Abstract
Background
Early neonatal death accounts for a significant number of under-5 mortality worldwide. However, the problem is under-researched and under-reported in low-income and middle-income countries, particularly in Ethiopia. The magnitude of mortality during the early neonatal period and associated factors should be studied for designing appropriate policies, and strategies that could help tackle the problem. Hence, this study aimed to determine the prevalence and identify factors associated with early neonatal mortality in Ethiopia.
Methods
This study was conducted by using data from Ethiopian Demographic and Health Survey 2016. A total of 10 525 live births were enrolled in the study. A multilevel logistic regression model was used to identify determinants of early neonatal mortality. Adjusted OR (AOR) at a 95% CI was computed to assess the strength and significance of the association between outcome and explanatory variables. Factors with a p<0.05 were declared statistically significant.
Results
The national prevalence of early neonatal mortality in Ethiopia was 41.8 (95% CI 38.1 to 45.8) early neonatal deaths per 1000 live births. The extreme ages of pregnancy (under 20 years (AOR 2.7, 95% CI 1.3 to 5.5) and above 35 years (AOR 2.4, 95% CI 1.5 to 4)), home delivery (AOR 2.4, 95% CI 1.3 to 4.3), low birth weight (AOR 3.3, 95% CI 1.4 to 8.2) and multiple pregnancies (AOR 5.3, 95% CI 4.1 to 9.9) were significantly associated early neonatal mortality.
Conclusions
This study revealed a higher prevalence of early neonatal mortality as compared with prevalence in other low-income and middle-income countries. Thus, it is determined to be essential to design maternal and child health policies and initiatives with a priority on the prevention of early neonatal deaths. Emphasis should be given to babies born to mothers at extreme ages of pregnancy, to those born of multiple pregnancies delivered at home and to low birthweight babies.
Keywords: Nursing, Nursing Care, Neonatology, Mortality, Health services research
WHAT IS ALREADY KNOWN ON THIS TOPIC
Early neonatal deaths account for a significant amount of under-5 mortality.
Neonates are more susceptible to maternal and environmental factors early within the first week of life.
Early neonatal deaths are understudied and under-reported in low-income and middle-income countries.
WHAT THIS STUDY ADDS
Neonates born with low weights were more likely to die within seven weeks of life.
More than a single birth outcome was significantly associated with early neonatal death.
Home delivery without skilled birth attendants could increase the likelihood of early neonatal deaths.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The prevalence of early neonatal mortality in this study was significantly high.
The progress made to reduce early neonatal deaths has lagged behind other achievements.
Early newborn mortality is still a major public health concern, indicating a pressing need for care.
Introduction
Neonatal mortality reduction has made significant progress over the past few decades, but for the majority of developing nations, it still poses a significant issue.1 Nearly 44% of the anticipated 6.3 million child deaths globally in 2032 happened during the neonatal period (first 28 days of life), and a further 75% of these neonatal deaths occurred during the first week of life (early neonatal period).2 The early neonatal period accounts for about 33% of all under-5 deaths worldwide, whereas the remaining 67% occur during the next days of their 5-year period.2 Studies on the magnitude and risk factors of early neonatal mortality are critical for developing policies that are targeted at reducing mortality.
Although childhood mortality has decreased by 50% globally, progress towards the Millennium Development Goal to reduce early neonatal death has lagged behind other achievements and is now a significant contributor to overall mortality in children under the age of 5.3 Ethiopia has made tremendous success in reducing maternal, under-5 and infant death rates over the past two decades because of Ministry of Health’s strong leadership in implementing the Health Sector Transformation Plan, coordination of efforts and major investments by the government, development partners and the community in the health system.4 The under-5 mortality rate has declined by 71% from the 1990 baseline of 204 per 1000 live births to 59 per 1000 live births in 2019.5 However, neonatal mortality declined from 58 deaths per 1000 live births in 2000 to 38 deaths per 1000 births in 2016.6 This reduction in child mortality was primarily among children in their postneonatal lives, indicating that Ethiopia’s progress in reducing neonatal deaths has been less successful. Despite a decrease in the mortality rate for children under the age of 5, more than half of these deaths (189 000) every year occur during the neonatal period.4
As evidenced by previous studies, low status of maternal education,7 delivery without a skilled care provider,8 complications during pregnancy,9 birth weight,10 11 cultural practices,12 wealth index,13 difficulty to afford for healthcare,14 and lack of basic antenatal and postnatal care utilisation2 15 were the factors responsible for mortality in the neonatal period in developing countries like Ethiopia.
