Abstract
Objectives
Axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) may have a profound impact on sleep and health-related quality of life. The aim of this study was to assess sleep quality and quality of life and determine associated factors in patients treated with spondyloarthritides (SpA).
Methods
Cross-sectional questionnaire-based assessment of sleep behaviour, quality of life, functional impairment and depression (Regensburg Insomnia Scale, WHO Quality of Life questionnaire, Funktionsfragebogen Hannover questionnaire, Beck Depression Inventory II, Patient health questionnaire 9) and retrospective medical chart analysis of a monocentric cohort of 330 patients with SpA (n=168 PsA and n=162 axSpA).
Results
46.6% of patients with SpA demonstrated abnormal sleep behaviour. Linear regression models showed HLA-B27 positivity, Bath Ankylosing Spondylitis Disease Activity Index, depressive symptoms, functional capacity and disease duration to be predictive of insomnia symptoms in axSpA, respectively, depressive symptoms, female sex and Disease Activity Score 28 in patients with PsA. Patients with unrestful sleep had a significantly reduced health-related quality of life (p<0.001) as well as significantly more depressive symptoms (p<0.001). Satisfaction with health was rated significantly lower (p<0.001), indicating poor sleep as a burden on general well-being.
In particular, female patients had a significantly worse sleep quality with a prolonged sleep latency (p=0.009), increased sleep disturbances (p=0.014) and unrestful sleep (p<0.001) as well as a reduced physical and mental health-related quality of life (p=0.015, p<0.001) and more depressive symptoms (p=0.015).
Conclusion
Despite treatment, many patients with SpA demonstrate abnormal sleep behaviour with symptoms of insomnia and a reduced quality of life with significant differences between male and female patients. An interdisciplinary and holistic approach may be needed to address unmet needs.
Keywords: Arthritis, Psoriatic; Spondylitis, Ankylosing; Therapeutics; Patient Reported Outcome Measures
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with spondyloarthritis (SpA) may suffer from disturbed sleep.
Poor sleep may significantly affect the quality of life and patients with SpA view fatigue and sleep problems as important health concerns.
WHAT THIS STUDY ADDS
Patients with SpA continue to experience abnormal sleep behaviour despite treatment. This effect was especially pronounced in women. We identified the following factors associated with sleep disturbances: depressive symptoms, functional capacity, female sex and axial involvement.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Awareness of risk factors and sex differences will help to identify patients, who may benefit from additional interventions, such as patient education, exercise or light therapy or cognitive behavioural therapy techniques and, thus, improve patient-centric outcomes.
Introduction
Spondyloarthritides (SpA) may lead to functional impairment and disability, especially if treated insufficiently.1 Both, axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) may have a profound impact on sleep quality and health-related quality of life (HRQOL).2 3 Early awakening constitutes a hallmark of inflammatory back pain4 and pain and stiffness are often implicated in poor sleep in patients with SpA.5 In patients with PsA sleep disturbances may be further aggravated by skin involvement with itchiness and pain.6
Poor sleep may have a profound effect on QOL and earlier studies reported that patients with SpA consider sleep improvement a priority in treatment.2 7 It has furthermore been suggested that poor sleep may lead to reduced physical activity, which may in turn worsen pain and disease activity.8 Apart from pain, low mood or depression and anxiety have been implicated as factors contributing to poor sleep quality and sleep disturbances, both in patients with SpA as well as in the general population.9 Poor sleep may furthermore lead to daytime dysfunction, which may include fatigue or sleepiness with reduced energy and motivation, irritation, reduced concentration and memory impairment.10
Persistent sleep disturbances, usually defined as lasting >4 weeks, and associated daytime dysfunction constitute hallmarks of insomnia.11 The latest edition of the international classification of sleep disorders defines insomnia as ‘persistent difficulty with sleep initiation, duration, consolidation or quality that occurs despite adequate opportunity and circumstances for sleep, and results in some form of daytime impairment’.12 Insomnia has a prevalence of approximately 10% in the general population, depending on the employed diagnostic criteria,13 and thus constitutes a common problem and major healthcare burden.10 14 In particular, insomnia has been associated with a reduced physical health status, missed workdays and increased healthcare usage.15 16 The term psychophysiological (PI) insomnia refers to the concept of insomnia being perpetuated by both, psychological and physiological factors,17 18 underlining the importance of psychological factors such as stress, rumination and heightened focus on sleep or anxiety of insomnia in the establishment and perpetuation of insomnia.19
The pathophysiology of sleep disturbances in patients with SpA remains ill-defined and only few studies have investigated sleep behaviour and associated factors in SpA. The aim of this project was to assess sleep quality and behaviour as well as HRQOL in a monocentric cohort of 330 patients with SpA and identify factors associated with sleep disturbance.
Materials and methods
Patient cohort and data collection
Three hundred and thirty patients comprising 162 patients with axSpA and 168 patients with PsA were recruited from the Rheumatology outpatient clinic of the University Medical Center Freiburg, Freiburg, Germany, and assessed by survey. Patients with AxSpA had to fulfil the modified New York or Assessment of SpondyloArthritis international Society (ASAS) criteria, patients with PsA the Classification of Psoriatic Arthritis criteria. Patients with AxSpA were treated according to 2016 ASAS-EULAR recommendations for the treatment of axSpA,20 patients with PsA according to GRAPPA 2015 treatment recommendations.21 Patients were consented according to International Conference on Harmonisation Good Clinical Practice guidelines.
Patients were assessed by survey with a cross-sectional approach using the following questionnaires: To assess sleep behaviour the Regensburg Insomnia Scale (RIS) was used.18 This validated self-rating scale comprises 10 items assessing different dimensions of insomnia, that is, sleep quality and quantity, focus on sleep/insomnia and medication intake. The scale was developed to assess PI insomnia with a number of items focusing on psychological aspects of sleep disturbances. RIS ≥13 (distinct PI symptoms of insomnia), respectively, RIS ≥25 points (severe PI symptoms of insomnia) were chosen as cut-offs as described in the original publication.18
QOL was assessed with the help of the short version of the WHO Quality of Life questionnaire (WHOQOL-Bref).22 This questionnaire comprises 26 items, grouped into the four dimensions of physical HRQOL, mental HRQOL, social relationships and environment. Mental health and depressive symptoms were assessed using the Patient health questionnaire 9 (Phq-9) and Beck Depression Inventory II (BDI-II).23–25 Phq-9 was developed as a nine-item screening instrument for the diagnosis of depression. BDI-II constitutes the revised version of BDI, and is validated to assess the severity of depressive symptoms in adults. To assess functional capacity, Funktionsfragebogen Hannover questionnaire was used, which assesses the functional capacity in everyday life.26 Relevant functional impairment is defined herein as functional capacity <60%. Non-response at item-level for questionnaires was between 0.6% and 11.2%.
Survey data were combined with clinical data from retrospective medical chart analysis. The correct diagnosis was reconfirmed and demographic and clinical data on age, disease duration, height, body weight, body mass index (BMI), comorbidities and therapy as well as HLA-B27 status were extracted. Laboratory values (C-reactive protein, CRP) were collected from the current outpatient clinical visit.
Whenever possible, missing data were handled with a full analysis approach.
Statistics
Descriptive statistics including mean and SD or percentages were calculated to summarise the demographic and disease characteristics of the study cohort. To compare continuous variables between groups, Student’s t-test was used in case of normal distribution, and Welch’s t-tests in case of unequal variance. To compare categorical variables between groups, χ2 tests were used.
