Abstract
The coexistence of anti-glomerular basement membrane (anti-GBM) disease with thrombotic microangiopathy (TMA) is rarely encountered, and the clinical characteristics of this phenomenon are not well known.
A 76-year-old Japanese woman with a history of idiopathic pulmonary disease was diagnosed with anti-GBM disease due to rapidly progressive glomerulonephritis and a positive anti-GBM antibody test result. We treated the patient with hemodialysis, glucocorticoids, and plasmapheresis. During treatment, the patient suddenly became comatose. TMA was then diagnosed because of thrombocytopenia and microangiopathic hemolytic anemia. The activity of a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 13 (ADAMTS-13) was retained at 48%. Although we continued the treatment, the patient died of respiratory failure. An autopsy revealed the cause of respiratory failure to be an acute exacerbation of interstitial pneumonia. The clinical findings of the renal specimen indicated anti-GBM disease; however, there were no lesions suggestive of TMA. A genetic test did not reveal an apparent genetic mutation of the atypical hemolytic uremic syndrome.
We conducted a literature review of past case reports of anti-GBM disease with TMA. The following clinical characteristics were obtained. First, 75% of the cases were reported in Asia. Second, TMA tended to appear during the treatment course for anti-GBM disease and usually resolved within 12 weeks. Third, ADAMTS-13 activity was retained above 10% in 90% of the cases. Fourth, central nervous system manifestations occurred in more than half of the patients. Fifth, the renal outcome was very poor. Further studies are required to understand the pathophysiology of this phenomenon.
Keywords: Anti-glomerular basement membrane disease, Thrombotic microangiopathy, Thrombotic thrombocytopenic purpura, Atypical hemolytic uremic syndrome
Introduction
Anti-glomerular basement membrane (anti-GBM) disease is a small-vessel vasculitis that affects the capillary beds of the kidneys and lungs. Most patients present with features of rapidly progressive glomerular nephritis (RPGN). Early treatment using plasma exchange (PE) and immunosuppression is essential as it is associated with positive renal outcomes [1].
Thrombotic microangiopathy (TMA) is characterized by thrombocytopenia, microangiopathic hemolytic anemia, and organ injury such as acute kidney injury [2]. In 2016, the Japanese Society of Nephrology and Japan Pediatric Society published guidelines and defined TMA as follows: TMA includes thrombotic thrombocytopenic purpura (TTP), Shiga toxin-producing Escherichia coli hemolytic uremic syndrome, atypical hemolytic uremic syndrome (aHUS), and secondary TMA [3, 4].
Autoimmune diseases, such as systemic lupus erythematosus, systemic sclerosis, antiphospholipid syndrome, and anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, have been reported to be the cause of secondary TMA [2, 3, 5]. Though there are some existing case reports of anti-GBM disease with TMA [6–20], the clinical features of this phenomenon have not been described extensively.
Herein, we presented an autopsy case of anti-GBM disease with TMA along with a literature review highlighting the clinical features of anti-GBM disease with TMA.
Case report
A 76-year-old Japanese woman presented to the emergency department with a fever. Three years prior to admission, the patient had been diagnosed with idiopathic pulmonary fibrosis, and follow-up was continued. Her medical history included hypertension and chronic kidney disease stage 3a. She was treated with 40 mg telmisartan. She had no family history of renal failure. One month prior to admission, she visited the hospital regularly, and her general condition was good. Her serum creatinine level was 0.82 mg/dL. One week prior to admission, she had developed a fever and loss of appetite with no diarrhea, bloody stools, or respiratory symptoms. On admission, vital signs were as follows: temperature, 36.5 °C; blood pressure, 108/69 mmHg; pulse, 68 beats/min; respiratory rate, 22 breaths/min; and oxygen saturation, 98% on room air. Physical examination was unremarkable, except for fine crackles in both lower lungs. Serum creatinine and C-reactive protein levels were 6.98 mg/dL and 22.68 mg/dL, respectively. A leukocyte urine test was positive. As a screening test before hospitalization, a COVID-19 antigen test was performed, and the result was negative. As urinary tract infection and prerenal kidney injury were suspected, ceftriaxone 2 g/day was administered, and fluid resuscitation was started. After starting ceftriaxone, the urine culture results were obtained and were negative.