In spite of the fact that deaths of neonates in the early days after delivery account for a significant amount of under-5 mortality, early neonatal deaths are understudied and under-reported in low-income and middle-income countries, particularly in Ethiopia. Furthermore, neonates are more susceptible to maternal and environmental factors early within the first week of life (the time when most of the extra uterine life adjustments happen); however, there is a paucity of information on the prevalence and contributing variables of early neonatal mortality in Ethiopia. Therefore, having a thorough grasp of the issue at a national level could aid decision-makers in developing a comprehensive strategy against the factors leading to neonatal death as soon as possible.
Methods
Study design, study area and period
A secondary analysis of data from the Ethiopian Demographic Health Survey 2016 was used to conduct a population-based cross-sectional study. The study was carried out in federal democratic republic of Ethiopia. Nine regional states (Tigray, Afar, Amhara, Oromia, Somali, Benishangul, SNNPR, Gambela and Harari) and two city administrations (Addis Ababa and Dire Dawa) make up the country.16 Ethiopia today has a three-tiered healthcare delivery system with primary, secondary and tertiary levels of care. The primary level of care consists of primary hospitals, health centres and health posts at the community level. For an average population of 100 000 people, a primary hospital serves as a referral location for health centres and provides emergency, inpatient and ambulatory services. The secondary healthcare system contains a general hospital that serves as a referral hub for primary hospitals as well as a training ground for public health officials, nurses and emergency surgeons. A tertiary healthcare system includes a specialised hospital, a referral centre for general hospitals.17 18
Data source, study population and sampling technique
The Ethiopian Demographic and Health Survey (EDHS) 2016, a nationally representative sample, was the data source for this investigation. A sampling frame of 84 915 enumeration areas (EAs) from the 2007 Ethiopian Population and Housing Census (PHC) served as the basis for choosing the EAs for the EDHS. The survey employed a two-stage stratified cluster sampling technique. In the first stage, a total of 645 EAs (of which 443 were in rural areas) were selected; in the second stage, an average of 28 residences per cluster were chosen. Any additional survey information can be found in the 2016 EDHS report.19 This study used the Kids Records dataset, and the study population was all live births.
Patient and population involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research, since the study used secondary data.
Study variables
Outcome variable
The outcome variable for this study was early neonatal mortality, which was dichotomised as ‘yes’=1 for neonates who died within 1 week of life and ‘no’=0 for neonates who were alive within 1 week of life.
Explanatory variables
Since the EDHS data are hierarchical, two-level explanatory variables (individual and community) were taken into account. The individual level (first level) factors include maternal age, maternal education, place of delivery, birth weight, type of pregnancy, mode of delivery, household wealth quantile (wealth index), antenatal care (ANC) utilisation and pregnancy complications. Region, residency, ANC use in the community and community women’s education make up the community level (second level) variables. The community levels of ANC utilisation and education were aggregated from the individual-level factors, ANC utilisation and maternal education, respectively.
Optimal ANC is defined as a woman with a normal pregnancy having at least four antenatal visits and more in the event of difficulties or complications.20
Birth weight is stratified into low birth weight, normal birth weight and high birth weight if the baby weighs 2500grammes (g), 2500–3999 g and ≥4000 g, respectively.21 22
Wealth index was classified as poorer, poorest, middle, richer and richest in EDHS 2016.23
Maternal educational status was categorised as no formal education, primary, secondary and higher education in our study and by the DHS programme.24
Data management and model selection
Following extraction from the source, data were entered, coded, cleaned, recoded and analysed using Stata V.14.0. In the EDHS data, variables are nested by clusters, and those within the same cluster show more similarities than those within separate clusters. As a result, the assumption independence of observation and equal variance across clusters violated to use the conventional logistic regression model. This implies that using a sophisticated model to take into account between-cluster factors is necessary. Taking this into consideration, multilevel mixed effect logistic regression was employed to identify the determinants of early neonatal mortality.
Multilevel mixed effect logistic regression follows four models; null model (with no predictors), mode I (only individual level factors), model II (only community factors) and model III (both individual-level and community-level factors).25 The model without exposure variables (null model) was used to check the variability of early neonatal death across the cluster. The association of individual-level variables with the outcome variable (model I) and the association of community-level variables with the outcome variable (model II) were assessed. In the final model (model III), the association of both individual and community-level variables was fitted simultaneously with the outcome (early neonatal mortality).
