Abstract
Introduction
Clinically important upper gastrointestinal bleeding is conventionally defined as bleeding accompanied by haemodynamic changes, requiring red blood cell transfusions or other invasive interventions. However, it is unclear if this clinical definition reflects patient values and preferences. This protocol describes a study to elicit views from patients and families regarding features, tests, and treatments for upper gastrointestinal bleeding that are important to them.
Methods and analysis
This is a sequential mixed-methods qualitative-dominant multi-centre study with an instrument-building aim. We developed orientation tools and educational materials in partnership with patients and family members, including a slide deck and executive summary. We will invite intensive care unit (ICU) survivors and family members of former ICU patients to participate. Following a virtual interactive presentation, participants will share their perspectives in an interview or focus group. Qualitative data will be analysed using inductive qualitative content analysis, wherein codes will be derived directly from the data rather than using preconceived categories. Concurrent data collection and analysis will occur. Quantitative data will include self-reported demographic characteristics. This study will synthesise the values and perspectives of patients and family members to create a new trial outcome for a randomised trial of stress ulcer prophylaxis. This study is planned for May 2022 to August 2023. The pilot work was completed in Spring 2021.
Ethics and dissemination
This study has ethics approval from McMaster University and the University of Calgary. Findings will be disseminated via manuscript and through incorporation as a secondary trial outcome on stress ulcer prophylaxis.
Trial registration number
Keywords: INTENSIVE & CRITICAL CARE, Protocols & guidelines, Adult intensive & critical care
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The protocol describes a rigorous process for building a measure which reflects patient preferences.
The protocol was developed in partnership with patient and family members.
Proposed participants are those with personal or caregiving experience of the adult intensive care unit (ICU).
Patient partners may differ in demographic and experiential traits from the general ICU population.
Introduction
Patient and family engagement occurs through an active partnership forged among patients, families, clinicians and researchers to improve both health and care.1 Through their lived experience, patients and families provide a unique perspective on various aspects of research, including investigational priorities.2 Their engagement can lead to better outcomes and improved satisfaction for patients and families, and cost savings for the healthcare system.3 For patients in the intensive care unit (ICU) who are usually unable to participate in their own care due to the severity of their illness, partnering with ICU survivors and family members is garnering increased attention.4–6
Ethically and scientifically compelling, patient involvement in critical care research can build on a proliferation of strategies for meaningful involvement of patient partners in health research to help ensure that the study outcomes are relevant and meaningful to future patients.6–10 In service of this tenet, there is a need to create a measure of upper gastrointestinal bleeding that is important to patients and their families. In critically ill patients, minor bleeding is extremely common, but major bleeding is rare, as documented using an ICU-specific bleeding instrument capturing bleeding from any body site.11 Bleeding from the upper gastrointestinal tract is a well-known complication of critical illness. Early investigations in the ICU setting examining the epidemiology, risk factors and consequences of upper gastrointestinal bleeding often use an outcome of ‘clinically important bleeding’ which was developed from the practitioner’s perspective. The criteria were based on aberrant physiology and associated required interventions,12 13 modified to distinctly incorporate vasopressors.14 15 Clinically important upper gastrointestinal bleeding is defined as overt bleeding in the absence of other causes with one of the following features: (1) spontaneous decrease in systolic blood pressure (SBP) or diastolic blood pressure of >20 mm Hg within 24 hours of upper gastrointestinal bleeding, (2) an orthostatic increase in heart rate >20 beats/min and a decrease in SBP of >10 mm Hg, (3) initiation of vasopressors or increase in their infusion rate of >20%, (4) a decrease in haemoglobin of > 20 g/L in 24 hours or (5) transfusion of >2 units of red blood cells within 24 hours of bleeding. While this definition has been used in several large studies, it does not take into account the views of patients and/or their families.
Other definitions and classifications of bleeding from any site are available, such as those of the WHO16 and International Society of Hemostasis and Thrombosis17 and the HEmorrhage Measurement (HEME) tool that was specifically developed to classify bleeding in critically ill patients.11 However, none of these definitions or tools are focused on bleeding from the gastrointestinal tract, and none have been developed with patient and family input. Incorporating patient and family perspectives is crucial to ensure that bleeding research not only acknowledges, but intentionally incorporates patient preferences and experiences—whether they are aware of, or personally experienced or observed this type of bleeding.
