Abstract
Introduction
Historic disruption in health infrastructure combined with data from a recent vaccine coverage survey suggests there are likely significant immunity gaps to vaccine preventable diseases and high risk of outbreaks in Timor-Leste. Community-based serological surveillance is an important tool to augment understanding of population-level immunity achieved through vaccine coverage and/or derived from prior infection.
Methods and analysis
This national population-representative serosurvey will take a three-stage cluster sample and aims to include 5600 individuals above 1 year of age. Serum samples will be collected by phlebotomy and analysed for measles IgG, rubella IgG, SARS-CoV-2 antispike protein IgG, hepatitis B surface antibody and hepatitis B core antigen using commercially available chemiluminescent immunoassays or ELISA. In addition to crude prevalence estimates and to account for differences in Timor-Leste’s age structure, stratified age-standardised prevalence estimates will be calculated, using Asia in 2013 as the standard population. Additionally, this survey will derive a national asset of serum and dried blood spot samples which can be used for further investigation of infectious disease seroepidemiology and/or validation of existing and novel serological assays for infectious diseases.
Ethics and dissemination
Ethical approval has been obtained from the Research Ethics and Technical Committee of the Instituto Nacional da Saúde, Timor-Leste and the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, Australia. Co-designing this study with Timor-Leste’s Ministry-of-Health and other relevant partner organisations will allow immediate translation of findings into public health policy, which may include changes to routine immunisation service delivery and/or plans for supplementary immunisation activities.
Keywords: EPIDEMIOLOGIC STUDIES, Diagnostic microbiology, Epidemiology, Public health, STATISTICS & RESEARCH METHODS
Strengths and limitations of this study.
This serosurvey will use a three-stage cluster sample strategy to ensure findings are representative of individuals >1 year of age in Timor-Leste.
Serum samples will be analysed using validated assays and internationally recognised serological cut-offs which relate to vaccine-preventable disease correlates of protection.
All procedures will be undertaken in Timor-Leste, representing an opportunity for local research and laboratory capacity building.
Information on possible immunosuppressive conditions or treatments which may impact on serological responses to infection and vaccination will not be collected.
Serological targets for most pathogens do not distinguish between serological responses to infection and vaccination.
Introduction
The Democratic Republic of Timor-Leste (Timor-Leste) achieved independence in 2002. It is a half-island nation located between Australia and Indonesia with a population of 1.3 million people. The Expanded Programme on Immunisation (EPI) began circa 1989 when Timor-Leste was still an Indonesian province. In 1999, there was a significant disruption in healthcare infrastructure, including the near cessation of routine vaccine delivery. After independence was regained in 2002, the EPI was reinstated as part of a national vaccination programme, initially with single-dose measles vaccine. Hepatitis B vaccination in infancy (three doses) was introduced in 2007. Birth dose hepatitis B vaccine was introduced in 2016, along with combined measles–rubella (MR) vaccination (two doses). Vaccination against SARS-CoV-2 began in adults in April 2021 and children above 12 years of age in October 2021.
The most comprehensive recent assessment of routine childhood vaccination coverage in Timor-Leste was a survey undertaken in 2018 which used a combination of maternal history and vaccination card review to confirm doses of vaccines given in the first and second years of life. This study found variable uptake between different vaccines and across geographic regions, large differences between ‘valid’ and ‘crude’ estimated vaccine coverage, and highlighted the need for further investigation of population immunity to vaccine-preventable diseases (VPDs, see table 1).1
Table 1.
Routine Timor-Leste vaccination schedule in 2022 and estimated coverage in 2018
| Vaccine | Recommended age of administration | Introduced | Crude estimated coverage (2018 national vaccine coverage survey) (95% CI)* (%) | Valid estimated coverage (2018 national vaccine coverage survey) (95% CI)† (%) |
| BCG | At birth | Pre-1999 | 94.7 (91.7–97.0) | 68.8 (61.3–75.0) |
| HepB0 | 2016 | 66.2 (58.5–73.0) | 55.0 (46.9–63.0) | |
| bOPV0 | 2016 | 80.4 (74.0–86.0) | 60.8 (52.2–69.0) | |
| DTwP‐Hib‐HepB1 | 6 weeks | 2007 | 91.8 (87.8–95.0) | 74.6 (68.4–80.0) |
| bOPV1 | 2016 | 91.8 (87.8–95.0) | 75.5 (69.4–81.0) | |
| RV1 | 2019 | Not part of routine vaccination in 2018 | ||
| DTwP‐Hib‐HepB2 | 10 weeks | 2007 | 87.4 (82.6–91.0) | 72.8 (66.7–78.0) |
| bOPV2 | 2016 | 87.8 (83.0–91.0) | 73.2 (66.9–79.0) | |
| RV2 | 2019 | Not part of routine vaccination in 2018 | ||
| DTwP‐Hib‐HepB3 | 14 weeks | 2007 | 83.3 (78.0–87.0) | 75.0 (68.8–80.0) |
| bOPV3 | 2016 | 83.3 (78.0–87.0) | 75.3 (69.0–81.0) | |
| RV3 | 2019 | Not part of routine vaccination in 2018 | ||
| IPV | 2016 | 80.6 (74.1–86.0) | 71.5 (64.6–77.0) | |
| MR1 | 9 months | 2016 | 77.3 (71.5–82.0) | 60.5 (54.0–67.0) |
| DTwP4 | 18 months | 2016 | 54.8 (46.5–63.0) | 16.0 (12.1–21.0) |
| MR2 | 2016 | 54.4 (46.1–62.0) | 12.5 (9.0–17.0) | |
| DT | 6 years or school entry | 2016 | Not measured in 2018 survey | |
| TT1-5 | Unimmunised pregnant women | Pre-1999 | 68.2 (62.4–74.0) | |
| SARS-CoV-2 | Adults and children above 12 years of age | Adults: April 2021 Children: October 2021 |
Not part of routine vaccination in 2018 |
*Crude coverage is defined as all doses, whether valid or not, by any documented evidence or verbal history at the time of the survey.
