Abstract
Introduction
Women with hypertensive disorders of pregnancy (HDP) have an increased risk of cardiovascular disease. Whether HDP is also associated with later‐life dementia has not been fully explored.
Methods
Using the Utah Population Database, we performed an 80‐year retrospective cohort study of 59,668 parous women.
Results
Women with, versus without, HDP, had a 1.37 higher risk of all‐cause dementia (95% confidence interval [CI]: 1.26, 1.50) after adjustment for maternal age at index birth, birth year, and parity. HDP was associated with a 1.64 higher risk of vascular dementia (95% CI: 1.19, 2.26) and 1.49 higher risk of other dementia (95% CI: 1.34, 1.65) but not Alzheimer's disease dementia (adjusted hazard ratio = 1.04; 95% CI: 0.87, 1.24). Gestational hypertension and preeclampsia/eclampsia showed similar increased dementia risk. Nine mid‐life cardiometabolic and mental health conditions explained 61% of HDP's effect on subsequent dementia risk.
Discussion
Improved HDP and mid‐life care could reduce the risk of dementia.
1. INTRODUCTION
The worldwide burden of late onset Alzheimer's disease (AD) and related dementias (ADRD) has been shown to disproportionately affect women in several prior studies. 1 Findings from the Framingham Heart Study reported that the lifetime risk of AD and ADRD after age 45 was 1 in 5 for women compared to 1 in 10 for men. 2 Women's longer life span only partially explains this risk difference. 3 The search for causes of AD must include this sex‐based disparity. Life course experiences unique to women, such as pregnancy‐related conditions, may increase the risk of dementia. 4 , 5
Hypertensive disorders of pregnancy (HDP) include preeclampsia; eclampsia; hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome; and gestational hypertension. These disorders are common, complicating 3% to 8% 6 , 7 of all pregnancies with approximately 1 in 7 women affected by HDP through her reproductive years. 7 Moreover, the prevalence of HDP has increased over the last 5 years. 8 HDP is the leading cause of maternal and perinatal morbidity and mortality. 9 , 10
An exaggerated inflammatory response leading to endothelial dysfunction is a known pathophysiologic finding in clinically apparent HDP. This suggests that this underlying mechanism may predispose pregnant women with HDP to other chronic conditions. 11 , 12 , 13 , 14 , 15 Research over the last decade has found that women who develop HDP are at increased risk for later cardiovascular and metabolic disorders. 16 Consequently, the American Heart Association now includes HDP as a risk factor for future cardiovascular disease. 14 Whether HDP is additionally linked to later‐life adverse neurologic conditions, specifically AD and vascular dementia, is beginning to be addressed. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Recent laboratory investigations have demonstrated shared disruptions in cell biological mechanisms including endoplasmic reticulum stress, the unfolded protein response, and autophagy in preeclampsia and AD. 25 Furthermore, the preeclampsia gene STOX1 has been found to be abundantly expressed in the brains of individuals diagnosed with late‐onset AD. 26 Researchers are also exploring the role of chronic inflammation and endothelial dysfunction as the underlying pathology of vascular dementia. 27
Despite the compelling argument that HDP could increase dementia risk, several studies have examined the effect of HDP on AD, vascular dementia, and ADRD with conflicting findings. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Inconsistencies in results among studies may arise from (1) failure to distinguish between dementia and HDP subtypes and (2) small sample size with insufficient power leading to potential type II errors. 17 , 18 , 19 , 20 Additionally, prior studies have addressed the impact of mid‐life intervening factors through simple adjustment. 19 , 21 , 24 Adjustment for mediating factors has been shown to lead to bias. Alternative methods that can quantify the direct and indirect effects of mid‐life mediating factors on the relationship between HDP and ADRD are preferred. These methods are in line with, not conflating, confounders and mediators. They can also inform potential interventions that might thwart ADRD onset or progression after HDP. To overcome weaknesses of prior work, we conducted a large retrospective cohort study in which information about mid‐life conditions was available to estimate the risk of HDP on dementia and dementia subtypes. We formally assessed the effects of HDP on later life dementia risk, both directly and mediated through mid‐life cardiometabolic/mental health diseases. 28 , 29 , 30
2. METHODS
2.1. Data sources
We used the Utah Population Database (UPDB) to identify a retrospective cohort of individuals who gave birth from 1939 through 2013. We restricted our cohort to individuals living in Utah until age 45 to ensure sufficient follow‐up time for observing fertility history. 31 , 32 We followed individuals for dementia outcome assessments available through 2019 from medical records and death certificates. Our dataset includes sex but not information on gender identity, an inherent limitation of our dataset. 33
The UPDB has been previously described in detail, 34 , 35 , 36 , 37 as has our prior research on HDP and long‐term mortality risk. 17 , 37 The UPDB contains detailed information on > 11 million individuals with linked demographic and medical information (Figure S1 in supporting information). Study approvals were obtained from the Resource for Genetic and Epidemiologic Research, a special review panel authorizing access to the UPDB and the University of Utah Institutional Review Board.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature using PubMed and Embase. Of six prior studies examining hypertensive disorders of pregnancy (HDP) and dementia, three found HDP to increase dementia risk, one found HDP to have a protective effect against dementia, and two found no effect.
Interpretation: We found that women with a history of HDP had a 1.4‐fold higher risk of all‐cause dementia, 2‐ to 3‐fold higher risk of vascular dementia, but null association with Alzheimer's disease. There was no appreciable difference in risk among women with gestational hypertension versus preeclampsia/eclampsia. Nine mid‐life cardiometabolic and mental health conditions explained 61% of HDP's effect on dementia risk.
