Abstract
The tegument is the structure between the envelope and nucleocapsid of herpesvirus particles. Viral (and cellular) proteins accumulate to create the layers of the tegument. Some Epstein-Barr virus (EBV) tegument proteins are conserved widely in Herpesviridae, but others are shared only by members of the gamma-herpesvirus subfamily. As the interface to envelope and nucleocapsid, the tegument functions in virion morphogenesis and budding of the nucleocapsid during progeny production. When a virus particle enters a cell, enzymes such as kinase and deubiquitinase, and transcriptional activators are released from the virion to promote virus infection. Moreover, some EBV tegument proteins are involved in oncogenesis. Here, we summarize the roles of EBV tegument proteins, in comparison to those of other herpesviruses.
Keywords: EBV, Tegument, Envelopment, Egress, Infectivity, Oncogenesis
Abbreviations
- EBV
Epstein-Barr virus
- IE
immediate-early
- HSV
herpes simplex virus
- VZV
varicella zoster virus
- HCMV
human cytomegalovirus
- HHV
human herpesvirus
- KSHV
Kaposi's sarcoma-associated herpesvirus
- NK
natural killer
- PPIs
protein-protein interactions
- BKRF4
BamHI-K fragment rightward reading frame 4
- CATC
capsid-associated tegument complex
- CVSCs
capsid vertex-specific components
- ORF
open reading frame
- BCL2
B-cell lymphoma 2
- UL/US:
unique long/unique short
- RL/RS
repeat long/repeat short
- VP
virion protein
- ICP
infected cell protein
- gB
glycoprotein B
- CDT1
chromatin licensing and DNA replication factor 1
- NF-κB
nuclear factor-kappa B
- IRF3
interferon regulatory factor 3
- RIG-I:
retinoic acid-inducible gene-I
- cGAS
cyclic GMP-AMP synthase
- TGN
trans-Golgi network
- AP-1
activator protein 1
- MAPK/MEK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- JAK/STAT
Janus kinase/signal transducer and activator of transcription
- AGO2
Argonaute 2
- CDK
cyclin-dependent kinase
- PML-NB
promyelocytic leukemia protein-nuclear body
- ND10
nuclear domain 10
- ATRX
alpha thalassemia/mental retardation syndrome, X-linked
- ERK
extracellular signal-regulated kinase
- RSK
ribosomal S6 kinase
- eIF4B
eukaryotic translation initiation factor 4B
- USP7
ubiquitin-specific protease 7
- ATF4
activating transcription factor 4
- SRPK2
serine/arginine protein-specific kinase 2
- MHV-68
murine gammaherpesvirus 68
- TK
thymidine kinase
- RR
ribonucleotide reductase
- APOBEC3B
apolipoprotein B mRNA editing enzyme catalytic subunit 3B
- LMP1
latent membrane protein 1
- EBNA1
Epstein-Barr virus nuclear antigen 1
- TNF-α:
tumor-necrosis factor α
- IL-6
interleukin 6
- VEGF
vascular endothelial growth factor
- RB
retinoblastoma
- TERT
telomere reverse transcriptase
- FGF
fibroblast growth factor
- HK2
hexokinase 2
- PKM2
pyruvate kinase 2
- LDHA1
lactate dehydrogenase A1
- GLUT1
glucose transporter 1
- HLA
human leukocyte antigen
- PD-L1
programmed cell death ligand 1
1. Introduction
Herpesviruses have double-stranded linear DNA genomes in icosahedral containers composed of viral capsid proteins. The nucleocapsid is wrapped in a lipid bilayer envelope, from which glycoproteins protrude. The space and/or the components between the nucleocapsid and envelope is termed the tegument [1]. The tegument of herpesviruses is mainly composed of viral proteins, with some cellular proteins. Tegument proteins do not comprise a solid structure like the nucleocapsid and, thus, a portion of the tegument structure can be released into the cytoplasm when the viral envelope fuses with the plasma membrane or endosomal membrane [2]. Some types of tegument proteins form interfaces with the exterior of the nucleocapsid (the inner tegument), and some others with membrane proteins on the interior surface of the envelope (outer tegument). Other tegument proteins form a complex network that connects the inner and outer tegument proteins.
Herpesvirus infections comprise latent and lytic phases [3,4]. In the latent phase, the virus exists as a circular double-stranded DNA molecule in the nucleus of a host cell, expressing only a small number of viral genes. This is an endurant type of infection, where the virus minimizes viral antigen presentation to avoid host immunity. By contrast, the lytic phase is an active type of infection, in which all viral genes are expressed, viral DNA is replicated, and progeny virus particles are produced. The lytic cycle starts with expression of a handful of viral genes, named immediate-early (IE) genes. IE genes are activators of viral gene expression and induce the expression of early viral genes. The proteins encoded by early genes, including those needed for nucleotide metabolism and DNA replication, amplify the viral genome in replication compartments in the host cell nucleus. The remaining viral lytic genes are categorized as late, and most encode viral structural proteins i.e., capsid, tegument, and glycoproteins. Capsid proteins assemble into an icosahedral architecture, into which a viral genome is incorporated in the nucleus. Because the resultant nucleocapsid is too large to pass through nuclear pores, it must traverse the nuclear double membrane [5,6]. First, the nucleocapsid buds into the inner nuclear membrane (primary/initial envelopment). Some tegument proteins are attached to the exterior of the nucleocapsid in the nucleus before the initial envelopment. The temporal envelope fuses with the outer nuclear membrane (de-envelopment) to release nucleocapsid into the cytoplasm. The nucleocapsid buds into a cytoplasmic membrane structure (possibly derived from the TGN or endosome) [7]. The other tegument proteins are incorporated during this secondary envelopment. The cytoplasmic membrane structure finally fuses with the plasma membrane, and a mature progeny virion is released. The released virion attaches to a receptor on the plasma membrane and enters a cell. Two routes have been indicated for herpesvirus entry [8]. Route 1 involves fusion of the viral envelope with the plasma membrane and release of nucleocapsid and tegument proteins into the cytoplasm. In route 2, the virion is engulfed by an endosome, and the viral envelope fuses with the endosomal membrane to release nucleocapsid and tegument proteins. Released tegument proteins play a regulatory role in newly infected cells and promote virus infectivity. Other tegument proteins attached to the incoming nucleocapsid support nuclear transportation of the nucleocapsid. The linear viral DNA genome enters the nucleus, where the genome is circularized and gains histones to become an episome.
Herpesviruses are categorized as alpha-, beta-, and gamma-herpesviruses. To date, nine human herpesviruses have been identified. Herpes simplex virus type 1 (HSV-1), HSV-2, and varicella zoster virus (VZV) belong to the alpha-herpesvirus group. HSV-1 and 2 cause herpes labialis, keratitis, encephalitis, and genital herpes. Chicken pox in children is attributable to initial VZV infection, and the same virus later causes shingles. The beta-herpesviruses include human cytomegalovirus (HCMV), human herpesvirus type 6A (HHV-6A), HHV-6B, and HHV-7. HCMV causes interstitial pneumonia, retinitis, and congenital HCMV infection, and HHV-6B and 7 are agents of exanthema subitem. Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) are gamma-herpesviruses. Initial EBV infection in children is associated with no obvious symptoms but can lead to infectious mononucleosis if initially infected during/after adolescence. Furthermore, EBV is associated with several cancers, such as Burkitt lymphoma, Hodgkin lymphoma, post-transplant lymphoproliferative disorder, T/NK cell lymphoma, chronic active EBV infection, nasopharyngeal carcinoma, and gastric carcinoma. KSHV is also an oncogenic virus, being the cause of Kaposi sarcoma, primary effusion lymphoma, and multicentric Castleman disease.
Some EBV tegument proteins are conserved across the Herpesviridae, but others—including BKRF4, BLRF2, and BNRF1—are unique to gamma-herpesviruses [9]. In this review, we summarize the structures and functions of EBV tegument proteins by comparison with other herpesviruses. Detailed electron microscopic analysis of the tegument structure of the whole EBV virion has not been performed, but protein-protein interactions (PPIs) analyses showed a plausible meshwork structure. Such complex PPIs hamper functional analysis of tegument proteins; following knockout of a tegument gene, loss of the encoded protein may affect incorporation of other tegument proteins. The multiple overlapping PPIs of tegument proteins may compensate for the loss of a tegument gene and obscure the phenotype of the mutant virus. Nevertheless, we and others have performed phenotypic analyses of EBV knockouts to assess the functions of tegument genes. We here summarize the findings of those analyses.
2. Composition of EBV tegument proteins
Mass spectrometry of purified EBV particles from culture medium detected some proteins homologous to the tegument proteins of alpha- and beta-herpesviruses [10]. Tegument proteins conserved across the Herpesviridae include BBLF1 (homolog of HSV UL11, HCMV UL99, and KSHV ORF38), BBRF2 (UL7, UL103, and ORF42), BGLF1 (UL17, UL93, and ORF32), BGLF2 (UL16, UL94, and ORF33), BGLF4 (UL13, UL97, and ORF36), BOLF1 (UL37, UL47, and ORF63), BPLF1 (UL36, UL48, and ORF64), BSRF1 (UL51, UL71, and ORF55), and BVRF1 (UL25, UL77, and ORF19) (Table 1). Because BGLF1 and BVRF1 are involved in viral-genome packaging, they are sometimes described as capsid proteins. However, BGLF1 and BVRF1 form the capsid-associated tegument complex (CATC; alternatively capsid vertex-specific components [CVSCs]) along with BPLF1, and so can be categorized as tegument components. Three tegument proteins, BKRF4 (homolog of KSHV ORF45), BLRF2 (ORF52), and BNRF1 (ORF75), are unique to gamma-herpesviruses (Table 1). KSHV homologs of these genes, ORFs45, 52, and 75, are incorporated into the tegument fraction [11,12]. Viral proteins that may be incorporated into the tegument include BALF1 (possibly related to KSHVORF16), BALF2 (homolog of HSV UL29, HCMV UL57, and KSHV ORF6), BGLF3.5 (homolog of HSV UL14 and KSHV ORF35, but not conserved in HCMV), BMRF1 (homolog of HSV UL42, HCMV UL44, and KSHV ORF59), BORF2 (UL39, UL45, and ORF61), BRLF1 (KSHV ORF50), BRRF2 (KSHV ORF48), and BXLF1 (HSV UL23 and KSHV ORF21) (Table 2). Because BDLF2 (homolog of KSHV ORF27) is a glycosylated type II membrane protein [13], it is likely to be an envelope, rather than a tegument, protein. Because some of these possible proteins, such as BALF2 and BMRF1, are abundantly expressed in cells, they may be incorporated into virions non-specifically. Several cellular proteins were also detected in EBV virions, such as ACTIN, TUBULIN, COFILIN, HSP70, and HSP90 [10].
Table 1.
Tegument proteins of EBV.
| EBV | alias name | HSV | HCMV | KSHV | Role in EBV lifecycle | Role in other herpesviruses |
|---|---|---|---|---|---|---|
| BBLF1 | MyrP | UL11 | UL99/pp28 | ORF38 | increase progeny production by improving egress [54,104], relocalize BGLF2 [51] | secondary envelopment [105] |
| BBRF2 | UL7 | UL103 | ORF42 | increase progeny production by improving infectivity [48], stabilize BSRF1 [48] | secondary envelopment, egress, and cell-to-cell spread [[100], [101], [102]] | |
| BGLF1 | CATC | UL17 | UL93 | ORF32 | function not reported | cleavage/packaging [64] |
| BGLF2 | MyrPBP | UL16 | UL94 | ORF33 | increase progeny production by improving infectivity and egress [51,109], signal modification [[109], [110], [111], [112], [113]] | secondary envelopment, cell-to-cell spread [60,[121], [122], [123], [124]], nuclear egress [125,126] |
| BGLF4 | vCDK | UL13 | U97 | ORF36 | increase progeny production [142], late gene expression [[143], [144], [145]], initial envelopment [135] | viral gene expression, replication, progeny production, cell-to-cell spread [[148], [149], [150], [151]] |
| BOLF1 | LTPBP | UL37 | UL47 | ORF63 | increase progeny production by improving infectivity [41] | secondary envelopment [39,70,91], transport [[92], [93], [94]], deamidase activity [95,96] |
| BPLF1 | LTP, CATC | UL36 | UL48 | ORF64 | has de-Ub activity [76], increase viral DNA synthesis, progeny production [76,78,86], and oncogenesis [90] | has de-Ub activity [77], assembly, secondary envelopment [70], nuclear transportation, nuclear targeting of viral DNA [[71], [72], [73], [74], [75]] |
| BSRF1 | PalmP | UL51 | UL71 | ORF55 | increase progeny production [14], relocalize BBRF2 [48] | maturation, secondary envelopment, egress, and cell-to-cell spread [50,98] |
| BVRF1 | CATC | UL25 | UL77 | ORF19 | function not reported | packaging [65,66], nuclear egress [36,68,69], and uncoating [67] |
| BKRF4 | ORF45 | increase progeny production by improving infectivity [57], inhibit DDR [159,160], relocalize BGLF2 [57] | involved in replication [164], assembly, envelopment, egress [165,166], transport [167], signal modification [[168], [169], [170], [171], [172], [173], [174], [175], [176]] | |||
| BLRF2 | ORF52 | increase progeny production by improving infectivity [22] | increase progeny production by improving infectivity [179], inhibit cGAS [[180], [181], [182]] | |||
| BNRF1 | MTP | ORF75 | increase infectivity by increasing viral gene expression, especially in primary B cells [62] | disrupt ATRX and increase viral gene expression [158] |
The numbers in the “Roles” column indicate reference numbers.
Table 2.