†Valid coverage includes only the doses of vaccines that were given on or after the minimum date of eligibility and requires a vaccination record (either home based or health facility) with a documented date.
BCG, BCG Calmette-Guérin; bOPV0, bivalent oral polio vaccine; DT, diphtheria and tetanus vaccine; DTwP‐Hib‐HepB, diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type B; HepB, hepatitis B; IPV, inactivated poliovirus vaccine; MR, measles and rubella vaccine; RV, rotavirus vaccine; TT, tetanus toxoid vaccine.
For many pathogens, including measles, rubella, hepatitis B and SARS-CoV-2, specific IgG antibodies can be detected in the blood for many years, and sometimes lifelong, following infection or vaccination. In some cases, a specific quantity and/or quality of antibody in individuals’ sera has been associated with protection from infection on subsequent exposure. Presence above antibody cut-off levels can in some contexts infer protection, although how much such levels correlate with protection varies on a range of factors.2 3 Nonetheless, community-based serological surveillance is an important tool to augment understanding of population level immunity achieved through vaccine coverage over many years and/or immunity to VPDs derived from prior infection.4 The results of serosurveys can be used to guide supplementary immunisation activities (SIAs), and tailor routine immunisation service delivery. There have been no previous community-based studies estimating VPD seroprevalence in Timor-Leste. However, targeted seroprevalence studies sampling healthcare workers in Timor-Leste have identified lower than expected seropositivity against measles, high seropositivity against SARS-CoV-2 and a high prevalence of active hepatitis B infection.5 6
This paper describes the protocol for a first and comprehensive national population-representative serosurvey of multiple VPDs. The survey is also designed to derive a national asset of serum and dried blood spot (DBS) samples which can be used for further investigation of infectious disease seroepidemiology in Timor-Leste.
Methods and analysis
Aim
To determine the seroprevalence of measles, rubella, hepatitis B and SARS-CoV-2 among individuals of different age strata in Timor-Leste.
Design
Population-representative, national cross-sectional serological survey.
Setting
Recruitment and data collection will occur in the community (within households) across Timor-Leste. Laboratory analysis will occur at Laboratório Nacional da Saúde (LNS) in Dili, Timor-Leste.
Sampling methods
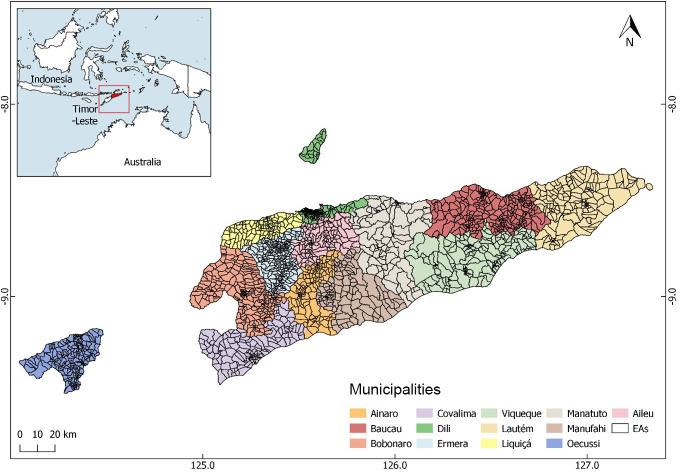
Timor-Leste is made up of 12 municipalities and one Special Region (Região Administrativa Especial de Oecusse Ambeno), some of which are divided into sub-municipalities (Posto administrativos). Atauro is a 14th municipality but through legacy is included as part of the Municipality of Dili. Each (sub-)municipality is divided into sucos (villages), which are further divided into aldeias (hamlets). In 2015, a national census took place in Timor-Leste. All households in the country were visited in-person, assigned a household number and global positioning (GPS) coordinates, and grouped into 2320 ‘enumeration areas’ (EAs, the boundaries of which roughly correspond to those of each aldeia, see figure 1).
Figure 1.
Map of Timor-Leste showing enumeration areas (EAs) within municipalities.