Random effects or measures of variation of the outcome variables were estimated by the median OR (MOR), intraclass correlation coefficient (ICC) and proportional change in variance (PCV). The MOR is the median value of the OR between the area of the highest risk and the area of the lowest risk for early neonatal mortality when two clusters are randomly selected, using clusters as a random variable; . On the other hand, the ICC, which is calculated as , indicates the proportion of between cluster variations in early neonatal mortality. Furthermore, the PCV demonstrates the variation in the prevalence of early neonatal mortality explained by factors and calculated as ; where Vnull=variance of the null model and VC=cluster level variance.25–27
The fixed effects (measure of association) was used to estimate the association between the likelihood of early neonatal mortality and individual-level and community-level explanatory variables. It was assessed and the strength was presented using adjusted OR (AOR) and 95% CIs with a p<0.05.
Because of the nested nature of the model, deviation =−2(log-likelihood ratio) was used to compare models, and the model with the lowest deviance was selected as the best-fit model.
The variables used in the models were verified for multicollinearity by measuring the variance inflation factors, with the findings falling within acceptable limits.1–10
Result
Sociodemographic characteristics of the study variables
A total of 10 525 live births (5422 males and 5103 females) were included in the analysis. Nearly two-thirds (64.3%) of the babies were born to mothers with no formal education. The majority (81.3%) of the participants were born to mothers living in rural areas of the country, and 2147 (46.3%) births faced complications during pregnancy. About half (50.3%) of the babies were born to mothers living in a community with a low level of literacy (table 1).
Table 1.
Sociodemographic and economic characteristics of the study
| Variables | Response | Frequency (n) | % |
| Individual level variables | |||
| Maternal education | No formal education | 6772 | 64.3 |
| Primary | 2633 | 25.0 | |
| Secondary | 729 | 6.9 | |
| Higher | 391 | 3.7 | |
| Maternal age | 15–19 | 399 | 3.8 |
| 20–35 | 7612 | 72.3 | |
| 36–49 | 2514 | 23.9 | |
| Wealth index | Poor | 5711 | 54.3 |
| Middle | 1444 | 13.7 | |
| Rich | 3370 | 32.0 | |
| Sex of child | Male | 5422 | 51.5 |
| Female | 5103 | 48.5 | |
| Birth weight | Low | 2132 | 26.9 |
| Normal | 2803 | 35.4 | |
| High | 2984 | 37.7 | |
| Place of delivery | Health facility | 3370 | 32.0 |
| Home | 7155 | 68.0 | |
| Mode of delivery | CS | 305 | 2.9 |
| Vaginal | 10 220 | 97.1 | |
| Type of pregnancy | Single | 10 253 | 97.4 |
| Multiple | 272 | 2.6 | |
| Pregnancy complications | Yes | 2147 | 46.3 |
| No | 2491 | 53.7 | |
| ANC utilisation | Not optimal | 4542 | 64.0 |
| Optimal | 2552 | 36. | |
| Community-level variables | |||
| Place of residence | Urban | 1964 | 18.7 |
| Rural | 8561 | 81.3 | |
| Region | Tigray | 1009 | 9.6 |
| Afar | 1061 | 10.1 | |
| Amhara | 961 | 9.1 | |
| Oromia | 1569 | 14.9 | |
| Somali | 1504 | 14.3 | |
| Benishangul | 868 | 8.3 | |
| SNNPR | 1250 | 11.9 | |
| Gambela | 702 | 6.7 | |
| Harari | 603 | 5.7 | |
| Addis Ababa | 459 | 4.4 | |
| Dire Dawa | 539 | 5.1 | |
| Community ANC utilisation | Low | 5296 | 50.3 |
| High | 5229 | 49.7 | |
| Community educational level | Low | 5288 | 50.2 |
| High | 5237 | 49.8 | |
ANC, antenatal care; CS, caesarean section; SNNPR, Southern Nations Nationalities and Peoples Region.
Prevalence of early neonatal mortality in Ethiopia
The national prevalence of early neonatal mortality in Ethiopia was 41.8 (95% CI 38.1 to 45.8) early neonatal deaths per 1000 live births. The magnitude of urban and rural prevalence of early neonatal mortality in Ethiopia was found to be 25.5 and 45.6 deaths per 1000 live births, respectively. Somali (55.2 deaths per 1000 live births) and Addis Ababa (26.1 deaths per 1000 live births) regions had the highest and lowest rates of early neonatal mortality in Ethiopia, respectively (figure 1).
Figure 1.
