Abstract
Background
The implementation of immunological biomarkers for radiotherapy (RT) individualization in breast cancer requires consideration of tumor-intrinsic factors. This study aimed to investigate whether the integration of histological grade, tumor-infiltrating lymphocytes (TILs), programmed cell death protein-1 (PD-1), and programmed death ligand-1 (PD-L1) can identify tumors with aggressive characteristics that can be downgraded regarding the need for RT.
Methods
The SweBCG91RT trial included 1178 patients with stage I–IIA breast cancer, randomized to breast-conserving surgery with or without adjuvant RT, and followed for a median time of 15.2 years. Immunohistochemical analyses of TILs, PD-1, and PD-L1 were performed. An activated immune response was defined as stromal TILs ≥10% and PD-1 and/or PD-L1 expression in ≥1% of lymphocytes. Tumors were categorized as high-risk or low-risk using assessments of histological grade and proliferation as measured by gene expression. The risk of ipsilateral breast tumor recurrence (IBTR) and benefit of RT were then analyzed with 10 years follow-up based on the integration of immune activation and tumor-intrinsic risk group.
Results
Among high-risk tumors, an activated immune infiltrate was associated with a reduced risk of IBTR (HR 0.34, 95% CI 0.16 to 0.73, p=0.006). The incidence of IBTR in this group was 12.1% (5.6–25.0) without RT and 4.4% (1.1–16.3) with RT. In contrast, the incidence of IBTR in the high-risk group without an activated immune infiltrate was 29.6% (21.4–40.2) without RT and 12.8% (6.6–23.9) with RT. Among low-risk tumors, no evidence of a favorable prognostic effect of an activated immune infiltrate was seen (HR 2.0, 95% CI 0.87 to 4.6, p=0.100).
Conclusions
Integrating histological grade and immunological biomarkers can identify tumors with aggressive characteristics but a low risk of IBTR despite a lack of RT boost and systemic therapy. Among high-risk tumors, the risk reduction of IBTR conferred by an activated immune infiltrate is comparable to treatment with RT. These findings may apply to cohorts dominated by estrogen receptor-positive tumors.
Keywords: Clinical Trials, Phase III as Topic; Tumor Biomarkers; Tumor Microenvironment; Radiotherapy; Genome Instability
Background
Adjuvant radiotherapy (RT) after breast-conserving surgery (BCS) significantly decreases the incidence of ipsilateral breast tumor recurrence (IBTR).1 However, despite standard treatment, approximately 10% of patients experience an IBTR within 10 years of diagnosis, associated with an increased risk of subsequent distant metastasis and death.1 2 Patients with high-risk tumors may be recommended RT boost to eliminate residual microscopic tumor foci.3 The most widely accepted boost indication is young age.3 Furthermore, other characteristics of tumor aggressivity represent additional boost indications, although the definition varies between guidelines.3 4 RT de-escalation has so far focused on low-risk tumors. However, recent data indicate significant prognostic heterogeneity among patients with high-risk tumors, for example, young individuals with estrogen receptor (ER)-negative tumors.5 This is an area where immunological biomarkers show great potential.5 In light of the above, we believe it is highly relevant to study the possibility of RT de-escalation in high-risk groups.
CD8+T cells are considered the primary effector cell of the antitumoral immune response6 7 and react to protein products of mutated tumor genes (ie, neoantigens). T cells are regulated by the programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway and other immune checkpoints.8 9 Despite its inherent inhibitory effect on CD8+T cells, an active PD-1/PD-L1 pathway may correlate with an activated immune response and an improved prognosis among aggressive subtypes.10 Assessments of the PD-1/PD-L1 axis provide independent information in addition to tumor-infiltrating lymphocytes (TILs),11 but it is unknown if this can be used to improve RT individualization. We have previously shown that high stromal TILs may be associated with a reduced risk of IBTR and decreased RT benefits.12
Histological grade has long been an important prognostic factor in breast cancer and primarily measures proliferation and dedifferentiation.13 In a previous study, we found that a signature correlating strongly with histological grade could predict the prognostic effect of an activated immune infiltrate14—a characteristic we will henceforth refer to as immune responsiveness. Histological grade may thus represent tumor-intrinsic qualities that predict the biological implications of a local immune infiltrate. However, many tumors are classified as grade II, which does not provide useful clinical information.15 Previous studies indicate that subtype, in part, can determine immune responsiveness.6 Subtype correlates with proliferation, whose biological relevance is illustrated by the fact that it may explain most of the performance of prognostic breast cancer signatures.16 17 Because the luminal B subtype exhibits significant heterogeneity regarding proliferation,18 we do not believe that subtype alone is the optimal method to estimate immune responsiveness. This is supported by recent data indicating immunotherapy responsiveness among a subset of luminal B tumors.19 For this reason, we chose to use histological grade as a hypothesized marker of immune responsiveness in this study.
This study aimed to investigate whether an integrated analysis of TILs, the PD-1/PD-L1 signaling pathway, and histological grade can identify immune-responsive tumors from a cohort dominated by luminal tumors and inform RT de-escalation. These biomarkers are already being evaluated in clinical practice, and an increased understanding of their interaction for determining RT benefit and immune responsiveness may improve the treatment of patients with breast cancer. Using our previously developed gene expression signature predicting immune responsiveness,14 we also attempted to stratify grade II tumors into high-risk and low-risk groups with hypothesized different benefits of a local immune infiltrate. We hypothesized that high-risk tumors with an activated immune response could be downgraded in terms of locoregional treatment.