Possible tegument proteins of EBV.
| EBV | alias name | HSV | HCMV | KSHV | Role in EBV lifecycle | Role in other herpesviruses |
|---|---|---|---|---|---|---|
| BALF1 | vBCL2 | (ORF16) | inhibit apoptosis [198] | viral gene expression, replication, progeny production, inhibit apoptosis in KSHV [200] | ||
| BALF2 | ssDNABP | UL29 | UL57 | ORF6 | essential for viral DNA synthesis, transcriptional activator [201,202] | essential for viral DNA synthesis [9] |
| BGLF3.5 | UL14 | ORF35 | no obvious function [188] | increase progeny production in HSV [16,190] | ||
| BMRF1 | EA-D | UL42 | UL44 | ORF59 | essential for viral DNA synthesis [201,205] | essential for viral DNA synthesis [9] |
| BORF2 | RR | UL39 | UL45 | ORF61 | increase progeny production [214], increase P53 [108], inhibit APOBEC3B [214,215], involved in nucleotide metabolism [9] | increase progeny production in non-dividing cells [218], involved in nucleotide metabolism, inhibit APOBEC3B in HSV [219] |
| BRLF1 | Rta | ORF50 | transcriptional activator [222], stabilize BORF1 [19] | transcriptional activator in KSHV [223] | ||
| BRRF2 | ORF48 | increase progeny production [224] | not reported | |||
| BXLF1 | TK | UL23 | ORF21 | no obvious function in cultured cell [211], nucleotide biosynthesis [210] | increase progeny production by improving infectivity in KSHV [213], nucleotide biosynthesis |
The numbers in the “Roles” column indicate reference numbers.
Note that all of the 12 tegument proteins in Table 1 and 4 out of the 8 possible tegument proteins in Table 2 were readily detected by mass spectrometry in virion [10], although BALF1, BGLF3.5, BRLF1, and BRRF2 proteins in Table 2 were below the threshold. BALF1 is a viral homolog of the anti-apoptotic protein, BCL2. Although not identified as a tegument protein, BALF1 is included in this table because overexpressed BALF1 protein was detected in EBV virions by western blotting [14], as was KSHV vBCL2 (ORF16) [15]. BGLF3.5 has not been detected in EBV virion, but its homologs HSV UL14 [16,17] and KSHV ORF35 [18] are identified in virions. Endogenous BRRF2 protein was detectable by western blotting of EBV virion using our antibody [14], and its KSHV homolog ORF48 protein is also identified by mass spectrometry in the virion fraction [18]. BRLF1 protein is recently identified in EBV virion fraction by western blotting [19], and the KSHV homolog ORF50 is detected by western blotting of KSHV virion proteins [11].
3. PPI network of EBV tegument proteins, and with nucleocapsid and envelope proteins
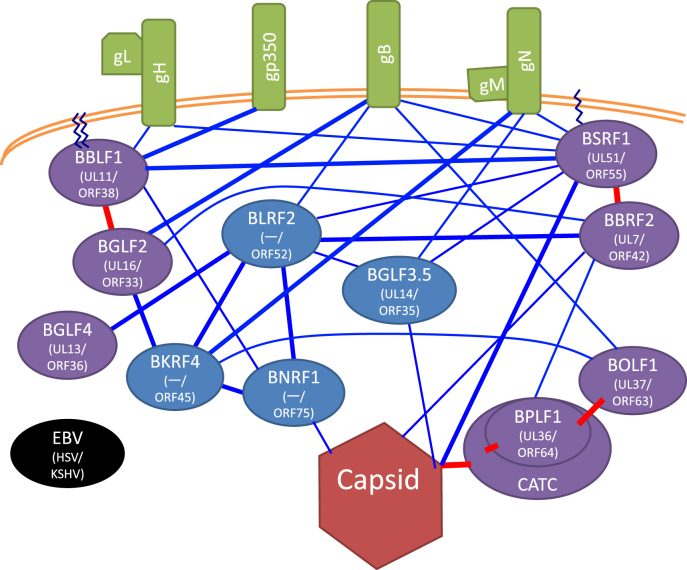
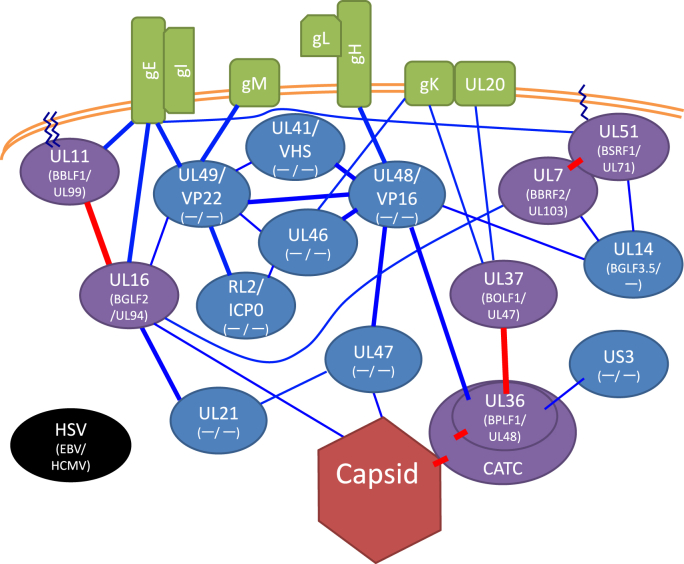
Tegument proteins form a complex PPI network that attaches the viral envelope to the nucleocapsid. We prepared a simplified diagram of the EBV PPI network based on prior reports [[20], [21], [22], [23]] (Fig. 1). For comparison, a PPI network centering on HSV tegument proteins was also generated (Fig. 2) [2,7,[24], [25], [26], [27]]. Most PPIs have been detected in lysates (by immunoprecipitation) or cells (yeast two-hybrid or complementation assay of split marker), but not in the tegument. Nevertheless, these interactions are important because layers of tegument proteins are formed during envelopment in cells. Unfortunately, no similar schema for HCMV is available possibly because of the large number of tegument components [[28], [29], [30]].
Fig. 1.
Simplified EBV virion protein network.
Network formed by protein-protein interactions (PPIs) of tegument proteins (oval), nucleocapsid (dark red hexagon at bottom), and envelope proteins (green box). Purple and blue ovals, conserved and non-conserved tegument proteins, respectively. EBV gene names together with those of their homologs in HSV and KSHV are noted. Saw-toothed line, post-translational lipid modifications—palmitoylation and myristoylation. A copy of BGLF1, two copies of BVRF1, and two copies of BPLF1 comprise the capsid-associated tegument complex (CATC). Red lines indicate interactions conserved across herpesviruses. Thick lines indicate interactions that were previously reported in at least one species of human herpesviruses in addition to EBV, or that were found only in EBV but validated by two or more methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Simplified HSV virion protein network.
Network formed by protein-protein interactions (PPIs) of tegument proteins (oval), nucleocapsid (dark red hexagon at bottom), and envelope proteins (green box). Purple and blue ovals, conserved and non-conserved tegument proteins, respectively. HSV gene names together with those of their homologs in EBV and HCMV are shown. Saw-toothed line, post-translational lipid modifications—palmitoylation and myristoylation. A copy of UL17, two copies of UL25, and two copies of UL36 comprise the CATC. Red lines indicate interactions conserved across herpesviruses. Thick lines indicate interactions that were previously reported in more than one papers. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A significant number of EBV tegument proteins are not conserved in subfamilies other than gamma-herpesviruses (blue ovals in Fig. 1), and many HSV tegument components are not present in other subfamilies (blue ovals in Fig. 2). So, the gamma-herpesviruses are likely to have evolved separately from the alpha- and beta-herpesviruses, and the tegument composition may reflect cell tropism, mode of infection, and disorders. For instance, the HSV and HCMV teguments have powerful transcriptional activators of IE genes (UL48/VP16 and UL82/pp71, respectively), but there is no counterpart of these genes in EBV. Their absence may explain the typically latent form of infection with EBV.
The nucleocapsid vertex-CATC-BOLF1/UL37-membrane protein axis PPI is preserved across subfamilies (Fig. 1, Fig. 2). Indeed, high-resolution cryogenic electron microscopy of EBV [31,32] showed that the capsid vertex-tegument interface encompasses CATC. Interestingly, EBV CATC, composed of BGLF1, BVRF1, and BPLF1, crowns more efficiently at the portal vertex rather than penton vertices, suggesting a more important role for CATC in EBV genome containment. CATC infrequently binds to penton vertices of KSHV [33], but HSV-1 CATC occupies most portal and penton vertices [34]. The HCMV nucleocapsid is associated with fewer copies of CATC at penton vertices, but its pentons and hexons are fixed by a unique tegument protein, UL32/pp150 [35]. The binding of BPLF1 and BOLF1 homologs is highly conserved in the family (Fig. 1, Fig. 2) [21,27,[36], [37], [38]], suggesting the importance of their interaction. BOLF1 links to glycoprotein B (gB) (Fig. 1) [21], whereas HSV-1 UL37 interacts with gK and UL20 (Fig. 2) [39]. EBV BPLF1 is linked to BBRF2 [40], BOLF1 associates with BKRF4 (Fig. 1) [41], and HSV-1 UL36 binds to UL48 [42] and US3 [43] (Fig. 2).
The complex of two tegument proteins, BSRF1 and BBRF2, is important in the EBV lifecycle, because these have homologs in other herpesviruses [14,44,45]. The UL51 protein of HSV-1 is post-translationally modified by palmitoylation [46], which facilitates its membrane association. Although palmitoylation of EBV BSRF1 and HCMV UL71 proteins has not been confirmed, it is likely because the modified N-terminal cysteine is conserved and associated with membranes, particularly of the Golgi apparatus [14,47]. EBV BSRF1 associates with many viral proteins, besides BBRF2, including gB, gH [40], gN, BLRF2 [22], and BGLF3.5 [14] (Fig. 1). BBRF2 associates with BGLF2 [48], BLRF2, BPLF1, and BcLF1 (major capsid protein [MCP]) [40] (Fig. 1). The HSV UL51 protein binds to UL14 [49] and gE [50]. The binding of UL7 to UL14 and UL16 proteins has been reported (Fig. 2) [11].
Another well-conserved tegument interaction is that of the EBV BBLF1-BGLF2 complex (Fig. 1) [51]; interactions of HSV UL11-UL16 [52] and HCMV UL99-UL94 [53] have also been reported. BBLF1 and its counterparts are post-translationally modified by palmitoylation and myristoylation [[54], [55], [56]]. BBLF1 is a small protein, which interacts with BNRF1, gH and gp350 [[20], [21], [22]]. BGLF2 associates with BBRF2, BKRF4, and gB (Fig. 1) [21,22,48,57]. HSV UL11 binds with gE [58], and UL16 binds to gE [59], UL49 [60], UL7 [21], UL21 [61], and UL35 (small capsid) [21] (Fig. 2).
HSV UL21, 41, 46, 47, 48, 49, and RL2 are present only in alpha-herpesviruses. In EBV, the loss of these tegument proteins can be compensated for by gamma-herpesvirus-specific proteins, BKRF4, BNRF1, and BLRF2. BKRF4 is linked to BGLF2, BLRF2, BOLF1, BNRF1, and gN [22,41,57]. BNRF1 interacts with tegument proteins, BKRF4, BBLF1, and BLRF2, and possibly BFRF3 (small capsid) [[20], [21], [22]]. We reported that BNRF1 interacts with capsid proteins [22], and the suppression by BNRF1 gene knockout of nucleocapsid nuclear transport in B cells also suggests an interaction with the nucleocapsid [62]. BLRF2 binds to many tegument proteins, BGLF4, BKRF4, BNRF1, BSRF1, BBRF2, BGLF3.5, and to gB [20,22], and is thus hypothesized to be a tegument hub [22].
EBV BGLF3.5 is a homolog of HSV UL14 but has no counterpart in HCMV. It interacts with BSRF1, as HSV UL14 interacts with UL51 [14,22]. It also associates with BLRF2 and BBRF1, the portal protein [22]. BGLF4 is linked to BLRF2 [22].
The detailed PPIs of EBV tegument components are shown in Fig. 3 [[20], [21], [22]]. EBV BALF1 is a homolog of the anti-apoptotic host protein, BCL2. It associates with multiple membrane (gH, gP350, gB, BDLF3, and gN), tegument (BLRF2, BNRF1, BALF2, BSRF1, and BORF2), and capsid proteins (BBRF1) [14,[20], [21], [22]]. Being a type II membrane protein, BDLF2 has a transmembrane domain and forms a complex with an envelope protein, BMRF2 [13,63]. It also interacts with BSRF1 and BRLF1 [22]. BALF2 and BMRF1 are required for lytic viral genome DNA synthesis and may be incorporated into virions via interactions with other tegument proteins (BALF1, BLRF2, BNRF1, and BGLF3.5 for BALF2; BKRF4, and BLRF2 for BMRF1) [22]. BRLF1 is associated with BDLF2, BXLF1, and a capsid component, BORF1 [19,20,22]. BXLF1 is linked to BKRF4, BGLF4, BLRF2, BRLF1, and a capsid protein, BBRF1 [22]. BORF2 and BRRF2 each have one interacting partner, BALF1 and gN, respectively [20,22].
Fig. 3.
Detailed EBV virion protein network.