A three-stage cluster random sample will be taken. First, a prespecified number of EAs will be randomly selected from all EAs in the country, with probability proportionate to municipality population. Second, within each participating EA, a prespecified number of households will be randomly selected from all households in that EA. Third, all occupants at participating households who meet eligibility criteria will be invited into the study.
A household will be defined as a dwelling unit that consists of a person or a group of related or unrelated persons, who live together in the same dwelling unit or informal shelter and who are considered as one unit and share a cooking area.
Eligibility criteria
Household members will be eligible to participate if they are ≥1 year of age and they (or their parent/guardian) provide consent to participate in the study. Individuals who report current illness which is compatible with Coronavirus disease (COVID-19), and those who report any of the following conditions will be excluded:
Needle phobia.
Anaemia.
Skin condition affecting phlebotomy sites.
Bleeding disorder.
Additionally, individuals who cannot communicate verbally in Tetun, Portuguese or English will be excluded. Individuals under 1 year of age were excluded because maternal transfer of antibodies may affect interpretation of serology results and because this age group is more challenging to sample.
Sample size
A sample size was calculated with reference to the WHO Reference Manual for Vaccination Coverage Cluster Surveys.7 The investigator group considered which age groups would represent the most important subcategories for estimating VPD seroprevalence. This process took into account the local vaccine programme history (so serological findings can be related to estimated uptake), existing data on vaccine coverage including the referenced vaccine coverage survey (so that data from each source can be triangulated), programmatic considerations relating to potential intervention(s) in case immunity-gaps are identified, and various logistical and technical considerations.
VPD seroprevalence estimates which were considered of particular importance to specific age strata include the following:
Measles in children under 5 years of age because they are most likely to suffer serious sequelae from infection when compared with other age groups8 and between 5 and 14 years of age because outbreaks can occur and/or amplify in schools and other settings where groups of children from different households congregate.
Rubella in women 15–40 years of age because future pregnancies may be at risk of congenital rubella syndrome (CRS). It is anticipated that the rubella virus is circulating in Timor-Leste as there has only been a recent introduction of rubella vaccination and there is very little surveillance for CRS.
SARS-CoV-2 in children 1–12 years of age because seropositivity is likely to represent naturally acquired infection as this group are not eligible for vaccination in Timor-Leste, which will indicate the extent of local transmission which has occurred.
Hepatitis B (surface antibody, HBsAb and core antibody, HBcAb) in children under 5 and 5–14 years of age because hepatitis B birth vaccination was introduced approximately 5 years ago and comparison of these groups will give an indication of uptake.
Therefore, each of the following age groups was considered separate strata to provide age-specific seroprevalence results of sufficient precision: 1–4, 5–14, 15–24, 25–40 and 41+ years.
An effective sample size was calculated (ie, the sample size required if undertaking a simple random sample) of 280 for each stratum. This used the WHO Reference Manual for Vaccination Coverage Cluster Surveys7 and was based on an expected seroprevalence of 50% (because this provided the most conservative estimate) and a precision of ±6% (because this was precise enough to adequately inform decision-making and small enough to provide a feasible sample size for the financial and human resources available) for the 95% CI.
Without local data on which to confidently base estimates of design effect, a conservative design effect of 4 was estimated, to ensure a sufficient sample to provide precise results. This provided a sample size for each strata of 1120 individuals, and a total survey sample size of 5600.
Based on national census data from 2015 the average number of individuals in each household was 5.7.9 Population projections for 2021 estimated the proportion of individuals in Timor-Leste belonging to each age strata to be 9.6%, 24.0%, 21.9%, 20.2% and 21.5%, respectively. As such, the expected number of required households to sample sufficient individuals from the smallest age strata (1–4 years) was calculated as:
1120/(0.096×5.7)= 2047
Household response rate was assumed to be 85%, based on consensus opinion of local investigators who had been involved in previous community surveys in Timor-Leste. The number of households which will be targeted is therefore calculated as:
2047/0.85 =2408
This lead to 112 EAs being selected (with probability proportionate to municipality population), and 23 households being randomly selected from each EA.
Fieldwork procedures
Municipalities will be visited sequentially depending on various logistical considerations including weather and road conditions, availability of staff, vehicles, and accommodation, and local municipal and health leader preference.
First, the study team leader will make a ‘coordination visit’ to the municipality during which they will explain the study procedures, receive permission to conduct the study, and discuss travel routes and gaining access to each EA with the following individuals:
Municipality Administrator (at Municipality Office; one per municipality).
Director of Municipality Health Service (at Municipality Health Service Office; one per municipality).
Sub-Municipality Administrator (at Administrative Post Offices; one for each sub-municipality being visited).
Head of Community Health Centre (at Community Health Centre Office; one for each sub-municipality being visited).
Chief of Suco (at Suco Office;one for every suco being visited).
Commander of Police in Suco (at Suco Police Station; one per suco being visited, at the discretion of the Chief of Suco).
Chief of Aldeia (at Aldeia Office; one for every aldeia being visited).
If any of these individuals are not available in-person during the coordination visit attempts will be made to contact them by telephone or through WhatsApp.