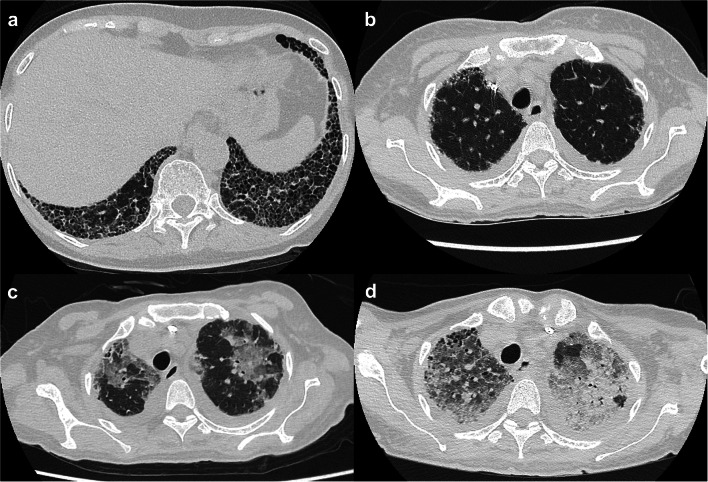
Six days after admission, the patient was unable to eat anything and referred to the nephrology department because of worsening renal function. The following parameters were observed (Table 1): serum creatinine, 10.31 mg/dL; blood urea nitrogen, 106 mg/dL; sodium, 128 mmol/L; potassium, 4.2 mmol/L; chloride, 94 mmol/L; C-reactive protein, 12.76 mg/dL; C3, 65.5 mg/dL (normal range: 73–138 mg/dL); C4, 22.7 mg/dL (normal range: 11–31 mg/dL). Blood plasma prothrombin time/international normalized ratio (PT-INR) was 1.27, fibrinogen was 167 mg/dL, and D-dimer was 11.2 µg/dL. The levels of myeloperoxidase ANCA and anti-GBM antibody were 22.5 U/mL and 858 U/mL, respectively. Urinalysis revealed a red blood cell count of 50–99/HPF; urine protein was 1.9 g/day. Lung computed tomography (CT) revealed a honeycomb lung, but no new lesions were observed (Fig. 1a, b). We diagnosed the patient with RPGN caused by anti-GBM disease and started treatment with methylprednisolone (mPSL) 60 mg/day along with PE. In addition, hemodialysis was initiated, and the patient’s loss of appetite improved following hemodialysis.
Table 1.