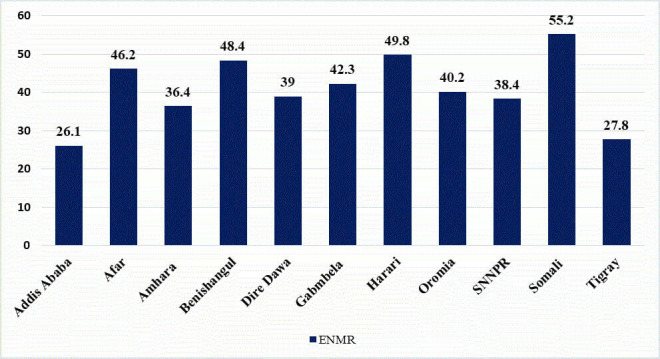
Regional prevalence of early neonatal mortality in Ethiopia. ENMR, early neonatal mortality rate; SNNPR, Southern Nations Nationalities and Peoples Region.
Random effect and model fitness
A null model was run to determine whether the data supported the decision to assess randomness at the community level. The results of the null model showed that the likelihood of early neonatal mortality varied significantly among different communities (variance=0.3 and p<0.0001). The ICC value of the null model indicates that 9% of the variation in early neonatal mortality was due to variation between the clusters (conversely, 91% of the variation is due to within-cluster variation). In the null model, the odds of early neonatal mortality were 1.7 times variable between higher and lower risk clusters (table 2).
Table 2.
Random effect and model fit statistics of early neonatal mortality in Ethiopia
| Parameter | Null model | Model I | Model II | Model III |
| Variance | 0.3 | 0.2 | 0.2 | 0.1 |
| ICC | 9% | 5.7% | 6.9% | 4% |
| MOR | 1.7 | 1.5 | 1.6 | 1.4 |
| PCV | Reference | 38.9 | 25.1 | 67.4 |
| LLR | −1818.8 | −408.4 | −1802.1 | −403.6 |
| Deviance | 3637.5 | 816.8 | 3604.2 | 807.2 |
ICC, intracluster correlation; MOR, median OR; PCV, proportional change in variance; LLR, loglikelihood ratio.
The value of intraclass correlation in model I pointed out that 5.7% of the diversities in the early death of neonates were responsible for the differences across communities. We then created model II by taking just community-level variables to the null model. The value of ICC from model II indicated that distinctions among clusters were responsible for 6.9% of the differences in early neonatal mortality. In the final model (model III), nearly 67% variation in odds of early neonatal mortality was attributed to both individual and community level factors (PCV=67.4%) and the odds of early neonatal mortality were 1.4 times variable between low and high-risk clusters (table 2).
Factors associated with early neonatal mortality
In the final best-fitted model (model III) of multivariable multilevel logistic regression, maternal ages under 20 and above 35, home delivery, low birth weight, and multiple pregnancies were significantly associated with early neonatal mortality (table 3).
Table 3.
Multivariable multilevel logistic regression analysis of individual-level and community-level factors associated with early neonatal mortality in Ethiopia, EDHS 2016
| Individual and community-level factors | Model I AOR (95% CI) | Model II AOR (95% CI) | Model III AOR (95% CI) | |
| Maternal education | No formal education | 0.6 (0.2 to 2.0) | 0.5 (0.2 to 1.7) | |
| Primary | 0.99 (0.3 to 3.1) | 0.9 (0.3 to 2.8) | ||
| Secondary | 1.61 (0.5 to 5.2) | 1.5 (0.5 to 4.7) | ||
| Higher | 1 | 1 | ||
| Maternal age | 15–19 | 2.8 (1.4 to 5.6) | 2.7 (1.3 to 5.5)* | |
| 20–35 | 1 | 1 | ||
| 36–49 | 2.3 (1.4 to 3.7) | 2.4 (1.5 to 4.0)* | ||
| Wealth index | Poor | 1.28 (0.8 to 2.2) | 1.4 (0.8 to 2.6) | |
| Middle | 0.8 (0.4 to 1.6) | 0.8 (0.4 to 1.8) | ||
| Rich | 1 | 1 | ||
| ANC utilisation | Not optimal | 0.9 (0.6 to 1.6) | 0.9 (0.6 to 1.5) | |
| Optimal | 1 | 1 | ||
| Place of delivery | Heath facility | 1 | 1 | |
| Home | 2.3 (1.3 to 4.1) | 2.4 (1.3 to 4.3)* | ||
| Birth weight | Low | 3.1 (1.3 to 7.6) | 3.3 (1.4 to 8.2)* | |
| Normal | 1 | 1 | ||
| High | 3.0 (1.1 to 8.6) | 2.7 (0.9 to 7.6) | ||
| Type of pregnancy | Single | 1 | 1 | |
| Multiple | 5.7 (4.3 to 10.3) | 5.3 (4.1 to 9.9)* | ||
| Mode of delivery | CS | 1.5 (0.6 to 3.6) | 1.6 (0.7 to 3.8) | |
| Vaginal | 1 | Pregnancy | ||
| Pregnancy complications | Yes | 0.8 (0.5 to 1.2) | 0.8 (0.5 to 1.3) | |
| No | 1 | 1 | ||
| Place of residence | Urban | 1 | 1 | |
| Rural | 1.9 (1.3 to 2.9) | 0.9 (0.5 to 2.1) | ||