In order to determine factors independently associated with unrestful sleep/insomnia, multivariate linear and logistic regression analyses were employed. Multivariate models were calculated using stepwise backwards elimination after adjusting for demographic and disease factors as indicated. The statistical level of significance was set at p<0.05. All statistical calculations were performed using Jamovi V.2.0.0.0. (The jamovi project. jamovi. (V.2.2) (computer software). Retrieved from https://www.jamovi.org).27
Results
Patient cohort
A total of 330 patients with SpA (168 patients with PsA and 162 patients with axSpA) were included in this study. Of these, 147 (44.5%) were women and 183 (55.5%) men. Mean age was 57.4 years in the PsA and 49.0 years in the axSpA subgroup (p<0.001). Mean disease duration did not differ significantly between patients with PsA and axSpA (10.8 vs 11.8 years, p=0.343). Epidemiological data as well as affected disease domains are summarised in table 1.
Table 1.
Patients’ characteristics
| Total (n=330) | PsA (n=168) | axSpA (n=162) | P value | |
| Age, years, mean (SD) | 53.3 (14.0) | 57.4 (12.4) | 49.0 (14.3) | <0.001 |
| BMI, kg/m2, mean (SD) | 27.3 (6.15) | 27.4 (5.43) | 27.1 (6.92) | 0.652 |
| Smokers, n (%) (n=311) | 69 (22.2) | 27 (16.3) | 42 (29.0) | 0.007 |
| Male, n (%)/female, n (%) | 183 (55.5)/147 (44.5) | 89 (53.0)/79 (47.0) | 94 (58.0)/68 (42.0) | 0.356 |
| HLA-B27, n (%) (n=244) | 128 (52.5) | 23 (24.2) | 105 (70.5) | <0.001 |
| Disease domains: | ||||
| Axial involvement, n (%) | 220 (66.9) | 58 (34.7) | 162 (100) | <0.001 |
| Peripheral arthritis, n (%) | 273 (83.2) | 162 (96.4) | 111 (69.4) | <0.001 |
| Enthesitis, n (%) | 134 (40.7) | 73 (43.5) | 61 (37.9) | 0.305 |
| Dactylitis, n (%) | 84 (25.5) | 74 (44.0) | 10 (6.2) | <0.001 |
| Skin psoriasis, n (%) | 180 (54.5) | 160 (95.2) | 20 (12.3) | <0.001 |
| Nail involvement, n (%) | n.a. | 90 (53.6) | n.a. | n.a. |
| CRP, mg/L, mean (SD) (n=328) | 6.10 (11.7) | 5.38 (5.19) | 6.85 (15.8) | 0.255 |
| Disease duration, years, mean (SD) | 11.3 (9.29) | 10.8 (7.62) | 11.8 (10.7) | 0.343 |
| Functional capacity (FFbH), % (n=325) | 79.1 | 78.0 | 80.1 | 0.357 |
| Significant functional impairment (FFbH <60%), n (%) (n=325) | 55 (17.0) | 33 (20.2) | 22 (13.7) | 0.115 |
| BASDAI, mean (SD) (n=183) | 3.61 (2.31) | 2.91 (1.91) | 3.71 (2.36) | 0.113 |
| DAS28, mean (SD) | n.a. | 2.32 (0.91) | n.a. | n.a. |
| Comorbidities: (n=330) | ||||
| Inflammatory bowel disease | 16 (4.8) | 6 (3.6) | 10 (6.2) | 0.271 |
| Chronic lung disease, n (%) | 41 (12.4) | 13 (8.0) | 28 (16.7) | 0.017 |
| Chronic kidney disease, n (%) | 31 (9.5) | 12 (7.4) | 19 (11.3) | 0.224 |
| Cardiovascular disease, n (%) | 29 (8.9) | 22 (13.6) | 7 (4.3) | 0.005 |
| Diabetes mellitus, n (%) | 35 (10.6) | 14 (8.6) | 21 (12.5) | 0.255 |
| Malignancy (previous or current), n (%) | 27 (8.2) | 15 (9.3) | 12 (7.1) | 0.483 |
| Therapy: | ||||
| csDMARD, n (%) | 114 (34.8) | 84 (50.0) | 30 (18.5) | <0.001 |
| bDMARD, n (%) | 224 (68.1) | 102 (60.7) | 123 (75.9) | 0.003 |
| Anti-TNF, n (%) | 144 (43.8) | 54 (52.9) | 91 (74.0) | <0.001 |
| Anti-IL-17, n (%) | 66 (20.1) | 37 (36.3) | 30 (24.4) | 0.509 |
| Combination bDMARD+csDMARD | 72 (21.9) | 46 (27.4) | 26 (16.1) | <0.001 |
| Glucocorticoids, n (%) | 29 (8.9) | 15 (8.9) | 14 (8.6) | 0.983 |
| NSAID monotherapy, n (%) | 35 (10.6) | 10 (6.0) | 25 (15.4) | 0.005 |
| No current therapy, n (%) | 26 (7.9) | 18 (10.7) | 8 (4.9) | 0.052 |
The number (n) for individual items is given in the left column for parameters, for which data from individual patients were missing. P values refer to comparison of patients with PsA and axSpA.
axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARD, biological DMARD; BMI, body mass index; CRP, C-reactive protein; csDMARD, conventional synthetic disease modifying antirheumatic drug; DAS28, Disease Activity Score 28; DAS28, Disease Activity Score 28; FFbH, Funktionsfragebogen Hannover questionnaire; IL, interleukin; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; TNF, tumour necrosis factor.
Axial involvement occurred in 100% of patients with axSpA and 34.7% of patients with PsA, peripheral arthritis in 69.4% of patients with axSpA and 96.4% of patients with PsA and dactylitis in 6.2% versus 44% of patients with axSpA and PsA (all p<0.001). There were no significant differences in occurrence of enthesitis between the axSpA and PsA subgroups.
HLA-B27 status was available for 244 patients and was positive in 52.5% of patients, with a higher rate of HLA-B27 positivity in patients with axSpA compared with patients with PsA (70.5% vs 24.2%, p<0.001).
At the time of assessment, 75.9% of the patients with axSpA and 60.7% of the patients with PsA received a biological disease modifying antirheumatic drug therapy, while 18.5%, respectively, 50% of the patients with axSpA and PsA had a conventional synthetic DMARD therapy. 15.4% of the patients with axSpA and 6% of the patients with PsA were on non-steroidal anti-inflammatory drug monotherapy. 8.9% of patients received glucocorticoids with a mean daily dose of 6.05 mg.
Sleep behaviour
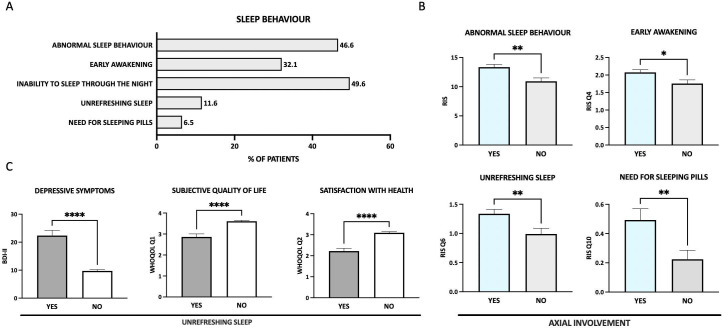
In this cohort of 330 patients with SpA, a total of 46.6% demonstrated abnormal sleep behaviour (RIS ≥13, see figure 1 and table 2). Patients with axSpA reached on average 13.1 points on the RIS, suspicious for PI insomnia, whereas patients with PsA reached on average 12.0 points (within the normal spectrum), though differences between both groups were not statistically significant (p=0.128). 4.8% of patients with PsA and axSpA had severe symptoms of PI insomnia (RIS ≥25, p=0.978).
Figure 1.