Methods
Study population
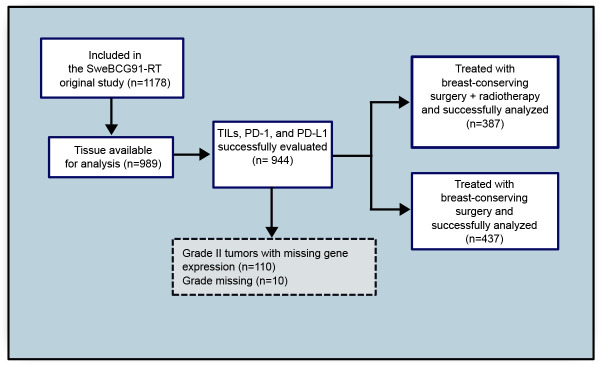
Patients from the SweBCG91RT trial were analyzed.20 21 In summary, 1178 patients with lymph node-negative (N0) stage I or IIA breast cancer were randomly assigned between 1991 and 1997 to BCS with or without whole-breast RT and followed for a median time of 15.2 years(online supplemental file 3) (figure 1). No patient had a positive surgical margin. Systemic adjuvant therapy was given per regional guidelines at the time. In total, 7% of patients received endocrine treatment, 1% received chemotherapy, and 0.4% received both endocrine therapy and chemotherapy. Tumor blocks were recollected and tumor subtyping was performed according to the St Gallen International Breast Cancer Conference (2013) Expert Panel on tissue microarray (TMA) slides as described previously.22 In short, tumors were classified as luminal A–like (ER-positive, progesterone receptor (PgR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, and Ki-67 low), luminal B–like (ER-positive, PgR-negative or Ki-67 high, and HER2-negative), HER2-positive (HER2-positive, any ER and PgR status, any Ki-67) and triple-negative (ER-negative, PgR-negative, HER2-negative, and any Ki-67). Analyses were performed on treatment-naïve formalin-fixed paraffin-embedded (FFPE) tumor samples. Invasive carcinoma was histologically confirmed by a board-certified pathologist. Included patients did not differ from excluded patients except for histological grade and tumor size. Excluded patients had slightly smaller tumors of a lower histological grade (online supplemental table S1).
Figure 1.
Consort diagram of included patients. Tumor blocks from patients included in the original SweBCG91RT trial were recollected. TILs and histological grade were scored on whole tissue sections and PD-1/PD-L1 were scored on TMAs. PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; RT, radiotherapy; TILs, tumor-infiltrating lymphocytes; TMA, tissue microarray.
jitc-2022-006618supp003.pdf (59.3KB, pdf)
jitc-2022-006618supp001.pdf (1.1MB, pdf)
The original trial and follow-up study were conducted per the Declaration of Helsinki. Oral informed consent was obtained from all patients before performing human investigations for the original trial and this follow-up study, and was determined appropriate and approved by the Ethical Review Board.
Data sharing
Gene expression data has been deposited in the Gene Expression Omnibus under accession number GSE119295. Due to regulations of the ethical review board and laws related to patient privacy, all clinical information has not been made publicly available.
Immunohistochemistry evaluations
Stromal TILs were evaluated on whole tissue H&E-stained sections as described previously.12 In short, TILs were evaluated as semicontinuous values (0%, 1–9%, 10–49%, 50–74%, 75–100%) by two board-certified pathologists, who were blinded to the outcome, until consensus was reached.12 Evaluations of PD-1 and PD-L1 were performed on TMAs by two board-certified pathologists using the Cell Marque (NAT105) and Ventana (SP142) antibodies. Two cores per marker were evaluated, and the highest value per marker was chosen, given that TMA evaluations of immune checkpoint proteins tend to underestimate the degree of positive staining.23 Staining of ≥1% of lymphocytes was defined as positive, as this is the cut-off used in clinical practice to determine PD-L1 positivity24 (an image of positive staining can be found in the online supplemental file 1). Staining protocols are included in the online supplemental file 1. We defined an activated immune infiltrate as TILs ≥10% and positive staining for at least one of PD-1 or PD-L1 (figure 2). We based this on previous literature indicating that TILs and immune checkpoint molecule expression provide independent information, complementing each other.25 Consequently, combining TILs with checkpoint molecule expression measurements may allow for identifying the most immunogenic tumors compared with either marker alone.26
Figure 2.
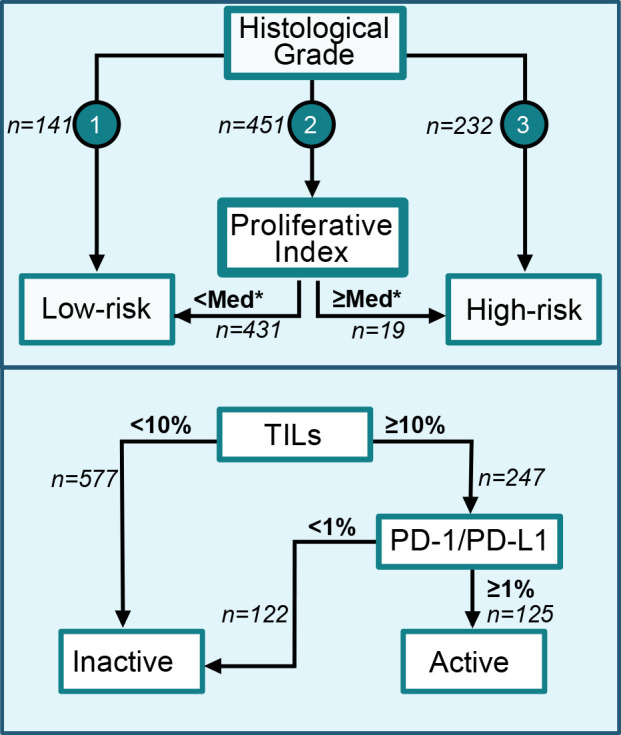
Flow charts for the classification of tumors into low-risk and high-risk tumor-intrinsic groups as well as of immune infiltrates as activated or inactivated/absent. *The median score of grade III tumors was used as the cut-off to classify grade II tumors as low-risk or high-risk. PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; TILs, tumor-infiltrating lymphocytes.
Tumor-intrinsic risk group assessment
We then divided patients into low-risk and high-risk groups depending on histological grade and the previously developed Proliferative Index signature.14 Histological grade I was classified as low-risk and grade III as high-risk. In our previous study, Proliferative Index demonstrated a strong correlation with histological grade and proliferation, and could predict the immune responsiveness of tumors. We hypothesized that grade II tumors are heterogeneous and can be reclassified into high-risk or low-risk as previously suggested.27 Most tumors of the SweBCG91RT cohort were previously classified as grade II. Since the literature indicates that an immune infiltrate’s prognostic effect in low-risk, ER-dominated, cohorts is either absent or unfavorable, we hypothesized that the majority of grade II tumors should be classified as low-risk and not immune-responsive.28–30 This hypothesis was further supported by the fact that the Proliferative Index of grade II tumors resembled grade I tumors more than grade III tumors (online supplemental figure S1). We, therefore, hypothesized that grade II tumors were more similar to grade I tumors regarding the biological implications of an immune infiltrate. To accurately reclassify grade II tumors based on their hypothesized immune responsiveness, we set the cut-off for high-risk grade II tumors at the median Proliferative Index of grade III tumors. The remainder of grade II tumors were classified as low-risk (figure 2). The high cut-off was further motivated by the fact that we did not want to dilute the hypothesized effect size of the high-risk group.