| Region | Tigray | 0.6 (0.3 to 1.4) | 1.2 (0.3 to 4.7) | |
| Afar | 0.9 (0.4 to 2.0) | 1.6 (0.3 to 7.4) | ||
| Amhara | 0.8 (0.4 to 1.7) | 1.1 (0.3 to 4.7) | ||
| Oromia | 0.8 (0.4 to 1.6) | 3.0 (0.8 to 11.5) | ||
| Somali | 1.2 (0.5 to 2.4) | 2.6 (0.6 to 10.7) | ||
| Benishangul | 0.9 (0.4 to 2.1) | 1.3 (0.3 to 6.1) | ||
| SNNPR | 0.8 (0.4 to 1.7) | 1.9 (0.5 to 7.3) | ||
| Gambela | 0.9 (0.4 to 1.9) | 1.7 (0.4 to 6.9) | ||
| Harari | 1.1 (0.5 to 2.4) | 2.4 (0.6 to 9.5) | ||
| Addis Ababa | 1 | 1 | ||
| Dire Dawa | 1.1 (0.5 to 2.3) | 2.4 (0.6 to 9.7) | ||
| Community ANC utilisation | Low | 1.3 (0.9 to 1.7) | 0.8 (0.5 to 1.4) | |
| High | 1 | 1 | ||
| Community educational level | Low | 0.8 (0.7 to 1.1) | 1.1 (0.6 to 2.0) | |
| High | 1 | 1 | ||
*Significantly associated (p<0.05).
ANC, antenatal care; AOR, adjusted OR; CS, caesarean section; EDHS, Ethiopian Demographic and Health Survey; SNNPR, Southern Nations Nationalities and Peoples Region.
In comparison to babies born to mothers in age groups 20–35, early neonatal mortality was 2.7 and 2.4 times more likely to happen among babies born to mothers in extreme ages of pregnancy; under 20 years (AOR 2.7, 95% CI 1.3 to 5.5) and above 35 years (AOR 2.4, 95% CI 1.5 to 4.0), respectively. The odds of early neonatal mortality were 2.4 times higher among babies born at home (AOR 2.4, 95% CI 1.3 to 4.3) than babies delivered at a health facilities with a skilled birth attendant. Low birthweighted babies were 3.3 times more likely to die in the first week of life compared with those born with normal birth weight (AOR 3.3, 95% CI 1.4 to 8.2). The occurrence of early neonatal death was 5.31 times more likely among neonates born fm a multiple pregnancies compared with those born from a singleton pregnancy (AOR 5.3, 95% CI 4.1 to 9.9) (table 3).
Discussion
Early neonatal deaths contribute significantly to childhood mortality in low-income and middle-income countries such as Ethiopia. This study revealed the prevalence and determinants of early neonatal mortality in Ethiopia. In this study, the national prevalence of early neonatal mortality was 41.8 (95% CI 38.1 to 45.8) early neonatal deaths per 1000 live births. This prevalence was higher than in a previous study in Ethiopia (28.5 per 1000 live births).28 The higher prevalence of the current findings reflects the less successful progress of Ethiopia in reducing early neonatal mortality.
The prevalence in our study was also higher than the prevalence of findings in African countries, such as Nigeria and Afghanistan, with 32 and 14 early neonatal deaths per 1000 live births, respectively.2 29 The higher prevalence of early neonatal mortality in this study than previous findings in Nigeria and Afghanistan could be due to differences in healthcare systems across the countries. In comparision to Ethiopia, Nigeria and Afghanistan have higher rates of hospital beds and physicians per 1000 inhabitants. We contend that Ethiopia has a higher rate of early neonatal mortality when compared with other low-income and middle-income countries, which suggests the need for a plan that places a higher priority on preserving the lives of newborns, especially in rural areas of the country.
In our study, early neonatal mortality was more prevalent in rural than urban areas of the country. This may be because health services are less accessible and available in rural areas than in urban ones due to poor transportation, and rural residents’ low access to information about perinatal health service utilisation.
Following multivariable multilevel mixed effect logistic regression; maternal ages under 20 and above 35, delivery at home, low birthweight and multiple pregnancies were significantly associated with early neonatal mortality in Ethiopia.