Network formed by protein-protein interactions (PPIs) of all tegument proteins (oval), nucleocapsid (dark red hexagon at the bottom), and envelope proteins (green box). Purple and blue ovals, conserved and non-conserved tegument proteins, respectively. Orange ovals show possible tegument components not included in Fig. 1. EBV gene names are provided in ovals. Saw-toothed line, post-translational lipid modifications—palmitoylation and myristoylation. A copy of BGLF1, two copies of BVRF1, and two copies of BPLF1 comprise the capsid-associated tegument complex (CATC). Red lines indicate interactions conserved across herpesviruses. Thick lines indicate interactions that were previously reported in at least one species of human herpesviruses in addition to EBV, or that were found only in EBV but validated by two or more methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Roles of EBV tegument proteins in the virus lifecycle
4.1. The CATC: BGLF1 (homolog of HSV UL17, HCMV UL93, and KSHV ORF32), BVRF1 (UL25, UL77, and ORF19), and BPLF (UL36, UL48, and ORF64)
The EBV CATC, composed of one BGLF1, two BVRF1, and two BPLF1 proteins, fully occupies the five sitting sites at the portal vertex of the EBV nucleocapsid, whereas up to two CATCs occupy the five available CATC-binding sites at penton vertices [31,32]. In HSV, not only the portal but also the penton vertices are fully crowned by CATC [34]. So, the role of EBV CATC may be slightly different from that of HSV CATC, at least in terms of structural dynamics. Still, it must be emphasized that the CATC of all herpesviruses links the nucleocapsid and tegument proteins.
The roles of EBV BGLF1 and BVRF1 are unclear, but can be inferred from other herpesviruses, such as HSV-1. HSV UL17 (homolog of BGLF1) is essential for viral genome DNA cleavage and packaging [64]. UL25 (homolog of BVRF1) is required for packaging but not for cleavage [65,66]. Interestingly, the UL25 is also involved in viral genome uncoating immediately after infection [67] and nuclear egress of the nucleocapsid via interaction with the nuclear egress complex [36,68,69]. So, EBV BGLF1 and BVRF1 may be involved in viral genome cleavage and packaging, and possibly also viral genome containment, nuclear export, and nucleocapsid uncoating.
EBV BPLF1 and its homologs have been investigated extensively. HSV UL36 links nucleocapsid and tegument proteins. Indeed, knockout of the UL36 gene in HSV-1 caused cytoplasmic accumulation of unenveloped nucleocapsids, implicating UL36 in secondary envelopment [70]. In addition, HSV-1 UL36 is reported to play multiple roles in nuclear transport and viral DNA release into the nucleus [[71], [72], [73], [74], [75]]. The N-terminal part of BPLF1 encodes de-ubiquitination and de-neddylation activities, which are conserved across the family [76,77]. BPLF1 reduces the activity of viral ribonucleotide reductase [78], targets polymerase eta and Rad18 and deregulates the DNA damage tolerance pathway to increase progeny production [[79], [80], [81]], promotes viral replication by inducing accumulation of the licensing factor CDT1 [76], inhibits cullin-RING ligases [82], targets topoisomerase II [83], downregulates innate immunity (NF-κB and IRF3 pathways) [[84], [85], [86], [87]], and inhibits selective autophagy by targeting the autophagy receptor [88]. Caspase-1 cleaves BPLF1 and enhances nuclear localization of catalytically active BPLF1 during the lytic cycle [89]. Because of these functions, knockdown or knockout of BPLF1 markedly reduces viral DNA synthesis and progeny production [76,78,86,90]. Interestingly, BPLF1-disrupted EBV exhibited reduced B-cell transformation activity in cell culture and suppressed tumor formation in a humanized model [90].
4.2. BOLF1 (homolog of HSV UL37, HCMV UL47, and KSHV ORF63)
To date, only one study has focused on the role of EBV BOLF1 [41]. Disruption of BOLF1 had little or no effect on viral gene expression or DNA replication. The knockout virus produced progeny at a level similar to the wild-type, but the progeny had reduced infectivity.
HSV UL37, a homolog of BOLF1, is implicated in secondary envelopment [70]. UL37 mediates the interaction of CATC with the gK/UL20 complex on the membrane, both of which are important for secondary envelopment [39,91]. It is also involved in nucleocapsid transport [[92], [93], [94]]. Also, UL37 has deamidase activity, by which it blocks antiviral responses by RIG-I and cGAS [95,96]. By contrast, KSHV ORF63 suppresses innate immunity by inhibiting inflammasome signaling [97].
4.3. BSRF1 (homolog of HSV UL51, HCMV UL71, and KSHV ORF55)
BSRF1 is post-translationally modified by palmitoylation and associates with viral envelope or membrane. The phenotype of BSRF1-knockout EBV was indistinguishable from wild-type EBV in HEK293 cells, but knockdown of BSRF1 in B95-8 cells significantly decreased infectious progeny production [14]. Expression of BSRF1 promoted BBRF2 transport from the nucleus to the cytoplasm [48].
HSV UL51 is also modified by palmitoylation, which is required for its membrane localization [46]. UL51 is implicated in viral maturation, secondary envelopment, egress, and intercellular spread [50,98]. UL51 is needed for proper localization of UL7 to cytoplasmic membranes [45]. It also associates with gE and UL14, which promote proper localization and envelopment, respectively [49,50]. In addition, UL51 phosphorylation affects viral nuclear egress and intercellular spread [99].
4.4. BBRF2 (homolog of HSV UL7, HCMV UL103, and KSHV ORF42)
Knockout of the EBV BBRF2 gene reduced progeny production without affecting viral gene expression and DNA replication [48]. The number of progeny produced was not reduced, but their infectivity was decreased. Co-expression of BBRF2 protected its interacting partner, BSRF1, from proteasome-dependent degradation, and co-expression of BSRF1 is needed for proper localization of BBRF2 [48], indicating the importance of the PPI.
Loss of the UL7 gene, a homolog of BBRF2, in HSV results in reduced virus replication and smaller plaques [[100], [101], [102]]. A UL7 deletion mutant of pseudorabies virus, an animal alpha-herpesvirus, had defects in secondary envelopment and egress [103].
4.5. BBLF1 (homolog of HSV UL11, HCMV UL99, and KSHV ORF38)
BBLF1 is a membrane-associated protein modified by palmitoylation and myristoylation [54]. Myristoylation of BBLF1 is crucial for its stabilization and localization to the TGN [54]. BBLF1 interacts with BGLF2, thereby mediating proper cytoplasmic localization of BGLF2 to the TGN [51]. BBLF1 knockdown reduced EBV progeny production [54]. We found that, rather than being secreted extracellularly, BBLF1-null mutant virus progeny remained associated with the host cell. Because BBLF1 knockout and wild-type viruses produced similar numbers of infectious progeny in total, BBLF1 is likely to promote extracellular egress without affecting processes at virion maturation [104].
All EBV BBLF1 homologs in other herpesviruses undergo palmitoylation and myristoylation. HSV UL11 is involved in secondary envelopment [105] and so knockout of the encoding gene reduces progeny production and causes cytoplasmic accumulation of unenveloped nucleocapsids. Its associations with UL16 and gE are implicated in secondary envelopment [58,106,107].
4.6. BGLF2 (homolog of HSV UL16, HCMV UL94, and KSHV ORF33)
EBV BGLF2 arrests the cell cycle at G1/S phase by inducing p21 [108]. It also induced AP-1 signaling by activating the P38 MAPK and JNK signaling pathways, thereby promoting EBV lytic replication [109,110]. In addition, BGLF2 inhibits NF-κB [111] and interferon-induced JAK/STAT pathway [112,113] signaling, to subvert antiviral innate immunity and induce the viral lytic cycle. It was also reported to de-regulate miRNA functions by targeting AGO2 [114]. BGLF2 is incorporated not only into virions but also exosomes, and exosomal BGLF2 facilitates de novo EBV infection [115]. Disruption of the BGLF2 gene had little effect on viral gene expression and DNA synthesis, but extracellular production of infectious progeny was suppressed by about 10-fold and the cell-associated progeny level was also decreased [51,109]. So, the BGLF2 gene improves not only virus egress but also the infectivity of secreted virions [109]. BGLF2 interacts with BBLF1 and BKRF4 [51,57]. When expressed alone, BGLF2 localizes to the nucleus and cytoplasm, but its co-expression with BBLF1 recruited BGLF2 to the TGN [51], and addition of BKRF4 re-localized BGLF2 to the nucleus and perinuclear region [57]. MHV-68 ORF33 is associated not only with cytoplasmic but also with nuclear nucleocapsids [116], suggesting that BGLF2 associates with nucleocapsids in the nucleus before primary envelopment. Other reports on KSHV ORF33 suggest roles for BGLF2 in the cytoplasm [117,118].
UL16-null mutant of HSV-1 exhibits about a 10-fold reduction in virus yield [119], and that of HSV-2 has 50-100-fold progeny loss [120]. Disruption of the UL16 gene in HSV-1 is associated with cytoplasmic accumulation of nucleocapsids [60]. HSV-1 UL16 is localized to the nucleus and cytoplasm, and interacts with nucleocapsid [121,122] and membrane proteins, including UL11 [123] and gE [59]. These findings, in conjunction with information from other herpesviruses, implicate UL16 in secondary envelopment and intercellular spread [124]. However, it must also be noted that HSV-2 UL16 plays an important role in the nuclear egress of nucleocapsids [125,126], suggesting that the roles of UL16 homologs are different depending on the virus species. In addition, modification of signaling pathways, e.g., MAPK, NF-κB, and JAK/STAT, has not been reported for HSV UL16 or KSHV ORF33.
4.7. BGLF4 (homolog of HSV UL13, HCMV UL97, and KSHV ORF36)
The BGLF4 gene encodes a serine/threonine protein kinase conserved across the family Herpesviridae, which has substrate similarity with host cyclin-dependent kinase (CDK). It phosphorylates many viral proteins, such as BGLF4, BMRF1, EBNA1, EBNA2, BXLF1, and BGLF2 [[127], [128], [129], [130]], as well as host proteins, such as EEF1D, P27, CHK1, RAD51, TIP60, and SAMHD1 [[131], [132], [133], [134]]. It induces disassembly of nuclear lamina [135], regulates microtubule dynamics [136], suppresses IRF3 signaling [137], mediates premature condensation of chromosomes [138], blocks host chromosomal DNA replication [139,140], and modulates nuclear pore complexes [141]. Compared to wild-type virus, progeny production by BGLF4-knockout EBV was reduced by about an order of magnitude [142]. The BGLF4 gene may modulate the expression of some late genes and initial envelopment at the nuclear membrane [[143], [144], [145]]. Furthermore, the BGLF4 gene, but not its thymidine kinase BXLF1, is needed for inhibition of virus replication by ganciclovir or acyclovir [146].
HSV UL13 phosphorylates substrates targeted by host CDKs, like BGLF4 [147], and plays roles in the expression of a subset of viral genes, viral replication, progeny production, intercellular spread, and evasion of host immunity [[148], [149], [150], [151]].
4.8. BNRF1 (homolog of KSHV ORF75)
The major tegument protein BNRF1 is conserved only among gamma-herpesviruses. It is expressed in latent cells, too, and is targeted by CD4+ and CD8+ T cells [152,153]. Disruption of the BNRF1 gene had little effect on EBV replication and progeny production. However, upon infection to primary B cells, viral gene expression was significantly repressed by the disruption, thereby suppressing B-cell transformation [62]. The group therefore speculated that nuclear transport of the nucleocapsid was inhibited by disruption of the BNRF1 gene. Later, however, other group showed that BNRF1 is involved in transcriptional activation of viral genes upon infection; BNRF1 targets PML-NB (also known as ND10) and disrupts the antiviral histone chaperone complex Daxx-ATRX, thereby increasing the expression of viral genes immediately after infection [154,155]. In addition, BNRF1 destabilizes the SMC5/6 cohesin complex to increase viral DNA replication [156] and induces centrosome amplification and thus chromosomal instability [157].
KSHV ORF75 disrupts ATRX to antagonize PML-NB-mediated intrinsic immunity, thus inducing viral gene expression [158]. Also, ORF75-null KSHV fails to produce IE genes, making ORF75 essential for viral replication, unlike EBV BNRF1.
4.9. BKRF4 (homolog of KSHV ORF45)
BKRF4 is an EBV late phosphoprotein [57] conserved among gamma-herpesviruses, albeit at low similarity. Knockout of the BKRF4 gene decreased progeny levels, possibly by reducing infectivity, but had no effect on viral gene expression and DNA replication [57]. Upon lytic induction of infected cells, BKRF4 localizes to the nucleus and peri-nuclear region. When expressed alone, BKRF4 and BGLF2 localize to the nucleus and cytoplasm, respectively, and co-expression of BKRF4 with BGLF2 re-localizes BGLF2 to the nucleus [57]. Interestingly, BGLF2 activates AP-1 signaling, an effect repressed by co-expression of BKRF4 [109]. BKRF4 associates with many other tegument proteins (Fig. 3), suggesting it to be a hub in the tegument meshwork [22]. BKRF4 inhibits the DNA damage response, suggesting involvement in oncogenesis [159]. Mutagenesis and structural analyses revealed that BKRF4 inhibits the DNA damage response by binding to partially unfolded nucleosomes [160]. In addition, BKRF4 has histone chaperone activity [161].
KSHV ORF45 is a tegument protein [12,18] with different functions to those of BKRF4 [162,163]. It is involved in viral replication [164]; assembly/envelopment/egress [165,166]; nucleocapsid transport [167]; and modification of the IRF7 [168,169], inflammasome [170], ERK/RSK [171,172], eIF4B [173], USP7/P53 [174,175], and ATF4 [176] signaling pathways. Modification of these signaling pathways by KSHV ORF45 is reminiscent of EBV BGLF2, but not their counterpart in EBV, BKRF4.
4.10. BLRF2 (homolog of KSHV ORF52)
The EBV BLRF2 gene encodes a gamma-herpesvirus-specific tegument protein expressed with late kinetics. The protein localizes to the nucleus and nuclear rim in transfected or infected cells [22]. Viral gene expression and viral DNA replication were not affected by the BLRF2 knockout, but production of infectious progeny into the culture medium was mildly but significantly dropped, possibly because of decreased infectivity [22]. A dimerization motif in the middle of BLRF2 mediates its self-association [177] and formation of this homodimer enhances protein stability [22]. The C-terminus of BLRF2 has a motif for phosphorylation by SRPK2, which is linked to progeny production [178]. Interestingly, BLRF2 also associates with many other tegument proteins and, thus, is likely to be a hub protein [22].
