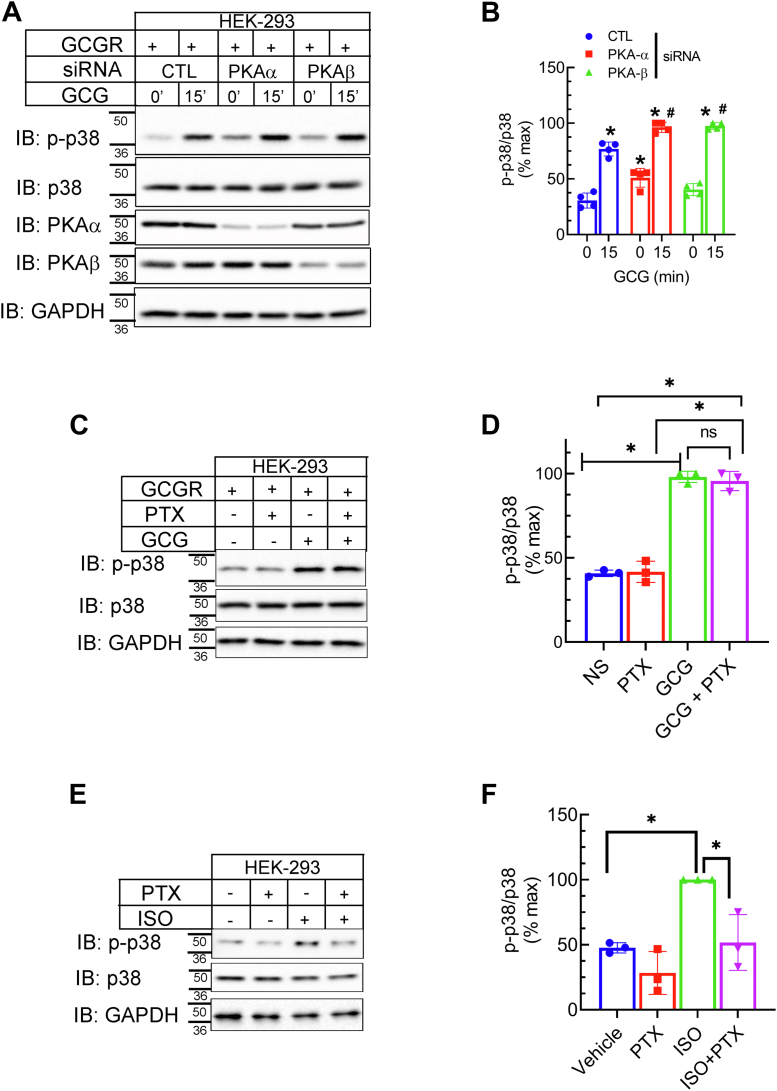
Prior studies have shown that p38 MAPK activity induced by β2AR agonist-stimulation proceeds in a biphasic manner, where the initial activation of p38 MAPK is βarr1-dependent, while the later phase is G protein/cAMP/PKA dependent (43). Therefore, to further address the mechanism of p38 MAPK activation via the GCGR, we silenced the expression of PKA isoforms, PKAα and PKAβ using previously validated siRNA (44, 45). While we obtained >80 to 85% reduction of respective PKA isoform with siRNA transfections, agonist-induced p38 MAPK activation was significantly increased compared with cells transfected with control siRNA (Fig. 5, A and B). Additionally, pretreatment with the PKA inhibitor, 6-22, also augmented p38 MAPK phosphorylation by ∼35% when compared with samples that were not treated with the inhibitor (Fig. S3, A and B). These data suggest that cAMP/PKA pathway might be inhibitory to GCGR-induced p38 MAPK activity. Using the same PKA knockdown experimental samples, we tested if GCGR-induced CREB activity is dependent on PKA expression (Fig. S2, E and F). We obtained a ∼40% decrease in the glucagon induced activation of CREB with the silencing of PKAα and no reduction with PKAβ knockdown as compared with CREB activity in control knockdown samples (Fig. S2, E and F). Additionally, pretreatment of cells with the PKA inhibitor 6-22 significantly reduced CREB activation (Fig. S3, C and D) with GCGR agonist-stimulation. Accordingly, we infer that in HEK-293 cells, PKA activity may impede GCGR-induced p38 MAPK activity and that PKAα is involved in GCGR-induced CREB activation.
Figure 5.
PKA and Gi are not critical for GCGR-induced p38 MAPK activation.A, HEK-293 cells stably expressing GCGR were transfected with siRNA targeting no mRNA (CTL), PKAα, or PKAβ. Forty-eight hours post-transfection the cells were serum-starved, stimulated ± GCG for 15′, and lysates were immunoblotted as indicated. B, phospho-p38 bands were normalized to cognate p38 bands and summarized as a percent of experimental maximum from four independent experiments ∗p < 0.05 versus respective 0 min; #p < 0.05 versus CTL, 15’; two-way ANOVA, Holm-Šídák's multiple comparisons test. C, HEK-293 cells expressing GCGR were treated ± 100 ng/ml pertussis toxin (PTX) for 16 h and then stimulated ± 100 nM GCG for 15’. Whole cell extracts were immunoblotted for indicated proteins. D, quantification of phospho-p38 was performed as in (B). ∗p < 0.05 as indicated, two-way ANOVA, Holm-Šídák's multiple comparisons test. E and F, assay and analyses were conducted as in C and D but cells were stimulated with 100 nM isoproterenol (ISO) to trigger endogenous β2AR-induced p38 activation. The mobility of molecular weight markers (kDa) is shown beside each blot panel. GCGR, glucagon receptor; MAPK, mitogen-activated protein kinase.
As MAPK activity generally triggered by GPCRs and by βarr-dependent mechanisms is sensitive to Bordetella pertussis toxin (PTX) treatment and linked with the recruitment and/or activation of the inhibitory Gi/o proteins (16, 46, 47, 48, 49) we also assessed the effect of PTX preincubation on GCGR-induced p38 MAPK activity (Fig. 5, C and D). Glucagon-induced p38 MAPK activity remained unchanged in cells pretreated with PTX, suggesting that GCGR-induced p38 MAPK phosphorylation is independent of Gi/o activity. Since the extent of PTX sensitivity of MAPK relies on the clonal properties of HEK-293 cells (50), we also tested the same cells that we used for glucagon stimulation, to assess p38 MAPK activity triggered by endogenously expressed β2ARs. Isoproterenol-stimulated phosphorylation of p38 MAPK was reduced by 60% in the presence of PTX, confirming that β2AR-induced p38 activity involves Gi proteins (Fig. 5, E and F). Overall, our data show that GCGR-induced p38 MAPK activation is not promoted by either Gs/PKA or Gi proteins in HEK-293 cells.
Diverse roles of βarr in promoting desensitization, trafficking, and deubiquitination in the overall framework of βarr bias at the GCGR
βarrs were originally discovered for their ability to block G protein coupling and dampen second messenger responses triggered by GPCR activation (10, 15). To dissect the role of βarr1 and βarr2 in dampening GCGR-induced cAMP generation, we undertook a gain-of-function approach, by reconstituting the expression of individual βarr in a βarr1/2 null background (16). We also used corresponding parental cells to evaluate the cAMP response in cells with endogenous βarrs (16). Compared with cAMP generated in the parental cells, the signals from CRISPR βarr1/2 KO were significantly increased, as evident from the increase in E-max; however, in the KO cells in which βarr expression was rescued, cAMP generation closely matched the pattern obtained in the parental cells (Fig. S4, A and B). We also confirmed the expression of endogenous, and exogenous βarrs as well as that of GCGR-WT, in all samples (Fig. S4C). These data indicate that βarr1 and βarr2 have a redundant role in desensitizing G protein-mediated cAMP signaling triggered by GCGR activation.