Aligned with the International Association of Public Participation principles,18 we will collaborate with ICU survivors and family members in research with the dual purpose of learning from their experiences and integrating their perspectives on which aspects of upper gastrointestinal bleeding during critical illness are most important to them. We understand ‘patient important bleeding events’ to be those that would, in the absence of any other benefits, lead patients to consider receiving an intervention to treat the bleed, or are associated with appreciable harm, distress, burden or personal cost.17 19 However, some aspects of bleeding that concern clinicians may not concern patients in the ICU who are generally unaware of adverse events due to their impaired consciousness. For example, receipt of inotropes or vasopressors may not be as meaningful to ICU patients as to clinicians, as critically ill patients are typically unaware that the infusion represents a form of advanced life support. By contrast, transfusions may be more concerning to patients than clinicians, especially if they are not fully informed of contemporary blood product safety.20
In critical care medicine, there is a dearth of research directly informed by legitimate public engagement, representing untapped potential.6 This Patient Important Bleeding Study will engage patients and families to create a definition of what matters most to them regarding tests and treatments used for upper gastrointestinal bleeding.21 Results will directly inform the definition of patient-important bleeding which is a secondary outcome in an ongoing international trial comparing stress ulcer prophylaxis with pantoprazole versus placebo—the Re-EValuating the Inhibition of Stress Erosions (REVISE) trial—the primary outcome of which is clinically important upper gastrointestinal bleeding.22
Objective, question and hypothesis
The overall objective of this study is to elicit the views of patients and families regarding features, tests and treatment for upper gastrointestinal bleeding that are important to them. The research question is ‘What are the most concerning tests and treatments to patients and families in the event of an upper gastrointestinal bleed that occurs in the ICU?’. Our hypothesis is that patients and families will be concerned about some bleeding tests and treatments (eg, invasive procedures), while they will be comfortable with others (eg, vasopressor infusion into a pre-existing intravenous access), even if this represents increased treatment intensity. We also hypothesise that regardless of their views regarding particular tests or treatments, they will be concerned if bleeding results in a longer hospital stay or if a patient dies with or from bleeding.
Design
This is a sequential mixed-methods, qualitative-dominant, multi-centre study with an instrument-building aim.23 24 In this protocol manuscript, we describe the collection and analysis of qualitative data used to build an instrument, operationalised as a multicomponent definition of patient-important bleeding. This instrument will be used to collect quantitative data for an outcome in patients enrolled in an international Randomized Controlled Trial of stress ulcer prophylaxis (REVISE trial). Pilot work began in 2021 and trial completion is anticipated in August 2023.
Participants
Adult patients >18 years of age who were admitted to ICU >72 hours and family members of adult ICU patients in ICU for >72 hours (unlinked), regardless of bleeding experience, ICU survival, health literacy or professional healthcare training. Eligible patient participants must have been discharged from hospital after their episode of critical illness. Individuals will be excluded if they have prohibitive communication challenges (eg, serious psychological or psychiatric illness that prevents the individual from consenting to participate in research or providing their perspective, insufficient ability to read and speak English or other languages for which a research staff or family interpreter exists). All experiential data will be self-reported, consistent with best practices in qualitative research. To avoid confounding by previous participation in related research, we will exclude patients enrolled in REVISE and family members of patients enrolled in REVISE.
We are purposeful in our decision not to make personal experience with upper gastrointestinal bleeding an inclusion criterion; most patients and families who encounter this type of bleeding do so for the first time and will make judgments about the importance of that outcome from that perspective. By recruiting participants who have not experienced or witnessed bleeding, but who imagine themselves in this situation, we will identify a participant population most similar to patients and families who will encounter this clinical scenario. However, personal experience with gastrointestinal bleeding from a patient’s perspective, and bearing witness to gastrointestinal bleeding from a family perspective is not an exclusion criterion, to reflect a range of perspectives for this study.
Sampling strategies
Multiple perspectives will be sought by sampling ICU survivors and family members of critically ill patients with diverse demographics and life experiences across several jurisdictions. Qualitative research uses non-probabilistic sampling approaches to obtain information-rich and relevant perspectives that respond to the research question.25 26 We will use criterion sampling to identify possible participants who satisfy our inclusion and exclusion criteria. We will use convenience sampling based on contacts of our investigative clinical team. We will use chain referral (snowball) sampling to identify other possible participants working as hospital-based or research-associated patient or family partners. The initial sample will use a maximum variation approach so that analysis of preliminary data may identify relevant experiential or demographic traits which should be explored with further criterion sampling.