The KSHV tegument protein ORF52 is expressed with late kinetics. Its null mutant showed reduced virion production and infectivity [179]. Importantly, the null mutant failed to pack other tegument proteins, including ORF33 and ORF45 [179]. KSHV ORF52 antagonizes the host DNA sensor cGAS, thereby promoting immune evasion [[180], [181], [182]]. ORF52 homologs in other gamma-herpesviruses, including EBV BLRF2, inhibit the antiviral response [182]. ORF52 is structurally similar to the HSV tegument protein VP22 (UL49) (Fig. 2), and both are involved in microtubule reorganization and immune evasion [[183], [184], [185], [186]], suggesting that non-homologous genes in alpha- and gamma-herpesviruses encode products with similar functions.
4.11. BGLF3.5 (homolog of HSV UL14 and KSHV ORF35)
EBV BGLF3.5 is conserved in alpha- and gamma-, but not in beta-, herpesviruses [187]. BGLF3.5 associates with other tegument proteins, such as BSRF1 and BLRF2. BGLF3.5 is likely to be a tegument gene, although its product has not been identified in EBV virion, possibly because of its small size. Knockout of BGLF3.5 did not affect virus replication or progeny production in HEK293 cells [188].
HSV UL14 is a homologous tegument protein [189] that increases the progeny titer by about one order of magnitude [16,190]. It has multiple functions, such as chaperone-like activity [191], protection from apoptosis [192], and aiding nuclear transport of VP16 and nucleocapsids [190,193]. KSHV ORF35 was detected in purified virions [18]. Knockout of ORF35 in KSHV slightly decreased viral gene expression, viral DNA synthesis, and progeny titer compared to the wild-type [194]. In MHV-68, viral gene expression and viral DNA synthesis were unaffected, but production of infectious progeny was decreased by the knockout [195].
4.12. BALF1 (possibly related to KSHVORF16)
EBV encodes two homologs of the host anti-apoptotic protein BCL2: BHRF1 and BALF1 [196,197]. Knockout of either or their simultaneous disruption had little effect on virus multiplication and progeny production. However, knockout of either moderately decreased B cell transformation efficiency, and their simultaneous disruption almost abolished B cell transformation [198], indicating that both vBCL2 proteins are required for B cell transformation. BALF1 reportedly stimulates autophagy [199]. We reported that it interacts with BSRF1 and is incorporated into the tegument [14].
KSHV has one BCL2 homolog, ORF16, the absence of which decreases viral gene expression, DNA synthesis, and progeny production [200]. Intriguingly, KSHV ORF16 protein interacts with ORF55, a homolog of the EBV tegument protein BSRF1, and mediates the incorporation of tegument proteins into virions [15].
4.13. BALF2 (homolog of HSV UL29, HCMV UL57, and KSHV ORF6)
BALF2 encodes a single-stranded DNA-binding protein essential for lytic viral DNA synthesis [201,202], and has been detected in purified EBV virions [10]. Some BALF2 proteins localize to the cytoplasm during secondary envelopment [203]. BALF2 interacts with a CATC component, BVRF1 [203], which may mediate the transport of BALF2 protein to newly formed nucleocapsids. The HSV and KSHV homologs of BALF2, UL29 and ORF6, respectively, have also been detected in purified virions [18,189,204].
4.14. BMRF1 (homolog of HSV UL42, HCMV UL44, and KSHV ORF59)
Like BALF2, the BMRF1 protein of EBV is required for viral DNA replication [201,205]. BMRF1 increases the activity of the DNA polymerase catalytic subunit, BALF5 [206]. BMRF1 also activates transcription [[207], [208], [209]]. BMRF1 associates with BKRF4 and BLRF2 [22], and possibly with BGLF4, by which it is phosphorylated [128]. EBV BMRF1 and HSV UL42 proteins were detected in purified virions [10,189], but KSHV ORF59 was not [204].
4.15. BXLF1 (homolog of HSV UL23 and KSHV ORF21)
BXLF1 encodes a thymidine kinase (TK), which is a component of the thymidine salvage pathway of nucleotide biosynthesis [210]. Disruption of the gene had little effect on virus multiplication in cell culture [211]. The protein has been detected in EBV particles [10], but its importance is unknown.
The alpha- and gamma-herpesviruses, but not the beta-herpesviruses, have TK genes. HSV TK (UL23) and KSHV TK (ORF21) have been detected in the virion tegument fraction [18,189,204]. Disruption of HSV UL23 moderately decreased virus replication [212]. Knockout of KSHV ORF21 had no effect on viral gene expression and DNA synthesis, but markedly suppressed infectious progeny production, presumably by decreasing infectivity [213]. Upon infection, ORF21 protein in the tegument is released into the cytoplasm, where it stimulates MEK signaling to enhance infectivity.
4.16. BORF2 (homolog of UL39, HCMV UL45, and KSHV ORF61)
The BORF2 gene of EBV encodes the large subunit of ribonucleotide reductase (RR), which is involved in nucleotide biosynthesis. A BORF2-null mutant virus exhibited lower progeny production than the wild-type [214]. Although the physiological role of BORF2 in the tegument is unknown, expression of the BORF2 gene increases the proportion of cells at G1/S phase by increasing the P53 protein level [108]. In addition, BORF2 associates with, and thereby suppresses the activity of, APOBEC3B, to maintain genomic integrity [214,215], which increases the number of cells in G1/S phase [216].
The large subunit of the RR gene is preserved in HSV, CMV, and KSHV, but HCMV UL45 lacks enzymatic activity [217]. HSV UL39 is non-essential for multiplication in cell lines but required for efficient replication in non-dividing cells [218]. EBV BORF2, HSV UL39, and KSHV ORF61 inhibit the restriction factor, APOBEC3B [219]. HCMV UL45 [220] and HSV UL39, but not KSHV ORF61 protein, have been detected in virions [204], together with the RR small subunit [189]. Two-hybrid analysis indicated that HCMV UL45 protein serves as a tegument network hub, and has many interactions [221].
4.17. BRLF1 (homolog of KSHV ORF50)
BRLF1 is one of the two IE genes encoded by EBV implicated in viral reactivation. This transcriptional activator is required for lytic initiation, particularly in differentiated epithelial cells [222]. BRLF1 protein is incorporated into the tegument [19] and binds a capsid triplex protein, BORF1, thereby preventing its ubiquitin-dependent degradation in transfected cells [19].
Homologs of EBV BRLF1 are found only in gamma-herpesviruses. KSHV ORF50, also known as K-Rta, is a key lytic activator [223], which, unlike EBV BRLF1, has not been detected in purified virions [204].
4.18. BRRF2 (homolog of KSHV ORF48)
The BRRF2 gene product is a late phosphoprotein. BRRF2 localizes to the cytoplasm and its knockout decreased progeny production [224]. Its homologs are present only in gamma-herpesviruses. KSHV ORF48, a homolog of BRRF2, was detected in virions [18], but its function is unclear.
5. Roles of EBV tegument proteins in oncogenesis
There are at least 10 hallmarks of cancer—evasion of apoptosis, self-sufficiency in growth signals, insensitivity to anti-growth signals, tissue invasion and metastasis, limitless replicative potential, sustained angiogenesis, dysregulation of cellular energetics, avoidance of immune destruction, tumor-promoting inflammation, and genome instability and mutation [225]. Latent genes of EBV, such as LMP1, LMP2A, EBNA1, EBNA2, EBNA3A, EBNA3C, and some viral microRNAs, have oncogenic activity, but accumulating pieces of evidence indicate that lytic genes are also involved in oncogenesis [[226], [227], [228], [229]]. Below we summarize the EBV genes linked to oncogenesis.
EBV has two BCL2 homologs, BHRF1 and BALF1, that contribute to evasion of apoptosis [198], and BALF1 may be present in the tegument [14]. Self-sufficiency in growth signals is conferred mainly by latent genes, LMP1 and LMP2A, which activate CD40 and BCR signaling, respectively, in a ligand-independent manner [230]. BZLF1 or other viral factors, like LMP1, induce the production of chemokines and cytokines (such as TNF-α, IL-6, IL-8, VEGF, and IL-10) that activate growth signals [[231], [232], [233]]. BGLF2 and BRLF1 induce MAPK signaling [109,110,234]. Insensitivity to anti-growth signals includes overcoming cell-cycle checkpoints by disabling proteins such as P53, and CDK inhibitors. The latent genes, LMP1, EBNA1, and EBNA3C have been reported to deregulate P53 [235,236]. Transcription of CDK inhibitors, such as P16, P21 and P27, is repressed by the latent genes EBNA3A and EBNA3C [[237], [238], [239], [240]]. Having CDK-like kinase activity, BGLF4 phosphorylates and inactivates P27 [131] and RB [241]. Regarding tissue invasion and metastasis, the EBV genes LMP1, LMP2A, EBNA1, EBNA3C, and BZLF1 induce the epithelial-to-mesenchymal transition and the emergence of cancer stem cells, and encourage migration and invasion [[242], [243], [244], [245], [246]]. Maintenance of telomere length by telomere reverse transcriptase (TERT) is linked to limitless replicative potential. EBV LMP1 induces the TERT gene and maintains telomere synthesis [247,248]. LMP1, LMP2A, BZLF1, and BRLF1 induce pro-angiogenic factors, including VEGF and FGF, thereby mediating sustained angiogenesis [[249], [250], [251], [252], [253]]. The EBV latent gene, LMP1, causes deregulation of cellular energetics, increasing glycolysis and lactate production by inducing HK2, PKM2, LDHA1, and GLUT1 [251,[254], [255], [256], [257]]. EBV encodes many latent and lytic genes linked to avoidance of immune destruction [258,259]. For instance, EBNA2, BZLF1, BGLF5, BILF1, and BNLF2A downregulate antigen presentation by HLA [[260], [261], [262], [263], [264], [265]]. EBNA2 and LMP1 repress the expression of PD-L1 [266]. Innate immunity is repressed by several latent and lytic proteins, including EBNA1, EBNA2, LMP1, LMP2A, BGLF5, BPLF1, BOLF1, BGLF4, BRLF1, BGLF2, and BCRF1 (vIL-10) [86,112,113,137,[267], [268], [269], [270], [271], [272], [273], [274], [275], [276]]. Intrinsic immune mechanisms, such as epigenetic silencing and PML-NB-mediated suppression, are reversed by EBNA1, EBNA2, EBNA3, BZLF1, BRLF1, and BNRF1 [156,[277], [278], [279], [280]]. With respect to tumor-promoting inflammation, the production of cytokines and chemokines is induced by EBV genes, as mentioned above. Finally, the EBV genes BZLF1, BORF2, and BGLF4 dysregulate P53 and CDK inhibitors, leading to genome instability and mutation [139,281,282]. LMP1, EBNA1, EBNA2, EBNA3C, BRLF1, BNRF1, BKRF4, BPLF1, and BGLF5 disrupt genomic integrity [157,159,281,[283], [284], [285], [286], [287], [288]].
6. Conclusion
We summarized the interactions and roles of EBV tegument proteins. The tegument proteins of the Herpesviridae have several conserved properties and functions whereas others are unique to EBV. The phenotypes of EBV knockout mutants are typically analyzed in HEK293 cells, but some processes of the EBV lifecycle are difficult to evaluate in the cell line, e.g., secondary envelopment. This may be because EBV replication is less efficient than HSV, and nucleocapsids cannot be easily detected in HEK293 cells. Alternatively, HEK293 cells might be conducive to secondary envelopment even in the absence of one or more tegument genes. BSRF1 knockout virus had a phenotype similar to wild-type virus in HEK293 cells, but knockdown of the BSRF1 gene reduced progeny virus production in B95-8 cells [14]. Therefore, EBV gene functions should be analyzed in the virus's natural host cell type, such as B cells, for further evaluation.
EBV tegument genes are good candidates for viral attenuation, for development of live vaccines, because their disruption does not cause complete inactivation and so does not prevent the production of other viral antigens, e.g., glycoproteins. Such development requires detailed analyses of knockout viruses, including in animal models.
Author statement
Takayuki Murata: Conceptualization; Data curation; Funding acquisition; Project administration; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing.
Funding sources
This work was supported by Japan Agency for Medical Research and Development (JP21wm0325042) and the Takeda Science Foundation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I thank H. Kimura, T. Watanabe, Y. Sato, and other lab members for discussions.
Data availability
No data was used for the research described in the article.
