Abstract
Background:
Evidence regarding the impact of perinatal ureteropelvic junction obstruction (UPJO) and surgical correction during infancy, on somatic growth are scarce. Understanding these impacts could help advise parents and aid in treatment decision making.
Objectives:
To assess the impact of unilateral UPJO and surgical correction on somatic growth in infants diagnosed antenatally and treated during infancy.
Design:
A retrospective bi-institutional analysis of somatic growth in patients under 2 years who underwent dismembered pyeloplasty for the treatment of UPJO was conducted.
Methods:
We evaluated patients who were diagnosed with unilateral hydronephrosis during pre-natal ultrasound screening for detection of fetal anomalies between May 2015 and October 2020. The height and weight of patients who were diagnosed with UPJO were recorded at the age of 1 month, time of surgery, and 6 months after surgery. Standard deviation scores (SDSs) for height and weight were calculated and compared.
Results:
Forty-eight patients under the age of 2 years were included in the analysis. Median age and weight at pyeloplasty were 6.9 months and 7.5 kg. At 1 month, the median SDS for weight in the entire cohort was –0.30 [interquartile range (IQR): –1.0 to 0.63] and the median SDS for height was –0.26 (IQR: –1.08 to 0.52). In 22.9% of patients (11/48), weight and height were below –1 age-appropriate standard deviations, and 6.3% (3/48) were below –2 standard deviations, suggesting growth restriction. When comparing SDS for the entire cohort, there was no significant difference corelated to measurement time or effect of surgery. In the growth restricted cohort, we found a significant improvement in linear growth for height, which was demonstrated between birth and surgery as well as after surgery.
Conclusion:
Infants with unilateral UPJO diagnosed antenatally as a single anomaly may be at an increased risk of somatic growth restriction in comparison with the general population. In children with growth restriction at time of birth, height seems to improve regardless of surgical treatment. Pyeloplasty during infancy does not seem to negatively affect somatic growth. These findings can be used to counsel parents regarding the potential effects of UPJO and pyeloplasty.
Keywords: growth, pediatrics, pyeloplasty, robotic surgery
Introduction
Ureteropelvic junction obstruction (UPJO) is one of the most common pediatric genitourinary anomalies that causes antenatal hydronephrosis (AH). Pediatric urinary obstruction is associated with potential urinary tract infection (UTI), renal scarring, and functional deterioration and has been shown to have implications on renal function during adulthood.1–3 Due to the potential harmful effects of the obstruction, most patients are followed up closely after birth with regard to indications for active treatment. As a substantial proportion of patients will experience improvement or resolution of hydronephrosis, corrective surgery, that is, pyeloplasty, is reserved for patients with worsening of hydronephrosis [Society for Fetal Urology (SFU) classification grade 3 or 4], deterioration of renal function, or complications. 4 The role of pyeloplasty in preventing renal function deterioration has been shown5,6 and is considered the gold standard for treatment. However, the timing of surgery and surgical approach have been a matter of debate. In recent years, several studies have shown renal functional advantages in performing early pyeloplasty in patients under the age of 1 year.7–9 The performance of pyeloplasty in infants has demonstrated safety, feasibility, and excellent long-term outcomes.10–13
As severe presentation of UPJO in the antenatal and infancy period carries reduced future renal function, it could potentially have systemic implications as well. The negative impact of renal disease and congenital urinary tract abnormalities on somatic growth during childhood has been shown in several studies.14–16 Potential pathological mechanisms for growth failure in these patients are recurrent infections, tubulointerstitial dysfunctions, anorexia, and increased catabolism. 17 Most studies examining somatic growth in urinary tract anomalies have focused on vesicoureteral reflux (VUR) and demonstrated the beneficial effect of treatment on physical somatic development.17–19
The aim of this study was to assess the impact of severe unilateral UPJO as a single anomaly on the somatic growth of infants and to examine the effect of minimally invasive pyeloplasty. We hypothesized that infants born with severe unilateral UPJO would display growth restriction that will be partially reversible after surgical treatment.
