Abstract
Background/objectives
The role of palliative care in the support of patients with neuromuscular disorders (NMDs) is generally recognised in spite of the scarcity of condition-specific evidence in the literature.
Methods
We have focussed specifically on palliative and end-of-life care for patients whose neuromuscular disease has an impact on their respiratory function. Reviewing the literature, we have examined where existing palliative care knowledge can be applied to the specific challenges faced by patients with NMDs, identifying where lessons learnt during the management of one condition may need to be judiciously applied to others.
Results
We highlight lessons for clinical practice centring on six themes: management of complex symptoms; crisis support; relief of caregiver strain; coordination of care; advance care planning; and end of life care.
Conclusions
The principles of palliative care are well suited to addressing the complex needs of patients with NMDs and should be considered early in the course of illness rather than limited to care at the end of life. Embedding relationships with specialist palliative care services as part of the wider neuromuscular multidisciplinary team can facilitate staff education and ensure timely referral when more complex palliative care problems arise.
Keywords: Neuromuscular disorders, palliative, end-of-life, assisted ventilation, motor neuron disease, muscular dystrophy
Introduction
Neuromuscular disorders (NMDs) are a heterogeneous group of conditions resulting in dysfunction of muscles, either directly, or through pathology afflicting peripheral nerves or neuromuscular junctions. 1 Aside from motor neurone disease (MND), the main areas of published NMD research in Europe concern muscular dystrophy and myotonic dystrophy. The extent of morbidity, disability and premature mortality in NMDs is highly variable and condition-specific. However, deleterious effects on the respiratory system are frequently seen. While some conditions such as Amotrophic Lateral Sclerosis progress rapidly over short years and lead directly to respiratory failure, others like Duchenne Muscular Dystrophy progress over three to four decades, with chronic respiratory insufficiency leading to increased morbidity and mortality, and impaired quality of life.2,3
It is well recognised that optimising respiratory function in this population may not only improve quality of life, but can also reduce morbidity and prolong life.4–7 Effective secretion management and timely ventilatory support can have a significant impact on overall wellbeing and survival. It is however important to recognise that for many NMDs progression continues in spite of interventions and it is essential that health care teams identify and address patients’ changing needs and priorities as their condition deteriorates.
Palliative care is defined as the provision of active, holistic care (control of physical and psychological symptoms and social and spiritual problems) for patients with life-limiting illness and their families by generalist and specialist providers.8–11 The primary objective is to improve quality of life through the prevention and relief of suffering by means of early identification and impeccable assessment. Palliative care is often an appropriate care approach at any point following diagnosis of a life-limiting condition. It can be delivered alongside treatment focussed on prolonging life, and should be an approach adopted by the whole multidisciplinary team supporting the patient and their family. 12 Palliative care includes but is not limited to end-of-life care, which generally refers to care provided in the last days to weeks of life. All healthcare providers have a role in ensuring patients receive good end-of-life care. 12
The majority of palliative care is delivered by community and hospital multidisciplinary teams who are not specialists in the field. Generalist palliative care should provide holistic assessment of patients’ needs including basic symptom control and psychological, social, spiritual, and practical support. Healthcare professionals should also coordinate care, signpost to services and conduct open and sensitive communication with the person, their family, and professional staff. Specialist advice should be sought and referral to specialist teams should occur when necessary.9,13
Specialist palliative care offers additional expertise to manage complex palliative care problems that cannot be dealt with by generalist services. They include multidisciplinary specialist palliative care teams who provide assessment, advice, and care in community, hospice and hospital settings. Specialist in-patient facilities (for example hospice beds) provide care for people with complex problems that cannot be managed adequately in other settings. Bereavement support services may also be provided.9,10 Services may be statutory or voluntary. However globally, the funding and provision of specialist palliative care services varies widely. 12
Palliative care is an essential service, now identified as a basic human right. 14 Despite this, it is estimated that only 14% of those who need palliative care, receive it. 10 Experiences during the coronavirus pandemic have highlighted the need to address inequities in experiences of, and access to, palliative care.15,16 People with non-malignant disease, including NMDs, are particularly likely to miss out on vital support. 12
Prognostic uncertainty is often cited as a barrier to palliative care delivery. As such, there is much debate regarding the assessment of prognosis and the optimal timing of palliative care for patients with NMDs.5,17–20 However, recent literature argues that palliative care provision should be provided on the basis of need not prognosis. 21
It is widely acknowledged that people with life-limiting NMDs have palliative care needs.22,23 Janicsh et al. (2020) identify six key aspects of care that require consideration to ensure optimal palliative care delivery for patients with Duchene Muscular Dystrophy;
1. Management of complex symptoms
2. Crisis support
3. Relief of Caregiver Strain
4. Coordination of care
5. Advance care planning
6. End of life care 24
This is echoed in wider literature describing the needs of people with other NMDs. As such, we will present learning points from the current literature using these themes whilst considering implications for the planning and delivery of palliative care.
Methods
A search strategy was developed using three main search concepts:
1. NMDs (including specific conditions: Spinal Muscle Atrophy, MND, Amyotrophic Lateral Sclerosis, Duchenne Muscular dystrophy, Myaesthenia Gravis, Myotonic dystrophy, Pompe disease)
2. Palliative and end of life care
3. Respiratory failure/ventilator support
Primary studies of any design published in English and concerning patients, carers or healthcare staff, were included. Given the lack of primary studies in this field, peer-reviewed papers such as review articles, descriptive, theoretical or clinical opinion articles were also considered.
A systematic search was conducted within the following databases:
1. PUBMED/MEDLINE
2. CINAHL
3. Cochrane Database of Systematic Reviews
Supplementary searching was carried out using the reference lists of included studies, and searching specific themes that emerged from the included studies (e.g. Management of specific symptoms, caregiver burden etc.).