References
- 1.Haarr L., Skulstad S. The herpes simplex virus type 1 particle: structure and molecular functions. Review article. APMIS. 1994;102(5):321–346. doi: 10.1111/j.1699-0463.1994.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith G.A. Navigating the cytoplasm: delivery of the alphaherpesvirus genome to the nucleus. Curr. Issues Mol. Biol. 2021;41:171–220. doi: 10.21775/cimb.041.171. [DOI] [PubMed] [Google Scholar]
- 3.Murata T., et al. Molecular basis of Epstein-Barr virus latency establishment and lytic reactivation. Viruses. 2021;13(12) doi: 10.3390/v13122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston C.M., Efstathiou S. In: Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A., et al., editors. 2007. Molecular basis of HSV latency and reactivation. (Cambridge) [Google Scholar]
- 5.Crump C. Virus assembly and egress of HSV. Adv. Exp. Med. Biol. 2018;1045:23–44. doi: 10.1007/978-981-10-7230-7_2. [DOI] [PubMed] [Google Scholar]
- 6.Mettenleiter T.C., Klupp B.G., Granzow H. Herpesvirus assembly: an update. Virus Res. 2009;143(2):222–234. doi: 10.1016/j.virusres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Owen D.J., Crump C.M., Graham S.C. Tegument assembly and secondary envelopment of alphaherpesviruses. Viruses. 2015;7(9):5084–5114. doi: 10.3390/v7092861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madavaraju K., et al. Herpes simplex virus cell entry mechanisms: an update. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.617578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murata T. Encyclopedia of EBV-encoded lytic genes: an update. Adv. Exp. Med. Biol. 2018;1045:395–412. doi: 10.1007/978-981-10-7230-7_18. [DOI] [PubMed] [Google Scholar]
- 10.Johannsen E., et al. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. U. S. A. 2004;101(46):16286–16291. doi: 10.1073/pnas.0407320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechtel J.T., Winant R.C., Ganem D. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2005;79(8):4952–4964. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu F.X., Yuan Y. The ORF45 protein of Kaposi's sarcoma-associated herpesvirus is associated with purified virions. J. Virol. 2003;77(7):4221–4230. doi: 10.1128/JVI.77.7.4221-4230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore M., Hutt-Fletcher L.M. The BDLF2 protein of Epstein-Barr virus is a type II glycosylated envelope protein whose processing is dependent on coexpression with the BMRF2 protein. Virology. 2009;383(1):162–167. doi: 10.1016/j.virol.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagi Y., et al. Initial characterization of the Epstein(-)Barr virus BSRF1 gene product. Viruses. 2019;11(3) doi: 10.3390/v11030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Q., et al. Novel role of vBcl2 in the virion assembly of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2018;92(4) doi: 10.1128/JVI.00914-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham C., et al. Herpes simplex virus type 1 gene UL14: phenotype of a null mutant and identification of the encoded protein. J. Virol. 2000;74(1):33–41. doi: 10.1128/jvi.74.1.33-41.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada K., et al. Identification and characterization of the UL14 gene product of herpes simplex virus type 2. J. Gen. Virol. 1999;80(Pt 9):2423–2431. doi: 10.1099/0022-1317-80-9-2423. [DOI] [PubMed] [Google Scholar]
- 18.Nabiee R., et al. An update of the virion proteome of kaposi sarcoma-associated herpesvirus. Viruses. 2020;12(12) doi: 10.3390/v12121382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H.H., et al. Rta is an Epstein-Barr virus tegument protein that improves the stability of capsid protein BORF1. Biochem. Biophys. Res. Commun. 2020;523(3):773–779. doi: 10.1016/j.bbrc.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Calderwood M.A., et al. Epstein-Barr virus and virus human protein interaction maps. Proc. Natl. Acad. Sci. U. S. A. 2007;104(18):7606–7611. doi: 10.1073/pnas.0702332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fossum E., et al. Evolutionarily conserved herpesviral protein interaction networks. PLoS Pathog. 2009;5(9) doi: 10.1371/journal.ppat.1000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara Y., et al. Comprehensive analyses of intraviral Epstein-Barr virus protein-protein interactions hint central role of BLRF2 in the tegument network. J. Virol. 2022;96(14) doi: 10.1128/jvi.00518-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathish N., Wang X., Yuan Y. Tegument proteins of Kaposi's sarcoma-associated herpesvirus and related gamma-herpesviruses. Front. Microbiol. 2012;3:98. doi: 10.3389/fmicb.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diefenbach R.J. Conserved tegument protein complexes: essential components in the assembly of herpesviruses. Virus Res. 2015;210:308–317. doi: 10.1016/j.virusres.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez Duran A., et al. Protein interactions and consensus clustering analysis uncover insights into herpesvirus virion structure and function relationships. PLoS Biol. 2019;17(6) doi: 10.1371/journal.pbio.3000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez Duran A., Grunewald K., Topf M. Conserved central intraviral protein interactome of the herpesviridae family. mSystems. 2019;4(5) doi: 10.1128/mSystems.00295-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vittone V., et al. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 2005;79(15):9566–9571. doi: 10.1128/JVI.79.15.9566-9571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalejta R.F. Tegument proteins of human cytomegalovirus. Microbiol. Mol. Biol. Rev. 2008;72(2):249–265. doi: 10.1128/MMBR.00040-07. (table of contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith R.M., Kosuri S., Kerry J.A. Role of human cytomegalovirus tegument proteins in virion assembly. Viruses. 2014;6(2):582–605. doi: 10.3390/v6020582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y.Q., Zhao X.Y. Human cytomegalovirus primary infection and reactivation: insights from virion-carried molecules. Front. Microbiol. 2020;11:1511. doi: 10.3389/fmicb.2020.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z., et al. CryoEM structure of the tegumented capsid of Epstein-Barr virus. Cell Res. 2020;30(10):873–884. doi: 10.1038/s41422-020-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W., et al. Structures of capsid and capsid-associated tegument complex inside the Epstein-Barr virus. Nat. Microbiol. 2020;5(10):1285–1298. doi: 10.1038/s41564-020-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong D., et al. DNA-packing portal and capsid-associated tegument complexes in the tumor herpesvirus KSHV. Cell. 2019;178(6):1329–1343 e12. doi: 10.1016/j.cell.2019.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai X., Zhou Z.H. Structure of the herpes simplex virus 1 capsid with associated tegument protein complexes. Science. 2018;(6384):360. doi: 10.1126/science.aao7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., et al. Structural basis for genome packaging, retention, and ejection in human cytomegalovirus. Nat. Commun. 2021;12(1):4538. doi: 10.1038/s41467-021-24820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klupp B.G., et al. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 2002;76(6):3065–3071. doi: 10.1128/JVI.76.6.3065-3071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozen R., et al. Virion-wide protein interactions of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2008;82(10):4742–4750. doi: 10.1128/JVI.02745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tullman J.A., et al. Recovery of an HMWP/hmwBP (pUL48/pUL47) complex from virions of human cytomegalovirus: subunit interactions, oligomer composition, and deubiquitylase activity. J. Virol. 2014;88(15):8256–8267. doi: 10.1128/JVI.00971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jambunathan N., et al. Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment. J. Virol. 2014;88(11):5927–5935. doi: 10.1128/JVI.00278-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He H.P., et al. Structure of Epstein-Barr virus tegument protein complex BBRF2-BSRF1 reveals its potential role in viral envelopment. Nat. Commun. 2020;11(1):5405. doi: 10.1038/s41467-020-19259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masud H., et al. The BOLF1 gene is necessary for effective Epstein-Barr viral infectivity. Virology. 2019;531:114–125. doi: 10.1016/j.virol.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Ko D.H., Cunningham A.L., Diefenbach R.J. The major determinant for addition of tegument protein pUL48 (VP16) to capsids in herpes simplex virus type 1 is the presence of the major tegument protein pUL36 (VP1/2) J. Virol. 2010;84(3):1397–1405. doi: 10.1128/JVI.01721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniel G.R., Pegg C.E., Smith G.A. Dissecting the herpesvirus architecture by targeted proteolysis. J. Virol. 2018;92(17) doi: 10.1128/JVI.00738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortiz D.A., Glassbrook J.E., Pellett P.E. Protein-protein interactions suggest novel activities of human cytomegalovirus tegument protein pUL103. J. Virol. 2016;90(17):7798–7810. doi: 10.1128/JVI.00097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roller R.J., Fetters R. The herpes simplex virus 1 UL51 protein interacts with the UL7 protein and plays a role in its recruitment into the virion. J. Virol. 2015;89(6):3112–3122. doi: 10.1128/JVI.02799-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nozawa N., et al. Subcellular localization of herpes simplex virus type 1 UL51 protein and role of palmitoylation in Golgi apparatus targeting. J. Virol. 2003;77(5):3204–3216. doi: 10.1128/JVI.77.5.3204-3216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dietz A.N., et al. A tyrosine-based trafficking motif of the tegument protein pUL71 is crucial for human cytomegalovirus secondary envelopment. J. Virol. 2018;92(1) doi: 10.1128/JVI.00907-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masud H., et al. Epstein-barr virus BBRF2 is required for maximum infectivity. Microorganisms. 2019;7(12) doi: 10.3390/microorganisms7120705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oda S., et al. The interaction between herpes simplex virus 1 tegument proteins UL51 and UL14 and its role in virion morphogenesis. J. Virol. 2016;90(19):8754–8767. doi: 10.1128/JVI.01258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roller R.J., et al. The herpes simplex virus 1 UL51 gene product has cell type-specific functions in cell-to-cell spread. J. Virol. 2014;88(8):4058–4068. doi: 10.1128/JVI.03707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hung C.H., et al. Interaction between BGLF2 and BBLF1 is required for the efficient production of infectious epstein-Barr virus particles. Front. Microbiol. 2019;10:3021. doi: 10.3389/fmicb.2019.03021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loomis J.S., Courtney R.J., Wills J.W. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2003;77(21):11417–11424. doi: 10.1128/JVI.77.21.11417-11424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips S.L., et al. Interaction between the human cytomegalovirus tegument proteins UL94 and UL99 is essential for virus replication. J. Virol. 2012;86(18):9995–10005. doi: 10.1128/JVI.01078-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu Y.F., et al. Characterization and intracellular trafficking of Epstein-Barr virus BBLF1, a protein involved in virion maturation. J. Virol. 2012;86(18):9647–9655. doi: 10.1128/JVI.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacLean C.A., Clark B., McGeoch D.J. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J. Gen. Virol. 1989;70(Pt 12):3147–3157. doi: 10.1099/0022-1317-70-12-3147. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez V., Sztul E., Britt W.J. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-golgi-intermediate compartment. J. Virol. 2000;74(8):3842–3851. doi: 10.1128/jvi.74.8.3842-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masud H., et al. Epstein-barr virus BKRF4 gene product is required for efficient progeny production. J. Virol. 2017;91(23) doi: 10.1128/JVI.00975-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farnsworth A., Wisner T.W., Johnson D.C. Cytoplasmic residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD. J. Virol. 2007;81(1):319–331. doi: 10.1128/JVI.01842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeh P.C., et al. Direct and specific binding of the UL16 tegument protein of herpes simplex virus to the cytoplasmic tail of glycoprotein E. J. Virol. 2011;85(18):9425–9436. doi: 10.1128/JVI.05178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starkey J.L., et al. Elucidation of the block to herpes simplex virus egress in the absence of tegument protein UL16 reveals a novel interaction with VP22. J. Virol. 2014;88(1):110–119. doi: 10.1128/JVI.02555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harper A.L., et al. Interaction domains of the UL16 and UL21 tegument proteins of herpes simplex virus. J. Virol. 2010;84(6):2963–2971. doi: 10.1128/JVI.02015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feederle R., et al. Epstein-Barr virus BNRF1 protein allows efficient transfer from the endosomal compartment to the nucleus of primary B lymphocytes. J. Virol. 2006;80(19):9435–9443. doi: 10.1128/JVI.00473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loesing J.B., et al. Epstein-Barr virus BDLF2-BMRF2 complex affects cellular morphology. J. Gen. Virol. 2009;90(Pt 6):1440–1449. doi: 10.1099/vir.0.009571-0. [DOI] [PubMed] [Google Scholar]
- 64.Salmon B., et al. The herpes simplex virus type 1 U(L)17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J. Virol. 1998;72(5):3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heming J.D., Conway J.F., Homa F.L. Herpesvirus capsid assembly and DNA packaging. Adv. Anat. Embryol. Cell Biol. 2017;223:119–142. doi: 10.1007/978-3-319-53168-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McNab A.R., et al. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J. Virol. 1998;72(2):1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preston V.G., et al. The UL25 gene product of herpes simplex virus type 1 is involved in uncoating of the viral genome. J. Virol. 2008;82(13):6654–6666. doi: 10.1128/JVI.00257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Draganova E.B., et al. Structural basis for capsid recruitment and coat formation during HSV-1 nuclear egress. Elife. 2020;9 doi: 10.7554/eLife.56627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeshima K., et al. Identification of the capsid binding site in the herpes simplex virus 1 nuclear egress complex and its role in viral primary envelopment and replication. J. Virol. 2019;93(21) doi: 10.1128/JVI.01290-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desai P.J. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 2000;74(24):11608–11618. doi: 10.1128/jvi.74.24.11608-11618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abaitua F., et al. A single mutation responsible for temperature-sensitive entry and assembly defects in the VP1-2 protein of herpes simplex virus. J. Virol. 2011;85(5):2024–2036. doi: 10.1128/JVI.01895-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abaitua F., et al. A Nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore. J. Virol. 2012;86(17):8998–9014. doi: 10.1128/JVI.01209-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Copeland A.M., Newcomb W.W., Brown J.C. Herpes simplex virus replication: roles of viral proteins and nucleoporins in capsid-nucleus attachment. J. Virol. 2009;83(4):1660–1668. doi: 10.1128/JVI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jovasevic V., Liang L., Roizman B. Proteolytic cleavage of VP1-2 is required for release of herpes simplex virus 1 DNA into the nucleus. J. Virol. 2008;82(7):3311–3319. doi: 10.1128/JVI.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts A.P., et al. Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1. J. Virol. 2009;83(1):105–116. doi: 10.1128/JVI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gastaldello S., et al. A deneddylase encoded by Epstein-Barr virus promotes viral DNA replication by regulating the activity of cullin-RING ligases. Nat. Cell Biol. 2010;12(4):351–361. doi: 10.1038/ncb2035. [DOI] [PubMed] [Google Scholar]