Materials and methods
A retrospective analysis of somatic growth patterns in patients under the age of 2 years who underwent unilateral robotic or laparoscopic dismembered pyeloplasty at two academic medical centers between May 2015 and October 2020 was performed. All patients were diagnosed during pre-natal ultrasound screening for detection of fetal anomalies and were followed up according to the urinary tract dilation (UTD) group recommendations. 20 UPJO was diagnosed postnatally using a combination of ultrasound and diuretic renography. The study was approved by the Shamir Medical Center ethics committee and performed in accordance with the Declaration of Helsinki (IRB 0317-19-ASF). Surgical indications for intervention were SFU grade 3–4 hydronephrosis, obstructing pattern on diuretic renography with impaired function (<40%), deterioration of kidney function; or febrile UTI with concomitant hydronephrosis.
We excluded patients with bilateral renal anomalies or concomitant anomalies such as skeletal birth defects, genetic syndromes, or systemic chronic disease that can potentially affect somatic parameters. One patient who had a surgical failure and required a re-do pyeloplasty was also excluded from the analysis.
All patients underwent robotic (n = 41) or laparoscopic (n = 7) dismembered pyeloplasty with insertion of a Double J (DJ) stent or a Pippi-sally nephroureterostomy according to surgeon preference. DJ stents or nephroureterostomies were removed 2–4 weeks after surgery.
Post-operative assessment included serial renal ultrasounds and MAG3 diuretic renography. A successful outcome was defined as improved hydronephrosis on renal ultrasound and a non-obstructive pattern on renography during follow-up.
Pre-operative, operative, and post-operative assessment included age at surgery; sex; laterality; pre- and post-operative imaging documenting degree of hydronephrosis; split renal function; T1/2; operative time; complications graded according to Clavian-Dindo classification; use of stents; and length of follow-up.
Patient’s height and weight were recorded at 1 month, day of surgery, and 6 months after surgery in order to assess somatic growth. Standard deviation scores (SDSs) for height and weight were calculated as the number of standard deviations from the mean for appropriate age and sex on the basis of the World Health Organization (WHO) child growth standard. 21 We compared the SDS regarding patient’s height and weight at three different time points as stated above. We performed a sub-group analysis comparing a group of patients who were below one standard deviation for height and weight (SDS ⩽ –1) at the age of 1 month with all other patients.
All statistical analyses were performed on SPSS statistics© v.25 software (IBM, Armonk, NY, USA). Variables are expressed as median with interquartile range (IQR) for continuous variables or n (%) for categorical variables. We used the Student’s t-test for comparison of normally distributed continuous variables and the Mann–Whitney U-test for non-normally distributed variables. The Pearson chi-square or Fisher’s exact test were used for comparison of categorical variables.
The variations of the SDS throughout the study period were analyzed using a one-way repeated-measures analysis of variance (ANOVA) and were represented by means of box plot graphs. Post hoc analysis was performed using a pairwise comparison between measurements with a Bonferroni correction for multiple comparisons. Statistical significance was defined as p < 0.05.
Results
During the study period, 48 patients under the age of 2 years fulfilled the study inclusion criteria and were included in the analysis. All patients underwent unilateral minimally invasive dismembered pyeloplasty for UPJO. Patient demographic, and pre- and post-operative data are presented in Table 1.
Table 1.