Statistical methods
Time to IBTR as the first event within 10 years from diagnosis was used as the primary endpoint. The aims were to analyze the interaction between an activated immune response and tumor-intrinsic risk group (high-risk or low-risk) on the risk of IBTR and its implications for the benefit from RT. A likelihood-ratio test between regression models with and without an interaction term was used to test the interaction effect. A p value<0.05 was considered significant. P values reported for other analyses, which were not part of the main hypothesis, were not adjusted for multiple hypothesis testing and should be interpreted with caution. Hazard ratios (HRs) with 95% confidence intervals (CIs) presented in tables and the results section were calculated with cause-specific Cox proportional hazards regression to reflect the biological effect of an activated immune infiltrate depending on tumor-intrinsic risk groups in the presence of competing risks. Other recurrences and deaths were considered competing risks for IBTR. Cumulative incidences were used to describe 10-year IBTR rates. Figures of cumulative incidences were created according to the method of Fine and Gray31 and based on the Cox models of subhazards, producing subdistribution HRs. P values for differences in cumulative incidences between compared groups were denoted as PCIF in the plots. Age, tumor size, ER status, and RT were tested in univariable analysis and, if significant, included in multivariable analysis.
The proportional hazards assumption was checked using the Schoenfeld residuals. It was violated for histological grade and RT. Therefore, estimates for these variables should be regarded as the mean effect over the 10-year follow-up period. Due to the violation of the proportional hazards assumption, we also included analyses with a follow-up time of 5 years in the supplement (online supplemental tables S2–S4). The results of these analyses were similar to those presented in the main manuscript, and the proportional hazards assumption was not violated.
Stata V.17.0 was used for analysis (StataCorp. 2017, Stata: Release 17, Statistical Software, StataCorp).
Results
Demographics
In total, 148 (15.4%) tumors were classified as grade I, 573 (59.8%) as grade II, and 237 (24.7%) as grade III. We calculated the previously developed signature, Proliferative Index, and centered and standardized the scores to have a mean of 0 and an SD of 1. We then used the Proliferative Index to classify grade II tumors as high-risk or low-risk (figure 2). Grade I tumors had a median Proliferative Index of −0.70, grade II tumors −0.43, and grade III tumors 1.03 (online supplemental figure S1). A total of 19 (3.3%) of the 573 grade II tumors had a Proliferative Index equal to or higher than the median of grade III tumors and were classified as high-risk.
In total, 139 (55.4%) of high-risk tumors had high TILs (≥10%), 62 (24.7%) had a high PD-1 expression (≥1%), and 101 (40.2%) had a high PD-L1 expression (≥1%) (table 1). A total of 96 (38.2%) tumors were classified as having an activated immune response (TILs ≥10% and PD-1 and/or PD-L1 ≥1%). A total of 75 (36.1%) high-risk tumors were ER negative, 232 (92.4%) tumors were of grade III, and 19 (7.6%) were of grade II (table 1, online supplemental table S5). Tumors with TILs ≥10% and PD-1/PD-L1 expression ≥1% generally had higher TILs than tumors with TILs ≥10% but without PD-1/PD-L1 expression (online supplemental table S6).
Table 1.
Demographics of included patients
| Variables | Low-risk group | High-risk group | ||||
| No RT | RT | Total | No RT | RT | Total | |
| TILs | ||||||
| Low | 240 (81.4%) | 225 (80.9%) | 465 (81.2%) | 63 (44.4%) | 49 (45.0%) | 112 (44.6%) |
| High | 55 (18.6%) | 53 (19.1%) | 108 (18.8%) | 79 (55.6%) | 60 (55.0%) | 139 (55.4%) |
| PD-1 | ||||||
| Low | 267 (90.5%) | 258 (92.8%) | 525 (91.6%) | 109 (76.8%) | 80 (73.4%) | 189 (75.3%) |
| High | 28 (9.5%) | 20 (7.2%) | 48 (8.4%) | 33 (23.2%) | 29 (26.6%) | 62 (24.7%) |
| PD-L1 | ||||||
| Low | 255 (86.4%) | 256 (92.1%) | 511 (89.2%) | 89 (62.7%) | 61 (56.0%) | 150 (59.8%) |
| High | 40 (13.6%) | 22 (7.9%) | 62 (10.8%) | 53 (37.3%) | 48 (44.0%) | 101 (40.2%) |
| Immune activation | ||||||
| Active* | 20 (6.8%) | 9 (3.2%) | 29 (5.1%) | 50 (35.2%) | 46 (42.2%) | 96 (38.2%) |
| Inactive/absent† | 275 (93.2%) | 269 (96.8%) | 544 (94.9%) | 92 (64.8%) | 63 (57.8%) | 155 (61.8%) |
| Subtype | ||||||
| HER2-positive‡ | 6 (2.2%) | 9 (3.5%) | 15 (2.8%) | 19 (16.2%) | 19 (21.6%) | 38 (18.5%) |
| Luminal A | 193 (71%) | 175 (67.8%) | 368 (69.4%) | 26 (22.2%) | 18 (20.5%) | 44 (21.5%) |
| Luminal B | 70 (25.7%) | 72 (27.9%) | 142 (26.8%) | 37 (31.6%) | 27 (30.7%) | 64 (31.2%) |
| Triple-negative | 3 (1.1%) | 2 (0.8%) | 5 (0.9%) | 35 (29.9%) | 24 (27.3%) | 59 (28.8%) |
| ER status | ||||||
| Negative | 7 (2.6%) | 5 (1.9%) | 12 (2.3%) | 42 (35.3%) | 33 (37.1%) | 75 (36.1%) |
| Positive | 266 (97.4%) | 254 (98.1%) | 520 (97.7%) | 77 (64.7%) | 56 (62.9%) | 133 (63.9%) |
| PgR status | ||||||