In this study, the odds of early neonatal mortality were higher among babies born to mothers aged under 20 years and above 35 years compared with babies born to mothers in age groups 20–35. Previous studies also had similar findings to ours.1 30 31 An association with being under 20 years of age may be explained by the possibility that the woman was not yet fully developed physically or physiologicaly for pregnancy. A lack of experience with child care may be an issue in addition to physical or physiological immaturity. Furthermore, because younger mothers are more likely to have preterm deliveries, low birthweight babies and babies with congenital abnormalities, early neonatal deaths are more common.32 The association between early neonatal mortality and maternal age over 35 years could be explained by higher rates of low or high birth weight and pregnancy complications among older mothers.1
Regarding the place of delivery, early neonatal mortality was more likely to happen among newborn babies born at home when compared with newborns born at a health facility. This was consistent with previous studies.33 It could be because babies delivered at home by a non-professional birth attendant may not have had access to all components of essential newborn care and immediate postpartum care services. This finding indicates a need to improve health facility delivery in Ethiopia to increase the chance of survival for newborns. Institutional deliveries are preferable in cases of delivery complications because both mothers and babies benefit from professional care and access to life-saving tools at healthcare facilities.34 35 In addition, the presence of a professional team at a health facility could offer another advantage to reducing a potential delay and enhancing early management of obstetric complications and birth asphyxia.34
In line with previous findings,11 31 36 this study found that babies born with low birth weight were more likely to die in the first week of life compared with those born with normal birth weight. The increased risk of mortality among newborns with low birth weight may be attributed to families’ and mothers’ poor healthcare-seeking behaviour for neonatal health issues.37 38 The lack of child healthcare facilities and their poor quality may also increase the danger of death for low birthweight neonates before 1 week of age.39 40
According to the result of this finding, multiple pregnancy was significantly associated with early neonatal mortality. The occurrence of early neonatal death was more likely among neonates born from multiple pregnancies compared with those born from singleton pregnancy. This was supported by previous studies.41 It could be a plausible reason that babies born from multiple pregnancies usually have growth restrictions, low Apgar scores and extremely low birth weights.42–44 In addition, multiple pregnancies are more likely to be complicated during pregnancy, labour and after birth.44
The use of nationally representative large sample data is virtue of this study. However, since the study was based on the EDHS, it could be limited in that mothers sometimes report the age at death of their children incorrectly during the survey, which might affect estimates of early neonatal mortality. The study was also limited in that the data used for this study is 7 years old, and data points may have changed in a rapidly low-income and middle-income country.
Conclusion
This study revealed a higher prevalence of early neonatal mortality as compared with prevalence in other low-income and middle-income countries. Thus, it is determined to be essential to design maternal and child health policies and initiatives with a priority on the prevention of early neonatal deaths. Extreme ages of pregnancy, multiple pregnancies, low birth weight and home delivery were determinants of early neonatal mortality. Therefore, emphasis should be given to babies born to mothers at extreme ages of pregnancy, to those born of multiple pregnancies delivered at home, and to low birthweight babies.
Supplementary Material
Footnotes
Contributors: TTT conceived and designed the study, run the analysis and drafted the manuscript. TTT takes the responibility for overall content as guaranter. All the authors checked the analysis and made substantial contributions to reviewing the design of the study and the drafted manuscript. All authors critically reviewed the manuscript for important intellectual content and contributed to the final approval of the version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Kibria GMA, Burrowes V, Choudhury A, et al. Determinants of early neonatal mortality in Afghanistan: an analysis of the demographic and health survey 2015. Global Health 2018;14:1–12. 10.1186/s12992-018-0363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahiru T. Determinants of early neonatal mortality in Nigeria: results from 2013 Nigeria DHS. J Pediatr Neonatal Care 2015;2. Available http://medcraveonline.com/JPNC/Volume2-Issue5.php 10.15406/jpnc.2015.02.00089 [DOI] [Google Scholar]