Sleep behaviour. (A) Overview of different items describing sleep behaviour. (B) Patients with axial involvement (light blue bars) show more abnormal sleep behaviour with early awakening, unrefreshing sleep and need for sleeping pills to fall asleep. (C) Patients with unrefreshing sleep (shown in dark grey) have more depressive symptoms, a worse subjective quality of life and a lower satisfaction with their own health. *p≤0.05; **p≤0.01; ***, p≤0.001; ****p≤0.0001. BDI-II, Beck Depression Inventory II; RIS, Regensburg Insomnia Scale; WHOQOL-Bref, WHO Quality of Life questionnaire.
Table 2.
Sleep, depressive symptoms and quality of life
| Total (n=330) | PsA (n=168) | axSpA (n=162) | P value | |
| RIS score, mean (SD) (n=313) | 12.5 (6.54) | 12.0 (6.5) | 13.1 (6.54) | 0.128 |
| RIS ≥13 (distinct PI symptoms of insomnia), n (%) | 146 (46.6) | 74 (44.0) | 72 (49.7) | 0.321 |
| RIS ≥25 (severe PI symptoms of insomnia), n (%) | 15 (4.8) | 8 (4.8) | 7 (4.8) | 0.978 |
| Sleep depth component, mean (SD) | 1.99 (0.91) | 1.92 (0.95) | 2.07 (0.86) | 0.147 |
| Sleep quantity component, mean (SD) | 0.91 (0.73) | 0,90 (0.74) | 0.92 (0.72) | 0.830 |
| Fearful focus on insomnia component, mean (SD) | 1.16 (1.19) | 1.10 (1.14) | 1.24 (1.25) | 0.281 |
| Hypnotics and daytime function component, mean (SD) | 1.08 (0.81) | 1.01 (0.74) | 1.16 (0.88) | 0.116 |
| Inability to sleep through the night most or all nights, n (%) | 154 (49.6) | 78 (47.0) | 76 (52.8) | 0.309 |
| Waking too early most or all nights, n (%) | 99 (32.1) | 47 (28.3) | 52 (36.6) | 0.120 |
| Need for sleeping pills most or all nights, n (%) | 20 (6.5) | 8 (4.8) | 12 (8.3) | 0.215 |
| Unrefreshing sleep most or all nights, n (%) | 36 (11.6) | 18 (10.9) | 18 (12.4) | 0.680 |
| WHOQOL-Bref: (n=327) | ||||
| Physical HRQOL, D1, mean (SD) | 60.5 (19.5) | 59.95 (19.32) | 61.12 (19.67) | 0.587 |
| Mental HRQOL, D2, mean (SD) | 67.7 (17.3) | 67.75 (16.85) | 67.62 (17.73) | 0.946 |
| Social QOL, D3, mean (SD) | 68.0 (19.7) | 67.19 (19.85) | 68.92 (19.53) | 0.429 |
| Environmental QOL, D4, mean (SD) | 77.4 (13.4) | 76.83 (12.68) | 77.96 (14.06) | 0.448 |
| Depressive symptoms: (n=307) | ||||
| BDI-II score, mean (SD) | 11.2 (9.4) | 11.51 (9.31) | 10.93 (9.52) | 0.591 |
| No depression, n (%) | 142 (46.0) | 74 (44.85) | 68 (47.89) | } 0.297 |
| Minimal or mild depression, n (%) | 115 (37.5) | 63 (38.18) | 52 (36.62) | |
| Moderate-to-severe depression, n (%) | 50 (16.3) | 28 (16.97) | 22 (15.49) |
The n number for individual items is given in the left column for parameters, for which data from individual patients were missing. P values refer to comparison of patients with psoriatic arthritis and axial spondyloarthritis.
BDI-II, Beck Depression Inventory II (see methods); D, domain; HRQOL, health-related quality of life; PI, psychophysiological; QOL, quality of life; RIS, Regensburg Insomnia Scale; WHOQOL-Bref, WHO Quality of Life questionnaire.
Both, patients with PsA and axSpA reached the highest scores in the sleep factor area of ‘poor sleep depth’, followed by ‘fearful focus on insomnia’ and ‘hypnotics and poor daytime function’ areas (see table 2). The mean reported sleep duration was 5–6 hours, with 12.9% of patients reporting sleeping 4 or less hours per night. Patients reported needing on average 20–40 min to fall asleep, whereas 6.47% had a sleep latency of >1 hour (normal range 10–20 min). 49.6% of patients reported not being able to sleep through the night during most nights, with 6.5% taking sleeping pills on a regular basis. 32.1% complained about waking up too early and 11.6% about feeling unrefreshed most mornings. 26.5% of patients indicated rarely or never feeling truly fit.
Compared with patients with PsA, patients with axSpA reached higher average scores for the item ‘I wake up too early’ (2.11 vs 1.84, p=0.038), otherwise there were no significant differences regarding sleep behaviour between axSpA and PsA in general. However, patients with axial involvement (axSpA/axial psoriatic arthritis (axPsA), n=162/58) reported significantly more symptoms of insomnia (p=0.002), awakening too early (p=0.016) and feeling unrefreshed in the morning (p=0.006) despite 74.9% receiving biological treatment (figure 1B). Furthermore, patients with axial involvement significantly more often reported taking sleeping pills (p=0.007). Both, insomnia symptoms and early awakening correlated significantly with BASDAI (Bath Ankylosing Spondylitis Disease Activity Index, p<0.001 each).
Patients on biological therapy had a shorter reported sleep duration (p=0.011) and felt on average less fit (p=0.049) than patients without biological therapy. Overall sleep behaviour, sleep latency, early awakening, however, did not differ significantly.
Depressive symptoms and QOL
Patients reporting unrestful sleep had significantly more depressive symptoms (p<0.001) as well as a highly reduced physical and mental HRQOL (both p<0.001). Satisfaction with health was rated significantly lower (p<0.001), indicating poor sleep as a significant burden on general well-being (figure 1C).
On average, patients reached a mean score of 11.2 points on the BDI-II scale, equivalent to minimal depression. 46% of patients did not show signs of depression, whereas 37.5% had minimal or mild depressive symptoms and 16.3% moderate-to-severe signs of depression. There were no significant differences between patients with PsA and axSpA.
Furthermore, QOL was assessed by WHOQOL-Bref questionnaire for the domains subjective QOL, physical and mental HRQOL, social relationships and environmental QOL. There were no significant differences between PsA and axSpA patients regarding QOL and satisfaction with health. Compared with published normal values28 patients with SpA, however, had a reduced physical and mental HRQOL, as well as a lower score in the social relationship domain, whereas environmental QOL was not affected. QOL was inversely correlated with measures of poor sleep quality, such as sleep latency and sleep disturbances (each p<0.001) as well as with signs of depression (p<0.001, figure 2).
Figure 2.
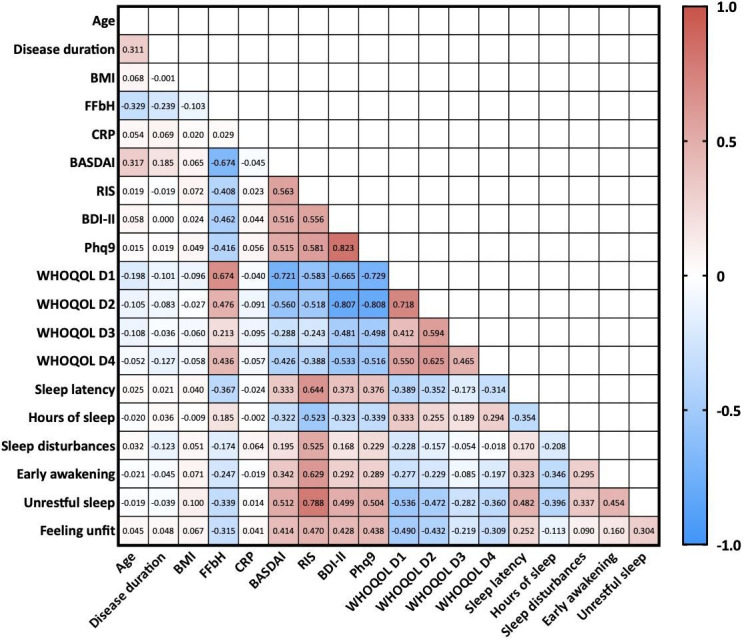
Pearson’s correlations between clinical data and parameters of sleep quality and quality of life. Numbers within the graph represent Pearson’s R values. Red colour indicates positive correlation, blue negative correlation. BASDI, Bath Ankylosing Spondylitis Disease Activity Index; BDI-II, Beck Depression Inventory II; BMI, body mass index; CRP, C-reactive protein; FFbH, Funktionsfragebogen Hannover questionnaire; Phq-9, Patient health questionnaire 9; QOL, quality of life; RIS Regensburg Insomnia Scale score; WHOQOL-Bref, WHO Quality of Life domain 1 (=physical health-related QOL), D2=mental health-related QOL, D3=social QOL, D4=environmental QOL.