To invite participants, we will engage pre-existing patient and family partners involved in the Patient and Community Engagement Research (PaCER) group, seeking contact using existing mailing lists and social media groups, including those of the Alberta SPOR (Strategy for Patient-Oriented Research) Support Unit. We will use similar strategies to invite potential participants associated with the Canadian Critical Care Trials Group (CCCTG) Patient and Family Partnership Committee.27 In Kingston, London, Toronto, Ottawa and Hamilton, our team of clinical investigators will email potential participants drawn from existing patient and family partners who are affiliated with their healthcare organisations or studies. The invitational emails will contain information about the study and ask potential participants to contact the investigators if interested.
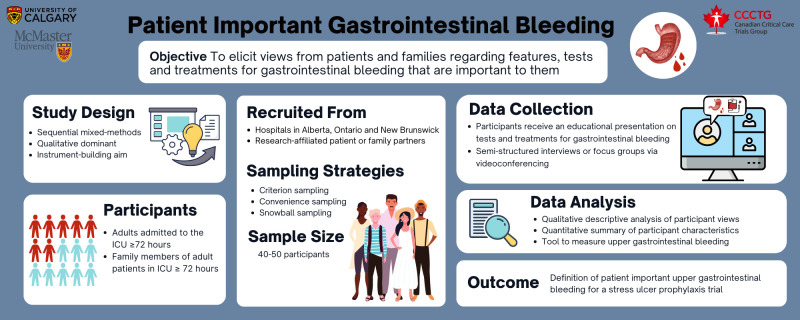
We created an infographic to depict the study methods to share with potential participants, particularly those who are already research partners in other studies (figure 1).
Figure 1.
Protocol infographic.
Sample size
The sample size projection is based on our estimate that approximately 40–50 individual participants will be needed to reach data saturation. This method of assessing sufficiency of qualitative data requires periodic assessment by multiple individuals who reach consensus through discussion on whether existing data adequately answers the research question and allows the researchers to offer a consistent explanation for all relevant perspectives.28 The final sample size will be confirmed as data collection progresses, but the theory of information power indicates we will likely need a large sample due to the heterogeneity of experiences, relatively little direct experience with the phenomenon of interest, and the lack of an underpinning explanatory theory to explain what aspects of upper gastrointestinal bleeding are likely to be meaningful to patients and families.29 Data saturation will be assessed periodically by five investigators through a review of transcripts and coding reports, and audit trail examination; a description of this process will be included in the final manuscript. Feasibility of enrolment will be met when at least 15 patients and at least 15 family members are recruited, with representation from several regions, strong representation from each decision-maker (patient, family), and at least 80% participation for invited individuals.
Preparatory work
In preparation for this study, in Calgary, we developed the orientation and education tools, refined with input from a patient partner, family partner, bedside ICU nurse and three research staff not involved in the project. In Hamilton, informal in-person discussions with eight ICU patients who experienced gastrointestinal bleeding helped to plan the scope of questions for the interview and focus group guide. A mock interview with a patient partner and a five-person mock focus group in Calgary informed the content, order and pacing of the questions, as well as the degree of detail and terminology.
Orientation and education tools
Informed input from patients and families requires a basic understanding of the various presentations of upper gastrointestinal bleeding, possible physiologic changes, diagnostic tests and therapeutic interventions. In partnership with patients and family members, we developed a slide deck containing approximately 20 images of upper gastrointestinal bleeding, tests and treatments as a companion to the verbal presentation that will orient participants preceding each interview or focus group. Each test and treatment are described in terms of how commonly it is used, its purpose, and possible discomforts or side effects. We also created a two-page written summary describing upper gastrointestinal bleeding, tests and treatments in text directed at grade 8 reading level. Thus, we will use written visual and oral approaches to depict and discuss the phenomena tailored to a lay audience, prior to the interviews and focus groups.
