Abstract
Introduction
Both B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-pro-BNP) are used to identify patients at risk of perioperative vascular events, but prognostic thresholds have been established in a large prospective cohort for NT-pro-BNP only. We designed this study to inform perioperative risk interpretation of BNP values. Our primary objective is to validate a formula to convert BNP to NT-pro-BNP concentrations before non-cardiac surgery. The secondary objective is to determine the association between BNP categories (established based on conversion from NT-pro-BNP categories) and a composite outcome of myocardial injury after non-cardiac surgery (MINS) and vascular death.
Methods and analysis
This is a single-centre, prospective cohort study in patients undergoing non-cardiac surgery who are >65 years old, Revised Cardiac Risk Index ≥1 or >45 years old with significant cardiovascular disease. BNP and NT-pro-BNP will be measured preoperatively, and troponin measurements will be analysed on postoperative days 1, 2 and 3. MINS and vascular death will be ascertained up to 30 days after surgery. The primary analyses will compare measured NT-pro-BNP values to those predicted by an existing formula (from a non-surgical population) based on BNP concentrations and patient characteristics, and recalibrate and update the formula with additional variables. Secondary analyses will estimate the relationship between categories of measured BNP (corresponding to established NT-pro-BNP thresholds) and the composite of MINS and vascular death. The target sample size of 431 patients is based on our primary analysis (assessing the conversion formula).
Ethics and dissemination
Ethics approval has been obtained by the Queen’s University Health Sciences Research Ethics Board, and all participants will provide informed consent for participation in the study. The results will be submitted for publication in conferences and in a peer-reviewed journal, and will inform perioperative vascular risk interpretation of preoperative BNP.
Trial registration number
Keywords: Adult anaesthesia, SURGERY, CARDIOLOGY, INTERNAL MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a pragmatic study design using existing preoperative assessment guidelines to recruit patients.
Our primary analysis will examine the relationship between B-type natriuretic peptide (BNP) and N-terminal-pro-BNP concentrations in the perioperative setting.
This study may be underpowered to examine our secondary outcome, a composite outcome of myocardial injury after non-cardiac surgery (MINS) and vascular death.
Measuring troponin based on baseline cardiovascular risk on postoperative days 1, 2 and 3 may underestimate the overall incidence of MINS during the first 30 days postoperatively.
Introduction
Background and rationale
Each year, millions of patients worldwide die within 30 days of non-cardiac surgery, with a large proportion of deaths attributed to complications of cardiac ischaemia.1–3 While a subset of patients with myocardial injury in the perioperative period develop ischaemic features that have classically been associated with myocardial infarction, 84%–93% of patients have no identifiable ischaemic symptoms.4 5 Myocardial injury after non-cardiac surgery (MINS) includes both myocardial infarction as well as the more subtle presentation of myocardial injury diagnosed during or within 30 days after non-cardiac surgery by a rise in troponin.5–7 There is a growing body of evidence demonstrating a strong and independent relationship between MINS and major vascular complications and mortality in the postoperative period, even in the absence of ischaemic features.8
Given the challenge of identifying a frequently clinically silent injury that has been found to be an independent predictor of both morbidity and mortality after surgery, much effort has focused on determining which patients are most likely to suffer MINS. Several risk factors for the development of MINS have been identified, including Revised Cardiac Risk Index (RCRI) score >1, age >65 or age 45–64 with significant cardiovascular disease.9 Cardiac biomarkers, including brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-pro-BNP), offer a non-invasive and low cost method of refining preoperative risk stratification, providing incremental prognostic information when added to traditional multivariate prediction models such as RCRI.9–11
Previous guidelines have recommended a BNP threshold of >92 mg/L or NT-pro-BNP threshold of >300 ng/L, to predict increased risk of MINS.9 While risk stratification using BNP remains dichotomous, four prognostically important NT-pro-BNP risk categories have recently been established that (A) predict elevated risk of MINS and vascular death at a lower thresholds than previously identified and (B) classify risk into four distinct categories to further guide risk assessment and discussions.10 Thus, despite its wide use, a large knowledge gap exists when using BNP for perioperative risk assessment, and there is a need to better define the relationship between BNP, NT-pro-BNP and perioperative risk.