- 77.Schlieker C., et al. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 2005;79(24):15582–15585. doi: 10.1128/JVI.79.24.15582-15585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitehurst C.B., et al. The Epstein-Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J. Virol. 2009;83(9):4345–4353. doi: 10.1128/JVI.02195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dyson O.F., Pagano J.S., Whitehurst C.B. The translesion polymerase pol eta is required for efficient Epstein-Barr virus infectivity and is regulated by the viral deubiquitinating enzyme BPLF1. J. Virol. 2017;91(19) doi: 10.1128/JVI.00600-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar R., Whitehurst C.B., Pagano J.S. The Rad6/18 ubiquitin complex interacts with the Epstein-Barr virus deubiquitinating enzyme, BPLF1, and contributes to virus infectivity. J. Virol. 2014;88(11):6411–6422. doi: 10.1128/JVI.00536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitehurst C.B., et al. Epstein-Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase eta recruitment to DNA damage sites. J. Virol. 2012;86(15):8097–8106. doi: 10.1128/JVI.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gastaldello S., et al. Herpes virus deneddylases interrupt the cullin-RING ligase neddylation cycle by inhibiting the binding of CAND1. J. Mol. Cell Biol. 2012;4(4):242–251. doi: 10.1093/jmcb/mjs012. [DOI] [PubMed] [Google Scholar]
- 83.Li J., et al. The Epstein-Barr virus deubiquitinating enzyme BPLF1 regulates the activity of topoisomerase II during productive infection. PLoS Pathog. 2021;17(9) doi: 10.1371/journal.ppat.1009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta S., et al. Herpesvirus deconjugases inhibit the IFN response by promoting TRIM25 autoubiquitination and functional inactivation of the RIG-I signalosome. PLoS Pathog. 2018;14(1) doi: 10.1371/journal.ppat.1006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta S., et al. 14-3-3 scaffold proteins mediate the inactivation of trim25 and inhibition of the type I interferon response by herpesvirus deconjugases. PLoS Pathog. 2019;15(11) doi: 10.1371/journal.ppat.1008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saito S., et al. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-kappaB signaling during productive replication. J. Virol. 2013;87(7):4060–4070. doi: 10.1128/JVI.02020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Gent M., et al. Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling. PLoS Pathog. 2014;10(2) doi: 10.1371/journal.ppat.1003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yla-Anttila P., Gupta S., Masucci M.G. The Epstein-Barr virus deubiquitinase BPLF1 targets SQSTM1/p62 to inhibit selective autophagy. Autophagy. 2021;17(11):3461–3474. doi: 10.1080/15548627.2021.1874660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gastaldello S., et al. Caspase-1 promotes Epstein-Barr virus replication by targeting the large tegument protein deneddylase to the nucleus of productively infected cells. PLoS Pathog. 2013;9(10) doi: 10.1371/journal.ppat.1003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitehurst C.B., et al. Knockout of Epstein-Barr virus BPLF1 retards B-cell transformation and lymphoma formation in humanized mice. mBio. 2015;6(5) doi: 10.1128/mBio.01574-15. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grzesik P., et al. Functional domains of the herpes simplex virus type 1 tegument protein pUL37: the amino terminus is dispensable for virus replication in tissue culture. Viruses. 2019;11(9) doi: 10.3390/v11090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krautwald M., et al. Translocation of incoming pseudorabies virus capsids to the cell nucleus is delayed in the absence of tegument protein pUL37. J. Virol. 2009;83(7):3389–3396. doi: 10.1128/JVI.02090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pasdeloup D., et al. Herpesvirus tegument protein pUL37 interacts with dystonin/BPAG1 to promote capsid transport on microtubules during egress. J. Virol. 2013;87(5):2857–2867. doi: 10.1128/JVI.02676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richards A.L., et al. The pUL37 tegument protein guides alpha-herpesvirus retrograde axonal transport to promote neuroinvasion. PLoS Pathog. 2017;13(12) doi: 10.1371/journal.ppat.1006741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J., et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe. 2018;24(2):234–248 e5. doi: 10.1016/j.chom.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao J., et al. A viral deamidase targets the helicase domain of RIG-I to block RNA-induced activation. Cell Host Microbe. 2016;20(6):770–784. doi: 10.1016/j.chom.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gregory S.M., et al. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331(6015):330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nozawa N., et al. Herpes simplex virus type 1 UL51 protein is involved in maturation and egress of virus particles. J. Virol. 2005;79(11):6947–6956. doi: 10.1128/JVI.79.11.6947-6956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kato A., et al. Roles of the phosphorylation of herpes simplex virus 1 UL51 at a specific site in viral replication and pathogenicity. J. Virol. 2018;92(18) doi: 10.1128/JVI.01035-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albecka A., et al. Dual function of the pUL7-pUL51 tegument protein complex in herpes simplex virus 1 infection. J. Virol. 2017;91(2) doi: 10.1128/JVI.02196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanaka M., Sata T., Kawaguchi Y. The product of the Herpes simplex virus 1 UL7 gene interacts with a mitochondrial protein, adenine nucleotide translocator 2. Virol. J. 2008;5:125. doi: 10.1186/1743-422X-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu X., et al. The mutated tegument protein UL7 attenuates the virulence of herpes simplex virus 1 by reducing the modulation of alpha-4 gene transcription. Virol. J. 2016;13(1):152. doi: 10.1186/s12985-016-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fuchs W., et al. The UL7 gene of pseudorabies virus encodes a nonessential structural protein which is involved in virion formation and egress. J. Virol. 2005;79(17):11291–11299. doi: 10.1128/JVI.79.17.11291-11299.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Uddin M.K., Watanabe T., Arata M., Sato Y., Kimura H., Murata T. Epstein-barr virus BBLF1 mediates secretory vesicle transport to facilitate mature virion release. J. Virol. 2023 doi: 10.1128/jvi.00437-23. in press. I am afraid but this paper has not been published yet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baines J.D., Roizman B. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 1992;66(8):5168–5174. doi: 10.1128/jvi.66.8.5168-5174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loomis J.S., Courtney R.J., Wills J.W. Packaging determinants in the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2006;80(21):10534–10541. doi: 10.1128/JVI.01172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meckes D.G., Jr., Marsh J.A., Wills J.W. Complex mechanisms for the packaging of the UL16 tegument protein into herpes simplex virus. Virology. 2010;398(2):208–213. doi: 10.1016/j.virol.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paladino P., et al. Identification of herpesvirus proteins that contribute to G1/S arrest. J. Virol. 2014;88(8):4480–4492. doi: 10.1128/JVI.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Konishi N., et al. BGLF2 increases infectivity of epstein-Barr virus by activating AP-1 upon de novo infection. mSphere. 2018;3(2) doi: 10.1128/mSphere.00138-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu X., Cohen J.I. Epstein-barr virus (EBV) tegument protein BGLF2 promotes EBV reactivation through activation of the p38 mitogen-activated protein kinase. J. Virol. 2016;90(2):1129–1138. doi: 10.1128/JVI.01410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen T., et al. Epstein-Barr virus tegument protein BGLF2 inhibits NF-kappaB activity by preventing p65 Ser536 phosphorylation. FASEB J. 2019;33(9):10563–10576. doi: 10.1096/fj.201901196RR. [DOI] [PubMed] [Google Scholar]
- 112.Jangra S., et al. Suppression of JAK-STAT signaling by Epstein-Barr virus tegument protein BGLF2 through recruitment of SHP1 phosphatase and promotion of STAT2 degradation. J. Virol. 2021;95(20) doi: 10.1128/JVI.01027-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X., et al. Epstein-barr virus (EBV) tegument protein BGLF2 suppresses type I interferon signaling to promote EBV reactivation. J. Virol. 2020;94(11) doi: 10.1128/JVI.00258-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell A.M., et al. Epstein-Barr Virus BGLF2 commandeers RISC to interfere with cellular miRNA function. PLoS Pathog. 2022;18(1) doi: 10.1371/journal.ppat.1010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sato Y., et al. Epstein-Barr virus tegument protein BGLF2 in exosomes released from virus-producing cells facilitates de novo infection. Cell Commun. Signal. 2022;20(1):95. doi: 10.1186/s12964-022-00902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shen S., et al. Gammaherpesvirus tegument protein ORF33 is associated with intranuclear capsids at an early stage of the tegumentation process. J. Virol. 2015;89(10):5288–5297. doi: 10.1128/JVI.00079-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia X., et al. The interaction between tegument proteins ORF33 and ORF45 plays an essential role in cytoplasmic virion maturation of a gammaherpesvirus. J. Virol. 2022;96(22) doi: 10.1128/jvi.01073-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu J.J., et al. ORF33 and ORF38 of Kaposi's sarcoma-associated herpesvirus interact and are required for optimal production of infectious progeny viruses. J. Virol. 2016;90(4):1741–1756. doi: 10.1128/JVI.02738-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baines J.D., Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J. Virol. 1991;65(2):938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao J., Yan X., Banfield B.W. Comparative analysis of UL16 mutants derived from multiple strains of herpes simplex virus 2 (HSV-2) and HSV-1 reveals species-specific requirements for the UL16 protein. J. Virol. 2018;92(13) doi: 10.1128/JVI.00629-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meckes D.G., Jr., Wills J.W. Dynamic interactions of the UL16 tegument protein with the capsid of herpes simplex virus. J. Virol. 2007;81(23):13028–13036. doi: 10.1128/JVI.01306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nalwanga D., et al. The UL 16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology. 1996;226(2):236–242. doi: 10.1006/viro.1996.0651. [DOI] [PubMed] [Google Scholar]
- 123.Yeh P.C., Meckes D.G., Jr., Wills J.W. Analysis of the interaction between the UL11 and UL16 tegument proteins of herpes simplex virus. J. Virol. 2008;82(21):10693–10700. doi: 10.1128/JVI.01230-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carmichael J.C., Wills J.W. Differential requirements for gE, gI, and UL16 among herpes simplex virus 1 syncytial variants suggest unique modes of dysregulating the mechanism of cell-to-cell spread. J. Virol. 2019;93(15) doi: 10.1128/JVI.00494-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao J., et al. Differentiating the roles of UL16, UL21, and Us3 in the nuclear egress of herpes simplex virus capsids. J. Virol. 2020;94(13) doi: 10.1128/JVI.00738-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gao J., Hay T.J.M., Banfield B.W. The product of the herpes simplex virus 2 UL16 gene is critical for the egress of capsids from the nuclei of infected cells. J. Virol. 2017;91(10) doi: 10.1128/JVI.00350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Asai R., et al. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 2006;80(11):5125–5134. doi: 10.1128/JVI.02674-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen M.R., et al. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 2000;74(7):3093–3104. doi: 10.1128/jvi.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yue W., Gershburg E., Pagano J.S. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J. Virol. 2005;79(9):5880–5885. doi: 10.1128/JVI.79.9.5880-5885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu J., et al. Protein array identification of substrates of the Epstein-Barr virus protein kinase BGLF4. J. Virol. 2009;83(10):5219–5231. doi: 10.1128/JVI.02378-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Iwahori S., et al. Phosphorylation of p27Kip1 by Epstein-Barr virus protein kinase induces its degradation through SCFSkp2 ubiquitin ligase actions during viral lytic replication. J. Biol. Chem. 2009;284(28):18923–18931. doi: 10.1074/jbc.M109.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kato K., et al. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 2001;82(Pt 6):1457–1463. doi: 10.1099/0022-1317-82-6-1457. [DOI] [PubMed] [Google Scholar]
- 133.Li R., et al. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe. 2011;10(4):390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang K., Lv D.W., Li R. Conserved herpesvirus protein kinases target SAMHD1 to facilitate virus replication. Cell Rep. 2019;28(2):449–459 e5. doi: 10.1016/j.celrep.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee C.P., et al. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J. Virol. 2008;82(23):11913–11926. doi: 10.1128/JVI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen P.W., et al. Regulation of microtubule dynamics through phosphorylation on stathmin by Epstein-Barr virus kinase BGLF4. J. Biol. Chem. 2010;285(13):10053–10063. doi: 10.1074/jbc.M109.044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J.T., et al. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J. Virol. 2009;83(4):1856–1869. doi: 10.1128/JVI.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee C.P., et al. Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II. J. Virol. 2007;81(10):5166–5180. doi: 10.1128/JVI.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang Y.H., et al. Epstein-Barr virus BGLF4 kinase retards cellular S-phase progression and induces chromosomal abnormality. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kudoh A., et al. Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication. J. Virol. 2006;80(20):10064–10072. doi: 10.1128/JVI.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang C.W., et al. BGLF4 kinase modulates the structure and transport preference of the nuclear pore complex to facilitate nuclear import of Epstein-Barr virus lytic proteins. J. Virol. 2015;89(3):1703–1718. doi: 10.1128/JVI.02880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murata T., et al. Efficient production of infectious viruses requires enzymatic activity of Epstein-Barr virus protein kinase. Virology. 2009;389(1–2):75–81. doi: 10.1016/j.virol.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 143.El-Guindy A., et al. A locus encompassing the Epstein-Barr virus bglf4 kinase regulates expression of genes encoding viral structural proteins. PLoS Pathog. 2014;10(8) doi: 10.1371/journal.ppat.1004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feederle R., et al. The Epstein-Barr virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production. J. Virol. 2009;83(21):10877–10891. doi: 10.1128/JVI.00525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gershburg E., et al. Epstein-Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus. J. Virol. 2007;81(10):5407–5412. doi: 10.1128/JVI.02398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meng Q., et al. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J. Virol. 2010;84(9):4534–4542. doi: 10.1128/JVI.02487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawaguchi Y., Kato K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 2003;13(5):331–340. doi: 10.1002/rmv.402. [DOI] [PubMed] [Google Scholar]