Future directions: Improved HDP and mid‐life care could reduce the risk of dementia. Future research should consider controlling for young adult atherogenic profile to better understand whether HDP independently leads to later dementia or simply unmasks an underlying predisposition to dementia.
2.2. Study cohort
Individuals were included if they both had at least one singleton live birth in Utah (1939–2013) and also lived in Utah until at least 45 years of age. Additionally, to capture dementia outcome data adequately, we required that women in our study lived in Utah after 1996, died in Utah after 1979, or linked to a Center for Medicare Services record. We followed women for outcome assessments (Table 1) through 2019.
TABLE 1.
ICD‐9 and ICD‐10 codes used for identifying Alzheimer's disease and related dementia in administrative databases
| ICD‐9 | Definition | ICD‐10 | Definition |
|---|---|---|---|
| 331.0 | Alzheimer's disease | G30.0 | Alzheimer's disease with early onset |
| G30.1 | Alzheimer's disease with late onset | ||
| G30.9 | Alzheimer's disease, unspecified | ||
| ICD‐9 | Definition | ICD‐10 | Definition |
| 290 | Senile dementia, uncomplicated | G30.8 | Other Alzheimer's disease |
| 290.0 | Senile dementia, uncomplicated | F01 | Vascular dementia |
| 290.1 | Presenile dementia (brain syndrome w/presenile dementia) | F01.5 | Vascular dementia |
| 290.10 | Presenile dementia, uncomplicated | F01.50 | Vascular dementia without behavioral disturbance |
| 290.11 | Presenile dementia with delirium | F01.51 | Vascular dementia with behavioral disturbance |
| 290.12 | Presenile dementia with delusional features | F02 | Dementia in other diseases classified elsewhere |
| 290.13 | Presenile dementia with depressive features | F02.8 | Dementia in other diseases classified elsewhere |
| 290.2 | Senile dementia with delusional features | F02.80 | Dementia in other diseases classified elsewhere w/out behavioral disturbance |
| 290.20 | Senile dementia with delusional features | F02.81 | Dementia in other diseases classified elsewhere with behavioral disturbance |
| 290.21 | Senile dementia with depressive features | F03 | Unspecified dementia |
| 290.3 | Senile dementia with delirium | F03.9 | Unspecified dementia |
| 290.4 | Vascular dementia, uncomplicated | F03.90 | Unspecified dementia without behavioral disturbance |
| 290.40 | Vascular dementia, uncomplicated | F03.91 | Unspecified dementia with behavioral disturbance |
| 290.42 | Vascular dementia, with delusions | F10.27 | Alcohol dependence with alcohol‐induced persisting dementia |
| 290.43 | Vascular dementia, with depressed mood | F19.97 | Other psychoactive substance use, unspecified with persisting dementia |
| 291.2 | Alcohol‐induced persisting dementia | G10 | Huntington's dementia |
| 292.82 | Drug‐induced persisting dementia | G31.0 | Frontotemporal dementia |
| 294.0 | Amnestic syndrome | G31.01 | Pick's disease |
| 294.1 | Dementia in conditions classified elsewhere w/out behavioral disturbance | G31.09 | Other frontotemporal dementia |
| 294.10 | Dementia in conditions classified elsewhere | G31.83 | Dementia with Lewy bodies |
| 294.2 | Dementia, unspecified, without behavioral disturbance | ||
| 294.21 | Dementia, unspecified, with behavioral disturbance | ||
| 294.8 | Other specified organ brain syndrome (chronic) | ||
| 331 | Other cerebral degeneration | ||
| 331.2 | Senile degeneration of the brain | ||
| 331.82 | Dementia with Lewy bodies | ||
| ‘797’ | Senility without mention of psychosis |
Abbreviation: ICD, International Classification of Diseases.
As has been done in prior studies on HDP and subsequent disease risk within the UPDB, 17 , 38 we chose a matched cohort design to ensure an equal distribution among exposed and unexposed of the variables we believed to be confounding factors. 39 Prior research indicated that matching in cohort studies introduces no bias, albeit may impact efficiency. 40 Women exposed to HDP were matched 1:2 to unexposed women by 5‐year age groups, year of childbirth, and parity at the time of the index pregnancy. Women were excluded if they were missing data on a key variable that would preclude matching. They were also excluded if they died within 1 year of delivery, or were diagnosed with any of the following comorbidities prior to the index child's birth date: myocardial infarction, ischemic heart disease, heart failure, stroke, diabetes, or dementia. We did not exclude women with pre‐existing chronic hypertension at index pregnancy (n = 722 overall; n = 607 [3%] of HDP exposed women and n = 115 [0.3%] of unexposed women) so that we could compare estimates including and excluding pre‐pregnancy chronic hypertension as has been done in prior studies. The final study cohort included 59,668 women (19,989 women exposed to HDP and 39,679 women unexposed to HDP; Figure 1).
FIGURE 1.