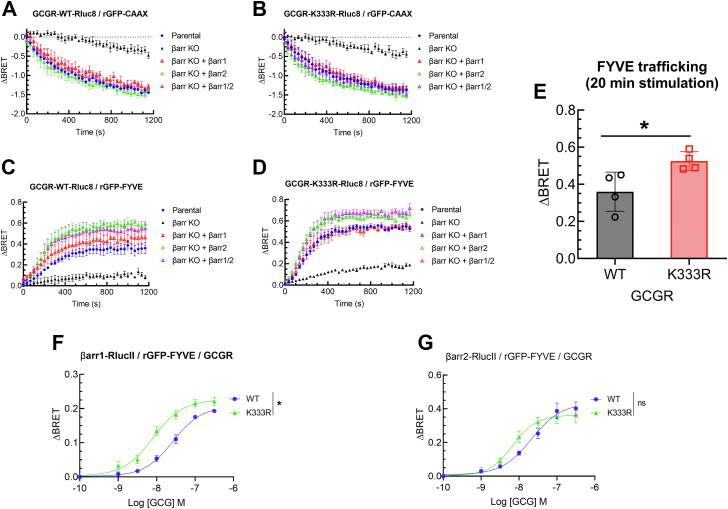
We next assessed the contribution of individual βarr isoforms in mediating internalization and endosomal trafficking of GCGR-WT and the ubiquitin-impaired GCGR-K333R. We generated RLuc8-tagged GCGR constructs and confirmed that their signaling profiles were comparable to that of untagged GCGR (Fig. S5). To measure internalization, we determined disappearance of the GCGR from the plasma membrane leading to a decrease in ebBRET with rGFP-CAAX (33) coexpressed in parental versus βarr1/2 KO CRISPR cells that were reconstituted with individual or both βarr1/2 constructs (Fig. 6, A and B). Internalization of GCGR-WT and of GCGR-K333R was impaired in the βarr1/2 KO cells, compared with parental cells or with cells reexpressing βarrs. The trafficking of GCGR-WT as well as GCGR-K333R to FYVE-endosomes was defective in βarr1/2 KO cells as compared with parental cells, and reconstitution with βarr1 and/or βarr2 led to more internalization than in parental cells (Fig. 6, C and D). Additionally, GCGR-K333R endosomal trafficking was significantly increased compared to that of GCGR-WT in parental cells (Fig. 6E). We next evaluated if the activation of GCGR-WT and GCGR-K333R induced differential recruitment of each βarr isoform to early endosomes. We measured agonist-induced ebBRET between RLucII-tagged βarr1 or βarr2, and rGFP-FYVE in cells expressing GCGR-WT, or GCGR-K333R (Fig. 6, F and G). Association of βarr1 with FYVE endosomes was significantly enhanced in cells expressing GCGR-K333R compared with WT (Fig. 6F). Recruitment of βarr2 with FYVE endosomes was also better with GCGR-K333R than GCGR-WT but failed to reach statistical significance as compared with the association induced by GCGR-WT (Fig. 6G). Taken together, these results suggest that the internalization of GCGR-K333R and localization in early endosomes is enhanced as compared with the GCGR-WT, and this trafficking is supported by the increased recruitment of βarr1 to the activated receptor in early endosomes.
Figure 6.
βarrestin-dependent internalization and early endosomal trafficking kinetics of GCGR-WT and GCGR-K333R. Enhanced bystander BRET (ebBRET) was measured between GCGR-Rluc8 (WT or K333R) and either the plasma membrane marker rGFP-CAAX (A and B) to monitor glucagon-induced receptor internalization, or the marker for early endosomes, rGFP-FYVE to monitor glucagon-induced receptor trafficking to early endosomes (C and D). In panels A–D, receptor trafficking was measured in βarr 1/2 CRISPR (SL) KO with and without βarr1 and/or βarr2 rescue and in cognate parental HEK-293 cells. In panels A–D, delta-BRET data are summarized as means ± SEM (N = 4). E, net BRET maximum obtained at 20 min of GCG stimulation in parental cells for WT and K333R Rluc8 tagged constructs with acceptors rGFP-FYVE. ∗p < 0.05, t test comparison. F and G, ebBRET between βarr1-RlucII and rGFP-FYVE or βarr2-Rluc II and rGFP-FYVE, upon stimulating for 15 min with GCG at indicated concentrations. ∗p < 0.05, two-way ANOVA, Tukey’s multiple comparisons test. ns, not significant. ebBRET, enhanced bystander bioluminescence resonance energy transfer; GCGR, glucagon receptor.
βarrs act as important adaptors for promoting ubiquitination and deubiquitination of GPCRs and non-GPCR proteins (17, 32, 51). To define the contribution of βarr in facilitating deubiquitination, we first tested the ubiquitination profile of GCGR-WT in the presence and in the complete lack of both βarr isoforms. We used three independent CRISPR βarr1/2 KO (16) and their cognate parental HEK-293 cells (labeled as HR, SL, or AI CRISPR) which were stably transfected with GCGR-WT (Fig. S6, A–F). In these assays glucagon-induced deubiquitination was obtained not only in all the three parental cells but also in the respective CRISPR βarr1/2 KO cells (Fig. S6, A–F). Interestingly, the GCGR protein band displays a retarded mobility in SDS gels with agonist stimulation (Fig. S6), which can be attributed to agonist-induced phosphorylation (52, 53). The exact role of GCGR phosphorylation in the context of agonist-induced deubiquitination remains to be defined. Correlating with the agonist-induced deubiquitination of GCGR obtained in the absence of βarrs, recruitment of cognate deubiquitinases (22), namely, USP33 and STAMBP to GCGRs occurs efficiently in the absence of βarr expression (Fig. S7). These data collectively suggest that although GCGRs in a deubiquitinated state favor βarr interaction and possess signaling bias, βarrs are not critical to induce a conformational change by facilitating deubiquitination.
Signaling and insulin secretion by GCGR-WT and GCGR-K333R in INS-1 β-cell line and isolated pancreatic islets
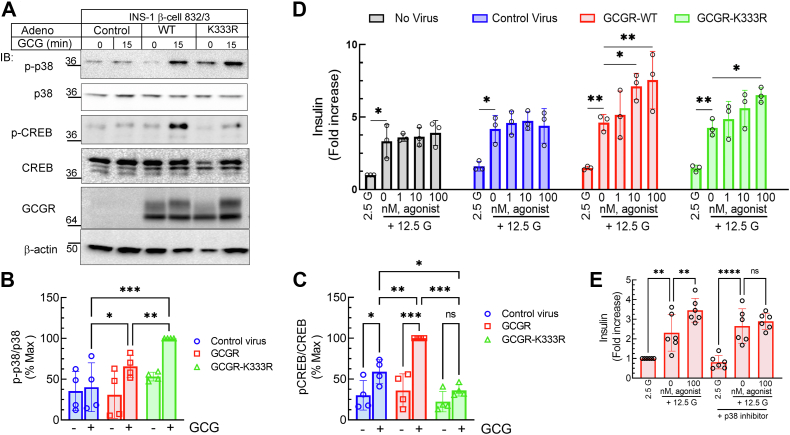
To evaluate whether the ubiquitin-driven signal bias obtained in HEK-293 cells is applicable to physiologically relevant systems, we expressed either GCGR-WT or GCGR-K333R using adenovirus in the β-cell line 832/3 (40, 54). The adenoviral vectors in which gene expression is controlled by rat insulin promoter (Fig. S8A) were generated by utilizing a recently developed versatile cloning platform (55). The transduction efficiency and protein expression levels of GCGR-WT and GCGR-K333R were equivalent as detected by imaging and Western blotting (Figs. 7A and S8, B and C). INS-1 β-cells express endogenous GCGRs (56), and glucagon-stimulation of cells infected with control adenovirus produced a weak response for p38 MAPK and ∼1.5 fold increase in CREB phosphorylation (Fig. 7, A–C). p38 MAPK activation was significantly increased by exogenous GCGR-K333R than GCGR-WT (Fig. 7, A and B). In contrast, the phospho-CREB induced by GCGR-K333R was significantly decreased compared to that provoked by GCGR-WT (Fig. 7, A and C). These results affirm the preferential coupling of the deubiquitinated GCGR K333R to p38 MAPK signaling versus Gs/PKA signaling that we obtained in HEK-293 cells to be prevalent in the β-cells.
Figure 7.