Pilot testing of education and orientation tools
Before finalising the written summary and slide deck, we obtained unstructured feedback until no new feasible ideas for improvement were obtained. From a pre-existing group of patient and family partners affiliated with the PaCER group, suggestions from two patients and two family members were captured with typed notes, coded and anonymised at source (online supplemental appendix text 1).
bmjopen-2022-070966supp001.pdf (88.6KB, pdf)
Interview and focus group guide development
Employing both interviews and focus groups allows triangulation of data collection methods,30 we developed a four-page interview and focus group guide using open-ended questions to elicit patient and family views of what matters most about this complication of critical illness. We started with in-person and e-discussions among the investigative team. We partnered with one former ICU patient associated with the PaCER group and one family advisor associated with the CCCTG Patient and Family Partnership Committee. While the guide focuses on asking questions about how participants evaluate particular tests and treatments, it also includes open-ended questions about what aspects or consequences of upper gastrointestinal bleeding matter most to participants. We anticipate that participants may raise concerns about bleeding-associated morbidity and mortality here (eg, death is likely to be identified as a patient-important outcome if it occurred due to bleeding).
Pilot testing of interview and focus group guides
We elicited feedback on the clarity, comprehensiveness and redundancy of the questions and prompts in the draft interview and focus group guides, modifying them per suggestions. This was achieved by a pilot interview with one former ICU patient and a pilot focus group of five family members in Calgary and Hamilton (six persons in total). Quantitative descriptors of pilot participants were anonymised and entered in an Excel V.16.6 database (Microsoft Corporation, Redmond, Washington). Feedback from the pilot interview and focus group was captured with typed notes, anonymised at source for future use, but was neither audiotaped nor transcribed (online supplemental appendix text 2).
bmjopen-2022-070966supp002.pdf (113.5KB, pdf)
Interviewer training
Two experienced qualitative interviewers in Hamilton and Calgary received training to harmonise their interviewing approach. We ensured calibration by having them use a common guide, both attending interviews and focus groups in the pilot phase, and discussing data collection at team meetings.
Main study
Qualitative data collection: individual interviews and focus groups
We will conduct individual interviews (45–60 min in duration) and focus groups (90–120 min in duration) with former patients or family members associated with healthcare institutions in Hamilton, Kingston, London, Ottawa, Toronto or Calgary and other cities as determined by snowball sampling methods. Focus groups will be comprised 2–5 patients or family members. All participants will receive a $25 gift card to thank them for their time.
One of two interviewers and one field note taker not involved in the REVISE trial will be present at each interview or focus group, along with the participant(s) and the investigator who will give the presentation. Following introductions, the interviewer will affirm consent and refer to the precirculated two-page document summarising tests and treatments. An orienting interactive slide presentation will follow, encouraging questions or clarifications on the content, after which the presenter will leave the videoconference. Although discussion about costs to the healthcare system may arise, we will clarify that our focus is not the cost of tests or treatments, or the economic consequences of bleeding.
The interview will be audio-recorded and transcribed verbatim. The field note taker will record observations during and after each interview or focus group. These notes will record non-verbal communication (eg, nodding in agreement with a verbal comment of another participant), reflect on process issues, and offer summaries of key ideas shared during the data collection session. At the end of each interview or focus group, we will ask participants to reflect on their research experience, which will also be incorporated into typed field notes, coded and anonymised at source.
Quantitative data collection
We will obtain quantitative data describing participants including age, sex, race, city of residence and any professional healthcare role. About the patient, we will collect the hospital name, reason for the patient’s ICU admission, and (if known to participant) whether the patient had experienced gastrointestinal bleeding in the ICU. We recognise that participants may not know if upper gastrointestinal bleeding developed in the ICU. Given that experiencing or witnessing a bleed may inform participant perspectives on bleeding, documenting a bleeding event is only relevant if the participant was aware of the bleeding. For this reason, we will not objectively verify whether the patient developed bleeding. For family members, we will document their relationship to the patient (eg, child, partner, sibling, friend), and corresponding information as above.
Analyses
Qualitative analyses
De-identified transcripts will be imported into NVivo (QSR International, Melbourne, Australia) for data management and analysis. We will conduct a qualitative descriptive analysis, aiming to create a descriptive summary of study findings, organised and presented in the language of the participants with minimal theoretical interpretation.31 Data will be analysed using qualitative content analysis, whereby codes are derived directly from the data rather than using preconceived categories.32 As data collection proceeds, new information and insights will be incorporated into data collection and analysis, making the processes reflexive and interactive.