Sex differences
In general, female patients reported significantly worse sleep than male patients (p<0.001), needing more time to fall asleep (p=0.009), not being able to sleep through the night (p=0.014) and feeling unrefreshed in the morning (p<0.001) as illustrated in figure 3. Furthermore, women demonstrated significantly more depressive symptoms (p=0.015) and also a significantly worse physical and mental HRQOL compared with male patients (p=0.015, respectively, p<0.001). In particular, moderate and severe depression tended to be more common in women than in men (p=0.054). Subjective overall QOL and satisfaction with health did not differ significantly. Despite similar disease duration (10.6 vs 11.9 years, p=0.209) and therapy, female patients furthermore had a significantly worse functional capacity (75.3% vs 85.2%, p=0.003).
Figure 3.
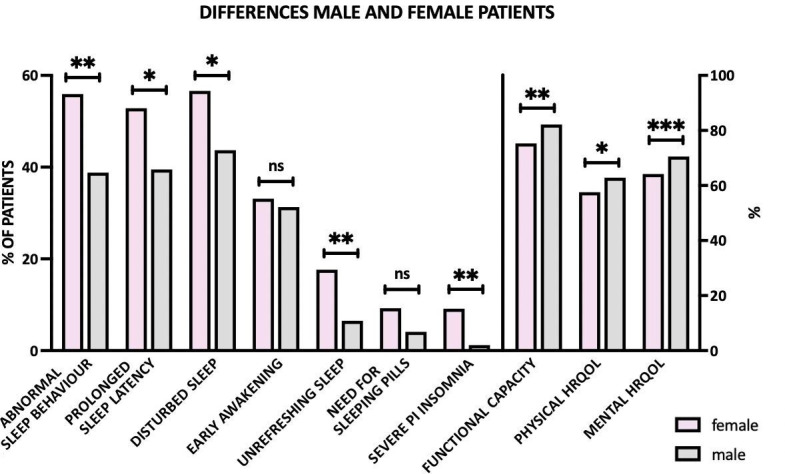
Sex differences in sleep behaviour, and quality of life. Female patients showed significantly more often abnormal sleep behaviour with prolonged sleep latency, disturbed sleep and unrefreshing sleep and had a significantly worse physical and mental health-related quality of life. ***p<0.001; **p<0.01; * <0.05; ns, not significant. HRQOL, health-related quality of life; PI, psychophysiological.
Female patients reached on average 14.2 points on RIS, suspicious for PI insomnia, whereas male patients reached on average 11.2 points (within the normal spectrum). In total, 9.1% of female patients showed signs of severe insomnia (RIS ≥25) compared with 1.2% of male patients (p=0.001). Women significantly more often indicated focusing on sleep and fear of insomnia (p<0.001, respectively, p=0.002), which constitute hallmarks of PI insomnia. Sex differences are also depicted in online supplemental table 1.
rmdopen-2022-002912supp001.pdf (53.7KB, pdf)
Factors associated with sleep disturbance
In order to identify factors associated with sleep disturbance, we stratified clinical data as well as questionnaire results by degree of sleep disturbance (RIS ≤12, ie, normal sleep behaviour; RIS 13–24, ie, abnormal sleep behaviour suggestive of insomnia; RIS ≥25, ie, symptoms of severe PI insomnia; table 3). We observed significant differences between the three groups for both axSpA and PsA regarding sex (p=0.0497, respectively, p=0.004), functional capacity/impairment (both p<0.0001 each), QOL (p<0.0001; p<0.0001; p=0.116, respectively, p=0.002; p=0.003, respectively, p<0.0001) and depressive symptoms (both p<0.0001): Female patients were significantly over-represented in the groups with insomnia symptoms. Furthermore, patients with higher RIS scores had a significantly worse functional capacity/more functional impairment, a worse QOL regarding physical and mental-HRQOL, social and environmental QOL as well as more depressive symptoms.
Table 3.
Stratification by RIS score
| RIS ≤12 | RIS 13–24 | RIS ≥25 | P value | |||||
| axSpA (n=73) | PsA (n=94) | axSpA (n=65) | PsA (n=66) | axSpA (n=7) | PsA (n=8) | axSpA | PsA | |
| Sex | ||||||||
| Male n (%) | 45 (61.6) | 59 (62.8) | 35 (53.8) | 29 (43.9) | 1 (14.3) | 1 (12.5) | 0.0497 | 0.004 |
| Female n (%) | 28 (38.4) | 35 (37.2) | 30 (46.2) | 37 (56.1) | 6 (85.7) | 7 (87.5) | ||
| Age in years, mean (SD) | 47.4 (14.6) | 57.7 (13.0) | 52.1 (13.9) | 57.0 (12.1) | 51 (14.4) | 57.5 (8.6) | 0.158 | 0.939 |
| Disease duration in years, mean (SD) | 12.0 (10.9) | 10.8 (7.5) | 12.0 (10.9) | 10.8 (7.8) | 6.7 (3.4) | 11.8 (7.9) | 0.445 | 0.942 |
| BMI, kg/m2, mean (SD) | 26.2 (5.6) | 27.0 (4.5) | 27.3 (5.1) | 28.0 (5.2) | 28.1 (5.0) | 24.2 (5.1) | 0.378 | 0.096 |
| Smokers, n (%) (n=311) | 20 (27.8) | 15 (16.3) | 19 (29.2) | 11 (16.7) | 2 (28.6) | 1 (12.5) | 0.982 | 0.955 |
| HLA-B27, n (%) (n=244) | 46 (68.7) | 11 (23.4) | 42 (70.0) | 12 (22.6) | 5 (83.3) | 1 (16.7) | 0.754 | 0.933 |
| Functional capacity (FFbH), % (n=325) | 86.3 | 84.5 | 75.9 | 71.3 | 61.1 | 58.0 | <0.0001 | <0.0001 |
| Significant functional impairment (FFbH <60%), n (%) (n=325) | 5 (6.9) | 8 (8.8) | 11 (16.9) | 20 (31.3) | 3 (42.9) | 5 (62.5) | 0.013 | <0.0001 |
| Disease domains: | ||||||||
| Axial involvement, n (%) | 73 (100.0) | 26 (28.0) | 65 (100.0) | 27 (40.9) | 7 (100.0) | 5 (62.5) | n.a. | 0.057 |
| Peripheral arthritis, n (%) | 47 (64.4) | 88 (93.6) | 47 (74.6) | 66 (100.0) | 7 (100.0) | 8 (100.0) | 0.092 | 0.086 |
| Enthesitis, n (%) | 24 (32.9) | 41 (43.6) | 26 (40.6) | 28 (42.4) | 5 (71.4) | 4 (50.0) | 0.116 | 0.919 |
| Dactylitis, n (%) | 6 (8.2) | 40 (42.6) | 1 (1.5) | 30 (45.5) | 2 (28.6) | 4 (50.0) | 0.011 | 0.881 |
| CRP, mg/L, mean (SD) (n=328) | 5.7 (6.6) | 5.7 (5.9) | 8.2 (23.6) | 4.9 (4.0) | 5.8 (6.2) | 5.2 (4.6) | 0.673 | 0.583 |
| Therapy: (n=329) | ||||||||
| csDMARD, n (%) | 14 (19.2) | 49 (52.7) | 14 (21.5) | 31 (47.0) | 2 (28.6) | 4 (57.1) | 0.821 | 0.730 |
| bDMARD, n (%) | 53 (73.6) | 56 (59.6) | 51 (78.5) | 40 (60.6) | 5 (71.4) | 6 (75.0) | 0.775 | 0.692 |
| Glucocorticoids, n (%) | 7 (10.0) | 5 (5.3) | 7 (10.8) | 10 (15.2) | 0 (0.0) | 0 (0.0) | 0.661 | 0.066 |
| NSAID monotherapy, n (%) | 14 (19.2) | 6 (6.4) | 9 (13.8) | 4 (6.1) | 1 (14.3) | 0 (0.0) | 0.692 | 0.764 |
| No current therapy, n (%) | 3 (4.2) | 10 (10.6) | 2 (3.1) | 8 (12.1) | 1 (14.3) | 0 (0.0) | 0.368 | 0.579 |