Signaling and insulin secretion by GCGR-WT and GCGR-K333R in INS-1 β-cell line.A, INS-1 β-cell line 832/3 was transduced with adenovirus encoding control, GCGR-WT or GCGR-K333R, and 48 h post-infection were stimulated with 10 nM GCG. Cells were solubilized and lysates were immunoblotted to detect phospho-p38, p38, phospho-CREB, CREB, MYC, and β-actin as indicated. The mobility of molecular weight markers (kDa) is shown beside each blot panel. The scatter plot with bar shown represents the quantification for the phosphorylation of p38 normalized to total p38 (B) and phospho-CREB normalized to CREB (C) as percentage of maximum signal in each experiment. ∗p< 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as indicated, two-way ANOVA, Holm-Šídák's multiple comparisons test. D, insulin secretory responses in 832/3 cells with no virus, control virus, GCGR-WT, or GCGR-K333R treated with 12.5 mM glucose alone or with GCGR selective agonist compound 44-0410 for 1 h. ∗p < 0.05, ∗∗p < 0.01, as indicated, two-way ANOVA Holm-Šídák's multiple comparisons test. E, INS-1 832/3 cells transduced with GCGR-WT virus were treated ± p38 inhibitor SB 203580 (100 nM, for 1 h) and then GSIS assay and analyses were performed as in panel D. ∗∗p< 0.01, ∗∗∗∗p < 0.001, as indicated, ANOVA, Holm-Šídák's multiple comparisons test. CREB, cAMP response element-binding protein; GCGR, glucagon receptor; GSIS, glucose-stimulated insulin secretion.
Glucagon stimulates insulin secretion through both the GLP-1R and GCGR, both expressed in islet β-cells and INS-1 cells, prompting us to utilize a GCGR specific agonist, 44-0410 to assess insulin secretion independent of GLP-1R activation (2). Prior studies in the β-cell line INS-1 832/3 have shown that endogenous GCGR expressed in these cells have negligible effect in potentiating glucose-stimulated insulin secretion (GSIS), and insulin secretion is mostly attributed to the activation of endogenous GLP-1R in INS-1 cells (12, 57). In keeping with these reports, while high glucose led to a significant increase in insulin secretion, there was no potentiation of this GSIS with increasing doses of 44-0410 in INS-1832/3 cells, with and without control adenovirus transduction (Fig. 7D). On the other hand, agonist-stimulation of overexpressed GCGR-WT and GCGR-K333R provoked significantly more insulin secretion than induced by high glucose conditions (Fig. 7D). Accordingly, in INS-1 832/3 despite the differences in signaling via the GCGR-WT and GCGR-K333R (Fig. 7, A–C), the effect on GSIS by both GCGR constructs was equivalent (Fig. 7D). To discern if p38 MAPK activation by GCGR-WT and GCGR-K333R (Fig. 7, A–C) is linked to the augmentation of GSIS induced by GCGR agonism (Fig. 7D), we pretreated INS-1 cells expressing these constructs with the p38 inhibitor SB 203580 or corresponding vehicle and assessed insulin secretion (Figs. 7E and S8D). Indeed, for both GCGR-WT and GCGR-K333R p38 inhibition eliminated the GCGR-induced augmentation of GSIS, while the inhibitor had no effect on GSIS itself (Figs. 7E and S8D). These results suggest that in INS-1 cells, the acute activation of p38 MAPK is important for GCGR-induced insulin secretion. Taken together these results support the notion that the observed agonist-promoted GSIS for both WT and K333R-GCGR likely results from p38 activation in the INS-1 β-cells.
Although the β-cell Gcgr is a less insulinotropic receptor compared to the β-cell Glp1r, it is still required for the full insulinotropic effects of native glucagon in vivo (2, 3). Importantly, the GCGR agonist 44-0410 fails to stimulate insulin secretion in pancreatic islets from Gcgrβcell−/− mice despite the presence of the Glp1r (2). Therefore, we utilized isolated islets from Gcgrβcell−/− islets transfected with either GCGR-WT or GCGR-K333R and 44-0410 agonist activation as a functional assay in primary β-cells to test the ability of either receptor to stimulate insulin secretion (Figs. 8 and S9). Although adenoviral transduction of INS-1 832/3 cells produced equivalent expression of GCGR-WT and GCGR-K333R (Fig 7A and S9), for reasons unknown there was repeatedly much lesser expression of GCGR-K333R than GCGR-WT in isolated islets; in most experimental replicates, GCGR-WT expression was 3-fold to 5-fold higher than the mutant (Figs. 8 and S9). While the transduction efficiency of GCGR-K333R was similar to that of GCGR-WT virus, immunostaining revealed weaker expression of the mutant than the WT (Fig. S9B). Remarkably, expression of either GCGR-WT or GCGR-K333R provoked an insulinotropic response to 44-0410 in a manner that was proportional to the amount of GCGR expression (Figs. 8A and S9). Since overall GCGR-WT expression was much higher than the mutant, we observed the greater rate of insulin secretion in response to 44-0410 than in samples expressing the mutant (Fig. S9, C and D). However, normalization of the insulin secretion response produced by 44-0410 agonist as a function of the level of GCGR showed the rate of insulin secretion was equivalent between GCGR-WT and GCGR-K333R (Fig. 8B). The response to glucose or KCl was also the same between groups (Fig. 8, C and D). Accordingly, these results suggest that despite its impaired G protein coupling, the deubiquitinated GCGR-K333R is functionally competent in promoting insulin secretion in pancreatic islets, which might proceed through βarrestin–mediated mechanisms.
Figure 8.
Signaling and insulin secretion by GCGR-WT and GCGR-K333R islets isolated from Gcgrβcell−/−mice.A, a correlation between insulin secretion rates in response to GCGR agonism by 44-0410 and the expression levels of GCGR. B, the area under the curves for the insulin secretion rates by WT and K333R induced by GCGR agonist stimulation (plot area between mins 22 and 73, Fig. S9C), in the presence of 12 mM glucose and normalized to the level of GCGR protein. C, the area under the curve for glucose-stimulated insulin secretion in islets transduced with WT or K333R virus (plot area between mins 6 and 22, Fig. S9C). D, the area under the curve for the KCl-stimulated insulin secretion in islets transduced with WT or K333R virus (plot area between mins 81 and 96, Fig. S9C). GCGR, glucagon receptor.
βarr1 isoform exclusively scaffolds p38 MAPK cascade promoted by GCGR agonist-stimulation
According to our analyses (Fig. 2) and that of others (58) both βarrs are effectively recruited to the GCGR, and furthermore both isoforms are able to transduce MAPK activation promoted by multiple GPCRs (14); hence the mechanism that constrains GCGR-mediated activation of p38 MAPK to be selective for βarr1 is perhaps an intrinsic property of the proteins assembling as a signaling complex (15). Therefore, we analyzed whether the scaffolding of p38 MAPK and MKK3 by the two βarr isoforms is different when the glucagon receptor is activated. We immunoprecipitated HA-tagged βarr1 and βarr2 from HEK-293 cells expressing either GCGR-WT or GCGR-K333R with and without agonist stimulation and analyzed binding of phospho-p38, p38, and MKK3 (Fig. 9, A–H). In these experiments, βarr1 but not βarr2 emerged as an efficient scaffold for phospho-p38 activated by the GCGR-WT. Notably, βarr1 formed complexes with phospho-p38 with GCGR activation but no agonist-promoted interaction was detected with βarr2 (Fig. 9, A–D). The immunoprecipitation assay conducted with cells expressing the GCGR-K333R, which is a βarr-biased mutant, presented a clear distinction for βarr1 scaffolding activity (Fig. 9, E–H). We not only detected a more robust association of phospho-p38 with βarr1 than with βarr2, but with the GCGR-K333R activation, βarr1 evidently showed increased agonist-induced binding with unphosphorylated p38, as well as with MKK3. Just as with the GCGR-WT activation, GCGR-K333R activation decreased βarr2 association with each of the above components that form a p38 MAPK scaffold. Accordingly, our data indicates that upon associating with the GCGR in its deubiquitinated state, βarr1 assumes an activated conformation that enables it to function as an exclusive and efficient scaffold to propagate p38 MAPK signaling (Fig. 9I).
Figure 9.