Five investigators will participate in the initial (open) coding, reading data to form a comprehensive list of codes. Specifically, we will use open coding, group discussion and reconciliation, to identify categories reflecting patient-important considerations (eg, familiarity, safety, effectiveness, invasiveness, etc) on which we will centre additional data collection and coding (focused coding). These considerations will be derived inductively from participant comments on bleeding characteristics, tests and treatments that matter most to them. For example, the familiarity of a test, or the effectiveness of the treatment might be identified as key patient-important considerations.
The next round of coding will involve deductively matching each consideration to participants’ expressions about each test or treatment. This focused framework coding will generate data about how each test or treatment is understood in relation to the general patient-important considerations. For example, at this stage we will be able to describe how participants perceive the safety of endoscopy as a test, and how they perceive the invasiveness of angioembolisation as treatment.
In the next round of coding, investigators will work to further categorise each test or treatment according to each consideration. For example, to what degree are participants concerned about the effectiveness of acid suppression? In this stage, we will also describe how consistently participants comment on each test or treatment in light of these considerations and assess the degree to which participants have convergent or divergent views.
Preliminary results will be shared with the broader group of interdisciplinary collaborators for further discussion (investigator triangulation). Results will also be shared with 2–4 patients and family participants via videoconference meeting to inquire about whether the findings resonate with their perspectives, exploring the credibility of the findings (member checking).33
Quantitative analyses
Data describing patient and family member characteristics, as described in the Quantitative Data Collection section, will be analysed using descriptive statistics, measures of central tendency and dispersion and proportions.
Data integration
The current study is designed with an instrument-building aim. Qualitative data will be translated into a measure for use as a secondary outcome of the ongoing REVISE trial. The planned translation of qualitative data into a secondary trial outcome will involve the creation of a binary variable for ‘patient-important bleeding’. The qualitative data analysis will inform a list of tests, treatments, or clinical outcomes which if experienced, constitute patient-important bleeding. If REVISE trial participants have had bleeding which led to the use of one of those tests or treatments, they will be deemed to have experienced patient-important gastrointestinal bleeding. In the absence of bleeding or absence of bleeding leading to test or treatment of concern to patients or family members, REVISE trial participants will be classified as not to have experienced patient-important gastrointestinal bleeding. If REVISE trial participants have had bleeding which directly resulted in death, and participants state that death is deemed to be a patient-important outcome, they will be deemed to have experienced patient-important gastrointestinal bleeding.
How will the results be used?
The findings from this study will have several implications. From the research perspective, results will be used to refine a novel secondary outcome of the ongoing REVISE trial, ensuring that the evidence produced by the trial will be patient and family-centred. The design could serve as a template for clinical research methodologists interested in meaningful citizen engagement in research. This new outcome will be useful for investigators recognising the importance of incorporating patient and family perspectives when designing studies on the incidence, risk factors, consequences, prevention and management of upper gastrointestinal bleeding in the ICU.
Bleeding rates in the literature may be more conditional on different bleeding definitions and assessment methods than on actual bleeding.34 Unclear and variable gastrointestinal bleeding definitions across studies over decades make inferences challenging when summarising studies about gastrointestinal bleeding rates, risk factors and consequences. This study will inform the interpretation of future randomised trials, systematic reviews, network meta-analyses35 and practice guidelines with an emphasis on the values of patients and families.
From the practice perspective, the results of this study will inform clinicians about how to better support patients and families to explain the characteristics of diagnostic and treatment options when upper gastrointestinal bleeding occurs in the ICU. From the educational perspective, our data will help clinical teachers understand how bleeding is perceived by patients and families, aiding conversations and counselling regarding tests and treatments for bleeding which are of greatest concern to them. From the health system perspective, the results of this study will further the goal of person-centred healthcare which honours patient and family values and perspectives as key evidence.
Patient and public involvement
As a mixed-methods study, whereby qualitative data are dominant and patient and family partnership is paramount, we have already engaged several ICU survivors and family members in completed pilot work. They have helped to develop the educational tools, improve the data collection instruments, and refine the interview guide. We will orient participants to the problem of upper gastrointestinal bleeding by a precirculated text summary and standardised slide deck that was cocreated by patient and family partners. To ensure that participants have an understanding of the ICU context, we will use three sampling strategies, including criterion sampling to recruit participants who have lived experience with critical illness but avoid an exclusive focus on participants with self-reported high health literacy. An experienced patient partner and family partner are study coinvestigators. Results will be shared with participants in two ways. First, all participants will receive an optional invitation to attend a member-checking session, where results will be shared and feedback solicited. This input may be used to further refine results. Final results will be disseminated to all participants via an infographic with accompanying one-page study brief.