| Negative | 41 (15.0%) | 48 (18.5%) | 89 (16.7%) | 59 (49.6%) | 48 (53.9%) | 107 (51.4%) |
| Positive | 232 (85.0%) | 211 (81.5%) | 443 (83.3%) | 60 (50.4%) | 41 (46.1%) | 101 (48.6%) |
| Histological grade | ||||||
| Grade I | 68 (23.1%) | 73 (26.3%) | 141 (24.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Grade II | 227 (76.9%) | 205 (73.7%) | 432 (75.4%) | 10 (7.0%) | 9 (8.3%) | 19 (7.6%) |
| Grade III | 0 (0%) | 0 (0%) | 0 (0%) | 132 (93.0%) | 100 (91.7%) | 232 (92.4%) |
| Endocrine therapy | ||||||
| No hormone therapy | 254 (92.7%) | 249 (95.8%) | 503 (94.2%) | 105 (88.2%) | 80 (88.9%) | 185 (88.5%) |
| Hormone therapy | 20 (7.3%) | 11 (4.2%) | 31 (5.8%) | 14 (11.8%) | 10 (11.1%) | 24 (11.5%) |
| Chemotherapy | ||||||
| No chemotherapy | 274 (100%) | 259 (99.6%) | 533 (99.8%) | 113 (95.0%) | 87 (96.7%) | 200 (95.7%) |
| Chemotherapy | 0 (0%) | 1 (0.4%) | 1 (0.2%) | 6 (5.0%) | 3 (3.3%) | 9 (4.3%) |
| IBTR within 5 years§ | ||||||
| No IBTR | 240 (81.4%) | 255 (91.7%) | 495 (86.4%) | 96 (67.6%) | 86 (78.9%) | 182 (72.5%) |
| IBTR | 37 (12.5%) | 7 (2.5%) | 44 (7.7%) | 27 (19.0%) | 8 (7.3%) | 35 (13.9%) |
| Censored | 18 (6.1%) | 16 (5.8%) | 34 (5.9%) | 19 (13.4%) | 15 (13.8%) | 34 (13.5%) |
| IBTR within 10 years§ | ||||||
| No IBTR | 206 (69.8%) | 221 (79.5%) | 427 (74.5%) | 80 (56.3%) | 69 (63.3%) | 149 (59.4%) |
| IBTR | 54 (18.3%) | 23 (8.3%) | 77 (13.4%) | 33 (23.2%) | 10 (9.2%) | 43 (17.1%) |
| Censored | 35 (11.9%) | 34 (12.2%) | 69 (12.0%) | 29 (20.4%) | 30 (27.5%) | 59 (23.5%) |
*Defined as TILs ≥10% and PD-L1 and/or PD-1 ≥1%.
†Defined as TILs <10% or TILs ≥10% but PD-L1 and PD-1 <1%.
‡Includes both ER-positive and ER-negative tumors.
§Reported as absolute frequencies rather than cumulative incidences.
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; IBTR, ipsilateral breast tumor recurrence; PD-1, programmed cell death protein-1 ; PD-L1, programmed death ligand-1 ; PgR, progesterone receptor; RT, radiotherapy; TILs, tumor-infiltrating lymphocytes.
In the low-risk group, high TILs were seen among 108 tumors (18.8%), high PD-1 expression among 48 (8.4%) tumors, and high PD-L1 expression among 62 (10.8%) tumors (table 1). In total, 29 (5.1%) tumors were classified as having an activated immune response. Among low-risk tumors, 12 (2.3%) were ER-negative, 141 (24.6%) of grade I, and 432 (75.4%) of grade II.
Prognostic effect
In total, 17.2% (13.1–22.5) of patients in the high-risk group and 13.7% (11.1–16.8) of patients in the low-risk group developed an IBTR within 10 years. High-risk tumors with an active immune response had an IBTR rate of 8.4% (4.3–16.1), while high-risk tumors without an active immune infiltrate had an IBTR rate of 22.8% (16.9–30.2). Among high-risk tumors, an activated immune infiltrate was associated with a reduced risk of IBTR in univariable (HR 0.34, 95% CI 0.16 to 0.73, p=0.006) and multivariable (HR 0.33, 95% CI 0.15 to 0.72, p=0.005) analysis (table 2).
Table 2.
Cox proportional hazard rate regression. Ten-year follow-up of ipsilateral breast tumor recurrence (IBTR) among low-risk and high-risk patients
| Variable | Low-risk group (n=573) | High-risk group (n=251) | ||||||||
| # of IBTR/ | Univariable Cox regression | P value | Multivariable Cox regression | P value | # of IBTR/ | Univariable Cox regression | P value | Multivariable Cox regression | P value | |
| # of patients | HR (95% CI) | HR (95% CI) | # of patients | HR (95% CI) | HR (95% CI) | |||||
| Immune system | ||||||||||
| Not activated | 71/544 | 1.0 | 1.0 | 35/155 | 1.0 | 1.0 | ||||
| Activated | 6/29 | 2.0 (0.87 to 4.6) | 0.100 | 1.8 (0.79 to 4.2) | 0.159 | 8/96 | 0.34 (0.16 to 0.73) | 0.006 | 0.33 (0.15 to 0.72) | 0.005 |
| Age (cont.) | 77/573 | 0.98 (0.95 to 1.0) | 0.102 | – | – | 43/251 | 0.97 (0.94 to 0.99) | 0.019 | 0.97 (0.94 to 0.99) | 0.016 |
| Tumor size (cont.) | 71/530 | 1.0 (0.96 to 1.1) | 0.739 | – | – | 39/208 | 0.98 (0.93 to 1.0) | 0.518 | – | – |
| ER | ||||||||||
| Negative | 4/9 | 1.0 | 1.0 | 11/85 | 1.0 | |||||
| Positive | 73/561 | 0.21 (0.08 to 0.56) | 0.002 | 0.17 (0.06 to 0.46) | 0.001 | 32/165 | 1.4 (0.72 to 2.8) | 0.302 | – | – |
| RT | ||||||||||
| No | 54/295 | 1.0 | 1.0 | 33/142 | 1.0 | 1.0 | ||||
| Yes | 23/278 | 0.41 (0.25 to 0.67) | <0.001 | 0.40 (0.25 to 0.66) | <0.001 | 10/109 | 0.36 (0.18 to 0.74) | 0.005 | 0.42 (0.20 to 0.85) | 0.017 |
ER, estrogen receptor; IBTR, ipsilateral breast tumor recurrence; RT, radiotherapy.
Low-risk tumors with an activated immune infiltrate had a 10-year IBTR rate of 20.9% (10.0–40.7) compared with an IBTR rate of 13.3% (10.7–16.5) among low-risk tumors without an activated immune infiltrate. No significant difference in risk IBTR among low-risk tumors was seen for an activated immune infiltrate (univariable: 2.0, 95% CI 0.87 to 4.6, p=0.100, multivariable: HR 1.8, 95% CI 0.79 to 4.2, p=0.159) compared with not having an activated immune infiltrate (HR 1.0) (table 2. The interaction between immunological activity and risk group was significant in univariable (p=0.005) and multivariable (p=0.007) analysis (table 3).