The principles of integrative narrative synthesis 25 were used to develop recommendations for practice based on the six themes identified above. 24
Results
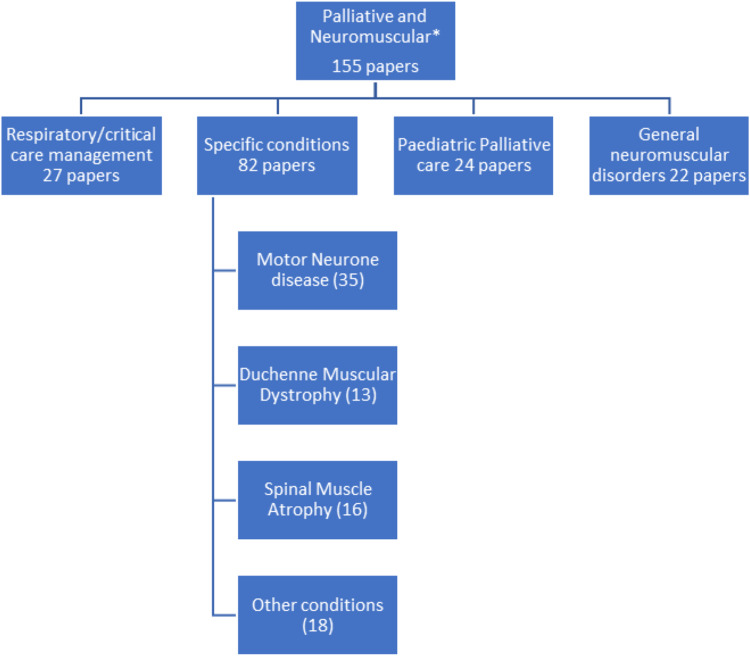
Initial literature searches identified 323 citations. Title and abstract review narrowed this search to 155 of which 41 papers represented original research. The breakdown of these papers to specific topics is illustrated in Figure 1.
Figure 1.
Illustrative breakdown of literature on palliative care in neuromuscular disorders.
Many papers were reviews, guidelines, or opinion pieces. Original research included service model and population studies as well as exploring advance care planning, specific aspects of symptom management and the patient/carer perspective.
The main messages from these papers are summarised below. Where appropriate, additional references detailing important national legislation have been included.
Discussion of palliative care themes
Management of complex symptoms
Multidisciplinary working is central to the management of the complex symptoms experienced by patients with NMDs to improve both quality of life and prognosis.26–30 The mainstay of managing symptoms is proactive identification and assessment of symptoms. This includes an understanding of the impact of the symptoms on the individual and enables a shared symptom management plan to be made which incorporates the patient’s priorities and goals. It is important to be aware of conditions associated with specific NMDs such as diabetes, epilepsy or cardiac problems as these can also have an impact on quality of life.
Respiratory symptoms such as cough, dyspnoea and troublesome secretions are generally expected and as such enquired about, proactively identified and managed. However, other common symptoms such as pain, fatigue, constipation and low mood are less frequently anticipated and therefore often remain un-addressed.31–36
Breathlessness
When managing breathlessness, the focus should be on both the maintenance of physical function and improving the patient’s ability to cope with their symptoms. A holistic approach to breathlessness should include management of any anxiety and other non-pharmacological measures. 37 Physiotherapy input and consideration of ventilatory support are an essential part of the management of breathlessness, however when distressing symptoms persist despite optimisation of respiratory function, low dose morphine has been shown to relieve breathlessness without further compromising respiratory function.38–40
Where assisted ventilation is used, some patients report symptoms including poor sleep, mouth dryness and problems associated with the mask/interface. Any problems should be identified and addressed to reduce the burden of this intervention.41,42
Cough and secretion management
NMDs can reduce ability to effectively clear secretions from the chest through weak respiratory muscles and reduced bulbar function. Physiotherapy input can support airway clearance techniques to the extent that the patient can tolerate. Non-invasive ventilation or mechanical insufflation-exsufflation devices may be appropriate. 7
Although most NMDs do not directly cause excessive production of oral secretions, poor oral and bulbar function, dysphagia and mouth breathing can make secretions difficult to clear. If secretions are sticky, inspissated or hard to clear, optimise cough function, ensure humidified oxygen is being used and consider mucolytic treatments such as carbocysteine. Where secretions are profuse and watery, ensure the patient is positioned to promote swallowing and consider anticholinergics such as hyoscine or glycopyronium. Alternative options include salivary gland botulinum toxin injections administered by specialist services.43,44 Caution should be used when drying secretions to avoid making them harder to clear through coughing. 45
Pain
Patients with NMDs often experience musculoskeletal and neuropathic pain.46,47 Proactive management of pain is essential to avoid development of chronic symptoms.48–51 A multidisciplinary approach can often help to address the causes of pain as well as maintaining function and enhance coping strategies. Where simple analgesia is insufficient, short courses of low dose opioids or neuropathic pain medication (e.g. Gabapentin, Duloxetine) may be needed, however long-term use of these drugs should be avoided if possible.
Towards the end of life, if pain is problematic, opioids may need to be titrated to manage this, however it remains important to address any physical problems that may be exacerbating pain. If the patient is unable to manage enteral preparations, topical patches or the subcutaneous route can be used.
Some NMDs can also cause gastrointestinal discomfort due to reduced motility and constipation. 52 This can also be exacerbated on ventilation through air swallowing causing gastric bloating. Gastrointestinal pain may respond to proactive management of gastro-oesophageal reflux, constipation and/or use of a prokinetic medication (domperidone or metoclopramide). For persistent problems dietetic input may be beneficial, and if the patient receives nutrition via feeding tube, this should be reviewed. An antimuscarinic such as hyoscine butylbromide may be helpful for smooth muscle pain or colic.