Patient demographic, and pre- and post-operative characteristics.
| Variable | Unilateral pyeloplasty (n = 48) | |
|---|---|---|
| Median age at surgery (months, IQR) | 6.9 (4.1–9.7) | |
| Median weight at surgery (kg, IQR) | 7.5 (6.2–8.9) | |
| Sex | ||
| Males/females | 36/12 | |
| Laterality | ||
| Right/left | 22/26 | |
| Median operative time in minutes (IQR) | 143 (120–185) | |
| No. of high-grade complications (%) | 3 (6.3) | |
| SFU degree | Pre-operative | Post-operative |
| 1 | 0% | 35.4% |
| 2 | 0% | 56.3% |
| 3 | 12.5% | 4.2% |
| 4 | 87.5% | 4.2% |
| Median kidney function (IQR) | Pre-operative 44% (41–52%) |
Post-operative 47% (45–53%) |
| Median follow-up in months (IQR) | 6.9 (6.2–8.9) | |
| Need for re-operation (n, %) | 1 (2.0) | |
IQR: interquartile range; SFU: Society for Fetal Urology.
Median age and weight at the time of operation for the whole cohort were 6.9 months and 7.5 kg. At the age of 1 month, the median SDS of weight in the entire cohort was –0.30 (IQR: –1.0 to 0.63) and the median SDS of height was –0.26 (IQR: –1.08 to 0.52). In 22.9% of patients (11/48), weight and height were below –1 age-appropriate standard deviations, and 6.3% (3/48) were below –2 standard deviations, suggesting growth restriction.
During the post-operative period, three patients (6.3%) developed Clavien-Dindo grade 3–5 complications. One patient required admission and treatment in the pediatric intensive care unit due to post-operative septic shock. One patient had omental hernia through the port wound after removal of a drain and needed wound closure. Another patient had a Pippi-sally nephroureterostomy stent that leaked and required an insertion of a DJ stent.
During follow-up after surgery, 44/48 (92%) patients had marked improvement in hydronephrosis. In the remaining four patients, SFU grades 3–4 were observed, and all had a non-obstructed MAG3 renogram. Median follow-up was 6.9 (6.2–8.9) months during which anthropometric measurements were registered.
When comparing a group of patients below –1 SD for height and weight at the age of 1 month (n = 11), that is, growth restricted group, and those with SDS > –1 (n = 37), there were no significant differences regarding pre-operative, operative, or post-operative clinical parameters (Table 2).
Table 2.
Comparison of patients with somatic SDS ⩽ –1 with patients with SDS > –1.
| Variable | Patients with somatic restriction
(SDS ⩽ –1) at 1 month (n = 11) |
Patients with somatic SDS > –1 at
1 month (n = 37) |
p | ||
|---|---|---|---|---|---|
| Median age at surgery (months, IQR) | 6 (5–11) | 7 (4–9) | 0.41 | ||
| Median weight at surgery (kg, IQR) | 6.5 (5.8–8.7) | 7.9 (6.5–9.5) | 0.43 | ||
| Sex | |||||
| Males/females | 8/3 | 28/9 | 0.84 | ||
| Laterality | |||||
| Right/left | 8/3 | 14/23 | 0.04 | ||
| Median operative time in minutes (IQR) | 141 (123.7–168.6) | 154 (120–194) | 0.44 | ||
| No. of high-grade complications (%) | 1 (9.1) | 2 (5.4) | 0.23 | ||
| SFU degree 3–4 (n) | Pre-operative | Post-operative | Pre-operative | Post-operative | |
| 11 | 1 | 37 | 3 | 0.34 | |
| Median kidney function (IQR) | Pre-operative | Post-operative | Pre-operative | Post-operative | 0.39 |
| 41% (38–45%) | 45% (41–49%) | 46% (41–50%) | 48% (45–54%) | ||
| Median follow-up in months (IQR) | 7.2 (6.4–8.5) | 6.8 (6.1–9.6) | 0.52 | ||
IQR: interquartile range; SFU: Society for Fetal Urology.
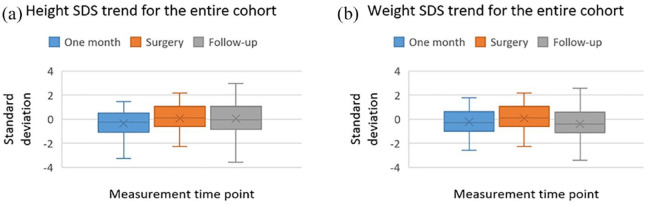
SDS for the entire cohort in the three time periods measured were not significantly difference corelated to measurement time or effect of surgery (Figure 1).