βarrestin1 isoform exclusively scaffolds p38 kinase cascade promoted by GCGR agonist-stimulation. HEK-293 cells stably expressing GCGR-WT (A–D) or GCGR-K333R (E–H) were transfected transiently with HA-tagged βarr1 or βarr2. Forty-eight hours post-transfection, cells were stimulated with 100 nM GCG for 15 min. Samples were immunoprecipitated using anti-HA affinity magnetic beads and both eluted proteins and cognate lysates were immunoblotted sequentially for the indicated proteins. Band signals for p-p38, p38, or MKK3 were normalized to respective βarr bands and presented in the scatter plots with bars in panels B, C, and D for GCGR-WT and panels F, G, and H for GCGR-K333R. ∗p < 0.05 between indicated samples, two-way ANOVA, and Tukey's multiple-comparison test. The mobility of molecular weight markers (kDa) is shown beside each blot panel. I, GCGR deubiquitination status potentiates bias toward βarr1-dependent p38 MAPK signaling. The left side of the schematic displays activation of GCGR-WT that retains ubiquitin tag before agonist activation. Glucagon stimulation promotes several immediate events: G protein activation, cAMP production, βarr recruitment, and internalization into early endosomes where the GCGR is rapidly deubiquitinated by USP33 and STAMBP (not shown). With the GCGR-WT, we observe equivalent potential to signal through Gs and βarr1-p38 signaling. However, our results suggest that Gs/PKA attenuates glucagon-induced p38 MAPK phosphorylation. The right side of the schematic displays activation of GCGR-K333R that is impaired in ubiquitination and retains a deubiquitinated state. Glucagon stimulation promotes similar trafficking itinerary as for the GCGR-WT but we observe enhanced mobilization of the receptor at early endosomes. The major differences from the WT pathway are not only a significantly decreased coupling to Gs leading to impaired cAMP production but also greater p38 MAPK scaffolding by βarr1 increasing the bias toward βarr1–dependent signal transduction. GCGR, glucagon receptor; MAPK, mitogen-activated protein kinase; STAMBP, signal transducing adaptor molecule–binding protein; USP33, ubiquitin-specific peptidase 33.
Discussion
Our results reveal a novel link between the ubiquitination profile and signal transduction mechanism of the GCGR: when ubiquitinated, the GCGR signals through G protein coupling as well as βarr recruitment, whereas in the deubiquitinated condition the signaling is biased to βarr1-dependent p38 MAPK activity (Fig. 9I). Our data suggests that ubiquitin-driven signaling at the GCGR engages K333 on the cytoplasmic face of transmembrane helix V and furthermore, crystal structure maps K333 at the interface of TM5’s collocation with alpha5 helix of G protein in the GCGR–Gαs protein complex (59). The exact molecular role of ubiquitin moieties in promoting GCGR-G protein coupling remains to be defined. While the GCGR-K333R is impaired in both ubiquitination and G protein coupling, βarr recruitment induced by this mutant was significantly increased compared with the WT GCGR as determined by ebBRET. Accordingly, ubiquitination at K333 may function as a molecular switch for engaging specific transducer pathway(s), which may be further fine-tuned by the balance between ubiquitinated and deubiquitinated GCGR species in cells.
GCGR-K333R overexpression in INS-1 cells promoted signal bias with increased p38 activity and decreased Gs/PKA dependent CREB activity compared to GCGR-WT overexpression. Prior studies consign cAMP/G protein activity as the sole trigger for glucagon-induced insulin secretion as well as CREB activation (60); however, insulin secretion induced by agonism of GCGR-K333R or GCGR-WT overexpression were equivalent in INS-1 cells and in islets. It is likely that when locked in a βarr-biased conformation, the GCGR may engage additional mechanisms aside from PKA activation to promote insulin release.
Bimodal activation of ERK1/2 activation via G protein and βarr–dependent mechanisms by various GPCRs has been an area of intense investigation for nearly 2 decades (14, 16). Indeed, a predominant focus of such studies has been on ERK1/2 signaling and its spatio-temporal regulation by βarr2 (33) and interestingly constitutive activation of ERK1/2 leads to sequestration of GPCRs at endosomes reducing their ability to signal through G proteins (61). Recent investigations on biased agonists of receptors in the secretin-glucagon family have evaluated βarr2 (not βarr1) recruitment and cAMP response and despite a favorable increase in cAMP by GCG-derived ligands, corresponding increases were not obtained for either GCGR-induced insulin secretion in INS-1 cells or glucose production in hepatocytes (57). Phenotyping of missense variants of the GCGR indicates that Gαs is the main signal transducer that preserves physiological role of the GCGR since defects in cAMP were associated with metabolic syndromes, although the signaling defect was most often associated with reduced binding capacity of the endogenous ligand to GCGR variant (58). Interestingly, the most common missense variant in the GCGR, G40S in the extra cellular domain, which has been linked with noninsulin-dependent diabetes and male adiposity in certain populations has preserved Gs/cAMP and βarr2 association but impaired βarr1 recruitment (58).
Together with or independently of GPCR activation, the βarr isoforms can play critical roles in insulin secretion by β-cells and in the pathogenesis of insulin resistance in vivo (62, 63, 64, 65). βarr1 associates with the GLP-1R and mediates agonist-induced signaling to cAMP, CREB, ERK and insulin receptor substrate 2, and augments GSIS in INS-1 cells (12). M3-muscarinic receptor-stimulated increase in insulin release is mediated by receptor phosphorylation/arrestin signaling independent of heterotrimeric G proteins and mediated by βarr1 activation of protein kinase D1 (66). β-cell βarr1 can enhance sulfonylurea-stimulated insulin secretion by promoting the activation of Epac2-Rap1 signaling that affects insulin vesicle trafficking (67). While there is increasing evidence for distinct roles of βarr isoforms in β-cell health, insulin release, and in hepatocyte glucose production, future elaborate studies are needed to determine the contributions of βarr1 and βarr2 in these paradigms as provoked by the biased GCGR-K333R.
Initially discovered as a protein tag for mobilizing unwanted proteins for degradation by 26S proteasomal machinery (68), ubiquitination has been shown to trigger a plethora of cellular effects (18, 69, 70, 71). The nonproteasomal functions of ubiquitination include protein–protein interaction, protein localization, kinase activation, and intracellular trafficking of membrane proteins. While ubiquitination of βarr2 has been linked with GPCR association, endocytosis, and scaffolding functions (32), the role of receptor ubiquitination in engaging MAPK signaling has also been reported for a few GPCRs (19). For the type 1 parathyroid hormone receptor (PTH1R), ubiquitination at the two mapped lysines does not regulate PTH-induced G protein coupling, trafficking, or degradation of the receptor but produces differences in the patterns of ERK and p38 phosphorylation induced by the βarr biased ligand PTH7-34 (26). Ubiquitination of protease-activated receptor 1 (PAR1) and the purinergic receptor P2Y1 engages the kinase TAB2 at endosomes, which can engender autophosphorylation of p38 MAPK (72). The atypical p38 activation by PAR1 does not involve βarr recruitment and is different from the canonical p38 activation by a three-tier kinase cascade that we have identified to be triggered by the GCGR, as facilitated by βarr1 recruitment, and scaffolding of MKK3 and p38.
Previous studies have shown that the actions of glucagon on lipid metabolism are mediated through p38 MAPK, AMPK, and peroxisome proliferator-activated receptor α dependent manner but independent of PKA activity (73, 74, 75). On the other hand, others have argued that chronic glucagon treatment (8 h) does not influence AMPK activity (76), suggesting that this signaling pathway may be more important for the acute, immediate response to glucagon agonism. Other studies have shown that p38 activity occurring in series with cAMP activation promotes hepatic gluconeogenesis by promoting transcription of peroxisome proliferator-activated receptor γ coactivator 1 as well as phosphorylation of CREB (77). Future studies are needed to define whether glucagon-dependent lipid and glucose homeostasis are regulated by the ubiquitin-dependent bias between Gs and βarr1 signaling. Akin to ERK1/2, p38 MAPK phosphorylates a wide variety of downstream substrates allowing its influence on aspects of cell growth, proliferation, and differentiation and the activation of p38 MAPK is balanced by multiple forms of positive and negative control (78). The roles of each βarrestin isoform in endocytosis and signaling (15, 79) and the relevance of endocytosis in biased signaling of the glucagon family receptors remains a complex issue that deserves future detailed investigations. Future studies that identify the set of specific p38 substrates regulated by βarr1 in GCGR signaling should help to elucidate how insulin release or gluconeogenesis could be regulated by ubiquitin-driven biased signaling. Furthermore, understanding the mechanisms that regulate GCGR has direct implications for novel GLP-1R/GCGR coagonists being developed for the treatment of type II diabetes.