| WHOQOL-Bref: (n=327) | ||||||||
| Physical HRQOL, D1, mean (SD) | 71.0 (18.4) | 68.2 (15.9) | 52.2 (15.5) | 51.7 (17.8) | 39.3 (15.3) | 31.3 (10.1) | <0.0001 | <0.0001 |
| Mental HRQOL, D2, mean (SD) | 74.6 (16.5) | 74.2 (13.3) | 62.3 (15.9) | 61.8 (15.5) | 43.5 (11.8) | 41.2 (22.3) | <0.0001 | <0.0001 |
| Social QOL, D3, mean (SD) | 72.7 (19.5) | 71.1 (20.3) | 66.1 (17.0) | 63.9 (17.5) | 67.3 (22.9) | 49.0 (20.6) | 0.116 | 0.002 |
| Environmental QOL, D4, mean (SD) | 81.9 (13.1) | 80.6 (11.1) | 75.4 (14.4) | 72.8 (12.7) | 62.7 (12.1) | 65.2 (14.0) | 0.0003 | <0.0001 |
| Depressive symptoms: (n=307) | ||||||||
| BDI-II score, mean (SD) | 7.0 (8.0) | 7.6 (6.1) | 14.0 (8.9) | 15.2 (9.2) | 24 (10.4) | 26.5 (13.8) | <0.0001 | <0.0001 |
P values refer to comparison of three groups RIS ≤12, RIS 13–24 and RIS ≥25 by analysis of variance or χ2 test.
axSpA, axial spondyloarthritis; bDMARD, biological DMARD; BMI, body mass index; CRP, C-reactive protein; csDMARD, conventional synthetic disease modifying antirheumatic drug; D, domain; FFbH, Funktionsfragebogen Hannover questionnaire; HRQOL, health-related quality of life; n, number; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; QOL, quality of life; RIS, Regensburg Insomnia Scale; WHOQOL-Bref, WHO Quality of Life questionnaire.
To further determine factors predicting symptoms of insomnia, linear regression analyses were conducted using stepwise backwards elimination, with RIS score as the dependent variable. Separate multivariate models were calculated for the axSpA and PsA subgroups. For axSpA, HLA-B27 positivity, disease activity assessed by BASDAI score and depressive symptoms were positively associated with insomnia symptoms (RIS score), whereas functional capacity and disease duration showed a significant negative association with the RIS score. The model was significant, explaining 55.5% of observed variance in RIS scores, adjusted R2=0.51, F(11, 114)=12.92, p<0.001. In contrast, for PsA, depressive symptoms, female sex and Disease Activity Score 28 (DAS28) predicted symptoms of insomnia whereas functional capacity and axial involvement did not contribute significantly to the model and thus were removed. The model explained 41.5% of variance in RIS score, adjusted R2=0.36, F(9, 98)=7.73, p<0.001. The results for individual predictors are shown in the bottom panel of table 4. An additional model comprising the whole SpA cohort can be found in online supplemental table 2.
Table 4.
Multiple linear regression analysis of factors associated with RIS score
| Predictors | β | 95% CI for β | B | SE | P value | |
| axSpA | HLA- B27 positivity | 0.62 | 0.32 to 0.93 | 4.16 | 1.02 | <0.001 |
| BASDAI | 0.31 | 0.13 to 0.49 | 0.90 | 0.26 | <0.001 | |
| Functional capacity (FFbH) | −0.28 | −0.47 to −0.09 | −0.11 | 0.04 | 0.004 | |
| Depressive symptoms (BDI-II) | 0.26 | 0.09 to 0.42 | 0.18 | 0.06 | 0.002 | |
| Disease duration | −0.23 | −0.39 to −0.08 | −0.15 | 0.05 | 0.003 | |
| R2=0.555, adjusted R2=0.512, F(11, 114)=12.92, p<0.001* | ||||||
| PsA | Depressive symptoms (BDI-II) | 0.50 | 0.34 to 0.67 | 0.40 | 0.07 | <0.001 |
| Female sex | 0.44 | 0.10 to 0.79 | 2.93 | 1.15 | 0.014 | |
| DAS28 | 0.22 | 0.05 to 0.40 | 1.63 | 0.65 | 0.003 | |
| R2=0.415, adjusted R2=0.361, F(9, 98)=7.73, p<0.001† | ||||||
*Linear regression adjusted for age, sex, BMI, smoking status, as well as comorbidities (chronic lung disease, chronic kidney disease).
†Linear regression adjusted for age, disease duration, BMI, smoking status, as well as comorbidities (chronic lung disease, chronic kidney disease).
axSpA, axial spondyloarthritis; B, estimate; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BDI-II, Beck Depression Inventory II; BMI, body mass index; DAS28, Disease Activity Score 28; FFbH, Funktionsfragebogen Hannover questionnaire; PsA, psoriatic arthritis; β, standardised estimate.
Psychophysiological insomnia and associated factors
In addition, a binomial logistic regression model was used to determine predictors independently associated with severe PI insomnia (RIS ≥25) employing stepwise backwards elimination. The model was adjusted for demographic and disease-related factors as well as comorbidities (disease duration, HLA-B27 status, axial involvement, age, BMI, smoking status, chronic lung disease, chronic kidney disease). Female sex (OR 100.20, p=0.004), functional impairment (OR 16.83, p=0.020) as well as depressive symptoms (BDI-II score, OR 1.16, p=0.002) could be shown to be independently associated with severe insomnia (table 5).
Table 5.
Multivariate logistic regression analysis of factors associated with severe (psychophysiological) insomnia (Regensburg Insomnia Scale ≥25)
| Predictors | OR | 95% CI | B | P value |
| Female sex | 100.20 | 4.20 to 2390.13 | 4.61 | 0.004 |
| Significant functional impairment (FFbH <60%) | 16.83 | 1.55 to 182.59 | 2.82 | 0.020 |
| Depressive symptoms (BDI-II) | 1.16 | 1.06 to 1.28 | 0.15 | 0.002 |
Multivariable logistic regression adjusted for diagnosis, disease duration, HLA-B27 status, axial involvement, age, body mass index, smoking status, chronic lung disease, chronic kidney disease.
BDI-II, Beck Depression Inventory II; FFbH, Funktionsfragebogen Hannover questionnaire.