Figure 1.
Box plots for height and weight of the entire cohort.
Patient’s weight and height SDS for the entire cohort, children with SDS > –1, and the restricted growth group are presented in Table 3.
Table 3.
Comparison of SDS during the study period.
| Variable | Age of 1 month | Time of surgery | 6 months follow-up | p |
|---|---|---|---|---|
| Entire cohort (n = 48) | ||||
| Median weight SDS (IQR) | –0.30 (–1.0 to 0.63) | –0.23 (–1.53 to 0.52) | –0.24 (–1.1 to 0.59) | 0.16 |
| Median height SDS (IQR) | –0.26 (–1.08 to 0.52) | 0.13 (–0.61 to 1.07) | –0.03 (–0.82 to 1.02) | 0.21 |
| Normal growth cohort (n = 37) | ||||
| Median weight SDS (IQR) | 0.26 (–0.74 to 0.71) | 0.02 (–0.79 to 0.55) | 0.09 (–0.82 to 0.73) | 0.49 |
| Median height SDS (IQR) | 0.13 (–0.26 to 0.87) | 0.43 (–0.1 to 1.18) | 0.31 (–0.7 to 1.21) | 0.37 |
| Restricted growth cohort (n = 11) | ||||
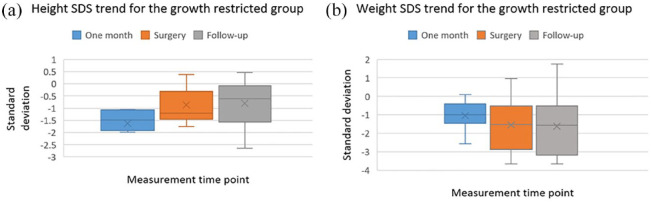
| Median weight SDS (IQR) | –1.05 (–1.53 to –0.54) | –1.54 (–2.88 to –0.61) | –1.56 (–3.11 to –0.63) | 0.45 |
| Median height SDS (IQR) | –1.49 (–1.92 to –1.08) | –1.20 (–1.54 to –0.31) | –0.65 (–1.63 to –0.15) | 0.04 |
When examining the growth restricted cohort (n = 11), we found a significant improvement in linear growth for height, which was demonstrated in the periods between birth and surgery (p = 0.029), as well as during follow-up after surgery (p = 0.04). Regarding weight, we observed a trend toward increased growth restriction (decline in linear growth) in the period between birth and surgery which remained stable after surgery. However, these trends were not statistically significant (Figure 2).
Figure 2.
Box plots for height and weight of the growth restricted group.
Discussion
In this retrospective study of children with severe hydronephrosis who underwent minimally invasive pyeloplasty, we demonstrated an increased tendency for growth restriction in comparison with the general population. Children with significant somatic growth impairment seem to demonstrate catch-up growth after birth reflected in SDS for length. Pyeloplasty during infancy did not seem to negatively affect somatic growth.