To our knowledge, the relationship between BNP and NT-pro-BNP has not been examined in the perioperative period. Although BNP and NT-pro-BNP are biologically related peptides in that they reflect the same cardiac hormonal activity, there is significant variability between BNP and NT-pro-BNP concentrations within individuals.12 This variability is predominantly explained by differences in their clearance pathways, as well as patient factors, including age, body mass index (BMI), renal function, anaemia and history of atrial fibrillation.12 Kasahara et al developed a BNP to NT-pro-BNP conversion formula that considers these variables among patients with heart failure.13 External validation of this conversion formula using an independent dataset is essential to understanding the performance and clinical utility of this formula and acceptance of the formula into clinical practice. Further, a validated conversion formula between BNP and NT-pro-BNP would enable adherence to the most evidence based perioperative risk stratification guidelines, regardless of whether BNP or NT-pro-BNP testing is available at each individual hospital or laboratory.
Study objectives
The primary objective of this study is to assess the performance of the BNP to NT-pro-BNP conversion formula13 when applied to an independent dataset in the perioperative period, and to correlate validated BNP levels with the recently established NT-pro-BNP categories that have been found to enhance perioperative risk prediction.10 The secondary objective of this study is to examine the association between BNP, NT-pro-BNP and combined MINS and vascular death in the first 30 days postoperatively.
Methods and analysis
Study design
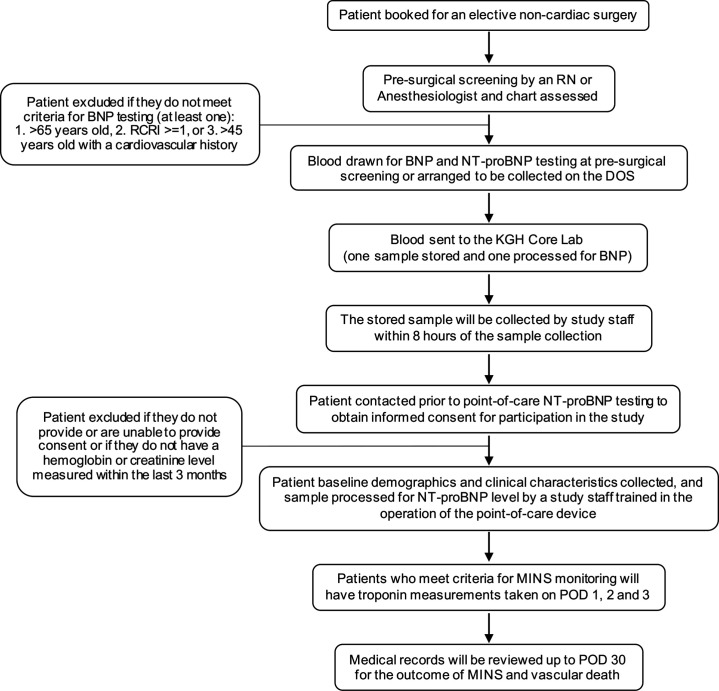
This is a single-centre, prospective study to determine the correlation and comparative thresholds between NT-pro-BNP and BNP values. This will involve simultaneous serum sampling for both BNP and NT-pro-BNP at the preoperative presurgical screening centre or the same day admission centre on the day of surgery at Kingston Health Sciences Centre in Kingston, Ontario. High sensitivity troponin I measurements will be taken on postoperative days (PODs) 1, 2 and 3 in patients who have an elevated BNP result based on local guidelines (>92 ng/L), and the composite outcome of MINS (high sensitivity troponin I>30 ng/L) and vascular death will be determined by an assessor blinded to the BNP/NT-pro-BNP results at POD 30. Figure 1 outlines the steps for patient recruitment and data collection.
Figure 1.
Steps for patient recruitment and data collection. BNP, B-type natriuretic peptide; DOS, day of surgery; KGH, Kingston General Hospital; MINS, myocardial injury after non-cardiac surgery; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; POD, postoperative day; RCRI, Revised Cardiac Risk Index; RN, registered nurse.
Patient recruitment and inclusion criteria
All patients who meet local guidelines for BNP testing will have an additional 5 mL of blood drawn in a heparinised tube and sent to the core laboratory. Eligibility for BNP testing includes patients undergoing elective non-cardiac surgery (under general or regional anaesthesia) requiring a minimum of one-night admission to hospital and are (A) >65 years old, (B) assigned an RCRI score of ≥1 or (C) >45 years old with significant cardiovascular disease (coronary artery disease, peripheral arterial disease, cerebrovascular disease, congestive heart failure, obstructive intracardiac disease such as severe aortic stenosis, severe mitral stenosis or severe hypertrophic obstructive cardiomyopathy). Patients will be contacted prior to processing the sample for NT-pro-BNP to obtain informed consent to participate in the study.
Exclusion criteria
Patients will be excluded if they do not provide or are unable to provide informed consent or if they do not have a baseline haemoglobin or creatinine level measured within the last 3 months.