- 3.Lehtonen L, Gimeno A, Parra-Llorca A, et al. Early neonatal death: a challenge worldwide. Seminars in Fetal and Neonatal Medicine 2017;22:153–60. 10.1016/j.siny.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 4.Fenta SM, Fenta HM. Risk factors of child mortality in Ethiopia: application of multilevel two-part model. PLoS One 2020;15:e0237640. 10.1371/journal.pone.0237640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argawu AS, Mekebo GG. Risk factors of under-five mortality in Ethiopia using count data regression models, 2021. Ann Med Surg (Lond) 2022;82:104764. 10.1016/j.amsu.2022.104764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibre G, Idriss-Wheeler D, Yaya S. Inequalities and trends in neonatal mortality rate (NMR) in Ethiopia: evidence from the Ethiopia demographic and health surveys, 2000–2016. PLoS One 2020;15:e0234483. 10.1371/journal.pone.0234483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaya Y, Eide KT, Norheim OF, et al. Maternal and neonatal mortality in south-west Ethiopia: estimates and socio-economic inequality. PLoS One 2014;9:e96294. 10.1371/journal.pone.0096294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinyemi JO, Bamgboye EA, Ayeni O. Trends in neonatal mortality in Nigeria and effects of bio-demographic and maternal characteristics. BMC Pediatr 2015;15:1–12. 10.1186/s12887-015-0349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titaley CR, Dibley MJ, Agho K, et al. Determinants of neonatal mortality in Indonesia. BMC Public Health 2008;8:1–15. 10.1186/1471-2458-8-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lona Reyes JC, Pérez Ramírez RO, Llamas Ramos L, et al. Neonatal mortality and associated factors in newborn infants admitted to a neonatal care unit. Arch Argent Pediatr 2018;116:42–8. 10.5546/aap.2018.eng.42 [DOI] [PubMed] [Google Scholar]
- 11.Smeeton NC, Rona RJ, Dobson P, et al. Assessing the determinants of stillbirths and early neonatal deaths using routinely collected data in an inner City area. BMC Med 2004;2:1–7. 10.1186/1741-7015-2-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolde HF, Gonete KA, Akalu TY, et al. Factors affecting neonatal mortality in the general population: evidence from the 2016 Ethiopian Demographic and Health Survey (EDHS)—multilevel analysis. BMC Res Notes 2019;12:1–6. 10.1186/s13104-019-4668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Reaching the every newborn national 2020 milestones: country progress, plans and moving forward 2017.
- 14.Amini Rarani M, Rashidian A, Khosravi A, et al. Changes in socio-economic inequality in neonatal mortality in Iran between 1995-2000 and 2005-2010: an Oaxaca decomposition analysis. Int J Health Policy Manag 2017;6:219–218. 10.15171/ijhpm.2016.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amouzou A, Ziqi M, Carvajal-Aguirre L, et al. Skilled attendant at birth and newborn survival in sub–Saharan Africa. J Glob Health 2017;7:020504. 10.7189/jogh.07.020504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CS Agency . The DHS program ICF. Rockville, Maryland, USA, 2017. [Google Scholar]
- 17.WHO . Primary health care systems (primasys): case study from Ethiopia: abridged version. World Health Organization, 2017. [Google Scholar]
- 18.Assefa Y, Gelaw YA, Hill PS, et al. Community health extension program of Ethiopia, 2003–2018: successes and challenges toward universal coverage for primary healthcare services. Global Health 2019;15:1–11. 10.1186/s12992-019-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Central Statistical Agency (CSA)[Ethiopia] and ICF . Ethiopia Demographic and Health Survey, Addis Ababa. Central Statistical Agency, 2016. [Google Scholar]
- 20.Belay AT, Fenta SM, Birhan Biresaw H, et al. The magnitude of optimal antenatal care utilization and its associated factors among pregnant women in South Gondar zone, Northwest Ethiopia: a cross-sectional study. Int J Reprod Med 2022;2022:1–10. 10.1155/2022/1415247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomella T, Cunningham M, Eya F, et al. Gestational age and birthweight classification. In: Neonatology: management, procedures, on-call problems, diseases, and drugs. 7th ed. New York: McGraw-Hill, 2013. [Google Scholar]
- 22.Khan W, Zaki N, Masud MM, et al. Infant birth weight estimation and low birth weight classification in United Arab Emirates using machine learning algorithms. Sci Rep 2022;12. 10.1038/s41598-022-14393-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bitew FH. Spatio-Temporal inequalities and predictive models for determinants of undernutrition among women and children in. Ethiopia: The University of Texas at San Antonio, 2020. [Google Scholar]