Somatic growth in children is a multi-factorial process with a complex interplay between genetic, nutritional, environmental, and hormonal causes. Growth regulation occurs by paracrine and endocrine mechanisms with secretion of growth hormone (GH), insulin-like growth factor-I (IGF-1), as well as thyroid hormone, glucocorticoids, androgens, and sex steroids.22–24
Pathological factors such as genetic disorders, renal disease, pre-natal factors, and malnutrition are known to negatively affect growth. 25 Several studies have examined the relation between renal impairment and growth retardation. In an early seminal study by West and Smith, 16 children with renal impairment, most of whom suffered from bilateral hydronephrosis, displayed reduced caloric intake, chronic acidosis, and growth retardation. Polito et al. 26 reported on growth patterns in 156 children with VUR and normal creatinine clearance. At the time of diagnosis, patients with bilateral VUR and signs of renal scarring had a significant decrease in the relative height and normal weight-for-height in comparison with normal children. In a follow-up study, they reported significant catch-up growth in children with severe VUR who were treated surgically. 27
Only few reports on growth patterns of children with UPJO have been published. Tapia et al. 28 reported on the systemic effects of pyeloplasty in 38 children with unilateral UPJO and found that in their cohort, 72% of children younger than 1 year were below the 50th percentile in height prior to treatment. After surgery, the distribution of heights became normal. They concluded that unilateral hydronephrosis systemically affects body growth and overall renal function, and that such abnormalities may be corrected after surgery. Chandrasekharam et al. 29 evaluated the impact of UTI and pyeloplasty on somatic growth in children diagnosed with symptomatic unilateral UPJO. In their study, out of 61 children who were reviewed, 26.2% displayed growth impairment, defined by the authors as an SDS of under –2 for height measurements. Patients who suffered recurrent UTI were more likely to display impaired somatic growth. The authors demonstrated catch-up growth in the children who were affected during the post-surgical follow-up.
Our study is unique in the sense that it reflects the impact of unilateral UPJO and its treatment in infants diagnosed antenatally. While our study cohort is comprised from children diagnosed with AH and surgically treated before the age of 2 years, both studies mentioned above examined older children (mean age at surgery of 2.5 and 6 years, respectively). Although our cohort demonstrated a higher percentage of patients below –1 SDS (22.9%), and –2 SDS (6.3%) in comparison with the normal population, the studies mentioned above presented a higher rate and severity in growth retardation. A cohort of older children who were diagnosed later might contribute to ongoing renal function deterioration and explain these differences. In that sense, we believe that our study better reflects the somatic impact in the contemporary diagnostic and treatment era.
In our cohort of growth restricted children, we found that in the period between birth and surgery, height improved significantly while there was a non-significant trend of weight restriction after birth that remained stable after surgery. These changes might imply an intra-uterine insult, related to the renal anomaly or other unknown factors which were diminished after labor. However, as such change can be multi-factorial, further research to elicit specific causes is needed. A different explanation to our findings could be that the short time interval for surgical treatment of these children allowed to display a negative change in weight but not in height, and if these children were not operated on, perhaps a decline in height would occur later on.
Interestingly, after surgery, continued growth in terms of height and stable weight was demonstrated. As weight, especially during the first years of life, can rapidly change in response to multiple factors, height is considered a better predictor of long-term somatic growth. As our follow-up period was relatively short (median of 6.9 months after surgery), catch-up growth for all parameters might be demonstrated only after longer follow-up.
In concordance with the existing literature, our study demonstrated a relation between unilateral UPJO as a single anomaly and an increased risk of somatic growth restriction. Such changes seem to be more moderate in the current era in comparison with earlier studies, possibly due to earlier diagnosis and treatment.
The limitations of this study include its retrospective nature, relatively small cohort size, and short follow-up period. As growth during infancy can be affected by multiple parameters such as genetic, socioeconomic, and environmental changes, these factors which were not assessed in this study could potentially skew our results. However, the use of an SDS from growth charts, which compare the study cohort with the normal pediatric population, eliminates this bias. Another limitation is the lack of global renal function assessment as we measured only the affected kidney function by way of renal scintigraphy. Therefore, we were not able to conclude possible associations between global kidney function and somatic growth.
To the best of our knowledge, ours is the first study to examine this relation in a contemporary cohort of children, diagnosed and treated early with minimally invasive surgery during infancy.
Conclusion
Infants with unilateral UPJO diagnosed antenatally as a single anomaly may be at an increased risk of somatic growth restriction in comparison with the general population. In children with growth restriction at time of birth, height seems to improve regardless of surgical treatment. Pyeloplasty during infancy does not seem to negatively affect somatic growth. These findings can be used to counsel parents regarding the potential effects of UPJO and pyeloplasty.