Data collection, serum sampling and storage
Informed consent will be obtained by telephone. Once consent is obtained, study staff will interview patients and review medical charts to collect baseline demographics and pertinent clinical characteristics, including age, sex, height, weight, history of diabetes, hypertension, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, chronic atrial fibrillation or flutter, cancer, severe aortic stenosis, severe mitral stenosis, severe hypertrophic obstructive cardiomyopathy, preoperative estimated glomerular filtration rate, haemoglobin and type of surgery (including vascular, gastrointestinal, urological, gynaecological, orthopaedic and neurological surgery), as well as urgency category. Tubes of blood will be drawn by the clinical nurse simultaneously for lab-based BNP (Abbott) analysis, as well as point-of-care (POC) NT-pro-BNP (Roche) analysis. The Abbott BNP test and Roche POC NT-pro-BNP test are immunoassays for the quantitative determination of BNP and NT-pro-BNP in venous blood, respectively. The NT-pro-BNP samples will be sent to an onsite wet lab for storage at room temperature until testing. The study staff will then process the samples (within 8 hours of collection of the blood) using a POC NT-pro-BNP kit. All staff involved in this study will receive specific training in patient screening and consent, as well as dedicated training in the calibration and operation of the POC NT-pro-BNP kit and cartridges.
Postoperatively, blood will be drawn for troponin I analysis on the surgical wards on PODs 1, 2 and 3 in patients with an elevated BNP. We expect that some patients will be discharged after one overnight admission prior to troponin testing on PODs 2 and 3. These patients will be included in the analysis, but their protocol-driven troponin measurements will be truncated at the last measurement performed before their discharge.
Electronic medical records and supporting documents will be reviewed up to POD 30 by study staff blinded to the BNP and NT-pro-BNP results to ascertain postoperative troponin level, mortality status and cause of death. To establish the relationship between BNP, NT-pro-BNP and MINS that is due to an ischaemic aetiology, clinical notes and laboratory data will be assessed for evidence of a non-ischaemic aetiology of postoperative troponin elevation (eg, sepsis, pulmonary embolus, atrial fibrillation, cardioversion, chronic elevation). Vascular death will be defined as death thought to be secondary to a vascular cause, including a fatal cardiac arrest, myocardial infarction, congestive heart failure, stroke, aortic dissection or rupture, major bleed, pulmonary embolus, other cause thought to be vascular in nature or death due to unknown cause. Table 1 provides an overview of the study schedule and the measures to be collected. The study staff will monitor the data to ensure protocol compliance and timely completion of the study procedures. Data will be deidentified and access to records and data will be limited to study staff. Patient enrolment for the study began in May 2022, and we plan to complete data collection and analysis by May 2023.
Table 1.
Study schedule
| Assessment no | 1 | 2 | 3 | 4 | 5 | |
| Assessment timing | Day −90 to 0 before surgery | Day 1 after surgery on ward | Day 2 after surgery on ward | Day 3 after surgery on ward | Day 30 after surgery +30 days to allow time to review postoperative investigations and supporting information | |
| Clinical history | Assessment | |||||
| Eligibility criteria | X | |||||
| Informed consent | X | |||||
| Demographics | X | |||||
| Past medical history | X | |||||
| Height | X | |||||
| Weight | X | |||||
| Surgery type | X | |||||
| Laboratory tests | ||||||
| Haemoglobin | X | |||||
| Serum creatinine | X | |||||
| BNP | X | |||||
| Point-of-care NT-pro-BNP | X | |||||
| hsTnI | X | X | X | |||
| Clinical outcomes | ||||||
| Death | X | |||||
| Vascular death | X | |||||
| MINS (including MI and isolated-ischaemic troponin elevation) | X | |||||
| Stroke | X | |||||
| Cardiac arrest | X | |||||
| Major bleed | X | |||||
| Congestive heart failure | X | |||||
| Coronary artery revascularisation | X | |||||
| Pulmonary embolus | X | |||||
| Pneumonia | X | |||||
| Sepsis | X | |||||
| New atrial fibrillation | X |
BNP, B-type natriuretic peptide; hsTnI, high sensitivity troponin I; MI, myocardial infarction; MINS, myocardial injury after non-cardiac surgery; NT-pro-BNP, N-terminal pro-BNP.
Data analysis
Participants’ baseline characteristics will be presented using mean ±SD and frequency (%) for continuous and categorical variables, respectively.