Second, a ‘study visit’ will be made by a whole study team, consisting of a team leader (usually non-clinical), three research nurses and two drivers. Additionally, at the discretion of the Municipality Administrator, Sub-Municipality Administrator and/or Head of Community Health Centre, one or two local government representatives and/or one or two Community Health Centre representatives may join the study visits. It is anticipated that these individuals will primarily observe study procedures. Any involvement in participant recruitment, data collection or sample collection will be directly supervised by the appropriate study team member.
Navigation and maps
Selected households will be identified using electronic tablets which will have GPS capability and Google Earth software installed. Keyhole Markup Language (KML) files with GPS coordinates for all selected households in each EA will be preloaded onto the tablets, such that they can be used without mobile/internet connectivity. KML files are generated in QGIS. Each household location is verified using a Google Satellite base map. Study teams will also carry printed colour copies of bespoke maps for each EA, which will show the location of households in relation to roads, paths and landmarks. These will be produced using ArcMap V.10.4.1 and will include ESRI imagery base map, showing the location of main roads and the selected households coded by letters. The standard operating procedure (SOP) for location of households is shown in online supplemental appendix 1.
bmjopen-2022-071381supp001.pdf (101.4KB, pdf)
Data collection
Study teams will approach the household occupants and introduce themselves. The occupants will be asked to identify an (acting) head-of-household. If this individual is not available (or if no occupants are present), the study team will arrange to return twice to the household, and at least once on a separate day until a head-of-household is present.
Data will be collected using structured interview questionnaires. Responses will be entered into electronic tablets which will have REDCap installed. This is a secure web platform for building and managing online databases which allows offline data entry.10 Questionnaire data will be uploaded to the REDCap secure server hosted at Menzies School of Health Research, Charles Darwin University, Darwin, Australia.
Three bespoke data collection tools have been developed (see online supplemental appendices 2–4):
bmjopen-2022-071381supp002.pdf (45.1KB, pdf)
bmjopen-2022-071381supp003.pdf (39.3KB, pdf)
bmjopen-2022-071381supp004.pdf (18.2KB, pdf)
Household questionnaire. This will be completed first. Demographic data on all household occupants (whether they are present at the time or not), will be collected by interviewing the head-of-household.
Participant questionnaire. This will be completed if/when any household occupants agree to participate. Each will be assigned a unique identification number (participant ID number) and relevant demographic, clinical and vaccine-related data will be collected. Participants will not be asked to provide written documentation of vaccines received because a low proportion of participants in the recent vaccine coverage survey had retained this.1
Unable to complete questionnaire. This will only be completed if the household questionnaire cannot be completed (ie, if a head-of-household was not present or not willing to provide demographic data after three household visits). The reason for non-completion will be recorded in free-text.
Sample collection and handling
Research nurses with training and experience in adult and paediatric phlebotomy will collect primary blood samples using appropriate infection prevention control procedures and safe management of sharps. Participants >5 years of age will undergo venepuncture using either a standard hypodermic or a winged butterfly needle with a syringe attached. Venous blood will then be injected directly into a gel serum separator tube (SST). Participants between 1 and 5 years of age (and those who do not consent to venepuncture but provide consent for a finger prick) may undergo capillary blood sampling through finger prick technique, in which case drops of blood will be applied directly to a paediatric gel SST. The method used will be determined on a case-by-case basis by the research nurse in the field. Table 2 shows sample volumes and collection techniques for participants in different age groups.
Table 2.
Sample volumes and collection techniques for primary sample collection by age group
| Age group | Method of blood collection | Equipment | Collection container | Target sample volume (mL) |
| 1–5 years | Venepuncture* Finger prick* |
23G butterfly needle, venous blood aspirated into 5 mL syringe Lancet, capillary blood drops transferred directly into collection tube |
5 mL SST tube 2 mL paediatric SST tube |
5 2 |
| 6–15 years | Venepuncture | 21–23G needle or 23G butterfly needle, venous blood aspirated into 5 mL syringe | 5 mL SST tube | 5 |
| Adults | Venepuncture | 21–23G needle, venous blood aspirated into 10 mL syringe | 2×5 mL SST tube | 10 |
*Determined in field by phlebotomist on case-by-case basis.
SST, serum separator tube.
Primary blood samples will be kept at ambient temperature out of direct sunlight and allowed to clot for a maximum of 8 hours (ie, 1 day of fieldwork, but usually much less). They will then undergo centrifugation at 1500 RCF for 10 minutes and the resulting separated serum samples will be kept at 4°C using a portable refrigerator with battery power backup. They will be transported to LNS within 5 days of sample collection and will undergo primary serological analysis within 2 weeks of sample collection.