Laboratory findings on hospital day 6
| Complete blood count | Serum biochemistry | Serology | ||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 10,970 | /μL | Urea | 106 | mg/dL | IgG | 1258 | mg/dL |
| Hb | 9.5 | g/L | Creatinine | 10.31 | mg/dL | IgA | 169 | mg/dL |
| Plt | 16.5 | × 109/L | eGFR | 3 | IgM | 67 | mg/dL | |
| Venous blood gas analysis | Sodium | 128 | mmol/L | Rheumatoid factor | 5 | IU/ml | ||
| pH | 7.33 | Potassium | 4.2 | mmol/L | Anti-nuclear antibody | < 40 | ||
| pO2 (room air) | 40.9 | mmHg | Chloride | 94 | mmol/L | MPO-ANCA | 22.5 | U/mL |
| pCO2 | 33.6 | mmHg | CRP | 12.76 | mg/dL | PR3-ANCA | < 1.0 | U/mL |
| HCO3- | 17.2 | mmol/L | AST | 16 | U/L | Anti-GBM antibody | 858 > | U/mL |
| Urinalysis | ALT | 11 | U/L | CH50 | 43.3 | U/mL | ||
| RBC sediment | > 100 | HPF | CK | 203 | IU/L | C3 | 65.5 | mg/dL |
| UPCR | 1.9 | g/gCr | LDH | 262 | U/L | C4 | 22.7 | mg/dL |
WBC white blood cell, Hb hemoglobin, Plt platelet, pO2 partial pressure of oxygen, pCO2 partial pressure of carbon dioxide, HCO3−, bicarbonate, RBC sediment, red blood cell sediment, UPCR urinary protein creatinine ratio, eGFR estimated glomerular filtration rate, CRP C-reactive protein, AST aspartate aminotransferase, ALT alanine transaminase, CK creatine kinase, LDH lactate dehydrogenase, Ig immunoglobulin, MPO-ANCA myeloperoxidase-antineutrophil cytoplasmic antibody, PR-3 ANCA proteinase 3 antineutrophil cytoplasmic antibody, Anti-GBM antibody anti-glomerular basement membrane antibody, CH50 total complement activity
Fig. 1.
Chest computed tomography results. a Honeycombing is observed in both lower lobe lungs. b There are no new lesions in both upper lobe lungs. c New ground-glass opacity appears in both upper lobe lungs. d Both lung infiltration show worsening
Fifteen days after admission, the patient became comatose. Brain CT and magnetic resonance imaging revealed no abnormalities. Serum hemoglobin, platelet, and lactate dehydrogenase levels were 5.8 g/dL, 3.4 × 104 /μL, and 527 U/L, respectively (Fig. 2). Blood plasma PT-INR was 1.29, fibrinogen was 196 mg/dL, D-dimer was 4.9 µg/dL, and fibrin degradation products (FDP) was 9.1 µg/dL. Serum haptoglobin was undetectable, and schistocytes were detected on peripheral blood smears, suggesting the presence of TMA. The activity of a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 13 (ADAMTS-13) was obtained before the fourth PE session and retained at 48%. The ADAMTS-13 inhibitor test and serum antibodies against E. coli O157 lipopolysaccharide were negative. The results for anti-cardiolipin antibody, lupus anticoagulant, anti-β2-glycoprotein I antibody, anti-platelet factor 4 heparin antibodies, and direct and indirect Coombs test were all negative. Serum vitamin B12 and folic acid levels were maintained at normal levels. We tested hemolysis in citrated plasma using sheep red blood cells, which is an early diagnostic test for complement-mediated aHUS, and no apparent hemolysis was observed [21]. At this time, the red blood cell concentrate was transfused, and PE and intravenous administration of mPSL were continued. Exacerbation and remission of consciousness were repeated until day 23, after which the patient developed clear awareness.
Fig. 2.
Levels of platelet and hemoglobin during the treatment course. RCC red cell concentrate, Pl, platelet, Hb hemoglobin, VCM vancomycin, ST sulfamethoxazole-trimethoprim, AZM azithromycin, MEPM meropenem, GBM glomerular basement membrane
Twenty-seven days after admission, the patient developed a fever of 37.8 °C, and blood culture was positive for methicillin-resistant Staphylococcus aureus. Catheter-related bloodstream infection was suspected, and subsequently, the hemodialysis catheter was replaced, mPSL dose was reduced to 20 mg, and intravenous vancomycin administration was initiated.
Thirty-eight days after admission, the respiratory status of the patient worsened and SpO2 was 88% (equivalent to room air). Lung CT revealed new diffuse ground-glass opacity (Fig. 1c). We diagnosed the patient with acute pneumonia and started treatment with oral sulfamethoxazole-trimethoprim, azithromycin, and intravenous meropenem. In addition, we increased the dose of intravenous mPSL to 1000 mg and continued it for 3 days, after which, the mPSL dose was reduced to 60 mg.