Experimental procedures
Reagents
Anti-FLAG M2 affinity agarose gel, N-ethylmaleimide, poly-lysine, Triton X-100, and BSA were purchased from Sigma. Lipofectamine 2000 was purchased from Thermo Fisher Scientific. Glosensor plasmid 22F and Luciferin reagent were from Promega Inc. The following IgGs were procured from the sources listed: mouse monoclonal c-Myc (catalog no. SC-40), rabbit polyclonal p38 (catalog no. SC-535), anti-PKAα (catalog no. sc-903), anti-PKAβ (catalog no. sc-904) from Santa Cruz Biotechnology, Inc; mouse monoclonal anti-β-actin (catalog no. A5441) from Sigma; anti-ubiquitin FK1 (BML-PW8805) from Enzo Life Sciences. Rabbit polyclonal anti–phospho-p44/42 ERK1/2 (catalog no. 9101), Rabbit polyclonal anti-ERK1/2 (catalog no. 9102), Rabbit polyclonal anti-phospho p38 (catalog no. 9102), MKK3 (catalog no. 8535), TAB1 (catalog no. 3226), STAMBP (catalog no. 5245), rabbit polyclonal Myc tag (catalog no. 2272), rabbit monoclonal GAPDH (horseradish peroxidase conjugate, catalog no. 3683) from Cell Signaling Technology, rabbit polyclonal anti-USP33 (A300-925A) from Bethyl Laboratories. Horseradish peroxidase-conjugated secondary antibodies were purchased from GE Biosciences, Cell Signaling Technology and Bethyl Laboratories, Inc. Alexa Fluor 488 or 594–conjugated secondary antibodies were obtained from Invitrogen and used at a dilution of 1:500 for immunofluorescence labeling. PKA inhibitor fragment (6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22) amide was from Bachem Americas Inc; p38 inhibitor SB 203580 was from Millipore Sigma.
Cell lines and plasmids
HEK-293 cells obtained from American Type Culture Collection were cultured in minimal essential media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. INS-1 832/3 cells were generously provided by Dr Christopher Newgard and were cultured according to published protocols (40, 54). Parental and CRISPR β-arr1/2 KO cells were cultured as reported before (16). GCGR-MYC-FLAG plasmid was purchased from Origene Technologies and GCGR-5KR-MYC-FLAG has been reported before (22). GCGR-K169R-MYC-FLAG, GCGR-K333R-MYC-FLAG, GCGR-K406R-MYC-FLAG, GCGR-K423R-MYC-FLAG, and GCGR-K451R-MYC-FLAG were generated by substituting lysine with arginine at position 169 or 333 or 406 or 423 or 451 using a QuikChange site-directed mutagenesis kit (Stratagene). Although each of the above mutant construct expressed to comparable levels of the WT construct as assessed by immunostaining of the MYC tag and by Western blotting of solubilized lysate proteins, we observed minor differences between different constructs in successive experiments. Thus, in some experiments GCGR-K168R detection was slightly at higher levels and GCGR-K333R and GCGR-K451R were detected at slightly lower levels as compared with GCGR-WT transfections. Gateway Technology was used to mobilize complementary DNA sequences of GCGR-MYC-FLAG and GCGR-K333R-MYC-FLAG, along with rat insulin promoter sequence and IRES-GFP insert into the adenoviral vector pAd/PL-DEST and recombinant adenoviral stocks were produced by using published methods (55). All plasmids were verified by DNA sequencing. Transfections were performed using Lipofectamine 2000 (Thermo Fisher Scientific) as per manufacture’s protocol.
Immunoprecipitation
HEK-293 cells expressing GCGR-WT or desired GCGR mutant were stimulated with glucagon after starvation for 1 h in serum-free media. Following stimulation cells were solubilized in ice-cold lysis buffer containing 50 mM Hepes (pH 7.5), 2 mM EDTA (pH 8.0), 250 mM NaCl, 10% (v/v) glycerol, and 0.5% (v/v) IGEPAL CA-630 (Sigma-Aldrich) or using radioimmunoprecipitation assay buffer (150 mM NaCl, 50 mM Tris, pH 8.0, 5 mM EDTA, 1% Nonidet P-40 [NP-40], and 0.5% deoxycholate), supplemented with phosphatase and protease inhibitors: 1 mM sodium orthovanadate, 10 mM sodium fluoride, 100 μM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, and 1 mM benzamidine; buffer was also supplemented with 10 mM N-ethylmaleimide. Lysates were centrifuged for 10 min at 13,000 rpm and protein was measured using Bradford reagent (Bio-Rad). Protein amount between 800 and 1500 μg was taken for setting up coimmunoprecipitation assays. Within each immunoprecipitation experiment, equivalent protein was used for all samples. The solubilized proteins were rotated end-over-end with the M2-FLAG-agarose (Sigma) or Ant-HA magnetic beads (Pierce) at 4 °C for overnight. The immunoprecipitated complexes were washed 3 to 4 times with cold lysis buffer and eluted in 2× Laemmli sample buffer.
Assessment of p38, ERK1/2, and CREB activation
HEK-293 cells requiring signaling analysis (GCGR stables, or cells with knockdown) were plated on 6-well dishes to be at <60% confluent next day. Twenty-four hours later cells were incubated in respective serum-free starvation media containing 10 mM Hepes, pH 7.5, and 0.1% BSA. One hour post-serum starvation, desired stimulation was performed and cells were harvested in 2× Laemmli sample buffer. Samples were centrifuged, cooled on ice, and then sonicated briefly before SDS-PAGE and immunoblotting.
Immunoblotting
Solubilized protein samples were resolved on 4 to 20% Tris Glycine gels or 10% custom acrylamide gels (ProtoGel, National Diagnostics) and then transferred on 0.2 μm Nitrocellulose membrane for Western blotting. For blocking of membrane and dilution of secondary antibodies, 5% (w/v) dried skim milk powder dissolved in Tween-Tris-buffered saline (0.2% (v/v) Tween 20, 10 mM Tris–HCl (pH 8.0), and 150 mM NaCl) was utilized, while primary antibodies were diluted in 5% (w/v) BSA prepared in TTBS. The enhanced chemiluminescence substrate, Super Signal West Pico Plus reagent was used to detect proteins through charge coupled device camera system (Bio-Rad Chemidoc-XRS). The quantification of protein bands was done by using Image Lab software (Bio-Rad; https://www.bio-rad.com/en-us/product/image-lab-software?ID=KRE6P5E8Z).
GloSensor assay for determining cAMP production
HEK-293 cells transiently transfected either with GCGR-WT, GCGR single lysine mutants or GCGR-5KR and GloSensor 22F plasmid (Promega) in 6-well dishes. Parallel transfections were set up in 6-well dishes and cells were used at the experiment end-point for preparing extracts that were subjected to SDS-PAGE and Western blot analysis to detect GCGR expression as well as for plating on confocal dishes to complete immunostaining and confocal detection of GCGR expression. For the cAMP assay, cells were detached 4 h post-transfection resuspended in clear mimimum essential medium containing 2% fetal bovine serum + 1% penicillin/streptomycin + 10 mM Hepes and reseeded in 96-well white clear bottomed plates that were previously coated with poly-D-lysine. Eighteen to twenty hours later, cells were washed with Hanks' Balanced Salt Solution (HBSS), GloSensor reagent diluted in HBSS was added and incubation was continued for 1 h at 26 °C. Subsequently, GloSensor reagent was replaced with 90 μl of HBSS supplemented with 10 mM Hepes, pH 7.5 and plates were subjected to a baseline preread for luminescence on a Synergy Neo2 plate reader driven by Gen5 Software (BioTek Instruments; https://www.agilent.com/en/product/microplate-instrumentation/microplate-instrumentation-control-analysis-software/imager-reader-control-analysis-software). Ten microliters of vehicle or agonist glucagon at desired concentration was added to respective wells and the plates were immediately read for luminescence at 26 °C.
Confocal microscopy
HEK-293 cells stably or transiently expressing GCGR construct were seeded on poly-D-lysine coated 20-mm confocal glass bottom dish. Cells were fixed using 5% formaldehyde diluted in Dulbecco's PBS (DPBS) for 20 min. Cells were then permeabilized for 20 min with 0.1% Triton X-100 in 2% BSA and subsequently incubated in anti-MYC 9E10 (Santa Cruz Biotechnology) primary antibody, at 4 °C for overnight. Cells were stained by incubating with secondary antibody conjugated to Alexa fluorophore 488 or 594 at room temperature for 1 to 2 h. After cell fixation and antibody incubations, cells were washed with DPBS. Two percent BSA prepared in DPBS was used for making permeabilizing solution and antibody dilutions. Confocal images were captured with LSM-510 META confocal microscope with filter settings for the respective fluorophores; excitation was at 488 nm (Alexa 488) and 561 nm (Alexa 594).