- 148.Coulter L.J., et al. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 1993;74(Pt 3):387–395. doi: 10.1099/0022-1317-74-3-387. [DOI] [PubMed] [Google Scholar]
- 149.Koyanagi N., et al. Herpes simplex virus-1 evasion of CD8+ T cell accumulation contributes to viral encephalitis. J. Clin. Invest. 2017;127(10):3784–3795. doi: 10.1172/JCI92931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Purves F.C., Ogle W.O., Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. U. S. A. 1993;90(14):6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou L., et al. Mechanism of herpesvirus protein kinase UL13 in immune escape and viral replication. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abbott R.J., et al. CD8+ T cell responses to lytic EBV infection: late antigen specificities as subdominant components of the total response. J. Immunol. 2013;191(11):5398–5409. doi: 10.4049/jimmunol.1301629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adhikary D., et al. The Epstein-Barr virus major tegument protein BNRF1 is a common target of cytotoxic CD4(+) T cells. J. Virol. 2020;94(15) doi: 10.1128/JVI.00284-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsai K., et al. Viral reprogramming of the Daxx histone H3.3 chaperone during early Epstein-Barr virus infection. J. Virol. 2014;88(24):14350–14363. doi: 10.1128/JVI.01895-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsai K., et al. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog. 2011;7(11) doi: 10.1371/journal.ppat.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yiu S.P.T., et al. Epstein-Barr virus BNRF1 destabilizes SMC5/6 cohesin complexes to evade its restriction of replication compartments. Cell Rep. 2022;38(10) doi: 10.1016/j.celrep.2022.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shumilov A., et al. Epstein-Barr virus particles induce centrosome amplification and chromosomal instability. Nat. Commun. 2017;8 doi: 10.1038/ncomms14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Full F., et al. Kaposi's sarcoma associated herpesvirus tegument protein ORF75 is essential for viral lytic replication and plays a critical role in the antagonization of ND10-instituted intrinsic immunity. PLoS Pathog. 2014;10(1) doi: 10.1371/journal.ppat.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ho T.H., et al. A screen for Epstein-Barr virus proteins that inhibit the DNA damage response reveals a novel histone binding protein. J. Virol. 2018;92(14) doi: 10.1128/JVI.00262-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J., et al. Epstein-Barr virus protein BKRF4 restricts nucleosome assembly to suppress host antiviral responses. Proc. Natl. Acad. Sci. U. S. A. 2022;119(37) doi: 10.1073/pnas.2203782119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu Y., et al. Epstein-barr virus tegument protein BKRF4 is a histone chaperone. J. Mol. Biol. 2022;434(19) doi: 10.1016/j.jmb.2022.167756. [DOI] [PubMed] [Google Scholar]
- 162.Atyeo N., Papp B. The ORF45 protein of Kaposi's sarcoma-associated herpesvirus and its critical role in the viral life cycle. Viruses. 2022;14(9) doi: 10.3390/v14092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liang Q., Zhu F. ORF45, a multifunctional immediate early and tegument protein of KSHV. J. Med. Virol. 2023;95(3) doi: 10.1002/jmv.28659. [DOI] [PubMed] [Google Scholar]
- 164.Zhu F.X., et al. Functional characterization of Kaposi's sarcoma-associated herpesvirus ORF45 by bacterial artificial chromosome-based mutagenesis. J. Virol. 2006;80(24):12187–12196. doi: 10.1128/JVI.01275-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gillen J., Zhu F. Disruption of the interaction between ORF33 and the conserved carboxyl-terminus of ORF45 abolishes progeny virion production of kaposi sarcoma-associated herpesvirus. Viruses. 2021;13(9) doi: 10.3390/v13091828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang X., et al. Mono-ubiquitylated ORF45 mediates association of KSHV particles with internal lipid rafts for viral assembly and egress. PLoS Pathog. 2015;11(12) doi: 10.1371/journal.ppat.1005332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sathish N., Zhu F.X., Yuan Y. Kaposi's sarcoma-associated herpesvirus ORF45 interacts with kinesin-2 transporting viral capsid-tegument complexes along microtubules. PLoS Pathog. 2009;5(3) doi: 10.1371/journal.ppat.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liang Q., et al. ORF45 of Kaposi's sarcoma-associated herpesvirus inhibits phosphorylation of interferon regulatory factor 7 by IKKepsilon and TBK1 as an alternative substrate. J. Virol. 2012;86(18):10162–10172. doi: 10.1128/JVI.05224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu F.X., et al. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. U. S. A. 2002;99(8):5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang X., et al. KSHV-encoded ORF45 activates human NLRP1 inflammasome. Nat. Immunol. 2022;23(6):916–926. doi: 10.1038/s41590-022-01199-x. [DOI] [PubMed] [Google Scholar]
- 171.Kuang E., et al. Activation of p90 ribosomal S6 kinase by ORF45 of Kaposi's sarcoma-associated herpesvirus and its role in viral lytic replication. J. Virol. 2008;82(4):1838–1850. doi: 10.1128/JVI.02119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuang E., Wu F., Zhu F. Mechanism of sustained activation of ribosomal S6 kinase (RSK) and ERK by kaposi sarcoma-associated herpesvirus ORF45: multiprotein complexes retain active phosphorylated ERK AND RSK and protect them from dephosphorylation. J. Biol. Chem. 2009;284(20):13958–13968. doi: 10.1074/jbc.M900025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kuang E., et al. Phosphorylation of eukaryotic translation initiation factor 4B (EIF4B) by open reading frame 45/p90 ribosomal S6 kinase (ORF45/RSK) signaling axis facilitates protein translation during Kaposi sarcoma-associated herpesvirus (KSHV) lytic replication. J. Biol. Chem. 2011;286(48):41171–41182. doi: 10.1074/jbc.M111.280982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alzhanova D., et al. The ORF45 protein of kaposi sarcoma-associated herpesvirus is an inhibitor of p53 signaling during viral reactivation. J. Virol. 2021;95(23) doi: 10.1128/JVI.01459-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gillen J., et al. A survey of the interactome of Kaposi's sarcoma-associated herpesvirus ORF45 revealed its binding to viral ORF33 and cellular USP7, resulting in stabilization of ORF33 that is required for production of progeny viruses. J. Virol. 2015;89(9):4918–4931. doi: 10.1128/JVI.02925-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun Q., et al. Upregulation of ATF4-LAMP3 Axis by ORF45 facilitates lytic replication of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2022;96(23) doi: 10.1128/jvi.01456-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Benach J., et al. Structural and functional studies of the abundant tegument protein ORF52 from murine gammaherpesvirus 68. J. Biol. Chem. 2007;282(43):31534–31541. doi: 10.1074/jbc.M705637200. [DOI] [PubMed] [Google Scholar]
- 178.Duarte M., et al. An RS motif within the Epstein-Barr virus BLRF2 tegument protein is phosphorylated by SRPK2 and is important for viral replication. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li W., et al. Kaposi's sarcoma-associated herpesvirus inhibitor of cGAS (KicGAS), encoded by ORF52, is an abundant tegument protein and is required for production of infectious progeny viruses. J. Virol. 2016;90(11):5329–5342. doi: 10.1128/JVI.02675-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bhowmik D., et al. Cooperative DNA binding mediated by KicGAS/ORF52 oligomerization allows inhibition of DNA-induced phase separation and activation of cGAS. Nucleic Acids Res. 2021;49(16):9389–9403. doi: 10.1093/nar/gkab689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bhowmik D., et al. Structural basis of higher order oligomerization of KSHV inhibitor of cGAS. Proc. Natl. Acad. Sci. U. S. A. 2022;119(33) doi: 10.1073/pnas.2200285119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu J.J., et al. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe. 2015;18(3):333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hew K., et al. VP22 core domain from Herpes simplex virus 1 reveals a surprising structural conservation in both the Alpha- and Gammaherpesvirinae subfamilies. J. Gen. Virol. 2015;96(Pt 6):1436–1445. doi: 10.1099/vir.0.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Loftus M.S., Verville N., Kedes D.H. A conserved leucine zipper motif in gammaherpesvirus ORF52 is critical for distinct microtubule rearrangements. J. Virol. 2017;91(17) doi: 10.1128/JVI.00304-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maruzuru Y., et al. Role of the DNA binding activity of herpes simplex virus 1 VP22 in evading AIM2-dependent inflammasome activation induced by the virus. J. Virol. 2021;95(5) doi: 10.1128/JVI.02172-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu G., et al. Viral tegument proteins restrict cGAS-DNA phase separation to mediate immune evasion. Mol. Cell. 2021;81(13):2823–2837 e9. doi: 10.1016/j.molcel.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 187.Mills R., et al. Improving gene annotation of complete viral genomes. Nucleic Acids Res. 2003;31(23):7041–7055. doi: 10.1093/nar/gkg878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Watanabe T., et al. Roles of Epstein-Barr virus BGLF3.5 gene and two upstream open reading frames in lytic viral replication in HEK293 cells. Virology. 2015;483:44–53. doi: 10.1016/j.virol.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 189.Loret S., Guay G., Lippe R. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 2008;82(17):8605–8618. doi: 10.1128/JVI.00904-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yamauchi Y., et al. The UL14 tegument protein of herpes simplex virus type 1 is required for efficient nuclear transport of the alpha transinducing factor VP16 and viral capsids. J. Virol. 2008;82(3):1094–1106. doi: 10.1128/JVI.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yamauchi Y., et al. Herpes simplex virus type 2 UL14 gene product has heat shock protein (HSP)-like functions. J. Cell Sci. 2002;115(Pt 12):2517–2527. doi: 10.1242/jcs.115.12.2517. [DOI] [PubMed] [Google Scholar]
- 192.Yamauchi Y., et al. Herpes simplex virus UL14 protein blocks apoptosis. Microbiol. Immunol. 2003;47(9):685–689. doi: 10.1111/j.1348-0421.2003.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 193.Yamauchi Y., et al. The UL14 protein of herpes simplex virus type 2 translocates the minor capsid protein VP26 and the DNA cleavage and packaging UL33 protein into the nucleus of coexpressing cells. J. Gen. Virol. 2001;82(Pt 2):321–330. doi: 10.1099/0022-1317-82-2-321. [DOI] [PubMed] [Google Scholar]
- 194.Bergson S., et al. The Kaposi's-sarcoma-associated herpesvirus orf35 gene product is required for efficient lytic virus reactivation. Virology. 2016;499:91–98. doi: 10.1016/j.virol.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 195.Hikita S.I., Yanagi Y., Ohno S. Murine gammaherpesvirus 68 ORF35 is required for efficient lytic replication and latency. J. Gen. Virol. 2015;96(12):3624–3634. doi: 10.1099/jgv.0.000310. [DOI] [PubMed] [Google Scholar]
- 196.Mabuchi S., et al. Role of Epstein-Barr virus C promoter deletion in diffuse large B cell lymphoma. Cancers. 2021;13(3) doi: 10.3390/cancers13030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marshall W.L., et al. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J. Virol. 1999;73(6):5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Altmann M., Hammerschmidt W. Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol. 2005;3(12):e404. doi: 10.1371/journal.pbio.0030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shao Z., et al. Epstein-barr virus BALF0 and BALF1 modulate autophagy. Viruses. 2019;11(12) doi: 10.3390/v11121099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gelgor A., et al. Viral bcl-2 encoded by the Kaposi's sarcoma-associated herpesvirus is vital for virus reactivation. J. Virol. 2015;89(10):5298–5307. doi: 10.1128/JVI.00098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fixman E.D., Hayward G.S., Hayward S.D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 1992;66(8):5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tsurumi T., et al. Epstein-Barr virus single-stranded DNA-binding protein: purification, characterization, and action on DNA synthesis by the viral DNA polymerase. Virology. 1996;222(2):352–364. doi: 10.1006/viro.1996.0432. [DOI] [PubMed] [Google Scholar]
- 203.Chao T.Y., et al. Subcellular distribution of BALF2 and the role of Rab1 in the formation of Epstein-Barr virus cytoplasmic assembly compartment and virion release. Microbiol. Spectr. 2023;11(1) doi: 10.1128/spectrum.04369-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu F.X., et al. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 2005;79(2):800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Neuhierl B., Delecluse H.J. The Epstein-Barr virus BMRF1 gene is essential for lytic virus replication. J. Virol. 2006;80(10):5078–5081. doi: 10.1128/JVI.80.10.5078-5081.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsurumi T., et al. Functional interaction between Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit in vitro. J. Virol. 1993;67(12):7648–7653. doi: 10.1128/jvi.67.12.7648-7653.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nakayama S., et al. Epstein-Barr virus polymerase processivity factor enhances BALF2 promoter transcription as a coactivator for the BZLF1 immediate-early protein. J. Biol. Chem. 2009;284(32):21557–21568. doi: 10.1074/jbc.M109.015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Salamun S.G., et al. The Epstein-Barr virus BMRF1 protein activates transcription and inhibits the DNA damage response by binding NuRD. J. Virol. 2019;93(22) doi: 10.1128/JVI.01070-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Su M.T., et al. The SWI/SNF chromatin regulator BRG1 modulates the transcriptional regulatory activity of the Epstein-Barr virus DNA polymerase processivity factor BMRF1. J. Virol. 2017;91(9) doi: 10.1128/JVI.02114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Littler E., et al. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986;5(8):1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shimizu N., Yoshiyama H., Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J. Virol. 1996;70(10):7260–7263. doi: 10.1128/jvi.70.10.7260-7263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Field H.J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob. Agents Chemother. 1980;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yamaguchi T., et al. Kaposi's sarcoma-associated herpesvirus ORF21 enhances the phosphorylation of MEK and the infectivity of progeny virus. Int. J. Mol. Sci. 2023;24(2) doi: 10.3390/ijms24021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cheng A.Z., et al. Epstein-Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity. Nat. Microbiol. 2019;4(1):78–88. doi: 10.1038/s41564-018-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shaban N.M., et al. Cryo-EM structure of the EBV ribonucleotide reductase BORF2 and mechanism of APOBEC3B inhibition. Sci. Adv. 2022;8(17) doi: 10.1126/sciadv.abm2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yockteng-Melgar J., et al. G(1)/S cell cycle induction by Epstein-Barr virus BORF2 is mediated by P53 and APOBEC3B. J. Virol. 2022;96(18) doi: 10.1128/jvi.00660-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lembo D., Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem. Sci. 2009;34(1):25–32. doi: 10.1016/j.tibs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 218.Goldstein D.J., Weller S.K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166(1):41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 219.Cheng A.Z., et al. A conserved mechanism of APOBEC3 relocalization by herpesviral ribonucleotide reductase large subunits. J. Virol. 2019;93(23) doi: 10.1128/JVI.01539-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Patrone M., et al. The human cytomegalovirus UL45 gene product is a late, virion-associated protein and influences virus growth at low multiplicities of infection. J. Gen. Virol. 2003;84(Pt 12):3359–3370. doi: 10.1099/vir.0.19452-0. [DOI] [PubMed] [Google Scholar]