Fatigue
Patients with NMDs often experience fatigue, which significantly affects their quality of life. It is often multifactorial and as such management should be patient-centred and multi-modal, addressing aspects including sleep, diet and exercise as well as additional contributing factors such as depression or pain.53,54
Depression and anxiety
An increased prevalence of neuropsychiatric disorders has been associated with several NMDs. Some of these may be related to the underlying pathology of the specific NMD, (e.g. somnolence and apathy in Myotonic Dystrophy) while others may be related to the impact of the condition on function or social interaction. Psychiatric symptoms have a disproportionate impact on quality of life and should be explored and treated proactively.55–58
Crisis support and parallel planning
There is an increasing focus in the literature on disease-modifying treatments that aim to slow or stop the progression of specific neuromuscular conditions. The prognosis of Duchenne Muscular Dystrophy has significantly changed with the use of steroids, and new drugs such as Nusinersen for Spinal Muscle Atrophy promise a new outlook for many patients. The availability, cost and long term impact of many of these treatments is not fully known, and palliative care is likely to continue to have a role in supporting patients even when “curative” treatment is being pursued, where there is significant symptom burden or uncertainty about prognosis.
Some patients with NMDs are at risk of very rapid deterioration and death which may be perceived to be unexpected. This is often precipitated by an acute illness causing respiratory compromise. During such a deterioration, it may be difficult to identify whether the patient is likely to die, become increasingly disabled or respond to treatment and regain their previous level of function. While initial treatment may be appropriately intensive and aimed at full recovery the clinical team need to consider the overall trajectory of the patient’s condition and ensure, where possible, the patient and their family are aware of their situation and have the opportunity to discuss their preferences. The concept of parallel planning should be considered, whilst patients and their families may wish to hope for the best outcome, it may be helpful to plan for the worst. 23
Janisch et al. 24 identify that the prevalence of emergency hospital admissions remains consistent across all age ranges of people with Duchene muscular dystrophy, even as death approaches. They suggest that palliative care could have a role in supporting communication and decision-making during an acute illness, particularly when the outcome is uncertain. When a patient does not want further admission or active intervention, palliative care services may also contribute to care planning and community input to ensure that the patient remains well supported with good symptom management in their own home. 24
In considering palliative care in NMDs, it is also important to note that patients with slowly progressive or stable NMDs may develop another life-threatening condition such as a cancer diagnosis. Some patients who are dying with (as opposed to from) their primary NMD may have additional needs for support as their overall condition deteriorates. They may also need to discuss the impact of the new condition and its treatment with the specialist teams supporting their NMD to ensure decision-making is fully informed.
Relief of caregiver strain
The impact of the carer role in NMDs has been widely acknowledged. 59 As patients with MND deteriorate, there is an increase in carer burden.32,60–62 Kaub-Wittemer et al. (2003) highlight that 30% caregivers of ventilated MND patients rated their quality of life lower than their patient’s. 63 Similarly there is an increasing body of evidence on the physical, emotional and financial impacts on parents caring for a young person with a life-limiting condition, both during their life and in bereavement.64–66
It is essential to consider the psychological and practical needs of the family and carers. For younger patients, support for siblings is also important, acknowledging their often-unrecognised role as carers as well as their need for psychological support. 67
Collaboration between health and social care teams should identify care needs and sources of practical support which may include paid carers. Where available, respite provision may provide periods of practical relief. This model has become well established in children’s hospices, where complex care needs can be safely supported. 68 Unfortunately for young adults in many healthcare settings age-appropriate respite with suitably skilled staff is not available, which further increases the impact on informal and family carers.
Where a condition is inherited, the patient may have other affected family members. They may value additional psychological support. If a family member’s condition causes physical or learning disability, care is needed to ensure that information and support is accessible and appropriate for them.
Coordination of care
In addition to practical needs, both patients and carers comment on the value of good communication with knowledgeable professionals and access to information and coordinated care.69,70 Coordinated care is the responsibility of all teams supporting patients with NMDs, and should include community and social care providers, as well as different medical specialties.
An increasing number of young people with childhood NMDs such as Duchenne Muscular Dystrophy transition to adult services,71,72 often at a point where their condition is progressing. Their need for developmentally appropriate and coordinated support in adult services is crucial.73–75 The approach to communication with young adults should adapt as the young person’s needs and ability to understand and participate in healthcare decisions develops. Planning for, and coordinating timely transition to adult services is important even when the young person’s prognosis is uncertain since they are even more likely to need a well-considered plan if their health is deteriorating when they reach adulthood. McFarlane et al. (2022) highlight the role adult palliative care services can play in support of this patient group as part of the multi-specialty team. 20
Advance care planning
Much has been written about advance care planning both in NMDs and more generally. 76 The process may enable a well-informed patient to make and record shared decisions about their future care. This can be particularly helpful when progression of the NMD may involve cognitive impairment or loss of the ability to communicate. Where a patient has cognitive impairment or a learning disability it remains important to involve them in decisions to the extent that they are able to participate. Advance care planning may enable the patient to participate in decision-making at a time that they are medically stable and have the support and time needed to optimise their participation.
In conditions such as MND, the relatively rapid rate of deterioration (2–4 years from diagnosis to death) 7 and progressive symptom burden leads teams to prepare the patient for further change, whilst responding to new needs and introducing discussions and decisions around supportive interventions. This proactive approach lends itself well to conversations about advance care planning, ensuring patients understand the benefits and burdens of different options and have an opportunity to express their preferences for end of life care.77,78
Ideally, advance care planning around specific interventions should begin prior to the point that the intervention is being considered. It is good practice when introducing the role of a new treatment or intervention to ensure the patient understands that the balance of benefits and burdens, and their experiences and opinions may change with time; and that they can choose to discontinue the treatment at any point. It can also be helpful to plan times to review their wishes around this, normalising these conversations as routine practice.