Table 3.
Cox proportional hazard rate regression. Ten-year follow-up of ipsilateral breast tumor recurrence (IBTR)
| Variables | No. of IBTRs/no. of patients | Univariable Cox regression | Multivariable Cox regression | ||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Combination of immune group and risk group | |||||
| Not activated, low risk | 71/544 | 1.0 | 1.0 | ||
| Not activated, high risk | 35/155 | 2.1 (1.4 to 3.1) | <0.001 | 1.9 (1.3 to 2.9) | 0.002 |
| Activated, low risk | 6/29 | 2.0 (0.87 to 4.6) | 0.105 | 1.8 (0.76 to 4.0) | 0.189 |
| Activated, high risk | 8/96 | 0.68 (0.33 to 1.4) | 0.306 | 0.63 (0.30 to 1.3) | 0.217 |
| Interaction | 0.005* | 0.007* | |||
| Age (cont.) | 120/824 | 0.97 (0.95 to 0.99) | 0.005 | 0.97 (0.96 to 0.99) | 0.003 |
| Tumor size (cont.) | 110/738 | 1.01 (0.97 to 1.04) | 0.761 | – | |
| ER status | |||||
| Negative | 15/94 | 1.0 | – | ||
| Positive | 105/726 | 0.78 (0.46 to 1.35) | 0.376 | – | |
| RT | |||||
| No | 87/437 | 1.0 | 1.0 | ||
| Yes | 33/387 | 0.39 (0.26 to 0.58) | <0.001 | 0.41 (0.27 to 0.61) | <0.001 |
*Likelihood-ratio test.
ER, estrogen receptor; IBTR, ipsilateral breast tumor recurrence; RT, radiotherapy.
Benefit from RT
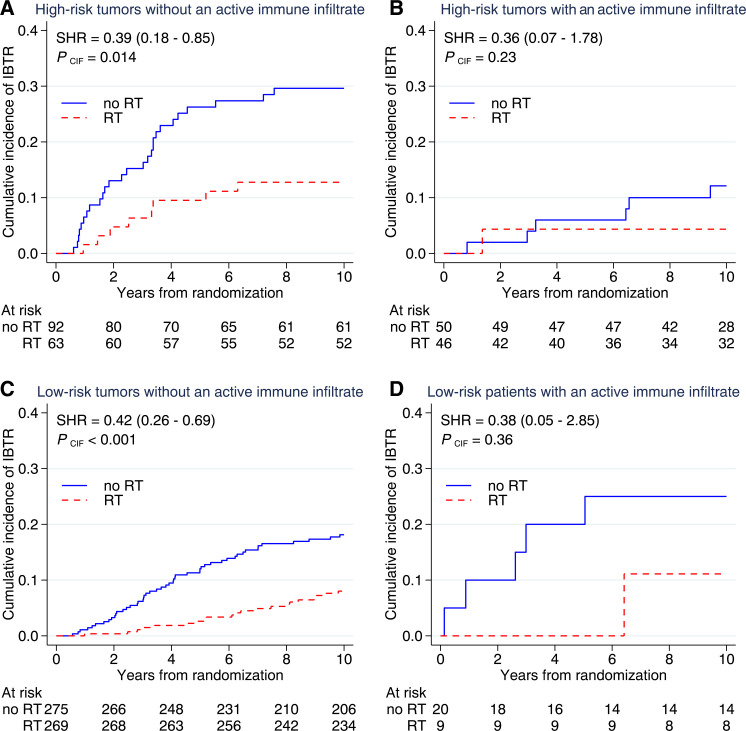
A non-significant benefit from RT was seen among high-risk tumors with an activated immune infiltrate (HR 0.34, 95% CI 0.07 to 1.67, p=0.182), while a significant benefit was observed among high-risk tumors without an activated immune infiltrate (HR 0.40, 95% CI 0.18 to 0.88, p=0.022). Among low-risk tumors with an activated immune infiltrate, the estimates for RT benefit (HR 0.40, 95% CI 0.05 to 3.44, p=0.403) were similar to those of low-risk tumors without an activated immune infiltrate (HR 0.42, 95% CI 0.25 to 0.69, p=0.001).
Figure 3 illustrates the cumulative incidences depending on RT, immune activation, and tumor-intrinsic risk group. High-risk tumors with an activated immune response had a 10-year incidence of IBTR of 12.1% (5.6–25.0) without RT and 4.4% (1.1–16.3) with RT. This can be contrasted against high-risk tumors with an absent immune response, where the 10-year incidence of IBTR was 29.6% (21.4–40.2) without RT and 12.8% (6.6–23.9) with RT. Low-risk tumors with an activated immune response had a 10-year IBTR incidence of 25.0% (11.2–50.0) without RT and 11.1% (1.6–56.7) with RT, while low-risk tumors without an activated immune infiltrate had a 10-year incidence of IBTR of 18.1% (14.0–23.3) without RT and 8.4% (5.6–12.5) with RT.
Figure 3.
Cumulative incidences among high-risk and low-risk tumors with and without an activated immune response. High-risk tumors were defined as histological grade III or histological grade II with a high Proliferative Index. An activated immune response was defined as tumor-infiltrating lymphocytes ≥10% and ≥1% of lymphocytes positive for programmed cell death protein-1 and/or programmed death ligand-1. IBTR, ipsilateral breast tumor recurrence; RT, radiotherapy; SHR, subdistribution hazard ratio; CIF, cumulative incidence function.
Exploratory analyses
As a post hoc exploratory analysis, we compared the high-risk groups with TILs 10–49% and 50–100% to investigate a potential dose-response relationship. Unirradiated patients with TILs 10–49% had a 10-year cumulative IBTR incidence of 15% (0.07–0.29). Unirradiated patients with TILs 50–100% had a lower, but not significantly different, cumulative IBTR incidence of 13% (0.05–0.31) (online supplemental figure S2).
Finally, to verify the stability of the results, we re-ran the main analyses excluding patients treated with systemic therapy. The findings remained stable (online supplemental tables S7 and S8).