RNA interference
Double-stranded siRNA oligonucleotides for control nontargeting sequence or targeting TAB1, MKK3, PKAα, PKAβ, βarr1, or βarr2 were purchased from Dharmacon Inc as described (16). Sequences of siRNA oligonucleotides were as follows: control nontargeting sequence: 5′-AAUUCUCCGAACGUGUCACGU-3′; human βarrestin1: 5′-AAAGCCUUCUGCGCG-GAGAAU-3′; human βarrestin 2: 5′ -AAGGA-CCGCAAAGUGUUUGUG-3′; human PKAα: 5′-CGUCCUGACCUUUGAGUAU-3′; human PKAβ: 5′-GGUCACAGACUUUGGGUUU-3′; human TAB1: 5′CGCAAUUGCCAGAGGGAGU3′; human MKK3: 5′UGGACAAGUUCUACCGG-AA-3′. rat βarrestin1: 5′-AGCCUUCUGUGCUGAGAAC-3′; and . rat βarrestin2: 5′-UGGAGUAGACUUUGAGAUU-3′. For siRNA experiments early passage cells at the confluence of 40 to 50% were transfected with 20 μg of siRNA using Lipofectamine 2000 in respective serum-free medium. After 4 h of transfection cells were supplemented with complete media and incubated at 37 °C for 48 h before assay was performed.
INS-1 cell assays
INS-1 832/3 cells were cultured according to published protocols (40, 54). Cells were seeded on 12-well Corning BioCoat poly-D-Lysine coated dishes and 24 h later were transduced with Ad-RIP-β-Gal-IRES-GFP (5 μl/ml), Ad-RIP-GCGR-IRES-GFP (5 μl/ml), or Ad-RIP-GCGR-K333R-IRES-GFP (10 μl/ml) to obtain equivalent multiplicity of infection for different constructs. Four hours after infection, media was replaced with complete growth medium and cells were allowed to recover for 48 h. Cells were serum-starved and stimulated with vehicle or agonist for 15 min and solubilized extracts were analyzed for desired protein expression by Western blotting. For measuring insulin secretion, cells were washed with PBS and then incubated in HBSS buffer (114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mM MgSO4, 20 mM Hepes, 2.5 mM CaCl2, 0.2% BSA, pH 7.2) containing 2.5 mM glucose. After 1 h, cells in duplicate wells were further treated for 1 h for the following conditions: low glucose (2.5 mM), high glucose (12.5 mM), or high glucose + agonist 44-0410 agonist (1 nM, 10 nM, 100 nM). Cell supernatant was carefully collected and assayed for insulin secretion with the Lumit Insulin Immunoassay Kit (Promega #CS3037A01). Insulin content in each sample was normalized to total protein. The monolayers of cells were solubilized in 2 × Laemmli sample buffer and total protein in each sample was determined using Pierce 660 nm Protein Assay Reagent supplemented with Ionic Detergent Compatibility Reagent (Pierce) according to the manufacturer’s protocol.
Islet isolation and perifusion
All animal experiments were performed in accordance with protocols approved by Duke University Institutional Animal Care and Use Committee.
Gcgrβcell−/− mice were generated as described (2). Briefly, mice with LoxP sites in the Gcgr allele (Gcgrfl) were crossed with MIP-CreERT mice and administered tamoxifen for four consecutive days to generate Gcgrβcell−/− mice. Islets were isolated from mice at least 4 weeks after tamoxifen administration. Islet isolation was performed using a histopaque gradient as described (2). Immediately after isolation, islets from a single mouse were incubated with either Ad-RIP-GCGR-IRES-GFP (5 μl/ml) or Ad-RIP-K333R-IRES-GFP (10 μl/ml) in RPMI for 24 h. Islets were then allowed to recover for 48 to 72 h in RPMI before being perifused. After recovery, 75 islets were handpicked into KRPH buffer (140 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 1 mM NaH2PO4, 1 mM MgSO4, 2 mM NaHCO3, 5 mM Hepes, and 0.1% BSA; pH = 7.4) containing 2.7 mM glucose and 100uL Bio-Gel P4 Media (Bio-Rad). Islets were equilibrated for 48 min and then perifused in experimental conditions shown in Fig S9. Insulin secretion was assessed by Lumit Immunoassay (Promega) and assayed using the EnVision plate reader (PerkinElmer; https://www.perkinelmer.com/Product/envision-hts-plate-reader-2105-0010).
BRET assays
HEK-293 cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% newborn calf serum, 100 units of penicillin, and 100 μg/ml streptomycin. Transient transfections were performed on suspended cells at a density of 0.4 million cells/ml using 25 kDa linear PEI as transfecting agent, at a ratio of 4:1 PEI/DNA. ebBRET experiments were performed as reported before (33).
Briefly, for βarrestin1/2 recruitment, cells were transfected with βarrestin1/2-RlucII (BRET donor) and rGFP-CAAX (BRET acceptor) along with each GCGR construct (WT, single lysine, or 5KR mutant construct). For Gs engagement, cells were transfected with Rluc8-miniGs (BRET donor) and rGFP-CAAX (BRET acceptor) to monitor the translocation of mini-Gs protein to active receptor at the plasma membrane. For receptor trafficking, parental HEK-293 cells or βarrestin1/2 KO cells were transfected with WT or mutant forms of GCGR-MYC-FLAG fused to Rluc8 (GCGR-MYC-FLAG-Rluc8) along with either rGFP-CAAX (plasma membrane) or rGFP-FYVE (early endosomes).
For ebBRET readings, transfected cells were seeded in 96-well microplates (Greiner) (100 μl/well). Forty-eight hours later, Dulbecco’s Modified Eagle’s Medium media was removed and cells were washed with DPBS and replaced by HBSS. For concentration-response experiments, increasing concentrations of glucagon (GCG) were added and cells were incubated for 10 min before adding coelenterazine 400a (2.5 μM). BRET values were collected 5 min after coelenterazine addition. For kinetic experiments, Prolume Purple (2.5 μM) was added for 6 min before cell stimulation with 1 μM GCG or vehicle and BRET measurement was started immediately after and continued for the indicated times. BRET values were collected on a Tecan Spark multimode microplate reader equipped with filters for BRET2 (400/70 nm (donor) and 515/20 nm (acceptor)). The BRET signal was calculated as the ratio of light emitted by the energy acceptor over the light emitted by the energy donor and the agonist-promoted BRET was calculated by subtracting the BRET signal obtained in the presence of vehicle from the BRET signal obtained in the presence of agonist.
Statistical analyses
The quantification for all the experiments is presented as scatter plots with bar or as line graphs with means ± SEM from experimental replicates indicated in the figure legends. The type of statistical analysis and post hoc test used are included in each figure legend. We have used GraphPad PRISM version 9 (GraphPad Inc; https://www.graphpad.com/) and considered a p value of < 0.05 as significant.
Data availability
All data, associated methods, and sources of materials are available in the main text or in the supporting information.
Supporting information
This article contains supporting information.
Conflict of interest
M. B. is the president of the scientific advisory Board of Domain Therapeutics which licensed-in some of the BRET-based biosensors, used in this study. All other authors declare no competing interests.
Acknowledgments
We thank Wenli Zhang, Thomas Becker, Mette Johnson, and Pavitra Murali for their help and discussions. We are grateful to Caroline Ray for her generous help in generating Rluc8 tagged GCGR plasmid. We gratefully acknowledge Drs Howard Rockman and Christopher Newgard for generously providing cell lines and for helpful suggestions. We thank Drs Asuka Inoue and Stephane Laporte for generously sharing CRISPR cell lines and Dr Nevin Lambert for providing mini-Gs-Rluc8 plasmid. We express our gratitude to Drs Robert J. Lefkowitz and Brian Kobilka for generously sharing reagents. We dedicate this article to the memory of our dear colleague Dr Marc Caron whose generous help with reagents and access to equipment immensely helped this work.
Author contributions
S. K., B. S., M. E. C., K. E., and Y. B. investigation; S. K. S. conceptualization; K. K. resources; S. K., B. S., M. E. C., K. E., Y. B., A. J., and B. H. formal analysis; S. K. writing-original draft; S. K., B. S., M. E. C., K. E., D. A. D., J. E. C., M. B., and S. K. S. writing-review and editing; D. A. D., J. E. C., M. B., and S. K. S. supervision; D. A. D., J. E. C., M. B., and S. K. S. project administration; M. B. and S. K. S. funding acquisition.