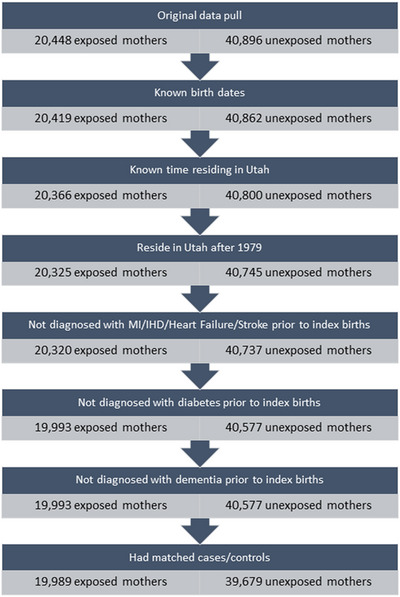
Flow chart of selecting study population. IHD, ischemic heart disease; MI, myocardial infarction
2.3. Exposure: hypertensive disorders of pregnancy
Using birth certificate data, we assigned a diagnosis of HDP (yes/no) to each affected pregnancy. HDP diagnoses included gestational hypertension, preeclampsia, HELLP syndrome, and eclampsia. Diagnoses were listed on the birth certificate for the affected pregnancy in the following manner: 1939 to 1977, text string; 1978 to 1988: International Classification of Diseases 9th revision codes; 1989 to present, check boxes with additional text. 17 , 34 When available, inpatient records were reviewed for exposed women (1996 to 2013). 17 If there was a discrepancy between hypertensive diagnoses listed in the inpatient records compared to the birth certificate, the inpatient record diagnoses were used in place of the birth certificate diagnoses. Unexposed women had pregnancies during the study period, but none were complicated by HDP. 17
2.4. Outcome: dementia
Our primary outcome of interest was all‐cause dementia captured via death certificates, inpatient hospital records, ambulatory surgery records, or emergency department records. We defined all‐cause dementia as having had a diagnosis of any dementia code (Table 1). We further classified dementia as AD, vascular dementia, or other/unspecified dementia including frontotemporal dementia, dementia with Lewy bodies, or all others. Our prior research indicates that UPDB administrative health records from 1996 to 2008 have 71% sensitivity and 81% specificity for all‐cause dementia diagnoses compared to gold‐standard research diagnoses. 41
2.5. Confounders
Potential confounders were informed by prior literature 21 and directed acyclic graphs. 42 Our primary models adjusted for index child's birth year, birth order, and mother's age at index childbirth. We additionally considered maternal education and marital status at index child's birth, and race/ethnicity as potential confounders.
2.6. Statistical analysis
We used Cox regression models to estimate unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for all‐cause dementia comparing women with and without a history of HDP. Additional models were run assessing dementia subtypes (vascular dementia, AD, or other related dementia). The dementia subtype analyses used dementia type based on first diagnosis. We also ran models assessing risk of dementia by HDP subtypes (preeclampsia/eclampsia or gestational hypertension compared to no HDP). Time from exposure to outcome was based on years since index birth. Follow‐up time was either censored at the date of the last observation of the woman, date of diagnosis of the dementia subtype, or death.
As has been done in prior research, 21 we used the Fine and Gray competing risk method 43 , 44 for analyzing associations with dementia subtypes. Additionally, fixed‐effect frailty models within the Cox regression framework were estimated; these models are based on clustering among siblings, obtained through birth record linkages, who share common time‐invariant genetic and early life environments. The missing indicator method was used to address missing data bias. The e‐value method was used to quantify potential bias due to unmeasured confounding. 45
Given the potentially long interval between pregnancies and subsequent dementia, there were several mediating factors that we considered. Using the inpatient discharge, ambulatory surgery, and emergency department records, we identified the following conditions (ever/never) among the total cohort that occurred after the index pregnancy during the follow‐up period (1996–2019): myocardial infarction, ischemic heart disease, heart failure, stroke, chronic kidney disease, diabetes, hypertension (primary or secondary), and depression/anxiety. A counterfactual approach to mediation analysis was applied to estimate the adjusted HR for the natural direct effect and natural indirect effect of HDP on future dementia mediated through these nine cardiometabolic/mental health factors that we assessed both one at a time and then combined (Figure S2 in supporting information). 29 , 30 Mediation analyses were adjusted for maternal age, birth year, and parity at time of index pregnancy. R statistical software was used for all analyses (R Foundation for Statistical Computing).
2.7. Sensitivity analyses
For comparison to prior research, several sensitivity analyses were conducted. First, we stratified our dementia outcome by early onset (diagnosed at <65 years) and late onset (diagnosed at ≥65 years). 46 , 47 Second, we stratified our data by pre‐pregnancy chronic hypertension status. Third, because of the importance of pre‐pregnancy body mass index (BMI) as a potential confounder, we conducted a sensitivity analysis that included BMI in our adjusted models. For this analysis, we restricted the sample to women whose index birth occurred in or after 1989, the year that pre‐pregnancy BMI began to be collected on birth certificates. Finally, given that preterm preeclampsia is a more severe phenotype and is more strongly associated with future cardiovascular disease risk, we stratified our data by preterm birth status. For this analysis, we were restricted to 1945 to 1977 during which a thorough review of birth certificates was conducted to identify preterm birth.
3. RESULTS
3.1. Cohort description
Among women with HDP, the majority were complicated with preeclampsia (63.0%), followed by gestational hypertension (33.5%), and eclampsia (3.5%). Among the total cohort, there were 5.3% of women with any dementia diagnosis during follow‐up. Other/unspecified dementia made up the majority of cases (66.0%), followed by AD (25.3%), vascular dementia (7.6%), dementia with Lewy bodies (1%), and frontotemporal dementia (0.2%).
The overall population of HDP‐exposed and ‐unexposed women comprised 9.4% Hispanic and 5.4% non‐White women, with mean birth year of 1982 and mean maternal age of 29 years. HDP‐exposed women, compared to non‐exposed women, were slightly more likely to be non‐White, Hispanic, born in Utah, have lower education, and be overweight or obese before pregnancy (Table 2). Women with a history of HDP had on average 1.15 pregnancies affected by HDP (standard deviation [SD] = 0.43).
TABLE 2.