Forty-one days after admission, the patient’s respiratory status worsened, and she was started on non-invasive positive pressure ventilation. She denied undergoing intubation.
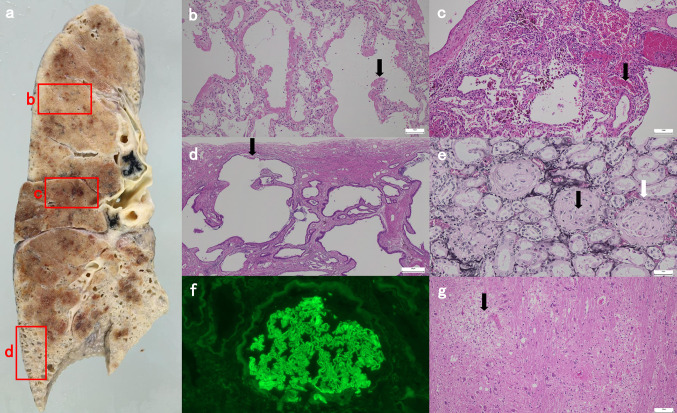
Forty-eight days after admission, her respiratory status worsened further, and the patient needed 100% fraction of inspiratory oxygen. CT revealed worsened diffuse ground-glass opacity (Fig. 1d). On this day, the patient died of respiratory failure. An autopsy was performed 3 h after the time of death. At autopsy, a gross finding of the cut surface of the lung showed various patterns of interstitial pneumonia (IP) (Fig. 3a). The lung specimen demonstrated diffuse alveolar damage with hyaline membrane formation, suggesting acute exacerbation of IP (Fig. 3b). Intra-alveolar hemorrhage with hemosiderin-laden macrophages was seen, indicating repetitive alveolar injury (Fig. 3c). The subpleural region of the lower lung showed a honeycomb pattern, which is one of the characteristic findings of usual IP (Fig. 3d). The kidney specimen revealed most glomeruli to be globally sclerosed and destruction of the Bowman’s layer (Fig. 3e). The degrees of interstitial fibrosis and tubular atrophy were moderate given that they were present in 50% of the sample. There were no lesions suggestive of TMA and glomerular crescent in the kidney specimen. Immunofluorescence analysis revealed immunoglobulin G deposition in a linear pattern along the glomerular capillaries (Fig. 3f). In the brain parenchyma, tiny foci of perivascular inflammatory cell infiltration with vacuolar degeneration were observed. There were microinfarctions in the cerebral white matter and pons. These microscopic lesions were suggestive of TMA (Fig. 3g). There were no lesions suggestive of active infection in the body.
Fig. 3.
Autopsy findings. a Gross autopsy of the cut surface of the lung. Immunohistochemical staining was performed on the tissue sections outlined in the red boxes. b Diffuse alveolar damage with hyaline membrane formation (black arrow) (H & E staining, bar: 100 μm). c Intra-alveolar hemorrhage indicating repetitive alveolar injury (black arrow) (H & E staining, bar: 100 μm) and d subpleural honeycomb change (black arrow) (H & E staining, bar: 500 μm). e Most glomeruli are globally sclerosed (black arrow) and destruction of the Bowman's layer (white arrow) is observed (periodic acid-methenamine silver staining, bar: 50 μm). f Immunofluorescence analysis shows linear immunoglobulin G deposition along the capillary walls. g Tiny foci of perivascular inflammatory cell infiltration with vacuolar degeneration (black arrow) are observed in the brain parenchyma. (H & E staining, bar: 100 μm). H & E, hematoxylin and eosin
Following the patient’s death, a genetic test result was obtained, and a c.1456G > T, p.Asp486Tyr variant was detected in the thrombomodulin (THBD) gene.
Discussion
We encountered a rare case of anti-GBM disease with TMA. Although we treated the patient with hemodialysis, glucocorticoids, and PE, the patient died of respiratory failure. An autopsy revealed that the cause of respiratory failure was an acute exacerbation of IP.