Funding and additional information
This work was supported by the National Institutes of Health (HL160029 to S. K. S.) as well as by a Seed Award funding support (to S. K. S. and J. E. C.) from the Edna and Fred L. Mandel Jr Foundation. Work conducted in Dr Bouvier’s laboratory is supported by a “Foundation Grant” form the Canadian Institute of Health Research to M. B. (FDN-148431). B. S. holds a studentship from the Fonds de Recherche en Santé du Québec. M. E. C. is supported by K01DK129417. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Kirill Martemyanov
Footnotes
Present address for Suneet Kaur: Laboratory of Signal Transduction, National Institute of Environmental Health Sciences, NIH, Research Triangle Park, North Carolina 27709, USA.
Supporting information
References
- 1.Brubaker P.L., Drucker D.J. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Recept. Channels. 2002;8:179–188. [PubMed] [Google Scholar]
- 2.Capozzi M.E., Svendsen B., Encisco S.E., Lewandowski S.L., Martin M.D., Lin H., et al. Beta cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svendsen B., Larsen O., Gabe M.B.N., Christiansen C.B., Rosenkilde M.M., Drucker D.J., et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 2018;25:1127–1134.e2. doi: 10.1016/j.celrep.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Capozzi M.E., D'Alessio D.A., Campbell J.E. The past, present, and future physiology and pharmacology of glucagon. Cell Metab. 2022;34:1654–1674. doi: 10.1016/j.cmet.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El K., Gray S.M., Capozzi M.E., Knuth E.R., Jin E., Svendsen B., et al. GIP mediates the incretin effect and glucose tolerance by dual actions on alpha cells and beta cells. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell J.E., Drucker D.J. Islet alpha cells and glucagon--critical regulators of energy homeostasis. Nat. Rev. Endocrinol. 2015;11:329–338. doi: 10.1038/nrendo.2015.51. [DOI] [PubMed] [Google Scholar]
- 7.Finan B., Capozzi M.E., Campbell J.E. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes. 2020;69:532–541. doi: 10.2337/dbi19-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galsgaard K.D., Pedersen J., Knop F.K., Holst J.J., Wewer Albrechtsen N.J. Glucagon receptor signaling and lipid metabolism. Front. Physiol. 2019;10:413. doi: 10.3389/fphys.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller R.A., Birnbaum M.J. Glucagon: acute actions on hepatic metabolism. Diabetologia. 2016;59:1376–1381. doi: 10.1007/s00125-016-3955-y. [DOI] [PubMed] [Google Scholar]
- 10.DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 11.Merlen C., Fabrega S., Desbuquois B., Unson C.G., Authier F. Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and gsalpha in rat liver. FEBS Lett. 2006;580:5697–5704. doi: 10.1016/j.febslet.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Sonoda N., Imamura T., Yoshizaki T., Babendure J.L., Lu J.C., Olefsky J.M. Beta-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6614–6619. doi: 10.1073/pnas.0710402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krilov L., Nguyen A., Miyazaki T., Unson C.G., Williams R., Lee N.H., et al. Dual mode of glucagon receptor internalization: role of PKCalpha, GRKs and beta-arrestins. Exp. Cell Res. 2011;317:2981–2994. doi: 10.1016/j.yexcr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn S., Shenoy S.K., Luttrell L.M., Lefkowitz R.J. SnapShot: beta-arrestin functions. Cell. 2020;182:1362–1362.e1. doi: 10.1016/j.cell.2020.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Peterson Y.K., Luttrell L.M. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol. Rev. 2017;69:256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrell L.M., Wang J., Plouffe B., Smith J.S., Yamani L., Kaur S., et al. Manifold roles of beta-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jean-Charles P.Y., Rajiv V., Shenoy S.K. Ubiquitin-related roles of beta-arrestins in endocytic trafficking and signal transduction. J. Cell Physiol. 2016;231:2071–2080. doi: 10.1002/jcp.25317. [DOI] [PubMed] [Google Scholar]
- 18.Jean-Charles P.Y., Snyder J.C., Shenoy S.K. Chapter one - ubiquitination and deubiquitination of G protein-coupled receptors. Prog. Mol. Biol. Transl. Sci. 2016;141:1–55. doi: 10.1016/bs.pmbts.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Dores M.R., Trejo J. Endo-lysosomal sorting of G-protein-coupled receptors by ubiquitin: diverse pathways for G-protein-coupled receptor destruction and beyond. Traffic. 2019;20:101–109. doi: 10.1111/tra.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchese A., Benovic J.L. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J. Biol. Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy S.K., McDonald P.H., Kohout T.A., Lefkowitz R.J. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 22.Kaur S., Chen Y., Shenoy S.K. Agonist-activated glucagon receptors are deubiquitinated at early endosomes by two distinct deubiquitinases to facilitate Rab4a-dependent recycling. J. Biol. Chem. 2020;295:16630–16642. doi: 10.1074/jbc.RA120.014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenoy S.K., Barak L.S., Xiao K., Ahn S., Berthouze M., Shukla A.K., et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J. Biol. Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenoy S.K., Lefkowitz R.J. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J. Biol. Chem. 2005;280:15315–15324. doi: 10.1074/jbc.M412418200. [DOI] [PubMed] [Google Scholar]
- 25.Grimsey N.J., Aguilar B., Smith T.H., Le P., Soohoo A.L., Puthenveedu M.A., et al. Ubiquitin plays an atypical role in GPCR-induced p38 MAP kinase activation on endosomes. J. Cell Biol. 2015;210:1117–1131. doi: 10.1083/jcb.201504007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q., Xiao K., Liu H., Song L., McGarvey J.C., Sneddon W.B., et al. Site-specific polyubiquitination differentially regulates parathyroid hormone receptor-initiated MAPK signaling and cell proliferation. J. Biol. Chem. 2018;293:5556–5571. doi: 10.1074/jbc.RA118.001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez G.A., Montminy M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 28.Delghandi M.P., Johannessen M., Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17:1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Reiter E., Ahn S., Shukla A.K., Lefkowitz R.J. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado-Peraza F., Ahn K.H., Nogueras-Ortiz C., Mungrue I.N., Mackie K., Kendall D.A., et al. Mechanisms of biased beta-arrestin-mediated signaling downstream from the cannabinoid 1 receptor. Mol. Pharmacol. 2016;89:618–629. doi: 10.1124/mol.115.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr R., 3rd, Schilling J., Song J., Carter R.L., Du Y., Yoo S.M., et al. Beta-arrestin-biased signaling through the beta2-adrenergic receptor promotes cardiomyocyte contraction. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4107–E4116. doi: 10.1073/pnas.1606267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenoy S.K., Lefkowitz R.J. Beta-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namkung Y., Le Gouill C., Lukashova V., Kobayashi H., Hogue M., Khoury E., et al. Monitoring G protein-coupled receptor and beta-arrestin trafficking in live cells using enhanced bystander BRET. Nat. Commun. 2016;7 doi: 10.1038/ncomms12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avet C., Mancini A., Breton B., Le Gouill C., Hauser A.S., Normand C., et al. Effector membrane translocation biosensors reveal G protein and betaarrestin coupling profiles of 100 therapeutically relevant GPCRs. Elife. 2022;11 doi: 10.7554/eLife.74101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Q., Okashah N., Inoue A., Nehme R., Carpenter B., Tate C.G., et al. Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 2018;293:7466–7473. doi: 10.1074/jbc.RA118.001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caron M.G., Barak L.S. A brief history of the beta-arrestins. Methods Mol. Biol. 2019;1957:3–8. doi: 10.1007/978-1-4939-9158-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn S., Wei H., Garrison T.R., Lefkowitz R.J. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J. Biol. Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 38.Oakley R.H., Laporte S.A., Holt J.A., Caron M.G., Barak L.S. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh E., Dwivedi H., Baidya M., Srivastava A., Kumari P., Stepniewski T., et al. Conformational sensors and domain swapping reveal structural and functional differences between beta-arrestin isoforms. Cell Rep. 2019;28:3287–3299.e6. doi: 10.1016/j.celrep.2019.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hohmeier H.E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 41.Trempolec N., Dave-Coll N., Nebreda A.R. SnapShot: p38 MAPK signaling. Cell. 2013;152:656–656.e1. doi: 10.1016/j.cell.