Discussion
Strengths
Additional study strengths include the methods which accord with increasingly recommended or required patient involvement in the design, conduct and dissemination of health research.3 36 37 The qualitative methods allow us to organise, clarify and summarise non-numerical data to build a definition of patient-important bleeding. Future findings will be grounded in the views of members of the public with lived critical care experience, rather than specialised practitioners. To maximise the generalisability of responses, this multicenter study will include participants reflecting hospital catchment areas in at least three Canadian provinces. A constructivist approach to qualitative inquiry permits patient and family member participants to share information about what truly matters to them, even when those perspectives might conflict with definitions of clinically important bleeding developed by clinicians and researchers.38 Multiple analysts with different interprofessional and interdisciplinary perspectives contribute to the usefulness and trustworthiness of this research.39
Limitations
Limitations of this study include no numerical measures of bleeding attributes or preference rankings, as we are eliciting views and values from patients and families using an open-ended, qualitative approach. The goal is not to exclusively characterise morbidity and mortality features of the bleed that are concerning (eg, short-term risk of death or long-term disability); indeed we assume that bleeding which leads to death or disability is very important to patients. Our main focus is on tests and treatments used to locate and limit the bleeding in order to add additional granularity to what patients find important about bleeding beyond the obvious consequences of dying with or from bleeding. Context is crucial here; outpatients and ward patients with acute or chronic illnesses may have different concerns than ICU patients (eg, they may be understandably more alarmed about minor bleeds compared with ICU survivors and their families). Thus, our results will not apply to bleeding from sites other than the gastrointestinal system, or to community-dwelling citizens or hospitalised patients who are not critically ill.
Future research implications
While the patient-important gastrointestinal bleeding definition derived from this study will serve as the quantitative instrument for this secondary outcome in the REVISE trial, results will also have implications for sample size calculations in future trials on this topic.40 Patients’ and clinicians’ views may differ when considering trade-offs related to bleeding. When the current study and the REVISE trial are complete, it would be worthwhile to explore patient and family perceptions about the balance of risks and benefits of pantoprazole prophylaxis in terms of bleeding, pneumonia, Clostridioides difficile and mortality. One study of physicians who treat atrial fibrillation and patients with, or at risk of, developing atrial fibrillation, explored the maximal increased risk of bleeding that respondents would tolerate with warfarin vs aspirin to achieve a reduction in stroke over 2 years.41 The variability in patient and physician values regarding trade-off between bleeds and strokes likely reflects differential aversion to anticoagulation-associated bleeding and stroke risks. Another study of diverse healthcare providers showed substantial variation in whether and when to restart oral anticoagulation after gastrointestinal bleeding.42
Ethics and dissemination
This study has Research Ethics Board (REB) approval at McMaster University (HiREB #9492), and the University of Calgary Conjoint Health Research Ethics Board (REB20-0120).
Potential adverse effects of patient engagement in research from patients’ perspectives identified in a recent systematic review related to frustrations with training, transportation or tokenism—or a false impression of inclusiveness, thereby devaluing patients’ input.9 Advice from our patient partner and family partner who are investigators on this study will ensure that we collaborate sensitively, avoid inauthentic engagement, ensure respectful communication, and offer compensation for their time.
Findings will be disseminated using an integrated knowledge translation framework. The integrated approach to knowledge translation is reflected in several patients and family members being integral to the pilot work. Furthermore, a CCCTG Patient and Family Partnership Committee family member and an experienced patient partner are coinvestigators who helped to design this study. End-of-study knowledge translation will include incorporating results to refine our placeholder definition of patient-important bleeding—presently overt bleeding resulting in invasive tests or treatments.
We will share findings at investigator, CCCTG and other meetings. Peer-review presentations at international conferences in critical care, gastroenterology and haematology will coincide with or precede open-access peer-review publications. We will translate findings into different languages for diverse audiences in traditional and social media. Our patient and family coinvestigators will help to create an infographic of our findings and clinician-facing educational materials to teach about procedural explanations for gastrointestinal bleeding.