A secondary DBS sample will be created: for participants who undergo venepuncture, the last 300–500 µL of venous blood in the syringe will be injected onto Whatman 903 filter paper marked with three 12 mm diameter circles. For participants who undergo finger-prick, additional drops of capillary blood will be applied directly from the finger to the filter paper. Once the circles are saturated with blood (typically using 100–150 µL blood for each circle), the filter paper will be dried at ambient temperature out of direct sunlight for 4 hours, then placed alongside a desiccant sachet into a plastic zip lock bag. The SOP for data and sample collection is shown in online supplemental appendix 5.
bmjopen-2022-071381supp005.pdf (103.4KB, pdf)
Sample analysis
Primary (serum) samples from all participants will be tested at LNS for rubella IgG (quantitative; considered positive if >10 IU/mL), SARS-CoV-2 antispike IgG (qualitative), hepatitis B core antibody (HBcAb, qualitative) and hepatitis B surface antibody (HBsAb, quantitative, considered positive if >10 mIU/mL) using Ortho Clinic Diagnostics chemiluminescent assays on the Vitros ECiQ platform, and for measles IgG using the Eurimmun ELISA assay (quantitative, positive if >120 IU/L). For quantitative assays, serological cut-offs which have been most commonly shown to correlate with protection from infection and/or those which are conventionally used in serosurveys and/or assessment of immunity have been chosen.11–13 Where data are somewhat conflicting, there is a lack of consensus supporting a correlate-of-protection, or considerable inter-assay variability in quantitative determination has been observed, secondary (exploratory) analyses using alternative cut-offs may also be undertaken (eg, 200 IU/L and/or 250 IU/L for measles IgG).3
Additionally, samples from participants residing in Dili municipality (excluding Atauro) will be tested for hepatitis B surface antigen (HBsAg, qualitative). This marker denotes active hepatitis B infection which may have significant health implications and will therefore only be tested in Dili municipality where there is a hepatology clinic in which participants can receive further assessment and follow-up. Any samples which are positive for HBsAg will also be tested for hepatitis B envelope antigen (HBeAg, qualitative) and hepatitis B envelope antibody (HBeAb, qualitative) testing using Ortho Clinic Diagnostics chemiluminescent assays on the Vitros ECiQ platform, and hepatitis B viral load (quantitative) using the Cepheid assay on the GeneXpert platform. All testing will be carried out according to manufacturers’ instructions and cited serological cut-off values. For qualitative assays, samples with borderline/indeterminate results will be considered negative, apart from HBsAg where the test will be repeated and both results reviewed alongside other hepatitis B results by an appropriately qualified clinical member of the research team who will decide whether the participant should be referred to the hepatology clinic for repeat sampling and clinical assessment.
Assays have been chosen based on their previous performance in seroprevalence studies, immediate availability for shipment to Timor-Leste, and local laboratory expertise in operating these types of assays (with ongoing capacity building for serological testing in LNS). The measles IgG assay is quantitative and calibrated against a WHO Standard (NIBSC, Anti-Measles serum, 3rd International Standard 97/648). It showed acceptable performance when assessed in a recent study of concordance between commercially available assays (concordance for samples with positive/negative status=90%/100%).14 The rubella IgG assay is quantitative and calibrated against a Centers for Disease Control and Prevention standard (Low Titer Rubella Standard) and a WHO standard (1st International Rubella IgG Standard). While concerns around standardisation of rubella IgG assays are noted,15 this assay showed acceptable performance when assessed in a recent study of concordance between automated immunoassays (concordance for samples with negative/positive status=90.6%/91.1%).16 The hepatitis B surface antibody assay is quantitative and showed high sensitivity (97.1%) and specificity (97.9%) when evaluated in a panel of sera from healthcare workers and patients.17 The SARS-CoV-2 anti-spike IgG assay has high sensitivity (93.3%, >21 days postinfection) and specificity (100%), which compares favourably to many other available immunoassays,18 and has been used in several serological surveillance studies.19–21
Provision of results to participants
The majority of testing in this study will be for antibodies against VPDs (either IgG or total antibody). Results will therefore only indicate whether an individual has been previously infected and/or vaccinated against each disease at some time in the past and will not provide information on current infection. While seronegative participants may be at risk of future infections (and may benefit from vaccination), individual notification of results and provision of vaccines to all seronegative study participants is not considered feasible in this large cross-sectional study. Instead, all participants will be advised of the benefits of routine vaccination and immunisation clinics in their area, as well as on any forthcoming SIAs which may occur as a result of this study.
Participants who test positive for HBsAg will be contacted by telephone to discuss their results and will be offered assessment including biochemical and radiological investigation of liver function and consideration of antiviral treatment in-line with international clinical guidelines.22 This approach has been successful and has been acceptable to participants in a smaller serological surveillance study including hepatitis B testing among healthcare workers in Timor-Leste.6
Sample storage
Primary (serum) samples will be stored at −80°C and secondary (DBS) samples will be stored at 4°C at LNS for 10 years. These may undergo additional serological analyses to further investigate infectious disease seroepidemiology in Timor-Leste and/or validating existing and novel serological assays for infectious diseases, pending successful funding application and appropriate ethical approval.