Demographic characteristics at time of index birth
| No HDP exposure | HDP exposure | |
|---|---|---|
| n = 39,679 | n = 19,989 | |
| Total pregnancies with HDP | NA | 1.15 ± 0.43 |
| Birth year of index child | 1981.5 ± 15.7 | 1981.5 ± 15.8 |
| Male child | 20,533 (51.7%) | 10,387 (52.0%) |
| Birth order | 2.4 ± 1.9 | 2.4 ± 1.9 |
| Pregnancy complications at index birth | ||
| Spontaneous pre‐term birth | 594 (1.5%) | 33 (0.2%) |
| Gestational diabetes | 282 (0.7%) | 291 (1.5%) |
| Mother's age | 28.5 ± 6.8 | 28.6 ± 6.8 |
| Maternal birth year (mean ± SD) | 1953.0 ± 14.7 | 1952.8 ± 14.8 |
| Race (White vs. non‐White) | ||
| Yes | 37,581 (94.7%) | 18,836 (94.2%) |
| No | 2084 (5.3%) | 1150 (5.8%) |
| Unknown | 14 (0.0%) | 3 (0.0%) |
| Ethnicity (Hispanic vs. non‐Hispanic) | ||
| Yes | 3523 (8.9%) | 1954 (9.8%) |
| No | 35,118 (88.5%) | 17,601 (88.1%) |
| Unknown | 1038 (2.6%) | 434 (2.2%) |
| Born in Utah | ||
| Yes | 11,720 (29.5%) | 6115 (30.6%) |
| No | 2421 (6.1%) | 1075 (5.4%) |
| Unknown | 25,538 (64.4%) | 12,799 (64.0%) |
| Mother's education | ||
| Less than HS | 2879 (7.3%) | 1601 (8.0%) |
| HS graduate | 10,166 (25.6%) | 5654 (28.3%) |
| Some college | 10,361 (26.1%) | 5166 (25.8%) |
| College graduate | 5304 (13.4%) | 2226 (11.1%) |
| Post college | 2717 (6.8%) | 1175 (5.9%) |
| Unknown | 8252 (20.8%) | 4167 (20.8%) |
| Mother's residence at time of index birth | ||
| Rural/frontier | 5458 (13.7%) | 2655 (13.3%) |
| Urban | 34,221 (86.2%) | 17,334 (86.7%) |
| Mother's BMI at time of index birth a (mean ± SD) | 24.0 ± 5.1 | 27.5 ± 6.7 |
| Underweight | 859 (2.2%) | 170 (0.9%) |
| Healthy | 8637 (21.8%) | 2869 (14.4%) |
| Overweight | 2718 (6.8%) | 1761 (8.8%) |
| Obese | 1618 (4.1%) | 2120 (10.6%) |
| Unknown | 25,847 (65.1%) | 13,069 (65.4%) |
| Father's age at time of index birth (mean ± SD) | 30.4 ± 7.3 | 30.4 ± 7.3 |
| Father's education at time of index birth b | ||
| Less than HS | 1905 (4.8%) | 1143 (5.7%) |
| HS graduate | 8052 (20.3%) | 4629 (23.2%) |
| Some college | 9039 (22.8%) | 4578 (22.9%) |
| College graduate | 5584 (14.1%) | 2412 (12.1%) |
| Post college | 5236 (13.2%) | 2088 (10.4%) |
| Unknown |
Note: n (%) unless otherwise specified.
Abbreviations: BMI, body mass index; HDP, hypertensive disorder of pregnancy; HS, high school; NA, not applicable; SD, standard deviation.
aAvailable ≥ 1989.
bAvailable ≥ 1968.
3.2. Primary findings
Women with a history of HDP had a higher risk of all‐cause dementia (HR = 1.37, 95% CI: 1.26, 1.50) compared to women without a history of HDP after adjustment for maternal age, year of childbirth, and parity (Table 3). Looking at dementia subtypes, the hazard was higher for vascular dementia (HR: 1.64, 95% CI: 1.19, 2.26) and other/unspecified dementia (HR: 1.49, 95% CI: 1.34, 1.65) but not for AD (HR: 1.04, 95% CI: 0.87, 1.24). Further adjustment for maternal education, marital status at index child's birth, and race/ethnicity did not appreciably alter the results.
TABLE 3.
Adjusted a hazard ratios for all‐cause dementia and dementia subtypes by history of hypertensive disorders of pregnancy
| History of HDP | All‐cause dementia | Vascular dementia | Alzheimer's disease | Other/unspecified dementia | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | ||
| Overall | Exposed | 827 | 1.37 (1.26, 1.50) | 55 | 1.64 (1.19, 2.26) | 178 | 1.04 (0.87, 1.24) | 594 | 1.49 (1.34, 1.65) |
| Unexposed | 1591 | 1 [Reference] | 97 | 1 [Reference] | 401 | 1 [Reference] | 1098 | 1 [Reference] | |
| Attained age by end of follow‐up | |||||||||
| <65 years | Exposed | 100 | 1.87 (1.49, 2.34) | 9 | 2.70 (1.01, 7.17) | 12 | 2.01 (0.99, 4.08) | 79 | 1.77 (1.38, 2.26) |
| Unexposed | 107 | 1 [Reference] | 5 | 1 [Reference] | 9 | 1 [Reference] | 93 | 1 [Reference] | |
| ≥65 years | Exposed | 727 | 1.30 (1.19, 1.43) | 46 | 1.41 (0.98, 2.04) | 166 | 0.99 (0.83, 1.19) | 515 | 1.44 (1.28, 1.61) |
| Unexposed | 1484 | 1 [Reference] | 92 | 1 [Reference] | 389 | 1 [Reference] | 1003 | 1 [Reference] | |
Abbreviations: CI, confidence interval; HDP, hypertensive disorder of pregnancy; HR, hazard ratio.
Adjusted for maternal age, birth year, and parity (1, 2, 3, 4, ≥5) at the time of the index pregnancy.