While this responsive and prepared approach remains relevant, the rate of change in function may be much slower in more slowly progressive conditions and may be interspersed by long periods of stability even while living with significant disability and requiring ventilator support. Although patients may prefer not to focus on their condition, it is important that clinicians are prepared to respond appropriately to changes in their health and patients are aware of what those changes might be. Increasingly condition-specific tools are being used to identify when palliative care should be considered. 17 Willis et al. (2022) describe how this can empower clinical teams working with patients with NMDs, using a traffic light system to identify patients who would benefit from palliative care input.23,79
Hiscock et al. (2019) emphasise both the importance of, and the difficulty in discussing the possibility of sudden death with young adults with Duchene muscular dystrophy in order to identify their wishes and preferences for resuscitation. Although clinicians may find this challenging, it remains important and it is essential that conversations are approached sensitively, and that discussions are communicated with the wider team to avoid unnecessary repetition. 80
End of life care
In UK, The Leadership Alliance for care of dying people 81 and the National Institute for Health and Care Excellence 82 have described essential elements of care in the last days of life as follows:
1. The possibility [of dying] is recognised.
2. Sensitive communication takes place between staff and the dying person, and those identified as important to them.
3. The dying person, and those identified as important to them, are involved in decisions about treatment and care to the extent that the dying person wants.
4. The needs of families and others identified as important to the dying person are actively explored, respected and met as far as possible.
5. An individual plan of care, which includes food and drink, symptom control and psychological, social and spiritual support, is agreed, coordinated and delivered with compassion.81,82
It is essential that teams supporting patients with NMDs, feel confident to recognise when their patients may be reaching the end of life, and are prepared to communicate with and care for the patient and their family. Person-centred care is crucial to good end of life care. Ongoing involvement of specialist neuromuscular teams should occur, ensuring the provision of condition-specific expertise and continuity of care.
In developing an individual plan of care with patients with NMDs specific consideration of the following may be needed:
Feeding and fluids. Many NMDs affect bulbar function, necessitating assisted nutrition and hydration via gastrostomy or nasogastric tube. Discussion with the patient should take place around their wishes and the benefits and burdens of continuing enteral food and fluids. If assisted feeding is not burdensome for the patient it may be appropriate to continue, however emphasis should be on patient’s overall wellbeing rather than focussed on weight maintenance.
As the patient reaches their last days of life, the benefit they gain from food or fluids is likely to reduce, and they may develop problems such as reflux, diarrhoea, oedema or respiratory secretions. In these circumstances it may be appropriate to stop food and fluids. This decision should be reviewed 12 hourly. 82
Management of symptoms. Despite an expanding evidence-base on symptom management, patients with NMDs are likely to receive poorer symptom control at the end of life than those dying with cancer.83,84 A recent qualitative study of bereaved carers demonstrated that unmanaged respiratory and psychological symptoms were frequently a significant cause of distress to both patients and their carers. 32 As discussed above, symptoms should be proactively addressed and reviewed regularly. If the enteral route is no longer appropriate, subcutaneous infusions may be needed.
Supporting communication. As the patient’s condition deteriorates their previous methods of communication may be less accessible. A personalised plan to facilitate their communication should be made, ideally with support from a Speech and Language therapist.
Assisted ventilation. For patients who use assisted ventilation end of life care must include consideration of ventilator management.
Any ventilator-associated symptoms that develop such as facial skin damage or inspissated secretions should be proactively managed.31,41,85,86 Changes to the interface, humidification and other patient support resources may help.
The patient and those close to them should be given an opportunity to discuss their wishes, concerns and expectations around ventilator use at the end of life, ideally well in advance. Fears and misunderstandings about dying on and off ventilators are widespread, and it is essential that any worries or misconceptions are addressed and that patients are aware that they have a choice regarding whether to continue ventilation or not.
Consideration should be given specifically to the person’s level of dependence on the ventilator and any symptoms they have experienced when stopping ventilation for short or longer periods. This is important information to ensure symptoms remain well managed whether the ventilator is continued or withdrawn.13,87
For patients who chose to continue with assisted ventilation at the end of life, the team should pay continued attention to their comfort. Changes to mask interface or pressures may be needed and ongoing support from ventilation specialist teams is recommended. At the point where the patient no longer appears to be deriving any symptomatic benefit from their ventilator, the interface may be removed providing this does not cause distress.
Withdrawal of ventilatory support
Supporting a request for withdrawal of ventilation requires sensitive communication from the point of initial request through to treatment withdrawal. Ensuring the patient is able to make an informed decision and to communicate their wishes is essential. Where a patient does not have mental capacity to participate in a decision, or where the patient is a child, decisions for withdrawal of ventilation must be based on the best interests of the patient, in consultation with clinical teams and family members. Clear communication and planning with the people close to the patient and the clinical teams and care-givers is also important. Such requests can also be ethically and morally challenging both to clinicians and the wider team. Whilst the UK legal position is clear that a patient with capacity has the right to decline or withdraw from a medical intervention, clinicians may have mixed feelings in practice.88–91 Patients will often die a short time after stopping ventilation, which can lead to a feeling of “causality” and the use of sedating and opioid medications to manage distress can cause anxiety about hastening death.