Fieldworker training
Field workers will undergo 1 week of formal in-person training in study procedures. Days 1–2 will be classroom based and will include sessions on ‘the study protocol’, ‘field team composition’ (structure, members, responsibilities), ‘logistics, technology and map reading’, ‘recruitment and consent’ and ‘collection of data using interview questionnaires’. Days 3–5 will be practical and will include demonstrations and training in adult and paediatric phlebotomy and finger-prick techniques, infection prevention control procedures, and the use of personal protective equipment. These skills will be assessed formatively throughout the training and summatively using pre-session and post-session assessments. Training will be delivered by PA, JF, NSSF and/or JY who are clinicians with experience of epidemiological and clinical research in Timor-Leste.
Laboratory team training
Laboratory training will occur in the Serology Department of LNS. Training will be delivered by PA and TO who have significant experience with ELISA and chemiluminescent techniques, including in Timor-Leste. The focus of training will be on assay verification and quality assurance, as well as procedures for sample processing, analysis and storage.
Data storage and handling
Field data will be stored in the REDCap secure server hosted at Menzies School of Health Research, Charles Darwin University, Darwin, Australia, until analysis. Laboratory data (ie, serology worksheets and results) will be stored on the password-protected LNS laboratory information system (SchuyLab) until analysis. Deidentified field and laboratory datasets will be downloaded and stored as password-protected databases on computer(s) at Menzies School of Health Research, Timor-Leste Office, Dili, Timor-Leste, where they will be linked using participant ID numbers and analysed. Only named investigators who are working directly on this project will have access to data.
Statistical analysis plan
Primary data analysis will occur at the end of the study, once all fieldwork is complete and all samples have been analysed. Interim analyses may also occur on reasonable request from the Timor-Leste Ministry of Health (MoH) or other partner organisation. As a multi-stage sampling survey design will be used to select participants, sampling weights will be calculated at each stage. These weights will reflect a participant’s inverse probability of selection at a particular stage, be it at the EA level, the household level or the householder level. Furthermore, these weights will subjected to both non-response adjustment and finite population correction. Measures of prevalence will be age-standardised using the standard population for Asia given by the International Network for the Demographic PNGIMR 2018, Papua New Guinea MIS 2016–2017.23 To account for this design, the ‘svy’ data commands in Stata (V.16) will be used for ally analyses. Characteristics of participants will be summarised using weighted descriptive statistics. Frequencies and proportions will be used to describe categorical distributions, while means and SD will be used to describe continuous variables. In the presence of non-normality, medians and IQRs will be reported. Univariable and multivariable binary logistic regression will be undertaken to model age, the independent variables of primary interest with the five VPD outcomes. In addition to this variable, other variables known to be risk factors of VPD, such as sex and travel history, will be subjected to a manual backward stepwise procedure. Variables with a p value ≥0.20 will not be retained. The Hosmer-Lemeshow test will be used to test the goodness of fit of each multivariable model. A p value <0.05 will be considered statistically significant with ORs, 95% CIs and p values calculated for age and sex.
Timing
This study will begin in January 2022 and is expected to end in December 2023.
Patient and public involvement
Engagement with members of the public, local administrative and health leaders has been central to the design of this study. It has resulted in procedures which will maximise participant choice and ensure any clinically relevant diagnoses made during the study are followed up.
Ethics and dissemination
Informed consent
Each prospective participant will receive a participant information sheet which will be printed in English and Tetum (online supplemental appendices 6 and 7). They will also be provided with a verbal explanation of the study rationale and procedures. This will include potential risks and benefits of sample collection, specific tests which their sample will undergo, the fact that they will not receive notification of any results (with the exception of a positive HBsAg, tested in Dili Municipality only) and the possibility that their sample will undergo additional analyses for evidence of communicable diseases during the next 10 years. They will be given up to 30 min to ask questions and decide whether they wish to participate, and will then provide informed, written consent by signing a consent form (online supplemental appendices 6 and 7). For individuals under 16 years of age, verbal assent will be sought, in addition to written consent from their parent or guardian. This study has received ethical approval from the Research Ethics and Technical Committee of the Instituto Nacional da Saúde, Timor-Leste (Reference: 875 MS-INS/DGE/IX/2021) and the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, Australia (Reference: 2021-4064).
bmjopen-2022-071381supp006.pdf (3MB, pdf)
bmjopen-2022-071381supp007.pdf (3MB, pdf)
Protocol amendments
Any modifications to the protocol which may impact on the conduct of the study will be documented in a formal protocol amendment and approved by both Research Ethics Committees prior to implementation of the changes. The Research Ethics Committees will also be notified of any minor corrections/clarifications or administrative changes to the protocol, which will be documented in a protocol amendment letter.
Adverse events
Data on adverse events will be collected throughout the study, with participants (or their parent/guardian) being informed of the risks of phlebotomy (including bruising, bleeding and infection), how to recognise these, and how to contact the study team if they occur. Adverse events will be reported to the principal investigator. In cases where infection or any other serious adverse event has occurred, the principle investigator will conduct a review of the study visit and decide whether any phlebotomy retraining or change in practice is required and/or whether recruitment to the study should be paused.