Women with preeclampsia/eclampsia at index pregnancy, compared to women with no prior history of HDP, had a 1.38 (95% CI: 1.26, 1.50) higher risk of all‐cause dementia, while women with gestational hypertension had a 1.36 (95% CI: 1.03, 1.79) higher risk (Table 4). Breaking down by dementia subtypes, women with a history of preeclampsia/eclampsia had a 1.58 (95% CI: 1.11, 2.24) and 1.51 (95% CI: 1.36, 1.68) higher risk of vascular dementia and other/unspecified dementia, respectively, while women with gestational hypertension had a 2.75 (95% CI: 0.90, 8.40) and 1.31 (95% CI: 0.96, 1.80) higher risk, respectively. HDP subtypes at index pregnancy were not associated with AD.
TABLE 4.
Adjusted a hazard ratios for all‐cause dementia and dementia subtypes by history of hypertensive disorders of pregnancy subtypes
| History of HDP | All‐cause dementia | Vascular dementia | Alzheimer's disease | Other/unspecified dementia | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | ||
| Preeclampsia/eclampsia | Exposed | 827 | 1.38 (1.26, 1.50) | 55 | 1.58 (1.11, 2.24) | 178 | 1.04 (0.87, 1.24) | 594 | 1.51 (1.36, 1.68) |
| Unexposed | 1596 | 1 [Reference] | 97 | 1 [Reference] | 401 | 1 [Reference] | 1098 | 1 [Reference] | |
| Gestational hypertension | Exposed | 75 | 1.36 (1.03, 1.79) | 7 | 2.75 (0.90, 8.40) | 16 | 1.18 (0.52, 2.68) | 51 | 1.31 (0.96, 1.80) |
| Unexposed | 132 | 1 [Reference] | 8 | 1 [Reference] | 28 | 1 [Reference] | 97 | 1 [Reference] | |
Abbreviations: CI, confidence interval; HDP, hypertensive disorder of pregnancy; HR, hazard ratio.
aAdjusted for maternal age, birth year, and parity (1, 2, 3, 4, ≥5) at the time of the index pregnancy.
For our mediation analyses, we found that after taking into account maternal age, year of childbirth, parity, and individual mid‐life cardiometabolic/mental health factors, women with a history of HDP had a total increased dementia risk between 39% and 88% (Table 5). Mid‐life stroke had the greatest indirect (mediating) effect, increasing risk by 20%. Overall, we found that up to 61% of HDP's effect on subsequent dementia risk could be explained by the nine mid‐life cardiometabolic/mental health factors we considered.
TABLE 5.
Effect of hypertensive disorders of pregnancy and all‐cause dementia taking into account potential mid‐life mediating factors assessed individually one at a time and then combined
|
Natural direct effect HR (95% CI) |
Natural indirect effect HR (95% CI) |
Total effect HR (95% CI) |
Proportion mediated | |
|---|---|---|---|---|
| Myocardial infarction | 1.40 (1.37, 1.43) | 1.09 (1.06, 1.12) | 1.53 (1.52, 1.54) | 22% |
| Ischemic heart disease | 1.40 (1.37, 1.44) | 1.08 (1.06, 1.11) | 1.52 (1.51, 1.53) | 19% |
| Heart failure | 1.38 (1.35, 1.42) | 1.07 (1.03, 1.10) | 1.47 (1.46, 1.48) | 17% |
| Stroke | 1.27 (1.25, 1.29) | 1.20 (1.18, 1.23) | 1.52 (1.52, 1.53) | 43% |
| Chronic kidney disease | 1.35 (1.32, 1.38) | 1.14 (1.11, 1.17) | 1.53 (1.52, 1.54) | 30% |
| Diabetes | 2.82 (0.83, 3.93) | 0.67 (0.43, 1.84) | 1.88 (1.51, 2.02) | NA |
| Hypertension | 1.21 (1.19, 1.24) | 1.14 (1.12, 1.17) | 1.39 (1.38. 1.40) | 41% |
| Anxiety | 1.38 (1.35, 1.41) | 1.10 (1.08, 1.13) | 1.52 (1.51, 1.53) | 23% |
| Depression | 1.49 (1.40, 1.57) | 1.02 (0.96, 1.08) | 1.51 (1.50, 1.52) | 4% |
| Overall | 1.12 (1.09, 1.15) | 1.19 (1.16, 1.22) | 1.33 (1.32, 1.34) | 61% |
Note: Adjusted for maternal age, birth year, and parity (1, 2, 3, 4, ≥5) at the time of the index pregnancy.
Abbreviations: CI, confidence interval; HR, hazard ratio.
3.3. Sensitivity analyses
We found that women with, compared to without, a history HDP had a higher risk of both early onset (adjusted HR [aHR] 1.87 95% CI: 1.49, 2.35) and late‐onset dementia (aHR 1.30, 95% CI: 1.19, 1.43). We found the magnitude of association for women with HDP exposure and pre‐pregnancy chronic hypertension was similar (aHR: 1.27, 95% CI: 0.89, 1.80) compared to HDP‐exposed women without pre‐pregnancy chronic hypertension (aHR: 1.38, 95% CI: 1.27, 1.38). Finally, in our subset analysis among women for whom we had pre‐pregnancy BMI information for index pregnancy (n = 20,752; 35% of the entire sample), we found that risk for all‐cause dementia increased to 2.31 (95% CI: 1.24, 4.32) among the HDP exposed after adjusting for maternal age, birth year, parity, and pre‐pregnancy BMI category (underweight, normal weight, overweight, obese; Table S1 in supporting information). Finally, compared to women with no HDP exposure, women with HDP and preterm birth had a 1.84 aHR (95% CI: 0.60, 5.60) while women with HDP and without preterm birth had a 1.37 aHR (95% CI: 1.26, 1.49).