Particularly in those fully dependent on respiratory support, ventilation withdrawal may lead to rapid onset severe breathlessness. 85 Medication to manage this includes opioids and Midazolam and can be given via the subcutaneous or intravenous route and should be titrated according to response. Experience is growing in paediatric and adult services around withdrawal of ventilator support in different settings including hospital, hospice and home.87,92–95
The Association of Palliative Medicine have produced clear guidelines on ventilator withdrawal for patients with MND, to achieve symptom control in the period between NIV withdrawal and death.13,96 In Paediatric palliative care, guidance has also been developed. 97 Both of these guidelines emphasise the importance of careful planning and communication, promotion of patient and parent choice, and ongoing psychosocial and bereavement support for family.
Conclusion
In the face of progressive conditions associated with a high symptom burden, the principles of palliative care are well suited to addressing the complex needs of patients with NMDs. While historically, adult palliative care has often been limited to the last months of a patient’s life, our findings demonstrate the benefits of this approach much earlier in the course of illness for NMDs.
It is essential that all clinicians recognise their role in providing palliative care for patients with NMDs and develop their skills and confidence in this area. Embedding specialist palliative care services as part of the wider neuromuscular multidisciplinary team can facilitate staff education as well as ensuring timely referral when more complex palliative care problems arise.
While our review highlights the breadth of the subject of palliative care in relation to NMDs, we also identified issues that lack in-depth research. As new life-prolonging technological and medical interventions are developed, it remains essential that research continues to further improve palliative care for patients with NMDs and their families.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Joanna Elverson https://orcid.org/0000-0002-0712-9208
References
- 1.Morrison B. Neuromuscular diseases. Semin Neurol 2016; 36(5): 409–418. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosino N, Carpenè N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J 2009; 34(2): 444–451. [DOI] [PubMed] [Google Scholar]
- 3.Landfeldt E, Thompson R, Sejersen T, et al. Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta-analysis. Eur J Epidemiol 2020; 35(7): 643–653. DOI: 10.1007/s10654-020-00613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boentert M, Wenninger S, Sansone V. Respiratory involvement in neuromuscular disorders. Current Opinion in Neurolog 2017; 30(5): 529–537. [DOI] [PubMed] [Google Scholar]
- 5.Shah N, Murphy P, Kaltsakas G. The adult multidisciplinary respiratory neuromuscular clinic. Breathe 2020; 16(3): 200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eagle M, Bourke J, Bullock R, et al. Managing duchenne muscular dystrophy–the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord 2007; 17(6): 470–475. [DOI] [PubMed] [Google Scholar]
- 7.Bourke S, Tomlinson M, Williams T, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5(2): 140–147. [DOI] [PubMed] [Google Scholar]
- 8.Association of Palliative Medicine, UK and Ireland [Internet] . Available from:The Association for Palliative Medicine - Vision and Values - APM Online.
- 9.National Institute for Health and Care Excellence (NICE) . Improving supportive and palliative care for adults with cancer. 2004. Available from:https://www.nice.org.uk/guidance/csg4/%0Aresources/improving-supportive-and-palliative-care-for-adults%02with-cancer-pdf-773375005
- 10.National Institute for Health and Care Excellence (NICE) . End of life care for adults: service delivery (C) evidence review: barriers to accessing end of life care services NICE guideline NG142. 2019. [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE) . End of life care for infants, children and young people with life-limiting conditions: planning and management. NICE guideline; NG61.: 2019. [PubMed] [Google Scholar]
- 12.The World Health Organization . Palliative care [internet]. 2020. Available from:https://www.who.int/news-room/fact-sheets/detail/palliative-care
- 13.Association for Palliative Medicine of Great Britain and Ireland . Withdrawal of assisted ventilation at the request of a patient with motor neurone disease guidance for professionals association for palliative medicine of great Britain and Ireland. 2015: 1–67. Available from:https://apmonline.org/wp-content/uploads/2016/03/Guidance-with-logos-updated-210316.pdf
- 14.NHS Confederation. NHS Providers . Health and care bill: joint briefing. The Kings Fund 2022; 35: 1–5. Available from:https://www.nhsconfed.org/sites/default/files/2022-01/Health-and-care-bill-joint–POD-local-reconfigs_0.pdf?utm_source=The_King%27s_Fund_newsletters_%28main_account%29&utm_medium=email&utm_campaign=12952212_NEWSL_HMP_2022-01-28&dm_i=21A8,7PLZO,5X00CF,VFIC [Google Scholar]
- 15.Oluyase AO, Hocaoglu MB, Cripps RL, et al. The challenges of caring for people dying from COVID-19 a multinationalobservational study of palliative and hospice services (CovPall). medRxiv 2020; 44(0): 1–23. Available from:https://medrxiv.org/cgi/content/short/2020.10.30.20221465 [Google Scholar]
- 16.Sleeman K, Cripps R, Murtagh FEM, et al. Change in activity of palliative care services during the Covid-19 pandemic: a multinational survey (CovPall). J Palliat Med 2022; 25: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang R, Wong Y. Prognostic indicators of neuromuscular disorders for palliative care referral. Ann Palliat Med 2018; 7(3): 335–338. [DOI] [PubMed] [Google Scholar]