Discussion
The integration of immunological and tumor-intrinsic factors enables the successful stratification of high-risk tumors regarding the risk of IBTR. The present study shows that this can generally be achieved with variables already used in the clinic and that aggressive tumors, including luminal subtypes, with an active immune response, have a low risk of IBTR even without RT boost and systemic therapy. High-risk tumors with activated immune infiltrates had the lowest rates of IBTR, highlighting the possibility to de-intensify locoregional treatment.
Research on biological predictors to inform RT de-escalation is ongoing. The recently published POLAR classifier may identify ER-positive HER2-negative tumors suited for RT omission.32 Genes associated with proliferation were associated with an increased risk of locoregional recurrence. The current study highlights a parallel de-escalation pathway on the opposite side of the proliferation spectrum, where traditionally regarded high-risk tumors, irrespective of ER status, can be downgraded if they benefit from an activated antitumor immune response. Patients with high-risk tumors with an activated immune infiltrate had a relatively low risk of IBTR unirradiated (12.1%) and irradiated (4.4%), despite standard RT (ie, without an RT boost) and a low frequency of systemic therapy. These tumors may have a delayed local and systemic dissemination preoperatively and inhibited regrowth of postoperative residual disease, reducing the need for RT treatment. With modern systemic treatment, the 10-year incidence of IBTR may be below 10% without RT. The findings align with another recent study showing that young patients with triple-negative breast cancer and high TILs have a surprisingly good prognosis without adjuvant therapy.5 Immune-responsive tumors with very high TIL levels (eg, ≥50%) may represent an RT omission group, while moderately increased TILs (eg, 10–49%) could justify RT boost omission. Although we hypothesize that low-risk tumors are best stratified for treatment de-escalation using proliferation measurements, the role of the immune response among these is not fully understood. We and others have previously shown that global measures of immune activation confer a favorable prognosis only among high-risk tumors.14 33 34 However, it cannot be excluded that activation of certain immune response subcomponents may still benefit low-risk tumors. For example, the humoral immune system may reduce the recurrence risk in luminal tumors,35 which conforms with findings of B-cell-related genes in POLAR predicting a favorable prognosis.32
We have previously shown that integrating tumor-intrinsic factors in the assessment of immunological biomarkers can improve the identification of high-risk tumors with different needs for RT. 14 CD8+T cells, the primary effector cell of antitumor immunity, recognize and are activated by neoantigens generated by tumor mutations.7 Therefore, tumor-intrinsic factors that correlate with proliferation and tumor mutational burden (TMB) may inform the likelihood that an immune infiltrate represents an active antitumoral immune response.34 Histological grade correlates with proliferation and TMB,36 and we hypothesized that histological grade might capture tumor-intrinsic qualities necessary to understand the biological influence of an immune infiltrate. In the SweBCG91RT cohort, PD-1 and/or PD-L1 were expressed by the majority of high-risk tumors with high TILs. Conversely, high TILs were less frequently associated with PD-1/PD-L1 expression among low-risk tumors, indicating that an immune infiltrate in these tumors has other biological implications. This is supported by studies showing an absent or unfavorable prognostic effect of immune infiltrates in low-risk tumors.28–30
Despite the overwhelming focus on triple-negative and HER2-positive subtypes in TILs research, most tumors with TILs are ER-positive.11 However, the lack of understanding of how TILs influence tumor progression among ER-positive tumors has prevented TILs from being used as a biomarker in this group.11 A better understanding may enable the implementation of immunotherapy on a subset of immunogenic ER-positive tumors.11 We found that the majority of tumors classified as high-risk, and deriving a significant benefit from an activated immune infiltrate, were ER-positive (63.9%), echoing the unmet potential for using TILs as a biomarker among these tumors.11 The International Immuno-Oncology Biomarker Working Group highlights the need for more research on TILs among ER-positive subtypes, stratifying analyses by luminal A and luminal B.11 However, our results indicate that there may exist heterogeneity within these subtypes, as all subtypes were relatively equally represented in the high-risk group. Our findings add a layer of complexity to previous observations37 by suggesting that it may not be subtype, but instead characteristics that can in part be approximated by subtype, that predict the biological influence of an immune infiltrate. These findings align with a previous study where luminal B tumors with aggressive tumor characteristics demonstrated immunotherapy responsiveness.19 We believe additional measures of tumor aggressiveness, such as histological grade or proliferation, are needed to accurately predict the implications of an immune infiltrate, particularly in the case of luminal B tumors, where the degree of proliferation can vary considerably.18 It remains to be determined if tumor-intrinsic characteristics predict immune responsiveness also in cohorts dominated by non-luminal subtypes.
Assessments of the PD-1/PD-L1 pathway are today used as biomarkers for immunotherapy in metastatic triple-negative breast cancer.38 Expression is associated with an improved prognosis,39 despite the inherent immunosuppressive effects, likely due to its association with an active immune response. PD-1 is expressed on activated T cells,40 and PD-L1, expressed by a wide range of cells, for example, T-regulatory cells and tumor cells, is upregulated by inflammatory signaling.41 Furthermore, measurements of the PD-1/PD-L1 pathway provide independent information in addition to TILs.26 For this reason, we used high TILs combined with the expression of PD-1 or PD-L1 to characterize an active immune response.
We used histological grade and a gene expression-based proliferation signature as tumor-intrinsic predictors of immune responsiveness.42 However, additional tumor characteristics should be considered. One such factor is HER2 status, which has emerged as a key biomarker in breast cancer. HER2-positive tumors are considered immunogenic, and anti-HER2 therapy functions partly by inducing an antitumoral immune response.42 43 Unfortunately, due to the low number of HER2-positive tumors and lack of anti-HER2 therapy, we did not try to answer whether HER2 positivity should be included as a variable predictive of immune responsiveness. Therefore, future studies should investigate whether HER2 positivity and additional tumor-intrinsic characteristics, such as TMB, provide independent information beyond histological grade and tumor proliferation regarding immune responsiveness.