Discussion
Within this study we assessed sleep quality and behaviour as well as HRQOL in a monocentric cohort of 330 patients with SpA and determined factors associated with sleep disturbance.
Despite treatment in accordance with current disease management guidelines and 68.1% of patients in our cohort receiving biologics, many patients with SpA continued to have impaired sleep and a reduced HRQOL. Patients with axSpA and PsA in this study generally had significantly more PI symptoms of insomnia than published for healthy controls (mean RIS scores for healthy controls 5.91, respectively, 5.83 points).18 29 In patients with axSpA, HLA-B27 positivity, disease activity (BASDAI), depressive symptoms, functional capacity and disease duration were significant predictors of reported PI symptoms of insomnia, whereas in PsA depressive symptoms, female sex as well as disease activity (DAS28) were significantly associated with insomnia symptoms.
We otherwise did not detect any significant differences between patients with PsA and axSpA regarding sleep behaviour apart from patients with axSpA reporting early awakening more often, though patients with axSpA showed tendentially more insomnia symptoms. This is in line with Cano-García et al’s observations, who also described sleep disorders to be comparable between patients with PsA and axSpA with however slightly more insomnia in the axSpA group.30
However, patients with axial involvement (axSpA/axPsA) reported significantly worse sleep and axial involvement was a predictor of insomnia symptoms on regression analysis. Inflammatory back pain with awakening in the early morning hours constitutes a clinical hallmark of axSpA as well as axPsA.4 31 Nocturnal pain and awakening may contribute significantly to sleep disturbance.32 Pain has been associated with poor sleep efficiency, that is, reduced proportion of time actually spent asleep or time spent in bed attempting sleep.33 In chronic lower back pain, greater pain severity was furthermore associated with greater sleep disturbance.34 Interestingly, however, axial involvement was not a significant predictor of insomnia symptoms in the PsA subgroup. This may be explained by the fact that approximately half of patients with axPsA have asymptomatic axial disease.35 36
For axSpA but not for PsA we furthermore identified HLA-B27 positivity as a predictor of insomnia symptoms. HLA-B27 positivity has been associated with earlier age of disease onset as well as increased severity and persistence of MRI inflammation of the sacroiliac joints and spine.37 38 While some studies furthermore reported an association with early structural disease progression, other studies did not support this hypothesis.37–39 Limsakul et al reported HLA-B27 positive patients to have less spinal flexibility, and postulated that HLA-B27 status may be associated with disease severity.40 However, also regarding disease activity, data are heterogeneous with some studies describing HLA-B27 positive patients to have higher disease activity scores,41 while other studies described the opposite.42 (In our cohort, we did not observe a significant association between HLA-B27 status and BASDAI score). Thus, the exact relationship between HLA-B27 positivity and disturbed sleep remains to be further elucidated and this result should be confirmed in other cohorts. In the PsA subgroup, HLA-B27 positivity was not a significant predictor of insomnia symptoms, however, data should be interpreted with caution as HLA-B27 status had only been assessed in just under 60% of patients with PsA.
Both, in the axSpA as well as in the PsA cohort, disease activity (BASDAI, respectively, DAS28) was associated with disturbed sleep. An association of poor sleep with disease activity has been described before in axSpA cohorts5 and has also been confirmed by polysomnography.43 For PsA, especially tender joint count and pain had previously been described to be associated with poor sleep.44 45
Furthermore, we observed a higher rate of women with sleep disturbances, with sex differences especially pronounced regarding severe insomnia. Hultgren et al also described sleep disturbances to be more prevalent in female patients with SpA.46 In general, insomnia has been described to occur more frequently in women.47 Reasons are thought to be multifactorial. It has been proposed that socioeconomic, physical and psychosocial factors might play a role47: Women are likely to have a lower income, and often are the primary caregiver in the family, thus experiencing more socioeconomic stress.48 49 Regarding physical factors, women in our study had a worse functional capacity, which is in line with other studies published50 and was associated with insomnia in our multivariate regression model. Furthermore, fibromyalgia, which constitutes a common comorbidity both in axSpA and PsA, occurs more commonly in women and is itself associated with a poor sleep efficiency.51 An association of insomnia with female sex hormones has been postulated, but so far could not be confirmed.52 Regarding psychosocial factors, also depression and anxiety have a higher prevalence in women.53 Psychiatric disorders, and especially anxiety and depression, are known to increase the risk of insomnia,54 while vice versa insomnia has also been described to increase the risk of depression.55 In our study, women had higher rates of PI insomnia, which is determined by both psychological and physiological factors, with psychological factors such as anxiety and fear of insomnia perpetuating the occurring sleep disturbances even though the initial cause of insomnia has been removed.56 The concept of PI insomnia may thus partly explain high rates of sleep disturbances despite anti-inflammatory therapy.
Sleep disturbances have a significant impact on QOL, especially if they lead to daytime dysfunction. Female patients also described a reduced HRQOL compared with male patients and overall, patients with PsA and axSpA had a reduced QOL compared with published normal values.28 Furthermore, sleep disturbances have been reported to be associated with an altered pain threshold, which has been attributed to alterations in central pain processing.57 58 The underlying mechanisms as well as their interplay are complex, however, inflammatory mediators such as CRP as well as cytokines seem to play a crucial role. Elevated CRP has been associated with increased nociception59 as well as with worse sleep quality.9 Additionally, it has been implicated that sleep loss or sleep deprivation leads to increased production of proinflammatory mediators, such as interleukin (IL)-1β, IL-6, IL-17A, tumour necrosis factor (TNF)-α and CRP.60 Irwin et al described sex differences with greater cellular immune activation in women compared with men on sleep loss,61 which may partly explain the observed sex differences. Several studies have furthermore described an improvement of sleep after initiation of anti-TNF treatment.62 63 However, in recent years it has come to light that men may have a greater benefit from anti-TNF therapy than women,64 65 which may provide another explanation for our observed sex differences under therapy.
Additionally, Gossec et al recently described female patients with PsA to experience greater disease impact than men with greater pain, fatigue and disability or impairment despite similar disease activity and therapy,66 mirroring our data. Further studies will be needed to gain a better understanding of these sex differences, both, on a pathophysiological disease level as well as on the clinical level.
In general, the fact that despite therapy according to disease management guidelines, 46.6% of patients still showed symptoms of insomnia highlights a gap between experienced symptoms and issues on the one hand and the physician’s assessment and available therapies on the other hand. Coates et al recently came to a similar conclusion that, while disease modifying antirheumatic drug therapies effectively manage inflammation and disease progression, patients continue to experience an impaired HRQOL, highlighting an unmet need.67 An interdisciplinary or holistic approach may be needed to address these unmet needs in the care of both, patients with PsA and axSpA. Non-pharmacological interventions to improve sleep may include patient education about sleep hygiene, exercise such as physical therapy and balneotherapy, bright-light therapy and cognitive behavioural therapy techniques.9 68 Improving sleep may also have beneficial synergistic effects on functional status and pain. Both, QOL and sleep quality should therefore be assessed in a regular manner during follow-up visits in the patient with SpA population to identify patients who might benefit from additional non-pharmacological interventions to improve sleep quality and thereby QOL.
In conclusion, in this retrospective analysis we assessed sleep quality and associated factors in a real-world monocentric cohort of 330 patients with SpA. Despite treatment, many patients with PsA and axSpA had a reduced HRQOL and impaired sleep with significant differences between male and female patients. We identified multiple factors associated with abnormal sleep behaviour. Awareness of these risk factors will assist physicians to monitor at-risk patients and identify those patients who will benefit from additional interventions. An interdisciplinary and holistic approach may be needed to address unmet needs in the care of patients with PsA and axSpA.
Acknowledgments
We thank all patients who participated in this study.