Strengths and limitations
This project will produce accurate, nationally representative seroprevalence data for multiple VPDs and relevant age groups, which has not been achieved in Timor-Leste previously. It has been codesigned by investigators at Menzies School of Health Research (Timor-Leste Office), the National Centre for Immunisation Research and Surveillance (Australia), the MoH (Timor-Leste), LNS (Timor-Leste) and the WHO (Timor-Leste Office) according to local research and public health priorities. This will allow immediate translation of findings into public health policy (including potentially changes to routine immunisation service delivery and/or plans for SIAs). Additionally, the survey will derive a national asset of serum and DBS samples which can be used for further investigation of infectious disease seroepidemiology in Timor-Leste and/or validating existing and novel serological assays for infectious diseases.24–28 Engagement with local administrative and health leaders and maximisation of participant choice and welfare have been central to the design, including ensuring all individuals diagnosed with active hepatitis B during the study have access to appropriate further investigation and follow-up.
Limitations include the collection of only a small number of VPD-related clinical variables. This decision was taken because the investigator group felt that conducting prolonged interview questionnaires including potentially sensitive health information may make some potential participants feel uncomfortable, and as such ease-of-questionnaire-administration and survey acceptability±uptake was prioritised. However, interpretation of serology can be affected by underlying immunosuppressive conditions (for example), and therefore absence of these data may affect study findings. The prevalence of such conditions in Timor-Leste is likely to be low, and therefore only to be a minor limitation to our study: Prevalence of HIV in Timor-Leste is estimated to be 0.2% in those aged 15–49 years, and chemotherapy for malignant conditions (except for corticosteroids) is largely unavailable. Another limitation is the exclusion of individuals who report current illness which is compatible with COVID-19. It is possible that this could lead to underestimation of SARS-CoV-2 seroprevalence. However, since acute illness is relatively short lived (<2 weeks for the majority of people) when compared with the duration of anti-S seropositivity (typically many months), this effect will likely be low, but will depend on the timing of fieldwork in relation to any local outbreaks of SARS-CoV-2 (ie, the prevalence of acute infection in relation to overall seroprevalence, at the time of survey). A final limitation relates to the choice of serological targets, which (except for hepatitis B) will not differentiate vaccine-derived from infection-derived immunity. For example, SARS-CoV-2 anti-S antibodies will be tested, but not antinucleocapsid antibodies. This decision was taken because targets related to population immunity (ie, those correlating with protection) were considered most important, regardless of its source and additional targets would be costly. Additionally, some whole-virus vaccines will likely be used in Timor-Leste, which cannot be differentiated with such methods.
Risks include the ongoing global outbreak of SARS-CoV-2 (which may delay/prohibit study visits), disruption of supply of field and laboratory consumables to Timor-Leste (which may delay/increase the cost of laboratory analysis), natural disasters such as flooding, and potential unwillingness of individuals to participate in provision of data and/or samples (which may affect recruitment, potentially disproportionately among children).
Dissemination/knowledge transition plan
After each interim analyses, results will be shared with Timor-Leste MoH partners in the form of an oral presentation and in a written report. Following completion of the study, results will be shared with Timor-Leste MoH, other partner organisations, and local administrative and health leaders for EAs where the study took place (Municipality Administrators, Directors of Municipality Health Services, Sub-Municipality Administrators, Heads of Community Health Centres, Chiefs of Sucos, Chief of Aldeia), in the form of a written report. Results will also be submitted for publication in peer-reviewed journals and presented at relevant international conferences.
Supplementary Material
Footnotes
Twitter: @thefrancis6
Contributors: PA, SLS, NM, SA, NS, CF, FdNM, NSSF, KM, JY and JRF conceived the study. PA, SLS, NM, MYT, SA, ADKD, NS, LA, CF, FdNM, CG reviewed existing literature and performed situation analysis. PA, MYT, SA, VS, LA, CG, IdCB determined the fieldwork procedures and designed data collection tools. PA, NG, TO, NS, EdS, LA, IdCB determined laboratory procedures. PA, MD, ADKD, NS, NSSF drafted the data and statistical analysis plan. NM, MYT, SA, NS, CF, FdNM, CG planned community engagement, obtained ethical approval and lead other regulatory aspects of the study. All authors reviewed and commented on the final manuscript.
Funding: This work was supported by the Department for Foreign Affairs and Trade, Australian Government (Complex Grant Agreement Number 75889).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Organization . Timor-leste: WHO and UNICEF estimates of immunization coverage: 2019 revision; 2021. 1–18.
- 2.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008;47:401–9. 10.1086/589862 [DOI] [PubMed] [Google Scholar]
- 3.Bolotin S, Hughes SL, Gul N, et al. What is the evidence to support a correlate of protection for measles? A systematic review. J Infect Dis 2020;221:1576–83. 10.1093/infdis/jiz380 [DOI] [PubMed] [Google Scholar]
- 4.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010;17:1055–65. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arkell P, Gusmao C, Sheridan SL, et al. Serological surveillance of healthcare workers to evaluate natural infection- and vaccine-derived immunity to SARS-CoV-2 during an outbreak in DILI, timor-leste. Int J Infect Dis 2022;119:80–6. 10.1016/j.ijid.2022.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gusmao C, Tanesi MY, Gomes N, et al. Seroprevalence and prevention of hepatitis B, measles, and rubella among healthcare workers in DILI, timor-leste. SSRN Journal 2022. 10.2139/ssrn.4186798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation . World Health Organization vaccination coverage cluster surveys: reference manual; 2018.