There are several weaknesses in the present study. First, our question involved post hoc analyses of subgroups, which reduces the power and should be viewed as hypothesis-generating. The low-risk group with an active immune infiltrate was small, why findings pertaining to this group should be interpreted cautiously. Second, many patients would have received a different therapy regimen had they been diagnosed today. No patients in the SweBCG91RT study received an RT boost, although some of them would be recommended a boost in the current situation. In addition, few patients received systemic treatment, which would likely have significantly reduced the risk of IBTR.44 Furthermore, systemic anti-HER2 therapy and chemotherapy treatment would probably have produced a differential benefit for patients, with highly proliferative immunogenic tumors showing the best response.45 46 While the above limits the generalizability of our findings, it also indicates that modern treatment would preferentially have reduced the risk of IBTR in the high-risk immunogenic group, further supporting de-escalation of RT as a valid strategy for these patients. Nevertheless, our findings apply primarily to a setting free of adjuvant systemic therapy. The high cut-off used to classify grade II tumors as high-risk resulted in only a minority of these tumors being classified as such and did not allow for thoroughly investigating immune responsiveness along the spectrum of tumor aggressiveness among grade II tumors. We used this high cut-off based on the hypothesis that most grade II tumors should be classified as low-risk and to avoid dilution of the hypothesized effect size of the high-risk group. We did not test additional cut-offs and cannot determine the proportion of grade II tumors likely to benefit from an immune infiltrate. This should be investigated in future studies. However, the finding that grade II tumors resemble grade I tumors more than grade III tumors in terms of a gene expression signature designed to measure immune responsiveness indicates that most should be classified as low-risk. Finally, the use of TMAs may miss tumor heterogeneity. Previous studies have shown that around three TMAs may be sufficient to categorize a tumor as having high or low TILs.47 Since we used four TMAs to assess the activity of the PD-1/PD-L1 axis, we believe the risk of missing tumor heterogeneity is reduced, although not eliminated.
There is a large variation in analytical sensitivity between different PD-L1 immunohistochemistry (IHC) assays, with SP142, used in the present study, shown to have poor sensitivity.48 Consequently, some tumors classified as PD-L1 negative were likely false negatives, indicating that findings should be interpreted cautiously. The optimal IHC assay identifying immunogenic tumors would preferably have a higher sensitivity than SP142. Furthermore, a potential added value to TILs of additional immunological markers, such as PD-1/PD-L1 expression,25 may be partly or entirely explained by an association with even higher TILs. Therefore, assessing TILs as a continuous variable on whole sections may be a sufficiently robust measurement to identify tumors with different immune activation degrees and tailor therapy accordingly.
In conclusion, high-risk tumors with an activated immune infiltrate have a surprisingly good prognosis in terms of local recurrences. The risk reduction regarding IBTR conferred by an activated immune infiltrate among these tumors may be comparable to treatment with RT. Therefore, we hypothesize that these patients do well with the de-escalation of RT treatment. Our findings likely apply to low-risk early breast cancer cohorts dominated by ER-positive tumors.
jitc-2022-006618supp002.pdf (232.8KB, pdf)
Acknowledgments
We wish to thank Kristina Lövgren for IHC stainings.
Footnotes
Contributors: AST: Conceptualization, Software, Supervision, Funding acquisition, Validation, Formal analysis, Investigation, Methodology, Visualization, Project administration, Writing—original draft, Writing—review and editing, Data curation. MS: Supervision, Writing—review, and editing. LT: Formal analysis, Writing—review and editing. EN: Resources, Writing—review and editing. FK: Resources, Writing—review and editing. AK: Supervision, Writing—review and editing. DL: Supervision, Writing—review and editing. EH: Formal analysis, Visualization, Methodology, Funding acquisition, Investigation, Validation, Writing—review and editing. PK: Supervision, Visualization, Funding acquisition, Resources, Methodology, Writing—review and editing, Guarantor.
Funding: This work was supported by the Swedish state under the agreement between the Swedish government and the county councils; ALF agreement Grant No. ALFGBG-965020; the Swedish Cancer Society (Cancerfonden) Grant No. Can- 21 1889-S; the King Gustav V Jubilee Clinic Foundation (Stiftelsen Jubileumsklinikens Forskningsfond mot Cancer) Grant No. 2021-351.
Competing interests: PK: Consulting or advisory role for AstraZeneca. Contract with PFS Genomics/Exact Sciences regarding genomic profiling. Co-inventor on patent applications. Contract with Prelude Dx. EH: Contract with PFS Genomics/Exact Sciences regarding genomic profiling. Co-inventor on patent applications. Contract with Prelude Dx. AST: Co-inventor on patent applications. Contract with Prelude Dx.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Gene expression data from the SweBCG91RT cohort has been made available in the Gene Expression Omnibus database (GSE119295). However, due to regulations of the ethical review board and of laws related to patient privacy, all clinical information has not been made publicly available. The IHC data used in this study is/are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Regional Ethical Review Board of Western Sweden (approval numbers 2010/127 and 2015/548). Participants gave informed consent to participate in the study before taking part.
References
- 1.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16. 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five national surgical adjuvant breast and bowel project protocols of node-negative breast cancer. J Clin Oncol 2009;27:2466–73. 10.1200/JCO.2008.19.8424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8–30. 10.1093/annonc/mdv298 [DOI] [PubMed] [Google Scholar]
- 4.Gulstene S, Raziee H. Radiation boost after adjuvant whole breast radiotherapy: does evidence support practice for close margin and altered fractionation? Front Oncol 2020;10:772. 10.3389/fonc.2020.00772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong VMT, Wang Y, Ter Hoeve ND, et al. Prognostic value of stromal tumor-infiltrating lymphocytes in young, node-negative, triple-negative breast cancer patients who did not receive (Neo) adjuvant systemic therapy. J Clin Oncol 2022;40:2361–74. 10.1200/JCO.21.01536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali HR, Provenzano E, Dawson S-J, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25:1536–43. 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]
- 7.Tran E, Robbins PF, Rosenberg SA. Final common pathway of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol 2017;18:255–62. 10.1038/ni.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catakovic K, Klieser E, Neureiter D, et al. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal 2017;15:1. 10.1186/s12964-016-0160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disis ML. Immune regulation of cancer. J Clin Oncol 2010;28:4531–8. 10.1200/JCO.2009.27.2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeong J, Lim JCT, Lee B, et al. Prognostic value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer 2019;7:34. 10.1186/s40425-019-0499-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Bairi K, Haynes HR, Blackley E, et al. The tale of tils in breast cancer: a report from the international immuno-oncology biomarker working group. NPJ Breast Cancer 2021;7:150. 10.1038/s41523-021-00346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovács A, Stenmark Tullberg A, Werner Rönnerman E, et al. Effect of radiotherapy after breast-conserving surgery depending on the presence of tumor-infiltrating lymphocytes: a long-term follow-up of the SweBCG91RT randomized trial. J Clin Oncol 2019;37:1179–87. 10.1200/JCO.18.02157 [DOI] [PubMed] [Google Scholar]