Regarding unmeasured confounding, the observed HR of 1.37 for all‐cause dementia and 1.64 for vascular dementia could be explained away by an unmeasured confounder that was associated with both the exposure and the outcome by an HR of 2.08‐fold and 2.66‐fold, respectively, above and beyond the measured confounders, but weaker confounding could not do so. The CI could be moved to include the null by an unmeasured confounder that was associated with both the exposure and the outcome by a HR of 1.83‐fold and 2.08‐fold, respectively, above and beyond the measured confounders, but weaker confounding could not do so.
4. DISCUSSION
4.1. Main findings
In this cohort of nearly 60,000 parous women followed retrospectively for up to 80 years, a history of HDP was associated with a 50% to 60% higher risk of vascular and other related dementias, but not AD. Findings were similar between women with a history of preeclampsia/eclampsia and gestational hypertension. In our mediation analyses, we found that mid‐life cardiometabolic and mental health factors (i.e., depression and anxiety) may explain > 60% of the association between HDP and subsequent dementia.
4.2. Comparison to prior research
Our findings are in line with the largest study to date, conducted in Denmark, which found that women with a history of HDP had a 1.53 higher HR (95% CI: 1.26, 1.85) for all‐cause dementia compared to women without HDP after adjusting for maternal birth year, parity, and region in which child was delivered. 21 The strongest association, similar to what we found, was between HDP and vascular dementia (aHR 3.46, 95% CI: 1.97, 6.10). Also similar to our findings, there was no appreciable change in estimates after excluding women with chronic hypertension; and both of our studies found increased risk of HDP with early‐ and late‐onset dementia. While both their and our study were adequately powered, comparison between studies is difficult given the differences in study time frame. Their study comprised predominately younger women with only 10% of women age ≥65 years at the end of the follow‐up. Consequently, the majority (71%) of their total dementia diagnoses (n = 1728, 0.1% of total sample) occurred among women < 65 years. In contrast, the majority (91%) of our total dementia diagnoses (n = 2418, 4.1% of total sample) occurred among women ≥65 years, as one would expect in a population‐based cohort followed long enough.
Our findings also align with another large population‐based study that was conducted in Sweden (1973–2013). 24 This study, similar to our study, investigated not only differences by dementia subtype, but also difference by HDP subtype (i.e., preeclampsia/eclampsia versus gestational hypertension). Overall, women with a history of HDP compared to those without had a 1.26 higher HR (95% CI: 1.07, 1.48) for all‐cause dementia. The association was strongest for vascular dementia (aHR: 3.02, 95% CI: 2.13, 4.31) and borderline for AD (aHR: 1.30, 95% CI: 0.97, 1.74) or other dementias (aHR: 1.11, 95% CI: 0.95, 1.33).
Similar to our study, both the Danish and Swedish studies took into account baseline chronic hypertension and intermediary cardiometabolic disorders that occurred during follow‐up. For both, this resulted in a slight attenuation in the estimates, but results remained strong and statistically significant. 21 , 24 Our formal mediation analysis suggests that while mid‐life cardiometabolic factors do partially explain the relationship between HDP and later dementia, they do not explain the full relationship. The direct effect for our strongest mediating factor, stroke, indicates that even if HDP‐exposed and HDP‐unexposed women had equal levels of stroke incidence, HDP‐exposed women would still have a 27% higher risk of dementia (95% CI: 25%, 29%) compared to women with no HDP exposure.
4.3. Strengths and limitations
Strengths of the study are the population‐based sample of women; the long observation period; linkage of birth records dating back to 1939 with electronic health records up through 2019; and our ability to consider important confounding, moderating, and mediating factors. We also acknowledge some limitations. First, we were limited in the number of factors we could appropriately adjust for in our mediation analyses. Further research that takes into account confounding of the exposure–mediator relationship and of the mediator–outcome relationship is warranted. Second, we recognize that using administrative health‐care records for dementia ascertainment may result in misclassification, especially in underdetection of dementia. 41 Worldwide there are barriers to receiving a diagnosis of a dementing disease, 48 an inherent limitation of studying dementia that impacts all research in this field. While higher precision and accuracy of dementia diagnoses in administrative health‐care records would maximize statistical power, in the trade‐off between sensitivity and specificity, having high specificity, which we have previously demonstrated in the UPDB, 41 is preferable for etiologic research in which minimizing false positives is key to reducing bias and distortion of risk estimates. Given our prior research showing vascular dementia to have higher specificity than AD or other related dementias, we cannot rule out that this difference may partially explain the stronger effect estimates we observed for vascular dementia.
Third, our HDP exposure is also susceptible to misclassification bias in the same direction as that of dementia, with higher specificity and lower sensitivity. Prior research comparing birth records to inpatient records found sensitivities ranging from 23% to 99% and specificities from 96% to 100%. 49
Fourth, we were restricted to only a subset of our data when looking at the confounding effect of BMI or in our stratified analyses assessing HDP accompanied by preterm labor, a more severe phenotype, due to availability of linked data, which led to decreased precision in our sensitivity analyses for these factors. Fifth, given the retrospective nature of our study, we were not able to follow up women who moved out of Utah during the study timeframe. However, given that women with and without HDP exposure had the same criteria for follow‐up and HDP exposure is unlikely to be associated with emigration, loss to follow‐up would most likely bias our results toward the null.
Finally, given that the majority of women in this study were non‐White (94%) and non‐Hispanic (88%), our findings are limited in their generalizability. Additional research in more US representative samples is needed before definitive conclusions can be made.