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Ge B., Gram H., Di Padova F., Huang B., New L., Ulevitch R.J., et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- 43.Gong K., Li Z., Xu M., Du J., Lv Z., Zhang Y. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J. Biol. Chem. 2008;283:29028–29036. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kommaddi R.P., Jean-Charles P.Y., Shenoy S.K. Phosphorylation of the deubiquitinase USP20 by protein kinase a regulates post-endocytic trafficking of beta2 adrenergic receptors to autophagosomes during physiological stress. J. Biol. Chem. 2015;290:8888–8903. doi: 10.1074/jbc.M114.630541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S.M., Jean-Charles P.Y., Abraham D.M., Kaur S., Gareri C., Mao L., et al. The deubiquitinase ubiquitin-specific protease 20 is a positive modulator of myocardial beta1-adrenergic receptor expression and signaling. J. Biol. Chem. 2019;294:2500–2518. doi: 10.1074/jbc.RA118.004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daaka Y., Luttrell L.M., Lefkowitz R.J. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 47.Zamah A.M., Delahunty M., Luttrell L.M., Lefkowitz R.J. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J. Biol. Chem. 2002;277:31249–31256. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Hanada K., Staus D.P., Makara M.A., Dahal G.R., Chen Q., et al. Galphai is required for carvedilol-induced beta1 adrenergic receptor beta-arrestin biased signaling. Nat. Commun. 2017;8:1706. doi: 10.1038/s41467-017-01855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith J.S., Pack T.F., Inoue A., Lee C., Zheng K., Choi I., et al. Noncanonical scaffolding of galphai and beta-arrestin by G protein-coupled receptors. Science. 2021;371 doi: 10.1126/science.aay1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefkowitz R.J., Pierce K.L., Luttrell L.M. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol. Pharmacol. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- 51.Jean-Charles P.Y., Zhang L., Wu J.H., Han S.O., Brian L., Freedman N.J., et al. Ubiquitin-specific protease 20 regulates the reciprocal functions of beta-arrestin2 in toll-like receptor 4-promoted nuclear factor kappaB (NFkappaB) activation. J. Biol. Chem. 2016;291:7450–7464. doi: 10.1074/jbc.M115.687129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stadel J.M., Nambi P., Shorr R.G., Sawyer D.F., Caron M.G., Lefkowitz R.J. Catecholamine-induced desensitization of Turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc. Natl. Acad. Sci. U. S. A. 1983;80:3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komolov K.E., Sulon S.M., Bhardwaj A., van Keulen S.C., Duc N.M., Laurinavichyute D.K., et al. Structure of a GRK5-Calmodulin complex reveals molecular mechanism of GRK activation and substrate targeting. Mol. Cell. 2021;81:323–339.e11. doi: 10.1016/j.molcel.2020.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronnebaum S.M., Jensen M.V., Hohmeier H.E., Burgess S.C., Zhou Y.P., Qian S., et al. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucose-stimulated insulin secretion from rodent islets. J. Biol. Chem. 2008;283:28909–28917. doi: 10.1074/jbc.M804665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haldeman J.M., Conway A.E., Arlotto M.E., Slentz D.H., Muoio D.M., Becker T.C., et al. Creation of versatile cloning platforms for transgene expression and dCas9-based epigenome editing. Nucleic Acids Res. 2019;47:e23. doi: 10.1093/nar/gky1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kieffer T.J., Heller R.S., Unson C.G., Weir G.C., Habener J.F. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology. 1996;137:5119–5125. doi: 10.1210/endo.137.11.8895386. [DOI] [PubMed] [Google Scholar]
- 57.Jones B., McGlone E.R., Fang Z., Pickford P., Correa I.R., Jr., Oishi A., et al. Genetic and biased agonist-mediated reductions in beta-arrestin recruitment prolong cAMP signaling at glucagon family receptors. J. Biol. Chem. 2021;296 doi: 10.1074/jbc.RA120.016334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Velden W.J.C., Lindquist P., Madsen J.S., Stassen R., Wewer Albrechtsen N.J., Holst J.J., et al. Molecular and in vivo phenotyping of missense variants of the human glucagon receptor. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2021.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilger D., Kumar K.K., Hu H., Pedersen M.F., O'Brien E.S., Giehm L., et al. Structural insights into differences in G protein activation by family A and family B GPCRs. Science. 2020;369 doi: 10.1126/science.aba3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandoval D.A., D'Alessio D.A. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol. Rev. 2015;95:513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 61.Paradis J.S., Ly S., Blondel-Tepaz E., Galan J.A., Beautrait A., Scott M.G., et al. Receptor sequestration in response to beta-arrestin-2 phosphorylation by ERK1/2 governs steady-state levels of GPCR cell-surface expression. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E5160–E5168. doi: 10.1073/pnas.1508836112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wess J. The two beta-arrestins regulate distinct metabolic processes: studies with novel mutant mouse models. Int. J. Mol. Sci. 2022;23:495. doi: 10.3390/ijms23010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luan B., Zhao J., Wu H., Duan B., Shu G., Wang X., et al. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–1149. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 64.Zhu L., Almaca J., Dadi P.K., Hong H., Sakamoto W., Rossi M., et al. Beta-arrestin-2 is an essential regulator of pancreatic beta-cell function under physiological and pathophysiological conditions. Nat. Commun. 2017;8 doi: 10.1038/ncomms14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pydi S.P., Barella L.F., Zhu L., Meister J., Rossi M., Wess J. Beta-arrestins as important regulators of glucose and energy homeostasis. Annu. Rev. Physiol. 2022;84:17–40. doi: 10.1146/annurev-physiol-060721-092948. [DOI] [PubMed] [Google Scholar]
- 66.Kong K.C., Butcher A.J., McWilliams P., Jones D., Wess J., Hamdan F.F., et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21181–21186. doi: 10.1073/pnas.1011651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barella L.F., Rossi M., Zhu L., Cui Y., Mei F.C., Cheng X., et al. Beta-cell-intrinsic beta-arrestin 1 signaling enhances sulfonylurea-induced insulin secretion. J. Clin. Invest. 2019;129:3732–3737. doi: 10.1172/JCI126309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 69.Komander D., Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 70.Yau R., Rape M. The increasing complexity of the ubiquitin code. Nat. Cell Biol. 2016;18:579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 71.Jean-Charles P.Y., Freedman N.J., Shenoy S.K. Chapter nine - cellular roles of beta-arrestins as substrates and adaptors of ubiquitination and deubiquitination. Prog. Mol. Biol. Transl. Sci. 2016;141:339–369. doi: 10.1016/bs.pmbts.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Burton J.C., Grimsey N.J. Ubiquitination as a key regulator of endosomal signaling by GPCRs. Front. Cell Dev. Biol. 2019;7:43. doi: 10.3389/fcell.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Longuet C., Sinclair E.M., Maida A., Baggio L.L., Maziarz M., Charron M.J., et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8:359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berglund E.D., Lee-Young R.S., Lustig D.G., Lynes S.E., Donahue E.P., Camacho R.C., et al. Hepatic energy state is regulated by glucagon receptor signaling in mice. J. Clin. Invest. 2009;119:2412–2422. doi: 10.1172/JCI38650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kimball S.R., Siegfried B.A., Jefferson L.S. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J. Biol. Chem. 2004;279:54103–54109. doi: 10.1074/jbc.M410755200. [DOI] [PubMed] [Google Scholar]
- 76.Huet C., Boudaba N., Guigas B., Viollet B., Foretz M. Glucose availability but not changes in pancreatic hormones sensitizes hepatic AMPK activity during nutritional transition in rodents. J. Biol. Chem. 2020;295:5836–5849. doi: 10.1074/jbc.RA119.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao W., Collins Q.F., Becker T.C., Robidoux J., Lupo E.G., Jr., Xiong Y., et al. p38 mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J. Biol. Chem. 2005;280:42731–42737. doi: 10.1074/jbc.M506223200. [DOI] [PubMed] [Google Scholar]
- 78.Canovas B., Nebreda A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021;22:346–366. doi: 10.1038/s41580-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gurevich V.V., Gurevich E.V. Arrestins: critical players in trafficking of many GPCRs. Prog. Mol. Biol. Transl. Sci. 2015;132:1–14. doi: 10.1016/bs.pmbts.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, associated methods, and sources of materials are available in the main text or in the supporting information.