- 8.Moss WJ. Measles. Lancet 2017;390:2490–502. 10.1016/S0140-6736(17)31463-0 [DOI] [PubMed] [Google Scholar]
- 9.Direccao Geral de Estatistica G of T-L . Population and housing census 2015: preliminary results. [Google Scholar]
- 10.REDCap . Available: https://www.project-redcap.org/ [Accessed 25 Aug 2022].
- 11.World Health Organisation . WHO immunological basis for immunization series module 7: measles. In: Immunological basis for immunization series. 2020: 18. [Google Scholar]
- 12.World Health Organization . The immunological basis for immunization series module 11: rubella. 2008. Available: https://apps.who.int/iris/bitstream/handle/10665/43922/9789241596848_eng.pdf;jsessionid=B50F1DE9E021388B2DBB9C7A8B76F6E5?sequence=1 [Accessed 13 Sep 2022].
- 13.World Health Organization . The immunological basis for immunization series module 22: hepatitis B; 2011.
- 14.Tischer A, Andrews N, Kafatos G, et al. Standardization of measles, mumps and rubella assays to enable comparisons of seroprevalence data across 21 European countries and Australia. Epidemiol Infect 2007;135:787–97. 10.1017/S0950268807008266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimech W, Grangeot-Keros L, Vauloup-Fellous C. Standardization of assays that detect anti-rubella virus IgG antibodies. Clin Microbiol Rev 2016;29:163–74. 10.1128/CMR.00045-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimech W, Arachchi N, Cai J, et al. Investigation into low-level anti-rubella virus IgG results reported by commercial immunoassays. Clin Vaccine Immunol 2013;20:255–61. 10.1128/CVI.00603-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huzly D, Schenk T, Jilg W, et al. Comparison of nine commercially available assays for quantification of antibody response to hepatitis B virus surface antigen. J Clin Microbiol 2008;46:1298–306. 10.1128/JCM.02430-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of 16 serological SARS-cov-2 immunoassays in 16 clinical laboratories. J Clin Microbiol 2021;59:2596–616. 10.1128/JCM.02596-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santarelli A, Lalitsasivimol D, Bartholomew N, et al. The seroprevalence of SARS-CoV-2 in a rural Southwest community. J Am Osteopath Assoc 2021;121:199–210. 10.1515/jom-2020-0287 [DOI] [PubMed] [Google Scholar]
- 20.Ng DL, Goldgof GM, Shy BR, et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun 2020;11:4698. 10.1038/s41467-020-18468-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin DK, Nesbitt DJ, Yang J, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in a cohort of New York city Metro blood donors using multiple SARS-cov-2 serological assays: implications for controlling the epidemic and "reopening." PLoS One 2021;16:e0250319. 10.1371/journal.pone.0250319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lampertico P, Agarwal K, Berg T, et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 23.Sankoh O, Sharrow D, Herbst K, et al. The indepth standard population for low- and middle-income countries, 2013. Global Health Action 2014;7:23286. 10.3402/gha.v7.23286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arkell P, Angelina J, do Carmo Vieira A, et al. Integrated serological surveillance of acute febrile illness in the context of a lymphatic filariasis survey in timor-leste: a pilot study using dried blood spots. Trans R Soc Trop Med Hyg 2022;116:531–7. 10.1093/trstmh/trab164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basile AJ, Horiuchi K, Panella AJ, et al. Multiplex microsphere immunoassays for the detection of IgM and IgG to arboviral diseases. PLoS One 2013;8:e75670. 10.1371/journal.pone.0075670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyson J, Tsai W-Y, Tsai J-J, et al. A high-throughput and multiplex microsphere immunoassay based on non-structural protein 1 can discriminate three flavivirus infections. PLoS Negl Trop Dis 2019;13:e0007649. 10.1371/journal.pntd.0007649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornace KM, Senyonjo L, Martin DL, et al. Characterising spatial patterns of neglected tropical disease transmission using integrated sero-surveillance in northern Ghana. PLoS Negl Trop Dis 2022;16:e0010227. 10.1371/journal.pntd.0010227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Hall T, Ssewanyana I, et al. Optimisation and standardisation of a multiplex immunoassay of diverse Plasmodium falciparum antigens to assess changes in malaria transmission using sero-epidemiology. Wellcome Open Res 2019;4:26. 10.12688/wellcomeopenres.14950.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071381supp001.pdf (101.4KB, pdf)
bmjopen-2022-071381supp002.pdf (45.1KB, pdf)
bmjopen-2022-071381supp003.pdf (39.3KB, pdf)
bmjopen-2022-071381supp004.pdf (18.2KB, pdf)
bmjopen-2022-071381supp005.pdf (103.4KB, pdf)
bmjopen-2022-071381supp006.pdf (3MB, pdf)
bmjopen-2022-071381supp007.pdf (3MB, pdf)