4.4. Conclusion
In summary, our results indicate that HDP is associated with subsequent dementia, especially for vascular dementia. It is remarkable that increased risk of vascular dementia was detectable, despite the smaller number of individuals identified with this specific cause of dementia. Because we found that mid‐life cardiovascular health significantly mediates the risk, future research should evaluate the effects of targeted surveillance and interventions in women who have experienced HDP. While we were able to take into account key sociodemographic confounding factors, future epidemiologic research should additionally consider controlling for young adult atherogenic profile. Doing so will help women's health researchers better understand whether HDP independently leads to later dementia or simply unmasks an underlying predisposition to later dementing disease. Improved detection of cognitive impairment and improved diagnostic precision with wide use of biomarkers in the future will improve our ability to understand the mechanisms of dementia risk. Such research can then better inform current guidelines on whether women with HDP should be better monitored and receive increased surveillance with a view to early care to mitigate cardiovascular and dementia risk over the rest of the lifespan.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Study approvals were obtained from the Resource for Genetic and Epidemiologic Research, a special review panel authorizing access to the UPDB and the University of Utah Institutional Review Board (UU IRB). The UU IRB approved the study on 03/22/2019 (IRB_00116984) and deemed the study exempt with a waiver of consent due to the study being retrospective in nature using historical records within the Utah Population Database for statistical analysis only.
Supporting information
Supplementary Information
Supplementary Information
ACKNOWLEDGMENTS
We thank the Pedigree and Population Resource of Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance, and support of the Utah Population Database (UPDB). We also acknowledge partial support for the UPDB through grant P30 CA2014 from the National Cancer Institute, University of Utah and from the University of Utah's program in Personalized Health and Center for Clinical and Translational Science. Research was also supported by the NCRR grant, “Sharing Statewide Health Data for Genetic Research” (R01 RR021746, G. Mineau, PI) with additional support from the Utah Department of Health and the University of Utah. This work was supported by the National Institute on Aging (NIA) grants “Hypertensive Disorders of Pregnancy and Subsequent Risk of Vascular Dementia, Alzheimer's Disease, or Related Dementia: A Retrospective Cohort Study Taking into Account Midlife Mediating Factors” (Project K01AG058781; PI: Karen Schliep), “Early Life Conditions, Survival, and Health: A Pedigree‐Based Population Study” (Project: R01AG022095; PI: Ken Smith), and “Population neuroscience of sex differences in the Alzheimer's disease biomarker cascade: the role of cerebral small vessel disease” (Project K01AG071849; PI: C. Elizabeth Shaaban).
Schliep KC, Shaaban CE, Meeks H, et al. Hypertensive disorders of pregnancy and subsequent risk of Alzheimer's disease and other dementias. Alzheimer's Dement. 2023;15:e12443. 10.1002/dad2.12443
REFERENCES
- 1. Mielke MM. Sex and gender differences in Alzheimer's disease dementia. Psychiatric Times. 2018;35(11):14. [PMC free article] [PubMed] [Google Scholar]
- 2. Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid‐adult life. Alzheimers Dement. 2015;11(3):310‐320. 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clinical Neurosci. 2016;18(4):437‐446. 10.31887/DCNS.2016.18.4/cepperson [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rocca WA, Mielke MM, Vemuri P, Miller VM. Sex and gender differences in the causes of dementia: a narrative review. Maturitas. 2014;79(2):196‐201. 10.1016/j.maturitas.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300‐307. 10.2337/dc15-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1‐7. 10.1016/j.ejogrb.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 7. Garovic VD, White WM, Vaughan L, et al. Incidence and long‐term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75(18):2323‐2334. 10.1016/j.jacc.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuehn BM. Hypertensive Disorders in Pregnancy Are on the Rise. JAMA. 2022;327(24):2387. 10.1001/jama.2022.9510 [DOI] [PubMed] [Google Scholar]
- 9. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987‐2004. Am J Hypertens. 2008;21(5):521‐526. doi:ajh200820 [pii] 10.1038/ajh.2008.20 [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy‐induced hypertension. Epidemiol Rev. 1997;19(2):218‐232. [DOI] [PubMed] [Google Scholar]
- 11. Louis J, Saade G. Pregnancy as a window to future health. Introduction. Semin Perinatol. 2015;39(4):253. 10.1053/j.semperi.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 12. Beeri MS, Ravona‐Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci. 2009;11(2):201‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self‐reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol. 2007;197(4):365e1‐6. doi: S0002‐9378(07)00820‐4 [pii] 10.1016/j.ajog.2007.06.044 [DOI] [PubMed] [Google Scholar]
- 14. Hypertension in pregnancy . Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122‐1131. 10.1097/01.AOG.0000437382.03963.88 [DOI] [PubMed] [Google Scholar]
- 15. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114(5):961‐970. 10.1097/AOG.0b013e3181bb0dfc00006250-200911000-00003 [pii] [DOI] [PubMed] [Google Scholar]
- 16. Wu R, Wang T, Gu R, et al. Hypertensive disorders of pregnancy and risk of cardiovascular disease‐related morbidity and mortality: a systematic review and meta‐analysis. Cardiology. 2020;145(10):633‐647. 10.1159/000508036 [DOI] [PubMed] [Google Scholar]