- 221.Phillips S.L., Bresnahan W.A. Identification of binary interactions between human cytomegalovirus virion proteins. J. Virol. 2011;85(1):440–447. doi: 10.1128/JVI.01551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ali A., et al. Rta is the principal activator of Epstein-Barr virus epithelial lytic transcription. PLoS Pathog. 2022;18(9) doi: 10.1371/journal.ppat.1010886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Staudt M.R., Dittmer D.P. The Rta/Orf50 transactivator proteins of the gamma-herpesviridae. Curr. Top. Microbiol. Immunol. 2007;312:71–100. doi: 10.1007/978-3-540-34344-8_3. [DOI] [PubMed] [Google Scholar]
- 224.Watanabe T., et al. The C-terminus of Epstein-Barr virus BRRF2 is required for its proper localization and efficient virus production. Front. Microbiol. 2017;8:125. doi: 10.3389/fmicb.2017.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 226.Manners O., et al. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr. Opin. Virol. 2018;32:60–70. doi: 10.1016/j.coviro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Murata T., et al. Oncogenesis of CAEBV revealed: intragenic deletions in the viral genome and leaky expression of lytic genes. Rev. Med. Virol. 2020;30(2):e2095. doi: 10.1002/rmv.2095. [DOI] [PubMed] [Google Scholar]
- 228.Rosemarie Q., Sugden B. Epstein-barr virus: how its lytic phase contributes to oncogenesis. Microorganisms. 2020;8(11) doi: 10.3390/microorganisms8111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yap L.F., et al. Functional implications of Epstein-Barr virus lytic genes in carcinogenesis. Cancers. 2022;14(23) doi: 10.3390/cancers14235780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kang M.S., Kieff E. Epstein-Barr virus latent genes. Exp. Mol. Med. 2015;47(1):e131. doi: 10.1038/emm.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Germini D., et al. Oncogenic properties of the EBV ZEBRA protein. Cancers. 2020;12(6) doi: 10.3390/cancers12061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang L., Ning S. New look of EBV LMP1 signaling landscape. Cancers. 2021;13(21) doi: 10.3390/cancers13215451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yoshizaki T., et al. Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci. 2018;109(2):272–278. doi: 10.1111/cas.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lee Y.H., et al. Activation of the ERK signal transduction pathway by Epstein-Barr virus immediate-early protein Rta. J. Gen. Virol. 2008;89(Pt 10):2437–2446. doi: 10.1099/vir.0.2008/003897-0. [DOI] [PubMed] [Google Scholar]
- 235.Chatterjee K., et al. The interplay between Epstein-Bar virus (EBV) with the p53 and its homologs during EBV associated malignancies. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Frappier L. Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses. 2012;4(9):1537–1547. doi: 10.3390/v4091537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jiang S., et al. Epstein-Barr virus nuclear antigen 3C binds to BATF/IRF4 or SPI1/IRF4 composite sites and recruits Sin3A to repress CDKN2A. Proc. Natl. Acad. Sci. U. S. A. 2014;111(1):421–426. doi: 10.1073/pnas.1321704111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Parker G.A., Touitou R., Allday M.J. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene. 2000;19(5):700–709. doi: 10.1038/sj.onc.1203327. [DOI] [PubMed] [Google Scholar]
- 239.Skalska L., et al. Induction of p16(INK4a) is the major barrier to proliferation when Epstein-Barr virus (EBV) transforms primary B cells into lymphoblastoid cell lines. PLoS Pathog. 2013;9(2) doi: 10.1371/journal.ppat.1003187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tursiella M.L., et al. Epstein-Barr virus nuclear antigen 3A promotes cellular proliferation by repression of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. PLoS Pathog. 2014;10(10) doi: 10.1371/journal.ppat.1004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kuny C.V., et al. Cyclin-dependent kinase-like function is shared by the beta- and gamma- subset of the conserved herpesvirus protein kinases. PLoS Pathog. 2010;6(9) doi: 10.1371/journal.ppat.1001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gaur N., et al. Epstein-Barr virus latent antigens EBNA3C and EBNA1 modulate epithelial to mesenchymal transition of cancer cells associated with tumor metastasis. Tumour Biol. 2015;36(4):3051–3060. doi: 10.1007/s13277-014-2941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kondo S., et al. Epstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J. Virol. 2011;85(21):11255–11264. doi: 10.1128/JVI.00188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kong Q.L., et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6(6) doi: 10.1371/journal.ppat.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lan Y.Y., et al. Epstein-Barr virus Zta upregulates matrix metalloproteinases 3 and 9 that synergistically promote cell invasion in vitro. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lin Z., et al. Epstein-Barr virus-encoded latent membrane protein 2A promotes the epithelial-mesenchymal transition in nasopharyngeal carcinoma via metastatic tumor antigen 1 and mechanistic target of rapamycin signaling induction. J. Virol. 2014;88(20):11872–11885. doi: 10.1128/JVI.01867-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mei Y.P., et al. siRNA targeting LMP1-induced apoptosis in EBV-positive lymphoma cells is associated with inhibition of telomerase activity and expression. Cancer Lett. 2006;232(2):189–198. doi: 10.1016/j.canlet.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 248.Terrin L., et al. Latent membrane protein 1 of Epstein-Barr virus activates the hTERT promoter and enhances telomerase activity in B lymphocytes. J. Virol. 2008;82(20):10175–10187. doi: 10.1128/JVI.00321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hong G.K., et al. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 2005;79(22):13984–13992. doi: 10.1128/JVI.79.22.13984-13992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kondo S., et al. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 2006;66(20):9870–9877. doi: 10.1158/0008-5472.CAN-06-1679. [DOI] [PubMed] [Google Scholar]
- 251.Lo A.K., et al. Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J. Pathol. 2015;237(2):238–248. doi: 10.1002/path.4575. [DOI] [PubMed] [Google Scholar]
- 252.Sakakibara S., Tosato G. Regulation of angiogenesis in malignancies associated with Epstein-Barr virus and Kaposi's sarcoma-associated herpes virus. Future Microbiol. 2009;4(7):903–917. doi: 10.2217/fmb.09.49. [DOI] [PubMed] [Google Scholar]
- 253.Ye J., et al. Blockage of store-operated Ca(2+) entry antagonizes Epstein-Barr virus-promoted angiogenesis by inhibiting Ca(2+) signaling-regulated VEGF production in nasopharyngeal carcinoma. Cancer Manag. Res. 2018;10:1115–1124. doi: 10.2147/CMAR.S159441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jiang Y., et al. Repression of Hox genes by LMP1 in nasopharyngeal carcinoma and modulation of glycolytic pathway genes by HoxC8. Oncogene. 2015;34(50):6079–6091. doi: 10.1038/onc.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Piccaluga P.P., et al. Epstein-barr virus-induced metabolic rearrangements in human B-cell lymphomas. Front. Microbiol. 2018;9:1233. doi: 10.3389/fmicb.2018.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Xiao L., et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33(37):4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang J., et al. mTORC2-mediated PDHE1alpha nuclear translocation links EBV-LMP1 reprogrammed glucose metabolism to cancer metastasis in nasopharyngeal carcinoma. Oncogene. 2019;38(24):4669–4684. doi: 10.1038/s41388-019-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Albanese M., Tagawa T., Hammerschmidt W. Strategies of Epstein-Barr virus to evade innate antiviral immunity of its human host. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.955603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ressing M.E., et al. Immune evasion by Epstein-Barr virus. Curr. Top. Microbiol. Immunol. 2015;391:355–381. doi: 10.1007/978-3-319-22834-1_12. [DOI] [PubMed] [Google Scholar]
- 260.Fares S., et al. Distinct roles of extracellular domains in the Epstein-Barr virus-encoded BILF1 receptor for signaling and major histocompatibility complex class I downregulation. mBio. 2019;10(1) doi: 10.1128/mBio.01707-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Horst D., et al. EBV protein BNLF2a exploits host tail-anchored protein integration machinery to inhibit TAP. J. Immunol. 2011;186(6):3594–3605. doi: 10.4049/jimmunol.1002656. [DOI] [PubMed] [Google Scholar]
- 262.Li D., et al. Down-regulation of MHC class II expression through inhibition of CIITA transcription by lytic transactivator Zta during Epstein-Barr virus reactivation. J. Immunol. 2009;182(4):1799–1809. doi: 10.4049/jimmunol.0802686. [DOI] [PubMed] [Google Scholar]
- 263.Quinn L.L., et al. The missing link in Epstein-Barr virus immune evasion: the BDLF3 gene induces ubiquitination and downregulation of major histocompatibility complex class I (MHC-I) and MHC-II. J. Virol. 2016;90(1):356–367. doi: 10.1128/JVI.02183-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ressing M.E., et al. Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products. Semin. Cancer Biol. 2008;18(6):397–408. doi: 10.1016/j.semcancer.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 265.Su C., et al. EBNA2 driven enhancer switching at the CIITA-DEXI locus suppresses HLA class II gene expression during EBV infection of B-lymphocytes. PLoS Pathog. 2021;17(8) doi: 10.1371/journal.ppat.1009834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Murata T. Human herpesvirus and the immune checkpoint PD-1/PD-L1 pathway: disorders and strategies for survival. Microorganisms. 2021;9(4) doi: 10.3390/microorganisms9040778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bentz G.L., et al. Epstein-Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-beta. Virology. 2010;402(1):121–128. doi: 10.1016/j.virol.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fathallah I., et al. EBV latent membrane protein 1 is a negative regulator of TLR9. J. Immunol. 2010;185(11):6439–6447. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 269.Jochum S., et al. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8(5) doi: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jog N.R., et al. Epstein Barr virus interleukin 10 suppresses anti-inflammatory phenotype in human monocytes. Front. Immunol. 2018;9:2198. doi: 10.3389/fimmu.2018.02198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shah K.M., et al. The EBV-encoded latent membrane proteins, LMP2A and LMP2B, limit the actions of interferon by targeting interferon receptors for degradation. Oncogene. 2009;28(44):3903–3914. doi: 10.1038/onc.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Spender L.C., et al. Direct and indirect regulation of cytokine and cell cycle proteins by EBNA-2 during Epstein-Barr virus infection. J. Virol. 2001;75(8):3537–3546. doi: 10.1128/JVI.75.8.3537-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Valentine R., et al. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol. Cancer. 2010;9:1. doi: 10.1186/1476-4598-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.van Gent M., et al. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J. Immunol. 2011;186(3):1694–1702. doi: 10.4049/jimmunol.0903120. [DOI] [PubMed] [Google Scholar]
- 275.Westhoff Smith D., et al. The Epstein-Barr virus oncogene EBNA1 suppresses natural killer cell responses and apoptosis early after infection of peripheral B cells. mBio. 2021;12(6) doi: 10.1128/mBio.02243-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wood V.H., et al. Epstein-Barr virus-encoded EBNA1 regulates cellular gene transcription and modulates the STAT1 and TGFbeta signaling pathways. Oncogene. 2007;26(28):4135–4147. doi: 10.1038/sj.onc.1210496. [DOI] [PubMed] [Google Scholar]
- 277.Buschle A., Hammerschmidt W. Epigenetic lifestyle of Epstein-Barr virus. Semin. Immunopathol. 2020;42(2):131–142. doi: 10.1007/s00281-020-00792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Salsman J., et al. Genome-wide screen of three herpesviruses for protein subcellular localization and alteration of PML nuclear bodies. PLoS Pathog. 2008;4(7) doi: 10.1371/journal.ppat.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sivachandran N., Cao J.Y., Frappier L. Epstein-Barr virus nuclear antigen 1 Hijacks the host kinase CK2 to disrupt PML nuclear bodies. J. Virol. 2010;84(21):11113–11123. doi: 10.1128/JVI.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.West M.J. Chromatin reorganisation in Epstein-Barr virus-infected cells and its role in cancer development. Curr. Opin. Virol. 2017;26:149–155. doi: 10.1016/j.coviro.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 281.Wu C.C., et al. Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010;38(6):1932–1949. doi: 10.1093/nar/gkp1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yang J., et al. Epstein-Barr virus BZLF1 protein impairs accumulation of host DNA damage proteins at damage sites in response to DNA damage. Lab. Invest. 2015;95(8):937–950. doi: 10.1038/labinvest.2015.69. [DOI] [PubMed] [Google Scholar]
- 283.Gruhne B., et al. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 2009;106(7):2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gruhne B., Sompallae R., Masucci M.G. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28(45):3997–4008. doi: 10.1038/onc.2009.258. [DOI] [PubMed] [Google Scholar]
- 285.Huang S.Y., et al. Epstein-Barr virus BRLF1 induces genomic instability and progressive malignancy in nasopharyngeal carcinoma cells. Oncotarget. 2017;8(45):78948–78964. doi: 10.18632/oncotarget.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jha H.C., et al. Epstein-Barr virus essential antigen EBNA3C attenuates H2AX expression. J. Virol. 2014;88(7):3776–3788. doi: 10.1128/JVI.03568-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu M.T., et al. Epstein-Barr virus latent membrane protein 1 induces micronucleus formation, represses DNA repair and enhances sensitivity to DNA-damaging agents in human epithelial cells. Oncogene. 2004;23(14):2531–2539. doi: 10.1038/sj.onc.1207375. [DOI] [PubMed] [Google Scholar]
- 288.Pan S.H., et al. Epstein-Barr virus nuclear antigen 2 disrupts mitotic checkpoint and causes chromosomal instability. Carcinogenesis. 2009;30(2):366–375. doi: 10.1093/carcin/bgn291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.