- 18.Tripodoro V, De Vito E. What does end stage in neuromuscular diseases mean? key approach-based transitions. Curr Opin Support Palliat Care 2015; 9: 361–368. [DOI] [PubMed] [Google Scholar]
- 19.Ackrivo J, Hansen-flaschen J, Wileyto EP, et al. Development of a prognostic model of respiratory insufficiency or death in amyotrophic lateral sclerosis. Eur Respir J 2019; 53(4): 1802237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macfarlane M, Willis T, Easthope-Mowatt Y, et al. Adult neuromuscular disorders: a joint palliative/neuromuscular clinic. BMJ Support Palliat Care 2022; 12: e279–e280. [DOI] [PubMed] [Google Scholar]
- 21.Petrova M, Wong G, Kuhn I, et al. Timely community palliative and end-of-life care: a realist synthesis. BMJ Support Palliat Care 2021: 2021: 003066. [DOI] [PubMed] [Google Scholar]
- 22.de Visser M, Oliver D. Palliative care in neuromuscular diseases. Curr Opin Neurol 2017; 30(6): 686–691. [DOI] [PubMed] [Google Scholar]
- 23.Willis D, Willis T, de Visser M, et al. Neuromuscular Disorders and Palliative Care in Adults. Emergencies in Neuromuscular Disorders. Berlin, Germany: Springer, 2022. DOI: 10.1007/978-3-030-91932-0_16. [DOI] [Google Scholar]
- 24.Janisch M, Boehme K, Thiele S, et al. Tasks and interfaces in primary and specialized palliative care for Duchenne muscular dystrophy - a patients’ perspective. Neuromuscul Disord 2020; 30(12): 975–985. [DOI] [PubMed] [Google Scholar]
- 25.Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme. Lancaster, UK: Lancaster University, 2006, p. 2006. [Google Scholar]
- 26.Ng L, Khan F, Mathers S. Multidisciplinary care for adults with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2009; 4: CD007425. [DOI] [PubMed] [Google Scholar]
- 27.Carter G, Joyce N, Abresch A, et al. Using palliative care in progressive neuromuscular disease to maximize quality of life. Phys Med Rehabil Clin N Am 2012; 23(4): 903–909. [DOI] [PubMed] [Google Scholar]
- 28.Traynor BJ, Alexander M, Corr B, et al. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996–2000. J Neurol Neurosurg Psychiatry 2003; 74(9): 1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta A, Jackson N, Wiedau-Pazos M. Palliative care consults in an inpatient setting for patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care 2021; 38(9): 1091–1098. [DOI] [PubMed] [Google Scholar]
- 30.Lau FS, Brennan FP, Gardiner MD. Multidisciplinary management of motor neurone disease. Aust J Gen Pract 2018; 47(9): 593–597. [DOI] [PubMed] [Google Scholar]
- 31.Hobson E, McDermott C. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat Rev Neurol 2016; 12(9): 526–538. [DOI] [PubMed] [Google Scholar]
- 32.McVeigh C, Donaghy C, McLaughlin B, et al. Palliative care for patients with motor neurone disease and their bereaved carers: a qualitative study. BMC Palliat Care 2019; 18(1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarmet M, Kabani A, Maragakis NJ, et al. Appetite and quality of life in amyotrophic lateral sclerosis: a scoping review. Muscle Nerve 2022; 66(6): 653–660. [DOI] [PubMed] [Google Scholar]
- 34.Houwen-van Opstal SLS, Heutinck L, Jansen M, et al. Occurrence of symptoms in different stages of duchenne muscular dystrophy and their impact on social participation. Muscle Nerve 2021; 64(6): 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoser B, Bilder DA, Dimmock D, et al. The humanistic burden of pompe disease: are there still unmet needs? a systematic review. BMC Neurol 2017; 17(1): 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelinas D, Parvin-Nejad S, Phillips G, et al. The humanistic burden of myasthenia gravis: a systematic literature review. J Neurol Sci 2022; 437: 120268. [DOI] [PubMed] [Google Scholar]
- 37.Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opin Support Palliat Care 2011; 5(2): 77–86. [DOI] [PubMed] [Google Scholar]
- 38.Allcroft P. Breathlessness in motor neurone disease: a review of the current strategies and gaps in the evidence. Curr Opin Support Palliat Care 2014; 8(3): 213–217. [DOI] [PubMed] [Google Scholar]
- 39.Barnes H, Mcdonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 2016; 3(3): CD011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon ST, Higginson IJ, Booth S, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev 2016; 10(10): CD007354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewhurst F, Elverson J, Mccleery A, et al. Ventilator dependence complications in motor neuron disease. BMJ Support Palliat Care 2020; 2020: 002560. [DOI] [PubMed] [Google Scholar]
- 42.Baxter S, Baird W, Thompson S, et al. The initiation of non-invasive ventilation for patients with motor neuron disease: patient and carer perceptions of obstacles and outcomes. Amyotroph Lateral Scler Front Degener 2013; 14(2): 105–110. [DOI] [PubMed] [Google Scholar]
- 43.Yu YC, Chung CC, Tu YK, et al. Efficacy and safety of botulinum toxin for treating sialorrhea: a systematic review and meta-analysis. Eur J Neurol 2022; 29(1): 69–80. [DOI] [PubMed] [Google Scholar]
- 44.Harbottle J, Carlin H, Payne-Doris T, et al. Developing an intrasalivary gland botox service for patients receiving long-term non-invasive ventilation at home: a single-centre experience. BMJ Open Respir Res 2022; 9(1): e001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGeachan AJ, Hobson E V., Al-Chalabi A, et al. A multicentre evaluation of oropharyngeal secretion management practices in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener 2017; 18(1–2): 1–9. [DOI] [PubMed] [Google Scholar]