Primary analysis
The performance of the BNP to NT-pro-BNP conversion formula (below) will be evaluated.13 A predicted NT-pro-BNP value will be calculated for each patient using the conversion formula (Formula I) determined by Kasahara et al.13 The correlation between measured and predicted NT-pro-BNP will be graphically displayed in a scatter plot and Bland-Altman plot. Passing-Bablok regression will be used to estimate calibration slope and intercept (along with their 95% CIs). Pearson’s correlation coefficient (r), the coefficient of determination (r2) and the root mean squared error (RMSE) will also be estimated to quantify the agreement between NT-pro-BNP values predicted by the formula and measured values.
Formula I
NT-pro-BNPpredicted = 10ˆ(2.05+0.907 × log10 BNP (in pg/mL) − 0.00522 × age + 0.00283 × body mass index (in kg per m2) − 0.00866 × haemoglobin (in g/dL) − 0.0422 × CCr + 0.000530 × CCr2 − 0.00000214 × CCr3 − 0.00000278 × max (0 [CCr–56.5))3 + 0.00000621 × max (0 (CCr–72.4))3 − 0.00000133 × max (0 (CCr–93.7))3 + 0.0164 (if female) + 0.194 (if with atrial fibrillation or flutter).
where CCr=creatinine clearance in mL per minute calculated by the Cockroft-Gault formula based on serum creatinine, age, sex and weight.14
To assess performance across key thresholds, weighted kappa will be calculated to quantify the agreement between predicted and observed NT-pro-BNP categories <100 pg/mL, 100 to <200 pg/mL, 200 to <1500 pg/mL or ≥1500 pg/mL. Sensitivity, specificity and predictive values will be calculated to quantify the ability of predicted NT-pro-BNP≥300 to detect a measured NT-pro-BNP≥300 vs <300 pg/mL.
To improve the equation, the relationship between log-transformed NT-pro-BNP will be modelled with the same predictor variables as in the above equation (log-transformed BNP, age, sex, creatinine clearance, BMI and presence of atrial fibrillation or atrial flutter). The model will be extended with an interaction term between creatinine clearance, age and BNP to determine if the interaction term improves the precision of the NT-pro-BNP estimates. Passing-Bablok regression will be used to estimate calibration slope and intercept (along with their 95% CIs) in 1000 bootstrap iterations and the conversion equation will be recalibrated with these values. We will assess the agreement between measured NT-pro-BNP and predicted from this equation with a Bland-Altman plot, a scatter plot, r, r2 and RMSE. Using the updated conversion formula, we will then assess weighted kappa for four-category agreement between predicted and measured NT-pro-BNP across categories <100 pg/mL, 100 to <200 pg/mL, 200 to <1500 pg/mL and ≥1500 pg/mL, and sensitivity, specificity and predictive values for binary classification (NT-pro-BNP≥300 vs <300 pg/mL).
Secondary analysis
The conversion formula will be used to determine the BNP concentrations that correspond with previously established NT-pro-BNP risk thresholds 100 pg/mL, 200 pg/mL and 1500 pg/mL10 (with other covariates set to their median values) and categorise BNP according to the corresponding thresholds. In two separate logistic regression models, we will estimate the association (ie, ORs and their 95% CIs) between the composite outcome of MINS or vascular death up to postoperative day 30 and (separately) (1) measured NT-pro-BNP categorised as <100 pg/mL, 100 to <200 pg/mL, 200 to <1500 pg/mL or ≥1500 pg/mL and (2) BNP categorised at the corresponding thresholds based on the final equation derived in the primary analysis. We will not adjust these analyses for other variables. The purpose of this analysis is to confirm that the categories based on the BNP thresholds derived from the conversion equation have similar trend of association with vascular events as established categories of NT-pro-BNP.
Sample size
Sample size calculation for a prediction model of a continuous outcome15 was based on the primary analysis to validate and update the formula for predicting NT-pro-BNP from measured BNP and patient characteristics. Since similar equations achieve r2 values of approximately 0.8 on average, we estimate that 431 patients would be required to develop a reproducible equation based on 31 parameters (ie, 13.9 participants per parameter estimated, including the variables and interactions listed, and flexible transformations of continuous variables and their interactions) assuming a stringent requirement of minimal overfitting bias (shrinkage parameter=0.96), mean log10 NTpro-BNP value of 2.05 and SD of 0.67 (estimated based on data from a large cohort study), and a 7.5% multiplicative margin of error acceptable for calculation of the intercept. We performed this sample size calculation using the R package pmsampsize V.1.1.2.
Patient and public involvement
There was no patient involvement in the development of the study design.