Although glomerular crescent was not observed in the autopsied kidney specimen, anti-GBM disease was diagnosed due to the clinical course of RPGN, elevated levels of serum anti-GBM antibody, destruction of the Bowman’s layer in most glomeruli, and linear staining of immunoglobulin G along the glomerular capillaries as observed in the immunofluorescence analysis.
TMA was diagnosed based on anemia, the elevation of serum LDH level, a notable decrease in serum haptoglobin level, the presence of schistocytes on a peripheral blood smear, thrombocytopenia, and AKI [3].
Several cases of anti-GBM disease with TMA have been reported [6–20]; however, the clinical characteristics of this phenomenon remain unclear. Therefore, we conducted a literature review on this phenomenon (Table 2). One report was excluded because it did not include detailed clinical description of the patient [22]. Although there might have been publication bias, the following clinical characteristics were acknowledged.
Table 2.
Summary of reported cases with anti-glomerular basement membrane disease and thrombotic microangiopathy
| References | Age Sex |
Authors’ institutions |
Coexisting conditions |
TMA on admission |
ADAMTS-13 activity |
CNS manifestations | Treatment | Hematologic outcome |
Renal outcome |
Survival outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Li et al. 2004 [6] | 79 F | China | N/A | No | No data | No data | PE, CS, CY | No data | No data | Died |
| Stallworthy et al. 2006 [7] | 63 M | New Zealand | N/A | No | No data | No | PE | Improve | HD | Survived |
| Terryn et al. 2007 [8] | 37 M | Belgium | N/A | No | No data | Seizure | PE, CS, VCR | Improve | HD → KT | Survived |
| Kobayashi et al. 2009 [9] | 67 F | Japan | MPO-ANCA | No data | 48% | No data | CS, CY | No data | no data | Died |
| Gowrishankar et al. 2009 [10] | 38 M | India | N/A | Yes | No data | Headache | PE, CS | No data | HD | Survived |
| Watanabe et al. 2010 [11] | 61 F | Japan | MPO-ANCA | No | 35% | Brain infarction | PE, CS | No improve | HD | Died |
| Torok et al., 2010 [12] | 43 M | USA | N/A | Yes | 17% | Confusion | PE, CS, CY | Improve | no RRT | Survived |
| Miki et al. 2012 [13] | 68 M | Japan | HIT | No | 70% | No | PE, CS | Improve | HD | Survived |
| Cabrera et al. 2013 [14] | 27 M | Spain | N/A | No | < 1% | Seizure | PE, CS, CY | improve | HD | Survived |
| Momose et al. 2015 [15] | 54 M | Japan | Fibrillary GN | Yes | 32% | PRES, brain hemorrhage | PE, CS | Improve | HD | Survived |
| Manabe et al. 2017 [16] | 59 M | Japan | PR-3ANCA | Yes | 19.80% | Disturbed consciousness | PE, CS | No data | HD | Survived |
| Yu et al. 2017 [17] | 41 F | China | N/A | No | 74% | No | PE, CS | Improve | HD | Survived |
| Alirezaei et al. 2018 [18] | 25 M | Iran | N/A | No | 99.10% | Seizure | PE, CS, CY | Improve | HD | Survived |
| Malviya et al. 2021 [19] | 53 M | India | N/A | No | No data | Seizure | PE, CS, CY | Improve | HD | No data |
| Torigoe et al. 2021 [20] | 48 F | Japan | APS | No | No data | PRES | PE, CS, CY | No data | HD | Survived |
| Our case | 76 F | Japan | MPO-ANCA | No | 48% | Coma | PE, CS | No improve | HD | Died |
ADAMTS-13 a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 13, APS antiphospholipid syndrome, CNS central nervous system, CS corticosteroid, CY cyclophosphamide, F female, Fibrillary GN Fibrillary glomerulonephritis, HD hemodialysis, KT kidney transplant, M male, MPO-ANCA myeloperoxidase-antineutrophil cytoplasmic antibody, N/A not applicable, PE plasma exchange, PR-3 ANCA proteinase 3 antineutrophil cytoplasmic antibody, PRES posterior reversible encephalopathy syndrome, RRT renal replacement therapy, TMA thrombotic microangiopathy, VCR vincristine