Acknowledgments
None.
Footnotes
ORCID iD: Eyal Kord  https://orcid.org/0000-0001-7361-1636
https://orcid.org/0000-0001-7361-1636
Contributor Information
Eyal Kord, Department of Urology, Shamir Medical Center, Sackler Faculty of Medicine, Tel-Aviv University, P.O. Box 70300, Zerifin, Israel.
Binyamin B Neeman, Departments of Urology & Pediatric Urology, Shaare Zedek Medical Center, Faculty of Medicine, Hebrew University, Jerusalem, Israel.
Dolev Perez, Departments of Urology & Pediatric Urology, Shaare Zedek Medical Center, Faculty of Medicine, Hebrew University, Jerusalem, Israel.
Boris Chertin, Departments of Urology & Pediatric Urology, Shaare Zedek Medical Center, Faculty of Medicine, Hebrew University, Jerusalem, Israel.
Amnon Zisman, Department of Urology, Shamir Medical Center, Sackler Faculty of Medicine, Tel-Aviv University, Zerifin, Israel.
Amos Neheman, Department of Urology, Shamir Medical Center, Sackler Faculty of Medicine, Tel-Aviv University, Zerifin, Israel.
Declarations
Ethics approval and consent to participate: This study was approved by the Shamir Medical Center Ethics Committee and the Shaare Zedek Medical Ethics Committee (IRB 0317-19-ASF) and was performed in accordance with the Declaration of Helsinki. Written informed consent was waived as measurements were recorded as part of routine clinical evaluations.
Consent for publication: Not applicable.
Author contributions: Eyal Kord: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Binyamin B Neeman: Data curation; Investigation; Resources.
Dolev Perez: Data curation; Investigation; Resources.
Boris Chertin: Conceptualization; Supervision; Writing – review & editing.
Amnon Zisman: Investigation; Supervision; Writing – review & editing.
Amos Neheman: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Study data will be available upon request and considerations of patient confidentiality.
References
- 1.Calderon-Margalit R, Golan E, Twig G, et al. History of childhood kidney disease and risk of adult end-stage renal disease. N Eng J Med 2018; 378: 428–438. [DOI] [PubMed] [Google Scholar]
- 2.Peters CA. Obstruction of the fetal urinary tract. J Am Soc Nephrol 1997; 8: 653–663. [DOI] [PubMed] [Google Scholar]
- 3.Wühl E, van Stralen KJ, Verrina E, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol 2013; 8: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passoni NM, Peters CA. Managing ureteropelvic junction obstruction in the young infant. Front Pediatr 2020; 8: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inugala A. Long term outcomes following pyeloplasty for unilateral pelviureteric junction obstruction in paediatric patients. Int Surg J 2021; 8: 3055–3059. [Google Scholar]
- 6.Koyle MA, Ehrlich RM. Management of ureteropelvic junction obstruction in neonate. Urology 1988; 31: 496–498. [DOI] [PubMed] [Google Scholar]
- 7.Babu R, Rathish VR, Sai V. Functional outcomes of early versus delayed pyeloplasty in prenatally diagnosed pelvi-ureteric junction obstruction. J Pediatr Urol 2015; 11: 63.e1–65.e1. [DOI] [PubMed] [Google Scholar]
- 8.Nordenström J, Koutozi G, Holmdahl G, et al. Changes in differential renal function after pyeloplasty in infants and children. J Pediatr Urol 2020; 16: 329.e1–329.e8. [DOI] [PubMed] [Google Scholar]