Supplementary Material
Acknowledgments
This Patient Important Bleeding Study could not be completed without the patients and family members who agreed to share their time and perspectives. We are grateful to the Patient and Community Engagement Research Partners including Nadine Foster and Bonnie Sept who piloted the interview and focus group guide and provided feedback on the two-page orienting document and slide deck presentation. We appreciate the preparatory work for this project by Nubia Zepeda and Carmen Hiploylee from the University of Calgary Critical Care Research Group, as well as Alyson Takaoka, Mary Copland and Nicole Zytaruk at McMaster University. We thank the Research Coordinators and Investigators of the REVISE trial and the Canadian Critical Care Trials Group for their encouragement on this project, as well as Dr Danae Tassy for suggestions on this manuscript. We would like to acknowledge the thoughtful comments from BMJ Open reviewers Drs Riccardo Levi, Stefano Skurzak, Riccardo Marmo and Xingshun Qi.
Footnotes
Twitter: @icuresearch, @brambo43, @Ball, @MGVanstone
Correction notice: This article has been corrected since it was published. Canadian Critical Care Trials Group has been added in the author list. Middle initials have been added in author names.
Contributors: All authors contributed towards the original research idea and provided input into the design of the study. DJC, ST, MES, KDK, MVa designed the protocol, drafted and revised the manuscript.
Funding: This study was funded by a grant from the Hamilton Academic Health Sciences Organization (Grant No. #HAH-22-009).
Competing interests: The following individuals are investigators for the randomised trial REVISE: DJC, JCD, GHG, WA, KEAB, JCM, JGM, SF, AMD, JAM, BR, IB, TM, DJN, SWE. We have no other conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Patient and public involvement section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Carman KL, Dardess P, Maurer M, et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff 2013;32:223–31. 10.1377/hlthaff.2012.1133 [DOI] [PubMed] [Google Scholar]
- 2. Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, et al. Patient and service user engagement in research: A systematic review and synthesized framework. Health Expect 2015;18:1151–66. 10.1111/hex.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Canadian Institutes for health research . strategy for patient oriented research: patient engagement framework. 2014. [Google Scholar]
- 4. Farrier CE, Stelfox HT, Fiest KM. In the pursuit of partnership: Patient and family engagement in critical care medicine. Curr Opin Crit Care 2019;25:505–10. 10.1097/MCC.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 5. Burns KEA, Jacob SK, Aguirre V, et al. Stakeholder engagement in trial design: Survey of visitors to critically ill patients regarding preferences for outcomes and treatment options during Weaning from mechanical ventilation. Ann Am Thorac Soc 2016;13:1962–8. 10.1513/AnnalsATS.201606-445OC [DOI] [PubMed] [Google Scholar]
- 6. Burns KEA, Misak C, Herridge M, et al. Patient and family engagement in the ICU. Untapped opportunities and underrecognized challenges. Am J Respir Crit Care Med 2018;198:310–9. 10.1164/rccm.201710-2032CI [DOI] [PubMed] [Google Scholar]
- 7. Vanstone M, Canfield C, Evans C, et al. Towards conceptualizing patients as partners in health systems: a systematic review and descriptive synthesis. Health Res Policy Syst 2023;21:1–14. 10.1186/s12961-022-00954-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sacristan JA, Aguaron A, Avendaño C, et al. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence n.d.;2016:631. 10.2147/PPA.S104259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res 2014;14:1–9. 10.1186/1472-6963-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burns KEA, McDonald E, Debigaré S. Patient and family engagement in patient care and research in Canadian intensive care units: a national survey. Can J Anesth/J Can Anesth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold DM, Donahoe L, Clarke FJ, et al. Bleeding during critical illness: A prospective cohort study using a new measurement tool. Clin Invest Med 2007;30:E93–102. 10.25011/cim.v30i2.985 [DOI] [PubMed] [Google Scholar]
- 12. Cook D, Guyatt G, Marshall J, et al. A comparison of Sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med 1998;338:791–7. 10.1056/NEJM199803193381203 [DOI] [PubMed] [Google Scholar]
- 13. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med Overseas Ed 1994;330:377–81. 10.1056/NEJM199402103300601 [DOI] [PubMed] [Google Scholar]
- 14. Krag M, Marker S, Perner A, et al. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med 2018;379:2199–208. 10.1056/NEJMoa1714919 [DOI] [PubMed] [Google Scholar]