First, 75% of the cases were reported from Asia. Anti-GBM disease is reportedly well-recognized in Asian populations; however, the incidence of anti-GBM disease in each region is unknown given that it is a rare disease [1]. Yu et al. hypothesized that there is a possibility of a common genetic susceptibility between anti-GBM disease and TMA [17]. In our case, THBD c.1456G > T, p.Asp486Tyr variant was detected through a genetic test. This variant was previously reported as a disease-related variant for aHUS [23]. However, according to the Tohoku Megabank database, the prevalence of this mutation is relatively high (minor allele frequency; 0.0091) in a Japanese population (https://jmorp.megabank.tohoku.ac.jp/202206/). Given the high prevalence, we were unable to identify the clinical significance of the THBD variant in our case. To date, there is only one previous case of anti-GBM disease with TMA in which a gene mutation of aHUS was detected [17]; thus, the reason for the coexistence of both diseases and 75% of cases being reported from Asia are unknown, and further studies are warranted to understand the relationship between anti-GBM disease with TMA and genetic factors.
Second, TMA tended to appear during the treatment course for anti-GBM disease and usually resolved within 12 weeks. Of a total of 15 cases, TMA did not occur on admission in 11 cases, but appeared a few days to more than a month after the diagnosis of anti-GBM disease. Although the reason for the varied timing was unknown, it was suggested that anti-GBM disease activates the lectin pathway of the complement cascade, and the subsequent over-activated complement system causes secondary TMA [17]. In the literature review, we confirmed 11 hematologic outcomes, and TMA resolved in 9 patients within 12 weeks.
Third, ADAMTS-13 activity was retained above 10% in 90% of the cases. From our literature review, 9 out of the 10 cases indicated that ADAMTS-13 activity was more than 10%. The diagnosis of TTP is reportedly confirmed by a severe deficiency (< 10%) of ADAMTS-13 activity [24, 25]. Thus, anti-GBM disease with TMA may be distinguishable from TTP using ADAMTS-13 activity, but there is one limitation. In our case, we obtained a sample of ADAMTS-13 before the fourth PE session; therefore, there is a possibility of falsely elevated ADAMTS-13 activity [24]. In this regard, of a total of 18 patients with TTP, ADAMTS-13 activity was reported to be less than 10% before the fourth PE session in 14 cases [26]. In most cases reported in the literature, it could not be confirmed when the sample of ADAMTS-13 activity was obtained. As shown in our case, TMA usually appears during the treatment course for anti-GBM disease; thus, there is a possibility that ADAMTS-13 activity was measured after the start of PE in other case reports.
Fourth, central nervous system (CNS) manifestations occurred in more than half of the patients. Approximately 60% of the patients were reported to have had neurologic symptoms at presentation of TTP [25], and half of the aHUS patients had neurologic symptoms [27]. In a previous study, CNS involvement in TTP was defined, ranging from headache to coma, including neurological dysfunction, convulsion, and clouding of consciousness [28]. In this literature review, 11 patients had CNS manifestations. In our case, the exacerbation and remission of consciousness were repeated. Brain autopsy revealed microangiopathy, which was consistent with TMA, and we finally diagnosed that the patient’s CNS manifestation was due to TMA.
Fifth, the renal outcome was very poor. Of a total of 14 cases, 13 patients (92.9%) required renal replacement therapy, and there were no cases in which renal replacement therapy could be discontinued. In our case, the patient required hemodialysis before the onset of TMA. Although there were no renal lesions compatible with TMA in our case, seven case reports indicated renal lesions compatible with TMA. It is possible that information bias may be present; therefore, further detailed analysis of renal lesions is required.