- 9.Tabari AK, Atqiaee K, Mohajerzadeh L, et al. Early pyeloplasty versus conservative management of severe ureteropelvic junction obstruction in asymptomatic infants. J Pediatr Surg 2020; 55: 1936–1940. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs J, Luithle T, Warmann SW, et al. Laparoscopic surgery on upper urinary tract in children younger than 1 year: technical aspects and functional outcome. J Urol 2009; 182: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 11.Kawal T, Srinivasan AK, Shrivastava D, et al. Pediatric robotic-assisted laparoscopic pyeloplasty: does age matter? J Pediatr Urol 2018; 14: 540.e1–540.e6. [DOI] [PubMed] [Google Scholar]
- 12.Piaggio LA, Franc-Guimond J, Noh PH, et al. Transperitoneal laparoscopic pyeloplasty for primary repair of ureteropelvic junction obstruction in infants and children: comparison with open surgery. J Urol 2007; 178: 1579–1583. [DOI] [PubMed] [Google Scholar]
- 13.Turner RM, 2nd, Fox JA, Tomaszewski JJ, et al. Laparoscopic pyeloplasty for ureteropelvic junction obstruction in infants. J Urol 2013; 189: 1503–1507. [DOI] [PubMed] [Google Scholar]
- 14.Seidel C, Schaefer F, Schärer K. Body growth in urinary tract malformations. Pediatr Nephrol 1993; 7: 151–155. [DOI] [PubMed] [Google Scholar]
- 15.Uttley WS, Paxton J, Thistlethwaite D. Urinary concentrating ability and growth failure in urinary tract disorders. Arch Dis Child 1972; 47: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West CD, Smith WC. An attempt to elucidate the cause of growth retardation in renal disease. AMA J Dis Child 1956; 91: 460–476. [DOI] [PubMed] [Google Scholar]
- 17.Polito C, La Manna A, Mansi L, et al. Body growth in early diagnosed vesicoureteric reflux. Pediatr Nephrol (Berlin, Germany) 1999; 13: 876–879. [DOI] [PubMed] [Google Scholar]
- 18.Merrell RW, Mowad JJ. Increased physical growth after successful antireflux operation. J Urol 1979; 122: 523–527. [DOI] [PubMed] [Google Scholar]
- 19.Sutton R, Atwell JD. Physical growth velocity during conservative treatment and following subsequent surgical treatment for primary vesicoureteric reflux. Br J Urol 1989; 63: 245–250. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen HT, Benson CB, Bromley B, et al. Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). J Pediatr Urol 2014; 10: 982–998. [DOI] [PubMed] [Google Scholar]
- 21.Borghi E, de Onis M, Garza C, et al. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat Med 2006; 25: 247–265. [DOI] [PubMed] [Google Scholar]
- 22.Hunziker EB. Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microscop Res Tech 1994; 28: 505–519. [DOI] [PubMed] [Google Scholar]
- 23.Murray PG, Clayton PE. Endocrine control of growth. Am J Med Genetic Part C, Sem Med Genetic 2013; 163c: 76–85. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson O, Marino R, De Luca F, et al. Endocrine regulation of the growth plate. Horm Res 2005; 64: 157–165. [DOI] [PubMed] [Google Scholar]
- 25.van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev 2003; 24: 782–801. [DOI] [PubMed] [Google Scholar]
- 26.Polito C, La Manna A, Capacchione A, et al. Height and weight in children with vesicoureteric reflux and renal scarring. Pediatr Nephrol 1996; 10: 564–567. [DOI] [PubMed] [Google Scholar]
- 27.Polito C, Marte A, Zamparelli M, et al. Catch-up growth in children with vesico-ureteric reflux. Pediatr Nephrol 1997; 11: 164–168. [DOI] [PubMed] [Google Scholar]
- 28.Tapia J, Gonzalez R. Pyeloplasty improves renal function and somatic growth in children with ureteropelvic junction obstruction. J Urol 1995; 154: 218–222. [PubMed] [Google Scholar]
- 29.Chandrasekharam VV, Srinivas M, Charles AR, et al. Urinary-tract infection affects somatic growth in unilateral symptomatic hydronephrosis. Pediatric Surg Int 2002; 18: 451–454. [DOI] [PubMed] [Google Scholar]