- 46.Jacques MF, Stockley RC, Bostock EI, et al. Frequency of reported pain in adult males with muscular dystrophy. PLoS One 2019; 14(2): e0212437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morís G, Wood L, FernáNdez-Torrón R, et al. Chronic pain has a strong impact on quality of life in facioscapulohumeral muscular dystrophy. Muscle Nerve 2018; 57(3): 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva T, Massetti T, Monteiro C, et al. Pain characterization in duchenne muscular dystrophy. Arq Neuropsiquiatr 2016; 74(9): 767–774. [DOI] [PubMed] [Google Scholar]
- 49.Abe Y, Miyashita M, Ito N, et al. Attitude of outpatients with neuromuscular diseases in Japan to pain and use of analgesics. J Neurol Sci 2008; 267(1–2): 22–27. [DOI] [PubMed] [Google Scholar]
- 50.Brettschneider J, Kurent J, Ludolph A, et al. Drug therapy for pain in amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2013; 2013: CD005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Vliet J, Tieleman A, Verrips A, et al. Qualitative and quantitative aspects of pain in patients with myotonic dystrophy type 2. J Pain 2018; 19(8): 920–930. [DOI] [PubMed] [Google Scholar]
- 52.Lo Cascio CM, Goetze O, Latshang TD, et al. Gastrointestinal dysfunction in patients with duchenne muscular dystrophy. PLoS One 2016; 11(10): e0163779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McElhiney M, Rabkin J, Gordon P, et al. Prevalence of fatigue and depression in ALS patients and changeover time. J Neurol Neurosurg Psychiatry 2009; 80(10): 1146–1149. [DOI] [PubMed] [Google Scholar]
- 54.Lou JS, Weiss MD, Carter GT. Assessment and management of fatigue in neuromuscular disease. Am J Hosp Palliat Med 2010; 27(2): 145–157. [DOI] [PubMed] [Google Scholar]
- 55.Pascual-Morena C, Cavero-Redondo I, Reina-Gutiérrez S, et al. Prevalence of neuropsychiatric disorders in duchenne and becker muscular dystrophies: a systematic review and meta-analysis. Arch Phys Med Rehabil 2022; 103: 2444–2453. [DOI] [PubMed] [Google Scholar]
- 56.Miller JN, Kruger A, Moser DJ, et al. Cognitive deficits, apathy, and hypersomnolence represent the core brain symptoms of adult-onset myotonic dystrophy type 1. Front Neurol 2021; 12: 700796–700810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Dowd D, Bostock E, Smith D, et al. Psychological parameters impact health-related quality of life in mental and physical domains in adults with muscular dystrophy. Neuromuscul Disord 2021; 31(4): 328–335. [DOI] [PubMed] [Google Scholar]
- 58.Lapin B, Mate K, Li Y, et al. Subjective health perception prioritizes psychological well-being over physical function in advanced ALS: a multigroup structural equation modeling analysis. J Neurol Sci 2022; 442: 120442. [DOI] [PubMed] [Google Scholar]
- 59.Landfeldt E, Edström J, Buccella F, et al. Duchenne muscular dystrophy and caregiver burden: a systematic review. Dev Med Child Neurol 2018; 60(10): 987–996. [DOI] [PubMed] [Google Scholar]
- 60.Baxter SK, Baird WO, Thompson S, et al. The impact on the family carer of motor neurone disease and intervention with noninvasive ventilation. J Palliat Med 2013; 16(12): 1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hecht M, Graesel E, Tigges S, et al. Burden of care in amyotrophic lateral sclerosis. Palliat Med 2003; 17(4): 327–333. [DOI] [PubMed] [Google Scholar]
- 62.Dawson S, Kristjanson LJ. Mapping the journey: family carers’ perceptions of issues related to end-stage care of individuals with muscular dystrophy or motor neurone disease. J Palliat Care 2003; 19(1): 36–42. [PubMed] [Google Scholar]
- 63.Kaub-Wittemer D, Steinbüchel Nv, Wasner M, et al. Quality of life and psychosocial issues in ventilated patients with amyotrophic lateral sclerosis and their caregivers. J Pain Symptom Manage 2003; 26(4): 890–896. [DOI] [PubMed] [Google Scholar]
- 64.Verberne LM, Kars MC, Meeteren AYNS, et al. Parental experiences and coping strategies when caring for a child receiving paediatric palliative care a qualitative study. Eur J Pediatr 2019; 178(7): 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher V, Atkin K, Fraser LK. The health of mothers of children with a life-limiting condition: a qualitative interview study. Palliat Med 2022; 36(9): 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koch KD, Jones BL. Supporting parent caregivers of children with life-limiting illness. Children 2018; 5(7): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lövgren M, Sejersen T, Kreicbergs U. Parents’ experiences and wishes at end of life in children with spinal muscular atrophy types I and II. J Pediatr 2016; 175: 201–205. [DOI] [PubMed] [Google Scholar]
- 68.Fraser LK, Aldridge J, Manning S, et al. Hospice provision and usage amongst young people with neuromuscular disease in the United Kingdom. Eur J Paediatr Neurol 2011; 15(4): 326–330. [DOI] [PubMed] [Google Scholar]
- 69.Hjorth E, Kreicbergs U, Sejersen T, et al. Parents’ advice to other parents of children with spinal muscular atrophy: two nationwide follow-ups. J Child Heal Care 2022; 26(3): 407–421. [DOI] [PubMed] [Google Scholar]
- 70.Hjorth E, Kreicbergs U, Sejersen T, et al. Bereaved parents more satisfied with the care given to their child with severe spinal muscular atrophy than nonbereaved. J Child Neurol 2019; 34(2): 104–112. [DOI] [PubMed] [Google Scholar]
- 71.Fraser LK, Childs AM, Miller M, et al. A cohort study of children and young people with progressive neuromuscular disorders: clinical and demographic profiles and changing patterns of referral for palliative care. Palliat Med 2012; 26(7): 924–929. [DOI] [PubMed] [Google Scholar]