Footnotes
Contributors: NV, REV and JT designed and supervised the study and gave critical input. ER, A-MK, SF, IJ, CG, NF and NV recruited and consented patients, provided clinical information and cared for the patients enrolled in this study. NF, ER, RL, ACV and NV performed data analysis and interpretation. NF and NV wrote the manuscript. All authors read and approved the final manuscript. NV is the guarantor of this study.
Funding: Parts of this study were financially supported by an unrestricted grant by Novartis Pharma GmbH, Germany.
Competing interests: NV: Speaker honoraria: AbbVie, Novartis, UCB, Bristol-Myers-Squibb, Pfizer; Advisory Boards: AbbVie, Novartis, UCB; Research grants: Bristol-Myers-Squibb, Novartis, Pfizer. JT: Speaker honoraria: GSK, BMS, Astra-Zeneca, Abbvie, UCB, Lilly; Advisory Boards: Novartis, GSK, Astra-Zeneca, Lilly. Grant/research support from: BMS, Novartis. RV: Speaker fees: AbbVie, Amgen, BMS, Boehringer-Ingelheim, GSK, Janssen-Cilag, Hexal, Novartis, Pfizer, Roche; Advisory boards: AbbVie, Amgen, Boehringer-Ingelheim, BMS, GSK, Janssen-Cilag, Hexal, Neutrolis, Novartis, Sanofi, Takeda; Unrestricted research grants: Amgen, BMS, Novartis, Pfizer. NF received travel grants from AbbVie, Janssen, Sobi.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
Ethics committee of the University of Freiburg, Germany, ethics protocols 190/17 and 37/17. Participants gave informed consent to participate in the study before taking part.
References
- 1.Braun J, Sieper J. Ankylosing Spondylitis. Lancet 2007;369:1379–90.:S0140-6736(07)60635-7. 10.1016/S0140-6736(07)60635-7 [DOI] [PubMed] [Google Scholar]
- 2.Ward MM. Health-Related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis & Rheumatism 1999;12:247–55. [DOI] [PubMed] [Google Scholar]
- 3.Ward MM. Quality of life in patients with Ankylosing Spondylitis. Rheum Dis Clin North Am 1998;24:815–27, 10.1016/s0889-857x(05)70043-0 [DOI] [PubMed] [Google Scholar]
- 4.Rudwaleit M, Metter A, Listing J, et al. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006;54:569–78. Available http://doi.wiley.com/10.1002/art.v54:2 10.1002/art.21619 [DOI] [PubMed] [Google Scholar]
- 5.Wadeley A, Clarke E, Leverment S, et al. Sleep in Ankylosing Spondylitis and non-radiographic axial Spondyloarthritis: Associations with disease activity, gender and mood. Clin Rheumatol 2018;37:1045–52. 10.1007/s10067-018-3984-7 [DOI] [PubMed] [Google Scholar]
- 6.Nowowiejska J, Baran A, Lewoc M, et al. The assessment of risk and predictors of sleep disorders in patients with Psoriasis—A questionnaire-based cross-sectional analysis. J Clin Med 2021;10:664. 10.3390/jcm10040664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiberg T, Lie E, van der Heijde D, et al. Sleep problems are of higher priority for improvement for patients with ankylosing spondylitis than for patients with other inflammatory arthropathies. Annals of the Rheumatic Diseases 2011;70:872–3. 10.1136/ard.2010.133793 [DOI] [PubMed] [Google Scholar]
- 8.Deodhar A, Gensler LS, Magrey M, et al. Assessing physical activity and sleep in axial Spondyloarthritis: Measuring the gap. Rheumatol Ther 2019;6:487–501. 10.1007/s40744-019-00176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Zhang S, Zhu J, et al. Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: a case-control study. Arthritis Res Ther 2012;14:R215. 10.1186/ar4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev 2010;14:379–89. 10.1016/j.smrv.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry 2019;18:337–52. Available https://onlinelibrary.wiley.com/toc/20515545/18/3 10.1002/wps.20674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International classification of sleep disorders . 3 rd ed. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 13.Ohayon MM, Reynolds CF. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International classification of sleep disorders (ICSD). Sleep Med 2009;10:952–60. 10.1016/j.sleep.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Léger D. Public health and insomnia: economic impact. Sleep 2000;23 Suppl 3:S69–76. [PubMed] [Google Scholar]
- 15.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract 2002;51:229–35. [PubMed] [Google Scholar]
- 16.Kuppermann M, Lubeck DP, Mazonson PD, et al. Sleep problems and their correlates in a working population. J Gen Intern Med 1995;10:25–32. 10.1007/BF02599573 [DOI] [PubMed] [Google Scholar]
- 17.Perlis ML, Giles DE, Mendelson WB, et al. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997;6:179–88. 10.1046/j.1365-2869.1997.00045.x [DOI] [PubMed] [Google Scholar]
- 18.Crönlein T, Langguth B, Popp R, et al. Regensburg insomnia scale (RIS): a new short rating scale for the assessment of psychological symptoms and sleep in insomnia; study design: development and validation of a new short self-rating scale in a sample of 218 patients suffering from insomnia and 94 healthy controls. Health Qual Life Outcomes 2013;11:65. 10.1186/1477-7525-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baglioni C, Spiegelhalder K, Lombardo C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev 2010;14:227–38. 10.1016/j.smrv.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 21.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and Psoriatic arthritis 2015 treatment recommendations for Psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. 10.1002/art.39573 [DOI] [PubMed] [Google Scholar]
- 22.WHODoMH . WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version 1996.
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kühner C, Bürger C, Keller F, et al. Reliability and validity of the revised Beck depression inventory (BDI-II. Results from German samples]. Nervenarzt 2007;78:651–6. [DOI] [PubMed] [Google Scholar]
- 25.Hautzinger M. [The Beck Depression Inventory in clinical practice]. Nervenarzt 1991;62:689–96. [PubMed] [Google Scholar]
- 26.Kohlmann T, Raspe H. Hannover functional questionnaire in ambulatory diagnosis of functional disability caused by backache. Rehabilitation 1996;35:I–VIII. [PubMed] [Google Scholar]
- 27.The jamovi project.: jamovi, 2021. Available: https://www.jamovi.org
- 28.Hawthorne G, Herrman H, Murphy B. Interpreting the WHOQOL-BrèF: preliminary population norms and effect sizes. Soc Indic Res. [Google Scholar]
- 29.Roloff T, Haussleiter I, Meister K, et al. Sleep disturbances in the context of neurohormonal dysregulation in patients with bipolar disorder. Int J Bipolar Disord 2022;10. 10.1186/s40345-022-00254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cano‐García L, Mena‐Vázquez N, Manrique Arija S, et al. Psychological factors associated with sleep disorders in patients with axial spondyloarthritis or psoriatic arthritis: a multicenter cross‐sectional observational study. J Clin Nurs 2021;30:266–75. 10.1111/jocn.15546 [DOI] [PubMed] [Google Scholar]
- 31.Sieper J, van der Heijde D, Landewé R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the assessment of spondyloarthritis International Society (ASAS). Ann Rheum Dis 2009;68:784–8. 10.1136/ard.2008.101501 [DOI] [PubMed] [Google Scholar]
- 32.Husak AJ, Bair MJ. Chronic pain and sleep disturbances: A pragmatic review of their relationships, Comorbidities, and treatments. Pain Med 2020;21:1142–52. 10.1093/pm/pnz343 [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Fu T, Wang Y, et al. Sleep disturbances in ankylosing spondylitis: a systematic review and meta-analysis. Psychol Health Med 2019;24:911–24. 10.1080/13548506.2019.1574357 [DOI] [PubMed] [Google Scholar]