- 13.Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 2010;12:207. 10.1186/bcr2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenmark Tullberg A, Sjöström M, Niméus E, et al. Integrating tumor-intrinsic and Immunologic factors to identify Immunogenic breast cancers from a low-risk cohort: Results from the randomized Swebcg91Rt trial. Clin Cancer Res 2023;29:1783–93. 10.1158/1078-0432.CCR-22-2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. the value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 2002;41:154–61. 10.1046/j.1365-2559.2002.14691.x [DOI] [PubMed] [Google Scholar]
- 16.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 2008;10:R65. 10.1186/bcr2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buus R, Sestak I, Kronenwett S, et al. Molecular Drivers of Onco type DX, Prosigna, EndoPredict, and the Breast Cancer Index: A TransATAC Study. J Clin Oncol 2021;39:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Martinez A, Pascual T, Perrone G, et al. Limitations in predicting PAM50 intrinsic subtype and risk of relapse score with Ki67 in estrogen receptor-positive HER2-negative breast cancer. Oncotarget 2017;8:21930–7. 10.18632/oncotarget.15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieci MV, Guarneri V, Tosi A, et al. Neoadjuvant chemotherapy and immunotherapy in luminal B-like breast cancer: results of the phase II GIADA trial. Clin Cancer Res 2022;28:308–17. 10.1158/1078-0432.CCR-21-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malmström P, Holmberg L, Anderson H, et al. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: a randomised clinical trial in a population with access to public mammography screening. Eur J Cancer 2003;39:1690–7. 10.1016/s0959-8049(03)00324-1 [DOI] [PubMed] [Google Scholar]
- 21.Killander F, Karlsson P, Anderson H, et al. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. fifteen-year results from the swedish breast cancer group randomised trial, swebcg 91 RT. Eur J Cancer 2016;67:57–65. 10.1016/j.ejca.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 22.Sjöström M, Lundstedt D, Hartman L, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol 2017;35:3222–9. 10.1200/JCO.2017.72.7263 [DOI] [PubMed] [Google Scholar]
- 23.Sobral-Leite M, Van de Vijver K, Michaut M, et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018;7:e1509820. 10.1080/2162402X.2018.1509820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 25.Loi S, Michiels S, Adams S, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol 2021;32:1236–44. 10.1016/j.annonc.2021.07.007 [DOI] [PubMed] [Google Scholar]
- 26.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019;5:74–82. 10.1001/jamaoncol.2018.4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 2006;98:262–72. 10.1093/jnci/djj052 [DOI] [PubMed] [Google Scholar]
- 28.Sobral-Leite M, Salomon I, Opdam M, et al. Cancer-immune interactions in ER-positive breast cancers: PI3K pathway alterations and tumor-infiltrating lymphocytes. Breast Cancer Res 2019;21:90. 10.1186/s13058-019-1176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Foulkes WD, Leung S, et al. Prognostic significance of foxp3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 2014;16:432. 10.1186/s13058-014-0432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson A, Yu NY, Iftimi A, et al. Clinical and molecular characteristics of estrogen receptor-positive ultralow risk breast cancer tumors identified by the 70-gene signature. Int J Cancer 2022;150:2072–82. 10.1002/ijc.33969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 32.Sjöström M, Fyles A, Liu F-F, et al. Development and validation of a genomic profile for the omission of local adjuvant radiation in breast cancer. J Clin Oncol 2023;41:1533–40. 10.1200/JCO.22.00655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M, Böhm D, von Törne C, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 2008;68:5405–13. 10.1158/0008-5472.CAN-07-5206 [DOI] [PubMed] [Google Scholar]
- 34.Miller LD, Chou JA, Black MA, et al. Immunogenic subtypes of breast cancer delineated by gene classifiers of immune responsiveness. Cancer Immunol Res 2016;4:600–10. 10.1158/2326-6066.CIR-15-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klopfenstein Q, Derangère V, Arnould L, et al. Evaluation of tumor immune contexture among intrinsic molecular subtypes helps to predict outcome in early breast cancer. J Immunother Cancer 2021;9:e002036. 10.1136/jitc-2020-002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budczies J, Bockmayr M, Denkert C, et al. Classical pathology and mutational load of breast cancer-integration of two worlds. J Pathol Clin Res 2015;1:225–38. 10.1002/cjp2.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-Infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 38.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019;7:278. 10.1186/s40425-019-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L, Wang Y, Wu Q, et al. Association between levels of tumor-infiltrating lymphocytes in different subtypes of primary breast tumors and prognostic outcomes: a meta-analysis. BMC Womens Health 2020;20:194. 10.1186/s12905-020-01038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gros A, Robbins PF, Yao X. PD-1 identifies the patient-specific CD8⁺ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014;124:2246–59. 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep 2019;29:3766. 10.1016/j.celrep.2019.11.113 [DOI] [PubMed] [Google Scholar]
- 42.Griguolo G, Pascual T, Dieci MV, et al. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer 2019;7:90. 10.1186/s40425-019-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mortenson ED, Fu Y-X. Adaptive immune responses and HER2/neu positive breast cancer. Curr Pathobiol Rep 2013;1:37–42. 10.1007/s40139-012-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019;394:2155–64. 10.1016/S0140-6736(19)32514-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: big 02-98. J Clin Oncol 2013;31:860–7. 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 46.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the finher trial. Ann Oncol 2014;25:1544–50. 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 47.Lee ATJ, Chew W, Wilding CP, et al. The adequacy of tissue microarrays in the assessment of inter- and intra-tumoural heterogeneity of infiltrating lymphocyte burden in leiomyosarcoma. Sci Rep 2019;9:14602. 10.1038/s41598-019-50888-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sompuram SR, Torlakovic EE, ’t Hart NA, et al. Quantitative comparison of PD-L1 IHC assays against NIST standard reference material 1934. Mod Pathol 2022;35:326–32. 10.1038/s41379-021-00884-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006618supp003.pdf (59.3KB, pdf)
jitc-2022-006618supp001.pdf (1.1MB, pdf)
jitc-2022-006618supp002.pdf (232.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Gene expression data from the SweBCG91RT cohort has been made available in the Gene Expression Omnibus database (GSE119295). However, due to regulations of the ethical review board and of laws related to patient privacy, all clinical information has not been made publicly available. The IHC data used in this study is/are available from the corresponding author upon reasonable request.