- 72.Cook KA, Bergeron K. Palliative care for young adults with life-limiting conditions: public health recommendations. BMJ Support Palliat Care 2022; 12(e2): E256–E263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quinlivan R, Messer B, Murphy P, et al. Adult North Star Network (ANSN): consensus guideline for the standard of care of adults with duchenne muscular dystrophy. J Neuromuscul Dis 2021; 8(6): 899–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willis LD. Transition from pediatric to adult care for young adults with chronic respiratory disease. Respir Care 2020; 65(12): 1916–1922. [DOI] [PubMed] [Google Scholar]
- 75.Rapley T, Farre A, Parr J, et al. Transition Collaborative Group. The transition collaborative GroupCan we normalise developmentally appropriate health care for young people in UK hospital settings? an ethnographic study. BMJ Open 2019; 9: e029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Health Service . The Universal Principles for Advance Care Planning has been jointly published by a coalition of the partners in response to the CQC, report ‘protect, connect R– decisions about living and dying well’. Universal Principles for Advance Care Planning (ACP) Contents: 2022. Leeds, UK: National Health Service, 2021: 1–22. [Google Scholar]
- 77.Murray L, Butow PN. Advance care planning in motor neuron disease: a systematic review. Palliat Support Care 2016; 14(4): 411–432. [DOI] [PubMed] [Google Scholar]
- 78.Murray L, Butow PN, White K, et al. Advance care planning in motor neuron disease: a qualitative study of caregiver perspectives. Palliat Med 2016; 30(5): 471–478. [DOI] [PubMed] [Google Scholar]
- 79.Willis TA, MacFarlane M, Vithlani R, et al. Neuromuscular diseases and advance care plans: traffic light system. BMJ Support Palliat Care. 2021 Mar;11(1):116. DOI: 10.1136/bmjspcare-2020-002336. Epub 2020 Jun 8. PMID: 32513680. [DOI] [PubMed] [Google Scholar]
- 80.Hiscock A, Barclay S. “It’s a hard conversation to have”. Healthcare professionals’ views concerning advance care discussions with young people affected by life-limiting neuromuscular diseases: an interview study. BMJ Support Palliat Care 2019; 9(1): e9. [DOI] [PubMed] [Google Scholar]
- 81.Leadership Alliance of the Care of Dying People. One chance to get it right: improving people’s experience of care in the last few days and hours of life. Publications Gateway Reference 01509. 2014, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/323188/One_chance_to_get_it_right.pdf [Google Scholar]
- 82.NICE . End of life care for adults. November 2011 (2017 update). Available from:http://publications.nice.org.uk/quality-standard-for-end-of-life-care-for-adults-qs13
- 83.Eljas Ahlberg E, Axelsson B. End-of-life care in amyotrophic lateral sclerosis: a comparative registry study. Acta Neurol Scand 2021; 143(5): 481–488. [DOI] [PubMed] [Google Scholar]
- 84.Ng L, Khan F, Young CA. Symptomatic treatments for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2017; 2017(1): CD011776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faull C, Wenzel D. Mechanical ventilation withdrawal in motor neuron disease: an evaluation of practice. BMJ Support Palliat Care 2020; 12: e752–e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wenzel D, Bleazard L, Pepper C, et al. Non-invasive advanced respiratory support in end-of-life care and symptom management: systematic review. BMJ Support Palliat Care 2022; 2022: 003905. [DOI] [PubMed] [Google Scholar]
- 87.Messer B, Armstrong A, Doris T, et al. Requested withdrawal of mechanical ventilation in six patients with motor neuron disease. BMJ Support Palliat Care 2020; 10(1): 10–13. [DOI] [PubMed] [Google Scholar]
- 88.Cullum K, Madani C, Cutler E, et al. The lived experience of respiratory therapists during withdrawal of advanced life-sustaining therapies at end of life in the ICU. Respir Care 2022; 67(12): 1568–1577. [DOI] [PubMed] [Google Scholar]
- 89.Kaur R, Chen E, Faizi A, et al. Emotional impact of compassionate extubation on respiratory therapists and nurses: a pilot study. Can J Respir Ther 2022; 58(58): 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orr S, Efstathiou N, Baernholdt M, et al. ICU clinicians’ experiences of terminal weaning and extubation. J Pain Symptom Manag 2022; 63(5): e521–e528. [DOI] [PubMed] [Google Scholar]
- 91.Phelps K, Regen E, Oliver D, et al. Withdrawal of ventilation at the patient’s request in MND: a retrospective exploration of the ethical and legal issues that have arisen for doctors in the UK. BMJ Support Palliat Care 2017; 7(2): 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaur R, Harmon E, Joseph A, et al. Palliative ventilator withdrawal practices in an inpatient hospice unit. Am J Hosp Palliat Med 2022; 0(0): 104990912211298. [DOI] [PubMed] [Google Scholar]
- 93.Woodruff A, Bingham S, Jarrah R, et al. A framework for pediatric intensivists providing compassionate extubation at home. Pediatr Crit Care Med 2021; 22(5): 454–461. [DOI] [PubMed] [Google Scholar]
- 94.Postier A, Catrine K, Remke S. Interdisciplinary pediatric palliative care team involvement in compassionate extubation at home: from shared decision-making to bereavement. Children 2018; 5(3): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laddie J, Craig F, Brierley J, et al. Withdrawal of ventilatory support outside the intensive care unit: guidance for practice. Arch Dis Child 2014; 99(9): 812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faull C, Oliver D. Withdrawal of ventilation at the request of a patient with motor neurone disease: guidance for professionals. BMJ Supportive and Palliative Care. 2016;6:144–6. DOI: 10.1136/bmjspcare-2016-001139. [DOI] [PubMed] [Google Scholar]
- 97.McNamara K. A care pathway to support extubation within a children’s palliative care framework. BMJ Support Palliat Care 2011; 1(2): 206–206. [Google Scholar]