- 34.Saravanan A, Tell D, Mathews H, et al. (121) pain, sleep disturbances, fatigue, mood changes, and underlying inflammation: a study of patients with chronic low back pain (CLBP). The Journal of Pain 2019;20:S6–7. 10.1016/j.jpain.2019.01.040 [DOI] [Google Scholar]
- 35.Queiro R, Belzunegui J, González C, et al. Clinically asymptomatic axial disease in Psoriatic Spondyloarthropathy. A retrospective study. Clin Rheumatol 2002;21:10–3. 10.1007/s100670200003 [DOI] [PubMed] [Google Scholar]
- 36.Chandran V, Barrett J, Schentag CT, et al. Axial Psoriatic arthritis: Update on a longterm prospective study. J Rheumatol 2009;36:2744–50. 10.3899/jrheum.090412 [DOI] [PubMed] [Google Scholar]
- 37.Chung HY, Machado P, van der Heijde D, et al. Hla-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis 2011;70:1930–6. 10.1136/ard.2011.152975 [DOI] [PubMed] [Google Scholar]
- 38.Arévalo M, Gratacós Masmitjà J, Moreno M, et al. Influence of HLA-B27 on the Ankylosing Spondylitis phenotype: Results from the REGISPONSER database. Arthritis Res Ther 2018;20:221. 10.1186/s13075-018-1724-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M, Xu M, Pan X, et al. Epidemiological comparison of clinical manifestations according to HLA-B*27 carrier status of Chinese ankylosing spondylitis patients. Tissue Antigens 2013;82:338–43. Available http://doi.wiley.com/10.1111/tan.2013.82.issue-5 10.1111/tan.12186 [DOI] [PubMed] [Google Scholar]
- 40.Limsakul N, Chiowchanwisawakit P, Permpikul P, et al. Younger age of Onset and uveitis associated with HLA-B27 and delayed diagnosis in Thai patients with axial Spondyloarthritis. Sci Rep 2021;11:13536. 10.1038/s41598-021-93001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Wang Y, Peng L, et al. Comparison of clinical features in HLA-B27 positive and negative patients with axial spondyloarthritis: results from a cohort of 4,131 patients. Front Med n.d.;7. 10.3389/fmed.2020.609562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbaum JT, Weisman MH, Hamilton H, et al. HLA-B27 is associated with reduced disease activity in axial Spondyloarthritis. Sci Rep 2021;11:12331. 10.1038/s41598-021-91829-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdulaziez O, Asaad T. Sleep problems in ankylosing spondylitis: polysomnographic pattern and disease related variables. The Egyptian Rheumatologist 2012;34:59–65. 10.1016/j.ejr.2012.02.001 [DOI] [Google Scholar]
- 44.Haugeberg G, Hoff M, Kavanaugh A, et al. Psoriatic arthritis: Exploring the occurrence of sleep disturbances, fatigue, and depression and their correlates. Arthritis Res Ther 2020;22:198. 10.1186/s13075-020-02294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W. Sleep disorders in patients with psoriatic arthritis and psoriasis. Rheumatology 2018;56:301–6. 10.5114/reum.2018.79501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hultgren S, Broman JE, Gudbjörnsson B, et al. Sleep disturbances in outpatients with ankylosing spondylitisa questionnaire study with gender implications. Scand J Rheumatol 2000;29:365–9. [DOI] [PubMed] [Google Scholar]
- 47.Zeng L-N, Zong Q-Q, Yang Y, et al. Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Front Psychiatry 2020;11:577429. 10.3389/fpsyt.2020.577429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lallukka T, Sares-Jäske L, Kronholm E, et al. Sociodemographic and socioeconomic differences in sleep duration and insomnia-related symptoms in Finnish adults. BMC Public Health 2012;12:565. 10.1186/1471-2458-12-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KA, Kryger MH. Women and sleep. Journal of Women's Health 2008;17:1189–90. 10.1089/jwh.2007.0574 [DOI] [PubMed] [Google Scholar]
- 50.Tournadre A, Pereira B, Lhoste A, et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res 2013;65:1482–9. 10.1002/acr.22001 [DOI] [PubMed] [Google Scholar]
- 51.Prados G, Miró E, Martínez MP, et al. Fibromyalgia: gender differences and sleep-disordered breathing. Clin Exp Rheumatol 2013;31:S102–10. [PubMed] [Google Scholar]
- 52.Morssinkhof MWL, van Wylick DW, Priester-Vink S, et al. Associations between sex hormones, sleep problems and depression: a systematic review. Neuroscience & Biobehavioral Reviews 2020;118:669–80. 10.1016/j.neubiorev.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 53.Piccinelli M, Wilkinson G. Gender differences in depression. British Journal of Psychiatry 2000;177:486–92. 10.1192/bjp.177.6.486 [DOI] [PubMed] [Google Scholar]
- 54.Johnson EO, Roth T, Schultz L, et al. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics 2006;117:e247–56. 10.1542/peds.2004-2629 [DOI] [PubMed] [Google Scholar]
- 55.Li L, Wu C, Gan Y, et al. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry 2016;16:375. 10.1186/s12888-016-1075-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.4th ed. ed2008Fisch BJ. Neurological aspects of sleep.
- 57.Lee YC, Chibnik LB, Lu B, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: A cross-sectional study. Arthritis Res Ther 2009;11:R160. 10.1186/ar2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee YC, Lu B, Edwards RR, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum 2013;65:59–68. 10.1002/art.37733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain States. Physiol Rev 2002;82:981–1011. 10.1152/physrev.00011.2002 [DOI] [PubMed] [Google Scholar]
- 60.Hurtado-Alvarado G, Pavón L, Castillo-García SA, et al. Sleep loss as a factor to induce cellular and molecular inflammatory variations. Clin Dev Immunol 2013;2013:801341. 10.1155/2013/801341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun 2010;24:54–7. 10.1016/j.bbi.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deodhar A, Braun J, Inman RD, et al. Golimumab reduces sleep disturbance in patients with active Ankylosing Spondylitis: Results from a randomized, placebo-controlled trial. Arthritis Care Res (Hoboken) 2010;62:1266–71. 10.1002/acr.20233 [DOI] [PubMed] [Google Scholar]
- 63.Rudwaleit M, Gooch K, Michel B, et al. Adalimumab improves sleep and sleep quality in patients with active ankylosing spondylitis. J Rheumatol 2011;38:79–86. 10.3899/jrheum.100213 [DOI] [PubMed] [Google Scholar]
- 64.Rusman T, Ten Wolde S, Euser SM, et al. Gender differences in retention rate of tumor necrosis factor alpha inhibitor treatment in Ankylosing Spondylitis: A retrospective cohort study in daily practice. Int J Rheum Dis 2018;21:836–42. 10.1111/1756-185X.13271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mease PJ, Gladman DD, Merola JF, et al. Potential impact of sex and BMI on response to therapy in psoriatic arthritis: post hoc analysis of results from the SEAM-PSA trial. J Rheumatol 2022;49:885–93. 10.3899/jrheum.211037 [DOI] [PubMed] [Google Scholar]
- 66.Gossec L, Walsh JA, Michaud K, et al. Women with psoriatic arthritis experience higher disease burden than men: findings from a real-world survey in the United States and Europe. J Rheumatol 2023;50:192–6. 10.3899/jrheum.220154 [DOI] [PubMed] [Google Scholar]
- 67.Coates LC, Orbai A-M, Azevedo VF, et al. Results of a global, patient-based survey assessing the impact of Psoriatic arthritis discussed in the context of the Psoriatic arthritis impact of disease (Psaid) questionnaire. Health Qual Life Outcomes 2020;18:173. 10.1186/s12955-020-01422-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Altan L, Bingöl U, Aslan M, et al. The effect of Balneotherapy on patients with Ankylosing Spondylitis. Scand J Rheumatol 2006;35:283–9. 10.1080/03009740500428806 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002912supp001.pdf (53.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request.