Ethics and dissemination
The study protocol has been approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (REB) and all participants will provide informed consent for enrollment in the study. Patients will be contacted by the study staff over the phone to obtain informed consent to participate in research using a standardised script. Any future modifications to the study protocol will be communicated with publication of the study results. The study protocol involves (A) one additional vial of blood drawn preoperatively (not requiring an additional needle stick) and (B) postoperative troponin screening on PODs 1, 2 and 3 in those patients with an elevated preoperative BNP (as per current Canadian Cardiovascular Society perioperative recommendations9). Patients with elevated troponin will be managed as per usual standard of care.9
Participants will be informed that there are no direct benefits to enrolling in this study, as there is no planned intervention that differentiates care from non-study patients. The expressed benefit of this study is to future patients presenting for elective non-cardiac surgery, as this study plans to further establish the translation between two common preoperative, non-invasive cardiac biomarkers (BNP and NT-pro-BNP) known to improve perioperative cardiac risk prediction and monitoring.
As future studies emerge and refine cardiovascular risk prediction using either BNP or NT-pro-BNP, a validated BNP to NT-pro-BNP conversion formula may have several critical applications in the preoperative period, including (1) using the best available evidence to inform perioperative risk discussions regardless of whether BNP or NT-pro-BNP testing is available at a specific hospital, (2) informing clinical decisions for individual patients (including the type of surgery, the type of anaesthesia, whether additional preoperative consults are required or the level of monitoring required postoperatively) and (3) better informing patients and their families about their perioperative risk. Importantly, this promotes comprehensive perioperative risk discussions and is an important step towards full informed consent tailored to individual patients.
Supplementary Material
Footnotes
Contributors: JL, JP, DD, PSR, PJD, BK and TD contributed to the study concept and design, acquisition of the data or critical revision of the manuscript for important intellectual content. All authors approved the version of the manuscript submitted for publication.
Funding: This work was supported by Clinical Teachers Association of Queen’s University (CTAQ) grant number 6035639.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: A 7 day cohort study. Lancet 2012;380:1059–65. 10.1016/S0140-6736(12)61148-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: A Modelling strategy based on available data. Lancet 2008;372:139–44. 10.1016/S0140-6736(08)60878-8 [DOI] [PubMed] [Google Scholar]
- 3.Smilowitz NR, Gupta N, Ramakrishna H, et al. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017;2:181–7. 10.1001/jamacardio.2016.4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642–51. 10.1001/jama.2017.4360 [DOI] [PubMed] [Google Scholar]
- 5.Botto F, Alonso-Coello P, Chan MTV, et al. Myocardial injury after noncardiac surgery: A large, International, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120:564–78. 10.1097/ALN.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 6.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MTV, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295–304. 10.1001/jama.2012.5502 [DOI] [PubMed] [Google Scholar]
- 7.Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: A systematic review and meta-analysis. Cardiol Rev 2019;27:267–73. 10.1097/CRD.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruetzler K, Khanna AK, Sessler DI. Myocardial injury after noncardiac surgery: Preoperative, intraoperative, and postoperative aspects, implications, and directions. Anesth Analg 2020;131:173–86. 10.1213/ANE.0000000000004567 [DOI] [PubMed] [Google Scholar]
- 9.Duceppe E, Parlow J, MacDonald P, et al. Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33:17–32. 10.1016/j.cjca.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Duceppe E, Heels-Ansdell D, Devereaux PJ. Preoperative N-terminal pro-B-type natriuretic peptide and cardiovascular events after noncardiac surgery. Annals of Internal Medicine 2020;172:843. 10.7326/L20-0269 [DOI] [PubMed] [Google Scholar]
- 11.Roshanov PS, Sessler DI, Chow CK, et al. Predicting myocardial injury and other cardiac complications after elective noncardiac surgery with the revised cardiac risk index: The VISION study. Can J Cardiol 2021;37:1215–24. 10.1016/j.cjca.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 12.Rørth R, Jhund PS, Yilmaz MB, et al. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail 2020;13. 10.1161/CIRCHEARTFAILURE.119.006541 [DOI] [PubMed] [Google Scholar]
- 13.Kasahara S, Sakata Y, Nochioka K, et al. Conversion formula from B-type natriuretic peptide to N-terminal proBNP values in patients with cardiovascular diseases. Int J Cardiol 2019;280:184–9. 10.1016/j.ijcard.2018.12.069 [DOI] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 15.Riley RD, Snell KIE, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: Part I – continuous outcomes. Stat Med 2019;38:1262–75. 10.1002/sim.7993 Available: http://doi.wiley.com/10.1002/sim.v38.7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.