This study’s limitation was that it was difficult to completely rule out the diagnosis of DIC. However, the blood coagulation system on the day of TMA onset did not suggest DIC (FDP and fibrinogen were 9.1 μg/dL and 196 mg/dL, respectively) [29]. Further, the plasma level of D-dimer was elevated only slightly in the disease course. (Fig. 2).
In summary, we described a rare case of anti-GBM disease with TMA. The THBD c.1456G > T, p.Asp486Tyr variant was detected through a genetic test, but we could not identify the clinical significance of this variant in our case. Furthermore, we conducted a literature review and presented the following clinical characteristics of anti-GBM disease with TMA. First, 75% of the cases were reported in Asia. Second, TMA tended to appear during the treatment course for anti-GBM disease and usually resolved within 12 weeks. Third, ADAMTS-13 activity was retained above 10% in 90% of the cases. Fourth, CNS manifestations occurred in more than half of the patients. Fifth, the renal outcome was very poor. Further studies are required to gain a comprehensive understanding of the pathophysiology of this phenomenon.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from the participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol. 2017;12:1162–1172. doi: 10.2215/CJN.01380217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brocklebank V, Wood KM, Kavanagh D. Thrombotic Microangiopathy and the Kidney. Clin J Am Soc Nephrol. 2018;13:300–317. doi: 10.2215/CJN.00620117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato H, Nangaku M, Hataya H, et al. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20:536–543. doi: 10.1007/s10157-016-1276-6. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Nangaku M, Okada H, Kagami S. Controversies of the classification of TMA and the terminology of aHUS. Clin Exp Nephrol. 2018;22:979–980. doi: 10.1007/s10157-017-1524-4. [DOI] [PubMed] [Google Scholar]
- 5.Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 6.Li FK, Tse KC, Lam MF, et al. Incidence and outcome of antiglomerular basement membrane disease in Chinese. Nephrology. 2004;9:100–104. doi: 10.1111/j.1440-1797.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 7.Stallworthy E, Yehia M. Thrombotic microangiopathy in a patient with anti-glomerular basement membrane antibody disease. Nephrology (Carlton) 2006;11:375–376. doi: 10.1111/j.1440-1797.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 8.Terryn W, Benoit D, Van Loo A, et al. Goodpasture’s syndrome associated with autoimmune thrombotic thrombocytopenic purpura an unusual case. Nephrol Dial Transplant. 2007;22:3672–3673. doi: 10.1093/ndt/gfm483. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Inokuma S. Intrapulmonary hemorrhage in collagen-vascular diseases includes a spectrum of underlying conditions. Intern Med. 2009;48:891–897. doi: 10.2169/internalmedicine.48.1760. [DOI] [PubMed] [Google Scholar]
- 10.Gowrishankar S, Patro A, Maitra S. Anti-GBM antibody disease sans crescents with thrombotic microangiopathy. NDT Plus. 2009;2:282–284. doi: 10.1093/ndtplus/sfp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe H, Kitagawa W, Suzuki K, et al. Thrombotic thrombocytopenic purpura in a patient with rapidly progressive glomerulonephritis with both anti-glomerular basement membrane antibodies and myeloperoxidase anti-neutrophil cytoplasmic antibodies. Clin Exp Nephrol. 2010;14:598–601. doi: 10.1007/s10157-010-0312-1. [DOI] [PubMed] [Google Scholar]
- 12.Torok N, Niazi M, Al Ahwel Y, Taleb M, Taji J, Assaly R. Thrombotic thrombocytopenic purpura associated with anti-glomerular basement membrane disease. Nephrol Dial Transplant. 2010;25:3446–3449. doi: 10.1093/ndt/gfq437. [DOI] [PubMed] [Google Scholar]