- 17. Theilen LH, Fraser A, Hollingshaus MS, et al. All‐cause and cause‐specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128(2):238‐244. 10.1097/AOG.0000000000001534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abheiden CN, van Doornik R, Aukes AM, van der Flier WM, Scheltens P, de Groot CJ. Hypertensive disorders of pregnancy appear not to be associated with Alzheimer's disease later in life. Dement Geriatr Cogn Dis Extra. 2015;5(3):375‐385. 10.1159/000439043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andolf EG, Sydsjo GC, Bladh MK, Berg G, Sharma S. Hypertensive disorders in pregnancy and later dementia: a Swedish National Register Study. Acta Obstet Gynecol Scand. 2017;96(4):464‐471. 10.1111/aogs.13096 [DOI] [PubMed] [Google Scholar]
- 20. Nelander M, Cnattingius S, Akerud H, Wikstrom J, Pedersen NL, Wikstrom AK. Pregnancy hypertensive disease and risk of dementia and cardiovascular disease in women aged 65 years or older: a cohort study. BMJ Open. 2016;6(1):e009880. 10.1136/bmjopen-2015-009880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basit S, Wohlfahrt J, Boyd HA. Pre‐eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018;363:k4109. 10.1136/bmj.k4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adank MC, Hussainali RF, Oosterveer LC, et al. Hypertensive disorders of pregnancy and cognitive impairment: a prospective cohort study. Neurology. 2021;96(5):e709‐e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fields JA, Garovic VD, Mielke MM, et al. Preeclampsia and cognitive impairment later in life. Am J Obstet Gynecol. 2017;217:74.e1‐74.e11. 10.1016/j.ajog.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andolf E, Bladh M, Moller L, Sydsjo G. Prior placental bed disorders and later dementia: a retrospective Swedish register‐based cohort study. BJOG. 2020;127(9):1090‐1099. 10.1111/1471-0528.16201 [DOI] [PubMed] [Google Scholar]
- 25. Cheng SB, Nakashima A, Sharma S. Understanding pre‐eclampsia using Alzheimer's etiology: an intriguing viewpoint. Am J Reprod Immunol. 2016;75(3):372‐381. 10.1111/aji.12446 [DOI] [PubMed] [Google Scholar]
- 26. van Dijk M, van Bezu J, Poutsma A, et al. The pre‐eclampsia gene STOX1 controls a conserved pathway in placenta and brain upregulated in late‐onset Alzheimer's disease. J Alzheimers Dis. 2010;19(2):673‐679. doi: W821425524825H33 [pii] 10.3233/JAD-2010-1265 [DOI] [PubMed] [Google Scholar]
- 27. Tian Z, Ji X, Liu J. Neuroinflammation in vascular cognitive impairment and dementia: current evidence, advances, and prospects. Int J Mol Sci. 2022;23(11):6224. 10.3390/ijms23116224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. Z Evid Fortbild Qual Gesundhwes. 2016;115‐116:33‐48. Das RECORD‐Statement zum Berichten von Beobachtungsstudien, die routinemassig gesammelte Gesundheitsdaten verwenden. 10.1016/j.zefq.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford University Press; 2015;xvi:706. [Google Scholar]
- 30. VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. 2016;37:17‐32. 10.1146/annurev-publhealth-032315-021402 [DOI] [PubMed] [Google Scholar]
- 31. Omura JD, McGuire LC, Patel R, et al. Modifiable risk factors for Alzheimer disease and related dementias among adults aged ≥45 years – United States, 2019. Morb. 2022;71(20):680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahmoudi E, Lin P, Kamdar N, Gonzales G, Norcott A, Peterson MD. Risk of early‐ and late‐onset Alzheimer disease and related dementia in adults with cerebral palsy. Dev Med Child Neurol. 2022;64(3):372‐378. 10.1111/dmcn.15044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rioux C, Weedon S, London‐Nadeau K, et al. Gender‐inclusive writing for epidemiological research on pregnancy. J Epidemiol Community Health. 2022;76(9):823‐827. 10.1136/jech-2022-219172 [DOI] [PubMed] [Google Scholar]
- 34. Esplin MS, Fausett MB, Fraser A, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344(12):867‐872. 10.1056/NEJM200103223441201 [DOI] [PubMed] [Google Scholar]
- 35. Smith KR, Mineau GP, Bean LL. Fertility and post‐reproductive longevity. Soc Biol. 2002;49(3‐4):185‐205. [PubMed] [Google Scholar]
- 36. Smith KR, Mineau GP. The Utah Population Database. The legacy of four decades of demographic research. Hist Life Course Stud. 2021;11:48‐73. [Google Scholar]
- 37. Smith KR, Fraser A, Reed DL, et al. The Utah Population Database. A model for linking medical and genealogical records for population health research. Histl Life Course Stud. 2022;12:58‐77. [Google Scholar]
- 38. Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long‐term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. 2018;219(1):107.e1‐107.e6. 10.1016/j.ajog.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Graaf MA, Jager KJ, Zoccali C, Dekker FW. Matching, an appealing method to avoid confounding? Nephron Clin Pract. 2011;118(4):c315‐c318. [DOI] [PubMed] [Google Scholar]
- 40. Lash TV TL, Haneuse S, Rothman KJ. Modern Epidemiology. 3rd ed. vol 4th Edition. Wolters Kluwer; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schliep KC, Ju S, Foster NL, et al. How good are medical and death records for identifying dementia? Alzheimers Dement. 2021;18(10):1812‐1823. 10.1002/alz.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. 10.1097/EDE.0b013e318225c2be [DOI] [PubMed] [Google Scholar]
- 43. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94(446):496‐509. [Google Scholar]
- 44. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244‐256. 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167(4):268‐274. [DOI] [PubMed] [Google Scholar]
- 46. Sirkis DW, Bonham LW, Johnson TP, La Joie R, Yokoyama JS. Dissecting the clinical heterogeneity of early‐onset Alzheimer's disease. Mol Psychiatry. 2022;27(6):2674‐2688. 10.1038/s41380-022-01531-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wattmo C, Wallin AK. Early‐ versus late‐onset Alzheimer's disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res Ther. 2017;9(1):70. 10.1186/s13195-017-0294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilkinson T, Ly A, Schnier C, et al. Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement. 2018;14(8):1038‐1051. 10.1016/j.jalz.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roberts CL, Bell JC, Ford JB, Hadfield RM, Algert CS, Morris JM. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27(3):285‐297. 10.1080/10641950701826695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information