- 24.Neves PAR, Barros AJD, Gatica-Domínguez G, et al. Maternal education and equity in breastfeeding: trends and patterns in 81 low- and middle-income countries between 2000 and 2019. Int J Equity Health 2021;20:1–13. 10.1186/s12939-020-01357-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommet N, Morselli D. Correction: keep calm and learn Multilevel logistic modeling: a simplified three-step procedure using STATA, R, mPLUS, and SPSS. Int Rev Soc Psychol 2017;30:229–30. 10.5334/irsp.162 [DOI] [Google Scholar]
- 26.Eshete T, Kumera G, Bazezew Y, et al. Determinants of inadequate minimum dietary diversity among children aged 6–23 months in Ethiopia: secondary data analysis from Ethiopian Demographic and Health Survey 2016. Agric & Food Secur 2018;7:1–8. 10.1186/s40066-018-0219-8 [DOI] [Google Scholar]
- 27.Tesema GA, Mekonnen TH, Teshale AB. Individual and community-level determinants, and spatial distribution of institutional delivery in Ethiopia, 2016: spatial and Multilevel analysis. PLoS One 2020;15:e0242242. 10.1371/journal.pone.0242242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinnon B, Harper S, Kaufman JS, et al. Distance to emergency obstetric services and early neonatal mortality in Ethiopia. Trop Med Int Health 2014;19:780–90. 10.1111/tmi.12323 [DOI] [PubMed] [Google Scholar]
- 29.Kibria GMA, Burrowes V, Choudhury A, et al. Determinants of early neonatal mortality in Afghanistan: an analysis of the demographic and health survey 2015. Global Health 2018;14:47. 10.1186/s12992-018-0363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y-N, Choi D-W, Kim DS, et al. Maternal age and risk of early neonatal mortality: a national cohort study. Sci Rep 2021;11:1–9. 10.1038/s41598-021-80968-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suparmi S, Chiera B, Pradono J. Low birth weights and risk of neonatal mortality in Indonesia. Health Science Journal of Indonesia 2016;7:113–7. 10.22435/hsji.v7i2.5587.113-117 [DOI] [Google Scholar]
- 32.Kang G, Lim JY, Kale AS, et al. Adverse effects of young maternal age on neonatal outcomes. Singapore Med J 2015;56:157–63. 10.11622/smedj.2014194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajaari J. Impact of place of delivery on neonatal mortality in rural Tanzania. Value in Health 2013;16:A209–10. 10.1016/j.jval.2013.03.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronsmans C, Scott S, Qomariyah SN, et al. Professional assistance during birth and maternal mortality in two Indonesian districts. Bull World Health Organ 2009;87:416–23. 10.2471/blt.08.051581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Titaley CR, Dibley MJ, Roberts CL. Type of delivery attendant, place of delivery and risk of early neonatal mortality: Analyses of the 1994–2007 Indonesia demographic and health surveys. Health Policy Plan 2012;27:405–16. 10.1093/heapol/czr053 [DOI] [PubMed] [Google Scholar]
- 36.Engmann C, Matendo R, Kinoshita R, et al. Stillbirth and early neonatal mortality in rural central Africa. Int J Gynaecol Obstet 2009;105:112–7. 10.1016/j.ijgo.2008.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelaw YA, Biks GA, Alene KA. Effect of residence on mothers’ health care seeking behavior for common childhood illness in Northwest Ethiopia: a community based comparative cross – sectional study. BMC Res Notes 2014;7:1–8. 10.1186/1756-0500-7-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolola T, Gezahegn T, Addisie M. Health care seeking behavior for common childhood illnesses in Jeldu district, Oromia regional state, Ethiopia. PLoS One 2016;11:e0164534. 10.1371/journal.pone.0164534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyene W, Jira C, Sudhakar M. Assessment of quality of health care in Jimma zone, Southwest Ethiopia. Ethiopian J Health Sci 2011. [PMC free article] [PubMed] [Google Scholar]
- 40.Getachew A, Ricca J, Cantor D. Quality of care for prevention and management of common maternal and newborn complications: a study of Ethiopia’s hospitals. Baltimore: Jhpiego, 2011: 1–9. [Google Scholar]
- 41.Bellizzi S, Sobel H, Betran AP, et al. Early neonatal mortality in twin pregnancy: findings from 60 low- and middle-income countries. J Glob Health 2018;8. 10.7189/jogh.08.010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel JP, Torloni MR, Seuc A, et al. Maternal and perinatal outcomes of twin pregnancy in 23 low-and middle-income countries. PLoS One 2013;8:e70549. 10.1371/journal.pone.0070549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizwan N, Abbasi RM, Mughal R. Maternal morbidity and perinatal outcome with twin pregnancy. J Ayub Med Coll Abbottabad 2010;22:105–7. [PubMed] [Google Scholar]
- 44.Nwankwo TO, Aniebue UU, Ezenkwele E, et al. Pregnancy outcome and factors affecting vaginal delivery of twins at University of Nigeria teaching Hospital, Enugu. Niger J Clin Pract 2013;16:490. 10.4103/1119-3077.116895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