- 13.Miki T, Akimoto T, Sugase T, et al. Anti-Glomerular basement membrane glomerulonephritis complicated by thrombocytopenia. Intern Med. 2012;51:3395–3399. doi: 10.2169/internalmedicine.51.8507. [DOI] [PubMed] [Google Scholar]
- 14.Vega-Cabrera C, Del Peso G, Bajo A, et al. Goodpasture’s syndrome associated with thrombotic thrombocytopenic purpura secondary to an ADAMTS-13 deficit. Int Urol Nephrol. 2013;45:1785–1789. doi: 10.1007/s11255-012-0279-9. [DOI] [PubMed] [Google Scholar]
- 15.Momose A, Nakajima T, Chiba S, et al. A case of fibrillary glomerulonephritis associated with thrombotic microangiopathy and anti-glomerular basement membrane antibody. Nephron Extra. 2015;5:30–38. doi: 10.1159/000371802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manabe S, Banno M, Nakano M, et al. A case of PR3-ANCA-positive anti-GBM disease associated with intrarenal arteritis and thrombotic microangiopathy. CEN Case Rep. 2017;6:39–45. doi: 10.1007/s13730-016-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu XJ, Han SS, Wang SX, et al. Anti-glomerular basement membrane glomerulonephritis with thrombotic microangiopathy: a case report. Immunol Res. 2017;65:769–773. doi: 10.1007/s12026-017-8918-y. [DOI] [PubMed] [Google Scholar]
- 18.Alirezaei A, Bakhtiyari M, Zare E. Anti-Glomerular basement membrane disease with thrombotic thrombocytopenic purpura. CRCP. 2019;3:106–112. [Google Scholar]
- 19.Malviya PB, Modigonda S, Maitra S, Gowrishankar S. Anti-glomerular basement membrane disease with atypical associations. Saudi J Kidney Dis Transpl. 2021;32:227–231. doi: 10.4103/1319-2442.318529. [DOI] [PubMed] [Google Scholar]
- 20.Torigoe M, Obata Y, Kitamura M, Hara S, Fukuoka J, Nishino T. Anti-glomerular basement membrane disease with antiphospholipid syndrome. Intern Med. 2021;60:2255–2260. doi: 10.2169/internalmedicine.4943-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida Y, Miyata T, Matsumoto M, et al. A novel quantitative hemolytic assay coupled with restriction fragment length polymorphisms analysis enabled early diagnosis of atypical hemolytic uremic syndrome and identified unique predisposing mutations in Japan. PLoS One. 2015;10:e0124655. doi: 10.1371/journal.pone.0124655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stave GM, Croker BP. Thrombotic microangiopathy in anti-glomerular basement membrane glomerulonephritis. Arch Pathol Lab Med. 1984;108:747–751. [PubMed] [Google Scholar]
- 23.Yun JW, Oh J, Lee KO, et al. Distinct genetic profile with recurrent population-specific missense variants in Korean adult atypical hemolytic uremic syndrome. Thromb Res. 2020;194:45–53. doi: 10.1016/j.thromres.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Scully M, Cataland S, Coppo P, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312–322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 25.Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129:2836–2846. doi: 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- 26.Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion. 2015;55:18–24. doi: 10.1111/trf.12762. [DOI] [PubMed] [Google Scholar]
- 27.Jamme M, Raimbourg Q, Chauveau D, et al. Predictive features of chronic kidney disease in atypical haemolytic uremic syndrome. PLoS One. 2017;12:e0177894. doi: 10.1371/journal.pone.0177894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto M, Bennett CL, Isonishi A, et al. Acquired idiopathic ADAMTS13 activity deficient thrombotic thrombocytopenic purpura in a population from Japan. PLoS One. 2012;7:e33029. doi: 10.1371/journal.pone.0033029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asakura H, Takahashi H, Uchiyama T, et al. Proposal for new diagnostic criteria for DIC from the Japanese society on thrombosis and hemostasis. Thromb J. 2016;14:1–13. doi: 10.1186/s12959-016-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]