- 15. Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 2015;41:833–45. 10.1007/s00134-015-3725-1 [DOI] [PubMed] [Google Scholar]
- 16. Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207–14. Available http://doi.wiley.com/10.1002/1097-0142(19810101)47:1<>1.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- 17. Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost 2010;8:2063–5. 10.1111/j.1538-7836.2010.03975.x [DOI] [PubMed] [Google Scholar]
- 18. IAP2 Spectrum of Public Participation . International Association for Public Participation. Louisville, CO: International Association for Public Participation, 2018. [Google Scholar]
- 19. Guyatt G, Montori V, Devereaux PJ, et al. Patients at the center: in our practice, and in our use of language. ACP J Club 2004;140:A11. 10.7326/ACPJC-2004-140-1-A11 [DOI] [PubMed] [Google Scholar]
- 20. Davis RE, Vincent CA, Murphy MF. Blood transfusion safety: the potential role of the patient. Transfus Med Rev 2011;25:12–23. 10.1016/j.tmrv.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 21. ClinicalTrials.gov . Patient important gastrointestinal bleeding in the ICU Clinicaltrials.Gov2022, 2022. Available: https://clinicaltrials.gov/ct2/show/NCT05506150?cond=patient+important+bleeding&draw=2&rank=1
- 22. ClinicalTrials.gov . Re-EValuating the inhibition of stress erosions (revise), 2017. Available: https://clinicaltrials.gov/ct2/show/NCT03374800
- 23. Tashakkori A, Teddlie C, Teddlie CB. Mixed methodology: combining qualitative and quantitative approaches: SAGE 1998.
- 24. Creswell JW, Fetters MD, Ivankova NV. Designing a mixed methods study in primary care. Ann Fam Med 2004;2:7–12. 10.1370/afm.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patton MQ. Qualitative evaluation checklist. Evaluation checklists project 2003;21:1–13. [Google Scholar]
- 26. Rapley T. Sampling strategies in qualitative research. The SAGE handbook of qualitative data analysis;2014:49–63. [Google Scholar]
- 27. Canadian critical care Trials Group. Canadian Critical Care Trials Group 2022. https://www.ccctg.ca/ [Google Scholar]
- 28. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893–907. 10.1007/s11135-017-0574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753–60. [DOI] [PubMed] [Google Scholar]
- 30. Golafshani N. Understanding reliability and validity in qualitative research. The qualitative report 2003;8:597–607. [Google Scholar]
- 31. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health 2000;23:334–40. Available http://doi.wiley.com/10.1002/1098-240X(200008)23:4<>1.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 32. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 33. Birt L, Scott S, Cavers D, et al. Member checking: a tool to enhance Trustworthiness or merely a NOD to validation? Qual Health Res 2016;26:1802–11. 10.1177/1049732316654870 [DOI] [PubMed] [Google Scholar]
- 34. Heddle NM, Cook RJ, Webert KE, et al. Methodologic issues in the use of bleeding as an outcome in transfusion medicine studies. Transfusion 2003;43:742–52. 10.1046/j.1537-2995.2003.00418.x [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Ge L, Ye Z, et al. Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: an updated systematic review and network meta-analysis of randomized trials. Intensive Care Med 2020;46:1987–2000. 10.1007/s00134-020-06209-w [DOI] [PubMed] [Google Scholar]
- 36. National Institute for health research . Patient and public involvement in health and social care research: a Handbook for researchers. 2018. [Google Scholar]
- 37. Patient Centered Outcomes Research Institute . Suggest a patient-centered research question, 2012. Available: https://www.pcori.org/engagement/engage-us/suggest-patient-centered-research-question
- 38. Crotty MJ. The foundations of social research: meaning and perspective in the research process. The foundations of social research;1998:1–256. [Google Scholar]
- 39. Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res 1999;34:5 Pt 2:1189–208. [PMC free article] [PubMed] [Google Scholar]
- 40. van Baarle FEHP, van de Weerdt EK, Suurmond B, et al. Bleeding assessment and bleeding severity in Thrombocytopenic patients undergoing invasive procedures. Transfusion 2020;60:637–49. 10.1111/trf.15670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: Observational study commentary: Varied preferences reflect the reality of clinical practice. BMJ 2001;323:1218. 10.1136/bmj.323.7323.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Little DHW, Robertson T, Douketis J, et al. Management of Antithrombotic therapy after gastrointestinal bleeding: A mixed methods study of Health‐Care providers. J Thromb Haemost 2021;19:153–60. 10.1111/jth.15111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-070966supp001.pdf (88.6KB, pdf)
bmjopen-2022-070966supp002.pdf (113.5KB, pdf)